Abstract
Wild boar (Sus scrofa) and red deer (Cervus elaphus) are the main large game species hunted in Europe. They can also be a source of zoonotic infections for cohabiting humans. The purpose of this systematic review was to examine the spatiotemporal tendencies and sanitary profiles of surveys on zoonotic diseases of wild boars and red deer in Europe in 15 years (2006–2020). Through the search strategy “((sus scrofa OR wild boar OR cervus elaphus OR red deer) AND (zoonosis OR zoonot* OR infectious disease))” in Pubmed and ScienceDirect databases, 1419 articles were assessed in February of 2021. Pursuing the inclusion criteria: species of interest – wild boar and red deer, established zoonosis and presence of natural infection and the exclusion filters: European study, specified a timeline (2006–2020), printed in English and with open-access. To conduct this systematic review, 194 European surveys issued in indexed journals were included after revising all abstracts and eliminating 323 unrepeated articles.
Geographically, dissimilarity in the pattern of distribution of surveys was uttered. In the short term, the pattern of the number of publications about zoonotic diseases in wild boars and red deer oscillates, but with an increasing tendency over 15 years under study. When examining the sanitary profile of the eligible surveys, the focus is mostly on zoonoses such as Hepatitis E virus, Toxoplasmosis, Trichinellosis, Salmonellosis and Tuberculosis.
With the high growth in the population of these large game species in Europe and the previous gaps in their sanitary profile, the number of surveys has been endorsed in the defined 15 years period. Based on the One Health concept and prioritizing the issue of the occurrence of zoonoses as a matter of Public Health, there must be increasing apprehension about that and enhanced knowledge about their potential risk for veterinarians, hunters and other agents involved in the hunting sector.
Keywords: Biogeography, Hunting, One health, Public health, Zoonosis
Highlights
-
•
Publications on large game's zoonoses have increased over the years, focusing on southern Europe.
-
•
The zoonoses that stand out are mostly foodborne diseases such as Hepatitis E and Toxoplasmosis.
-
•
The potential risk of these zoonoses is highlighted for veterinarians, game managers and hunters.
1. Introduction
In Europe, there is a large number of wild species considered a large game. Though the most hunted and studied by the scientific community are the wild boar (Sus scrofa) and the red deer (Cervus elaphus). The population of these two species is extensively allocated throughout Europe. But in central and southern Europe, they are the most abundant and equally hunted species [1,2].
The tremendousexpansion of these species leads scientists more and more to explore and study the penalties of this dissemination, both ecologically and epidemiologically [3]. Several infectious agents mingle in the wild boar and red deer populations, about which little is known and can cause several extra-wildlife problems for livestock and for humans [4]. An evident close human-wildlife-cattle interface makes it clear that numerous diseases that can mingle freelyon this border [5]. The fact that the epidemiology of several infectious diseases in large game species is unknown conducts to significant concern about the scientific community of the infectious agents' circulation [6]. Zoonoses, as transmissible diseases to humans, is a major Public Health worry in the referenced interface due to the closeness these wild species (e.g., wild boar and badgers) progressively have with the semi-urban and urban habitats [7,8]. Outdoor activities, human settlements in natural areas, and socioeconomic and food habits changes are responsible for the increased risk of humans acquiring zoonotic pathogens from large game species [4,5,9]. This risk increases to a great extent when the linked risk is about game professionals, namely veterinarians and hunters [4,10,11].
Both wild boar and red deer are described as hosts and disseminators of some zoonotic agents such as: Hepatitis E virus, Influenza virus, Salmonella sp., Campylobacter sp., Brucella sp., Leptospira sp.; among others. An emerging zoonotic disease outbreak related to the human–large game interaction is difficult to predict. However, the associated and attributable risk should be the target of information and preventive procedures [4,12,13,14]. Effective monitoring of wildlife zoonotic infectious diseases helps to implement public and animal health control strategies in one One Health perspective [13].
Over the studied 15 years, the behaviour, spreading, and sanitary status of these species have been of interest to the scientific community. This systematic review aims to assess the European scientific spatiotemporal tendencies on zoonosis in wild boar and red deer over 15 years (2006–2020 period). And so, this information clarifies which zoonoses have the most impact on these populations.
2. Material and methods
2.1. Sources of data
The articles were referredreferred to using the online scientific databases - Pubmed and ScienceDirect – during February of 2021. Each article was selected using the search expression “((sus scrofa OR wild boar OR cervus elaphus OR red deer) AND (zoonosis OR zoonot* OR infectious disease))”. No filter was applied, such as language or date interval, in the primary search.
2.2. Inclusion and exclusion criteria
The abstracts of all articles were attained and analysed. After the primary analysis, it was excluded the multiple publication count. The included articles follow the inclusion criteria: (i) species of interest - wild boar and red deer, (ii) confirmed zoonosis and (iii) presence of natural infection; and the exclusion filters were applied: (i) Language (English, Spanish or Portuguese writing articles), (ii) based geography of the article (only Europe), (iii) stipulated timeline (2006–2020) and (iv) open access articles.
2.3. Data extraction
The aim/objective was prudently read and analysed, and the material and methods sections of each article included regardless of the size and statistical significance of the sample. The data extracted were about: the year of publication, the localization, studied species, wild or captive characterization, study period, sample, learned zoonosis, used diagnose test, attained prevalence, and expressed concern about the zoonotic potential of the agent studied.
3. Results
The search identified 1419 articles from the two databases (Pubmed and ScienceDirect). In the first showing of all abstracts, 1051 articles were excluded, remaining 368. Of these 368 screenings in all databases, 45 were repeated and were excluded. To the remaining 323 articles, the primary exclusion filters were applied: 35 were excluded due to timeline (out of period 2006–2020), and six were excluded due to no stipulated language. In a second screening of all abstracts to inclusion criteria, 22 articles were excluded. With secondary exclusion filters screening to full-review the articles: 69 were excluded due to based geography (out of Europe), and 2 were no open access full articles.
So, 194 articles were identified for a full review to the Systematic Review (Fig. 1) and are summarised in Table 1.
Fig 1.
Schematic information of the Systematic Review process.
Table 1.
