Abstract
Introduction
In 2020, the first Dutch West Nile virus (WNV) infected birds were detected through risk-targeted surveillance of songbirds. Retrospective testing of patients with unexplained neurological disease revealed human WNV infections in July and August 2020. Bird ringers are highly exposed to mosquito bites and possibly avian excrements during ringing activities. This study therefore investigates whether bird ringers are at higher risk of exposure to WNV and Usutu virus (USUV).
Methods
Dutch bird ringers were asked to provide a single serum sample (May – September 2021) and to fill out a survey. Sera were screened by protein microarray for presence of specific IgG against WNV and USUV non-structural protein 1 (NS1), followed by focus reduction virus neutralization tests (FRNT). Healthcare workers (2009–2010), the national immunity cohort (2016–2017) and blood donors (2021) were used as control groups without this occupational exposure.
Results
The majority of the 157 participating bird ringers was male (132/157, 84%) and the median age was 62 years. Thirty-seven participants (37/157, 23.6%) showed WNV and USUV IgG microarray signals above background, compared to 6.4% (6/94) in the community cohort and 2.1% (2/96) in blood donors (p < 0.01). Two seroreactive bird ringers were confirmed WNV or USUV positive by FRNT. The majority of seroreactive bird ringers travelled to EU countries with reported WNV human cases (30/37, 81%) (p = 0.07). No difference was observed between bird ringers with and without previous yellow fever vaccination.
Discussion
The higher frequency of WNV and/or USUV IgG reactive bird ringers indicates increased flavivirus exposure compared to the general population, suggesting that individuals with high-exposure professions may be considered to complement existing surveillance systems. However, the complexity of serological interpretation in relation to location-specific exposure (including travel), and antibody cross-reactivity, remain a challenge when performing surveillance of emerging flaviviruses in low-prevalence settings.
Keywords: Usutu virus, West Nile virus, Bird ringers, Antibody, IgG, One health
Highlights
-
•
23.6% of bird ringers had WNV and/or USUV IgG microarray signals above background.
-
•
This frequency of IgG signals was higher compared to general population controls.
-
•
Two bird ringers were confirmed positive for either USUV or WNV (FRNT).
-
•
Location of exposure is challenged by different ringing locations and travel.
1. Introduction
Emerging vector-borne viruses are rapidly increasing in incidence and geographic range, underlining the need for outbreak preparedness including targeted surveillance systems in regions at risk [[1], [2], [3]]. In August 2020, the first local detection of West Nile virus (WNV), in a common whitethroat (Curruca communis), was detected in the central region of the Netherlands [4]. A few months later this was followed by the first human WNV case without any recent travel history outside the country [5]. In addition, further investigation and retrospective analysis of cases with unknown neuroinvasive disease led to the detection of additional human WNV cases likely infected in the Netherlands already in July and August 2020 [6].
WNV infection is a zoonosis, and the flavivirus is transmitted in an enzootic cycle between Culex mosquitoes and birds. Following the bite of an infected mosquito, mammals can also become infected [7]. WNV was first isolated in Uganda in 1937 [8], and since then spread across the Northern Hemisphere in the past three decades [9,10]. WNV is now found on an annual basis in several European countries with the majority of cases reported in Southern-European countries. The majority of human infections (80%) remain asymptomatic while 20% of cases can develop symptoms such as headache and muscle pain i.e., West Nile fever (WNF). Although progression to severe neuroinvasive disease is rare (<1%), older and immunocompromised people have an increased risk of developing symptoms such as meningitis or encephalitis (West Nile neuroinvasive disease or WNND) [7,11]. Usutu virus (USUV) is a flavivirus closely related to WNV, and both belong to the Japanese encephalitis virus (JEV) serocomplex [12]. USUV was originally isolated from South Africa in 1959 and since then has been widely detected in Africa among mosquitoes and birds, and occasionally in humans [13,14]. 1996, USUV was introduced in Europe [15] where around 25 symptomatic human infections have been described in literature since 2008 [16,17]. Of these, the majority were documented cases of USUV-related neuroinvasive infection [15]. Although clinical manifestations of WNV and USUV seem to be similar [18,19], the detection of USUV cases is far less common [20]. USUV has been circulating extensively in birds in the Netherlands at least since 2016 [[21], [22], [23]], and in 2018, USUV-RNA and antibodies were also found in blood donor screenings in the Netherlands [20].
Considering that <1% of human WNV infections results in neurological manifestations, the few WNND cases detected in the Netherlands in 2020, may imply that a larger proportion of the population was likely infected that year. This showed the need to further investigate the potential human exposure in areas where positive WNV and/or USUV birds were found in order to assess the risk of human infection. This may strengthen our knowledge of local virus circulation, especially for viruses mainly characterized by mild or no disease including WNV and USUV.
In the Netherlands, animal surveillance is performed in order to monitor the presence of diseases, including zoonoses [4,24]. In particular, combined with bird ringing activities, wild birds are caught and sampled to detect the possible introduction and spread of emerging viruses such as arboviruses [4]. Bird ringers involved in these activities are likely a high-risk group for exposure to arboviruses: bird catching activities occur in the bird's natural habitat where the ringers are extensively exposed to mosquito bites at the same time of day mosquitoes are active (i.e., dusk and down), increasing their risk of infection with mosquito-borne viruses. In addition, some of them are also actively involved in sampling of birds and are therefore in direct contact with blood and other avian fluids. Although mosquito bites are the most common route of viral transmission for flaviviruses, WNV infections following percutaneous inoculations have also been reported in literature [25,26].
For these reasons, in this study, we aim to investigate the possible occupational exposure risk of bird ringers to WNV and USUV compared to the general population, as well as exploring their potential role to supplement current arbovirus surveillance. We therefore carried-out a cross sectional serological screening of bird ringers to determine their possible exposure to WNV and USUV following the WNV outbreak in 2020. Finally, this study may help raise awareness in risk groups and, if more at risk, enhance prevention measures.
2. Methods & materials
2.1. Study design, data collection and sample collection
This cross-sectional observational study started in May 2021. Bird ringers, who carried out their activities in the Netherlands during spring, summer or early autumn 2020 (when the first WNV cases in the Netherlands were found), were invited to participate by the Netherlands Institute of Ecology (Centre for Avian Migration and Demography). After informed consent, a single blood sample of 4 mL was drawn at the local laboratory and sent to Leiden University Medical Center (LUMC) (Leiden, the Netherlands) for serum sample processing, followed by transport to the Erasmus Medical Center (EMC) (Rotterdam, the Netherlands) for laboratory testing. In addition, participants were asked to fill out a questionnaire to provide information on demographic data, exposure at the bird ringing sites, travel history, previous vaccinations against arboviruses such as tick-borne encephalitis virus (TBEV), yellow fever virus (YFV) and Japanese encephalitis virus (JEV), previous infections with flaviviruses, and possible complaints compatible with WNF or WNND experienced between April and November 2020. Complaints compatible with WNF were defined as flu and fever with at least one of the following symptoms: headache, rash, muscle aches, vomiting or diarrhea (case definition 1). Complaints compatible with WNND were defined as inflammation of the brain or meninges or flaccid paralysis or other nervous system disorder diagnosed by a medical doctor (case definition 2). Definitions above were based on CDC (Centers for Disease Control and Prevention) description of WNV symptoms [11]. A summary of the questions and reply options from the survey is shown as supplemental information.
This study (number P20.112) was approved by the Medical Research Ethics Committee of Leiden, The Hague, Delft in the Netherlands.
Bird ringer serum samples were collected between June and September 2021. Date of serum sample arrival at LUMC has been used as a proxy for date of serum sample collection since the latter was not available for all participants. The date of sample collection used in the analysis was therefore 1 to 2 days after actual sampling (or unknown).
3. External comparators
Three external control groups were used as comparator:
1) Healthy healthcare workers from two hospitals in the South of the Netherlands (N = 58) that participated in a longitudinal influenza vaccination study from November 2009 to June 2010 [27], when no WNV and/or USUV exposure was expected since the first Dutch USUV and WNV detections were detected in 2016 and 2020, respectively; 2) a population representative sample of participants from a national immune surveillance study with sera collected in 2016–2017 (N = 94, PIENTER-3 study, National Institute of Public Health) [28]; 3) Dutch age- and sex-matched blood donors (Sanquin) with sera collected in September 2021 representative for all 12 provinces in the Netherlands (N = 96, 8 per province).
Control group 1 was used as negative control and control groups 2 and 3 were used to estimate WNV and USUV seroprevalence in the general Dutch population between 2016 and 2021.
The healthcare worker influenza vaccination study was approved by the Medical Ethical Review Committee of the St. Elisabeth Hospital, Tilburg, the Netherlands and the Medical Ethical Review Committee of the University Medical Centre, Utrecht, the Netherlands [27]. The PIENTER-3 study was approved by the Medical Ethical Review Committee (METC), Noord-Holland, the Netherlands. Following Sanquin's ethical guidelines, Sanquin provided anonymized donor samples, originating from donors who permitted the use of the samples for research purposes.
