Abstract
Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli has been linked to both life-threatening hospital- and community-acquired infections across the globe. Here, we conducted a systematic review and meta-analysis to evaluate the prevalence of ESBL in E. coli isolated from humans, animals, and environments in Bangladesh. Following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, the current systematic review and meta-analysis was taken into account for studies published between 2010 and 2021 in peer-reviewed journals. The meta-analysis was performed on “R” version 4.2.2. A total of 36 studies were included in this systematic review and meta-analysis; among them, 22 were human, seven were animal, four were environmental, and three were multidisciplinary studies. The meta-analysis revealed that the pooled prevalence of ESBL-producing E. coli in Bangladesh was 21% (95% CI: 15%–27%). On the sample basis, the pooled prevalence of ESBL-producing E. coli in humans, animals, and environments was 17% (95% CI: 11%–23%), 22% (95% CI: 9%–34%), and 39% (95% CI: 16%–62%), respectively. All the pooled prevalence of ESBL-producing E. coli showed substantial heterogeneity (I2 > 75%; p < 0.05) among the selected studies. This systematic review reported 13 different types of resistance genes encoding ESBL, such as blaTEM-1 (37.5%), blaCMY (34.6%), blaCTX-M-1 (20.7%), blaCTX-M-15 (16.1%), blaTEM (12.3%), blaCTX-M and blaOXA (9.6%), blaOXA-1 (5.8%), blaampC (3.9%), blaSHV (3.8%), blaCMY-2 (2.3%), blaCTX-M-14 (1.3%), and blaCTX-M-9 (0.3%). Moreover, 39 types of epidemiologically important clones (including ST10 and ST131) were detected in ESBL-producing E. coli isolated from humans, animals, and environments in Bangladesh. To the best of our knowledge, this is the first systematic review and meta-analysis of integrated studies on ESBL-producing E. coli using the One Health approach in Bangladesh. The high prevalence of ESBL-producing E. coli, their resistance genes, and epidemiologically important clones in humans, animals, and environments highlights the importance of implementing comprehensive antimicrobial resistance (AMR) surveillance under a One Health perspective to mitigate the AMR consequences in Bangladesh.
Keywords: ESBL, E. coli, blaCTX-M, blaTEM, Clones, ST10, ST131, One Health, Bangladesh
Highlights
-
•
This is the first systematic review in Bangladesh, narrating ESBL-producing E. coli in One Health components in Bangladesh.
-
•
This review reports a high level of ESBL-producing E. coli from humans, animals, and environments in Bangladesh.
-
•
Thirteen types of resistant genes associated with ESBLs were identified in Bangladesh.
-
•
Thirty-nine types of important E. coli clones (including ST10 and ST131) were reported in Bangladesh.
-
•
The findings highlight research gaps for future AMR mitigation strategies (the One Health approach) in Bangladesh.
1. Introduction
As a major threat to public health, antimicrobial resistance (AMR) reduces the effectiveness of currently available antibiotic treatments for bacterial infections. Due to the ecological niche between patients, antimicrobial exposure, and hospital-adaptation, AMR microbes are commonly found in healthcare facilities [1]. The health of humans, animals, and environments is threatened by the consequences of AMR [2]. Moreover, it is abundantly obvious that AMR in livestock is intricately connected to their existence in humans and environments as well [3]. It is predicted that AMR would result in a significant number of deaths throughout the world [4]. It would also cause massive economic damages and a considerable drop in animal production globally [5].
In both humans and animals, Escherichia coli is one of the most common pathogens responsible for a wide variety of common bacterial diseases. Most of the cases, E. coli typically exists in the gut microbiota, but some E. coli strains are responsible for various severe infections, such as colibacillosis in poultry; mastitis in dairy cattle; urinary tract infection, neonatal meningitis, septicemia, etc. in humans [6,7]. This organism is also capable to develop more devastating effects because of its zoonotic characteristics [8]. Moreover, E. coli pathogen has the opportunity to be transferred to environmental settings from humans and animals.
It has been observed that the prevalence of AMR in E. coli has skyrocketed during the past several years [9]. Most commonly, bacteria produce beta-lactamase enzymes, which, by hydrolyzing the beta-lactam ring, can render the antibiotic ineffective [10]. The fast spread of enzyme-mediated resistance and the resulting rise of multidrug-resistant bacteria have earned it recognition as a global public health problem [11]. Extended-spectrum beta-lactamase (ESBL)-generating E. coli has emerged as a major health threat in both humans and animals. Since beta-lactam antibiotics are extensively used, E. coli has become resistant, resulting in widespread diseases in the community [12,13]. Infections generated by ESBL-producing E. coli pose a significant threat to healthcare settings because of the scarcity of effective empiric treatments [9].
The CTX-M, TEM, and SHV types of enzymes are the core components of ESBL and can be found in a wide variety of pathogens that are considered to be clinically significant around the world [14]. The majority of TEM and SHV types are thought to have originated from parent enzymes such as TEM-1, TEM-2, and SHV-1. This evolution occurred as a result of point mutations that occurred along the active sites of TEM and SHV sequences, which led to an increased spectrum of activity [9,15]. Moreover, there are around 220 different enzyme versions of CTX-M-lactamases. These enzymes are grouped together into five distinct groups, some of which are CTX-M-1, 2, 8, 9, and 25 [16]. Worldwide, ESBLs of the TEM, CTX-M, SHV, and OXA kinds have been reported often in E. coli isolated from humans, environments, animals, and foods derived from animals [[17], [18], [19], [20], [21]].
Widespread reports indicate that excessive and maybe inappropriate use of a variety of antibiotics is a major contributor to the spread of AMR in Bangladesh. The presence of ESBL-producing E. coli in humans, animals, and environments is a public health concern. Moreover, it is important to keep up-to-date data on ESBL-producing E. coli in health systems to minimize the consequences of ESBL-producers. In this study, we conducted a systematic review and meta-analysis on the current status of ESBL-producing E. coli in Bangladesh, taking into account studies conducted in humans, animals, and environments, in order to provide a detailed description and up-to-date information for professionals in a variety of fields.
2. Materials and methods
2.1. Review protocols
The review followed the guidelines for systematic reviews found in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [22]. The review followed the procedures outlined in the PRISMA statement, which included (1) searching database systems for potentially pertinent articles; (2) determining the extent to which the articles are acceptable to the review; (3) assessing the relevancy of the articles; and (4) extracting, screening, and analyzing the data. The protocol was established before starting the meta-analysis.
2.2. Search protocols
We took a rigorous approach to finding original scientific articles that were published between January 2010 and December 2021 and discussed the occurrence and distribution of ESBL-producing E. coli in Bangladesh. We used several database systems, including PubMed, ScienceDirect, Web of Science, BanglaJol, Scopus, Google Scholar, ResearchGate, EMBASE, and Crossref, to conduct a comprehensive literature search. Search terms we used include: “Bangladesh”, “Escherichia coli”, “E. coli”, “Enterobacteriaceae”, “extended-spectrum beta-lactamase”, “extended-spectrum-beta-lactamase”, “extended-spectrum β-lactamase”, “extended-spectrum-β-lactamase”, “ESBL”, “ESBLs”, “double-disk synergy test”, “humans”, “animals”, “environments”, “TEM”, “CTX-M", “SHV”, “CMY”, “OXA”, “AMPc”, and “STs clones”. According to the study's objectives, a list of Boolean keywords was developed, including “AND” (for words in the same category) and “OR” (for words within a category). In addition to this, the reference lists of the articles that were chosen for further examination were provided in order to maximize the likelihood of acquiring other articles. During the survey, we downloaded articles directly from the journals or with the help of the Bangladesh Agricultural University Library Network (http://catalog.bau.edu.bd). The analysis included all articles that were eligible for inclusion. Two investigators (MS Islam and MT Rahman) performed all of the literature searches. All the authors re-examined the sources of all articles according to the international standards for systematic review.
2.3. Study inclusion and exclusion criteria
All the retrieved articles were verified before being included in the systematic review and meta-analysis. All the research articles that met the following standards were eligible for our systematic review and meta-analysis:
-
•
Only those articles that focused on ESBL-producing E. coli isolated from human, animal, and environmental samples.
-
•
Only those articles that had information on the sample size, total number of E. coli isolates, and total number of ESBL-producing E. coli isolates were included.
-
•
Only those articles that were peer-reviewed and published between January 2010 and December 2021 were included.
-
•
All the articles narrating the prevalence, occurrence, or characterization of ESBL-producing E. coli using both phenotypic and genotypic approaches.
The following criteria were used to compile the final list of articles. Articles were excluded from consideration if they met any one of the following criteria: (1) they were duplicates that had already been checked and dismissed; (2) they did not meet the inclusion criteria established; (3) they did not fall within the purview of our study; (4) they were published before 2010; (4) they were unavailable in full-text, or have only title and/or abstract; (5) they were unpublished, review articles or meta-analysis, conference or meeting abstracts.
2.4. Data extraction
Two authors (MS Islam and MT Rahman) took the initiative of going through the entire texts of the studies, taking into account the criteria for study eligibility, extracting reasonable data, and then entering the data into a spreadsheet. The other reviewers (AMMT Rahman and J Hassan) then double-checked these data. The data we extracted include the citation or title of the study, the name of the first author, the published year (2010−21), the study areas (districts), the study period, the sample categories and types, the sample size, the total number or prevalence of E. coli isolates, the total number or prevalence of ESBL-producing E. coli with their detection method (phenotypic and/or genotypic), the types of ESBL-encoding genes with their number or percentage, and the sequencing types of the isolates.
2.5. Statistical analysis
To perform data analysis, all the retrieved data were first imported into Excel-365 (Microsoft/Office 365, Redmond, WA, USA) and subsequently exported to R (version 4.2.2, The R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism (version 8.4.2, GraphPad Software, CA, USA) after sorting the data.