Overview of all publications analysed in this systematic review, by target species, type of agent, diseases and respective references obtained.
| Target species | Infectious agents | Zoonotic diseases of study | References |
|---|---|---|---|
| Only Wild Boar | Bacterial agents | Yersiniosis | Sanno et al., 2014; Arrausi-Subiza et al., 2016; Sanno et al., 2018; Morka et al., 2018; Fredriksson-Ahoma et al., 2020; Bonardi et al., 2020(b) |
| Brucellosis | Ruiz-Fons et al., 2006; Leuenberger et al., 2007; Montagnaro et al., 2010; Wu et al., 2011; Gregoire et al., 2012; Pilo et al., 2015; Bertelloni et al., 2020; Van Tulden et al., 2020 | ||
| Streptococcosis | Risco et al., 2015; Fernandez-Aguilar et al., 2018 | ||
| Erysipelothrix rhusiopathiae infection | Boadella et al., 2012(a) | ||
| Tuberculosis | García-Sanchez et al., 2007; Boadella et al., 2012(b); Broughan et al., 2013; Barasona et al., 2016; CheAmat et al., 2016; Santos et al., 2018; Varela-Castro et al., 2020; Ghielmetti et al., 2020 | ||
| Colibacillosis | Navarro-Gonzalez et al., 2013; Sanno et al., 2014; Navarro-Gonzalez et al., 2015; Szczerba-Turek et al., 2019 | ||
| Tularaemia | Otto et al., 2014; Moinet et al., 2016; Hestvik et al., 2019 | ||
| Borreliosis | Juricova & Hubálek, 2009; Faria et al., 2015 | ||
| Salmonellosis | Montagnaro et al., 2010; Mentaberre et al., 2013; Chiari et al., 2013; Navarro-Gonzalez et al., 2013; Sanno et al., 2014; Touloudi et al., 2015; Sanno et al., 2018; Ortega et al., 2020; Fredriksson-Ahoma et al., 2020; Petersen et al., 2020 | ||
| Campylobacteriosis | Navarro-Gonzalez et al., 2013; Navarro-Gonzalez et al., 2014 | ||
| Rhodococcosis | Witkowski et al., 2015 | ||
| Leptospirosis | Jansen et al., 2007; Montagnaro et al., 2010; Boqvist et al., 2012; Vale-Gonçalves et al., 2015; Zmudzik et al., 2016; Tagliabue et al., 2016; Cilia et al., 2020(a); Cilia et al., 2020(b); Cilia et al., 2020(c); Bertelloni et al., 2020 | ||
| Cutaneous diphtheria | Contzen et al., 2011; Eisenberg et al., 2014 | ||
| Anaplasmosis | Nahayo et al., 2014; Reiterova et al., 2016 | ||
| Antrax | Bagamian et al., 2014 | ||
| Aeromoniasis | Risco et al., 2013 | ||
| Viral agents | Influenza A | Kovalenko et al., 2017 | |
| Hepatitis E virus | Martelli et al., 2008; Kaci et al., 2008; de Deus et al., 2008; Reuter et al., 2009; Adlhoch et al., 2009; Kaba et al., 2010; Carpentier et al., 2012; Boadella et al., 2012(a); Oliveira-Filho et al., 2014; Burri et al., 2014; Mazzei et al., 2015; Montagnaro et al., 2015; Martinelli et al., 2015; Vina-Rodriguez et al., 2015; Ivanova et al., 2015; Serracca et al., 2015; Caruso et al., 2015; Jori et al., 2016; Zele et al., 2016; Mesquita et al., 2016; Rivero-Juarez et al., 2017; Risalde et al., 2017; DeSabato et al., 2018; Aprea et al., 2018; Porea et al., 2018; Weigand et al., 2018; Rivero-Juarez et al., 2018; Strakova et al., 2018; Wang et al., 2019; Caballero-Gomez et al., 2019; Kureljusic et al., 2020; Pierini et al., 2020; De Sabato et al., 2020; Bertelloni et al., 2020; Takova et al., 2020; Bonardi et al., 2020(a) | ||
| Rabies | Dascalu et al., 2019 | ||
| Influenza A | Kovalenko et al., 2017 | ||
| Parasitic agents | Toxoplasmosis | Ruiz-Fons et al., 2006; Antolová et al., 2007; Berger-Schoch et al., 2011(a); Berger-Schoch et al., 2011(b); Opsteegh et al., 2011; Jokelainen et al., 2012; Beral et al., 2012; Pastiu et al., 2013; Calero-Bernal et al., 2013; Coelho et al., 2014; Wallander et al., 2015; Touloudi et al., 2015; Slany et al., 2016; Calero-Bernal et al., 2016; Reiterova et al., 2016; Roqueplo et al., 2017; Gazzonis et al., 2018; Laforet et al., 2019; Santoro et al., 2019; Sgroi et al., 2020 | |
| Trichinellosis | Hurnikova & Dubinsky, 2009; Pozio et al., 2009; Szell et al., 2012; Jokelainen et al., 2012; Borza et al., 2012; Zivojinovic et al., 2013; Santrac et al., 2015; Nicorescu et al., 2015; Bandino et al., 2015; Kirjusina et al., 2015; Touloudi et al., 2015; Zamora et al., 2015; Karssin et al., 2016; Gazzonis et al., 2018; Antolová et al., 2020 | ||
| Sarcocystosis | Calero-Bernal et al., 2016; Imre et al., 2017; Gazzonis et al., 2019 | ||
| Alariosis | Gazzonis et al., 2018; Gavrilovic, 2019 | ||
| Equinococcosis | Umhang et al., 2014; Sgroi et al., 2019 | ||
| Piroplasmosis | Gimenez et al., 2009; Silaghi et al., 2014 | ||
| Rickettsiosis | Ortuño et al., 2006 | ||
| Cryptosporidiosis | Garcia-Presedo et al., 2013 | ||
| Ascaridiosis | Castagna et al., 2019; Petersen et al., 2020 | ||
| Sparganosis | Kolodziej et al., 2016 | ||
| Trichuriasis | Castagna et al., 2019 | ||
| Blastocystiosis | Russini et al., 2020 | ||
| Zoonotic nematods infection | Mizgajska-Wiktor & Jarosz, 2010; Petersen et al., 2020; Tamara et al., 2020 | ||
| Macracanthorhynchus hirudinaceus infection | Gassó et al., 2016 | ||
| Fungal agents | Enterocytozoonosis | Dashti et al., 2020 | |
| Only Red Deer | Bacterial agents | Q fever | Gonzalez-Barrio et al., 2015; Fernandez-Aguilar et al., 2016; Candela et al., 2017; San-Miguel Ayanz et al., 2017 |
| Chlamydiosis | Regenscheit et al., 2012; Di Francesco et al., 2012 | ||
| Brucellosis | Serrano et al., 2011; San-Miguel Ayanz et al., 2017 | ||
| Leptospirosis | San-Miguel Ayanz et al., 2017 | ||
| Anaplasmosis | Silaghi et al., 2011; Kiss et al., 2014; Di Domenico et al., 2016; Stigum et al., 2019; Razanske et al., 2019; Jouglin et al., 2019 | ||
| Borreliosis | Mysterud et al., 2019 | ||
| Tuberculosis | Queiros et al., 2016 | ||
| Viral agents | Hepatitis E virus | Neumann et al., 2016; Di Bartolo et al., 2017; Palombieri et al., 2020 | |
| Parasitic agents | Toxoplasmosis | De Craeye et al., 2011; Formenti et al., 2015; San Miguel et al., 2016 | |
| Mange | Oleaga et al., 2008 | ||
| Sarcocystidiosis | Basso et al., 2020 | ||
| Piroplasmosis | Zintl et al., 2011; Ebani et al., 2016; Razanske et al., 2019 | ||
| Zoonotic nematods infection | Gnat et al., 2015 | ||
| Wild Boar + Red Deer | Bacterial agents | Tuberculosis | Parra et al., 2006; Cunha et al., 2012; Madeira et al., 2017 |
