Abstract
Objectives
Artificial intelligence (AI) may be practical for image classification of small bowel capsule endoscopy (CE). However, creating a functional AI model is challenging. We attempted to create a dataset and an object detection CE AI model to explore modeling problems to assist in reading small bowel CE.
Methods
We extracted 18,481 images from 523 small bowel CE procedures performed at Kyushu University Hospital from September 2014 to June 2021. We annotated 12,320 images with 23,033 disease lesions, combined them with 6161 normal images as the dataset, and examined the characteristics. Based on the dataset, we created an object detection AI model using YOLO v5 and we tested validation.
Results
We annotated the dataset with 12 types of annotations, and multiple annotation types were observed in the same image. We test validated our AI model with 1396 images, and sensitivity for all 12 types of annotations was about 91%, with 1375 true positives, 659 false positives, and 120 false negatives detected. The highest sensitivity for individual annotations was 97%, and the highest area under the receiver operating characteristic curve was 0.98, but the quality of detection varied depending on the specific annotation.
Conclusions
Object detection AI model in small bowel CE using YOLO v5 may provide effective and easy‐to‐understand reading assistance. In this SEE‐AI project, we open our dataset, the weights of the AI model, and a demonstration to experience our AI. We look forward to further improving the AI model in the future.
Keywords: artificial intelligence, capsule endoscopy, diagnostic imaging, gastrointestinal tract, intestine small
INTRODUCTION
The small bowel is a 5–6 m long organ located between the stomach and the colon, making it difficult to observe directly. Since the initial report of capsule endoscopy (CE) in 2000, 1 the small bowel has increasingly been screened using CE rather than X‐ray radiography. 2 Current guidelines recommend prompt CE for patients with obscure gastrointestinal bleeding and those with Crohn's disease or celiac disease who have not yet received a confirmed diagnosis. 3 CE of the small bowel involves the acquisition of two to six frames per second for 2 to 8 hours, resulting in tens of thousands of frames being output as a video during a single examination. Clinicians require approximately one hour to view and interpret this video, which is more labor‐intensive and expensive than a standard upper gastrointestinal endoscopy. 4 Automated reading by artificial intelligence (AI) is currently the focus of research due to the significant effort required in CE and the risk of missing disease lesions. 5 In medicine, AI models have demonstrated considerable progress in detecting important findings in head computed tomography, 6 skin cancer, 7 and diabetic retinopathy, 8 as well as lung computed tomography images in patients with coronavirus disease. 9 In the field of lower gastrointestinal endoscopy, AI models have aided in the detection of colorectal polyps, 10 and some of these models are currently used in clinical practice.
Previously, reading aids in small bowel CE have been explored for the detection of bleeding through image coloration 11 and the use of support vector machines. 12 Techniques like support vector machines were machine learning methods. Nevertheless, conventional machine learning methods have encountered difficulties in selecting and extracting two‐dimensional image recognition features. Convolutional neural network emerged as a deep learning model that automatically extracts required feature vectors from the training data. 13 While these techniques are rooted in machine learning, the introduction of convolutional neural network has profoundly impacted the field of image recognition technology. In 2012, AlexNet, which utilizes convolutional neural network, was used in an object image recognition competition (the ImageNet Large Scale Visual Recognition Challenge) to classify 14 million images into 1000 classes. 13 Since then, many AI models have been demonstrated to be effective in clinical practice for image classification, 14 , 15 including the detection of Gastrointestinal bleeding, 16 protrusion, 17 and inflammatory bowel disease. 18 However, small bowel CE images often depict multiple disease lesions in a single shot, and it is challenging to create a practical model based on simple image classification alone. An object detection model that indicates the location of disease lesions in an image would be appropriate for the physician to understand, although the method to generate such a model is complex. As far as we know, AI models alone to identify multiple types of clinically necessary lesions from CE images with a high level of expertise have not been achieved. In this paper, we analyzed a large dataset and created an AI model, aiming to explore relevant modeling problems.
