Abstract
Akagera National Park and its surroundings are home to tsetse flies and a number of their mammalian hosts in Rwanda. A One-health approach is being used in the control and surveillance of both animal and human trypanosomosis in Rwanda. Determination of the infection level in tsetse flies, species of trypanosomes circulating in vectors, the source of tsetse blood meal and endosymbionts is crucial in understanding the epidemiology of the disease in animals and humans in the region.
Tsetse flies (n = 1101), comprising Glossina pallidipes (n = 771) and Glossina morsitans centralis (n = 330) were collected from Akagera park and surrounding areas between May 2018 and June 2019. The flies were screened for trypanosomes, vertebrate host DNA to identify sources of blood meal, and endosymbionts by PCR - High Resolution Melting analysis and amplicon sequencing. The feeding frequency and the feeding indices (selection index - W) were calculated to identify the preferred hosts. An overall trypanosome infection rate of 13.9% in the fly's Head and Proboscis (HP) and 24.3% in the Thorax and Abdomen (TA) were found. Eight trypanosome species were identified in the tsetse fly HP and TA, namely: Trypanosoma (T.) brucei brucei, T. congolense Kilifi, T. congolense savannah, T. vivax, T. simiae, T. evansi, T. godfreyi, T. grayi and T. theileri. We found no evidence of human-infective T. brucei rhodesiense. We also identified eighteen species of vertebrate hosts that tsetse flies fed on, and the most frequent one was the buffalo (Syncerus caffer) (36.5%). The frequently detected host by selection index was the rhinoceros (Diceros bicornis) (W = 16.2). Most trypanosome infections in tsetse flies were associated with the buffalo blood meal. The prevalence of tsetse endosymbionts Sodalis and Wolbachia was 2.8% and 4.8%, respectively. No Spiroplasma and Salivary Gland Hypertrophy Virus were detected. These findings implicate the buffaloes as the important reservoirs of tsetse-transmitted trypanosomes in the area. This contributes to predicting the main cryptic reservoirs and therefore guiding the effective control of the disease. The study findings provide the key scientific information that supports the current One Health collaboration in the control and surveillance of tsetse-transmitted trypanosomosis in Rwanda.
Keywords: One health, Glossina, Trypanosomes, Host preference, Endosymbionts, Akagera Park
Graphical abstract
Highlights
-
•
Eight animal infective trypanosome species were identified in tsetse flies examined
-
•
There is no evidence of human-infective T. brucei rhodesiense in tsetse flies of Akagera park region
-
•
The blood meal analysis incriminates the buffalo as the important cryptic reservoir in the area
-
•
The prevalence of endosymbionts was too low to assess efficiently their relationship with trypanosome infections
-
•
The study contributed to the elimination dossier of sleeping sickness as a Public Health Problem in Rwanda.
-
•
The findings provide the key scientific information that supports the existing One Health collaboration in Rwanda.
1. Introduction
Tsetse flies (Glossina spp.) are the biological vectors of African trypanosomes that cause diseases in both humans and animals [1]. The tsetse-transmitted trypanosomes are often linked to tsetse-infested protected areas inhabited by wildlife, which are reservoirs of many pathogens including trypanosomes [2]. For instance, previous findings from Akagera National Park (NP) at the human-wildlife-livestock interface have found trypanosome infections in tsetse flies and cattle [3,4], and in the last two decades, no cases of human sleeping sickness (T. brucei rhodesiense) have been reported [5,6]. Wild animals such as warthogs and buffaloes do not suffer from trypanosomosis as a result of their inherent capability of co-existing with trypanosome infections and therefore act as parasite reservoirs [[7], [8], [9], [10]]. Whereas wild reservoirs are not sampled, the xenomonitoring surveillance in tsetse fly vectors helps to uncover the diversity of trypanosomes and mammalian hosts, which is crucial to the understanding of the disease transmission dynamics [11]. Tsetse flies are exclusively hematophagous. For that reason, their dispersal, the source of their blood meal and their level of feeding preference are dependent on the availability of the hosts in the area [12,13].
Tsetse fly blood meal analysis is crucial in elucidating the relationship between these vectors and their preferred hosts, for estimating the risk of parasite transmission [14]. Tracking the feeding patterns of tsetse flies and the choice of host is a key element in understanding vector-host interactions, and subsequently predicting the reservoirs of trypanosomes in an area [15].
Symbionts impact on tsetse fly's physiology, including fecundity, vector competence, and nutrition [16]. The symbiont-trypanosome relationship is thought to modulate the vectorial competence of tsetse flies and may therefore have the potential for vector and disease control [17,18]. Tsetse flies can carry up to four different microorganisms, which are vertically transmitted, including the obligate Wigglesworthia, commensal Sodalis, parasitic Wolbachia, and the Salivary Gland Hypertrophy Virus (SGHV). Sodalis glossinidius, Wigglesworthia glossinidia (Enterobacteriaceae), and Wolbachia (Rickettsiaceae) are the main endosymbiotic bacterial species that reside in the tsetse fly gut as facultative symbionts. Apart from the obligatory Wigglesworthia, the rest of these symbionts are extensively studied and described and this list may increase over time [17].
Both Sodalis and Wigglesworthia spp. promote trypanosome infection in tsetse fly [[18], [19], [20], [21]], and could potentially be used as targets for vector control [[22], [23], [24]]. On the other hand, in infected arthropods, Wolbachia causes disorders in reproduction such as parthenogenesis and cytoplasmic incompatibility [25], thus impairing host fertility, lifespan, immunity, and development [26,27]. Spiroplasma was demonstrated to decrease vector competence in tsetse flies [28], and has been found in different tsetse fly species (Glossina (G.) fuscipes fuscipes, G. tachinoides, G. palpalis palpalis and G. pallidipes) of various African countries [[28], [29], [30]]. Both male and female G. fuscipes fuscipes flies infected with Spiroplasma present several phenotypes that would put them at a significant reproductive difficulty. These changes in sex–biased gene expression in the reproductive organs are likely to have a deep effect on the structure of the fly population. Therefore, Spiroplasma could be used to reduce trypanosome transmission in the tsetse fly [31]. The Salivary Gland Hypertrophy Virus (Hytrosaviridae family) replicates in the salivary gland inducing the hypertrophy of the same gland and thus causing reproductive malfunction [32,33]. The infection with this virus in salivary glands increases the vulnerability of the organ to trypanosome infection due to decreased immunity [33,34]. However, its potential use as a tool for biological control of tsetse is not yet clear [35].
Rwanda has a national One Health structure that involves different related stakeholders. The ministry of health leads; however, there is a One Health Multi-sectoral Coordination Mechanism (OH-MCM) composed of leaders from different sectors that coordinates the activities. Under the coordination mechanism, several Technical Working Groups provide specific technical expertise. The African Trypanosomosis technical working group involves the health sector, veterinary services, universities, local government, tourism and conservation board, national parks management and communities. Through this collaboration, Rwanda eliminated the rhodesiense sleeping sickness as Public Health Problem (PHP). In this study, we aimed to determine the trypanosome diversity, tsetse feeding preferences, and the endosymbionts in tsetse flies in and around Akagera NP of Rwanda; and to show their interactions in the epidemiology of tsetse-transmitted trypanosomes. Furthermore, the study aimed to support the current One Health collaboration in the control of tsetse-transmitted trypanosomosis in Rwanda by providing scientific information on vectors of the disease.
2. Materials and methods
2.1. Study area
The study was undertaken in the eastern province of Rwanda, with a focus on the Akagera National Park and surrounding areas. The region borders the United Republic of Tanzania to the East and Uganda to the North, and the study area is located in three neighbouring districts of Kayonza, Gatsibo, and Nyagatare (Fig. 1). The area is infested by two main tsetse species of the morsitans savannah group (subgenus Glossina) i.e. G. pallidipes (Austen 1903) and G. morsitans centralis (Machado 1970). These two species are sympatric in the Akagera region, with G. pallidipes being predominant. Tsetse populations are more abundant inside the Akagera NP than outside, and during the rainy season than the dry [36]. The park is home to a variety of wildlife including primates, large and small mammals, carnivores, and reptiles [37].
Fig. 1.
Collection sites (black dots) for tsetse flies.
2.2. Sample collection
The flies used here were collected during a previous parallel study [36]. Twenty sites were randomly selected (10 in the Akagera NP and 10 in the areas surrounding the park) in a longitudinal survey, taking into account the habitats potentially suitable for Glossina [38]. Tsetse flies were captured between May 2018 and June 2019 (Suppl.1) with biconical traps [39] [Vestergaard Frandsen, Switzerland]. Traps were deployed for six consecutive days and flies were collected every 48 h [40]. In each site, two traps were deployed at a distance of 200 m, and the flies collected from the two traps were later combined to represent a site. To improve the catching efficiency of a trap, we used a combination of baits comprising of 3 weeks-old cow urine and acetone, kept in odour-dispensing plastic bottles. Tsetse flies were morphologically identified as described in training manuals of the Food and Agriculture Organization (FAO) [41,42] using a stereomicroscope (Opta Tech SK392, Poland). Blood-fed and undamaged flies were individually preserved as dry carcasses in 2 ml Eppendorf tubes (Eppendorf AG, Hamburg-Germany) containing pieces of silica gel - coarse 6–20 mesh (Vardaan House, New Delhi, India) and separated by cotton wool. Molecular analysis was carried out at the molecular biology laboratories of the International Centre of Insect Physiology and Ecology (icipe, Nairobi, Kenya).
2.3. DNA extraction from tsetse fly samples
To determine which tissue type is most reliable for detecting trypanosomes and assessing the infections, each tsetse fly was cut into two parts comprising the head and proboscis (HP) and the thorax plus abdomen (TA) [15]. The technique is useful for determining the stage of infection depending on the species or subspecies. The two parts were separately handled, and their surface was sterilised by a quick soaking in 1% bleach, then in 70% ethanol, and allowed to dry on a paper towel to avoid external contaminants including Deoxyribonucleic Acid (DNA) particles. To cut, the scalpel blade was soaked in 70% ethanol and then wiped with a paper towel containing 2% bleach to avoid contamination between flies. The cut parts were placed in 1.5 ml Polymerase Chain Reaction (PCR) clean Eppendorf tubes (Eppendorf AG, Hamburg-Germany) with 2.0 mm zirconia beads (Stratech, UK) and crushed using Tissue Lyser II (Qiagen, Hilden- Germany) for 30 s at a frequency of 3000 rpm. A simplified arthropod genomic-DNA extraction protocol was used to isolate DNA from homogenates of respective tsetse fly parts as described by Margam [43]. Eppendorf BioSpectrometer (Enfield, CT, USA) was used to determine the purity and quantity of the DNA, whereas the integrity was determined using 1.5% agarose gel electrophoresis and visualisation under UV light.