4. Protein microarray
Collected serum samples were tested in the Laboratory for Virology at Erasmus University Medical Center for the presence of WNV and USUV specific IgG antibodies using a protein microarray. Protein microarray was performed as previously described in detail with a few modifications [29,30]. Slides were printed with WNV (Sino Biological) and USUV non-structural proteins 1 (NS1) (The Native Antigen company) proteins as well as JEV and TBEV NS1 (Immune Technology) to assess flavivirus antibody cross-reactivity. NS1 is considered less cross-reactive and previously showed limited antibody cross-reactivity in this protein microarray [30]. The optimal antigen concentrations were determined by checkerboard titration using positive confirmed (PCR and/or neutralization test) control sera of WNV, USUV and TBEV and were standardized between different batches of slides [29]. Positive control sera (including WNV, USUV and TBEV) were also taken along each test slide to monitor and correct for possible slide to slide variation. As a positive test control, we included the nucleoprotein (NP) influenza A antigen (H7N9, 2013, anhui, Sino Biological) that is influenza virus antibody cross-reactive and therefore can detect antibodies against most influenza virus subtypes. Since previous Influenza exposures are expected in all Dutch individuals of 18 years and older, the H7N9 NP antigen signals can be used as a process control. Slides were incubated in Blocker™ Blotto blocking buffer in TBS (Thermo Scientific) to prevent non-specific binding. For IgG antibody detection, slides were incubated with four-fold serially diluted sera ranging from 1:20 to 1:1280. IgG binding was detected by incubation with Alexa Fluor® 647 conjugated goat anti-human IgG-Fcγ (Jackson Immunoresearch). Slides were washed with PBS 0.05% TWEEN® 20 washing buffer (Sigma Aldrich) between incubation steps and signals were measured using the Tecan PowerScanner™ (IgG; 647 nm). Fluorescent intensity of individual spots was analyzed using ScanArray® Express software and the mean intensity of the fluorescent signals of two identical protein spots was calculated. For IgG, the fluorescent signals of the dilutions tested were used to calculate the half maximal effective concentration titers [29] using RStudio software, version 2022.12.0 [31]. Negative samples (< titer 20) were set to a titer of 10 in all figures. The USUV and WNV NS1 maximum titers of the negative control panel were used to determine the IgG reactive cut-off, which was set at a titer of ≥20.
4.1. Focus reduction neutralization test (FRNT)
To confirm protein array reactivity, serum samples from the bird ringer participants and blood donors (Sanquin) with signals for WNV and USUV NS1 were tested for the presence of neutralizing antibodies against WNV lineage 2 (B956, NCPV Porton Down #638, 2010) and USUV (Africa-3, Merula Turdus NL isolate) by FRNT as described with some modifications [32] As negative controls, 10 sera of IgG negative bird ringers with a YFV and/or TBEV vaccination history or without prior arbovirus vaccination, were also tested by FRNT. Briefly, sera were heat-inactivated for 30 min at 56 °C. sera were 2-fold serially diluted in Dulbecco modified Eagle medium (Lonza, LO BE12-733F) supplemented with NaHCO3 (Lonza, LO BE17-613E), HEPES buffer (Sartorius, BEBP17-737E), Penicillin/Streptomycin (Pen/Strep) (Capricorn, CA PS-B), L-glutamin (Capricorn, CA GLN-B), and 3% fetal bovine serum (Sigma Aldrich, F7524–500 mL) starting at a dilution of 1:10 in 60 μL.Thereafter, 60 μL of virus suspension (800 plaque forming units (FFU) (based on 24 h titrations) were added to each well. The end concentration of each well is 400 FFU in 120 μL. Plates were incubated for 1 h at 37 °C. Next, 100 μL of virus and serum mix was added to confluent monolayers of Vero cells (ATCC CCL-81) for USUV and WNV. USUV and WNV infected plates were incubated for 24 h at 37 °C before fixing in 4% paraformaldehyde (PFA) and permeabilizing in 70% ethanol. For staining, plates were treated with Triton X-100 in PBS (0,5% v/v, Merck, T8787-50ML) by adding 100 μL to the wells, and plates were incubated for 10 min at 37 °C. 100 μL of Blocker blotto in TBS (Life Technology, 37530) was subsequently added to the wells and incubated for 30 min at 37 °C. The cells were stained with polyclonal mouse anti-USUV NS1 antibody (1:10000, MyBioscource, MBS569354_1mg) or anti-WNV NS1 antibody (1:4000, IC12) (The Native Antigen Company, MAB12160–100) diluted in Blocker blotto, followed by secondary antibody staining with goat anti-mouse IgG(H + L) cross-adsorbed horseradish peroxidase (HRP) (1:6000, Invitrogen, A16072). After and in between primary and secondary antibody stainings, cells were incubated for 1 h (37 °C, 5% CO2) and washed with PBS. 50 μL of TrueBlue Peroxidase Substrate (KPL TrueBlue, 5510–0030, Seracare) was added to the wells and incubated in the dark at room temperature for 5–10 min. Plates were washed with PBS, air-dried and scanned by the CTL Immunospot scanner (S6 Ultimate-V Analyzer, CTL Analyzers LCC). The FRNT titer was calculated based on a 70% or greater reduction in infected cells counts. A reciprocal titer of ≥1:80 and a ≥ 4-fold difference between the FRNT titers of USUV and WNV was considered as a positive result. This was based on validation with testing PCR confirmed WNV and USUV sera (Table S1 and S2) as well as confirmed (PCR, VNT and/or EIA) ZIKV, JEV, DENV and TBEV sera to assess cross-reactivity (specificity: 87% (WNV), 92% (USUV); sensitivity: 95% (WNV), 80% (USUV); calculations are based on using a reciprocal titer of ≥1:80 as cut-off to determine true positives, false positives, true negatives and false negatives).
4.2. Serological interpretation
Bird ringer participants were considered confirmed positive when detected IgG protein array signals above background (negative control group) were confirmed by a positive FRNT result (with negative results for or 4-fold difference with either USUV or WNV). Samples were considered as possibly exposed to WNV when FRNT results were negative, but protein array WNV NS1 IgG signals are detected above background with no cross-reaction to or 4-fold difference with either USUV, JEV or TBEV. In the same way, participants with USUV NS1 protein array signals only were considered as possible exposure to USUV. Samples with cross-reactive protein array signals (< 4-fold difference) were given the possible flavivirus exposure interpretation.
4.3. Statistical and descriptive analysis
Descriptive statistics were used to summarize the characteristics of bird ringers and the serology results. Age, sex and USUV and/or WNV IgG signal results were compared to those of the different control groups using chi-square test and t-test for categorical and continuous variables, respectively.
Among bird ringers, we further assessed the rate of confirmed positive (by PRNT) or possibly exposed (to WNV, USUV or other flaviviruses) in relation to the microarray reactivity results. In addition, we explored time-bound antibody patterns using descriptive analysis.
Next, we explored risk factors for antibody reactivity and confirmed positive for WNV or USUV among bird ringers. Exposure included both local bird ringer activities and possible travel related exposure. Possible travel related exposure was based on visited countries and country specific information from ECDC reports [33] on locally-acquired WNV human infections. In order to assess differential exposure between bird ringers with positive and negative microarray signals, we used t-test for continuous variables and chi-square or Fisher's exact test, as appropriate, for categorical variables. Due to the small size of the different serological interpretation groups (WNV, USUV, flavivirus), only descriptive analysis was performed.
Analyses were performed using SPSS statistics version 25. All figures were made using RStudio [31] (version 2022.12.0, packages: ggpubr, ggplot2, reshape2, sp, sf, rgdal, dplyr, tidyr, raster) or QGIS mapping software.
5. Results
5.1. Study characteristics
From 28 May 2021 to 30 June 2021, Dutch bird ringers with an active license in 2020 (n = 580) were informed about the study, and 163 were interested in participating. Of the 163 bird ringers, one was excluded since inclusion criteria were not met. Out of 162 participants, 157 provided a serum sample and filled out the questionnaire (Fig. S1).
Of the 157 bird ringers, 151 (96.2%) fully completed the survey, five (3.2%) filled out 97% of the questions and one 39%. Between March and November 2021, the months with the highest proportion of active bird ringers were May, June and July, when 87.9%, 91.1% and 82.8% of participants reported to have performed ringing activities, respectively (Table S3). The majority (70%) reported to spend ≥4 h outdoors per day when engaging in bird ringing activities (Table 1 and S4). Out of 157 participants, 42 (26.8%) were involved in blood sampling of birds. Regarding mosquito bite preventive measures, 63.7% reported to never use mosquito repellent while ringing, but more than half (51.6%) reported to use other preventive measures, such as long sleeves always or most of the times. Previous vaccination against TBEV, JEV and YFV was reported by 8, 3 and 54 bird ringers, respectively. In total 124 reported to have travelled outside the Netherlands in the previous 5 years (79%) (Table 1 and S4). In particular, 108 bird ringers reported to have visited EU countries with reported locally-acquired WNV human infections (Table 1) [33]. Fifteen bird ringers reported to have experienced symptoms compatible with the case definition 1 between April and November 2020. However, three of them reported SARS-CoV-2 infections and two had different diagnoses unrelated to possible arboviral infection.