The meta-analysis was carried out in RStudio.v.2022.07.2 + 576 by utilizing the “metaprop” codes that are included in the meta (version 6.0–0) package [23] of the R program. We used the residual maximum likelihood approach for the random-effects model to compute the pooled prevalence and 95% confidence interval (CI) of ESBL-producing E. coli isolated from humans, animals, and environments in Bangladesh. The statistical heterogeneity that existed between the studies was examined with the help of the Cochran's Q test for the significance of heterogeneity and the inconsistency index (I2), where an I2 value of >75% and a significance level of <0.05 (p-value) were considered to indicate a significant statistical variation [24]. Forest plots were prepared using the “forest” codes in the meta package, and images were extracted from the “R” plot using the “jpeg” and “dev.off()” codes. In addition, following the Wilson/Brown Hybrid technique [25], the GraphPad Prism was used to compute the prevalence and 95% CI of ESBLs encoding genes in E. coli isolated from humans, animals, and environments.
The author, MS Islam, performed all the analyses.
3. Results
3.1. Details of the included studies
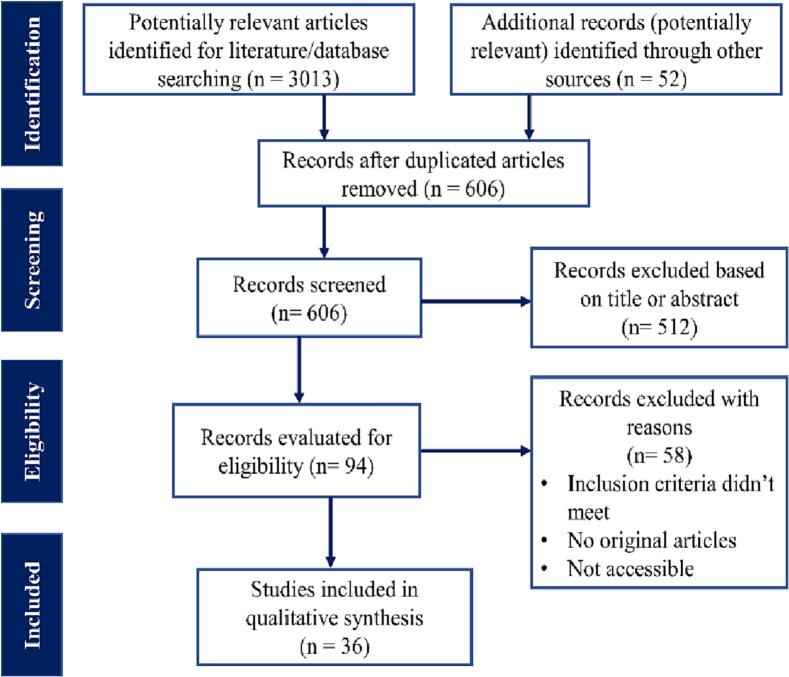
Following the PRISMA guidelines, a total of 3065 articles (3013 articles found in the selected database and 52 in other sources) were included for the initial screening. After removing duplicate articles and articles that deviated from the concept of the review or lacked details, 94 articles were selected for eligibility evaluation. Finally, a total of 36 scientific articles fulfilled the eligibility criteria to be included in our present systematic review and meta-analysis (Fig. 1).
Fig. 1.
A PRISMA flow diagram illustrating the process of selecting studies. We looked through several world-recognized online databases to find qualifying studies that reported ESBL-producing E. coli, and then we used predetermined search algorithms to find those studies. After combining the records and removing duplicates with the help of the Zotero software, the data were subjected to a screening based on the previously established eligibility criteria before being included in the systematic review and meta-analysis.
The study period of the selected articles was between 2003 and 2020, and the published period was between 2010 and 2021. Two articles [26,27] didn't mention the study period. Area-wise, these selected articles covered different districts of Bangladesh, among them, the highest studies were conducted in Dhaka district (21/36), followed by Rajshahi, Mymensingh, Sylhet, and Chottogram. Since both sample materials and scientific facilities were readily available in these regions, the majority of the studies were carried out there. These investigations were carried out in either a single district of Bangladesh or a cluster of districts. Amin et al. [28] conducted their study on samples collected from different districts of Bangladesh (Table 1).
Table 1.
Overall outcomes of studies published between 2010 and 2021 focusing ESBL-producing E. coli in humans, animals, and environments in Bangladesh.
| Study area | Study Period | Published year | Sample categories | Sample types (N) | No. of E. coli | No. of ESBL-producers | ESBL detection method | Genes encoding ESBL | STs | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Rajshahi | 2008 | 2010 | Humans | Wound swabs (125) | 19 | 11 | DD | – | – | [29] |
| Rajshahi | 2009 | 2012 | Wild ducks, ducks, chickens, geese | Cloacal swabs, feces (96) | 66 | 36 | DD, PCR | CTX-M1,9,14,15 | √ | [30] |
| Dhaka | 2008–09 | 2013 | Environments | Water (4) | 4 | 2 | DD | – | – | [31] |
| Dhaka | 2010–11 | 2013 | Humans | Wound swabs, urine (320) | 90 | 29 | DD, PCR | CTM-M, OXA | – | [32] |
| Dhaka | 2010–11 | 2013 | Humans | Urine, pus, sputum, swabs (200) | 114 | 61 | DD | – | – | [33] |
| Chattogram | 2010 | 2014 | Gull | Feces (150) | 85 | 29 | DD, PCR | CTX-M14,15 | √ | [34] |
| Cox's Bazar, Rangamati, Chattogram | 2011 | 2014 | Pigeons | Cloacal swabs (150) | 36 | 7 | DD, PCR | CTX-M15 | √ | [35] |
| Dhaka | 2011–12 | 2014 | Humans | Urine (300) | 112 | 36 | DD | – | – | [36] |
| Dhaka, Sylhet | 2003–07 | 2014 | Humans | Urine, surgical wound swab (339) | 339 | 40 | DD, PCR | TEM, CTX-M1,15, OXA1 | – | [37] |
| Dhaka | NM | 2014 | Humans | Urine (44) | 13 | 7 | DD | – | [26] | |
| Dhaka | 2011 | 2014 | Humans | Urine, pus, wound swab (354) | 120 | 50 | DD, PCR | SHV | – | [38] |
| Mymensingh | 2011 | 2014 | Humans | Urine, skin wound swabs (300) | 156 | 35 | DD, PCR | TEM, CTX-M, SHV | – | [39] |
| Dhaka | 2005 | 2015 | Humans | Urine (250) | 69 | 22 | DD | – | – | [40] |
| Dhaka, Moulavibazar, Sylhet, Rajshahi | 2010 | 2015 | Open Bill Stork, Environment | Feces, river water (170 + 8) | 76 + 8 | 2 + 4 | DD, PCR | CTX-M15 | √ | [41] |
| Mymensingh | 2011 | 2015 | Humans | Wound swab, pus (84) | 39 | 24 | DD | – | – | [42] |
| Dhaka | 2014 | 2016 | Humans | Urine (800) | 90 | 34 | DD, PCR | CTX-M15, OXA1 | √ | [43] |
| Dhaka | 2006–07 | 2016 | Humans | Pus, swab, exudates (125) | 61 | 6 | DD, PCR | TEM, CTX-M1, SHV, OXA | – | [44] |
| Dhaka | 2012 | 2016 | Humans, Poultry | Urine, feces (48 + 40) | 14 + 11 | 11 + 11 | DD, PCR | TEM, CTX-M, OXA | – | [45] |
| Dhaka | 2016 | 2018 | Humans | Urine (220) | 103 | 23 | DD | – | [46] | |
| Mymensingh | 2014–15 | 2018 | Humans | Urine, pus, swab of wound, vaginal, throat (375) | 233 | 100 | PCR | TEM1, CTX-M1,9, SHV, AMPc | √ | [47] |
| Tangail | 2016 | 2018 | Humans, chickens, cattle, Environments | stool, feces, soil/dirt (52 + 104 + 52) | 50 + 102 + 23 | 3 + 2 + 2 | DD, PCR | TEM, CTX-M, OXA1 | – | [48] |
| Dhaka | 2006 | 2018 | Humans | surgical and burn wound (182) | 45 | 17 | DD | – | – | [49] |
| Chandpur | 2017 | 2019 | Humans | Stool (100) | 82 | 74 | DD, PCR | TEM, CTX-M1, OXA1 | – | [50] |
| Dhaka, Bogura | 2014 | 2019 | Environments | Fecal sludge (34) | 26 | 22 | DD, PCR | TEM, CTX-M, OXA | – | [51] |
| Dhaka | 2014 | 2019 | Humans | Urine (200) | 89 | 23 | DD | – | – | [52] |
| Dhaka | 2012 | 2019 | Humans | Urine (90) | 41 | 13 | DD, PCR | TEM, CTX-M | – | [53] |
| Dhaka | NM | 2019 | Humans | Urine (59) | 45 | 18 | DD, PCR | CTX-M, SHV, OXA1, AMPc | – | [27] |
| Cox's Bazar | 2018 | 2020 | Environments | Water (421) | 384 | 66 | DD, PCR | TEM, CTX-M1,15 | – | [54] |
| Dhaka, Sylhet, Mymensingh, Chattogram, Rajshahi | 2019 | 2020 | Broilers, cockerel | Meat (113) | 86 | 74 | DD, PCR | TEM | – | [55] |
| Sylhet, Moulavibazar, Sunamganj, Habiganj | 2020 | 2020 | Broilers, layers | Meat swabs (600) | 381 | 53 | PCR | SHV | – | [56] |
| Sirajganj | 2020 | 2021 | Humans | Urine (589) | 127 | 103 | DD | – | – | [57] |
| Different districts | 2017–18 | 2021 | Poultry Environments | Water (300) | 300 | 183 | DD, PCR | TEM, CTX-M1, SHV, CMY2, OXA1 | – | [28] |
| Dhaka | 2019 | 2021 | Humans | Urine, sputum (100) | 100 | 25 | PCR | TEM, CTX-M15, SHV, OXA, AMPc | √ | [58] |
| Rajshahi | 2020 | 2021 | Broilers, Layers, Sonali | Cloacal swabs (60) | 37 | 13 | DD | – | – | [59] |
| Dhaka | 2015–19 | 2021 | Humans | Nasopharyngeal swab, wound swab, stool, blood (430) | 85 | 26 | PCR | TEM | – | [60] |
| Magura | 2019–20 | 2021 | Migratory birds | Feces (66) | 55 | 21 | DD, PCR | TEM, CTX-M, SHV, CMY | – | [61] |
Here, N = Sample size, ESBL = Extended-spectrum beta-lactamase, STs = Sequence types, DD = Disk diffusion method, PCR = Polymerase chain reaction, NM = Not mentioned.