| Yersiniosis | Syczylo et al., 2018 | ||
| Colibacillosis | Diaz-Sanchez et al., 2013; Cabal et al., 2017; Dias et al., 2019 | ||
| Salmonellosis | Diaz-Sanchez et al., 2013 | ||
| Anaplasmosis | Portillo et al., 2011; Vichova et al., 2014 | ||
| Campylobacteriosis | Diaz-Sanchez et al., 2013 | ||
| Viral agents | West Nile Virus | Boadella et al., 2012; Hubalek et al., 2017 | |
| Hepatitis E virus | Forgach et al., 2010; Rutjes et al., 2010; Larska et al., 2015; Roth et al., 2016; Kukielka et al., 2016; Thiry et al., 2017; Anheyer-Behmenburg et al., 2017; Spancerniene et al., 2018 | ||
| Tahyna virus infection | Camp et al., 2018 | ||
| Parasitic agents | Zoonotic nematods infection | Galecki et al., 2015 | |
| Giardiosis | Paziewska et al., 2007; Beck et al., 2011; Solarczyk et al., 2012 | ||
| Piroplasmosis | Tampieri et al., 2008; Zanet et al., 2014; Pereira et al., 2016; Kazimirova et al., 2018 | ||
| Cryptospotidiosis | Paziewska et al., 2007 | ||
| Toxoplasmosis | Waap et al., 2016; Bier et al., 2020; Barroso et al., 2020 |
3.1. Spatial pattern
Spatially (Fig. 2), 101 surveys (52%) are in south Europe (mainly Spain and Italy). Followed by Western and Central (n = 43) and East Europe (n = 36). North Europe presented fewer revised surveys (n = 14) in the analysed 15 years (period 2006–2020).
Fig. 2.
Number and percentage of surveys spatially distributed in Europe.
3.2. Temporal pattern
Temporally (Fig. 3), the pattern of several publications about zoonotic diseases in wild boar and red deer oscillates, but with an increasing leaning over the studied 15 years. In the period before 2011 (2006–2010), the maximum number was 6 (in 2009), but in the decade between2011 and2020, the number ranged from 10 to 26, with a clear peak in 2015–2016 (n = 25 in 2015 and n = 26 in 2016, with a total of n = 51, 26% of total).
Fig. 3.
Number of publications per year, between 2006 and 2020.
3.3. Species distribution
Of the 194 included surveys in the systematic review, three surveys obtained data from farmed animals with hunting purposes (1 about red deer and two about wild boar), 160 surveys were performed on hunted animals (118 about wild boar, 17 about red deer and 25 that included both species), 22 surveys only with data about alive wild animals (7 about red deer, 11 about wild boar and 3 surveys with both species) and 9 mixed surveys (wild and hunted animals) distributed as 6 about wild boar, one about red deer and 2 with both species.
There was a clear dominance of surveys carried out only in wild boar (138 surveys, 71%) to the detriment of studies in red deer (26 surveys, 13%). Red deer and wild boar are the most prominent hunting species at the European level; there are 30 surveys (16%) that include both species, focusing mainly on samples of hunted animals.
3.4. Sanitary profile
In the 138 analysed articles whose study target is only the wild boar: different zoonotic bacteria (n = 16), parasites (n = 14), viruses (n = 5) and fungi (n = 1) were found (Table 1).
The zoonotic bacterial agents are in higher numbers, with Salmonella and Leptospira as the most recurrent agents (10 surveys each). Following are infections caused by zoonotic parasitic agents. The most studied parasite in wild boar is Toxoplasma (20 surveys), followed by an approximation of Trichinella (15 surveys). Of the 5 different viral agents analysed, the Hepatitis E virus is the most studied in the scientific community with 36 surveys in wild boar as the only target species.
The articles found in this systematic review, whose study target is only the red deer are in minor numbers compared to the wild boar (n = 23). Regarding the distribution of agents analysed in the surveys, the scenario changes in relation to wild boar (6 different bacteria, 5 parasites and 1 virus). In the red deer surveys, the bacterial agent more studied is the Anaplasma sp. (6 surveys), the parasites are equally the Toxoplasma gondii, and the Piroplasm (3 surveys each), and the only viral agent studied is the Hepatitis E virus, with 3 surveys.
In reports with combined surveys (n = 30), both wild boar and red deer infectious agents were analysed: 6 different bacterial agents, 5 parasitic agents and 3 viral agents. Tuberculosis and Colibacillosis are the zoonotic bacterial infections more studied (3 surveys each). Piroplasmosis and Hepatitis E are the most surveyed parasitic and viral infections, respectively.
Briefly and concentrating the data (Table 1), in the 15 years (2006–2020), the scientific community focused its research efforts on European wild boar health, especially on 5 infectious diseases with zoonotic potential: Hepatitis E (44 surveys), Toxoplasmosis (23 surveys), Trichinellosis (15 surveys), Tuberculosis and Salmonellosis (11 surveys each). Even though the diversity of bacterial agents studied in wild boar is superior, with this systematic review, we can conclude that the highest efforts in surveys about wild boar's zoonotic agents are mainly concentrated on a viral agent, the Hepatitis E virus. Regarding red deer health, the scientific community focuses efforts on surveys of zoonotic agents of this cervid on 3 infectious diseases: Hepatitis E (11 surveys), Anaplasmosis (8 surveys) and Piroplasmosis (7 surveys). Similarly, in the case of the wild boar, the most examined agent is the Hepatitis E virus, for the scientific community in that studied 15 years.
In this systematic review, in the final output, only surveys about zoonotic agents were examined. Still, only in some articles, the zoonotic potential of the studied agent was approached. Few articles expressed a strong,concern about the zoonotic potential of the agent surveyed. Most of those that do are HEV surveys. The agents that are simultaneously foodborne zoonoses (e.g. HEV, Salmonella sp., Yersinia sp., Trichinella sp. and Toxoplasma sp.) are those in which the concern of zoonotic transmission is most evident.