METHODS
Data extraction and annotation
In the Small Bowel CE Examination with Object Detection AI Model (The SEE‐AI) project, we retrospectively analyzed anonymized videos from 954 patients who underwent small bowel CE (PillCam SB 3; Medtronic, Minneapolis, MN, USA) at Kyushu University Hospital from September 2014 to June 2021. Participants were given the opportunity to opt out of the study, which was approved by the Ethics Review Committee of Kyushu University (“Approval No. 2021–213”). Individual consent forms were not required because this was a retrospective study with anonymized data by the Ethics Committee. All included patients fasted for at least 12 h before undergoing small bowel CE. Most examinations were performed while the patients were hospitalized. Physicians with 3–5 years of clinical experience initially reviewed the medical reports, which were then reviewed by specialists with more than 7 years of experience to identify clinically important images. Image extraction was performed by RAPID for PillCam (Medtronic), a dedicated analysis software for the PillCam SB3 endoscopy system, which extracted 576‐pixel x 576‐pixel JPEG images from the captured videos. Because some patients had no disease lesions, images were ultimately extracted from 523 of 954 videos. For anonymization, each image's area outside the frame (containing personal data) was blackened. The dataset includes 18,481 images, 12,320 of which contain disease lesions and 6161 of which are normal mucosal images with various mucosal backgrounds. Figure 1 shows the extraction procedure, and Table 1 shows the disease backgrounds of patients in the dataset. All images were annotated by seven specialists using VOTT ver1.0.8 (Microsoft, Washington, WA, USA), with square bounding boxes and 12 annotation labels deemed clinically useful. All annotation labels were checked and any discrepancies were resolved through discussion among the specialists. The quantities of food residue and air bubbles in the gastrointestinal tract varied among individuals. However, no selection was based on image quality as long as the disease lesions were discernible. In anticipation of future data being utilized by the scientific community, on our public dataset we did not crop, deform, or change the brightness or saturation of the images. As a point of note, in YOLOv5, data augmentation can be used as an internal processing step, and we have employed standard augmentation techniques in creating our models.
FIGURE 1.
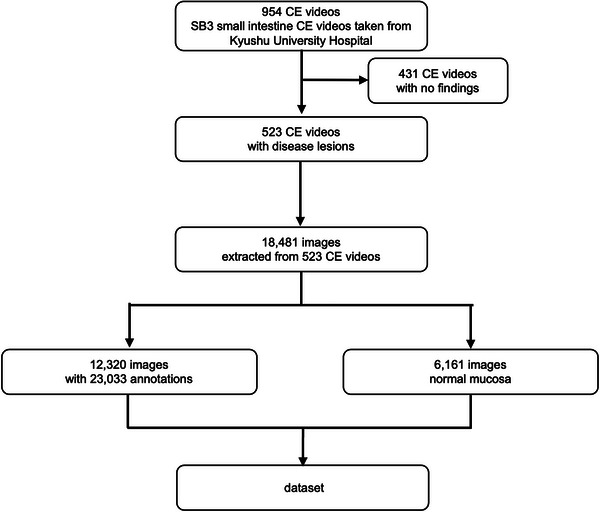
Image extraction procedure of the dataset. We used 523 of 954 collected capsule endoscopy (CE) videos as the dataset. This dataset contains 23,033 annotation labels.
TABLE 1.
Disease backgrounds included in the dataset (n = 523).
| Characteristics | ||
|---|---|---|
| No. of images | 18,481 | |
| With disease lesions | 12,320 | |
| No. of annotations | 23,033 | |
| Cause | ||
| Follicular lymphoma | 77 | |
| Crohn's disease | 58 | |
| Angiodysplasia | 39 | |
| MALT lymphoma | 22 | |
| Familial adenomatous polyposis | 23 | |
| Diffuse large B‐cell lymphoma | 17 | |
| Cowden syndrome | 17 | |
| Peutz‐Jeghers syndrome | 14 | |
| NSAIDs ulcer | 9 | |
| GIST, adenoma | 7 | |
| Amyloidosis | 6 | |
| IgA vasculitis, anastomotic ulcer | 3 | |
|
Small intestine cancer, Bechet's disease, intestinal tuberculosis, Protein‐losing gastroenteropathy, metastatic cancer, Arteriovenous malformation, adult T‐cell leukemia‐lymphoma, Eosinophil enteritis, chronic enteropathy associated with SLCO2A1 |
2 | |
|
Radioactive enteritis, ischemic enteritis, T‐cell lymphoma, Plasma cell tumor, Cryoglobulinemia, Intestinal emphysema, Polyarteritis nodosa, Scleroderma, Cavernous hemangioma, Olmesartan Associated Enteropathy, Cancer of unknown primary, Celiac disease, Sarcoma, Giardia, Ulcerative Colitis, Systemic lupus erythematosus, Neuroendocrine neoplasm, Cytomegalovirus enteritis |
1 | |
| Unknown disease name | 196 | |
Disease backgrounds of collected images in the dataset. MALT; mucosa associated lymphoid tissue, NSAIDs; non‐steroidal anti inflammatory drug, GIST; gastrointestinal stromal tumor.