2.4. Detection and identification of trypanosomes
Tsetse DNA was first analysed in pools of equal individual DNA volumes. A pool was made of three flies of the same trap, collection time, species and sex. In case a pool turned positive, the individual DNAs were re-examined to determine the individual infections. Trypanosomes were detected by using the ITS1_CF and ITS1_BR universal primers (Table 1) that target the trypanosome internal transcribed spacer region [44]. A conventional PCR was performed in 9800 Fast and Gene Amp PCR system 9700 thermocyclers (Applied biosystems by life technologies). The reaction was in a 10 μL volume containing 3 μL of nuclease-free water, 5 μL of 2× DreamTaq Green Master Mix (Thermo Fisher Scientific), 0.5 μL of each primer at 10 mM concentrations and 30 ng of DNA template. The thermocycling conditions were 95 °C for 3 min, 35 cycles: 95 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min and the final extension at 72 °C for 10 min. Trypanosome species were identified according to their respective band sizes and were later confirmed by DNA sequencing.
Table 1.
Primers used in this study.
| Target gene | Primer sequence (5′-3′) | Amplicon size (bp) | Primer source | |
|---|---|---|---|---|
| ITS 1 |
Trypanosomes CF |
CCGGAAGTTCACCGATATTG | 250 to 700 | (Njiru et al., 2005) |
| BR | TTGCTGCGTTCTTCAACGAA | |||
| T. brucei | TBR 1 | CGA ATG AAT ATT AAA CAA TGC GCA GT | 177 (repetitive) | (Welburn et al., 2001) |
| TBR 2 | AGA ACC ATT TAT TAG CTT TGT TGC | |||
| SRA | SRA A | GACAACAAGTACCTTGGCGC | 460 | (Gibson et al., 2002) |
| SRA E | TACTGTTGTTGTACCGCCGC | |||
| SRA | B537 | CCATGGCCTTTGACGAAGAGCCCG | 743 | (Welburn et al., 2001) |
| B538 | CTCGAGTTTGCTTTTCTGTATTTTTCCC | |||
| Blood meal sources | ||||
| Vertebrate 16S | Vert 16S F | GAGAAGACCCTRTGGARCTT | 250 | (Omondi et al.; 2015) |
| Vert 16S R | CGCTGTTATCCCTAGGGTA | |||
| Vertebrate cytochrome B | Cyt b F | CCCCTCAGAATGATATTTGTCCTCA | 380 | (Omondi et al., 2015) (Mejı et al.; 2012) (Boakye et al.; 1999) |
| Cyt b R | CATCCAACATCTCAGCATGATGAAA | |||
| Endosymbionts | ||||
| GPO gene | GPO l F | TGAGAGGTTCGTCAATGA | 564 | (Dale & Maudlin, 1999) |
| GPO I R | ACGCTGCGTGACCATTC | |||
| Wolbachia surface protein gene | wsp_F1 | GTCCAATARSTGATGARGAAAC | 714 | (Baldo et al., 2006); (Zhou & Neill, 1998) |
| wsp_R1 | CYGCACCAAYAGYRCTRTAAA | |||
| Wolbachia 16S rRNA gene | Wspec For | CATACCTATTCGAAGGGATAG | 438 | (Werren & Windsor, 2000) |
| Wspec Rev | AGCTTCGAGTGAAACCAATTC | |||
| Spiroplasma RPOB | RPOB3044F_ALL | ARTHTTACCADTDGAAGATATGCC | 300 | (Chepkemoi et al., 2017) |
| RPOB3380R_ALL | TGTARTTTRTCATCWACCATGTG | |||
| SGHV P74 gene | P74 2F | TGTCARATWAATTATCCMCGYGGTAA | 373 | (Abd-alla et al., 2011) |
| P74 2R | AARTCATCGCAATARTAYTTRTT | |||
2.5. Test for human infective T. brucei rhodesiense
All samples that were positive for sub-genus Trypanozoon were subsequently tested using primers that detect the Serum Resistance-Associated (SRA) gene, which is specific for T. brucei rhodesiense and confers resistance to survive in human serum. The SRA gene was PCR amplified by using B537/537 [45] and SRA A/E primers [46] (Table 1). The PCRs were performed in a ProFlex thermocycler (Applied Biosystems by Life technologies) in a 10 μL volume reaction containing 3 μL of nuclease-free water, 5 μL of 2× DreamTaq Green Master Mix (Thermo Fisher Scientific), 0.5 μL of each primer at 10 mM concentrations and 30 ng of DNA template.
The touchdown PCR conditions for B537/537 were 95 °C for 3 min, followed by 10 cycles of 94 °C for 20 s, 55 °C for 30 s and 72 °C for 1 min, followed by 25 cycles of 94 °C for 20 s, annealing at 63.8 °C for 30 s and extension at 72 °C for 1 min per cycle. The final extension was set at 72 °C for 7 min. The PCR conditions for SRA A/E were as follows: 95 °C for 3 min, 40 cycles of 95 °C for 1 min, 68 °C for 1 min and 72 °C for 1 min, with a final elongation step of 10 min at 72 °C.
2.6. Detection of endosymbionts in tsetse flies
The detection of endosymbionts followed the same pooling approach as for trypanosomes. Abdomen (A + T) DNA was used for Sodalis, Wolbachia and Spiroplasma, while both parts were used for SGHV since salivary glands are found both in the abdomen and mouthparts. The PCR amplification used the Glycerophosphate oxidase 1 (GPO1) F/R primer for Sodalis, WspF1/R1 and wspecF/R primers for Wolbachia, RPOB primer for Spiroplasma and P74 primer for SGHV. For each primer, the reaction was made of 10 μL comprising 6 μL nuclease-free water, 2 μL of 5× HOT FIREPol Blend Master Mix (Solis BioDyne, Estonia), 0.5 μL forward primer, 0.5 μL reverse primer and 30 ng DNA template). The amplifications were run by conventional PCRs in Gene Amp PCR system 9700 and 9800 Fast thermocyclers (Applied biosystems by life technologies). Positive controls for each symbiont were used. For SGHV, the positive control used was a synthetic P74 gene plasmid standard of SGHV (GenScript Inc. NJ, USA). The GPO1F/R thermocycling Conditions were 950C for 15 min, 35 cycles: 950C for 1 min, 550C for 1 min, 720C for 1 min, and final extension at 720C for 10 min. The thermocycling conditions for WspF1/R1 were 94 °C for 15 min, 37 cycles: 94 °C for 30 s, 59 °C for 45 s, 72 °C for 1 min 30 s, and final extension at 72 °C for 10 min. The Wspec primer used the following cycling conditions: 95 °C for 15 min, 2 cycles of 2 min at 95 °C, 1 min at 60 °C and 1 min at 72 °C, followed by 35 cycles of 30s at 95 °C, 1 min at 60 °C and 45 s at 72 °C, and a final extension at 72 °C for 5 min. The thermocycling conditions for RNA Polymerase Beta-subunit (RPOB) to detect Spiroplasma were 95 °C for 15 min, 35 cycles: 95 °C for 30 s, 55.9 °C for 30 s, 72 °C for 30 s, and final extension at 72 °C for 10 min. Finally, the SGHV P74 gene was amplified by using the following conditions: 95 °C for 15 min, 35 cycles: 95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and final extension at 72 °C for 7 min. 5 μL of the resulting PCR amplicons were resolved in 2% (w/v) ethidium bromide–stained agarose gels to determine successful amplification. The positive PCR amplicons were purified using Exo1-rSAP (New England BioLabs, inc. MA, US) according to the manufacturer's protocol and sequenced by Macrogen Inc. (Amsterdam, The Netherlands).
2.7. Identification of tsetse blood meal sources
Real-time quantitative PCR (qPCR) and two-gene High-Resolution Melting (HRM) Analysis were used to detect the blood meal source in flies. Primers targeting the genes for Cytochrome B (Cyt B) and 16S rRNA markers were used (Table 1). DNA samples extracted from the abdomens were used for blood meal analysis and reactions were prepared in a volume of 10 μL consisting of 6 μL of nuclease-free water, 2 μL of 5× Hot FIREPol EvaGreen HRM Mix (Solis BioDyne, Tartu, Estonia), 0.5 μL of each primer at 10 mM concentrations and 30 ng of DNA template. The Thermo-cycling and HRM analysis were performed in QuantStudio™ 3 system (Applied biosystems by Thermo Fisher Scientific). The PCR conditions for Cyt B were as follows: 95 °C for 15 min followed by 40 cycles of at 95 °C for 20 s, 56 °C for 20 s and 72 °C for 30 s. The final elongation was at 72 °C for 5 min. Dissociation was done from 70 °C to 99.9 °C at the rate of 0.5 °C/ s. For 16S primer, the PCR conditions were 95 °C for 15 min followed by 40 cycles of at 95 °C for 20 s, 56 °C for 20 s and 72 °C for 30 s. The final elongation was at 72 °C for 5 min. The dissociation was done from 70 °C to 99.9 °C at the rate of 0.5 °C/ s. QuantStudio™ Design & Analysis Software was used to analyse the melt curves and select representative samples for sequencing.
2.8. Blood meal feeding frequency and feeding indices
The feeding frequency was calculated as a proportion of blood meals from a particular host out of the total number of blood meals examined. The feeding index or selection index (Wi) is a population density-based selection ratio (probability of selection), computed using previously reported methods based on forage ratios [14,[47], [48], [49]]. To calculate the feeding indices, we used the aerial population count estimates that are conducted every 2 years in Akagera NP by the Akagera Management Company (AMC). We used the formula ; where W = feeding index (selection index) of a particular host; o = proportion of blood meal from a particular host out of the total blood meals from an area; and P= density of that host out of the total density of hosts found in an area. The value above 1 designates the host is more frequently selected than it would be through random selection. Hosts with values between 0 and 1 are avoided, or less frequently fed on than expected by chance.
2.9. Amplicon sequencing and BLASTn analysis
One hundred sixty-five PCR amplicons for representative positive samples (96 for trypanosomes and 69 for blood meal), were re-amplified in larger PCR reaction volumes (20 μL) and resolved in 2% ethidium-stained agarose gel electrophoresis. Samples with the discrete band were purified by Exonuclease 1-Shrimp Alkaline Phosphatase (Exo1-rSAP) (New England BioLabs, inc. MA, US) as instructed in the guidelines. The products with more than one band were excised and then purified by a QIAquick PCR purification kit (Qiagen, Germany). The purified amplicons were submitted for unidirectional sanger sequencing by Macrogen (Macrogen Inc., The Netherlands). The resultant sequence chromatograms from Sanger sequencing were processed using Geneious prime 2020.2.2 (Biomatters, New Zealand). BLASTn searches were used to identify homologous sequences of reference and sequence entries closely related to each of the individual sequences from this study [50]. All sequences were aligned using the MAFFT plugin in Geneious Prime version 2020.2.2 software (Biomatters) [51].