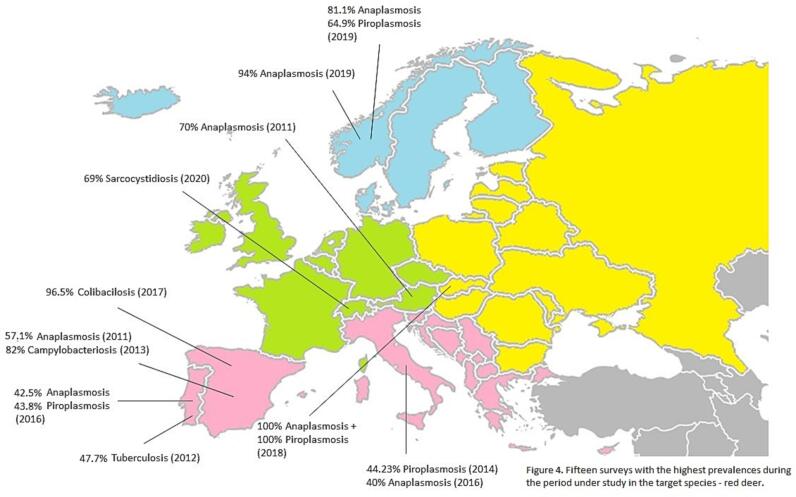
However, it should be taken into account that the number of scientific productions/surveys on certain diseases differs from those with the highest prevalence in the target country and species (Fig. 4, Fig. 5).
Fig. 4.
Fifteen surveys with the highest prevalences during the period under study in the target species - red deer.
Fig. 5.
Fifteen surveys with the highest prevalences during the period under study in the target species - wild boar.
4. Discussion
In overall research about the topic, only non-systematic literature reviews on wild boar and red deer zoonoses were found, this being the first on the subject. An increase over the 15 years studied that the number of publications on surveys of zoonoses in these two large game species (wild boar and red deer) has observed and is the majority on wild boar. Interest by the scientific community is growing, and the need to monitor these populations has become evident to prevent future outbreaks. And since the wild boar is the most abundant large game species in all of Europe and that is for many of the target zoonoses. They can be considered a sentinel species and so,the,most significant number of publications focus on this species.
With this systematic review of 15 years of scientific publications in this area, concise and schematic conclusions were drawn about which zoonoses cause the most concern and impact European populations of large game species from a One Health perspective.
Ruiz-Fons, in 2017 [4], in his review, mentioned viruses such as Hepatitis E virus, Japanese Encephalitis virus, Influenza virus and Nipah virus, and bacteria such as Salmonella sp., Shiga toxin-producing Escherichia coli, Campylobacter sp. and Leptospira sp. as the most prone to be transmitted from wild boar to humans. As mentioned in other literature reviews, foodborne pathogens are those of most zoonotic concern relatedto hunted animals, such as Hepatitis E virus, Salmonella sp. and Trichinella sp. [13,14]. This concern is mainly related to the possible consumption of unsafety meat from large game species [13], leading to diseases outbreaks with marked causes and facilitating the epidemiological investigation of zoonotic transmission. In this current systematic review, surveys about all these pathogens were found, but in lower numbers, except HEV. However, looking at Fig. 5, in the case of wild boar, these foodborne diseases are those that appear most in the top 15 surveys for the period 2006–2020 with the highest prevalence (e.g. Colibacillosis, Sarcocystiosis and Yersiniosis). But not the most studied, probably because their impact on wild populations is low in the eyes of the scientific community [6,9]. In the case of red deer, this disparity is not observed in relation to foodborne diseases, but in relation to tick-borne diseases. Since the vast majority of studies on red deer that reveal the highest prevalence are related to diseases such as Piroplasmosis and Anaplasmosis.
Regarding the most studied zoonotic agents, the HEV infection is described in several studies carried out in different European countries, which occurs sero studies and/or RNA extraction in different organic matrices. The actual reported prevalence of HEV in wild boar varies widely. With no apparent explanation and without being straightly related to the year of publication, time of year, country and diagnostic matrix used. For example, several studies published in 2015 in Italy show a distinct pattern of infection: 4 articles with different seroprevalences (56.2%; 40.7%; 10.2%; 4.9%) [[15], [16], [17], [18]] and 3 articles with different real prevalences tested in different matrices (1.90% and 3.7% RNA-positive in liver and 9.4% in faeces) [15,17,19]. Equally, it is recognized that HEV's prevalence is generally highest in the south of Europe (Mediterranean countries such as Italy, Portugal, and Spain). The surveys of the Mediterranean countries state the highest prevalence of HEV using molecular identification (PCR); the majority present relevant occurrence to 25% in diverse matrices such as liver, bile samples and serum [20]. The survey that stands out is precisely one in Italy in 2018, with 52.20% of positives using molecular identification (PCR) in 92 wild boar livers [21]. And about the HEV seroprevalence in these southern European countries, the results of the surveys reveal seroprevalences above 25% in almost all articles, and they even reach 56.2% (Italy, 2015), 57.6% and 59% (2 surveys in Spain, 2019) [17,22,23].
For studies that include this zoonosis during these 15 years, many were also carried out in central Europe (e.g. Germany, Poland, and Estonia). Although the overall results are of lower prevalence than those of southern countries, a survey carried out in Germany in 2009 reports the absolute value of HEV prevalence of 68.2% by molecular identification with PCR encompassing matrices in the study (liver, bile and blood) [24].
As mentioned above, in the results paragraph, surveys about HEV are those in which more references are made about the zoonotic potential of this agent and the consequent concerns about public health. In industrialized countries, Hepatitis E is now recognized as an emerging zoonosis. Embrace European Food Safety Authority (EFSA) data, autochthonous cases have increased over recent years in Europe and are mainly associated with HEV genotype 3 infections, with pigs and wild boars considered the main reservoirs [25]. Within this frame of reference, a human infection can occur through direct contact with infected animals or by ingesting raw or undercooked wild boar meat, being the liver the edible product with major risk since it is considered the primary target place of HEV replication in swine and an essential organ in the pathogenesis of the disease [26]. Aprea et al. [27] describe several foodborne transmissions of HEV genotypes 3 and 4 in industrialized countries, which were linked to the ingestion of uncooked deer and wild boar meat, and raw pork liver, like the one described by Rivero-Juarez et al., in 2017 [28].