To classify various abnormal lesions of the small bowel without any omission, we proposed 12 types of annotations. The specific annotation definitions were as follows:
Angiodysplasia: areas of erythema with suspected capillary lesions
Erosion: areas of mucosal damage such as erosions, ulcers, and notches
Stenosis: areas of constriction and rigidity
Lymphangiectasis: areas containing lymphatic vessels larger than a point
Lymph follicle: areas containing normal follicles and suspected lymphatic follicles
Submucosal tumor: areas resembling submucosal tumors
Polyp‐like: elevated lesions with a base or areas of suspected adenoma
Bleeding: areas of apparent hemorrhage, exclude bile‐colored intestinal fluid
Diverticula: areas of the suspected diverticulum
Redness: areas of redness and edema that may be related to inflammation
Foreign body: foreign objects other than food
Venous: areas with venous structures
AI modeling and test validation
We tested the technical quality of the dataset using YOLO v5 19 to evaluate the efficacy and limitations of our object detection AI model and annotation system. YOLO v5 is an open‐source object detection algorithm developed by Glenn Jocher (Ultralytics, Los Angeles, CA, USA) and released in June 2020. We can easily use YOLO v5 in Google Colaboratory. We trained YOLO v5x with larger parameters to train an object detection model using the dataset. The AI model was created using 17,085 of the 18,481 images in the dataset, comprising 11,043 abnormal images and 6,042 normal mucosa images with 20,629 annotated labels. The model was generated on a data center GPU (Tesla V100 SXM2 16 GB; NVIDIA, Santa Clara, CA, USA) and required over 40 hours of computation time. The starting weights for model creation were pre‐trained on COCO128, 20 with a batch size of eight. Yolov5 reads images through hyperparameters determining the augmentations to each image. The model's mean Average Precision becomes constant during the latter half of 200 epochs, and the model is stopped being created at 300 epochs to prevent overfitting. Assuming that each augmentation produces a unique augmented image, the total number of images employed for training is 18,481×300. We employed three‐fold cross‐validation. Intersection over union measures the overlap between the AI‐detected region and the truth annotation area. In our AI validation, an intersection over a union value >0.5 was considered indicative of a correct answer. We used precision, recall, and F1‐score as model performance indicators during AI creation, which is commonly used in object detection. The indicators are defined as follows.
Precision (i.e., positive predictive value)
The proportion of samples that test positive, calculated as true positives divided by the sum of true positives and false positives.
Recall (i.e., sensitivity, probability of detection, or true positive rate)
The proportion of positive samples that correctly test positive out of all positive samples, calculated as true positives divided by the sum of true positives and false negatives.
F1‐score
A measure of test accuracy, calculated as the harmonic mean of precision and recall using the formula; 2 x precision x recall divided by the sum of precision and recall.
We utilized the residual 1396 images from the dataset for test validation. This validation of the test was executed to evaluate the performance of the AI using data that was not employed during the AI's construction. The calculation of the receiver operating characteristic (ROC) curve and other metrics such as true positives, true negatives, false positives, false negatives, sensitivity, and specificity were determined by analyzing the annotations per frame in 1396 test‐validated images. When multiple annotations of the same type are present in a single image, the bounding box with the highest confidence rate is utilized for the calculation. The cut‐off value was determined based on the Youden Index, calculated by adding sensitivity and specificity while subtracting one. Typically, the cut‐off value is set at the highest point of the Youden Index.
RESULTS
Figure 2 contains representative images of the annotations and shows the distribution of annotation labels in the dataset. Figure 3a shows how many abnormal lesions were detected in one image, and 4083 images out of 18,481 images have multiple disease lesions. Figure 3b shows that some images have different types of annotations in one image. There are two different abnormalities in 1356 images and three types in 44 images. The combination of annotations in the same image is shown in Figure 3c. These results indicate that images of diffuse lesions containing multiple instances of the same type of annotation within the same image and entirely different annotation types are simultaneously included in CE images.
FIGURE 2.