Table 1.
Characteristics of the bird ringers included in the study (N = 157).
| Bird ringers (N = 157) | Frequency 157 |
Percentage 100 |
|---|---|---|
| Socio-demographics | ||
| Men | 132 | 84.1 |
| Women | 25 | 5.9 |
| Median age in years (min-max) | 62 (25–84) | |
| Activities |
Frequency 156 |
Percentage 100 |
| Hours spent for bird ringing activities each time | ||
| 1–2 h | 12 | 7.7 |
| 2–3 h | 35 | 22.4 |
| >4 h | 109 | 69.9 |
| Blood sampling |
Frequency 157 |
Percentage 100 |
| Yes | 42 | 26.8 |
| No | 115 | 73.2 |
| Visiting Utrecht province for bird ringing activities and/or outdoor activities | ||
| Yes | 50 | 31.8 |
| No | 107 | 68.2 |
| Mosquito nuisance and mosquito bite preventive measures | ||
| Use of mosquito repellent | ||
| Always/Most of the times | 9 | 5.7 |
| Sometimes | 25 | 15.9 |
| Rarely/Never | 123 | 78.3 |
| Use of other mosquito bite preventive measures (e.g. long sleeves) | ||
| Always/Most of the times | 81 | 51.6 |
| Sometimes | 19 | 12.1 |
| Rarely/Never | 57 | 36.3 |
| High mosquito nuisance experienced while ringing |
Frequency 156 |
Percentage 100 |
| Yes | 33 | 21.2 |
| No | 123 | 78.8 |
| Vaccination status |
Frequency 156 |
Percentage 100 |
| Tickborne encephalitis vaccination | ||
| Yes | 8 | 5.1 |
| No | 146 | 93.6 |
| I do not remember | 2 | 1.3 |
| Japanese encephalitis vaccination | ||
| Yes | 3 | 1.9 |
| No | 150 | 96.2 |
| I do not remember | 3 | 1.9 |
| Yellow fever vaccination | ||
| Yes | 54 | 34.6 |
| No | 96 | 61.5 |
| I do not remember | 6 | 3.8 |
| Travelling outside the Netherlands in the previous 5 years |
Frequency 156 |
Percentage 100 |
| Yes | 124 | 79.5 |
| No | 32 | 20.5 |
| Travel history | ||
| Europe | 114 | 73.1 |
| European countries with reported locally-acquired WNV human infections | 108 | 69.2 |
| United States | 18 | 11.5 |
| South America | 10 | 6.4 |
| Central America and the Caribbean | 12 | 7.7 |
| North and West Africa | 15 | 9.6 |
| East Africa | 8 | 5.1 |
| South Africa | 19 | 12.2 |
| West Asia | 10 | 6.4 |
| East Asia and South East Asia | 16 | 10.3 |
| South Asia | 8 | 5.1 |
| Australia | 4 | 2.6 |
| Pacific Islands | 4 | 2.6 |
The majority of the study population was male (84%) and the median age was 62 years (range 25–84 years) (Table 1). The mean age of bird ringers was higher compared to the general population representative and healthcare worker control groups (Table 2).
Table 2.
Characteristics of the control groups, i.e., Sanquin blood donors, general population (PIENTER) and negative healthcare worker panel.
| POPULATION |
BIRD RINGERS |
SANQUIN blood donors |
PIENTER |
HEALTHCARE WORKERS |
|||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | P Value | N (%) | P Value | N (%) | P Value | |
| Men | 132 (84.1) | 80 (83.3) | 0.88 | 49 (52.1) | <0.01⁎ | – | – |
| Age (mean) | 59.8 | 57.7 | 0.22 | 44.7 | <0.01⁎ | 41.1 | <0.01⁎ |
| USUV and/or WNV IgG signal | 37 (23.6) | 2 (2.1) | <0.01⁎ | 6 (6.4) | <0.01⁎ | 0 (0) | <0.01⁎ |
| Total | 157 | 96 | 94 | 58 |
= statistically significant results; p-values have been rounded to two decimal places.
5.2. WNV and USUV seroprevalence
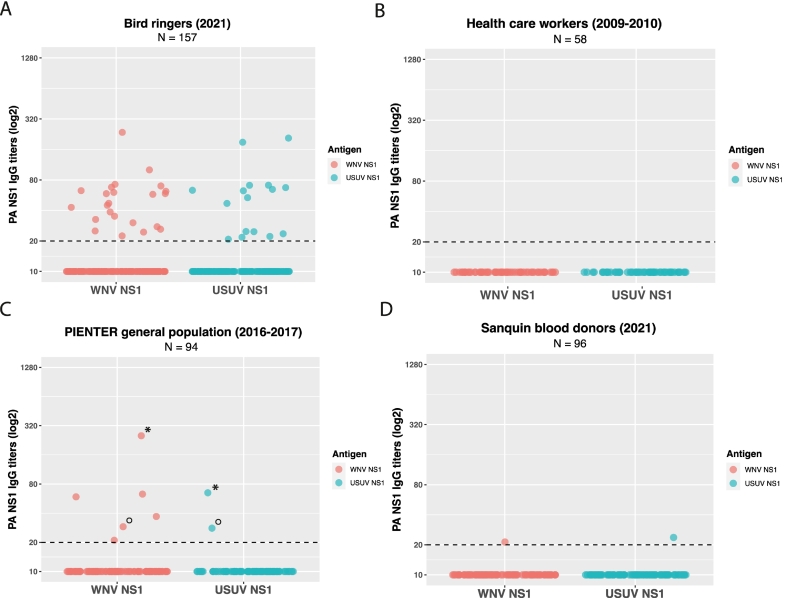
Out of 157 bird ringers, 37 participants (24%) showed USUV and/or WNV IgG signals above background (negative panel) on the protein array (Fig. 1A and B), of which the majority had specific-antibodies binding either USUV NS1 (12/37, 32.4%) or WNV NS1 (21/37, 56.8%) (Fig. S2). Three out of 37 participants had antibodies binding to two or three different flavivirus NS1 proteins, with the highest titer for USUV (1.9 to 2.9-fold difference) compared to the other signals (Fig. S2). One participant had cross-reactive antibodies binding almost equally to JEV and USUV NS1 (Fig. S2). The observed range of titers of USUV and/or WNV reactive bird ringers is similar to protein array WNV and USUV NS1 titers seen in sera of PCR confirmed blood donors, especially in blood donor sera approximately 200 days or more after infection (Table S1 and S2).
Fig. 1.
.IgG protein microarray signals of bird ringers and control groups.
WNV and USUV NS1 protein microarray IgG titers (log2 scale) for A) Bird ringers (N = 157, 2021), B) Health care workers (negative panel, N = 58, 2009–2010), C) Dutch general population survey (PIENTER, N = 94, 2016–2017), * and ° symbols indicate the same participants, and D) Dutch blood donors (Sanquin, N = 96, 2021).
Bird ringers had USUV and WNV binding IgG signals (37/157, 23.6%) more often compared to controls from the national immune surveillance PIENTER survey (6/94, 6.4%; p < 0.01) as well as the age- and sex-matched blood donor controls (2/96, 2.1%; p < 0.01) (Fig. 1A, C and D and Table 2). Of the 6 IgG signals found in the controls from the PIENTER surveillance survey, four were solely reactive with WNV NS1, and two showed binding to both WNV NS1 and USUV NS1 (Fig. 1C and Table 2). In blood donors, two IgG signals were reactive with either WNV NS1 or USUV NS1, although both signals were just above cut-off (Fig. 2D and Table 2).
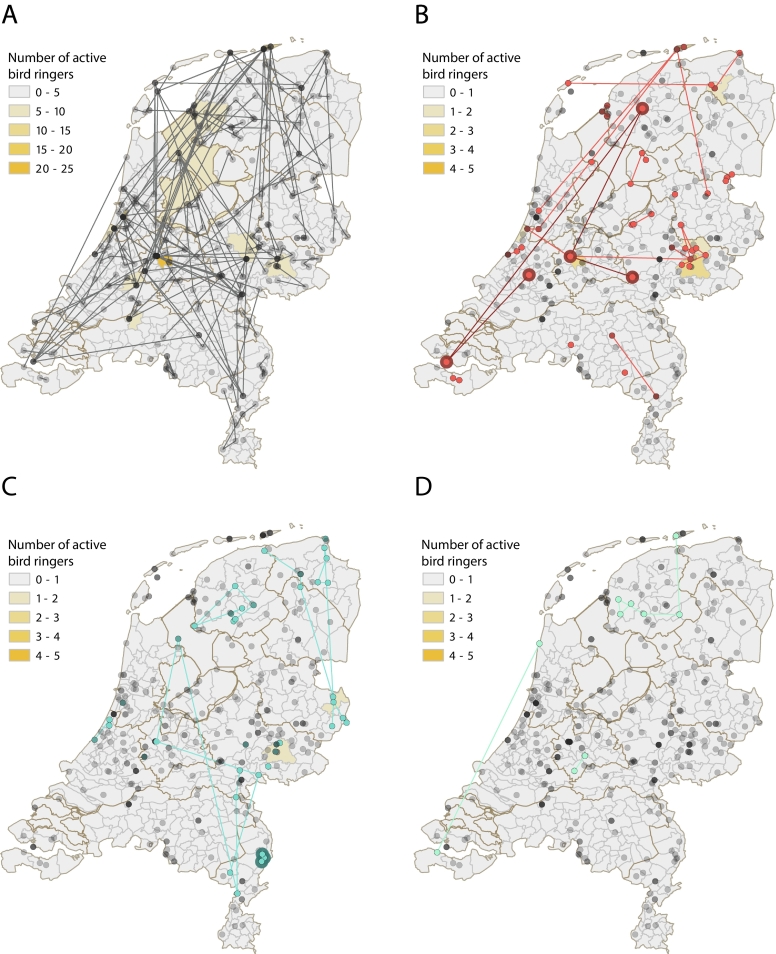
Fig. 2.
