Abstract
Dog-mediated rabies is responsible for tens of thousands of human deaths annually, and in resource-constrained settings, vaccinating dogs to control the disease at source remains challenging for various reasons. Currently, rabies elimination efforts rely on mass dog vaccination by the parenteral route. While oral rabies vaccination (ORV) of dogs is primarily considered a tool to increase herd immunity, particularly by targeting free-roaming and stray dogs, here, we are showcasing an ORV-only approach as an emergency response model. Using a third-generation vaccine and a standardized egg-flavored bait, we assessed the effectiveness and vaccination under field conditions in the Zambezi region of Namibia. During this trial, with four teams and within four working days, 3097 dogs were offered a bait, of which 88,0% were considered vaccinated. Teams managed to vaccinate, on average, over 20 dogs/h, despite using a door-to-door vaccination approach.
The favorable results both in terms of bait acceptance and successful vaccination as well as field applicability and effectiveness further support the great potential of ORV in dog rabies control programmes.
Keywords: Africa, Dogs, Namibia, Oral vaccination, Rabies, SPBN GASGAS
1. Introduction
Among the various mesocarnivorous and chiropteran rabies reservoir hosts [1,2], the domestic dog poses by far the greatest threat to global public health [3,4]. Vaccinating at least 70% of the targeted dog population against rabies would break the cycle of transmission within the dog population and from dogs to humans, saving the lives of several tens of thousands of people [5] who fall victim of this horrendous disease each year, primarily in low income countries in Africa and Asia [6].
Experience from Latin America and the Caribbean has shown that mass dog vaccinations and public awareness are key to success in the fight against canine rabies [7,8]. Therefore, it is not surprising that parenteral vaccination with inactivated vaccines is still considered the only approach to control canine-transmitted rabies on a large scale. However, depending on the socio-cultural background and the resulting attitudes towards local dog populations, implementing these organizationally costly techniques in resource-poor settings in other parts of the world can be extremely challenging. Unfortunately, there are hardly any success stories in controlling dog-mediated rabies outside the Americas. Achieving an appropriate threshold for herd immunity is certainly feasible in general terms [9,10]. However, the question remains to be answered as to how maintenance of herd immunity in epidemiologically important subpopulations, i.e., poorly accessible free-ranging dog populations, in African and Asian settings can be ensured [[11], [12], [13]]. One promising way to address this challenge may be oral rabies vaccination (ORV) [13,14].
The effectiveness of ORV in combating wildlife-transmitted rabies has been impressively demonstrated in western and central Europe and North America [[15], [16], [17]]. Although the concept of ORV was also initially tested for dogs by evaluating the efficacy of oral rabies vaccines [[18], [19], [20], [21], [22], [23], [24]] or the attractiveness and uptake of different baits [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34]], it remains unclear why research in this regard has continued at a slow pace. Thus, it is not surprising that oral vaccines have never been effectively used in dog rabies control programs and are still an undervalued tool for achieving dog rabies elimination [13,14].
In a previous field study, we demonstrated high bait acceptance and applicability of ORV in the Oshana and Omusati regions of Namibia [35] by using vaccine baits consisting of an highly immunogenic [[36], [37], [38], [39]] and safe [40,41] oral rabies vaccine and a universal, industrially produced egg-flavored bait (egg bait) [28,33,34] that meet international recommendations [42]. The vaccine construct was the first to also demonstrate efficacy according to requirements by both World Organization for Animal Health (WOAH) and the European Pharmacopoeia in a controlled animal experimental study [43]. However, with the exception of Thailand and Haiti [44,45], field data on the applicability and effectiveness of ORV under various socio-economic settings in Africa are still lacking.
Therefore, we set out to implement another ORV pilot field study to demonstrate the applicability of this approach in Namibia. Initially, the objectives of this study were to compare the effectiveness of mass dog vaccination using parenteral vaccines and a combination of the latter with ORV in the Zambezi region of Namibia. Veterinary control interventions have been hampered by a number of challenges, incl. Transport (availability of 4WD); competing disease priorities (Foot and mouth disease, Contagious bovine pleuropneumonia, etc.) and availability of field staff (not all staff members themselves were vaccinated against rabies), so that mass dog vaccination campaigns had been on hold since 2017. With cases reported both in animals and humans, we therefore initiated this research field study as an ORV-only approach, with a view to its application as an emergency response to rabies incidences or outbreaks.
2. Materials and methods
2.1. Study sites
The ORV field trial was conducted in the Zambezi region of Namibia's Northern Communal Areas (NCAs), in rural and suburban communities centered around the city of Katima Mulilo (Fig. 1). The terrain covers an area of 14,785 km2 and is mostly made up of swamps, floodplains, wetlands and woodland.
Fig. 1.