The two zoonoses in a large game most studied by the scientific community next to HEV, are two parasitic infections: Toxoplasmosis and Trichinellosis. Regarding the most recent surveys on Toxoplasmosis, three from 2020 stand out. Two encompass both target species of this systematic review and one only the wild boar. One in Germany [29], which reveals a seroprevalence of 39% in wild boar and 30.7% in red deer, and another survey in Spain [30], using the modified agglutination test (MAT), obtained seroprevalences of 24.4% in wild boar and 6.4% in red deer. The third highlighted article is one Italian survey in which molecular identification (PCR) was performed in several samples (263 brains, 310 hearts and 311 masseters) from 338 wild boars and obtained 39.60% of positives [31]. Toxoplasmosis is one of the most common parasitic zoonoses worldwide [32], being 6 billion people infected with this parasite [33]. As foodborne, humans may become infected by ingesting contaminated food and water by sporulated oocysts or ingesting cysts (bradyzoites) from infected tissues [34]. According to Gazzonis et al. [32], the ingestion of raw or undercooked meat of livestock containing tissue cysts is considered an important source of infection for humans. Still, the increased consumption of game meat, including wild boar and red deer, is expected to be measured as an emerging risk factor for Toxoplasma gondii infection in humans [32,35]. Furthermore, monitoring T. gondii infection has been indicated as a high priority, not only in domestic animals but also in the large game [36].
An additional parasitic zoonosis emphasised in this systematic review is Trichinellosis. The reported prevalence accessed by methods of artificial digestion of wild boar muscle is low across Europe, ranging from 0% to 2.5%, except for a survey in Serbia in 2013, whose prevalence is 11.7% [37]. The seroprevalence increase, and one survey in Greece in 2015 reports 6.4%, and another in Estonia in 2016 reports 17.4% of seropositivity [38,39]. Contempt the low prevalence of Trichinella sp. in wild boars meat in the surveys attained in this systematic review, wild boar meat is considered the second source of infection of this parasite for humans worldwide. Several European countries have reported human Trichinellosis originating from wild boar meat, such as Spain, Bulgaria and Romania. A misdiagnosis of this pathology in wild boars is described in the case of self-consumption of this wild meat, and therefore, the risk is so great that it will be considered an emerging Public Health case [40].
At last, the two bacterial infections most studied by the scientific community over these 15 years were Salmonellosis and Tuberculosis. Salmonella sp. is one of the most common zoonotic pathogens shared by wild animals and humans. Similarly, according to Gil-Molino et al. [41], wild boar has a a significant potential role as carriers of Salmonella sp. Moreover, this zoonotic foodborne pathogen was highly prioritised in wild boar meat safety assurance [36]. Unfortunately, in contrast to the abundance of literature on the prevalence of Salmonella sp. in humans and domestic pigs, data on Salmonella sp. in wild animals and meat are very limited. There was a concentration of published surveys on this pathology in large game species between 2010 and 2014, a minority after 2015, in which only 3 surveys were analysed. The gold standard diagnosis of Salmonellosis is the culture of faeces/intestinal content. In articles where this technique was applied, the prevalence varies between 0.8% and 49% (both studies in Spain in 2013) [42,43]. When surveys are based on seroprevalences, it ranges from 4.3% (in Greece in 2015) and 19.3% (in Spain in 2020) [38,44].
Sideways with Salmonellosis, the zoonosis of bacterial origin that most surveys present in this systematic review, is TB. This is an extremely important zoonosis in the large game [45]. Although the number of surveys found is lower than of HEV or Toxoplasmosis, over the last few years, TB has produced much interest in the scientific community, particularly due to its strong character of multi-host zoonosis. Regarding this zoonosis, in the open access literature, there are many studies on the prevalence of TB and its impact on large game species. Still, these studies are usually associated with the impact of the potential transmission at the interface between wildlife-livestock and humans, as well [46]. It is common sense that this is a disease with great economic impact, and in livestock, in several countries, there are eradication programs in place for many decades [47]. The scientists most committed to studying this phenomenon are those from southern European countries, namely Mediterranean countries such as Spain, Italy or Portugal. This tendency was also confirmed in the surveys eligible for this systematic review: of the 12 articles, 7 address Spanish data and 4 are Portuguese, mostly published between 2016 and 2018. Some report Tuberculosis-like lesions (TBL), with post-mortem inspection data, as a method of diagnosis, though this is not the true gold standard for TB [[48], [49], [50]]. But other surveys present molecular identification and bacterial culture data but are usually applied to selected samples after positivity to post-mortem inspection (e.g. 53.9% of TBL and 21.1% of confirmed TB positivity after culture of TBL samples) [51]. As for serodiagnosis, some surveys were included, ranging the seropositivity between 2.4% in Portugal [52] and 17% in Spain [53].
Tuberculosis is not a true foodborne disease; that is, in the literature allied with large game meat, it is not referred to as a zoonosis to be careful. But, considering that it can be transmitted by aerosols and contact with TBL during evisceration and initial examination, this has a unique role as a Public Health risk. It is a very important zoonosis for game managers, hunters, veterinarians and game meat slaughterhouse professionals [46,54].
Preventive measures are required. The biggest worry is usually linked to not consuming raw or undercooked meatto prevent foodborne infections such as Salmonellosis, Toxoplasmosis, Trichinellosis and Hepatitis E. However, hunters, game managers, veterinarians and large game meat consumers should be more counselled on handlingof wild meat safely. Not only, how to consume and the evisceration procedure and pre-treatment of the meat before cooking, but also the biosecurity measures to do this, too. Individual protection measures for that eviscerating, preparing the carcasses, and performing the initial examination/inspection should always be used to minimize the risk of transmission of zoonotic agents while handling the hunted animals' carcasses, as in the case of TB [55]. And when studies are performed on animals in-vivo, these individual protection measures are also essential.
5. Conclusions
Briefly, it can be concluded that in the 15-year period studied (2015–2020), the concern of the scientific community in relation to zoonoses that affect wild fauna, especially large game species such as wild boar and red deer, is evident and has a growing trend. Zoonotic agents such as HEV, TB, Salmonella sp., Trichinella sp., and Toxoplasma sp. as those that have been the subject of more studies, although they are not those that present the agents with the highest prevalence in the analysed studies. It is clear in some surveys, the concern and the in-depth study of the zoonotic power of these agents and the consequences of a potential outbreak for humans, being HEV that is evident.
Up till now, the consequences of zoonotic transmission between wild boar or red deer and humans did not have significance at a pandemic level. Still, this potential circumstance cannot be forgotten. Most of the zoonoses stated in this systematic review are multi-host, knowing that their flow in the ecosystem does not depend solely on a host and its interaction with humans. Several cases of zoonotic transmission are designated in the literature, but most are related to hunters or professionals in the hunting area, mostly through large game meat.