3.2. Methods detecting ESBLs
Twelve out of 36 studies used a phenotypic assay to detect ESBL-producing E. coli from human, animal, and environmental samples. Most laboratories employ one of three methods to identify bacteria: disc diffusion (double disc synergy test), minimum inhibitory concentration (MIC), or the VITEK system. Moreover, four studies employed genotypic assays (especially polymerase chain reaction assay), and the remaining 20 articles used both phenotypic and genotypic approaches to identify ESBL-producing E. coli (Table 1).
3.3. Study categories
The selected studies were categorized into three classes based on the sample origin: humans, animals, and environments. >60% (22/36) of the studies focused on human samples, followed by animal (19.4%, 7/36) and environmental (11.1%, 4/36) samples. Moreover, three (8.3%) were multidisciplinary studies, analyzing samples collected from two or three categories at the same time. Different types of animals were under consideration for sub-classification of animal samples, including chickens (broilers, layers, cockerels, and sonali), wild birds, ducks, geese, pigeons, and cattle. It is worth noting that several different species of farm animals were examined in certain published works. In addition to sample categories, sample types include urine, different wound swabs, pus, sputum, exudates, swabs from vagina, nasopharynx, and throat, stool, and blood (humans), feces, cloacal swabs, meat, and meat swabs (animals), and water from different sources, fecal sludge, soil, and dirt (environments). Moreover, studies were conducted on a total of 8054 samples, where 70.6% (n = 5686), 19.2% (n = 1549), and 10.2% (n = 819) of the samples were collected from humans, animals, and environments, respectively (Table 1).
3.4. Prevalence of E. coli
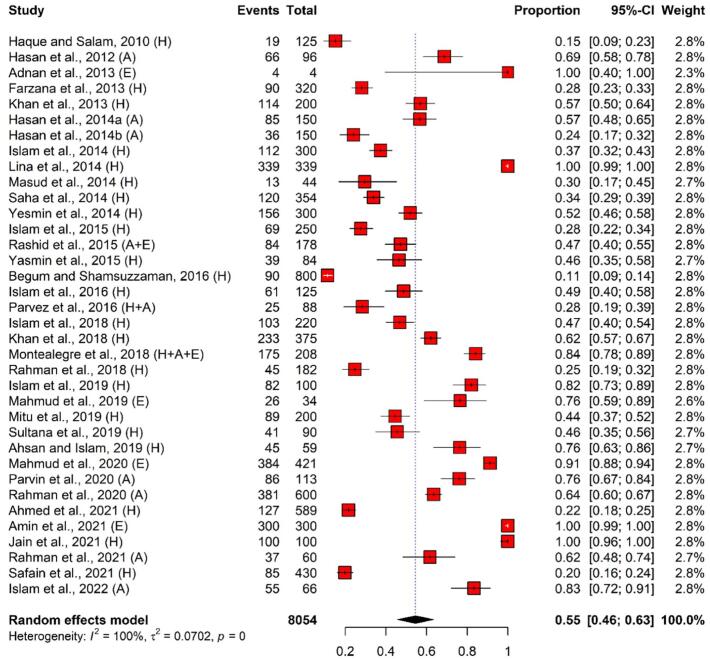
The total number of pooled E. coli isolates was 3916, where 2247 (57.4%), 935 (23.9%), and 734 (18.7%) of the isolates were identified in humans, animals, and environmental samples, respectively. According to the meta-analysis, the overall pooled prevalence of E. coli in the selected 36 scientific articles was 55% (95% CI: 46%–63%) with perfect heterogeneity (I2 = 100%, p < 0.001). Overall, the lowest and highest estimated prevalence of E. coli were 11% (95% CI: 9%–14%) and 100% (99%–100%), respectively. Sample-wise, the maximum and minimum prevalence of E. coli in human samples were 11% (95% CI: 9%–14%) and 100% (99%–100%), respectively; 24% (95% CI: 14%–32%) and 83% (95% CI: 72%–91%) in animal samples; and 76% (95% CI: 59%–89%) and 100% (95% CI: 99%–100%) in environmental samples, respectively (Fig. 2 and Table 1).
Fig. 2.
Forest plot depicting the prevalence of E. coli isolated from humans, animals, and environments in Bangladesh. The estimate of the prevalence was determined by combining the findings of 36 separate studies by applying a random-effects model to the data. An I2 value > 75% and a p-value < 0.05 were deemed statistically significant. The R program was used to analyse the data and generate the figure. Events = No. of positive E. coli isolated from each study, Total = Sample size of each study, CI = Confidence interval, H = Human, A = Animal, E = Environment.
3.5. Distribution of ESBL-producing E. coli
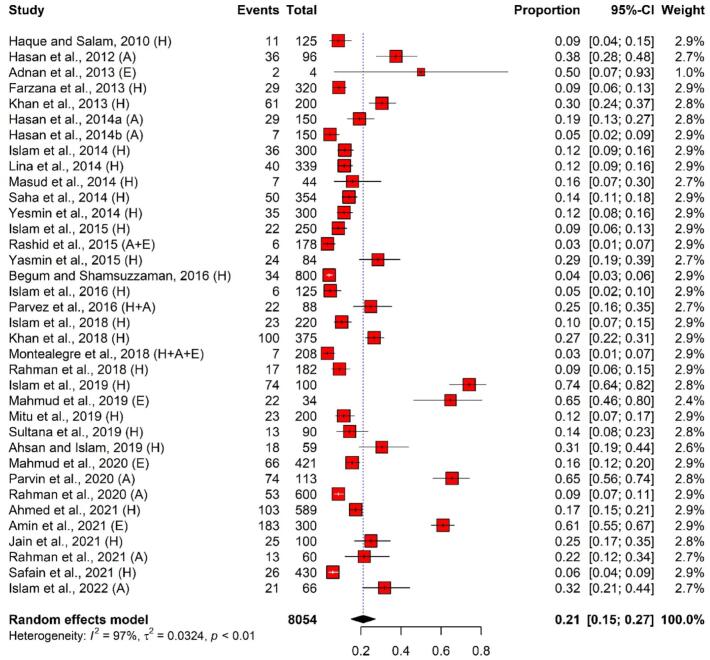
In total, the pooled number of ESBL-producing E. coli isolates was 1318 (isolated from 8054 samples), revealing a pooled prevalence of 21% (95% CI: 15%–27%). The pooled prevalence of ESBL-producing E. coli isolates among different selected studies showed significant heterogeneity (I2 = 97%, p < 0.01). Overall, the highest and lowest prevalence of ESBL-producing E. coli isolated from humans, animals, and environments was 3% (95% CI: 1%–7%) and 74% (95% CI: 64%–82%) (Fig. 3).
Fig. 3.
Forest plot depicting the prevalence of ESBL-producing E. coli isolated from One Health components in Bangladesh. The estimate of the prevalence was determined by combining the findings of 36 separate studies by applying a random-effects model to the data. An I2 value > 75% and a p-value < 0.05 were deemed statistically significant. The R program was used to analyse the data and generate the figure. Events = No. of positive ESBL-producing E. coli isolated from each study, Total = Sample size of each study, CI = Confidence interval, H = Human, A = Animal, E = Environment.
3.5.1. Studies focusing humans
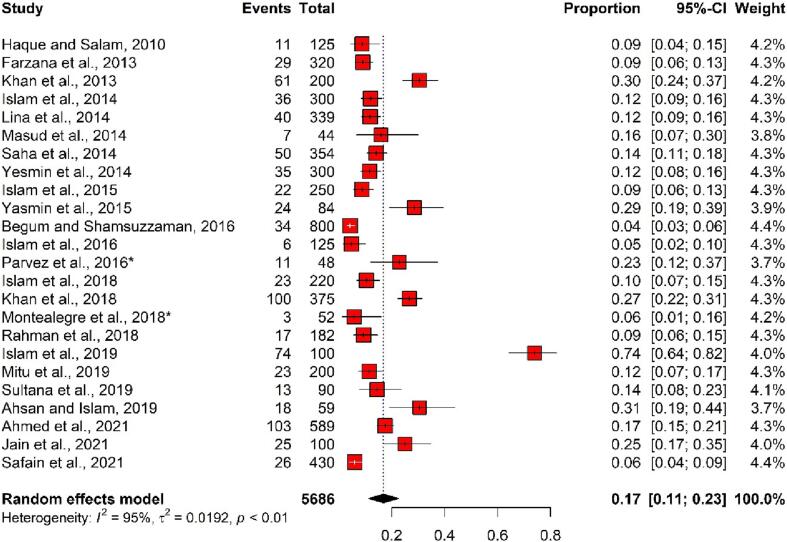
In human samples, the overall pooled number of ESBL-producing E. coli isolates was 791 (out of 5686 samples), and the pooled prevalence was 17% (95% CI: 11%–23%), with considerable heterogeneity (I2 = 95%, p < 0.01). In total, the prevalence of ESBL-producing E. coli in human samples ranged from 4% (95% CI: 3%–6%) to 74% (95% CI: 64%–82%) (Fig. 4).