2.10. Data analysis
The analysis was performed in Statistical Package for Social Sciences (SPSS) software (SPSS Inc., IL, USA). Pearson chi-square (χ2) test was used to compare the trypanosome infection rates and blood meal sources among different variables such as area and seasons. Logistic regression was used to determine the associations between endosymbionts and trypanosome infections in flies. The significance (p < 0.05) at a 95% confidence interval was considered.
3. Results
3.1. Trypanosome infections in flies
The overall infection rate was 13.9% (153/1101) in the Head and proboscis (HP) and 24.3% (268/1101) in Thorax and Abdomen (TA) (Table 2). Eight species and sub-species of trypanosomes were identified. For each tsetse specimen, body parts (Head + Proboscis and Thorax+ Abdomen) were analysed separately and are presented in parallel as HP/TA. Of the trypanosome species, T.brucei brucei accounted for 4.1/7.1%, T. congolense Kilifi (2.2/2.1%), T. congolense savannah (1.6/1.2%), T. evansi (0/0.9%), T. godfreyi (1.2/3.1%), T. grayi (0/1.08%), T. simiae (2.08/3.7%), T. theileri (0/2.08%) and T. vivax (5.2/3.7%). The study found some mixed infections in proportions of 2.2/0.8% (25/9), which are detailed in Table 2 below.
Table 2.
Comparison of trypanosome infections prevalence between tsetse fly species.
| Fly species | Sex | N | All |
Tbb |
Tck |
Tcs |
Te |
Tgod |
Tgr |
Tsim |
Tth |
Tv |
Mixed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP/TA | HP/TA | HP/TA | HP/TA | HP/TA | HP/TA | HP/TA | HP/TA | HP/TA | HP/TA | HP/TA | |||
| Gp | F | 456 | 66/113 | 17/37 | 13/11 | 8/4 | 0/0 | 5/19 | 0/4 | 8/15 | 0/8 | 24/18 | 8/3 |
| M | 315 | 45/92 | 15/33 | 6/6 | 3/5 | 0/2 | 6/11 | 0/6 | 6/13 | 0/12 | 16/8 | 5/4 | |
| Σ (%) | 771 | 111/205 (14.4/25.6%) | 32/70 (4.1/9%) | 19/17 (2.4/2.2%) | 11/9 (1.4/1.1%) | 0/2 (0/0.2%) | 11/30 (1.4/3.9%) | 0/10 (0/1.3%) | 14/28 (1.8/3.6%) | 0/20 (0/2.6%) | 40/26 (5.2/3.4%) | 13/7 (1.7/0.9%) | |
| p value | 0.915/0.172 | ||||||||||||
| Gmc | F | 195 | 23/39 | 6/8 | 3/3 | 2/2 | 0/6 | 3/3 | 0 | 5/8 | 0 | 11/9 | 7/0 |
| M | 135 | 19/23 | 7/0 | 3/3 | 5/3 | 0/2 | 0/2 | 0/2 | 4/5 | 0/3 | 7/6 | 5/2 | |
| Σ (%) | 330 | 42/63 (12.7/19%) | 13/8 (3/2.4%) | 6/6 (1.8/1.8%) | 7/5 (2.1/1.5%) | 0/8 (0/2.4%) | 3/5 (0.9/1.5%) | 0/2 (0/0.6%) | 9/13 (2.7/3.9%) | 0/3 (0/0.9%) | 18/15 (5.4/4.5%) | 12/2 (3.6/0.6%) | |
| p value | 0.168/0.064 | ||||||||||||
| Total (Gp + Gmc) (%) | 1101 | 153/268 (13.9/24.3%) | 45/78 (4.1/7.1%) | 25/23 (2.2/2.1%) | 18/14 (1.6/1.2%) | 0/10 (0/0.9%) | 14/35 (1.2/3.1%) | 0/12 (0/1.08%) | 23/41 (2.08/3.7%) | 0/23 (0/2.08%) | 58/41 (5.2/3.7%) | 25/9 (2.2/0.8%) | |
| p value | 0.962/0.089 | ||||||||||||
Gp = G. pallidipes, Gmc = G. morsitans centralis, NE = Number, HP/TA = Head + Proboscis / Thorax + Abdomen, F=Female, M = Male, Tbb = T. brucei brucei, Tck = T. congolense kilifi, Tcs = T. congolense savannah, Te = T. evansi, Tgod = T. godfreyi, Tgr = T. grayi, Tsim = T. simiae, Tth = T. theileri, Tv = T. vivax.
There were slightly higher infections in G. pallidipes (14.4/25.6%) than in G. morsitans centralis (12.7/19%, p = 0.962/0.089). 25 mixed infections were identified in HP (Tv + Tbb = 8, Tv + Tsim = 7, Tck + Tbb = 3, Tck + Tv = 2, Tcs + Tv = 2, Tbb + Tsim = 1, Tcs + Tbb = 1 and Tv + Tgod = 1), and 9 mixed infections in TA (Tbb + Tgod = 3, Tck + Te = 1, Tck + Tgr = 1, Tck + Tth = 1, Tck + Tv = 1, Tgod + Tsim = 1 and Tgod + Tsim = 1).
Table 3 shows the variations in trypanosome infections between different predictors. More trypanosome infections were observed in the wet season (16.3/26.2%) than in the dry season (10.02/21.4%), although the difference was not statistically significant in HP (p = 0.078) rather for TA (p = 0.018). Akagera NP accounted for (15.8/25.8%), compared to the interface area (11.5/22.5%), p = 0.025 / 0.000. At the interface, higher tsetse trypanosome infections rates were observed in Kayonza district (14.4/36.8%). The variation of infection prevalence across locations is shown in Fig. 2 and Suppl. 3, 4 and 5.
Table 3.
Comparison of infections between different predictors.
| Head and proboscis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Variable | N | All | Tbb | Tck | Tcs | Te | Tgod | Tgr | Tsim | Tth | Tv | Mixed |
| Season | Dry | 429 | 43 (10.02%) | 13 (3.03%) | 9 (2.09%) | 3 (0.6%) | 0 (0%) | 3 (0.6%) | 0 (0%) | 6 (1.3%) | 0 (0%) | 18 (4.1%) | 6 (1.3%) |
| Wet | 672 | 110 (16.3%) | 32 (4.7%) | 16 (2.3%) | 15 (2.2%) | 0 (0%) | 11 (1.6%) | 0 (0%) | 17 (2.5%) | 0 (0%) | 40 (5.9%) | 19 (2.8%) | |
| p value | 0.078 | ||||||||||||
| Area | Akagera NP | 600 | 95 (15.8%) | 35 (5.8%) | 16 (2.6%) | 9 (1.5%) | 0 (0%) | 10 (1.6%) | 0 (0%) | 13 (2.1%) | 0 (0%) | 37 (6.1%) | 20 (3.3%) |
| Interface | 501 | 58 (11.5%) | 10 (2%) | 9 (1.8%) | 9 (1.8%) | 0 (0%) | 4 (0.8%) | 0 0% | 10 (2%) | 0 (0%) | 21 (4.2%) | 5 (1%) | |
| p value | 0.025 | ||||||||||||
| District | Gatsibo | 15 | 2 (13.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6.6%) | 0 (0%) | 1 (6.6%) | 0 (0%) |
| Kayonza | 201 | 29 (14.4%) | 5 (2.4%) | 6 (3%) | 3 (1.5%) | 0 (0%) | 4 (2%) | 0 (0%) | 6 (3%) | 0 (0%) | 7 (3.5%) | 2 (1/3%) | |
| Nyagatare | 285 | 27 (9.5%) | 5 (1.7%) | 6 (2.1%) | 6 (2.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (1.05%) | 0 (0%) | 13 (4.5%) | 3 (0%) | |
| p-value | 0.645 | ||||||||||||
| Thorax and Abdomen | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Variable | N | All | Tbb | Tck | Tcs | Te | Tgod | Tgr | Tsim | Tth | Tv | Mixed |
| Season | Dry | 429 | 92 (21.4%) | 31 (7.2%) | 10 (2.3%) | 7 (1.6%) | 0 (0%) | 11 (2.5%) | 2 (0.4%) | 11 (2.5%) | 8 (1.8%) | 18 (4.1%) | 6 (1.3%) |
| Wet | 672 | 176 (26.2%) | 47 (7%) | 13 (1.9%) | 7 (1%) | 10(1.4%) | 24 (3.5%) | 10(1.4%) | 30 (4.4%) | 15(2.2%) | 23 (3.4%) | 3 (0.4%) | |
| p-value | 0.018 | ||||||||||||
| Area | Akagera NP | 600 | 155 (25.8%) | 53 (8.8%) | 13 (2.1%) | 8 (1.3%) | 8 (1.3%) | 21 (3.5%) | 12 (2%) | 27 (4.5%) | 12 (2%) | 4 (0.6%) | 3 (0.5%) |
| Interface | 501 | 113 (22.5%) | 25 (5%) | 10 (2%) | 6 (1.2%) | 2 (0.4%) | 14 (2.8%) | 0 (0%)) | 14 (2.8%) | 11(2.2%) | 37 (7.3%) | 6 (1.2%) | |
| p-value | 0.000 | ||||||||||||
| District | Gatsibo | 15 | 3 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) | 0 (0%) | 0 (0%) | 0(0%) |
| Kayonza | 201 | 74 (36.8%) | 12 (6%) | 5 (2.5%) | 3 (1.5%) | 0 (0%) | 14 (7%) | 0 (0%) | 7 (3.5%) | 8 (4%) | 31 (15.4%) | 6 (3%) | |
| Nyagatare | 285 | 36 (12.6%) | 13 (4.5%) | 5 (1.7%) | 3 (1.05%) | 2 (0.7%) | 0 (0%) | 0 (0%) | 4 (1.4%) | 3(1.05%) | 6 (2.1%) | 0 (0%) | |
| p-value | 0.645 / 0.033 | ||||||||||||
N = Number, T = Trypanosoma, Tbb = T. brucei brucei, Tck = T. congolense kilifi, Tcs = T. congolense savannah, Te = T. evansi, Tgod = T. godfreyi, Tgr = T. grayi, Tsim = T. simiae, Tth = T. theileri, Tv = T. vivax.
Fig. 2.
Prevalence of trypanosome infection in tsetse flies from various sampling locations of the studied area.
3.2. Detection of human-infective T. b. rhodesiense in tsetse flies
Forty-five tsetse fly samples isolated from the Head and Proboscis (HP) and seventy-eight consisting of Thorax and abdomen (TA) that were positive for T. b. brucei were subjected to further analysis using specific primers to sub-genus Trypanozoon, the T. brucei group [45]. All one hundred twenty-three samples were confirmed positive for Trypanosoma brucei Repeat (TBR). Conversely, none of them tested positive for the SRA gene by using either SRA A/E or B537/538 primers. We thus found no evidence of T. brucei rhodesiense in tsetse flies analysed.