Large game species may pose a risk to humans and other domestic animals, especially in areas of marked interface, as reported in many publications in southern Europe, especially in Mediterranean countries. This interface eases the interaction, transmission, and circulation of the most impactful zoonoses that cause more significant concern in large game populations, such as Tuberculosis, Hepatitis E, Salmonellosis, Toxoplasmosis and Trichinellosis, as concluded in the current systematic review.
The scientific community must adopt a surveillance, monitoring and prevention policy, not one reactive action. There are still many breaks in the knowledge of large game epidemiology and ecology of these marked zoonotic agents, which should on be the board of future research approaches. From the One Health perspective, it is essential to prevent and carry out predictive and innovative surveys to discover if some zoonotic agents circulate and not just study their prevalence after knowing that they already exist in the population of these two species of large game. According to the New Animal Health Law, “Prevention is better than cure”, and this should be a motto to use in this state, where innovative surveys and actions to raise awareness of game stakeholders should be promoted.
Author contributions
Conceptualization: AC and MMVP; Data curation: AC; Formal analysis and Methodology: AC and MMVP; Software: AC; Supervision: MMVP; Validation: MMVP; Roles/Writing - original draft: AC; Writing – review: MMVP & editing: AC and MMVP.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Portuguese Foundation for Science and Technology (FCT) [UIDB/CVT/00772/2020 and LA/P/0059/2020].
Data availability
No data was used for the research described in the article.
References
- 1.Sommer R.S., Zachos F.E., Street M., Jöris O., Skog A., Benecke N., Late N. Quaternary distribution dynamics and phylogeography of the red deer (Cervus elaphus) in Europe. Quat. Sci. Rev. 2008;27:714–733. doi: 10.1016/j.quascirev.2007.11.016. [DOI] [Google Scholar]
- 2.Consortium E., Croft S., Smith G., Acevedo P., Vicente J. Wild boar in focus: review of existing models on spatial distribution and density of wild boar and proposal for next steps. EFSA J. 2018;15(10) doi: 10.2903/sp.efsa.2018.EN-1490. [DOI] [Google Scholar]
- 3.Gortázar C., Acevedo P., Ruiz-Fons F., Vicente J. Disease risks and overabundance of game species. Eur. J. Wildl. Res. 2006;52:81–87. doi: 10.1007/s10344-005-0022-2. [DOI] [Google Scholar]
- 4.Ruiz-Fons F. A review of the current status of relevant zoonotic pathogens in wild swine (sus scrofa) populations: changes modulating the risk of transmission to humans. Transbound. Emerg. Dis. 2017;64:68–88. doi: 10.1111/tbed.12369. [DOI] [PubMed] [Google Scholar]
- 5.Wiethoelter A.K., Beltrán-Alcrudo D., Kock R., Mor S.M. Global trends in infectious diseases at the wildlife–livestock interface. PNAS. 2015;112:9662–9667. doi: 10.1073/pnas.1422741112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Fons F., Segalés J., Gortázar C. A review of viral diseases of the European wild boar: effects of population dynamics and reservoir role. Vet. J. 2008;176(2):158–169. doi: 10.1016/j.tvjl.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stillfried M., Gras P., Börner K., Göritz F., Painer J., Röllig K., Wenzler M., Hofer H., Ortmann S., Kramer-Schadt S. Secrets of success in a landscape of fear: urban wild boar adjust risk perception and tolerate disturbance. Front. Ecol. Evol. 2017;5:157. doi: 10.3389/fevo.2017.00157. [DOI] [Google Scholar]
- 8.Fernández-Aguilar X., Gottschalk M., Aragon V., Càmara J., Ardanuy C., Velarde R., Galofré-Milà N., Castillo-Contreras R., López-Olvera J.R., Mentaberre G., Colom-Cadena A., Lavín S., Cabezón O. Urban wild boars and risk for zoonotic Streptococcus suis, Spain. Emerg. Infect. Dis. 2018;24(6):1083–1086. doi: 10.3201/eid2406.171271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredriksson-Ahomaa M. Wild boar: a reservoir of foodborne zoonoses. Foodborne Pathog. Dis. 2019;16(3):153–165. doi: 10.1089/fpd.2018.2512. [DOI] [PubMed] [Google Scholar]
- 10.Ebani V.V., Rocchigiani G., Bertelloni F., Nardoni S., Leoni A., Nicoloso S., Mancianti F. Molecular survey on the presence of zoonotic arthropod-borne pathogens in wild red deer (Cervus elaphus) Comp. Immunol. Microbiol. Infect. Dis. 2016;47:77–80. doi: 10.1016/j.cimid.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Ozioko K.U., Okoye C.I., Obiezue R.N., Agbu R.A. Knowledge, attitudes, and behavioural risk factors regarding zoonotic infections among bushmeat hunters and traders in Nsukka, Southeast Nigeria. Epidemiol. Health. 2018;16 doi: 10.4178/epih.e2018025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnat S., Trościańczyk A., Nowakiewicz A., Majer-Dziedzic B., Ziółkowska G., Dziedzic R., Zięba P., Teodorowski O. Experimental studies of microbial populations and incidence of zoonotic pathogens in the faeces of red deer (Cervus elaphus) Lett. Appl. Microbiol. 2015;61:446–452. doi: 10.1111/lam.12471. [DOI] [PubMed] [Google Scholar]
- 13.Fredriksson-Ahomaa M., London L., Skrzypczak T., Kantala T., Laamanen I., Biström M., Maunula L., Gadd T. Foodborne zoonoses common in hunted wild boars. EcoHealth. 2020;17:512–522. doi: 10.1007/s10393-020-01509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes-Neves E., Abrantes A.C., Vieira-Pinto M., Muller A. Wild game meat—a microbiological safety and hygiene challenge? Curr. Clin. Microbiol. Rep. 2021;8:31–39. doi: 10.1007/s40588-021-00158-8. [DOI] [Google Scholar]
- 15.Caruso C., Modesto P., Bertolini S., Peletto S., Acutis P.L., Dondo A., Robetto S., Mignone W., Orusa R., Ru G., Masoero L. Serological and virological survey of Hepatitis E virus in wild boar populations in northwestern Italy: detection of HEV subtype 3e and 3f. Arch. Virol. 2015;160:153–160. doi: 10.1007/s00705-014-2246-5. [DOI] [PubMed] [Google Scholar]