Location in Africa and map of Namibia and the Zambezi region highlighted (red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As of the last census the region had a population of almost 91,000 [46]. The field trial area was selected after consultation with the Directorate of Veterinary Services (DVS) considering available infrastructure and logistics and based on results of a Knowledge, Attitude and Practice (KAP) study conducted (unpublished), indicating low vaccination coverage in certain regions due to a number of constraints (see above) and free-roaming hard-to-reach shepherd dogs. In addition, the Zambezi region shares a number of international borders with its neighbors Zambia, Angola, Botswana and Zimbabwe. As diseases spread irrespective of national borders, socio-economic and public health consequences are part and parcel.
2.2. Vaccine baits
Oral rabies vaccinations were conducted using 3rd generation oral rabies virus vaccine (Ceva Innovation Center GmbH, Dessau in Germany), consisting of the SPBN GASGAS vaccine virus strain, a genetically engineered derivate of SAD L16 derived from the vaccine strain SAD B19 which is licensed for foxes and raccoon dogs according to international standards (Freuling et al., 2019). The recombinant vaccine virus construct is distinguished from SAD B19 by the deletion of pseudogene ψ, the introduction of four recognition sequences for restriction enzymes and duplicate insertion of an identical altered glycoprotein [47]. The genes encoding for glycoprotein G contain the amino acid exchange Arg333 → Glu333 and Asn194 → Ser194 to eliminate residual pathogenicity and reduce the risks for compensatory mutations, respectively [48]. These alterations significantly enhance the safety profile of the vaccine virus [40]. A soft sachet filled with the liquid vaccine virus (3 mL, 108.2 FFU/mL) was incorporated in a universal industrial manufactured egg-flavored bait (egg bait) previously shown to be highly attractive to local free-roaming dogs [35].
2.3. Shipment, transportation and storage of vaccine baits
Vaccine baits were shipped according to IATA guidelines on dry ice (UN 1845) directly from the manufacturer to the importing company in Windhoek (Swavet, Windhoek, Namibia), using a commercial courier service. After temporary storage, the vaccine baits were cold-chain-transported to the field trial area in Zambezi region and stored in a central freezer and fridge at the site of the project location. Maintenance of the cold chain was monitored and documented using temperature data logger and integrated electronic measuring.
2.4. Vaccination teams
Four vaccination teams were working simultaneously, with each team consisting of at least three DVS staff members (state veterinary officer or animal health technician) to share the tasks of driving, vaccinating and data collection. Vaccination teams used four-by-four pickup trucks equipped with cool-boxes, cooler bags, gloves, trash bags hand disinfectants and information leaflets. Immediately prior to the field trial, a two-hour introductory workshop was conducted during which staff was trained on the objectives of the field trial, oral rabies vaccination, vaccine bait handling, safety issues, techniques for approaching free-roaming dogs, best practice on offering vaccine baits to dogs, and data collection.
2.5. Vaccinations
The field trial was carried out at the beginning of the rainy season during the second half of November 2022. During this time, vaccinations were performed over four full working days. Vaccination campaigns were planned in advance and announced via local radio one week ahead of the campaign, the evening before and on the morning at which the campaigns took place.
Vaccine baits were transferred to portable cool bags the evening before field use, allowing the baits to thaw before they were offered to the dogs. Baits left unused at the end of the vaccination day were kept at fridge temperatures (4–8 °C) and offered to dogs the next day to avoid repeated freezing and thawing of vaccine baits.
In Katima Mulilo, an urbanized community with formal and informal settlements, areas were selected for the individual teams and vaccine baits were distributed directly from 4WD trucks to free-roaming dogs during morning vaccination sessions between 07:00–09:00 a.m. Vaccination in other parts of the Zambezi region took place between 10:00 am and 5:00 pm and were conducted both at individual homesteads or villages where visually identified dogs were targeted. Dog owners were informed about ORV using a leaflet with ORV related information provided in both the official (English) as well as the local (Lozi) language that also contained an emergency contact phone number in case of any adverse events. Also, a rabies vaccination card for the dog was offered upon request. If possible, discarded baits or sachets were retrieved, collected in trash bags and disposed of as infective materials according to prevailing regulations on hazardous waste. Team debriefings and daily evaluations were held at the end of each vaccination day.
2.6. Data collection and vaccination monitoring
For collection of vaccination and survey data as well as project management, e.g. navigation within demarcated boundaries, sharing real-time team locations during roaming work and survey assessment (supplementary file), a smartphone application including the WVS web-based backend platform was used essentially as described [35,49].
2.7. Evaluations and statistical analysis
Animals were regarded as successfully vaccinated via the oral route if bait chewing and intensity (thoroughness) was observed and/or perforation of the sachet clearly visible. Any dog that swallowed the bait immediately, or walked away with it and could not be observed, or insufficiently chewed the bait without visible perforation of the sachet, was assigned an ‘unknown’ vaccination status. The status ‘non-vaccinated’ was assigned if the dog was not interested, the bait was only shortly taken up and immediately dropped with the bait casing and sachet still intact, or bait consumption was interrupted by external factors (interference by other dogs, humans, cars, etc).