3.3. Nucleotide BLAST analysis
The resultant sequences from this study were compared to similar sequences deposited in the GenBank database. The BLAST results are summarised in Table 4 below.
Table 4.
Sequence similarity between the study sequences and GenBank.
| Trypanosome species /subspecies | NCBI GenBank match | Origin | E-value | Similarity (%) |
|---|---|---|---|---|
| T. congolense Kilifi type | MK756200 | Nigeria | 0.0 | 97.85–98.43 |
| U22317.1 | Kenya | 0.0 | 97.46 | |
| MZ461917 | Kenya | 0.0 | 95.74 | |
| T. congolense Savannah type | LC492130 | Sudan | 0.0 | 99–100 |
| MK131987 | Zambia | 1e-132 | 99.62 | |
| U22315(IL1180) | Kenya | 0.0 | 91.68 | |
| MZ147874 | Chad | 0.0 | 95.83 | |
| T. brucei brucei | KR092361 | DR Congo | 8e-162 | 95.38 |
| KR092362 | Ivory Coast | 2e-173 | 97.28 | |
| KR092353 | Uganda | 2e-163 | 95.65 | |
| T. evansi | KX898423.1 | Iran | 2e-158 | 96.79 |
| T. godfreyi | MK131839.1 | Zambia | 1e-91 | 100 |
| T. grayi | MK656903.1 | Cameroon | 0.0 | 99.44 |
| T. simae | JN673387.1 | Tanzania | 5e-143 | 98.97 |
| MK132108.1 | Zimbabwe | 2e-126 | 98.85 | |
| T. theileri | JN673396.1 | Zambia | 1e-124 | 98.11 |
| T. vivax | DQ316041(IL3905 isolate) | Kenya | 3e-09 | 95.65 |
| KX584844.1 | Mozambique | 5e-42 | 93.13 | |
| MW689625 | Kenya | 5e-42 | 91.67–93.13 |
T. congolense sequences from this study showed similarity with two different strains, i.e.: T. congolense Kilifi-type and T. congolense Savannah type. The nucleotide sequences from this study were deposited in the GenBank database (Suppl 2).
3.4. Blood meal analysis results
Host DNA was found in 312 samples (312/367 = 85%). These comprise 50 cases of blood meal taken from different hosts (47 from 2 different hosts and 3 from 3 different hots) and 262 single feedings. Host DNA was missed in 25 samples (25/367 = 6.8%). In total, 18 species of mammalian hosts were identified (Table 5).
Table 5.
Tsetse fly blood meal sources, their host feeding frequency and GenBank similarities.
| SN | Hosts fed on | Feeding frequency | NCBI GenBank closest match & % identity (Vert 16S) | NCBI Genbank closest match & % identity (Cytochrome B) |
|---|---|---|---|---|
| 1 | African buffalo (Syncerus caffer) | 134/367 (36.5%) | JQ235547.1 (100%) | KX697546.1 (100%) |
| KX697546.1 (99.67%) | ||||
| JQ235542.1 (99.44%) | KX697512.1 (96.61%) | |||
| 2 | Common warthog (Phacochoerus africanus) | 52/367 (14.1%) | DQ409327.1 (99.46%) | – |
| KJ193171.1 (97.88%) | ||||
| KJ193171.1 (86.97%) | ||||
| 3 | Cattle (Bos taurus) | 39/367 (10.6%) | – | AY682375.1 (96.1%) |
| 4 | African savannah elephant (Loxodonta africana) | 32/367(8.7%) | AB443879.1 (96.33%) | KX697470.1 (97.88%) |
| 5 | Bushbuck (Tragelaphus scriptus) | 27/367 (7.3%) | JN632706.1 (87.68%) | HQ641317.1 (92.86%) |
| 6 | Human (Homo sapiens) | 21/367 (5.7%) | MK248422.2 (98.89%) | MT568795.1 (100%) |
| MN687316.1 (98.51%) | LC088149.1 (100%) | |||
| MT511085.1 (88.42%) | KX697544.1 (99.66%) | |||
| 7 | Olive baboon (Papio Anubis) | 17/367 (4.6%) | – | – |
| 8 | Black rhinoceros (Diceros bicornis) | 9/367 (2.4%) | MK909143.1 (97.28%) | – |
| 9 | Hippopotamus (Hippopotamus amphibious) | 7/367 (1.9%) | AP003425.1 (92.49%) | – |
| 10 | Impala (Aepyceros melampus) | 7/367 (1.9%) | – | – |
| 11 | Goat (Capra hircus) | 6/367 (1.6%) | – | – |
| 12 | Eland (Tragelaphus oryx) | 5/367 (1.3%) | KX697487.1 (82.12%) | – |
| 13 | Blue monkey (Cercopithecus mitis) | 4/367 (1.08%) | – | – |
| 14 | Giraffe (Giraffa camelopardalis tippl.) | 4/367 (1.08%) | – | – |
| 15 | Topi (Damaliscus lunatus) | 3/367 (0.8%) | – | – |
| 16 | Common Duiker (Sylvicapra grimmia) | 3/367 (0.8%) | – | – |
| 17 | Nile Tilapia (Oreochromis niloticus) | 2/367 (0.5%) | – | XM 003447436.5 (100%) |
| 18 | Plain zebra (Equus quagga) | 2/367 (0.5%) | – | – |
| 19 | Defassa waterbuck (Kobus ellipsiprymnus defassa) | 1/367 (0.27%) | – | – |
We could not find a similarity match with National Center for Biotechnology Information (NCBI) blast for 23 samples (25/367 = 6.2%), and the blood source could not be identified in 7 samples (7/367 = 1.9%). Table 6 shows how hosts were fed on, in comparison with the season, area, and tsetse fly species.
Table 6.
Feeding frequency associated with the season, area and tsetse fly species (Gp = G. pallidipes, Gmc = G. morsitans centralis)
| SN | Hosts fed on | Season |
Area |
Tsetse fly species |
|||
|---|---|---|---|---|---|---|---|
| Dry | Wet | Interface | Park | Gmc | Gp | ||
| 1 | Buffalo | 48 | 86 | 57 | 77 | 44 | 90 |
| 2 | Warthog | 17 | 35 | 16 | 36 | 13 | 39 |
| 3 | Cattle | 20 | 19 | 39 | 0 | 11 | 28 |
| 4 | Elephant | 14 | 18 | 24 | 8 | 7 | 25 |
| 5 | Bushbuck | 7 | 20 | 4 | 23 | 6 | 21 |
| 6 | Human | 9 | 12 | 15 | 6 | 4 | 17 |
| 7 | Baboon | 7 | 10 | 2 | 15 | 7 | 10 |
| 8 | Rhinoceros | 4 | 4 | 0 | 9 | 4 | 5 |
| 9 | Hippopotamus | 2 | 5 | 1 | 6 | 1 | 6 |
| 10 | Impala | 1 | 6 | 0 | 7 | 2 | 5 |
| 11 | Goat | 5 | 1 | 5 | 1 | 1 | 5 |
| 12 | Eland | 2 | 3 | 3 | 2 | 3 | 2 |
| 13 | Monkey | 1 | 3 | 0 | 4 | 3 | 1 |
| 14 | Giraffe | 0 | 4 | 1 | 3 | 2 | 2 |
| 15 | Topi | 2 | 1 | 0 | 3 | 0 | 3 |
| 16 | Duiker | 2 | 1 | 3 | 0 | 1 | 2 |
| 17 | Nile Tilapia | 1 | 1 | 0 | 2 | 0 | 2 |
| 18 | Plain Zebra | 2 | 0 | 0 | 2 | 0 | 2 |
| 19 | Waterbuck | 0 | 1 | 0 | 1 | 0 | 1 |
| p value | 0.571 | 0.000 | 0.090 | ||||
There was no statistical significance in feeding patterns between the seasons (p = 0.571). However, variations exist within host species. Cattle and goats were more fed on during the dry season, even for Topi, duiker, and zebra. Generally, the park contributed more blood meals than the interface (p-0.000). However, looking at the individual hosts, it is evident that the feeding on cattle, elephants, humans, goats and some wild animals like eland and duiker was increased at the interface than inside the park. Both G. m. centralis and G. pallidipes feed on the same hosts, G. pallidipes showed an increased feeding frequency, although not statistically significant (p = 0.090). Fig. 3 shows the blood-feeding preference in different localities of the park and the interface area.
Fig. 3.
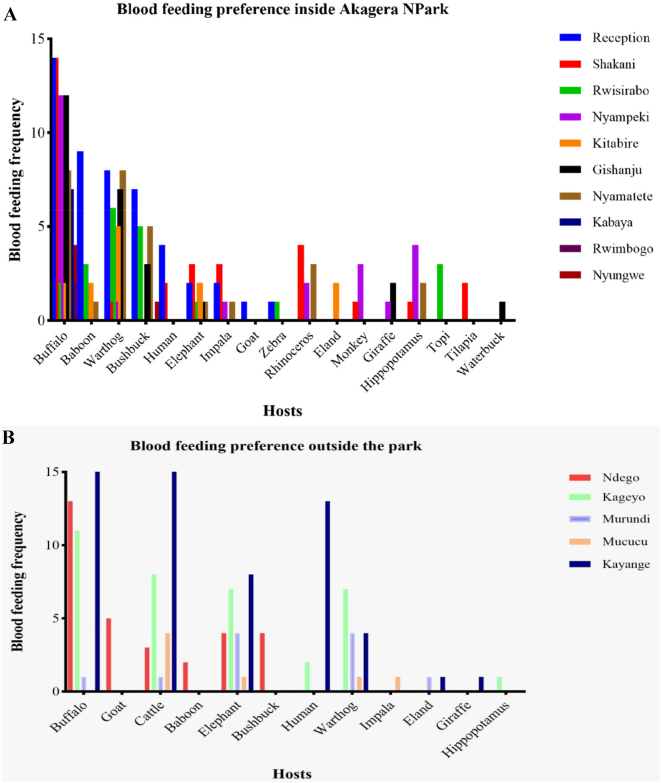
Hosts preference of tsetse flies inside Akagera NP (A) and at the interface (B).
There were higher feeding frequencies mainly on buffalo, warthog, and bushbuck in localities of park reception, Shakani, Nyampeki, Nyamatete and Gishanju. At the interface, the buffalo, cattle, elephant, human, and warthog were the most fed on frequently in localities of Kayange, Kageyo, and Ndego. Based on the animal population densities as per the aerial census, the feeding indices were analysed to see the most likely hosts to be preferred by tsetse flies. Table 7 shows the order of preference according to the calculated feeding indices.