Fig. 4.
Forest plot depicting the prevalence of ESBL-producing E. coli isolated from human samples in Bangladesh. The estimate of the prevalence was determined by combining the findings of 22 separate studies by applying a random-effects model to the data. An I2 value > 75% and a p-value < 0.05 were deemed statistically significant. The R program was used to analyse the data and generate the figure. Events = No. of positive ESBL-producing E. coli isolated from each study, Total = Sample size of each study, CI = Confidence interval, * = Study conducted with more than one sample category.
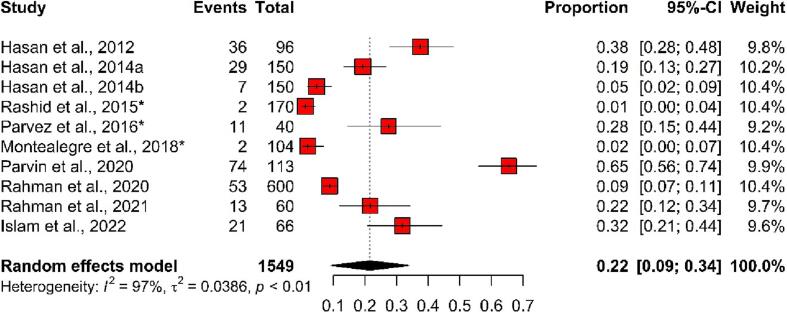
3.5.2. Studies focusing animals
Overall, 248 ESBL-producing E. coli isolates were detected in 1549 animal samples, and the pooled prevalence was 22% (95% CI: 9%–34%), with evidence of notable heterogeneity (I2 = 97%, p < 0.01) among the selected 36 studies. In total, the highest prevalence of ESBL-producing E. coli was detected in a study (65%, 95% CI: 56%–74%) which was conducted in 2020, whereas the lowest prevalence was detected in a study (1%, 95% CI: 0%–4%) which was carried out in 2015 (Fig. 5).
Fig. 5.
Forest plot depicting the prevalence of ESBL-producing E. coli isolated from animal samples in Bangladesh. The estimate of the prevalence was determined by combining the findings of ten separate studies by applying a random-effects model to the data. An I2 value > 75% and a p-value < 0.05 were deemed statistically significant. The R program was used to analyse the data and generate the figure. Events = No. of positive ESBL-producing E. coli isolated from each study, Total = Sample size of each study, CI = Confidence interval, * = Study conducted with more than one sample category.
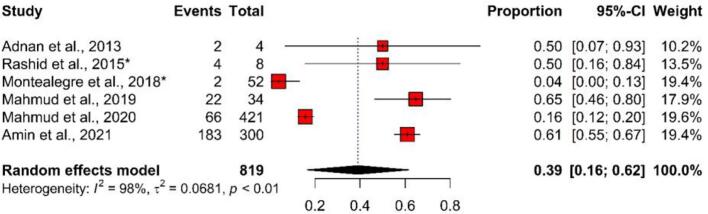
3.5.3. Studies focusing environments
In environmental samples, the overall pooled number of ESBL-producing E. coli isolates was 279 (out of 819 samples), and the pooled prevalence was 39% (95% CI: 16%–62%), with substantial heterogeneity (I2 = 98%, p < 0.01). In total, the prevalence of ESBL-producing E. coli in environmental samples ranged between 4% (95% CI: 0%–13%) and 65% (95% CI: 46%–80%) (Fig. 6).
Fig. 6.
Forest plot depicting the prevalence of ESBL-producing E. coli isolated from environmental samples in Bangladesh. The estimate of the prevalence was determined by combining the findings of six separate studies by applying a random-effects model to the data. An I2 value > 75% and a p-value < 0.05 were deemed statistically significant. The R program was used to analyse the data and generate the figure. Events = No. of positive ESBL-producing E. coli isolated from each study, Total = Sample size of each study, CI = Confidence interval, * = Study conducted with more than one sample category.
3.6. Prevalence of genes encoding ESBL-producing E. coli
A total of 13 types of genes conferring ESBL-producers were detected in E. coli isolated from humans, animals, and environments. The ESBL gene blaTEM-1 showed the highest prevalence (37.5%), followed by blaCMY (34.6%), blaCTX-M-1 (20.7%), blaCTX-M-15 (16.1%), blaTEM (12.3%), blaCTX-M and blaOXA (9.6%), blaOXA-1 (5.8%), blaampC (3.9%), blaSHV (3.8%), blaCMY-2 (2.3%), blaCTX-M-14 (1.3%), and blaCTX-M-9 (0.3%) (Table 2).
Table 2.
Prevalence of ESBL genes in E. coli isolated from human, animal, and environmental samples in selected studies (published between 2010 and 2021) in Bangladesh.
| Genes encoding ESBL | No. of studies | Total E. coli isolates | No. of positive ESBL genes (%) | Prevalence (%) | 95% CI (%) |
|---|---|---|---|---|---|
| blaTEM | 14 | 2230 | 275 | 12.3 | 11.0–13.8 |
| blaTEM-1 | 3 | 344 | 129 | 37.5 | 32.6–42.7 |
| blaCTX-M | 9 | 994 | 95 | 9.6 | 7.9–11.5 |
| blaCTX-M-1 | 9 | 1932 | 399 | 20.7 | 18.9–22.5 |
| blaCTX-M-9 | 5 | 1083 | 3 | 0.3 | 0.1–0.8 |
| blaCTX-M-14 | 2 | 151 | 2 | 1.3 | 0.2–4.7 |
| blaCTX-M-15 | 8 | 1184 | 191 | 16.1 | 14.2–18.3 |
| blaSHV | 14 | 1993 | 76 | 3.8 | 3.1–4.8 |
| blaCMY | 1 | 55 | 19 | 34.6 | 23.4–47.8 |
| blaCMY-2 | 2 | 684 | 16 | 2.3 | 1.5–3.8 |
| blaOXA | 5 | 302 | 29 | 9.6 | 6.8–13.5 |
| blaOXA-1 | 7 | 1415 | 82 | 5.8 | 4.7–7.1 |
| blaampC | 4 | 762 | 30 | 3.9 | 2.8–5.6 |
Here, ESBL = Extended-spectrum beta-lactamase, CI = Confidence interval.
3.7. Epidemiological important clones
Seven out of 36 included articles recorded different clones (n = 39) where the E. coli clone ST131 was predominantly found in the included studies (5/7). The clinically relevant clone ST10 and ST48 were also reported in ESBL-producing E. coli. Khan et al. [47] and Hasan et al. [34] detected 17 and 11 types of E. coli clones, respectively. Also, E. coli clones were found in all of the sample categories (humans, animals, and environments) (Table 3).
Table 3.
Sequence types (STs) clones found in Escherichia coli isolated from human, animal, and environmental samples in selected studies (published between 2010 and 2021) in Bangladesh.
| Study ID | Study period | Sample categories | Sample types | Sequence types (STs) |
|---|---|---|---|---|
| Hasan et al. [30] | 2009 | Wild ducks, ducks, chickens, geese | Cloacal swabs, feces | ST131, ST405, ST448, ST648, ST744 |
| Hasan et al. [34] | 2010 | Gull | Feces | ST10, ST48, ST131, ST345, ST349, ST648, ST853, ST1727, ST2687–2689 |
| Hasan et al. [35] | 2011 | Pigeons | Cloacal swabs | ST1408, ST3489–92 |
| Rashid et al. [41] | 2010 | Open Bill Stork, Environment | Feces, river water | ST10, ST46, ST156, ST2689, ST4016 |
| Begum and Shamsuzzaman [43] | 2014 | Humans | Urine | ST131 |
| Khan et al. [47] | 2014–15 | Humans | Urine, pus, swab of wound, vaginal, throat | ST38, ST73, ST88, ST101, ST127, ST131, ST155, ST167, ST224, ST405, ST410, ST466, ST754, ST851, ST1623, ST2104, ST2659, ST2851, ST6682 |
| Jain et al. [58] | 2019 | Humans | Urine, sputum | ST131 |
4. Discussion
ESBL-producing E. coli strains have been identified as a prominent multidrug-resistant pathogen that have been linked to serious infections acquired in hospitals and communities all over the world. The treatment of E. coli infections has become particularly difficult because of the widespread emergence of ESBL-forming strains, which has reduced the number of effective antimicrobial agents available. Like in other countries, ESBL-producing E. coli strains are being regularly reported in humans, animals, and environments in Bangladesh. In this systematic review, we did a comprehensive analysis of data on the occurrence of ESBL-producing E. coli isolated from human, animal, and environmental samples in Bangladesh. We also focused on designing strategies to minimize the consequences of AMR in the multidisciplinary categories (humans, animals, and environments) by employing the One Health approach. To our knowledge, this is the first systematic review and meta-analysis in Bangladesh narrating the distribution of ESBL-producing E. coli in all the components of One Health to comprehend the AMR issue from a one-health perspective.
4.1. Prevalence of E. coli in humans, animals, and environments
The present study showed a high pooled prevalence of E. coli in humans, animals, and environments, reporting E. coli in >50% of the multidisciplinary samples. Previously, Bastidas-Caldes et al. [11] conducted a similar type of systematic review and meta-analysis and reported E. coli in 17.2% of the samples from humans, animals, and environments in South America, which is lower than our present study. The discrepancies in the prevalence of E. coli might be due to differences in geographical characteristics (e.g., location, temperatures, humidity, etc.), sample size, types, study period, and others. This systematic review showed that E. coli was isolated from diversified types of samples, e.g., urine, blood, pus, swabs from different organs (human samples), feces, cloacal swabs, meat or meat swabs (animal samples), and wastewater, sewage, and fecal sludge (environmental samples). The presence of E. coli in humans, animals, and environments shows a significant concern for a wide range of health communities because these isolates have the potential to be transferred from one component to another and vice versa. However, the objective of this study was not to determine the presence of E. coli isolates in humans, animals, and/or environments. The main purpose of the present study was to evaluate the prevalence of ESBL-producing E. coli in humans, animals, and environments, which has been discussed in detail later.