- 16.Martinelli N., Pavoni E., Filogari D., Ferrari N., Chiari M., Canelli E., Lombardi G. Hepatitis E virus in wild boar in the central northern part of Italy. Transbound. Emerg. Dis. 2015;62:217–222. doi: 10.1111/tbed.12118. [DOI] [PubMed] [Google Scholar]
- 17.Mazzei M., Nardini R., Verin R., Forzan M., Poli A., Tolari F. Serologic and molecular survey for hepatitis E virus in wild boar (sus scrofa) in Central Italy. New Microb. New Infect. 2015;7:41–47. doi: 10.1016/j.nmni.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montagnaro S., De Martinis C., Sasso S., Ciarcia R., Damiano S., Auletta L., Iovane V., Zottola T., Pagnini U. Viral and antibody prevalence of hepatitis E in European wild boars (sus scrofa) and hunters at zoonotic risk in the Latium region. J. Comp. Pathol. 2015;153(1):1–8. doi: 10.1016/j.jcpa.2015.04.00. [DOI] [PubMed] [Google Scholar]
- 19.Serracca L., Battistini R., Rossini I., Mignone W., Peletto S., Boin C., Pistone G., Ercolini R., Ercolini C. Molecular investigation on the presence of Hepatitis E virus (HEV) in wild game in North-Western Italy. Food Environ. Virol. 2015;7:206–212. doi: 10.1007/s12560-015-9201-9. [DOI] [PubMed] [Google Scholar]
- 20.Mesquita J.R., Oliveira R.M.S., Coelho C., Vieira-Pinto M., Nascimento M.S.J. Hepatitis E virus in sylvatic and captive wild boar from Portugal. Transbound. Emerg. Dis. 2016;63:574–578. doi: 10.1111/tbed.12297. [DOI] [PubMed] [Google Scholar]
- 21.De Sabato L., Ostanello F., De Grossi L., Marcario A., Franzetti B., Monini M., Di Bartolo I. Molecular survey of HEV infection in wild boar population in Italy. Transbound. Emerg. Dis. 2018;65:1749–1756. doi: 10.1111/tbed.12948. [DOI] [PubMed] [Google Scholar]
- 22.Caballero-Gómez J., Jiménez-Ruiz S., Lopez-Lopez P., Vicente J., Risalde M.A., Cano-Terriza D., Frias M., Barasona J.A., Rivero A., García-Bocanegra I., Rivero-Juarez A. Emergent subtype of hepatitis E virus genotype 3 in wild boar in Spain. Transbound. Emerg. Dis. 2019;66(5):1803–1808. doi: 10.1111/tbed.13251. [DOI] [PubMed] [Google Scholar]
- 23.Wang H., Castillo-Contreras R., Saguti F., López-Olvera J.R., Karlsson M., Mentaberre G., Lindh M., Serra-Cobo J., Norder H. Genetically similar hepatitis E virus strains infect both humans and wild boars in the Barcelona area, Spain, and Sweden. Transbound. Emerg. Dis. 2019;66:978–985. doi: 10.1111/tbed.13115. [DOI] [PubMed] [Google Scholar]
- 24.Adlhoch C., Wolf A., Meisel H., Kaiser M., Ellerbrok H., Pauli G. High HEV presence in four different wild boar populations in East and West Germany. Vet. Microbiol. 2009;139(3–4):270–278. doi: 10.1016/j.vetmic.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 25.De Sabato L., Amoroso M.G., Ianiro G., Esposito C., De Grossi L., Fusco G., Barone A., Martini E., Ostanello F., Di Bartolo I. Detection of Hepatitis E virus in livers and muscle tissues of wild boars in Italy. Food Environ. Virol. 2020;12:1–8. doi: 10.1007/s12560-019-09405-0. [DOI] [PubMed] [Google Scholar]
- 26.Risalde M.A., Rivero-Juarez A., Romero-Palomo F., Frıas M., Lopez-Lopez P., Cano-Terriza D., Garcıa-Bocanegra I., Jimenez-Ruiz S., Camacho A., Machuca I., Gomez-Villamandos J.C., Rivero A. Persistence of hepatitis E virus in the liver of non-viremic naturally infected wild boar. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aprea G., Amoroso M.G., Di Bartolo I., D’Alessio N., Di Sabatino D., Boni A., Cioffi A., D’Angelantonio D., Scattolini S., De Sabato L., Cotturone G., Pomilio F., Migliorati G., Galiero G., Fusco G. Molecular detection and phylogenetic analysis of hepatitis E virus strains circulating in wild boars in south-central Italy. Transbound. Emerg. Dis. 2018;65:25–31. doi: 10.1111/tbed.12661. [DOI] [PubMed] [Google Scholar]
- 28.Rivero-Juarez A., Frias M., Martinez-Peinado A., Risalde M.A., Rodriguez-Cano D., Camacho A., García-Bocanegra I., Cuenca-Lopez F., Gomez-Villamandos J.C., Rivero A. Familial Hepatitis E outbreak linked to wild boar meat consumption. Zoonoses Public Health. 2017;64:561–565. doi: 10.1111/zph.12343. [DOI] [PubMed] [Google Scholar]
- 29.Bier N.S., Stollberg K., Mayer-Scholl A., Johne A., Nöckler K., Richter M. Seroprevalence of toxoplasma gondii in wild boar and deer in Brandenburg, Germany. Zoonoses Public Health. 2020;67:601–606. doi: 10.1111/zph.12702. [DOI] [PubMed] [Google Scholar]
- 30.Barroso P., García-Bocanegra I., Acevedo P., Palencia P., Carro F., Jiménez-Ruiz S., Almería S., Dubey J.P., Cano-Terriza D., Vicente J. Long-term determinants of the seroprevalence of toxoplasma gondii in a wild ungulate community. Animals. 2020;10(12):2349. doi: 10.3390/ani10122349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sgroi G., Viscardi M., Santoro M., Borriello G., D’Alessio N., Boccia F., Pacifico L., Fioretti A., Veneziano V., Fusco G. Genotyping of toxoplasma gondii in wild boar (sus scrofa) in southern Italy: epidemiological survey and associated risk for consumers. Zoonoses Public Health. 2020;67:805–813. doi: 10.1111/zph.12762. [DOI] [PubMed] [Google Scholar]
- 32.Gazzonis A.L., Villa L., Riehn K., Hamedy A., Minazzi S., Olivieri E., Zanzani S.A., Manfredi M.T. Occurrence of selected zoonotic food-borne parasites and first molecular identification of Alaria alata in wild boars (sus scrofa) in Italy. Parasitol. Res. 2018;117:2207–2215. doi: 10.1007/s00436-018-5908-5. [DOI] [PubMed] [Google Scholar]