Table 7.
Order of preference according to the feeding indices analysis.
| SN | Blood meal source | Total | Feeding frequency (%) | Gmc | Gp | Population by aerial count estimation | Feeding Index (selection index) |
|---|---|---|---|---|---|---|---|
| 1 | Rhinoceros | 9 | 2.4 | 4 | 5 | 25 | 16.2 |
| 2 | Elephant | 32 | 8.7 | 7 | 25 | 109 | 13.2 |
| 3 | Bushbuck | 27 | 7.3 | 6 | 21 | 121 | 10.04 |
| 4 | Warthog | 52 | 14.1 | 13 | 39 | 871 | 2.69 |
| 5 | Giraffe | 4 | 1.08 | 2 | 2 | 78 | 2.3 |
| 6 | Eland | 5 | 1.3 | 3 | 2 | 120 | 1.87 |
| 7 | Buffalo | 134 | 36.5 | 44 | 90 | 3456 | 1.74 |
| 8 | Duiker | 3 | 0.8 | 1 | 2 | 97 | 1.4 |
| 9 | Monkey | 4 | 1.08 | 3 | 1 | 455 | 0.4 |
| 10 | Olive baboon | 17 | 4.6 | 7 | 10 | 3255 | 0.23 |
| 11 | Topi | 3 | 0.8 | 0 | 3 | 682 | 0.2 |
| 12 | Hippopotamus | 7 | 1.9 | 1 | 6 | 1838 | 0.17 |
| 13 | Impala | 7 | 1.9 | 2 | 5 | 2414 | 0.13 |
| 14 | Zebra | 2 | 0.5 | 0 | 2 | 1936 | 0.05 |
| 15 | Waterbuck | 1 | 0.27 | 0 | 1 | 1050 | 0.04 |
| 16 | Cattle | 39 | 10.6 | 11 | 28 | – | – |
| 17 | Human | 21 | 5.7 | 4 | 17 | – | – |
| 18 | Goat | 6 | 1.6 | 1 | 5 | – | – |
| 19 | Nile Tilapia | 2 | 0.5 | 0 | 2 | – | – |
| 32 | 16,507 |
n = sample size; Gmc = Glossina morsitans centralis; Gp = Glossina pallidipes.
According to the feeding indices calculated based on each species' population density, rhinoceros, elephant, bushbuck, warthog, giraffe, eland, buffalo and duiker were likely the most preferred blood meal hosts by tsetse. The cattle, humans, goats, and Nile tilapia were excluded from this analysis as we could not find their population estimates during the study.
3.5. Tsetse blood-feeding preferences and the trypanosome infections
Eight (8) species of trypanosomes and nineteen (19) hosts DNA were identified in tsetse flies' abdomen samples. Flies infected with T. b. brucei had mainly fed on the buffalo, elephant, and warthog. Flies infected with T. congolense strains had predominantly fed on the buffalo. T. simiae infected flies fed primarily on the warthog while flies infected with T. vivax had fed on a wide host range including mainly the buffalo, warthog, elephant, and cattle. The detailed feeding frequency of infected flies on hosts is shown in Table 8.
Table 8.
The association between hosts' feeding frequency and trypanosome infections in tsetse flies.
| SN | Host/trypanosome species | Freq | Neg | Tbb | Tck | Tcs | Te | Tgod | Tgr | Tsim | Tth | Tv |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Buffalo | 134 | 81 | 12 | 9 | 10 | 4 | 4 | 1 | 3 | 3 | 7 |
| 2 | Warthog | 52 | 23 | 4 | 2 | 0 | 0 | 4 | 1 | 10 | 1 | 7 |
| 3 | Cattle | 39 | 32 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| 4 | Elephant | 32 | 18 | 5 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 5 |
| 5 | Bushbuck | 27 | 11 | 5 | 3 | 0 | 1 | 3 | 0 | 3 | 1 | 0 |
| 6 | Human | 21 | 15 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| 7 | Baboon | 17 | 12 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 |
| 8 | Rhinoceros | 9 | 6 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 |
| 9 | Hippopotamus | 7 | 4 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| 10 | Impala | 7 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | Goat | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | Eland | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | Monkey | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | Giraffe | 4 | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 15 | Topi | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | Duiker | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | Nile Tilapia | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | Zebra | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | Waterbuck | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Freq = feeding frequency, Neg = Negative for trypanosomes, T = Trypanosoma, Tbb = T. brucei brucei, Tck = T. congolense kilifi, Tcs = T. congolense savannah, Te = T. evansi, Tgod = T. godfreyi, Tgr = T.grayi, Tsim = T.simiae, Tth = T. theileri, Tv = T. vivax.
Infected flies fed less on some hosts such as duiker, Tilapia, topi, zebra, and waterbuck. The relationship between tsetse trypanosome infection and the frequently fed-on hosts is shown in Fig. 4.
Fig. 4.
Association between trypanosome infections in tsetse with their host feeding preferences.
The infections with T. b. brucei were much associated with tsetse blood meal from the buffalo, elephant and bushbuck. T. congolense strains and T. evansi were related to the buffalo blood meal whereas T. godfreyi was associated with buffalo, warthog and bushbuck. T. simiae was much linked to the warthog blood meal, while T. vivax was related to the blood meals from the buffalo, warthog, elephant and cattle. T. vivax has the widest range of hosts followed by T. b. brucei. The buffalo seems to contribute to all the trypanosome species. Cases of human blood meal were detected in localities with settlements or campsites (Fig. 5).
Fig. 5.

Map showing sampling sites of tsetse flies that fed on humans.
At the interface, cases of human blood meal were found in Karangazi and Kageyo localities of Nyagatare and Kayonza districts respectively. Inside the park, human blood in tsetse flies was found around the reception and the Shakani park rangers' campsite.
3.6. Endosymbionts and their relationship with trypanosome infections in flies
A total of 31 flies (31/1101, 2.8%) [(21 (2.7%) G. pallidipes and 10 (3%) G. m. centralis)] had Sodalis infection, among which only 18 flies (14 G. pallidipes and 4 G. m. centralis) were positive for trypanosome infections and other 13 were trypanosome negative. Contrary to our expectations, more Sodalis-negative flies were found to be positive for trypanosomes (250/268, 93.3%) compared to Sodalis-positive flies having trypanosome infections (18/268, 6.7%). This difference was statistically significant at p = 0.000, indicating that there was an inverse relationship between Sodalis and trypanosome infections in this study (Table 9). T. vivax has a lifecycle that occurs entirely in tsetse fly mouthparts. It was therefore not included in the analysis of symbiont-trypanosome associations.
Table 9.
Overview of the symbionts-trypanosomes association (G. = Glossina, Tryps = Trypanosome, + = positive, − = negative).
| Symbiont |
G. pallidipes |
G. morsitans centralis |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tryps + | Tryps - | P value | Tryps + | Tryps - | P value | Tryps + | Tryps - | P value | |
| Sodalis + | 14 | 7 | 0.000 | 4 | 6 | 0.102 | 18 | 13 | 0.000 |
| Sodalis - | 191 | 559 | 59 | 261 | 250 | 820 | |||
| Wolbachia + | 11 | 23 | 0.275 | 4 | 15 | 0.508 | 15 | 38 | 0.294 |
| Wolbachia - | 194 | 543 | 59 | 252 | 253 | 795 | |||
For Wolbachia, 53 flies out of 1101 were positive (53/1101, 4.8%) [(34 (4.4%) G. pallidipes and 19 (5.7%) G. m. centralis)]. Of these, 15 flies were Trypanosoma-positive (11 G. pallidipes and 4 G. m. centralis) and 38 flies were Trypanosome-negative (23 G. pallidipes and 15 G. m. centralis). No statistical significance was found (p = 0.294) between Wolbachia-positive flies with and without trypanosomes. Two cases of co-infection of Sodalis and Wolbachia were observed, both of them were trypanosome-positive, one for T. simiae and another for T. b. brucei. No Spiroplasma and SGH Virus were found in the samples analysed. Looking at distinct trypanosome species in relation to symbionts, a significant difference was noticed (Table 10) for Sodalis, not for Wolbachia.
Table 10.
Relationship between the presence of endosymbionts (Sodalis and Wolbachia) and the trypanosome infections in tsetse flies.
|
Sodalis |
Wolbachia |
||||||
|---|---|---|---|---|---|---|---|
| Sodalis/trypanosome | P value | Odds ratio | 95% CI | Wolbachia/trypanosome | P value | Odds ratio | 95% CI |
| Sod /Tbb | 0.000 | 8.601 | 3.546–20.864 | Wol / Tbb | 0.075 | 2.154 | 0.927–5.005 |
| Sod / Tck | 0.239 | 3.504 | 0.435–28.243 | Wol / Tck | 0.998 | – | – |
| Sod /Tcs | – | – | – | Wol / Tcs | 0.651 | 1.609 | 0.205–12.624 |
| Sod /Te | – | – | – | Wol / Te | 0.371 | 2.615 | 0.319–21.445 |
| Sod / Tgod | 0.998 | 4.401E-8 | – | Wol / Tgod | 0.998 | – | – |
| Sod / Tgrayi | 0.090 | 6.308 | 0.752–52.940 | Wol / Tgrayi | 0.487 | 2.092 | 0.261–16.767 |
| Sod / Tsim | 0.014 | 5.114 | 1.397–18.726 | Wol / Tsim | 0.897 | 1.101 | 0.256–4.735 |
| Sod / Tth | 0.001 | 9.960 | 2.620–37.855 | Wol / Tth | 0.331 | 2.092 | 0.472–9.279 |
The reference category is Negative. Sod = Sodalis, Wol = Wolbachia, /=versus, T = Trypanosoma, Tbb = T. brucei brucei, Tck = T. congolense kilifi, Tcs = T. congolense savannah, Te = T. evansi, Tgod = T. godfreyi, Tgr = T. grayi, Tsim = T. simiae, Tth = T. theileri.
There was a significant association between the presence of Sodalis and infections with T. brucei brucei, (p = 0.000, OR = 8.601). This means to get the cohabitation of Sodalis + and T. b. brucei was 8.601 times more likely to happen than getting Sodalis - and T. b. brucei. A significant association was also found for T. simiae (0.014, OR = 5.114) and T. theileri (p = 0.001. OR = 9.960). There was no association at all between Sodalis + and T. congolense savannah, T. evansi and T. godfreyi (p values very high or even not generated). On another hand, no single association was found between the presence of Wolbachia and trypanosome species. The majority of Wolbachia-positive flies were trypanosome-negative. This could be expected since the presence of Wolbachia is known to hamper the establishment of trypanosomes in a tsetse fly vector. T. vivax is not inclusive, as its life cycle does not occur in the abdomen. Fig. 6 illustrates the distribution of endosymbionts in the flies across various locations of the study area.