Data were uploaded daily to a cloud-based server and downloaded by evaluation supervisors as an Excel document Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA) for initial review. Subsequent analyses were performed using GraphPad Prism v9.0 (GraphPad Prism Software Inc., San Diego, USA). Spatial information was analyzed and displayed using QGIS Geographic Information System (QGIS.org, 2022.http://www.qgis.org).
2.8. Ethical and legal considerations
The ORV field trial was planned in close cooperation with the Namibian Directorate of Veterinary Services (DVS) within the Ministry of Agriculture, Water, Forestry and Land Reform (MAWLR). Import approval of the vaccine was conducted as described before [35]. Approval of the oral rabies field study in the Zambezi region and its ethical clearance was afforded in August 2022 by the chief veterinary officer of the Republic of Namibia in agreement with the registrar of the medicines at the Namibian Medicines Regulatory Council.
3. Results
Since the last rabies mass dog vaccination campaign was held in 2017, dog owners appreciated the possibility to have their dogs orally vaccinated and were extremely positive regarding the use of oral baits. Using ORV only, we distributed a total of 3097 baits in four days.
In various urban and suburban parts of Katima Mulilo, 750 dogs were offered a bait during the four days at vaccinations during morning hours (Fig. 2 A). Additionally, the morning after the 4 days officially scheduled for the field trial, 115 baits were distributed from two teams at vaccination rounds between 05:30 a.m. – 6:30 a.m. in previously omitted areas of Katima Mulilo.
Fig. 2.
A) Distribution of baits offered at four days during urban vaccinations. Dogs considered vaccinated are displayed as green dots (N = 624), dogs with an unknown status are displayed blue (N = 98) and non-vaccinated dogs (N = 28) are displayed as black dots. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In rural parts of the Zambezi region, 2347 baits were distributed, covering mostly areas accessible by roads (Fig. 2B).
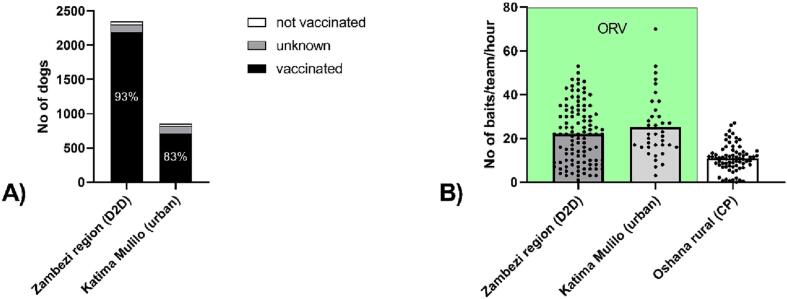
While in rural areas using door-to-door vaccinations, 93.1% of dogs offered a bait were considered vaccinated, in urban areas this was 83.1% (Fig. 3A). Thus, we successfully vaccinated at least 2725 dogs in just four days with four teams. The vaccination efficiency was slightly higher in urban areas with a mean of 25.2 dogs orally vaccinated per hour and team as opposed to rural areas with 22.1 dogs vaccinated per hour and team, while both was higher than during parenteral vaccinations in the Oshana region at central points (Fig. 3B).
Fig. 3.
Display of vaccinations and human settlements in the Zambezi-region, overlayed with a 4x4km grid. Insert: Estimation of vaccination coverage in grid cells with ORV using population counts from High Resolution Population Density Maps and a human:dog ratio of 5.
4. Discussion
Similar to other areas in developing countries, the Zambezi region of Namibia faces many challenges to development, e.g. an increasing population, and under-resourced public services, including veterinary and public health. Rabies is one of the burdens for the society with cases reported in animals and humans [50]. Veterinary control interventions have been hampered by a number of challenges, incl. Transport (availability of 4WDs); competing disease priorities (FMD,CBPP, etc.) and availability of field staff (not all staff members themselves are vaccinated against rabies), so that mass dog vaccination campaigns have been on hold since 2017. While the initial plan was to conduct ORV as a supplement to parenteral MDV, under these setting we initiated ORV-only as a research study, but also as an emergency response to a given rabies outbreak. The results of this field trial confirm the high acceptability of this method and the attractiveness of the egg-flavored bait. Interestingly, the percentage of dogs being assessed as vaccinated was >81%, higher than seen in other parts of Namibia [35], even without using additional gravy. Without any central point vaccinations and with an average distance travelled per team of 250 km per day, individual teams vaccinated >20 dogs/h on average (Fig. 4). This confirms similar data from an emergency vaccination in Windhoek, Namibia (supplementary file). Compared to parenteral vaccinations [51]., the effectiveness was much higher than encountered in the initial pilot phase of mass dog vaccination in the Oshana region of the NCAs (Fig. 4B). Currently ORV is considered only as a complementary tool to parenteral vaccination, particularly to increase herd immunity in hard-to-reach dogs. However, the results of our field data show that “complementary” could be widely interpreted to any situation and circumstance where this tool offers advantages to traditional parenteral vaccination, be it for epidemiological reasons or costs. While the cost of the vaccine is a minor component for parenteral vaccination campaigns [45], using the costs for rabies vaccinations in Namibia (Supplementary file) this benefit is only valid if >85 dogs are vaccinated per team and day (Fig. 5). If numbers, like in our case, of >188 dogs/team are vaccinated per day, even with higher costs for the vaccine, this approach would be more cost effective than the currently used parenteral vaccination. While we could not assess ORV in comparison with a combined approach, it was shown in Haiti, that the combination of door-to-door vaccinations and ORV increased the effectiveness and reduced the price per dog vaccinated [45].