.Locations of bird ringing activities in the Netherlands.
The color gradient (yellow) shows the number of active bird ringers per municipality of each exposure group and lines connect active bird ringing locations per bird ringer in the Netherlands, shown for A) All included participants (N = 157, grey), B) Possible (red) and confirmed (red with dark red encircled) WNV exposed bird ringers (N = 21), C) Possible (blue) or confirmed (blue with dark blue encircled) USUV exposed bird ringers (N = 13) and D) Possible flavivirus exposed bird ringers (N = 3, turquoise).
Two protein array reactive bird ringers had USUV or WNV neutralizing antibodies and therefore were confirmed positive for USUV or WNV infection, respectively (Fig. S2). A set of IgG negative bird ringer sera (negative controls), with or without prior arbovirus vaccination, were all FRNT negative or below cut-off (Table S5). The remaining 35 bird ringers with IgG protein array signals but negative or below cut-off in neutralization assays, were considered as possibly exposed to a specific flavivirus, or – in case of reactivity to more than one antigen (< 4-fold difference) – flavivirus in general (Fig. S2, for details see Methods). The USUV IgG reactive blood donor was confirmed positive for USUV by FRNT (Table S6).
WNV as well as USUV and flavivirus possible or confirmed exposures were found throughout the study period (Fig. S4). Among the participants seroreactive to WNV (WNV possible or confirmed exposure group), 61.9% (13/21) were sampled before mid-July and 38.1% (8/21) were sampled from the second half of July to September. This can also be seen in Fig. S5, showing a left-sided distribution and a median in the first half of July. Concerning USUV possible or confirmed exposures, only a few were found from June to the first half of July (3/12 25%), and 9 were found after this period (9/12, 75%) (Fig. S4 and 5). Both the confirmed WNV and USUV cases were found at the end of July (Fig. S4 and 5).
5.3. Possible risk factors and descriptive analysis of ringing locations and travel history
No significant differences in risk factor exposure between IgG positive and negative bird ringers have been found (Table 3). This included no observed difference between the percentage of vaccinated bird ringers against TBEV and YFV among the negative bird ringers (TBEV: 5.9%, YFV: 35.3%) compared to those with WNV and/or USUV IgG signals (TBEV: 2.7%, YFV: 32.4%) (Table 3). Comparing the travel history of bird ringers with and without possible or confirmed arbovirus exposure, a higher proportion of reactive bird ringers (30/37, 81%) travelled to EU countries with reported WNV human cases compared to the USUV and WNV negative bird ringers (78/119, 65.5%) although this was not statistically significant (p = 0.07) (Table 3).
Table 3.
Comparison between IgG positive and negative participants.
| WNV and/or USUV IgG positive N (%) 37 (23.6) |
WNV and/or USUV IgG negative N (%) 120 (76.4) |
P value | |
|---|---|---|---|
| Socio-demographics | |||
| Men | 32 (86.5) | 100 (83.3) | 0.65 |
| Age (mean) | 58 | 60.3 | 0.30 |
| Activities | N (%) | N (%) | 0.36 |
| 37 (23.7) | 119 (76.3) | ||
| Hours spent for bird ringing activities each time | |||
| 1–2 h | 1 (2.7) | 11 (9.2) | |
| 2–3 h | 10 (27) | 25 (21) | |
| >4 h | 26 (70.3) | 83 (69.7) | |
| Blood sampling | N (%) | N (%) | 0.37 |
| 37 (23.6) | 120 (76.4) | ||
| Yes | 12 (32.4) | 30 (25) | |
| No | 25 (67.6) | 90 (75) | |
| Visiting Utrecht province for bird ringing activities and/or outdoor activities | 0.92 | ||
| Yes | 11 (29.7) | 39 (32.5) | |
| No | 26 (70.3) | 81 (67.5) | |
| Beginners in 2020 | 1 (2.7) N (%) 31 (21.2) |
5 (4.2) N (%) 115 (78.8) |
1.00 |
| Ringing since | |||
| 1957–1967 | 1 (3.2) | 7 (6.1) | |
| 1969–1979 | 2 (6.5) | 8 (7.0) | |
| 1980–1990 | 7 (22.6) | 15 (13.0) | |
| 1992–2002 | 2 (6.5) | 26 (22.6) | |
| 2004–2014 | 10 (32.3) | 30 (26.1) | |
| 2015–2019 | 9 (29) | 29 (25.2) | |
| Mosquito nuisance and mosquito bite preventive measures | 0.94 | ||
| High mosquito nuisance experienced while ringing | |||
| Yes | 8 (21.6) | 25 (21) | |
| No | 29 (78.4) | 94 (79) | |
| Use of mosquito repellent | 0.21 | ||
| Always | 0 (0) | 1 (0.8) | |
| Most of the times | 1 (2.7) | 7 (5.8) | |
| Sometimes | 8 (21.6) | 17 (14.2) | |
| Rarely | 2 (5.4) | 21 (17.5) | |
| Never | 26 (70.3) | 74 (61.7) | |
| Use of other mosquito bite preventive measures (e.g. long sleeves) |
N (%) 37 (23.6) |
N (%) 120 (76.4) |
0.47 |
| Always | 13 (35.1) | 33 (27.5) | |
| Most of the times | 8 (21.6) | 27 (22.5) | |
| Sometimes | 6 (16.2) | 13 (10.8) | |
| Rarely | 2 (5.4) | 19 (15.8) | |
| Never | 8 (21.6) | 28 (23.3) | |
| Vaccination status | N (%) 37 (23.7) |
N (%) 119 (76.3) |
0.82 |
| Tickborne encephalitis vaccination | |||
| Years from vaccination | |||
| Yes | 1 (2.7) | 7 (5.9) | |
| <1 year | 3 (42.9) | ||
| 1–4 years | 2 (28.5) | ||
| 5–9 years | 1 (14.3) | ||
| >10 years | 1 (100) | 1 (14.3) | |
| No | 36 (97.3) | 110 (92.4) | |
| I do not remember | 0 | 2 (1.7) | |
| Japanese encephalitis vaccination | 0.26 | ||
| Years from vaccinationYears from vaccination | |||
| Yes | 2 (5.4) | 1 (0.8) | |
| >10 yeare | 2 (100) | 1 (100) | |
| No | 35 (94.6) | 115 (96.6) | |
| I do not remember | 0 | 3 (2.5) | |
| Yellow fever vaccination | 0.79 | ||
| Years from vaccination | 12 (32.4) | 42 (35.3) | |
| Yes | |||
| 1–4 years | 2 (16.6) | 6 (14.3) | |
| 5–9 years | 1 (8.4) | 15 (35.7) | |
| >10 years | 9 (75) | 21 (50) | |
| No | 23 (62.2) | 73 (61.3) | |
| I do not remember | 2 (5.4) | 4 (3.4) | |
| Travel history in the previous five years | N (%) 37 (23.7) |
N (%) 119 (76.3) |
|
| Europe | 30 (81.1) | 84 (70.6) | 0.21 |
| European countries with reported locally-acquired WNV human infections | 30 (81.1) | 78 (65.5) | 0.07 |
| United States | 6 (16.2) | 12 (10.1) | 0.38 |
| South America | 2 (5.4) | 8 (6.7) | 1.00 |
| Central America and the Caribbean | 1 (2.7) | 11 (9.2) | 0.30 |
| North and West Africa | 2 (5.4) | 13 (10.9) | 0.52 |
| East Africa | 1 (2.7) | 7 (5.9) | 0.68 |
| South Africa | 3 (8.1) | 16 (13.4) | 0.57 |
| West Asia | 1 (2.7) | 9 (7.6) | 0.45 |
| East Asia and South East Asia | 2 (5.4) | 14 (11.8) | 0.36 |
| South Asia | 1 (2.7) | 7 (5.9) | 0.68 |
| Australia | 1 (2.7) | 3 (2.5) | 1.00 |
| Pacific Islands | 0 (0) | 4 (3.4) | 0.57 |
Descriptive analysis shows that out of all municipalities, most bird ringers engaged in bird ringing in the Northern and central part of the Netherlands with the highest number of active bird ringers in the Utrecht municipality (N = 22) where WNV was found in 2020, whereas regions in the south are less well represented (Fig. 2A). Comparison of the geographical distribution of possibly and confirmed versus negative bird ringers did not specifically highlight a certain area at risk (Fig. 2B-D). However, due to the fact that each individual bird ringer is often active across the Netherlands, assessing information regarding specific areas at risk is not possible. Though, the municipalities Utrecht (N = 3, Utrecht), Schiermonnikoog (N = 3, Friesland) and Bronckhorst (N = 3, Gelderland) for the WNV possible or confirmed exposure group, and Dinkelland (N = 2, Overijssel) and Bronckhorst (N = 2, Gelderland) for the USUV possible or confirmed exposure group were most frequently visited (Fig. 2B-D). In total, almost 24% (5/21) of the possible or confirmed WNV exposed bird ringers visited the Utrecht province for ringing or outdoor activities (Table S7). The confirmed WNV infected bird ringer visited several regions for bird ringing activities, including Utrecht (Haarzuilens), Gelderland (Wageningen), Zuid-Holland, Zeeland and Friesland (Fig. 2B). Only several nearby locations within Limburg were visited for bird ringing by the USUV confirmed participant (Fig. 2C).