Fig. 6.
Occurrence and prevalence of endosymbionts in tsetse flies.
4. Discussion
G. pallidipes had a higher trypanosomal infection rate than G. m. centralis. The infection rate in the Thorax + Abdomen (TA) was higher than in the Head + Proboscis (HP). This was in agreement with other studies such as [15,52,53] and [54]. We attribute this difference to the fact that the abdomen is the site for the establishment of trypanosomes before maturation in the mouthparts [55]. Depending on the trypanosome species, a parasite may be found in the midgut and/or the salivary glands, both being anatomically located in the abdomen [56]. In addition, the presence of the parasite in the abdomen could originate from a recent blood meal coming from different infected hosts. The majority of infections are limited to the midgut and therefore unable to reach maturation in the mouthparts [57,58]. Not all the infections established in the fly mid-gut will be transmitted to the susceptible hosts [20].
These findings of this study corroborate that infection rates obtained after crushing the whole fly may overestimate infections. A case in point is the number of T. vivax present in the abdomen, which would have been counted as the infections if the whole fly had been crushed. Another example is an underestimation of T. congolense infections after considering the proboscis of only infected mid-guts. This may have an impact on the determination of the fly vector competence. The interpretation of the tsetse trypanosome infections should, therefore, be done carefully by taking the trypanosome location in the fly and the method used into consideration. Molecular detection targets parasite DNA, however, does not always indicate active infection, hence it likely overestimates tsetse fly infection rates [59,60]. The DNA does not discriminate between infective and immature forms, therefore, the presence of trypanosome DNA in the fly midgut does not necessarily designate an infection [61], and could also result from a recent blood meal [62]. In a recent parallel study on bovine trypanosomosis conducted in the same area, cattle were found to be infected by T. congolense savannah (10.7%), T. vivax (5.2%), T. brucei brucei (2%), and T. evansi (0.7%) [4]. Linking the above previous study with the current findings in tsetse infection, we would therefore surmise the high transmissibility of the T. congolense savannah strain in the region. This assumption is because T. congolense savannah was the most prevalent in cattle and it is not the case in tsetse fly vectors in the same area. Furthermore, the latter finding would corroborate that cattle are not the important feeding hosts for tsetse flies in the area despite their abundance.
Similarly to other regional studies, this study findings show that tsetse blood feeding in the Akagera region is closely associated with wild animals, even at the interface between the park and the agricultural areas with livestock farms and human settlements. Although the Akagera NP has a complete perimeter fence, a few wild animals were observed outside the park. Thus, tsetse flies collected at the interface feed on both wild and domestic hosts. Transmission of trypanosomes is therefore likely to occur by both sylvatic and domestic cycles between hosts. We recall that trypanosomes from sylvatic cycles are the most pathogenic to domestic animals [63]. Although cattle are the dominant livestock species around the park, cattle and goats contributed few blood meals at the interface as opposed to the wild animals. The same pattern was found in Kenya by Channumsin [64] and Ebhodaghe [65]. This could be linked to the farming practices being implemented in the area such as reducing the unwanted vegetation in farms; which could be the resting places for tsetse flies. On top of that, farmers are aware of the active hours of tsetse flies and try to avoid taking their animals to high-risk areas during that time. It is uncertain whether this is maybe simply because tsetse flies avoid livestock when more preferred hosts are available. Our study did not find evidence of seasonality influencing the feeding patterns in the area. However, variations exist within host species. Cattle and goats were more fed on during the dry season. We think there is a limited dispersal of wild hosts at the interface during the dry season. This would suggest that the tsetse bite and hence the transmission of the disease would be increased for livestock in the dry season. We found human DNA in tsetse blood meals as is the case for some other similar regional studies [30,65,66]. The human DNA might have come from the investigators while manipulating flies; or simply because flies fed on humans frequenting or residing in the area. Contact with human DNA could ensue during traps deployment, collection, or DNA extraction [67]. Whatever the case, these findings were observed in human-dwelling localities and it shows how humans are in close contact with tsetse flies. We found some Nile Tilapia blood meals, a fish species that is locally reared in inland lakes. Fish are aquatic and almost impossible to be fed on by a tsetse fly. We attribute this to the presence of a fishing site and fish cleaning process in Shakani around lake Ihema within the park. Flies may have encountered fish during those activities.
Our findings on the feeding preferences of G. pallidipes in the Akagera region agree with Auty [14], who studied it in the Serengeti National Park of Tanzania. Also in the Nguruman game reserve in Kenya, G. pallidipes fed mainly on the African elephant, warthogs, African buffalo, and baboons [68,69]. Multiple blood meal was observed, which increases the exposure to a diverse range of trypanosomes, therefore the transmission of the disease to a wide host range. Despite the mixed blood meal sources, we estimate that the likely important reservoirs for trypanosomes in the area are the buffalo, the warthog, the elephant, and the bushbuck. These associations are in agreement with trypanosome infections in wild hosts/ reservoirs in various studies [[7], [8], [9], [10]].
The Sodalis prevalence found in G. pallidipes (2.8%) was lower than what was found in Shimba hills (16%) [70] and in the Masai Mara National Reserve (6.3%) [30], both in Kenya. It is worth noting that the Sodalis density is known to vary according to the tsetse species and Sodalis genotype in question [71,72]; but also other factors like the geographic location, age and sex of the fly influence Sodalis to act on Glossina competence [73]. We recommend further identification of Sodalis genotypes circulating in tsetse populations of the Akagera region to decipher the trypanosome-symbionts associations in the area. The prevalence of Wolbachia in this study was 4.8%. The prevalence of Wolbachia normally ranges between 9.5 and 100% in the morsitans group of tsetse [74,75], which was not the case for this study. The use of different markers is recommended to maximize the detection. A deep identification of various Wolbachia haplotypes is suggested to better understand their relationship with trypanosomes. We did not find any Spiroplasma and SGH virus positives in all flies. Nevertheless, the presence of Spiroplasma occurs in specific populations, varying in locations and seasons [28]. Similarly, SGH Virus in the field-tsetse flies tends to vary according to the tsetse species and the location [16,76,77].
Both Sodalis and Wolbachia were more concentrated in Akagera NP than outside. To conclude, we suggest that the prevalence of endosymbionts (2.8% Sodalis and 4.8% Wolbachia) from this study were too low as opposed to trypanosome infections, to evaluate effectively their relationship. Additionally, having used the molecular method, we could not differentiate active infections from those coming with the blood meal. It is therefore challenging to conclude on the role of symbionts found on tsetse competence. The lack of sequencing of different bacterial genomes studied prevents us from seeing eventual associations between bacterial genotypes and the presence or absence of the parasite. Despite the low prevalence, there was a significant association between the presence of Sodalis and infections with T. brucei brucei, T. simiae and T. theileri. There was an expected tendency for an inverse relationship between Wolbachia-positive and trypanosome infections.
5. Conclusions
While no human infective trypanosome (T. brucei rhodesiense) was detected, both G. pallidipes and G. morsitans centralis host animal-infective trypanosomes, and are consequently potential vectors of the disease to susceptible hosts in the area. The risk of transmission increases during the rainy season. The presence of mechanically transmitted species of trypanosomes suggests the role of other biting flies in the epidemiology of trypanosomosis in the area. Vector control should therefore also target biting flies alongside the tsetse flies. The study shows that tsetse blood feeding is much associated with wild animals, implicating the buffalo as the main host both inside the park and at the interface. Other likely important reservoirs for trypanosomes in the area are the warthog, the elephant and the bushbuck. Conversely, according to the feeding indices, the rhino, elephant and bushbuck could be the most preferred hosts by tsetse flies. The risk of humans contracting trypanosome infections cannot be considered null as human DNA was detected in tsetse flies in some localities. It makes us understand that the risk of transmission still exists even though the disease in humans was eliminated. Sleeping sickness surveillance should include those areas for regular monitoring. Only Sodalis and Wolbachia were found in tsetse flies. The prevalence of endosymbionts was too low to assess efficiently their relationship with trypanosome infections. Nevertheless, the presence of Sodalis seemed to be associated with T. brucei brucei, T. simiae and T. theileri infections. There was a negative association between Wolbachia and trypanosome infections.
Beyond its scientific interest, this study has also direct implications for the ongoing efforts to control and eliminate trypanosomosis and it strengthened the One-Health collaboration at the national level. The findings support the existing collaboration in tsetse and trypanosomosis control and surveillance between different stakeholders through sharing scientific information. In particular, the study contributed to the dossier of HAT elimination as a Public Health Problem (PHP) in Rwanda, as it corroborated the notion that HAT risk in the area can be considered marginal [6]. In the area of animal disease control, the present study and the previous related ones [2,35] will also contribute to efforts aimed at developing atlases of tsetse and animal trypanosome infections at the national [78] and continental levels [79,80]. This spatially-explicit evidence is considered crucial to design effective strategies for the progressive control of the disease in livestock [81]. Generating the scientific information and sharing it with the relevant stakeholders are key to the successful use of the One Health approach to address trypanosomosis challenges in both animals and humans.
Funding
This study was supported by the United States Agency for International Development, as part of the Feed the Future initiative, under the CGIAR Fund, award number BFS G-11–00002, and the predecessor fund The Food Security and Crisis Mitigation II grant, award number EEM-G-00–04-00013. The funder supported the data collection phase I of this work. Rwanda Dairy Development Project, funded by the International Fund for Agricultural Development (IFAD)—project ID: 2000001195 through Rwanda Agriculture and Animal Resources Board for field support (phase II data collection) and laboratory facilities. The International Centre of Insect Physiology and Ecology (icipe), through a Dissertation Research Internship Programme (DRIP) (Postgraduate Training | icipe - International Centre of Insect Physiology and Ecology), Fellowship to RGS. All the molecular work and sequencing were conducted under this fellowship. This fellowship also received financial support from the German Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft fu¨r Internationale Zusammenarbeit (GIZ) ICTDL Project Contract Number 81235250 and Project Number 18.7860.2–001.00.
The funders are not involved in any step from the study design to the decision to submit the article for publication. Similarly, the views expressed herein do not necessarily reflect the official opinion of the donors.
Ethical approval
The ethical permission was granted by the ethics committees of the Faculty of Veterinary Medicine—University of Nairobi (REF: FVM BAUEC/2019/246) and the College of Agriculture and Veterinary Medicine–University of Rwanda (REF: 030/19/DRI).
Disclaimer
The boundaries and names shown and the designations used on the maps presented in this paper do not imply the expression of any opinion whatsoever on the part of FAO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate borderlines for which there may not yet be full agreement. The views expressed in this paper are those of the authors and do not necessarily reflect the views of FAO.