Fig. 4.
Vaccination assessment (A) and team efficiency (B) for ORV stratified per location, i.e. the rural Zambezi region where door-to-door (D2D) was performed, and urban Katima Mulilo, were free-roaming dogs were baited directly from the car. Additionally, the per team performance in Oshana is shown, where vaccines were give parenterally at central points (CP).
Fig. 5.
Display of the dynamic range of costs per dog vaccinated depending on the number of dogs vaccinated per team and day, and the use of oral vaccination compared to parenteral vaccination.
Unlike the previous field trial where large parts of the costs were incurred by establishing a central cold storage [35], here we used available storage capacities at the accommodation. Such approach is feasible if baits are to be used in a relative short time frame of a few weeks. Also, we used cheap cooling bags in the front of the car where temperatures we cooler due to air conditioning and never exceeded critical thresholds as demonstrated by data loggers (Supplementary material).
Another aspect for planning and conducting of ORV vaccination campaigns is the flexibility and independence. Training in ORV distribution and data-capturing was reduced to two hours based on previous experiences [35]. DVS-staff were employed for vaccination but it would not be a prerequisite to use veterinary personnel but also laymen under veterinary supervision. Whilst authorities and communities were generally informed prior to the vaccination campaign, no central points were communicated regarding the presentation of dogs for oral vaccination. This reduced the efficiency of the campaign, but it allowed for flexibility in conducting the vaccinations. For instance, owner consent was not required and specific oral vaccination certificates for dog owners were only provided upon request, in our case, for <10% of dogs vaccinated. Nevertheless, the use of central points for vaccination at village level under the ORV approach may further increase effectiveness.
Generally, involvement of all stakeholders is essential to create e.g. awareness and change behaviors, however, this inclusive approach has the potential to delay agreements and arrangements, slow down the actual dog ORV campaign and thereby creates additional costs. It is, of course, in the hands of countries' authorities and NGOs to establish which method or combination may be more effective under particular circumstances that may differ from country to country. Besides effectiveness, the overarching goal is to increase the level of herd immunity. During this field trial, we were restricted regarding the amount of baits and time available for vaccination. Therefore, we did not anticipate to reach an overall high vaccination coverage. However, when using gridded human population data as a proxy for dog population, we reached higher values than for MDV in the Oshana region [52]. In subsequent studies or field applications, it is envisaged to use mobile phone based or external GPS tracker, so that areas that were covered but without dogs could be identified.
Other limitations to our study are the timing of the campaign at the beginning of the rainy season. This complicated the accessibility of certain communities in rural areas and streets in Katima Mulilo and also decreased the number of available dogs for vaccinations since they were partly accompanying their owners to the cattle grazing areas in the wet plains. Public announcement via radio was undertaken, but we understood that additional notification using loud-speakers would increase compliance to present dogs for vaccination. Similarly, as with previous dog vaccination campaigns [51], school children, presenting dogs for vaccination, should be involved, preferentially during holidays. The planning and subsequent implementation of this field trial would have been impossible without the use of geographical information systems and mobile technology, as outlined before [10]. The employed mobile phone technology not only enabled the systematic direction of vaccination teams in the field, but equally allowed for rapid assimilation and analyses of data, so that team debriefings could be undertaken via mobile communication. The use of mobile phone technology principally enables the gathering of much more information more easily for subsequent assessments, e.g. of vaccine uptake or other epidemiologically interesting aspects [35]. In this study, however, we restricted the number of data entries to a minimum (see supplementary file), partly, because some questions to ORV had already been answered [35], and also to balance between more data analyses and a more realistic effectiveness of the method. For instance, based on the data on vaccination success using the egg-flavored bait, under these setting the simple bait distribution would be sufficient to derive vaccination data.
In the framework of this field trial, vaccine exposure to humans that would require intervention did not occur and corroborates previous results [35]. Unused baits were retrieved when possible, i.e. the handout-and-retrieve model was only partly used. Sporadically, baits were provided with detailed instructions on their use for dog owners that presented without their dogs. This may seem to increase the risk of an unintentional human contact with the vaccine, but was jointly considered negligible against the background of the high safety profile of this vaccine construct [42,53].