4.2. ESBL-producing E. coli from humans, animals, and environments
In this study, the prevalence of ESBL-producing E. coli isolated from humans, animals, and environments was estimated to be 21% across all of Bangladesh, which is similar to the estimates for Latin America (23.2%) [62] and Europe (17.2%) [62], but lower than those reported for South Asia (33%) [9], the Asia-Pacific region (38.2%) [63], and Africa (40.4%) [62], and higher than the estimates for North America (9.8%) [62] and South America (3%) [11]. The variations in the prevalence of ESBL-producing E. coli might be due to differences in geographical properties, sample categories and types, sample size, identification methods, study periods, etc. In addition, there may be a correlation between the high population density, the scarcity of healthcare resources, and the lack of established antimicrobial stewardship programs in Bangladesh, all of which contribute to the region's disproportionately high incidence of these infections. Islam et al. [9] also conducted a systematic review and meta-analysis, reporting 35% ESBL-producing E. coli in Bangladesh, however, they focused only on humans, excluded those studies that incorporated non-human specimens (e.g., animals, foods, environments, etc.). But in this systematic review and meta-analysis, we extracted data on ESBL-producing E. coli isolated from all the One Health components.
In this study, the pooled prevalence of ESBL-producing E. coli from humans, animals, and environments was 17%, 22%, and 39%, respectively, which is comparable to a previous study [11] that reported ESBL-producing E. coli in human (2.2%), animal (21.4%), and environmental (12.6%) samples. The presence of ESBL-producing E. coli in animals and environments poses a serious health risk because it can be transmitted to humans through contact with animals and contaminated environmental components or through consumption of contaminated animal products or environmental elements. Moreover, carriers, who are otherwise healthy, are a significant reservoir for the transmission of beta-lactamases and, as a result, play a role in disseminating the bacteria to other populations or environments. The epidemiology of bacterial resistance in human populations may reveal a previously unknown pattern if further studies include surveillance at these levels [11].
Studies that were part of the One Health initiative were considered multidisciplinary because they analyzed samples from multiple interfaces, including human-animal, animal-environment, environment-human, and human-animal-environment, respectively. This review only reported on three cross-disciplinary studies (8.3%; 3/36), including human-animal, animal-environment, and human-animal-environment-related studies. The small number of multidisciplinary studies was also reported in other systematic reviews conducted globally [11,64,65]. As Bangladesh and other regions of the world are struggling to conduct their research using One Health approaches, the health authorities should take initiatives to combat the consequences of ESBL. For example, the World Health Organization suggests international cooperation to implement multi-sectoral action plans, which could help countries like Bangladesh and other parts of the world use “One Health” approaches in their experiment designs [66].
4.3. ESBL genes in E. coli isolated from humans, animals, and environments
This systematic review recorded that the ESBL genes blaTEM and blaCTX-M genes were predominantly found among E. coli isolated from humans, animals, and environments in Bangladesh, which is supported by the previous studies [[67], [68], [69]]. These genes are typically found on the same large plasmids as the determinants of resistance that cause the bacteria to be resistant to multiple classes of antimicrobials [70]. Moreover, this review showed a high prevalence of the blaCTX-M-1 and blaCTX-M-15 genes in E. coli isolates from multidisciplinary samples, which has serious public health implications, because these types of genes are the predominant ESBL genes in humans [71]. This study also reported the presence of CTX-M-9-like enzymes (blaCTX-M-9 and blaCTX-M-14) in humans, animals, and environments, though these genes are directly or indirectly linked with animals [72]. In this systematic review, we found that two or more types of CTX-M type ESBLs co-existed in several studies, which indicates a serious issue in the treatment of infectious diseases caused by E. coli isolates. Since there are so many homologous regions between CTX-M strains, recombinant enzymes are a distinct possibility in a world where multiple CTX-M species coexist [73]. We hypothesize that the presence of multiple CTX-M types of genes in the isolates may indicate that infections caused by these isolates may be more challenging to treat due to the increased likelihood of ESBL expression occurring phenotypically [67]. According to the present study, other important ESBL genes, e.g., OXA, SHV, CMY, and ampC, were detected in animals, humans, and environments in Bangladesh, which also indicates a serious threat to health communities. The identification of ESBL genes in animals raises concerns about the environmental dissemination of the pathogen, which in turn could contribute to its transmission to farm workers and the wider community. Furthermore, the presence of ESBL genes in the environment has demonstrated the significance of these genes' spread in humans and animals, and vice versa.
4.4. Epidemiological important clones related to ESBL-producing E. coli
In this study, we found that seven out of 36 studies reported 39 types of E. coli clones in humans, animals, and environments in Bangladesh. Among them, the clone ST131 was predominantly (5/7) detected. ST131 is a highly virulent pathogenic clone of the extraintestinal pathogenic E. coli subgroup [74]. This type of clone is widely distributed in ESBL-producing E. coli around the globe [[75], [76], [77]]. The detection of this pandemic ST131 clone in ESBL-producing E. coli in humans, animals, and environments in Bangladesh is worrisome. The ST131 clone containing E. coli isolates are deemed to be highly pathogenic because of the variety of infections they produce in both community and hospital settings and the high number of genes linked with virulence that they carry [78]. In addition, these clones are widely recognized as a significant source of plasmids harboring genes for resistance to several antimicrobial agents, including ESBL-encoding genes blaTEM, blaCTX-M, and blaSHV [79]. The presence of other epidemiologically important E. coli clones in humans, animals, and environments indicates a serious public health issue. The issue of the rapid and continued existence of international high-risk clones in ESBL-producing E. coli at the multidisciplinary interface (humans, animals, and environments) needs to be viewed as a One Health challenge in Bangladesh.
4.5. Possible transmission pathways of ESBL-producing E. coli among One Health components
Although AMR is a concern for human health, this phenomenon has its origins at the interface between humans, animals, and the environment. As a result, resistant genes or bacteria find their way into the human food chain. There are many different ways that ESBL-producing E. coli can be transmitted. Animals, inanimate objects, and environments can directly and indirectly spread ESBL-producing E. coli and their related resistance genes to humans, as well as vice versa. Hospital communities are directly associated with the distribution of ESBL-producing E. coli to humans and environments via human-to-human transmission and contaminated sewage or water supplies, respectively. Wild birds can pick up ESBL-producing E. coli from their surroundings (especially surface-contaminated water) and act as vectors or transmitters of these organisms to humans and animals. Contaminated environmental components (e.g., water, food sources, etc.) may also directly transfer ESBL-producing E. coli to humans and animals through consumption of wastewater, foods, bathing, and others. Animals may also be responsible for the transmission of ESBL-producing E. coli to humans via direct contact and/or the food supply chain. Humans may contaminate animals and environments with ESBL-producing E. coli via direct contact with animals and transferring fecal sludge or waste to the environment. Fig. 7 depicts the possible transmission pathways of ESBL-producing E. coli to humans from various components of One Health process, as well as vice versa.
Fig. 7.
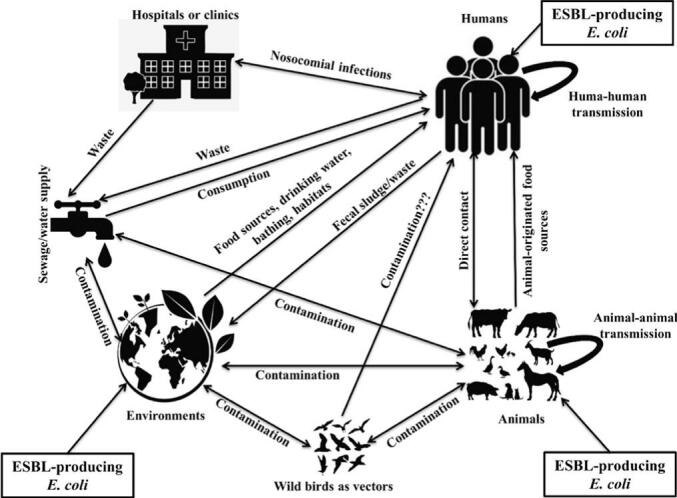
Possible transmission dynamics of ESBL-producing E. coli and their associated genes into humans via One Health components and vice versa.