CRediT authorship contribution statement
Richard S. Gashururu: Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Validation, Writing – original draft. Ndichu Maingi: Methodology, Supervision, Validation, Writing – review & editing. Samuel M. Githigia: Methodology, Supervision, Validation, Writing – review & editing. Dennis O. Getange: Data curation, Formal analysis, Software. Jean B. Ntivuguruzwa: Investigation, Methodology, Writing – original draft. Richard Habimana: Data curation, Formal analysis, Software. Giuliano Cecchi: Visualization, Validation, Data curation, Writing – review & editing. James Gashumba: Methodology, Supervision, Validation, Writing – review & editing. Joel L. Bargul: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. Daniel K. Masiga: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgments
-
•
We are thankful to the administration of the districts of Kayonza, Gatsibo, and Nyagatare for the permission to conduct the fieldwork;
-
•
African Parks through Akagera National Park management and Rwanda Development Board for the research permission;
-
•
We also acknowledge the technical contribution of J. Claude Mpayimana for data collection, Peter Odhiambo Otieno for DNA extraction, Dickens O. Ochieng for bench assistance, Kevin Kidambasi for the assistance in blood meal analysis, James Kabii for logistic support, Edward E. Makhulu for guidance in SGHV analysis, Gervais Habarugira for assistance in data presentation, and the rest of the Molecular biology and bioinformatics unit team in one way or another;
-
•
The Animal Production and Health Division of the Food and Agriculture Organization of the United Nations (FAO), in the framework of the Programme Against African Trypanosomosis (PAAT), for guidance and GIS expertise;
-
•
Finally yet importantly, Animal Resources Officers at the district and sector level, students and farmers for field facilitation, data collection and analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100550.
Appendix A. Supplementary data
Supplementary material 1 Dataset
Supplementary material 2 NCBI GenBank accession numbers generated from this study
Supplementary material 3 Distribution of Trypanosoma infections detected in Head and Proboscis (HP) of tsetse flies
Supplementary material 4 Distribution of Trypanosoma infections detected in Thorax and Abdomen (TA) of tsetse flies
Supplementary material 5 Non-pathogenic trypanosomes detected in tsetse body (TA)
Data availability
The datasets and additional files supporting the conclusions of this study are included in the article. Supplementary material 2 contains the nucleotide sequences from this study that were deposited in the GenBank database.
References
- 1.Jordan A.M. Tsetse flies as vectors of trypanosomes. Vet. Parasitol. 1976;2:143–152. [Google Scholar]
- 2.Lord J.S., et al. Geostatistical models using remotely-sensed data predict savanna tsetse decline across the interface between protected and unprotected areas in Serengeti, Tanzania. J. Appl. Ecol. 2018;55(4):1997–2007. doi: 10.1111/1365-2664.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihok S., Otieno L.H., Tarimo C.S. Trypanosome infection rates in tsetse flies (Diptera: Glossinidae) and cattle during tsetse control operations in the Kagera River region of Rwanda. Bull. Entomol. Res. 1992;82(3):361–367. [Google Scholar]
- 4.Gashururu R., et al. Occurrence, diversity and distribution of Trypanosoma infections in cattle around the Akagera National Park, Rwanda. PLoS Negl. Trop. Dis. 2021;15(12) doi: 10.1371/journal.pntd.0009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simarro P.P., et al. The atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases. Int. J. Health Geogr. 2010;9(1):57. doi: 10.1186/1476-072X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco J.R., et al. The elimination of human African trypanosomiasis: achievements in relation to WHO road map targets for 2020. PLoS Negl. Trop. Dis. 2022;16(1) doi: 10.1371/journal.pntd.0010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker J., Sachs R., Laufer I. Trypanosomes of wild mammals in an area northwest of the Serengeti National Park, Tanzania. Z Tropenmed Parasitol. 1967;18(3):280–284. [PubMed] [Google Scholar]
- 8.Connor R. The impact of nagana. Onderstepoort J. Vet. Res. 1994;61(4):379–383. [PubMed] [Google Scholar]
- 9.Reichard R. Area-wide biological control of disease vectors and agents affecting wildlife. Rev. Sci. Tech. l’OIE. 2002;21(1):179–185. doi: 10.20506/rst.21.1.1325. [DOI] [PubMed] [Google Scholar]
- 10.Mbaya A.W., Aliyu M.M., Ibrahim U.I. The clinico-pathology and mechanisms of trypanosomosis in captive and free-living wild animals: a review. Vet. Res. Commun. 2009;33(7):793–809. doi: 10.1007/s11259-009-9214-7. [DOI] [PubMed] [Google Scholar]
- 11.Garcia H.A., et al. Remarkable richness of trypanosomes in tsetse flies (Glossina morsitans morsitans and Glossina pallidipes) from the Gorongosa National Park and Niassa National Reserve of Mozambique revealed by fluorescent fragment length barcoding (FFLB) Infect. Genet. Evol. 2018;63:370–379. doi: 10.1016/j.meegid.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Chikowore G., et al. A pilot study to delimit tsetse target populations in Zimbabwe. PLoS Negl. Trop. Dis. 2017;11(5) doi: 10.1371/journal.pntd.0005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues C.M.F., et al. Genetic diversity of trypanosomes pathogenic to livestock in tsetse flies from the Nech Sar National Park in Ethiopia: a concern for tsetse suppressed area in southern Rift Valley? Infect. Genet. Evol. 2019;69:38–47. doi: 10.1016/j.meegid.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Auty H., et al. Quantifying heterogeneity in host-vector contact: tsetse (Glossina swynnertoni and G. pallidipes) host choice in Serengeti National Park, Tanzania. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0161291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odeniran P.O., Macleod E.T., Ademola I.O., Welburn S.C. Molecular identification of bloodmeal sources and trypanosomes in Glossina spp., Tabanus spp. and Stomoxys spp. trapped on cattle farm settlements in Southwest Nigeria. Med. Vet. Entomol. 2019;33(2):269–281. doi: 10.1111/mve.12358. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Weiss B.L., Aksoy S. Tsetse fly microbiota : form and function. Front. Cell. Infect. Microbiol. 2013;3(October):1–6. doi: 10.3389/fcimb.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiger A., Malele I., Abd-Alla A.M., Njiokou F. Blood feeding tsetse flies as hosts and vectors of mammals-pre-adapted African Trypanosoma: current and expected research directions. BMC Microbiol. 2018;18(S1):162. doi: 10.1186/s12866-018-1281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss B.L., Wang J., Maltz M.A., Wu Y., Aksoy S. Trypanosome infection establishment in the tsetse Fly gut is influenced by microbiome-regulated host immune barriers. PLoS Pathog. 2013;9(4) doi: 10.1371/journal.ppat.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herren J.K., et al. A microsporidian impairs plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat. Commun. 2020;11(1):2187. doi: 10.1038/s41467-020-16121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farikou O., et al. Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes—an epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infect. Genet. Evol. 2010;10(1):115–121. doi: 10.1016/j.meegid.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Hamidou I., et al. The bacterial flora of tsetse fly midgut and its effect on trypanosome transmission. J. Invertebr. Pathol. 2013;112:S89–S93. doi: 10.1016/j.jip.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Wamwiri F.N., Ndungu K., Thande P.C., Thungu D.K., Auma J.E., Ngure R.M. Infection with the secondary tsetse-endosymbiont Sodalis glossinidius (Enterobacteriales : Enterobacteriaceae) influences parasitism in Glossina pallidipes (Diptera : Glossinidae) J. Insect Sci. 2014;14(4) doi: 10.1093/jisesa/ieu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rio R.V.M., et al. Mutualist-provisioned resources impact vector competency. Am. Soc. Microbiol. 2019;10(3):1–14. doi: 10.1128/mBio.00018-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger A., Ponton F., Simo G. Adult blood-feeding tsetse flies, trypanosomes, microbiota and the fluctuating environment in sub-Saharan Africa. ISME J. 2015;9(7):1496–1507. doi: 10.1038/ismej.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam U., Medlock J., Brelsfoard C., Pais R., Lohs C. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse Fly Glossina morsitans. PLoS Pathog. 2011;7(12) doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glaser R.L., Meola M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kambris Z., et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6(10) doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider D.I., et al. Spatio-temporal distribution of Spiroplasma infections in the tsetse fly (Glossina fuscipes fuscipes) in northern Uganda. PLoS Negl. Trop. Dis. 2019;13(8) doi: 10.1371/journal.pntd.0007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doudoumis V., Blow F., Saridaki A., Augustinos A., Dyer N.A., Goodhead I. Challenging the Wigglesworthia, Sodalis, Wolbachia symbiosis dogma in tsetse flies : Spiroplasma is present in both laboratory and natural populations. Sci. Rep. 2017;7(4699):1–13. doi: 10.1038/s41598-017-04740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makhulu J.V., Edward E., Adunga V.O., Jeneby M., Kimathi E.M., Mararo E., Musa L.W., Abdulahi Ali. Tsetse blood-meal sources, endosymbionts and trypanosome-associations in the Maasai Mara National Reserve, a wildlife-human- livestock interface. PLoS Negl. Trop. Dis. 2021:1–18. doi: 10.1371/journal.pntd.0008267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son J.H., et al. Infection with endosymbiotic Spiroplasma disrupts tsetse (Glossina fuscipes fuscipes) metabolic and reproductive homeostasis. PLoS Pathog. 2021;17(9) doi: 10.1371/journal.ppat.1009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.HenryM E.M., İnce İkbal Agah, Boeren Sjef, Murungi Edwin K., Meki Irene K., Otieno Everlyne A., Nyanjom Steven R.G., van Oers Monique M., Vlak Just M., Abd-Alla Adly M.M. Comparative analysis of salivary gland proteomes of two Glossina species that exhibit differential Hytrosavirus pathologies. Frontiersin Microbiology. 2016;7(February):1–16. doi: 10.3389/fmicb.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kariithi H.M., Meki I.K., Boucias D.G., Abd-alla A.M.M. ScienceDirect Hytrosaviruses : current status and perspective. Curr. Opin. Insect Sci. 2017;22:71–78. doi: 10.1016/j.cois.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Orlov I., Drillien R., Spehner D., Bergoin M., Abd-alla A.M.M. Structural features of the salivary gland hypertrophy virus of the tsetse fl y revealed by cryo-electron microscopy and tomography. Virology. 2018;514(November 2017):165–169. doi: 10.1016/j.virol.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Demirbas-uzel G., et al. Susceptibility of tsetse species to Glossina pallidipes salivary gland hypertrophy virus (GpSGHV) Front. Microbiol. 2018;9(April):1–12. doi: 10.3389/fmicb.2018.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gashururu R.S., et al. An update on the distribution of Glossina (tsetse flies) at the wildlife-human-livestock interface of Akagera National Park, Rwanda. Parasit. Vectors. 2021;14(1):294. doi: 10.1186/s13071-021-04786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macpherson D.D.B. 2019. Report on an Aerial Wildlife Census of Akagera National Park, Rwanda. [Google Scholar]