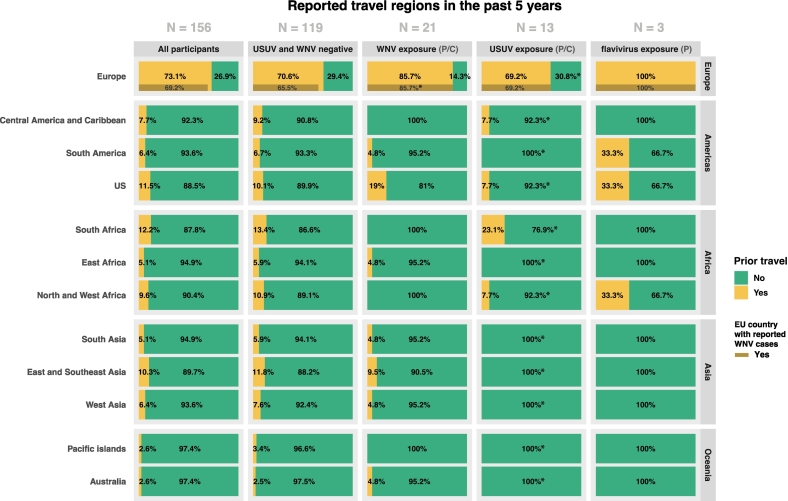
Analysis of the travel history of bird ringers shows that the group that travelled to EU countries with WNV circulation also includes the WNV confirmed case (Fig. 3). In addition, descriptive analysis shows a higher proportion of bird ringers with possible WNV, USUV or flavivirus exposure (6/37, 16.2%) reported to have travelled to the US compared to the negative participants (12/119, 10.1%) (Fig. 3). More specifically, most of the reactive bird ringers that travelled to the US [6] belonged to the possible WNV exposure group [4], accounting for 19% of the WNV exposure group (4/21, 19%) (Fig. 3). Interestingly, other regions more frequently visited compared to the USUV and WNV negative group, were South Africa (3/13, 23.1%) for the possible USUV exposure group as well as North and West Africa (1/3, 33.3%) for the possible flavivirus exposed group (Fig. 3). The USUV confirmed bird ringer did not travel to any countries abroad in the past five years (Fig. 3). Out of the six WNV and/or USUV reactive participants of the Dutch seroepidemiological PIENTER-3 study (2016–2017), four reported prior travelling abroad to the Americas (N = 2) and Asia (N = 2), whereas two reported never having travelled abroad (Table S8).
Fig. 3.
Detailed exploration of the travel locations of bird ringer interpretation groups
All reported travel locations of the past five years are summarized per area for each possible (P) or confirmed (C) exposure interpretation group. For Europe, the number of visited countries that reported human locally-acquired WNV circulation between 2011 and 2021 based on ECDC reports, are shown as a separate bar. One participant did not fill in the questionnaire regarding travel history and is therefore not showed in this figure. * Indicates the percentages of which the confirmed WNV or USUV exposed individuals belong to.
6. Discussion
Since the detection of USUV in 2016 and WNV in 2020 in the Netherlands, several studies have been done to estimate the prevalence and spread of these viruses in the country to some extent [4,6,20,21]. However, the extent of the introduction and the actual risk of exposure to USUV or WNV in the Dutch human population is currently unknown. In particular, certain individuals, such as bird ringers, might be at higher risk of arbovirus infections due to elevated time spent outdoor in mosquito-rich areas, and therefore potentially experience a higher exposure to mosquito bites. Therefore, in this study, we assessed the USUV and WNV seroprevalence among bird ringers considered as a possible high-risk group.
Using a multiplex protein microarray, we found low level USUV and/or WNV IgG signals in 23.6% of the 157 included participants with minimal antibody cross-reactivity (4/37, 10.8%). Although all IgG titers were above background compared to a group of healthcare workers sampled prior to the first USUV and WNV detection in the Netherlands, titers were relatively low [30], but comparable to the protein microarray titers found in PCR confirmed USUV and WNV reference sera, especially those from late timepoints of approximately 200 days or more after confirmed infection. These low IgG titers might be explained by antibody waning following a flavivirus exposure in the past, and/or asymptomatic infection which might have led to more rapid antibody waning or lower induced antibody titers at the start of infection [[34], [35], [36]]. The frequency of WNV and USUV IgG signals found in the bird ringers (37/157, 23.6%) was higher compared to both the population representative controls of 2016–2017 (6/94, 6.4%) as well as age-matched blood donor controls from September 2021 (2/96, 2.1%), suggesting increased (asymptomatic) exposure in bird ringers compared to the general population. Although the timing of sample collection was slightly different between the bird ringers and blood donors (i.e., June–September 2021 and September 2021, respectively), the difference in USUV and WNV IgG signal frequency is likely not explained by antibody waning in the blood donor control group considering that arbovirus IgG antibodies can typically persist for years [37]. This suggests that antibodies in September 2021 would likely have been detectable in the event of a past infection among the blood donor group. Four of the six persons with WNV and/or USUV IgG signals from the community cohort reported travelling abroad to the Americas or Asia, but two subjects reported no previous travel abroad and therefore suggests possible flavivirus exposure in the Netherlands in 2017 or earlier.
Only a small fraction of reactive sera had neutralizing functional antibodies. One explanation for this might be the usually observed lower sensitivity of virus neutralization assays compared to enzyme-linked immunosorbent assays (ELISA) including the protein microarray [30,38,39]. Also, functional and binding antibodies may wane at different rates, which could result in a group of protein microarray positive individuals that do not have detectable neutralizing antibodies anymore [[40], [41], [42], [43], [44], [45]]. Another possible explanation is that the protein microarray measures binding antibodies induced by exposure to other flaviviruses than tested here (USUV, WNV, JEV, TBEV), since flavivirus anti-NS1 cross-reactivity has been demonstrated to some extent, for instance between WNV and Dengue virus (DENV) NS1 [30,46]. This is not explained by vaccination: only 3 out of 37 (8.1%) IgG reactive bird ringers were vaccinated with either TBEV or JEV, and the vaccines used in the Netherlands are both inactivated vaccines meaning NS1-targeted antibodies are not expected to be produced after vaccination [[47], [48], [49], [50]], although this is not entirely clear [51]. In addition, no cross-reactivity in the protein array has been observed previously in YFV vaccinated individuals even though YFV is a live-attenuated vaccine, and therefore NS1 antibodies are elicited upon vaccination [30], Furthermore, the percentages of YFV vaccinated individuals in the IgG reactive bird ringer group and the negative group are not significantly different, indicating YFV vaccination is unlikely causing the USUV and/or WNV IgG signals seen.
Possible and confirmed WNV, USUV and flavivirus exposures were found across the Netherlands. However, since each bird ringer often rings birds at different locations and regions in the Netherlands, it was not possible to assess whether bird ringers in certain areas were more at risk of flavivirus exposure. Also, the majority of them had history of travel to known WNV endemic regions, such as the US, Africa and certain European countries [9,14,[52], [53], [54], [55], [56], [57]]. Furthermore, bird ringers are likely to get involved in bird watching and/or outdoor activities in nature-rich areas when travelling abroad, likely not only increasing their chances of exposure to mosquitos and the viruses they may carry, but also challenging defining the place and time of exposure. Only the confirmed USUV positive bird ringer appears confirmed to have been exposed in the Netherlands, as the person did not travel abroad in the past five years, suggesting local infection.
Despite the challenges in defining the possible place and time of arbovirus infection, this study suggests that bird ringers might be at higher risk of arbovirus exposure compared to the general population. For this reason, targeted surveillance of this group could be considered in order to detect early signals of arbovirus circulation. So far, human surveillance of arboviruses such as USUV or WNV mainly relies on the detection of neuroinvasive disease cases [6]. However, considering that the majority of USUV and WNV infections are asymptomatic and only <1% of infections causes neuroinvasive manifestations, syndromic surveillance would only detect human cases when the virus has already been circulating in the population for a longer period. Blood donor screening may also be used for surveillance purposes, however, this would be challenging in areas with a low-prevalence of the virus since blood donors are not considered a high-risk group for USUV or WNV exposure.