5. Conclusions
The acceptance of the method was overwhelming, and communities not reached during this study requested DVS to also vaccinate their dogs. Therefore, members of the Namibian DVS vaccinated a further 315 dogs during the following three days. The results of this field trial add to the body of evidence that ORV has the potential to be decisive tool in the fight against dog-mediated rabies.
CRediT authorship contribution statement
Conrad M. Freuling: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition. Frank Busch: Investigation, Project administration, Writing – review & editing. Mainelo Beatrice Shikongo: Investigation, Resources, Writing – review & editing. Nzwana Silume: Investigation, Writing – review & editing. Jolandie van der Westhuizen: Investigation, Writing – review & editing. Siegfried Khaiseb: Investigation, Writing – review & editing. Albertina Shilongo: Resources, Writing – review & editing. Thomas Müller: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgments
This study was financed through a project (HR-0068) by the World Organization for Animal Health (WOAH) based on funds from the German Federal Ministry for Economic Cooperation and Development. The authors would like to thank all dog owners from the study areas in the Zambezi region for their overwhelming willingness to participate in this field trial. We also thank all veterinary assistants during the study. We are particularly indebted to Giulia Manzetti, who documented the entire field trial via photos and video recordings.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100562.
Appendix A. Supplementary data
Supplementary material 1: Data from dog vaccinations in Windhoek, Namibia, and a screenshot of the WVS survey.
Supplementary material 2: Calculations for the costs per dog vaccinated
Data availability
Data will be made available on request.
References
- 1.Müller T., Freuling C.M. In: Rabies - Scientific Basis of the Disease and its Management. fourth ed. Fooks A.R., Jackson A.C., editors. Elsevier Academic Press; 2020. Rabies in terrestrial animals; pp. 195–230. [Google Scholar]
- 2.Rupprecht C.E., Barrett J., Briggs D., Cliquet F., Fooks A.R., Lumlertdacha B., Meslin F.X., Müller T., Nel L.H., Schneider C., Tordo N., Wandeler A.I. Can rabies be eradicated? Dev. Biol. (Basel) 2008;131:95–121. [PubMed] [Google Scholar]
- 3.Fooks A.R., Banyard A.C., Horton D.L., Johnson N., McElhinney L.M., Jackson A.C. Current status of rabies and prospects for elimination. Lancet. 2014;384:1389–1399. doi: 10.1016/S0140-6736(13)62707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Vol. 1012. 2018. WHO Expert Consultation on Rabies: Third report, WHO Tech Rep Ser; p. 195. [Google Scholar]
- 5.Morters M.K., Restif O., Hampson K., Cleaveland S., Wood J.L., Conlan A.J. Evidence-based control of canine rabies: a critical review of population density reduction. J. Anim. Ecol. 2013;82:6–14. doi: 10.1111/j.1365-2656.2012.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M., Barrat J., Blanton J.D., Briggs D.J., Cleaveland S., Costa P., Freuling C.M., Hiby E., Knopf L., Leanes F., Meslin F.X., Metlin A., Miranda M.E., Müller T., Nel L.H., Recuenco S., Rupprecht C.E., Schumacher C., Taylor L., Vigilato M.A., Zinsstag J., Dushoff J. Global alliance for rabies control partners for rabies, prevention, estimating the global burden of endemic canine rabies. PLoS Neglect Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freire de Carvalho M., Vigilato M.A.N., Pompei J.A., Rocha F., Vokaty A., Molina-Flores B., Cosivi O., Del Rio Vilas V.J. Rabies in the Americas: 1998-2014. PLoS Neglect Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigilato M.A.N., Clavijo A., Knobl T., Silva H.M.T., Cosivi O., Schneider M.C., Leanes L.F., Belotto A.J., Espinal M.A. Progress towards eliminating canine rabies: policies and perspectives from Latin America and the Caribbean: policies and perspectives from Latin America and the Caribbean. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013;368:20120143. doi: 10.1098/rstb.2012.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleaveland S., Thumbi S.M., Sambo M., Lugelo A., Lushasi K., Hampson K., Lankester F. Proof of concept of mass dog vaccination for the control and elimination of canine rabies. Rev. Sci. Tech. 2018;37:559–568. doi: 10.20506/rst.37.2.