- 33.Torgerson P.R., Macpherson C.N.L. The socioeconomic burden of parasitic zoonoses: global trends. Vet. Parasitol. 2011;182:79–95. doi: 10.1016/j.vetpar.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Santoro M., Viscardi M., Sgroi G., Alessio N.Dʼ., Veneziano V., Pellicano R., Brunetti R., Fusco G. Real-time PCR detection of toxoplasma gondii in tissue samples of wild boars (sus scrofa) from southern Italy reveals high prevalence and parasite load. Parasit. Vectors. 2019;12:335. doi: 10.1186/s13071-019-3586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slany M., Reslova N., Babak V., Lorencova A. Molecular characterization of toxoplasma gondii in pork meat from different production systems in the Czech Republic. Int. J. Food Microbiol. 2016;238:252–255. doi: 10.1016/j.ijfoodmicro.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 36.EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) The European Union One Health 2019 zoonoses report. EFSA J. 2021;19(2) doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zivojinovic M., Sofronic-Milosavljevic L.J., Cvetkovic J., Pozio E., Interisano M., Plavsic B., Radojicic S., Kulisic Z. Trichinella infections in different host species of an endemic district of Serbia. Vet. Parasitol. 2013;194(2–4):136–138. doi: 10.1016/j.vetpar.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 38.Touloudi A., Valiakos G., Athanasiou L.V., Birtsas P., Giannakopoulos A., Papaspyropoulos K., Kalaitzis C., Sokos C., Tsokana C.N., Spyrou V., Petrovska L., Billinis C. A serosurvey for selected pathogens in Greek European wild boar. Vet. Rec. Open. 2015;2 doi: 10.1136/vetreco-2014-000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kärssin A., Velström K., Gómez-Morales M.A., Saar T., Jokelainen P., Lassen B. Cross-sectional study of anti-Trichinella antibody prevalence in domestic pigs and hunted wild boars in Estonia. Vector-Borne Zoonotic Dis. 2016:604–610. doi: 10.1089/vbz.2016.1943. Sept2016. [DOI] [PubMed] [Google Scholar]
- 40.Vieira-Pinto M., Fernandes A.R.G., Santos M.H., Marucci G. Trichinella britovi infection in wild boar in Portugal. Zoonoses Public Health. 2021;68:103–109. doi: 10.1111/zph.12800. [DOI] [PubMed] [Google Scholar]
- 41.Gil-Molino M., Risco D., Gonçalves-Blanco P., Fernandez Llario P., Quesada-Molina A., García-Sánchez A., Cuesta-Gerveno J.M., Gómez-Gordo L., Martín-Cano F., Pérez-Martínez R., Varela-Fernández E., Rey-Pérez J. Outbreaks of antimicrobial resistant Salmonella Choleraesuis in wild boars piglets from central-western Spain. Transbound. Emerg. Dis. 2019;66:225–233. doi: 10.1111/tbed.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Díaz-Sánchez S., Sánchez S., Herrera-León S., Porrero C., Blanco J., Dahbi G., Blanco J.E., Mora A., Mateo R., Hanning I., Vidal D. Prevalence of Shiga toxin-producing Escherichia coli, Salmonella spp. and Campylobacter spp. in large game animals intended for consumption: relationship with management practices and livestock influence. Vet. Microbiol. 2013;163(3–4):274–281. doi: 10.1016/j.vetmic.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 43.Mentaberre G., Porrero M.C., Navarro-Gonzalez N., Serrano E., Domínguez L., Lavín S. Cattle drive Salmonella infection in the wildlife–livestock interface. Zoonoses Public Health. 2013;60:510–518. doi: 10.1111/zph.12028. [DOI] [PubMed] [Google Scholar]
- 44.Ortega N., Fanelli A., Serrano A., Martínez-Carrasco C., Escribano F., Tizzani P., Candela M. Salmonella seroprevalence in wild boar from Southeast Spain depends on host population density. Res. Vet. Sci. 2020;132:400–403. doi: 10.1016/j.rvsc.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Aranha J., Abrantes A.C., Gonçalves R., Miranda R., Serejo J., Vieira-Pinto M. GIS as an epidemiological tool to monitor the spatial–temporal distribution of tuberculosis in large game in a high-risk area in Portugal. Animals. 2021;11(8):2374. doi: 10.3390/ani11082374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De La Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis. 2006;86(2):77–109. doi: 10.1016/j.tube.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Schiller I., Oesch B., Vordermeier H.M., Palmer M.V., Harris B.N., Orloski K.A., Buddle B.M., Thacker T.C., Lyashchenko K.P., Waters W.R. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 2010;57:205–220. doi: 10.1111/j.1865-1682.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 48.Parra A., García A., Inglis N.F., Tato A., Alonso J.M., Hermoso de Mendoza M., Hermoso de Mendoza J., Larrasa J. An epidemiological evaluation of Mycobacterium bovis infections in wild game animals of the Spanish Mediterranean ecosystem. Res. Vet. Sci. 2006;80(2):140–146. doi: 10.1016/j.rvsc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Queirós J., Vicente J., Alves P.C., De La Fuente J., Gortazar C. Tuberculosis, genetic diversity and fitness in the red deer, Cervus elaphus. Infect. Genet. Evol. 2016;43:203–212. doi: 10.1016/j.meegid.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 50.Madeira S., Manteigas A., Ribeiro R., Otte J., Fonseca A.P., Caetano P., Abernethy D., Boinas F. Factors that influence mycobacterium bovis infection in Red Deer and wild boar in an epidemiological risk area for tuberculosis of game species in Portugal. Transbound. Emerg. Dis. 2017;64:793–804. doi: 10.1111/tbed.12439. [DOI] [PubMed] [Google Scholar]
- 51.Boadella M., Vicente J., Ruiz-Fons F., De La Fuente J., Gortázar Effects of culling Eurasian wild boar on the prevalence of mycobacterium bovis and Aujeszky's disease virus. Prevent. Vet. Med. 2012;107(3–4):214–221. doi: 10.1016/j.prevetmed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Santos N., Nunes T., Fonseca C., Vieira-Pinto M., Almeida V., Gortázar C., Correia-Neves M. Spatial analysis of wildlife tuberculosis based on a serologic survey using dried blood spots, Portugal. Emerg. Infect. Dis. 2018;24(12):2169–2175. doi: 10.3201/eid2412.171357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varela-Castro L., Alvarez V., Sevilla I.A., Barral M. Risk factors associated to a high Mycobacterium tuberculosis complex seroprevalence in wild boar (Sus scrofa) from a low bovine tuberculosis prevalence area. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abrantes A.C., Vieira-Pinto M. Challenges and insights regarding fenced large game populations and the new EU animal health law. EcoHealth. 2021;18:272–274. doi: 10.1007/s10393-021-01550-y. [DOI] [PubMed] [Google Scholar]
- 55.Vieira-Pinto M., Vinhas B., Coelho C. Initial examination of wild large game on the spot—importance and rules. J. Nutr. Ecol. Food Res. 2014;1:1–3. doi: 10.1166/jnef.2013.1047. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.