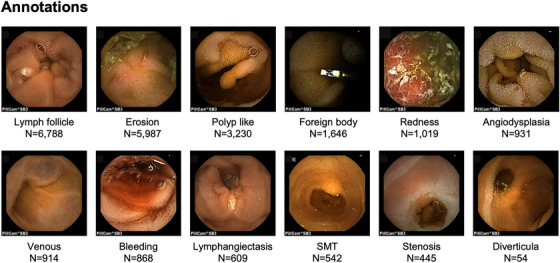
Type of annotations in the dataset. Representative images of the annotations and the distribution of annotation label numbers in the dataset; the number of labels per annotation ranged from 54 to 6788. The specific annotation definitions were as follows: Angiodysplasia; areas of erythema with suspected capillary lesions, Erosion; areas of mucosal damage such as erosions, ulcers, and notches, Stenosis; areas of constriction and rigidity, Lymphangiectasis; areas containing lymphatic vessels larger than a point, Lymph follicle; areas containing normal follicles and suspected lymphatic follicles, Submucosal tumor (SMT); areas resembling submucosal tumors, Polyp like; elevated lesions with a base or areas of suspected adenoma, Bleeding; areas of apparent hemorrhage, exclude sites that have become darker and are distant from the source of bleeding, Diverticula; areas of the suspected diverticulum, Redness; areas of redness and edema that may be related to inflammation, Foreign body; foreign objects other than food, Venous; areas with venous structures.
FIGURE 3.
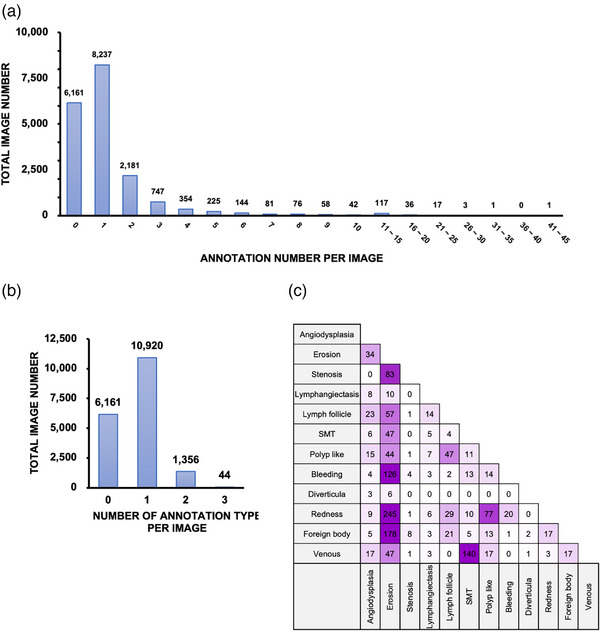
Features of annotations in the dataset. (a) Distribution of annotation labels per image and 4083 of 18,481 images have multiple lesions in one image. Approximately 2% of all images contained more than 10 annotation labels. (b) Distribution of annotation label types per image. There are two different abnormalities in 1356 images and three types in 44 images. (c) The number of annotation labels appears in the same image; for example, “erosion” and “redness” both tended to appear more often with other annotation label types.
Since the object detection AI model recognizes specific pathological lesions in an image, it is more appropriate than the classification AI model. The object detection AI model returns the location and confidence rate of the disease lesion. For videos, detection is repeated per frame. Figure 4 shows the relationships among precision, recall, F1‐score, and confidence rate in the model creation. In standard object detection, precision and recall are usually balanced; the cut‐off is often established where the F1‐score is highest. However, when the AI model is used in actual clinical practice, we envision that a specialist will review annotations made by the AI. The F1‐score increased rapidly at confidence rates of ≤0.1, then exhibited a gradual increase.
FIGURE 4.
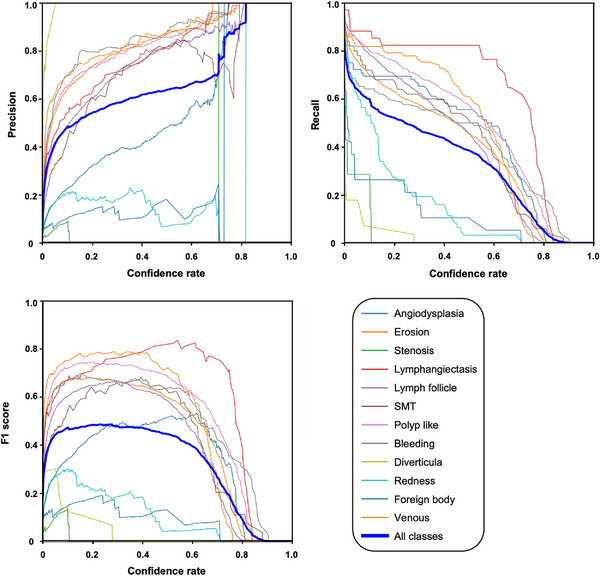
Relationship between Precision, Recall, F1 score, and confidence rate in creating AI model. The point with the highest F1 score had a confidence rate of 0.279.