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson A.D., Handel I.G., Shervell K., Roux T., Mayer D., Muyila S., Maruwo G.B., Nkhulungo E.M., Foster R.A., Chikungwa P., Chimera B., Bronsvoort B.M., Mellanby R.J., Gamble L. The vaccination of 35,000 dogs in 20 working days using combined static point and door-to-door methods in Blantyre, Malawi. PLoS Neglect Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lembo T., Hampson K., Kaare M.T., Ernest E., Knobel D., Kazwala R.R., Haydon D.T., Cleaveland S. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Neglect Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sambo M., Ferguson E.A., Abela-Ridder B., Changalucha J., Cleaveland S., Lushasi K., Mchau G.J., Nanai A., Nonga H., Steenson R., Johnson P.C., Hampson K. Scaling-up the delivery of dog vaccination campaigns against rabies in Tanzania. PLoS Neglect Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace R.M., Cliquet F., Fehlner-Gardiner C., Fooks A.R., Sabeta C.T., Setién A.A., Tu C., Vuta V., Yakobson B., Yang D.-K., Brückner G., Freuling C.M., Knopf L., Metlin A., Pozzetti P., Suseno P.P., Shadomy S.V., Torres G., Vigilato M.A.N., Abela-Ridder B., Müller T. Role of Oral rabies vaccines in the elimination of dog-mediated human rabies deaths. Emerg. Infect. Dis. 2020;26:e1–e9. doi: 10.3201/eid2612.201266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cliquet F., Guiot A.L., Aubert M., Robardet E., Rupprecht C.E., Meslin F.X. Oral vaccination of dogs: a well-studied and undervalued tool for achieving human and dog rabies elimination. Vet. Res. 2018;49:61. doi: 10.1186/s13567-018-0554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freuling C.M., Hampson K., Selhorst T., Schroder R., Meslin F.X., Mettenleiter T.C., Müller T. The elimination of fox rabies from Europe: determinants of success and lessons for the future. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013;368:20120142. doi: 10.1098/rstb.2012.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robardet E., Bosnjak D., Englund L., Demetriou P., Martín P.R., Cliquet F. Zero endemic cases of wildlife rabies (classical rabies virus, RABV) in the European Union by 2020: an achievable goal. Trop. Med. Infect. Dis. 2019;4:124. doi: 10.3390/tropicalmed4040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacInnes C.D., Smith S.M., Tinline R.R., Ayers N.R., Bachmann P., Ball D.G., Calder L.A., Crosgrey S.J., Fielding C., Hauschildt P., Honig J.M., Johnston D.H., Lawson K.F., Nunan C.P., Pedde M.A., Pond B., Stewart R.B., Voigt D.R. Elimination of rabies from red foxes in eastern Ontario. J. Wildl. Dis. 2001;37:119–132. doi: 10.7589/0090-3558-37.1.119. [DOI] [PubMed] [Google Scholar]
- 18.Baer G.M., Brooks R.C., Foggin C.M. Oral vaccination of dogs fed canine adenovirus in baits. Am. J. Vet. Res. 1989;50:836–837. [PubMed] [Google Scholar]
- 19.Rupprecht C.E., Shaddock J.S., Sanderlin D.W., Hanlon C.A., Niezgoda M., Schumacher C.L. Oral rabies vaccination of dogs. Isr. J. Vet. Med. 1998;34:228–239. [Google Scholar]
- 20.Rupprecht C.E., Hanlon C.A., Blanton J.D., Manangan J.S., Morrill P., Murphy S., Niezgoda M., Orciari L.A., Schumacher C.L., Dietzschold B. Oral vaccination of dogs with recombinant rabies virus vaccines. Virus Res. 2005;111:101–105. doi: 10.1016/j.virusres.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Aylan O., Vos A. Efficacy studies with SAD B19 in Turkish dogs. J. Etlik Vet. Microbiol. 1998;9:93–102. [Google Scholar]
- 22.Faber M., Dietzschold B., Li J. Immunogenicity and safety of recombinant rabies viruses used for Oral vaccination of stray dogs and wildlife. Zoonoses Public Health. 2009;56:262–269. doi: 10.1111/j.1863-2378.2008.01215.x. [DOI] [PubMed] [Google Scholar]
- 23.Yakobson B.A., King R., Sheichat N., Eventov B., David D. Assessment of the efficacy of oral vaccination of livestock guardian dogs in the framework of oral rabies vaccination of wild canids in Israel. Dev. Biol. (Basel) 2008;131:151–156. [PubMed] [Google Scholar]
- 24.Shuai L., Feng N., Wang X., Ge J., Wen Z., Chen W., Qin L., Xia X., Bu Z. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antivir. Res. 2015;121:9–15. doi: 10.1016/j.antiviral.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Frontini M.G., Fishbein D.B., Ramos J.G., Collins E.F., Torres J.M.B., Huerta G.Q., Rodriguez J., Belotto A.J., Dobbins J.G., Linhart S.B., Baer G.M. A field evaluation in Mexico of four baits for Oral rabies vaccination of dogs. Am. J. Trop. Med. Hyg. 1992;47:310–316. doi: 10.4269/ajtmh.1992.47.310. [DOI] [PubMed] [Google Scholar]