4.6. Mitigation of AMR consequences using One Health perspective approach
When it comes to global health issues, AMR is the issue that best exemplifies the One Health approach. Because of the imprudent and disproportionate use of antibiotics in a variety of fields, AMR is connected to all three components (humans, animals, and environments) of One Health [80]. The approach to One Health taken in the WHO Global Action Plan is pertinent and in line with the observations that have been made in other local and global action plans. A lot of work remains, however, until a comprehensive One Health plan is implemented on a national and international scale to mitigate AMR consequences [81]. As a result, the One Health approach is now fully incorporated into international initiatives to combat AMR. The following are some of the key strategies that the One Health approach recommends for combating AMR:
-
•
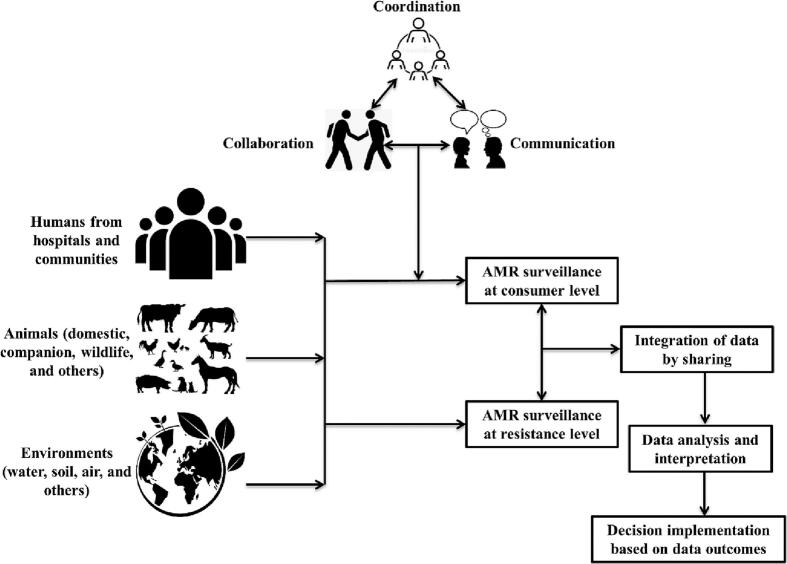
It is imperative that both national and global surveillance systems be put in place to detect the emergence of antimicrobial-resistant and pathogenically important microbial hazards in One Health components. AMR surveillance entails the following stages: (1) data collection (including information on antibiotic use and its effects after it reaches consumers); (2) data integration, analysis, and interpretation; and (3) decision-making and the subsequent rollout of any necessary corrective measures based on the results of the surveillance (Fig. 8).
-
•
The development of regulation in antimicrobial use and infection control in multiple sectors should be highlighted by a rigorous surveillance system.
-
•
A public-awareness program should be implemented to improve knowledge among people about the dangers of misuse and excessive use of antimicrobial agents. It is possible to lower the number of prescribed antimicrobials by conducting successful public education campaigns.
-
•
Expert veterinarians, human physicians, and environmental specialists must approve the use of any antimicrobial agents at any level. Without their consultation and approval, the use of all antimicrobial agents should be prohibited.
-
•
The research, development, and widespread implementation of vaccines and alternative treatments (via vaccines, probiotics, bacteriophages, etc.) should be encouraged.
-
•
Sanitation efforts at any level of the One Health program should be boosted in order to curb the spread of diseases, especially infectious diseases.
Fig. 8.
A framework of multidisciplinary surveillance program to combat AMR consequences in different components of One Health (humans, animals, and environments).
5. Limitations
Only peer-reviewed original articles were used in our systematic review. Our review excluded review articles, preprints, or theses that might have important information on ESBL-producing E. coli. Moreover, we had to exclude several peer-reviewed original articles because of the exclusion criteria. There is also an important note that even though the included articles were all judged to be of high quality based on their respective evaluation results, it is possible that a few of them appeared in predatory journals. Studies included in this review are trustworthy, and we base our conclusions not on the prestige of the journal in which they appeared but on the merits of the research presented therein.
6. Conclusion
As we know, this is the first systematic review and meta-analysis, reporting ESBL-producing E. coli isolated from all One Health components. This review reported a high prevalence of ESBL-producing E. coli in humans, animals, and environments. ESBL-producing E. coli in Bangladesh harbored a wide range of enzyme variants that are associated with ESBLs, including the clinically important ESBL TEM and CTX-M enzymes. The three most epidemiologically important clones, ST10, ST48, and ST131, were also detected in ESBL-producing E. coli isolated from humans, animals, and environments. The presence of ESBL-producing E. coli, their representative genes, and epidemiologically important clones in human, animal, and environmental samples indicates a serious issue in all the health communities in Bangladesh. The information gleaned from this meta-analysis can serve as the foundation for future monitoring initiatives that will significantly reduce potential scathing points in the transmission chain. Moreover, this systematic review emphasized how important it is to have complete AMR surveillance in Bangladesh to find out the exact scenario of ESBL-producing E. coli in humans, animals, and environments, and to speed up support for antimicrobial stewardship programs in Bangladesh. Finally, the One Health concept should be implemented in hospital communities, animal farms, and waste management plants to find out the transmission pathways of ESBL-producing E. coli, their resistant enzymes, and representative resistant genes. Multisectoral and multidisciplinary collaborations would be crucial to fulfill One Health principles and minimize the AMR consequences.
Funding
This research was supported by Bangladesh Agricultural University Research System (BAURES) (Project Number: 2022/12/BAU).
Ethical approval
Not applicable.
Declaration of Competing Interest
The author reports no conflicts of interest in this work.
Data availability
Data will be made available on request.
References
- 1.Islam M.S., Paul A., Talukder M., Roy K., Sobur M.A., Ievy S., Nayeem M.M.H., Rahman S., Nazir K.N.H., Hossain M.T., Rahman M.T. Migratory birds travelling to Bangladesh are potential carriers of multi-drug resistant Enterococcus spp., Salmonella spp., and Vibrio spp. Saudi J. Biol. Sci. 2021;28(10):5963–5970. doi: 10.1016/j.sjbs.2021.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy K., Islam M.S., Paul A., Ievy S., Talukder M., Sobur M.A., Ballah F.M., Khan M.S.R., Rahman M.T. Molecular detection and antibiotyping of multi-drug resistant Enterococcus faecium from healthy broiler chickens in Bangladesh. Vet. Med. Sci. 2022;8(1):200–210. doi: 10.1002/vms3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tawyabur M., Islam M.S., Sobur M.A., Hossain M.J., Mahmud M.M., Paul S., Hossain M.T., Ashour H.M., Rahman M.T. Isolation and characterization of multidrug-resistant Escherichia coli and Salmonella spp. From healthy and diseased turkeys. Antibiotics. 2020;9(11):770. doi: 10.3390/antibiotics9110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray C.J., Ikuta K.S., Sharara F., Swetschinski L., Aguilar G.R., Gray A., Han C., Bisignano C., Rao P., Wool E., Johnson S.C. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talukder M., Islam M.S., Ievy S., Sobur M.A., Ballah F.M., Najibullah M., Rahman M.B., Rahman M.T., Khan M.F.R. Detection of multidrug resistant Salmonella spp. from healthy and diseased broilers having potential public health significance. J. Adv. Biotechnol. Exp. Ther. 2021;4(2):248–255. [Google Scholar]
- 6.Ievy S., Islam M.S., Sobur M.A., Talukder M., Rahman M.B., Khan M.F.R., Rahman M.T. Molecular detection of avian pathogenic Escherichia coli (APEC) for the first time in layer farms in Bangladesh and their antibiotic resistance patterns. Microorganisms. 2020;8(7):1021. doi: 10.3390/microorganisms8071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam M.S., Nayeem M.M.H., Sobur M.A., Ievy S., Islam M.A., Rahman S., Kafi M.A., Ashour H.M., Rahman M.T. Virulence determinants and multidrug resistance of Escherichia coli isolated from migratory birds. Antibiotics. 2021;10(2):190. doi: 10.3390/antibiotics10020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman M.T., Sobur M.A., Islam M.S., Ievy S., Hossain M.J., El Zowalaty M.E., Rahman A.T., Ashour H.M. Zoonotic diseases: etiology, impact, and control. Microorganisms. 2020;8(9):1405. doi: 10.3390/microorganisms8091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam K., Heffernan A.J., Naicker S., Henderson A., Chowdhury M.A.H., Roberts J.A., Sime F.B. Epidemiology of extended-spectrum β-lactamase and metallo-β-lactamase-producing Escherichia coli in South Asia. Future Microbiol. 2021;16(7):521–535. doi: 10.2217/fmb-2020-0193. [DOI] [PubMed] [Google Scholar]
- 10.Zowawi H.M., Harris P.N., Roberts M.J., Tambyah P.A., Schembri M.A., Pezzani M.D., Williamson D.A., Paterson D.L. The emerging threat of multidrug-resistant gram-negative bacteria in urology. Nat. Rev. Urol. 2015;12(10):570–584. doi: 10.1038/nrurol.2015.199. [DOI] [PubMed] [Google Scholar]
- 11.Bastidas-Caldes C., Romero-Alvarez D., Valdez-Vélez V., Morales R.D., Montalvo-Hernández A., Gomes-Dias C., Calvopiña M. Extended-Spectrum Beta-lactamases producing Escherichia coli in South America: a systematic review with a one health perspective. Infect. Drug Resist. 2022:5759–5779. doi: 10.2147/IDR.S371845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj P., Singh N.S., Virdi J.S. Escherichia coli β-lactamases: what really matters. Front. Microbiol. 2016;7:417. doi: 10.3389/fmicb.2016.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker D., Sniatynski M.K., Mandrusiak D., Rubin J.E. Extended-spectrum β-lactamase producing Escherichia coli isolated from wild birds in Saskatoon, Canada. Lett. Appl. Microbiol. 2016;63(1):11–15. doi: 10.1111/lam.12589. [DOI] [PubMed] [Google Scholar]