On test validation, there were 1495 annotation types on 1396 test images. Of these, 1375 were detected, resulting in a sensitivity of 91%. Figure 5 shows representative images detected by this AI model, demonstrating multiple representative detections. The left image detected two different erosions, the middle image detected submucosal tumor and erosion at the top, and the right image detected erosion and bleeding (representative detection videos are also included in the Supporting Information). Figure 6 shows the ROC curve for each annotation, with eight of the 12 annotations demonstrating good detection with the area under the curves greater than 0.9. The highest area under the curve was 0.98 for “lymphangiectasia” and “venous.” Table 2 presents the details of the test validation conducted by the created AI model for each annotation type. “Lymphangiectasis,” “venous,” and “angiodysplasia” were well detected by the model. However, the sensitivity for “diverticula” and “stenosis” was found to be low compared to other labels, and many were missed.
FIGURE 5.
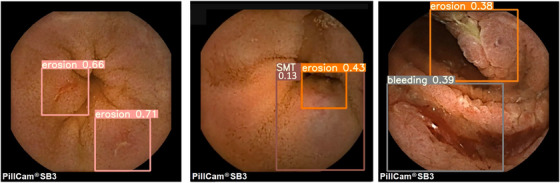
Representative images detected by created artificial intelligence (AI) model. Disease lesions detected by the AI are indicated on a rectangle, along with the confidence rate. The left image detected two different erosions, the middle image detected submucosal tumor (SMT) and erosion at the top, and the right image detected erosion and bleeding (representative detection videos are also included in the dataset).
FIGURE 6.
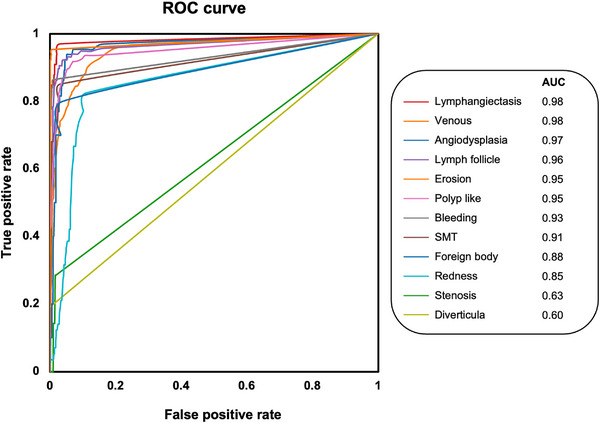
Receiver operating characteristic (ROC) curves for each annotation. The ROC curve is calculated by determining annotations per frame in 1396 test‐validated images. When multiple annotations of the same type are present in the same image, the bounding box with the highest confidence score is utilized in the calculation. The highest area under the ROC curve (AUC) was 0.98 for “venous” and “lymphangiectasis”.
TABLE 2.
Test validation results of the created artificial intelligence (AI) model.
| Venous | Lymphangi ectasis | Lymph follicle | Angio dysplasia | Bleeding | Foreign body | Polyp like | SMT | Erosion | Redness | Stenosis | Diverticula | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| True positives | 61 | 32 | 107 | 60 | 71 | 9 | 304 | 40 | 484 | 50 | 2 | 4 |
| True negatives | 1325 | 1321 | 1232 | 1263 | 1302 | 1335 | 994 | 1305 | 740 | 1191 | 1366 | 1375 |
| False positives | 7 | 42 | 48 | 68 | 13 | 51 | 74 | 44 | 141 | 148 | 23 | 0 |
| False negatives | 3 | 1 | 9 | 5 | 10 | 1 | 24 | 7 | 31 | 7 | 5 | 17 |
| Best confidence rate | 0.02 | 0.01 | 0.236 | 0.206 | 0.015 | 0.014 | 0.062 | 0.011 | 0.039 | 0.011 | 0.041 | 0.038 |
| Sensitivity | 0.953 | 0.97 | 0.922 | 0.923 | 0.877 | 0.9 | 0.927 | 0.851 | 0.94 | 0.877 | 0.286 | 0.19 |
| Specificity | 0.995 | 0.969 | 0.963 | 0.949 | 0.99 | 0.963 | 0.931 | 0.967 | 0.84 | 0.889 | 0.983 | 1 |
| Youden Index | 0.948 | 0.939 | 0.885 | 0.872 | 0.867 | 0.863 | 0.858 | 0.818 | 0.78 | 0.767 | 0.269 | 0.19 |
The true positives, true negatives, false positives, best confidence rate, sensitivity, specificity, and Youden Index were calculated by determining annotations per frame in 1,396 test validated images. These values are determined by the best confidence rate (cut‐off value). The sensitivity for “diverticula” was 0.19, and “stenosis” was 0.29, found to be low compared to other labels. SMT, submucosal tumor.