- 38.Ford J., Glasgow J.P., Johns D.L., Welch J.R. Transect Fly-rounds in field studies of Glossina. Bull. Entomol. Res. 1959;50(2):275–285. [Google Scholar]
- 39.Challier L.C. Cah ORSTOM Entomol Med Parasitol. Vol. 11. 1973. A new trap for capturing Glossina flies (Diptera: Muscidae), description and field trials; pp. 251–262. no. 11. [Google Scholar]
- 40.Nnko H.J., et al. Seasonal variation of tsetse fly species abundance and prevalence of trypanosomes in the Maasai steppe, Tanzania. J. Vector Ecol. 2017;42(1):24–33. doi: 10.1111/jvec.12236. [DOI] [PubMed] [Google Scholar]
- 41.Pollock J.N. Vol. 1. 1982. Training Manual for Tsetse control personnel - Tsetse biology, systematics and distribution; techniques. [Google Scholar]
- 42.Leak S., Ejigu D., Vreysen M. 2008. Collection of Entomological Baseline Data for Tsetse Area-Wide Integrated Pest Management Programmes. [Google Scholar]
- 43.Margam V.M., Gachomo E.W., Shukle J.H., Oluwole O.O., Seufferheld M.J., Kotchono S.O. A simplified arthropod genomic-DNA extraction protocol for polymerase chain reaction (PCR) -based specimen identification through barcoding. Mol. Biol. Rep. 2010;37:3631–3635. doi: 10.1007/s11033-010-0014-5. [DOI] [PubMed] [Google Scholar]
- 44.Njiru Z.K., et al. The use of ITS1 rDNA PCR in detecting pathogenic African trypanosomes. Parasitol. Res. 2005;95:186–192. doi: 10.1007/s00436-004-1267-5. [DOI] [PubMed] [Google Scholar]
- 45.Welburn S., et al. Identification of human-infective trypanosomes in animal reservoir of sleeping sickness in Uganda by means of serum-resistance-associated (SRA) gene. Lancet. 2001;358(9298):2017–2019. doi: 10.1016/s0140-6736(01)07096-9. [DOI] [PubMed] [Google Scholar]
- 46.Gibson W., Backhouse T., Griffiths A. The human serum resistance associated gene is ubiquitous and conserved in Trypanosoma brucei rhodesiense throughout east Africa1. Infect. Genet. Evol. 2002;1(3):207–214. doi: 10.1016/s1567-1348(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 47.Kilpatrick A.M., Daszak P., Jones M.J., Marra P.P., Kramer L.D. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B. 2006;(May):2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lardeux F., Loayza P., Bouchité B., Chavez T. Host choice and human blood index of Anopheles pseudopunctipennis in a village of the Andean valleys of Bolivia. Malar. J. 2007;14:1–14. doi: 10.1186/1475-2875-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamer G.L., et al. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80(2):268–278. [PubMed] [Google Scholar]
- 50.Altschul S.F., Gish W., Pennsylvania T., Park U. Basic local alignment search tool 2Department of computer science. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 51.Kearse M., et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isaac C., et al. Molecular identification of different trypanosome species and subspecies in tsetse flies of northern Nigeria. Parasit. Vectors. 2016:1–7. doi: 10.1186/s13071-016-1585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber J.S., et al. 2019. Genetic Diversity of Trypanosome Species in Tsetse Flies (Glossina spp.) in Nigeria; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Signaboubo D., et al. Diversity of tsetse flies and trypanosome species circulating in the area of Lake Iro in southeastern Chad. Parasit. Vectors. 2021:1–11. doi: 10.1186/s13071-021-04782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maudlin I., Holmes P., Miles M. CABI Publishing; Wallington, UK: 2004. The Trypanosomes. [Google Scholar]
- 56.Leak S.G.A. CABI Publishing; Nairobi: 1999. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomosis. [Google Scholar]
- 57.Nayduch D., Aksoy S. Refractoriness in tsetse flies (Diptera : Glossinidae) may be a matter of timing. J. Med. Entomol. 2007;44(4):660–665. doi: 10.1603/0022-2585(2007)44[660:ritfdg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 58.Roditi I. Interactions between trypanosomes and tsetse flies, currcent. Opin. Micribiol. 1998;11(4):345–351. doi: 10.1016/j.mib.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Farikou O., Njiokou F., Simo G., Asonganyi T., Cuny G., Geiger A. Tsetse fly blood meal modification and trypanosome identification in two sleeping sickness foci in the forest of southern Cameroon. Acta Trop. 2010;116(1):81–88. doi: 10.1016/j.actatropica.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Simo G., et al. Identification of different trypanosome species in the mid-guts of tsetse flies of the Malanga (Kimpese) sleeping sickness focus of the Democratic Republic of Congo. Parasit. Vectors. 2012;5(1):201. doi: 10.1186/1756-3305-5-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mekata H., et al. Virological and serological studies of porcine respiratory coronavirus infection on a Japanese farm. J. Vet. Med. Sci. 2008;70(9):923–928. doi: 10.1292/jvms.70.929. [DOI] [PubMed] [Google Scholar]
- 62.Macleod E.T., Darby A.C., Maudlin I., Welburn S.C. Factors affecting trypanosome maturation in tsetse flies. PLoS One. 2007;2(2) doi: 10.1371/journal.pone.0000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van den Bossche P., de La Rocque S., Hendrickx G., Bouyer J. A changing environment and the epidemiology of tsetse-transmitted livestock trypanosomiasis. Trends Parasitol. 2010;26(5):236–243. doi: 10.1016/j.pt.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Channumsin M., et al. Blood meal analysis of tsetse flies (Glossina pallidipes: Glossinidae) reveals higher host fidelity on wild compared with domestic hosts. Wellcome Open Res. 2021;6:213. doi: 10.12688/wellcomeopenres.16978.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ebhodaghe F.I., Okal M.N., Kalayou S., Bastos A.D.S., Masiga D.K. Tsetse Bloodmeal analyses incriminate the common warthog Phacochoerus africanus as an important cryptic host of animal trypanosomes in smallholder cattle farming communities in. Pathogens. 2021;10(1501) doi: 10.3390/pathogens10111501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bett B., et al. Estimation of tsetse challenge and its relationship with trypanosomosis incidence in cattle kept under pastoral production systems in Kenya. Vet. Parasitol. 2008;155(3–4):287–298. doi: 10.1016/j.vetpar.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 67.England E.C., Baldry D.A.T. Observations on the relative attractiveness to Glossina pallidipes of different animal baits, a tsetse trap, and a fly-round patrol. Bull. World Health Organ. 1972;47(6):789–793. [PMC free article] [PubMed] [Google Scholar]
- 68.Nyawira N.C. Egerton University; Kenya: 2009. Analysis of Tsetse Fly (Diptera: Glossinidae) Blood Meals Using Mitochondrial Cytochrome Genes for Vertebrate Host Identification. [Google Scholar]
- 69.Muturi C.N., et al. Tracking the feeding patterns of tsetse flies (Glossina genus) by analysis of Bloodmeals using mitochondrial cytochromes genes. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wamwiri F.N., et al. Wolbachia, Sodalis and trypanosome co-infections in natural populations of Glossina austeni and Glossina pallidipes. Parasit. Vectors. 2013;6(232):1–11. doi: 10.1186/1756-3305-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geiger A., et al. Vector competence of Glossina palpalis gambiensis for Trypanosoma brucei s.l. and genetic diversity of the symbiont Sodalis glossinidius. Mol. Biol. Evol. 2006;24(1):102–109. doi: 10.1093/molbev/msl135. [DOI] [PubMed] [Google Scholar]
- 72.Farikou O., Njiokou F., Cuny G., Geiger A. Microsatellite genotyping reveals diversity within populations of Sodalis glossinidius, the secondary symbiont of tsetse flies. Vet. Microbiol. 2011;150:207–210. doi: 10.1016/j.vetmic.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 73.Channumsin M., Ciosi M., Masiga D., Turner C.M.R., Mable B.K. Sodalis glossinidius presence in wild tsetse is only associated with presence of trypanosomes in complex interactions with other tsetse-specific factors. BMC Microbiol. 2018;18(S1):163. doi: 10.1186/s12866-018-1285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doudoumis V., et al. Detection and characterization of Wolbachia infections in laboratory and natural populations of different species of tsetse flies (genus Glossina) BMC Microbiol. 2012;12(Suppl. 1):1–13. doi: 10.1186/1471-2180-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doudoumis V., et al. Tsetse- Wolbachia symbiosis : comes of age and has great potential for pest and disease control. J. Invertebr. Pathol. 2013;(2012) doi: 10.1016/j.jip.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alam U., et al. Implications of microfauna-host interactions for trypanosome transmission dynamics in Glossina fuscipes fuscipes in Uganda. Appl. Environ. Microbiol. 2012;78(13) doi: 10.1128/AEM.00806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malele I.I., et al. Prevalence of SGHV among tsetse species of economic importance in Tanzania and their implication for SIT application. J. Invertebr. Pathol. 2013;112:S133–S137. doi: 10.1016/j.jip.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 78.Ngari N.N., Gamba D.O., Olet P.A., Zhao W., Paone M., Cecchi G. Developing a national atlas to support the progressive control of tsetse-transmitted animal trypanosomosis in Kenya. Parasit. Vectors. 2020;13(1):286. doi: 10.1186/s13071-020-04156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cecchi G., Paone M., Feldmann U., Vreysen M.J., Diall O., Mattioli R.C. Assembling a geospatial database of tsetse-transmitted animal trypanosomosis for Africa. Parasit. Vectors. 2014;7(1):39. doi: 10.1186/1756-3305-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cecchi G., Paone M., Argilés Herrero R., Vreysen M.J.B., Mattioli R.C. Developing a continental atlas of the distribution and trypanosomal infection of tsetse flies (Glossina species) Parasit. Vectors. 2015;8(1):284. doi: 10.1186/s13071-015-0898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diall O., et al. Developing a progressive control pathway for African animal Trypanosomosis. Trends Parasitol. 2017;33(7):499–509. doi: 10.1016/j.pt.2017.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Dataset
Supplementary material 2 NCBI GenBank accession numbers generated from this study
Supplementary material 3 Distribution of Trypanosoma infections detected in Head and Proboscis (HP) of tsetse flies
Supplementary material 4 Distribution of Trypanosoma infections detected in Thorax and Abdomen (TA) of tsetse flies
Supplementary material 5 Non-pathogenic trypanosomes detected in tsetse body (TA)
Data Availability Statement
The datasets and additional files supporting the conclusions of this study are included in the article. Supplementary material 2 contains the nucleotide sequences from this study that were deposited in the GenBank database.