In the control of vector-borne diseases, the development of tools and strategies to promote early warning surveillance and rapid response has been recognized as key to prevent current and future further spread of emerging or re-emerging viruses [1]. Sentinel systems using chickens or wild bird screening have proven its utility to detect (newly) emerging viruses [4,58,59]. However, to enhance early warning systems, a combination of efforts would be beneficial; preferably also including a human sentinel – which is not yet in place – aiming at covering both the human and animal side [60]. The addition of a human sentinel in early warning surveillance is valuable since it also provides information about the current risk of human exposure to (re-)emerging viruses. Integrated sentinel surveillance would allow early detection of emerging viruses and implementation of control measures such as blood safety interventions, enhanced vector and mosquito-bite prevention measures.
Considering that bird ringers are often active across the Netherlands and frequently travel abroad, they might not be the best sentinels - on their own - for location specific risk-assessment of WNV and USUV circulation. However, combined active surveillance of high-risk individuals such as bird ringers, along with wild bird, poultry and mosquito surveillance would enhance the current framework for early flavivirus detection and spread in the Netherlands based on a One Health approach [61].
Overall, this study underlines the complexity of interpreting seroepidemiological data in a low prevalence setting, where chances of false positive (reactive) results proportionally increase when the prevalence is low [62,63]. We show that bird ringers in the Netherlands likely are at higher risk of WNV and USUV infection, however, from the current cross-sectional design, we could not determine if and where (possible) exposure occurred in the Netherlands. This requires a longitudinal approach with repeated measurements among bird ringers, for instance just before, during and immediately after the Dutch arbovirus season in the Netherlands. Furthermore, for monitoring and early detection of localized flavivirus circulation in the Netherlands, this should be combined with other localized surveillance activities focusing on birds and mosquitos. In summary, with regards to early detection of emerging viruses, our findings suggest that individuals with high-exposure professions may be considered to complement existing surveillance systems.
The following are the supplementary data related to this article.
Figure S1 – Flowchart showing the number of included participants
Figure S2 – Heatmap of protein microarray antigen IgG and FRNT titers with exposure interpretation
Figure S3 – IgG protein microarray signals of bird ringers and control groups
Figure S4 – Timeline of found possible or confirmed WNV, USUV and flavivirus bird ringer exposures
Figure S5 – Sampling dates of exposure groups and all participants
Table S1 – PCR confirmed USUV blood donor reference sera
Table S2 – PCR confirmed WNV blood donor reference sera
Table S3 – Reported months and frequency of bird ringing activities
Table S4 – Characteristics of the bird ringers included in the study (N = 157) – extended version
Table S5 – FRNT results of protein microarray IgG negative bird ringer sera
Table S6 – FRNT results of protein microarray IgG reactive Sanquin blood
Table S7 – Comparison between possible and confirmed exposure groups
Table S8 – Travel history from the participants of the Dutch
Please note that the document including the supplemental information (survey) is attached as separate word document only
Funding
NWO: This work is part of the research programme One Health PACT with project number 109986, which is (partly) financed by the Dutch Research Council (NWO). RS and MK received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 874735 (VEO project).
CRediT authorship contribution statement
Chiara de Bellegarde de Saint Lary: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Louella M.R. Kasbergen: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Patricia C.J.L. Bruijning-Verhagen: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. Henk van der Jeugd: Conceptualization, Methodology, Resources, Validation, Writing – review & editing. Felicity Chandler: Investigation, Methodology, Validation, Writing – review & editing. Boris M. Hogema: Methodology, Resources, Validation, Writing – review & editing. Hans L. Zaaijer: Resources, Validation, Writing - review & editing. Fiona R.M. van der Klis: Resources, Validation, Writing – review & editing. Luisa Barzon: Resources, Validation, Writing – review & editing. Erwin De Bruin: Methodology, Validation, Writing – review & editing. Quirine Ten Bosch: Conceptualization, Methodology, Validation, Writing – review & editing. Marion P.G. Koopmans: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. Reina S. Sikkema: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. Leo G. Visser: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no financial and/or competing interests.
Acknowledgements
Sera of the healthcare workers - part of a longitudinal influenza vaccination study from November 2009 to June 2010 – were kindly provided by Dr. Inge Huijskens. We acknowledge the PIENTER-3 study team for the collection of samples (national immune surveillance study). The authors would like to thank Koen Wijnans for testing sera on the presence of neutralizing antibodies (FRNT70) and Corine Prins for supporting the data collection during the study. In addition, the authors would like to thank all study participants.
Data availability
The data is available in the manuscript and supplement. If additional data from the survey is needed, it can be requested unless this is confidential information of the participants.
References
- 1.Chala B., Hamde F. Emerging and Re-emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front Public Health. 2021 Oct 5;9 doi: 10.3389/fpubh.2021.715759. [Internet]. [cited 2022 Jun 10]. Available from: /pmc/articles/PMC8524040/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y., Tjaden N.B., Jaeschke A., Lühken R., Ziegler U., Thomas S.M., et al. Evaluating the risk for Usutu virus circulation in Europe: comparison of environmental niche models and epidemiological models. Int J Health Geogr [Internet] 2018;17(1) doi: 10.1186/s12942-018-0155-7. (Oct 12 [cited 2022 Jun 10]. Available from: /pmc/articles/PMC6186058/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubel F., Brugger K. Dynamics of infectious diseases according to climate change: the Usutu virus epidemics in Vienna. Game meat hygiene in focus [Internet] 2011:173–198. https://link.springer.com/chapter/10.3920/978-90-8686-723-3_14 [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [Google Scholar]
- 4.Sikkema R.S., Schrama M., Van Den Berg T., Morren J., Munger E., Krol L., et al. Detection of west nile virus in a common whitethroat (Curruca communis) and Culex mosquitoes in the Netherlands, 2020. Eurosurveillance. 2020 Oct 8;25(40):1–6. doi: 10.2807/1560-7917.ES.2020.25.40.2001704. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.40.2001704 [Internet]. [cited 2022 Jun 10]. Available from: [Internet]. [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eerste patiënt in Nederland met westnijlvirus | RIVM [Internet]. [cited 2022 Jun 10] 2020. https://www.rivm.nl/nieuws/eerste-patient-in-nederland-met-westnijlvirus Available from:
- 6.Vlaskamp D.R.M., Thijsen S.F.T., Reimerink J., Hilkens P., Bouvy W.H., Bantjes S.E., et al. First autochthonous human west nile virus infections in the Netherlands, July to August 2020. Eurosurveillance. 2020 Nov 1;25(46):1–4. doi: 10.2807/1560-7917.ES.2020.25.46.2001904. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.46.2001904 [Internet]. [cited 2022 Jun 10]. Available from: [Internet]. [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ECDC Factsheet about West Nile virus infection [Internet] 2023. https://www.ecdc.europa.eu/en/west-nile-fever/facts [cited 2022 Jun 10]. Available from:
- 8.Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. [Internet] 1940. https://www.cabdirect.org/cabdirect/abstract/19412700112?freeview=true [cited 2022 Jun 10]. Available from:
- 9.WHO West Nile virus [Internet]. [cited 2022 Jun 10] 2017. https://www.who.int/news-room/fact-sheets/detail/west-nile-virus Available from:
- 10.Sejvar J.J. West Nile Virus: An Historical Overview. Ochsner J [Internet] 2003 Jun;5(3):6. [cited 2022 Jun 10]. Available from: /pmc/articles/PMC3111838/ [PMC free article] [PubMed] [Google Scholar]
- 11.Symptoms, Diagnosis, & Treatment | West Nile Virus | CDC [Internet]. [cited 2022 Jul 27] 2022. https://www.cdc.gov/westnile/symptoms/index.html Available from:
- 12.Poidinger M., Hall R.A., Mackenzie J.S. Molecular characterization of the Japanese encephalitis Serocomplex of the Flavivirus genus. Virology. 1996 Apr 15;218(2):417–421. doi: 10.1006/viro.1996.0213. [DOI] [PubMed] [Google Scholar]
- 13.Wellehan J.F.X., Lierz M., Phalen D., Raidal S., Styles D.K., Crosta L., et al. Infectious disease. Current Therapy in Avian Medicine and Surgery. 2016 Jan;1:22–106. [Google Scholar]
- 14.Nikolay B., Diallo M., Boye C.S.B., Sall A.A. 11(11) 2011 Nov 11. Usutu Virus in Africa; pp. 1417–1423.https://www.liebertpub.com/doi/10.1089/vbz.2011.0631 https://home.liebertpub.com/vbz [Internet] [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PubMed] [Google Scholar]
- 15.Weissenböck H., Bakonyi T., Rossi G., Mani P., Nowotny N. Usutu virus, Italy, 1996. Emerg Infect Dis. 2013;19(2):274–277. doi: 10.3201/eid1902.121191. https://pubmed.ncbi.nlm.nih.gov/23347844/ [Internet]. [cited 2022 Jun 10]. Available from: [Internet]. [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zannoli S., Sambri V. West Nile Virus and Usutu Virus Co-Circulation in Europe: Epidemiology and Implications. Microorganisms. 2019;7:184. doi: 10.3390/microorganisms7070184. https://www.mdpi.com/2076-2607/7/7/184/htm [Internet]. 2019 Jun. Available from: [Internet]. 2019 Jun. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graninger M., Hubmer S., Riederer F., Kettner S., Hauk M., Auf T., et al. The first case of Usutu Virus Neuroinvasive disease in Austria, 2021. Open Forum Infect Dis [Internet] 2022 Jul 4;9(7) doi: 10.1093/ofid/ofac255. https://academic.oup.com/ofid/article/9/7/ofac255/6586374 [cited 2022 Jul 28]. Available from: [cited 2022 Jul 28]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giglia G., Agliani G., Oude Munnink B.B., Sikkema R., Mandara M.T., Lepri E., et al. Pathology and pathogenesis of eurasian blackbirds (Turdus merula) naturally infected with usutu virus. Viruses [Internet] 2021 Aug 1;13(8):1481. doi: 10.3390/v13081481. https://www.mdpi.com/1999-4915/13/8/1481/htm [cited 2023 Mar 16]. Available from: [cited 2023 Mar 16]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benzarti E., Garigliany M. In Vitro and In Vivo Models to Study the Zoonotic Mosquito-Borne Usutu Virus. Viruses. 2020;12:1116. doi: 10.3390/v12101116. https://www.mdpi.com/1999-4915/12/10/1116/htm [Internet]. 2020 Sep 30 [cited 2023 Mar 16];12(10):1116. Available from: [Internet]. 2020 Sep 30 [cited 2023 Mar 16];12(10):1116. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaaijer H.L., Slot E., Molier M., Reusken C.B.E.M. Koppelman MHGM. Usutu virus infection in Dutch blood donors. Transfusion (Paris) [Internet] 2019 Sep 1;59(9):2931–2937. doi: 10.1111/trf.15444. https://onlinelibrary.wiley.com/doi/full/10.1111/trf.15444 [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PubMed] [Google Scholar]
- 21.Oude Munnink B.B., Münger E., Nieuwenhuijse D.F., Kohl R., van der Linden A., Schapendonk C.M.E., et al. Genomic monitoring to understand the emergence and spread of Usutu virus in the Netherlands, 2016-2018. Sci Rep. 2020 Feb 18;10(1):2798. doi: 10.1038/s41598-020-59692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rijks J.M., Kik M., Slaterus R., Foppen R., Stroo A., Ijzer J., et al. Widespread Usutu virus outbreak in birds in The Netherlands, 2016. Eurosurveillance [Internet] 2016 Nov 10;21(45):30391. doi: 10.2807/1560-7917.ES.2016.21.45.30391. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2016.21.45.30391 [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merelsterfte door usutuvirus, vogelmalaria en parasieten | Dutch Wildlife Health Centre (DWHC) [Internet] 2023 Mar 15. https://dwhc.nl/merelsterfte-door-usutuvirus-vogelmalaria-en-parasieten/ cited. Available from:
- 24.Bergervoet S.A., Pritz-Verschuren S.B.E., Gonzales J.L., Bossers A., Poen M.J., Dutta J., et al. Circulation of low pathogenic avian influenza (LPAI) viruses in wild birds and poultry in the Netherlands, 2006–2016. Sci Rep. 2019 Dec 1;9(1) doi: 10.1038/s41598-019-50170-8. https://pubmed.ncbi.nlm.nih.gov/31548582/ [Internet]. [cited 2023 Mar 15]. Available from: [Internet]. [cited 2023 Mar 15]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venter M., Steyl J., Human S., Weyer J., Zaayman D., Blumberg L., et al. Transmission of West Nile Virus during Horse Autopsy. Emerg Infect Dis. 2010 Mar;16(3):573. doi: 10.3201/eid1603.091042. [Internet]. [cited 2022 Jun 10]. Available from: /pmc/articles/PMC3322023/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC Laboratory-Acquired West Nile Virus Infections --- United States. 2002. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5150a2.htm [Internet]. [cited 2022 Jul 13] Available from: [PubMed]
- 27.Huijskens E.G.W., Reimerink J., Mulder P.G.H., van Beek J., Meijer A., de Bruin E., et al. Profiling of Humoral Response to Influenza A(H1N1)pdm09 Infection and Vaccination Measured by a Protein Microarray in Persons with and without History of Seasonal Vaccination. PloS One. 2013 Jan 30;8(1) doi: 10.1371/journal.pone.0054890. [Internet]. [cited 2022 Jun 10]. Available from: /pmc/articles/PMC3554683/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PIENTER-onderzoek RIVM [Internet] 2022 Jun 10. https://www.rivm.nl/pienter-onderzoek cited. Available from:
- 29.Koopmans M., de Bruin E., Godeke G.J., Friesema I., van Gageldonk R., Schipper M., et al. Profiling of humoral immune responses to influenza viruses by using protein microarray. Clinical Microbiology and Infection [Internet] 2012 Aug 1;18(8):797–807. doi: 10.1111/j.1469-0691.2011.03701.x. http://www.clinicalmicrobiologyandinfection.com/article/S1198743X14634406/fulltext [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PubMed] [Google Scholar]
- 30.Cleton N.B., Godeke G.J., Reimerink J., Beersma M.F., van Doorn H.R., Franco L., et al. Spot the difference-development of a syndrome based protein microarray for specific serological detection of multiple flavivirus infections in travelers. PLoS Negl Trop Dis. 2015 Mar 13;9(3) doi: 10.1371/journal.pntd.0003580. https://pubmed.ncbi.nlm.nih.gov/25767876/ [Internet]. [cited 2022 Jun 10]. Available from: [Internet]. [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posit team . 2022. RStudio: Integrated Development Environment for R. Boston, MA. [Google Scholar]
- 32.GeurtsvanKessel C.H., Geers D., Schmitz K.S., Mykytyn A.Z., Lamers M.M., Bogers S., et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol [Internet] 2022 Mar 25;7(69):eabo2202. doi: 10.1126/sciimmunol.abo2202. https://pubmed.ncbi.nlm.nih.gov/35113647/ [cited 2023 Mar 15]. Available from: [cited 2023 Mar 15]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ECDC West Nile virus infections in humans, 2011–2021 [Internet] 2022 Jun 10. https://www.ecdc.europa.eu/en/publications-data/west-nile-virus-infections-humans-2011-2021 cited. Available from:
- 34.Pierro A., Gaibani P., Manisera C., Rossini G., Finarelli A.C., Ghinelli F., et al. Persistence of Anti-West Nile Virus-Specific Antibodies Among Asymptomatic Blood Donors in Northeastern Italy. 2013 Dec 5. https://www.liebertpub.com/doi/10.1089/vbz.2012.1157 https://home.liebertpub.com/vbz [Internet]. [cited 2022 Jun 10];13(12):892–3. Available from: [DOI] [PubMed]
- 35.Percivalle E., Cassaniti I., Sarasini A., Rovida F., Adzasehoun K.M.G., Colombini I., et al. West Nile or Usutu Virus? A Three-Year Follow-Up of Humoral and Cellular Response in a Group of Asymptomatic Blood Donors. Viruses. 2020;12(2) doi: 10.3390/v12020157. [Internet]. [cited 2022 Jun 10]. Available from: /pmc/articles/PMC7077259/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prince H.E., Tobler L.H., Yeh C., Gefter N., Custer B., Busch M.P. Persistence of West Nile Virus-specific antibodies in Viremic blood donors. Clin Vaccine Immunol [Internet] 2007 doi: 10.1128/CVI.00233-07. (Sep [cited 2023 Mar 15];14(9):1228. Available from: /pmc/articles/PMC2043320/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince H.E., Tobler L.H., Lapé-Nixon M., Foster G.A., Stramer S.L., Busch M.P. Development and Persistence of West Nile Virus-Specific Immunoglobulin M (IgM), IgA, and IgG in Viremic Blood Donors. J Clin Microbiol [Internet] 2005 Sep;43(9):4316. doi: 10.1128/JCM.43.9.4316-4320.2005. [cited 2023 Mar 15]. Available from: /pmc/articles/PMC1234148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dauphin G., Zientara S. West Nile virus: recent trends in diagnosis and vaccine development. Vaccine. 2007 Jul 26;25(30):5563–5576. doi: 10.1016/j.vaccine.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Beck C., Lowenski S., Durand B., Bahuon C., Zientara S., Lecollinet S. Improved reliability of serological tools for the diagnosis of West Nile fever in horses within Europe. 2017 Sep 15;11(9):e0005936. doi: 10.1371/journal.pntd.0005936. https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005936 PLoS Negl Trop Dis [Internet] [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rey F.A., Stiasny K., Vaney M., Dellarole M., Heinz F.X. The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design. EMBO Rep [Internet] 2018 Feb;19(2):206–224. doi: 10.15252/embr.201745302. https://pubmed.ncbi.nlm.nih.gov/29282215/ [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slon Campos J.L., Mongkolsapaya J. Screaton GR. The immune response against flaviviruses. Nat Immunol. 2018 Oct 17;19(11):1189–1198. doi: 10.1038/s41590-018-0210-3. https://www.nature.com/articles/s41590-018-0210-3 2018 19:11 [Internet]. [cited 2022 Jun 10]. Available from: 2018 19:11 [Internet]. [cited 2022 Jun 10]. Available from: [DOI] [PubMed] [Google Scholar]