DISCUSSION
In this study, we created an object detection CE AI model, which classified pathological abnormalities into 12 types of annotations. The ROC analysis shows areas under the curve greater than 0.9 for eight of the 12 annotations. The total sensitivity for the disease lesion was about 91%. More commonly observed annotations had better detection performance, resulting in total higher sensitivity. Nevertheless, AI models currently exhibit lower confidence when detecting “diverticula” and “stenosis.” Since we use open‐source and open our dataset, clinicians can reproduce and use our model.
Previous research has demonstrated that image classification AI models for single lesions in each small bowel CE image can be highly effective. 15 , 21 Since the image classification process is based on the whole visual feature of an image, image classification AI models are weak in pointing out multiple types of annotations in the same frame. Previous reports using image classification for CE AI rarely mention instances where different annotation types overlap in the same frame. 22 As shown in this study, CE images often contain multiple pathological lesions within the same frame. AI using object detection is useful to solve this problem. Object detection is an AI model type that identifies each image's location and type of annotations. For example, if a polyp causes gastrointestinal bleeding with an ulcer, endoscopic hemostasis may be required. Conversely, if the bleeding results from multiple open ulcers, the possibility of Crohn's disease should be considered, and prioritizing appropriate treatment is advisable. The model with multiple annotations is in line with actual clinical practice, and with further advances, it could become a user‐friendly reading aid. Exploratory use of object detection to identify anomalies in CE images has been reported in Single Shot MultiBox Detector and Retinanet. 23 , 24 Those reports attempted to detect three to four types of pathological lesions. It is currently uncertain to what extent classification of abnormalities is the most effective reading aid in small bowel CE AI for detecting abnormal images. Ding classified CE images extracted from 6970 individuals into 12 classes, including “normal,” for comprehensive abnormality detection. 15 When establishing annotations in our AI model, our goal was to classify all important abnormalities for reading; when possible, we sought to combine annotations that rarely need to be distinguished in clinical practice. We divided the pathological lesions into 12 categories, with “stenosis,” “diverticula,” and “redness” being particularly poorly detected by our AI. Regarding “stenosis,” it was challenging to determine whether the intestinal tract was constricted or actively engaged in peristalsis, and concerning “diverticula,” it was difficult to determine whether the image showed a lumen or a diverticulum. Object detection AI cannot account for the previous, and next frames, which we found makes it poor at detecting some disease lesions. For “redness,” it was difficult to draw clear borders; annotation boundaries were easily influenced by subjectivity and often overlapped with other annotation labels. The challenge of annotating such lesions with overlapping classes may contribute to the difficulty in creating high‐level object detection models. To increase the detection rate, more educational images are needed.
The validation of AI models should be carried out using standard images or videos. The creation of standard datasets for CE images will significantly contribute to the development of AI models. For instance, in skin cancer, datasets such as the Melanoma Detection Dataset 25 and The HAM 10,000 dataset 26 have been released and are readily accessible. For small bowel CE, several studies have attempted to create large datasets and developed comprehensive AI models for lesion detection. Many of these datasets are no longer available 27 or may only be obtainable upon request. 28 Our object detection model and dataset are implemented in the open source and are relatively easy to reproduce, use, and improve.
This study has several limitations. Our dataset was collected from images acquired using the PillCam SB 3. The color and luminosity may vary slightly depending on the manufacturer of the CE system; therefore, the AI model must be modified to detect images from CE devices other than the PillCam SB 3. For example, The Kvasir‐Capsule Dataset 29 is an existing publicly available large‐scale CE dataset; however, The Kvasir‐Capsule Dataset was acquired using an Olympus EC‐S10 (Olympus, Tokyo, Japan). Thus, it may be challenging to align our dataset with other datasets simply. Our dataset had fewer than 20 cases of most rare diseases, which represents an important limitation. Previous studies have indicated that AI can effectively classify celiac disease 30 and parasites. 31 Because there were very few patients with parasites or celiac disease in our hospital, our database is unsuitable for validating AI models trained to detect such diseases. Our test validation, in this case, suggests that our created AI is partially effective at detecting disease lesions and has the potential to serve as a reading aid. However, it has yet to be validated in clinical practice whether this AI leads to a reduction in reading costs or missed findings.