- 14.Paterson D.L., Bonomo R.A. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford P.A. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001;14(4):933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naas T., Oueslati S., Bonnin R.A., Dabos M.L., Zavala A., Dortet L., Retailleau P., Iorga B.I. Beta-lactamase database (BLDB)–structure and function. J. Enzyme Inhibit. Med. Chem. 2017;32(1):917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carattoli A. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 2008;14:117–123. doi: 10.1111/j.1469-0691.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 18.Day M.J., Hopkins K.L., Wareham D.W., Toleman M.A., Elviss N., Randall L., Teale C., Cleary P., Wiuff C., Doumith M., Ellington M.J. Extended-spectrum β-lactamase-producing Escherichia coli in human-derived and foodchain-derived samples from England, Wales, and Scotland: an epidemiological surveillance and typing study. Lancet Infect. Dis. 2019;19(12):1325–1335. doi: 10.1016/S1473-3099(19)30273-7. [DOI] [PubMed] [Google Scholar]
- 19.Singh A.S., Nayak B.B., Kumar S.H. High prevalence of multiple antibiotic-resistant, extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in fresh seafood sold in retail markets of Mumbai, India. Vet. Sci. 2020;7(2):46. doi: 10.3390/vetsci7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamaruzzaman E.A., Abdul Aziz S., Bitrus A.A., Zakaria Z., Hassan L. Occurrence and characteristics of extended-spectrum β-lactamase-producing Escherichia coli from dairy cattle, milk, and farm environments in peninsular Malaysia. Pathogens. 2020;9(12):1007. doi: 10.3390/pathogens9121007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghenea A.E., Zlatian O.M., Cristea O.M., Ungureanu A., Mititelu R.R., Balasoiu A.T., Vasile C.M., Salan A.I., Iliuta D., Popescu M., Udriștoiu A.L. TEM, CTX-M, SHV genes in ESBL-producing Escherichia coli and Klebsiella pneumoniae isolated from clinical samples in a county clinical emergency hospital Romania-predominance of CTX-M-15. Antibiotics. 2022;11(4):503. doi: 10.3390/antibiotics11040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 24.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11(2):193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 25.Brown L.D., Cai T.T., DasGupta A. Interval estimation for a binomial proportion. Stat. Sci. 2001;16(2):101–133. [Google Scholar]
- 26.Masud M.R., Afroz H., Fakruddin M. Prevalence of extended-spectrum β-lactamase positive bacteria in radiologically positive urinary tract infection. SpringerPlus. 2014;3(1):1–6. doi: 10.1186/2193-1801-3-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahsan S., Islam R. Beta-lactamase-producing Escherichia coli in Bangladesh: their phenotypic and molecular characteristics. Dhaka Univ. J. Biol. Sci. 2019;28(1):71–81. [Google Scholar]
- 28.Amin M.B., Saha S.R., Islam M.R., Haider S.A., Hossain M.I., Chowdhury A.H.K., Rousham E.K., Islam M.A. High prevalence of plasmid-mediated quinolone resistance (PMQR) among E. coli from aquatic environments in Bangladesh. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0261970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haque R., Salam M.A. Detection of ESBL producing nosocomial gram negative bacteria from a tertiary care hospital in Bangladesh. Pakistan J. Med. Sci. 2010;26(4):887–891. [Google Scholar]
- 30.Hasan B., Sandegren L., Melhus Å., Drobni M., Hernandez J., Waldenström J., Alam M., Olsen B. Antimicrobial drug–resistant Escherichia coli in wild birds and free-range poultry, Bangladesh. Emerg. Infect. Dis. 2012;18(12):2055. doi: 10.3201/eid1812.120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adnan N., Sultana M., Islam O.K., Nandi S.P., Hossain M.A. 2013. Characterization of ciprofloxacin resistant extended spectrum β-lactamase (ESBL) producing Escherichia spp. from clinical waste water in Bangladesh. [Google Scholar]
- 32.Farzana R., Shamsuzzaman S.M., Mamun K.Z., Shears P. Antimicrobial susceptibility pattern of extended spectrum beta-lactamase producing gram-negative bacteria isolated from wound and urine in a tertiary care hospital, Dhaka City, Bangladesh. Southeast Asian J. Trop. Med. Public Health. 2013;44(1):96–103. [PubMed] [Google Scholar]
- 33.Khan S.A., Feroz F., Noor R. Study of extended-spectrum β-lactamase-producing bacteria from urinary tract infections in Bangladesh. Tzu Chi Med. J. 2013;25(1):39–42. [Google Scholar]
- 34.Hasan B., Melhus Å., Sandegren L., Alam M., Olsen B. The gull (Chroicocephalus brunnicephalus) as an environmental bioindicator and reservoir for antibiotic resistance on the coastlines of the bay of Bengal. Microb. Drug Resist. 2014;20(5):466–471. doi: 10.1089/mdr.2013.0233. [DOI] [PubMed] [Google Scholar]
- 35.Hasan B., Islam K., Ahsan M., Hossain Z., Rashid M., Talukder B., Ahmed K.U., Olsen B., Kashem M.A. Fecal carriage of multi-drug resistant and extended spectrum β-lactamases producing E. coli in household pigeons, Bangladesh. Vet. Microbiol. 2014;168(1):221–224. doi: 10.1016/j.vetmic.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 36.Islam T.A.B., Shamsuzzaman S.M., Farzana A. Prevalence and antibiogram of ESBL producing gram negative bacilli isolated from urine in Dhaka Medical College Hospital, Bangladesh. Bangladesh J. Med. Microbiol. 2015;9(1):17–21. [Google Scholar]
- 37.Lina T.T., Khajanchi B.K., Azmi I.J., Islam M.A., Mahmood B., Akter M., Banik A., Alim R., Navarro A., Perez G., Cravioto A. Phenotypic and molecular characterization of extended-spectrum beta-lactamase-producing Escherichia coli in Bangladesh. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saha M.R., Jhora S.T., Khan T.M., Paul S., Chowdhury D. Detection of SHV gene from extended-spectrum beta-lactamases (ESBLs) producing isolates in a tertiary care hospital. Bangladesh J. Med. Microbiol. 2014;8(2):14–18. [Google Scholar]
- 39.Yesmin T., Hossain A., Paul S., Yusuf A., Sultana S., Gmowla G. Detection of extended-spectrum beta-lactamases producing genes among third generation cephalosporins sensitive bacteria strains from a medical college hospital in Bangladesh. J. Allergy Disord. Ther. 2014;1(1) [Google Scholar]
- 40.Islam M.S., Yusuf M.A., Begum S.A., Sattar A.A., Hossain A., Roy S. Extended-spectrum-beta-lactamase producing uropathogenic Escherichia coli infection in Dhaka, Bangladesh. Afr. J. Bacteriol. Res. 2015;7(1):1–7. [Google Scholar]
- 41.Rashid M., Rakib M.M., Hasan B. Antimicrobial-resistant and ESBL-producing Escherichia coli in different ecological niches in Bangladesh. Infect. Ecol. Epidemiol. 2015;5(1):26712. doi: 10.3402/iee.v5.26712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasmin T., Yusuf M.A., Sayam M.A.N., Haque R., Mowla G. Status of ESBL producing bacteria isolated from skin wound at a tertiary care hospital in Bangladesh. Adv. Infect. Dis. 2015;5(04):174. [Google Scholar]
- 43.Begum N., Shamsuzzaman S.M. Emergence of CTX-M-15 producing E. coli O25b-ST131 clone in a tertiary care hospital of Bangladesh. Malaysian J. Pathol. 2016;38(3):241. [PubMed] [Google Scholar]
- 44.Islam S.S., Malek M.A., Haque A.F., Talukder K.A., Akhter M.Z. Beta lactamase genes of extended spectrum beta lactamase producing Escherichia coli from anorectal sepsis cases in Bangladesh. Bangladesh J. Microbiol. 2013;30(1–2):23–29. [Google Scholar]
- 45.Parvez A.M.K., Marzan M., Liza S.M., Mou T.J., Azmi I.J. Prevalence of inhibitor resistant beta lactamase producing E. coli in human and poultry origin of Bangladesh. J. Bacteriol. Parasitol. 2016;7(271):2. [Google Scholar]
- 46.Islam M.B., Yusuf M.A., Afrin S., Bashar M.A. Prevalence of extended spectrum B-lactamases in Hospitalized Patents and Community Patients. Bangladesh J. Infect. Dis. 2018;5(2):61–64. [Google Scholar]
- 47.Khan E.R., Aung M.S., Paul S.K., Ahmed S., Haque N., Ahamed F., Sarkar S.R., Roy S., Rahman M.M., Mahmud M.C., Hossain M.A. Prevalence and molecular epidemiology of clinical isolates of Escherichia coli and Klebsiella pneumoniae harboring extended-spectrum beta-lactamase and carbapenemase genes in Bangladesh. Microb. Drug Resist. 2018;24(10):1568–1579. doi: 10.1089/mdr.2018.0063. [DOI] [PubMed] [Google Scholar]
- 48.Montealegre M.C., Roy S., Böni F., Hossain M.I., Navab-Daneshmand T., Caduff L., Faruque A.S.G., Islam M.A., Julian T.R. Risk factors for detection, survival, and growth of antibiotic-resistant and pathogenic Escherichia coli in household soils in rural Bangladesh. Appl. Environ. Microbiol. 2018;84(24) doi: 10.1128/AEM.01978-18. e01978–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman M., Sultana H., Mosawuir M.A., Akhter L., Yusuf M.A. Status of extended spectrum beta-lactamase (ESBL) producing Bacteria isolated from surgical and burn wound at tertiary Care Hospital in Dhaka City. Bangladesh J. Infect. Dis. 2018;5(1):21–26. [Google Scholar]
- 50.Islam M.A., Amin M.B., Roy S., Asaduzzaman M., Islam M.R., Navab-Daneshmand T., Mattioli M.C., Kile M.L., Levy K., Julian T.R. Fecal colonization with multidrug-resistant E. coli among healthy infants in rural Bangladesh. Front. Microbiol. 2019;10:640. doi: 10.3389/fmicb.2019.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmud Z.H., Shirazi F.F., Hossainey M.R.H., Islam M.I., Ahmed M.A., Nafiz T.N., Imran K.M., Sultana J., Islam M.S., Islam M.A., Islam M.S. Presence of virulence factors and antibiotic resistance among Escherichia coli strains isolated from human pit sludge. J. Infect. Dev. Count. 2019;13(03):195–203. doi: 10.3855/jidc.10768. [DOI] [PubMed] [Google Scholar]