- 26.Matter H.C., Kharmachi H., Haddad N., Ben Youssef S., Sghaier C., Ben Khelifa R., Jemli J., Mrabet L., Meslin F.X., Wandeler A.I. Test of three bait types for oral immunization of dogs against rabies in Tunisia. Am. J. Trop. Med. Hyg. 1995;52:489–495. doi: 10.4269/ajtmh.1995.52.489. [DOI] [PubMed] [Google Scholar]
- 27.Matter H.C., Schumacher C.L., Kharmachi H., Hammami S., Tlatli A., Jemli J., Mrabet L., Meslin F.X., Aubert M.F.A., Neuenschwander B.E., El Hicheri K. Field evaluation of two bait delivery systems for the oral immunization of dogs against rabies in Tunisia. Vaccine. 1998;16:657–665. doi: 10.1016/s0264-410x(97)00259-4. [DOI] [PubMed] [Google Scholar]
- 28.Gibson A.D., Mazeri S., Yale G., Desai S., Naik V., Corfmat J., Ortmann S., King A., Müller T., Handel I., Bronsvoort B.M., Gamble L., Mellanby R.J., Vos A. Development of a non-meat-based, mass producible and effective bait for Oral vaccination of dogs against rabies in Goa state, India. Trop. Med. Infect. Dis. 2019;4:118. doi: 10.3390/tropicalmed4030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuster P., Gülsen N., Neubert A., Vos A. Field trials evaluating bait uptake by an urban dog population in Turkey. J. Etlik Vet. Microbiol. 1998;9:73–81. [Google Scholar]
- 30.Estrada R., Vos A., de Leon R.C. Acceptability of local made baits for oral vaccination of dogs against rabies in the Philippines. BMC Infect. Dis. 2001;1:19. doi: 10.1186/1471-2334-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knobel D.L., Du Toit J.T., Bingham J. Development of a bait and baiting system for delivery of oral rabies vaccine to free-ranging African wild dogs (Lycaon pictus) J. Wildl. Dis. 2002;38:352–362. doi: 10.7589/0090-3558-38.2.352. [DOI] [PubMed] [Google Scholar]
- 32.Bergman D., Bender S., Wenning K., Slate D., Rupprecht C., Heuser C., DeLiberto T. Bait acceptability for delivery of oral rabies vaccine to free-ranging dogs on the Navajo and Hopi Nations. Dev. Biol. (Basel) 2008;131:145–150. [PubMed] [Google Scholar]
- 33.Bender S., Bergman D., Vos A., Martin A., Chipman R. Field studies evaluating bait acceptance and handling by dogs in Navajo nation. USA, Trop. Med. Infect Dis. 2017;2:17. doi: 10.3390/tropicalmed2020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasemsuwan S., Chanachai K., Pinyopummintr T., Leelalapongsathon K., Sujit K., Vos A. Field studies evaluating bait acceptance and handling by free-roaming dogs in Thailand. Vet. Sci. 2018;5 doi: 10.3390/vetsci5020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freuling C.M., Busch F., Vos A., Ortmann S., Lohr F., Hedimbi N., Peter J., Nelson H.A., Shoombe K., Shilongo A., Gorejena B., Kaholongo L., Khaiseb S., van der Westhuizen J., Dietze K., Geurtse G., Müller T. Oral rabies vaccination of dogs-experiences from a field trial in Namibia. PLoS Neglect Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freuling C.M., Eggerbauer E., Finke S., Kaiser C., Kaiser C., Kretzschmar A., Nolden T., Ortmann S., Schröder C., Teifke J.P., Schuster P., Vos A., Mettenleiter T.C., Müller T. Efficacy of the oral rabies virus vaccine strain SPBN GASGAS in foxes and raccoon dogs. Vaccine. 2019;37:4750–4757. doi: 10.1016/j.vaccine.2017.09.093. [DOI] [PubMed] [Google Scholar]
- 37.Freuling C.M., Kamp V.T., Klein A., Günther M., Zaeck L., Potratz M., Eggerbauer E., Bobe K., Kaiser C., Kretzschmar A., Ortmann S., Schuster P., Vos A., Finke S., Müller T. Long-term immunogenicity and efficacy of the Oral rabies virus vaccine strain SPBN GASGAS in foxes. Viruses. 2019;11 doi: 10.3390/v11090790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molini U., Hassel R., Ortmann S., Vos A., Loschke M., Shilongo A., Freuling C.M., Müller T. Immunogenicity of the Oral rabies vaccine strain SPBN GASGAS in dogs under field settings in Namibia. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.737250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leelahapongsathon K., Kasemsuwan S., Pinyopummintr T., Boodde O., Phawaphutayanchai P., Aiyara N., Bobe K., Vos A., Friedrichs V., Müller T., Freuling C.M., Chanachai K. Humoral immune response of Thai dogs after Oral vaccination against rabies with the SPBN GASGAS vaccine strain. Vaccines. 2020;8:573. doi: 10.3390/vaccines8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortmann S., Vos A., Kretzschmar A., Walther N., Kaiser C., Freuling C.M., Lojkic I., Müller T. Safety studies with the oral rabies virus vaccine strain SPBN GASGAS in the small Indian mongoose (Herpestes auropunctatus) BMC Vet. Res. 2018;14:90. doi: 10.1186/s12917-018-1417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortmann S., Kretzschmar A., Kaiser C., Lindner T., Freuling C.M., Schuster P., Müller T., Vos A. In vivo safety studies with SPBN GASGAS in the frame of Oral vaccination of foxes and raccoon dogs against rabies. Front. Vet. Sci. 2018;5:91. doi: 10.3389/fvets.