In summary, we make our data and model available to many researchers as a milestone in the technology. The supplement data includes a link to experience our object detection CE AI model on the web‐based Google Colab Notebook. We hope more facilities will collect CE images in the future, supporting the continued improvement and availability of CE AI diagnostic systems to endoscopists.
CONFLICT OF INTEREST STATEMENT
None.
The link to experience our object detection artificial intelligence and model weights is as follows; https://colab.research.google.com/drive/1mEE5zXq1U9vC01P‐qjxHR2kvxr_3Imz0?usp=sharing
Supporting information
Video S1 A sample video detecting submucosal tumor with erosions.
Video S2 A sample video detecting multiple erosions.
Video S3 A sample video detecting multiple lymph follicle.
Video S4 A sample video detecting foreign body (single tablet).
Video S5 A sample video detecting bleeding.
ACKNOWLEDGMENTS
We received no significant financial support for this work that could have influenced its outcome. We thank Angela Morben, DVM, ELS, and Ryan Chastain‐Gross, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
DATA AVAILABILITY STATEMENT
Our data are licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) License. The material is free to copy and redistribute in any medium or format; it can be remixed, transformed, and built upon for any purpose if appropriate credit is given. We have archived the dataset of the project at Kaggle (Google LCC, USA), the world's largest data science online community; DOI: 10.34740/kaggle/ds/1516536.
REFERENCES
- 1. Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature 2000; 405: 417. [DOI] [PubMed] [Google Scholar]
- 2. Hosoe N, Takabayashi K, Ogata H, Kanai T. Capsule endoscopy for small‐intestinal disorders: Current status. Dig Endosc 2019; 31: 498–507. [DOI] [PubMed] [Google Scholar]
- 3. Enns RA, Hookey L, Armstrong D et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology 2017; 152: 497–514. [DOI] [PubMed] [Google Scholar]
- 4. Cave DR, Hakimian S, Patel K. Current controversies concerning capsule endoscopy. Dig Dis Sci 2019; 64: 3040–7. [DOI] [PubMed] [Google Scholar]
- 5. Aoki T, Yamada A, Aoyama K et al. Clinical usefulness of a deep learning‐based system as the first screening on small‐bowel capsule endoscopy reading. Dig Endosc 2020; 32: 585–91. [DOI] [PubMed] [Google Scholar]
- 6. Chilamkurthy S, Ghosh R, Tanamala S et al. Deep learning algorithms for detection of critical findings in head CT scans: A retrospective study. Lancet 2018; 392: 2388–96. [DOI] [PubMed] [Google Scholar]
- 7. Esteva A, Kuprel B, Novoa RA et al. Dermatologist‐level classification of skin cancer with deep neural networks. Nature 2017; 542: 115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gulshan V, Peng L, Coram M et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. Jama 2016; 316: 2402–10. [DOI] [PubMed] [Google Scholar]
- 9. Zouch W, Sagga D, Echtioui A et al. Detection of covid‐19 from CT and chest X‐ray images using deep learning models. Ann Biomed Eng 2022; 50: 825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HH, Tseng VS. Accurate classification of diminutive colorectal polyps using computer‐aided analysis. Gastroenterology 2018; 154: 568–75. [DOI] [PubMed] [Google Scholar]
- 11. Lau PY, Correia PL. Detection of bleeding patterns in WCE video using multiple features. Annu Int Conf IEEE Eng Med Biol Soc 2007; 2007: 5601–4. [DOI] [PubMed] [Google Scholar]
- 12. Giritharan B, Yuan X, Liu J, Buckles B, Oh J, Tang SJ. Bleeding detection from capsule endoscopy videos. Annu Int Conf IEEE Eng Med Biol Soc 2008; 2008: 4780–3. [DOI] [PubMed] [Google Scholar]
- 13. Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. Commun ACM 2017; 60: 84–90. [Google Scholar]
- 14. Yu H, Yang LT, Zhang Q, Armstrong D, Deen MJ. Convolutional neural networks for medical image analysis: State‐of‐the‐art, comparisons, improvement and perspectives. Neurocomputing 2021; 444: 92–110. [Google Scholar]
- 15. Ding Z, Shi H, Zhang H et al. Gastroenterologist‐level identification of small‐bowel diseases and normal variants by capsule endoscopy using a deep‐learning model. Gastroenterology 2019; 157: 1044–54.e5. [DOI] [PubMed] [Google Scholar]