- 52.Mitu F.S., Al-Maruf M.A., Arpan M., Huda A.K.M.N., Khan S.A., Rahman M.M. Prevalence of extended spectrum beta-lactamase (ESBL) and AmpC beta-lactamase producing bacteria in urinary tract infection patients in Bangladesh. Malaysian J. Microbiol. 2019;15(3):204–212. [Google Scholar]
- 53.Sultana M., Parvez A.K., Sultana K.F., Mukharje S.K., Hossain M.A. Characterization of extended spectrum β-lactamase producing bactieria isolated from urinary tract infections. Bangladesh Med. Res. Counc. Bull. 2019;45(1):23–33. [Google Scholar]
- 54.Mahmud Z.H., Kabir M.H., Ali S., Moniruzzaman M., Imran K.M., Nafiz T.N., Islam M.S., Hussain A., Hakim S.A.I., Worth M., Ahmed D. Extended-spectrum beta-lactamase-producing Escherichia coli in drinking water samples from a forcibly displaced, densely populated community setting in Bangladesh. Front. Public Health. 2020;8:228. doi: 10.3389/fpubh.2020.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parvin M.S., Talukder S., Ali M.Y., Chowdhury E.H., Rahman M.T., Islam M.T. Antimicrobial resistance pattern of Escherichia coli isolated from frozen chicken meat in Bangladesh. Pathogens. 2020;9(6):420. doi: 10.3390/pathogens9060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahman M., Husna A., Elshabrawy H.A., Alam J., Runa N.Y., Badruzzaman A.T.M., Banu N.A., Al Mamun M., Paul B., Das S., Khairalla A.S. Isolation and molecular characterization of multidrug-resistant Escherichia coli from chicken meat. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-78367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed A.A., Hasan S.A., Juyee N.A., Islam M.S., Abedin M.Z. Patterns of extended-spectrum β-lactamase producing uropathogens detection in tertiary Hospital of Bangladesh. Am. J. Pure Appl. Biosci. 2021;3(2):29–34. [Google Scholar]
- 58.Jain P., Bepari A.K., Sen P.K., Rafe T., Imtiaz R., Hossain M., Reza H.M. High prevalence of multiple antibiotic resistance in clinical E. coli isolates from Bangladesh and prediction of molecular resistance determinants using WGS of an XDR isolate. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-02251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahman W., Hossain M., Ali M., Sultana T., Hossain K.M. Detection of extended-spectrum β-lactamase (ESBL) producing Escherichia coli in chickens. Turk. J. Vet. Res. 2023;5(2):73–79. [Google Scholar]
- 60.Safain S.K., Bhuyan G.S., Hassan Hasib S., Islam M.S., Mahmud-Un-Nabi M.A., Sultana R., Tasnim S., Noor F.A., Sarker S.K., Islam M.T., Rahat A. Genotypic and phenotypic profiles of antibiotic-resistant bacteria isolated from hospitalised patients in Bangladesh. Tropical Med. Int. Health. 2021;26(7):720–729. doi: 10.1111/tmi.13584. [DOI] [PubMed] [Google Scholar]
- 61.Islam M., Sobur M., Rahman S., Ballah F.M., Ievy S., Siddique M.P., Rahman M., Kafi M., Rahman M. Detection of blaTEM, blaCTX-M, blaCMY, and blaSHV genes among extended-spectrum beta-lactamase-producing Escherichia coli isolated from migratory birds travelling to Bangladesh. Microb. Ecol. 2022;83(4):942–950. doi: 10.1007/s00248-021-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z., Chen M., Yu Y., Pan S., Liu Y. Antimicrobial susceptibility among gram-positive and gram-negative blood-borne pathogens collected between 2012-2016 as part of the Tigecycline evaluation and surveillance trial. Antimicrob. Resist. Infect. Control. 2018;7(1):1–13. doi: 10.1186/s13756-018-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang Y.T., Coombs G., Ling T., Balaji V., Rodrigues C., Mikamo H., Kim M.J., Rajasekaram D.G., Mendoza M., Tan T.Y., Kiratisin P. Epidemiology and trends in the antibiotic susceptibilities of gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region, 2010–2013. Int. J. Antimicrob. Agents. 2017;49(6):734–739. doi: 10.1016/j.ijantimicag.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 64.O’Neal L., Alvarez D., Mendizábal-Cabrera R., Ramay B.M., Graham J. Community-acquired antimicrobial resistant Enterobacteriaceae in Central America: a one health systematic review. Int. J. Environ. Res. Public Health. 2020;17(20):7622. doi: 10.3390/ijerph17207622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Escher N.A., Muhummed A.M., Hattendorf J., Vonaesch P., Zinsstag J. Systematic review and meta-analysis of integrated studies on antimicrobial resistance genes in Africa—a one health perspective. Tropical Med. Int. Health. 2021;26(10):1153–1163. doi: 10.1111/tmi.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization (WHO) 2016. Global action plan on antimicrobial resistance.http://www.emro.who.int/healthtopics/drug-resistance/global-action-plan.html Available online: (accessed on November, 2022) [Google Scholar]
- 67.Gundran R.S., Cardenio P.A., Villanueva M.A., Sison F.B., Benigno C.C., Kreausukon K., Pichpol D., Punyapornwithaya V. Prevalence and distribution of blaCTX-M, blaSHV, blaTEM genes in extended-spectrum β-lactamase-producing E. coli isolates from broiler farms in the Philippines. BMC Vet. Res. 2019;15(1):1–8. doi: 10.1186/s12917-019-1975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sepp E., Andreson R., Balode A., Bilozor A., Brauer A., Egorova S., Huik K., Ivanova M., Kaftyreva L., Kõljalg S., Kõressaar T. Phenotypic and molecular epidemiology of ESBL-, AmpC-, and carbapenemase-producing Escherichia coli in northern and Eastern Europe. Front. Microbiol. 2019;10:2465. doi: 10.3389/fmicb.2019.02465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandit R., Awal B., Shrestha S.S., Joshi G., Rijal B.P., Parajuli N.P. Extended-spectrum β-lactamase (ESBL) genotypes among multidrug-resistant uropathogenic Escherichia coli clinical isolates from a teaching hospital of Nepal. Interdisc. Perspect. Infect. Dis. 2020;2020 doi: 10.1155/2020/6525826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J.H., Bae I.K., Hee Lee S. New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med. Res. Rev. 2012;32(1):216–232. doi: 10.1002/med.20210. [DOI] [PubMed] [Google Scholar]
- 71.Cantón R., González-Alba J.M., Galán J.C. CTX-M enzymes: origin and diffusion. Front. Microbiol. 2012;3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coque T.M. Increasing prevalence of ESBL-producing Enterobacreriaceae in Europe. Eurosurveillance. 2008;13:1–11. [PubMed] [Google Scholar]
- 73.He D., Partridge S.R., Shen J., Zeng Z., Liu L., Rao L., Lv L., Liu J.H. CTX-M-123, a novel hybrid of the CTX-M-1 and CTX-M-9 group β-lactamases recovered from Escherichia coli isolates in China. Antimicrob. Agents Chemother. 2013;57(8):4068–4071. doi: 10.1128/AAC.00541-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merino I., Porter S.B., Johnston B., Clabots C., Thuras P., Ruiz-Garbajosa P., Cantón R., Johnson J.R. Molecularly defined extraintestinal pathogenic Escherichia coli status predicts virulence in a murine sepsis model better than does virotype, individual virulence genes, or clonal subset among E. coli ST131 isolates. Virulence. 2020;11(1):327–336. doi: 10.1080/21505594.2020.1747799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louka C., Ravensbergen S.J., Ott A., Zhou X., García-Cobos S., Friedrich A.W., Pournaras S., Rosema S., Rossen J.W., Stienstra Y., Bathoorn E. Predominance of CTX-M-15-producing ST131 strains among ESBL-producing Escherichia coli isolated from asylum seekers in the Netherlands. J. Antimicrob. Chemother. 2021;76(1):70–76. doi: 10.1093/jac/dkaa395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tóth K., Tóth Á., Kamotsay K., Németh V., Szabó D. Population snapshot of the extended-spectrum β-lactamase-producing Escherichia coli invasive strains isolated from a Hungarian hospital. Ann. Clin. Microbiol. Antimicrob. 2022;21(1):1–10. doi: 10.1186/s12941-022-00493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahazu S., Sato W., Ayibieke A., Prah I., Hayashi T., Suzuki T., Iwanaga S., Ablordey A., Saito R. Insights and genetic features of extended-spectrum beta-lactamase producing Escherichia coli isolates from two hospitals in Ghana. Sci. Rep. 2022;12(1):1–11. doi: 10.1038/s41598-022-05869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicolas-Chanoine M.H., Bertrand X., Madec J.Y. Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014;27(3):543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jouini A., Klibi A., Elarbi I., Chaabene M.B., Hamrouni S., Souiai O., Hanachi M., Ghram A., Maaroufi A. First detection of human ST131-CTX-M-15-O25-B2 clone and high-risk clonal lineages of ESBL/pAmpC-producing E. coli isolates from diarrheic poultry in Tunisia. Antibiotics. 2021;10(6):670. doi: 10.3390/antibiotics10060670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velazquez-Meza M.E., Galarde-López M., Carrillo-Quiróz B., Alpuche-Aranda C.M. Antimicrobial resistance: one health approach. Vet. World. 2022;15(3):743–749. doi: 10.14202/vetworld.2022.743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tweldemedhin M., Muthupandian S., Gebremeskel T.K., Mehari K., Abay G.K., Teklu T.G., Dhandapani R., Paramasivam R., Asmelash T. Multidrug resistance from a one health perspective in Ethiopia: a systematic review and meta-analysis of literature (2015–2020) One Health. 2022;14 doi: 10.1016/j.onehlt.2022.100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.