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yale G., Lopes M., Isloor S., Head J.R., Mazeri S., Gamble L., Dukpa K., Gongal G., Gibson A.D. Review of Oral rabies vaccination of dogs and its application in India. Viruses. 2022;14:155. doi: 10.3390/v14010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bobe K., Ortmann S., Kaiser C., Perez-Bravo D., Gethmann J., Kliemt J., Körner S., Theuß T., Lindner T., Freuling C., Müller T., Vos A. Efficacy of Oral rabies vaccine baits containing SPBN GASGAS in domestic dogs according to international standards. Vaccines. 2023;11:307. doi: 10.3390/vaccines11020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chanachai K., Wongphruksasoong V., Vos A., Leelahapongsathon K., Tangwangvivat R., Sagarasaeranee O., Lekcharoen P., Trinuson P., Kasemsuwan S. Feasibility and effectiveness studies with Oral vaccination of free-roaming dogs against rabies in Thailand. Viruses. 2021;13:571. doi: 10.3390/v13040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Undurraga E.A., Millien M.F., Allel K., Etheart M.D., Cleaton J., Ross Y., Wallace R.M. Costs and effectiveness of alternative dog vaccination strategies to improve dog population coverage in rural and urban settings during a rabies outbreak. Vaccine. 2020;38:6162–6173. doi: 10.1016/j.vaccine.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Namibia Statistics Agency Namibia Inter-censal Demographic Survey 2016 Report. 2017. https://cms.my.na/assets/documents/NIDS_2016.pdf
- 47.Faber M., Pulmanausahakul R., Hodawadekar S.S., Spitsin S., McGettigan J.P., Schnell M.J., Dietzschold B. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J. Virol. 2002;76:3374–3381. doi: 10.1128/jvi.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faber M., Faber M.L., Papaneri A., Bette M., Weihe E., Dietzschold B., Schnell M.J. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J. Virol. 2005;79:14141–14148. doi: 10.1128/jvi.79.22.14141-14148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson A.D., Mazeri S., Lohr F., Mayer D., Burdon Bailey J.L., Wallace R.M., Handel I.G., Shervell K., Bronsvoort B.M.D., Mellanby R.J., Gamble L. One million dog vaccinations recorded on mHealth innovation used to direct teams in numerous rabies control campaigns. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hikufe E.H., Freuling C.M., Athingo R., Shilongo A., Ndevaetela E.-E., Helao M., Shiindi M., Hassel R., Bishi A., Khaiseb S., Kabajani J., van der Westhuizen J., Torres G., Britton A., Letshwenyo M., Schwabenbauer K., Mettenleiter T.C., Denzin N., Amler S., Conraths F.J., Müller T., Maseke A. Ecology and epidemiology of rabies in humans, domestic animals and wildlife in Namibia, 2011-2017. PLoS Neglect Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Athingo R., Tenzin T., Shilongo A., Hikufe E., Shoombe K.K., Khaiseb S., van der Westhuizen J., Letshwenyo M., Torres G., Mettenleiter T.C., Freuling C.M., Müller T. Fighting dog-mediated rabies in Namibia-implementation of a rabies elimination program in the northern communal areas. Trop. Med. Infect. Dis. 2020;5:12. doi: 10.3390/tropicalmed5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Athingo R., Tenzin T., Coetzer A., Hikufe E.H., Peter J., Hango L., Haimbodi T., Lipinge J., Haufiku F., Naunyango M., Kephas M., Shilongo A., Shoombe K.K., Khaiseb S., Letshwenyo M., Pozzetti P., Nake L., Nel L.H., Freuling C.M., Müller T., Torres G. Application of the GARC data logger—a custom-developed data collection device—to capture and monitor mass dog vaccination campaigns in Namibia. PLoS Neglect Trop. Dis. 2021;14 doi: 10.1371/journal.pntd.0008948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Head J.R., Vos A., Blanton J., Müller T., Chipman R., Pieracci E.G., Cleaton J., Wallace R. Environmental distribution of certain modified live-virus vaccines with a high safety profile presents a low-risk, high-reward to control zoonotic diseases. Sci. Rep. 2019;9:6783. doi: 10.1038/s41598-019-42714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1: Data from dog vaccinations in Windhoek, Namibia, and a screenshot of the WVS survey.
Supplementary material 2: Calculations for the costs per dog vaccinated
Data Availability Statement
Data will be made available on request.