- 16. Tsuboi A, Oka S, Aoyama K et al. Artificial intelligence using a convolutional neural network for automatic detection of small‐bowel angioectasia in capsule endoscopy images. Dig Endosc 2020; 32: 382–90. [DOI] [PubMed] [Google Scholar]
- 17. Saito H, Aoki T, Aoyama K et al. Automatic detection and classification of protruding lesions in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest Endosc 2020; 92: 144–51.e1. [DOI] [PubMed] [Google Scholar]
- 18. Barash Y, Azaria L, Soffer S et al. Ulcer severity grading in video capsule images of patients with Crohn's disease: An ordinal neural network solution. Gastrointest Endosc 2021; 93: 187–92. [DOI] [PubMed] [Google Scholar]
- 19. Ultralytics . Yolov5 [Internet]. Ultralytics. Accessed June 16, 2023. Available from: https://github.com/ultralytics/yolov5
- 20. Lin T‐Y, Maire M, Belongie S et al. Microsoft coco: Common objects in context. In: Fleet D, Pajdla T, Schiele B, Tuytelaars T (eds), Computer Vision – ECCV 2014, Springer, Cham: 2014. pp. 740–55. [Google Scholar]
- 21. Ding Z, Shi H, Zhang H et al. Artificial intelligence‐based diagnosis of abnormalities in small‐bowel capsule endoscopy. Endoscopy 2023; 55: 44–51. [DOI] [PubMed] [Google Scholar]
- 22. Phillips F, Beg S. Video capsule endoscopy: Pushing the boundaries with software technology. Transl Gastroenterol Hepatol 2021; 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Otani K, Nakada A, Kurose Y et al. Automatic detection of different types of small‐bowel lesions on capsule endoscopy images using a newly developed deep convolutional neural network. Endoscopy 2020; 52: 786–91. [DOI] [PubMed] [Google Scholar]
- 24. Wang S, Xing Y, Zhang L, Gao H, Zhang H. A systematic evaluation and optimization of automatic detection of ulcers in wireless capsule endoscopy on a large dataset using deep convolutional neural networks. Phys Med Biol 2019; 64: 235014. [DOI] [PubMed] [Google Scholar]
- 25. Garcia‐Arroyo JL, Garcia‐Zapirain B. Segmentation of skin lesions in dermoscopy images using fuzzy classification of pixels and histogram thresholding. Comput Methods Programs Biomed 2019; 168: 11–9. [DOI] [PubMed] [Google Scholar]
- 26. Tschandl P, Rosendahl C, Kittler H. The ham10000 dataset, a large collection of multi‐source dermatoscopic images of common pigmented skin lesions. Sci Data 2018; 5: 180161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koulaouzidis A, Iakovidis DK, Yung DE et al. Kid project: An internet‐based digital video atlas of capsule endoscopy for research purposes. Endosc Int Open 2017; 5: E477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leenhardt R, Li C, Le Mouel JP et al. Cad‐cap: A 25,000‐image database serving the development of artificial intelligence for capsule endoscopy. Endosc Int Open 2020; 8: E415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smedsrud PH, Thambawita V, Hicks SA et al. Kvasir‐capsule, a video capsule endoscopy dataset. Sci Data 2021; 8: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molder A, Balaban DV, Jinga M, Molder CC. Current evidence on computer‐aided diagnosis of celiac disease: Systematic review. Front Pharmacol 2020; 11: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He JY, Wu X, Jiang YG, Peng Q, Jain R. Hookworm detection in wireless capsule endoscopy images with deep learning. IEEE Trans Image Process 2018; 27: 2379–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 A sample video detecting submucosal tumor with erosions.
Video S2 A sample video detecting multiple erosions.
Video S3 A sample video detecting multiple lymph follicle.
Video S4 A sample video detecting foreign body (single tablet).
Video S5 A sample video detecting bleeding.
Data Availability Statement
Our data are licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) License. The material is free to copy and redistribute in any medium or format; it can be remixed, transformed, and built upon for any purpose if appropriate credit is given. We have archived the dataset of the project at Kaggle (Google LCC, USA), the world's largest data science online community; DOI: 10.34740/kaggle/ds/1516536.