- 42.Stettler K., Beltramello M., Espinosa D.A., Graham V., Cassotta A., Bianchi S., et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science (1979) 2016 Aug 19;353(6301):823–826. doi: 10.1126/science.aaf8505. https://www.science.org/doi/full/10.1126/science.aaf8505 [Internet]. [cited 2022 Jun 10]. Available from: [Internet]. [cited 2022 Jun 10]. Available from: [DOI] [PubMed] [Google Scholar]
- 43.Gao X., Wen Y., Wang J., Hong W., Li C., Zhao L., et al. Delayed and highly specific antibody response to nonstructural protein 1 (NS1) revealed during natural human ZIKV infection by NS1-based capture ELISA. BMC Infect Dis [Internet] 2018 Jun 14;18(1):1–7. doi: 10.1186/s12879-018-3173-y. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-018-3173-y [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freire M.C.L.C., Pol-Fachin L., Coêlho D.F., Viana I.F.T., Magalhães T., Cordeiro M.T., et al. Mapping putative B-cell Zika Virus NS1 epitopes provides molecular basis for anti-NS1 antibody discrimination between Zika and dengue viruses. ACS Omega [Internet] 2017 Jul 31;2(7):3913–3920. doi: 10.1021/acsomega.7b00608. https://pubs.acs.org/doi/full/10.1021/acsomega.7b00608 [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rastogi M., Sharma N., Singh S.K. Flavivirus NS1: A multifaceted enigmatic viral protein. Virol J [Internet] 2016 Jul 29;13(1):1–10. doi: 10.1186/s12985-016-0590-7. https://virologyj.biomedcentral.com/articles/10.1186/s12985-016-0590-7 [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thao T.T.N., De Bruin E., Phuong H.T., Thao Vy N.H., Van Den Ham H.J., Wills B.A., et al. Using NS1 Flavivirus Protein Microarray to Infer Past Infecting Dengue Virus Serotype and Number of Past Dengue Virus Infections in Vietnamese Individuals. J Infect Dis [Internet] 2021 Jun 15;223(12):2053–2061. doi: 10.1093/infdis/jiaa018. https://academic.oup.com/jid/article/223/12/2053/5713534 [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firbas C., Jilma B. Product review on the JE vaccine IXIARO. Hum Vaccin Immunother [Internet] 2015;11(2):411–420. doi: 10.4161/21645515.2014.983412. https://pubmed.ncbi.nlm.nih.gov/25621812/ [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubinski M., Beicht J., Gerlach T., Volz A., Sutter G., Rimmelzwaan G.F. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines (Basel) [Internet] 2020 Sep 1;8(3):1–45. doi: 10.3390/vaccines8030451. [cited 2022 Jun 10]. Available from: /pmc/articles/PMC7564546/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Japanse encefalitis | LCI richtlijnen [Internet] 2022 Jun 10. https://lci.rivm.nl/richtlijnen/japanse-encefalitis cited. Available from:
- 50.Tekenencefalitis | LCI richtlijnen [Internet] 2022 Jun 10. https://lci.rivm.nl/richtlijnen/tekenencefalitis cited. Available from:
- 51.Salat J., Mikulasek K., Larralde O., Formanova P.P., Chrdle A., Haviernik J., et al. Tick-Borne Encephalitis Virus Vaccines Contain Non-Structural Protein 1 Antigen and May Elicit NS1-Specific Antibody Responses in Vaccinated Individuals. Vaccines (Basel) [Internet] 2020 Mar 1;8(1):81. doi: 10.3390/vaccines8010081. [cited 2022 Jun 10]. Available from: /pmc/articles/PMC7157539/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.García-Carrasco J.M., Muñoz A.R., Olivero J., Segura M., Real R. An African West Nile virus risk map for travellers and clinicians. Travel Med Infect Dis. 2023 Mar;1(52) doi: 10.1016/j.tmaid.2022.102529. [DOI] [PubMed] [Google Scholar]
- 53.Mancuso E., Cecere J.G., Iapaolo F., Di Gennaro A., Sacchi M., Savini G., et al. West Nile and Usutu Virus Introduction via Migratory Birds: A Retrospective Analysis in Italy. Viruses [Internet] 2022 Feb 1;14(2) doi: 10.3390/v14020416. https://pubmed.ncbi.nlm.nih.gov/35216009/ [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mencattelli G., Ndione M.H.D., Rosà R., Marini G., Diagne C.T., Diagne M.M., et al. West Nile Virus in Africa: current epidemiological situation and knowledge gaps. Int J Infect Dis. 2022 Mar;1(116):S123. [Google Scholar]
- 55.Mencattelli G., Ndione M.H.D., Rosà R., Marini G., Diagne C.T., Diagne M.M., et al. Epidemiology of West Nile virus in Africa: An underestimated threat. PLoS Negl Trop Dis [Internet] 2022 Jan 1;16(1):e0010075. doi: 10.1371/journal.pntd.0010075. https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0010075 [cited 2022 Jun 10]. Available from: [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plante J.A., Burkhalter K.L., Mann B.R., Godsey M.S., Mutebi J.P., Beasley D.W.C. 2010. Co-Circulation of West Nile Virus Variants, Arizona, USA. (Emerg Infect Dis [Internet]. 2014 Feb [cited 2022 Jun 10];20(2):272. Available from: /pmc/articles/PMC3901498/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West Nile virus - PAHO/WHO | Pan American Health Organization [Internet] 2022 Jun 10. https://www.paho.org/en/topics/west-nile-virus cited. Available from:
- 58.Lim S.M., Geervliet M., Verhagen J.H., Müskens G.J.D.M., Majoor F.A., Osterhaus A.D.M.E., et al. Serologic evidence of West Nile virus and Usutu virus infections in Eurasian coots in the Netherlands. Zoonoses Public Health [Internet] 2018 Feb 1;65(1):96–102. doi: 10.1111/zph.12375. https://onlinelibrary.wiley.com/doi/full/10.1111/zph.12375 [cited 2022 Jul 13]. Available from: [cited 2022 Jul 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 59.Buckley A., Dawson A., Gould E.A. Detection of seroconversion to West Nile virus, Usutu virus and Sindbis virus in UK sentinel chickens. 2006. http://www.virologyj.com/content/3/1/71 [cited 2022 Jul 13]; Available from: [DOI] [PMC free article] [PubMed]
- 60.Achieving an Effective Zoonotic Disease Surveillance System - Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases - NCBI Bookshelf [Internet] 2022 Jul 26. https://www.ncbi.nlm.nih.gov/books/NBK215315/ cited. Available from:
- 61.De Best P., De Wit M., Streng K., Dellar M., Koopmans M. Emerging arboviral diseases. 2023 Mar 16. www.instagram.com/onehealthpact/ cited. Available from:
- 62.Parikh R., Mathai A., Parikh S., Sekhar G.C., Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol [Internet] 2008;56(1):45–50. doi: 10.4103/0301-4738.37595. (cited 2022 Jun 10]. Available from: /pmc/articles/PMC2636062/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Healy B., Khan A., Metezai H., Blyth I. Asad H. The impact of false positive COVID-19 results in an area of low prevalence. Clin Med (Lond) 2021 Jan 1;21(1):E54–E56. doi: 10.7861/clinmed.2020-0839. https://pubmed.ncbi.nlm.nih.gov/33243836/ [Internet]. [cited 2022 Jun 10]. Available from: [Internet]. [cited 2022 Jun 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 – Flowchart showing the number of included participants
Figure S2 – Heatmap of protein microarray antigen IgG and FRNT titers with exposure interpretation
Figure S3 – IgG protein microarray signals of bird ringers and control groups
Figure S4 – Timeline of found possible or confirmed WNV, USUV and flavivirus bird ringer exposures
Figure S5 – Sampling dates of exposure groups and all participants
Table S1 – PCR confirmed USUV blood donor reference sera
Table S2 – PCR confirmed WNV blood donor reference sera
Table S3 – Reported months and frequency of bird ringing activities
Table S4 – Characteristics of the bird ringers included in the study (N = 157) – extended version
Table S5 – FRNT results of protein microarray IgG negative bird ringer sera
Table S6 – FRNT results of protein microarray IgG reactive Sanquin blood
Table S7 – Comparison between possible and confirmed exposure groups
Table S8 – Travel history from the participants of the Dutch
Please note that the document including the supplemental information (survey) is attached as separate word document only
Data Availability Statement
The data is available in the manuscript and supplement. If additional data from the survey is needed, it can be requested unless this is confidential information of the participants.