Abstract
The disease anthrax occurs generally in herbivores and the causative organism (Bacillus anthracis) infects humans who come in contact with infected animals or their products. The persistence of anthrax spores for decades and its lethality contribute to its biowarfare potential. We conducted this systematic review along with risk mapping to investigate the spatio-temporal distribution, clinico-epidemiological, socio-behavioural and programmatic issues pertaining to anthrax in India over the last two decades.
Peer reviewed quantitative and qualitative studies and grey literature comprising weekly reports of the ‘Integrated Disease Surveillance Program’ (IDSP), were accessed for extracting data. IDSP data were used for geo-referencing of the villages of anthrax cases; Pseudo-absence was generated to fit a Bayesian Additive Regression Trees (BART) model to develop anthrax risk map.
The case fatality rate of cutaneous anthrax ranged from 2% to 38%, while the gastrointestinal and inhalational types were 100% fatal. Our synthesis revealed that human anthrax outbreaks in India were clustered around the eastern coastal regions. The states of Odisha, West Bengal, Andhra Pradesh and Jharkhand reported maximum number of outbreaks. Odisha reported a maximum number of 439 human anthrax cases since 2009, of which Koraput district contributed to 200 cases (46%). While handling or consumption of infected animal product were proximal drivers of these events, poverty, lack of awareness, traditional beliefs and local practices served as facilitatory factors. Other structural determinants were wild life-livestock interface, historical forest loss, soil pH, soil-water balance, organic carbon content, temperature, rainfall and humidity.
The programmatic issues identified through this review were lack of active surveillance, non-availability of diagnostic facility at the periphery, delayed reporting, absence of routine livestock vaccination and lack of adequate veterinary services. Interventions based on One-health approach in the country merit immediate policy and program attention; high risk zones for anthrax identified during present investigation, should be prioritized.
Keywords: Anthrax, Spatio-temporal distribution, Risk map, Bio-terrorism, Disease elimination science & health, Community engagement & Strategic Communication
Highlights
-
•
Human anthrax outbreaks were clustered around the eastern coastal regions in India.
-
•
Risk map shows hot spots of anthrax suitability in eastern and northeastern regions.
-
•
The states Odisha, Andhra Pradesh, West Bengal and Jharkhand had frequent outbreaks.
-
•
Predominant risk factor was handling or consuming dead or sick animals.
-
•
Poverty, socio-cultural practices & environmental factors exacerbated the risk.
1. Introduction
Herbivores get infected with anthrax from contaminated soil of grazing field and the causative organism (Bacillus anthracis) is transmitted to humans who come in contact with infected animals or their products. It is a zoonotic, multi-species bacterial disease the distribution of which in India is not well characterized. Bacillus anthracis is a gram-positive, endospore forming bacteria that can persist in soil for up to 200 years [1]; these endospores are highly contagious and resistant to heat, ultraviolet light, gamma radiation and many disinfectants, enabling them to be used as a potential agent for biowarfare [2].
A recent study mapping the distribution of anthrax across 70 countries [3] estimated that approximately 1.83 billion people lived within the regions of anthrax risk globally, mostly concentrated in rural rainfed systems throughout arid and temperate land across Asia, southern Europe, sub-Sahelian Africa, North America and parts of Australia [3]. India is an endemic country for animal anthrax with its largest population of livestock in the world, which leads to sporadic and seasonal outbreaks in humans [1].
Although the cases of B. anthracis infection have decreased globally, the persistence of its spores in nature for long and lethal effects of the disease make it the topmost zoonotic disease of public health concern [1]. According to the Centers for Disease Control and Prevention (CDC), anthrax belongs to ‘Category A' bioterrorism agent of highest priority [4]. The extent of human anthrax in India is poorly understood due to underreporting [5]. Against this background we conducted the current systematic review and explored issues pertaining to One-health initiatives [6] and human-animal-environment interfaces in the country.
The disease occurrence of anthrax in humans in India over the past two decades, its spatiotemporal distribution and environmental as well as socio-economic factors were kept in consideration. We analysed village level reports of anthrax as well to develop a predictive risk map.
2. Methodology
The present systematic review focused on human anthrax in India. Capturing the patterns and determinants of human anthrax cases in different geographical regions of the country remained as central considerations so that the existing public health programs can be appropriately informed.
2.1. Literature search
2.1.1. Search strategy
We used three bibliographic databases, namely PubMed, Embase, and Ovid, articles published in English during the past two decades (January 2000 to December 2021) were retrieved.The above mentioned broader topic was broken down to specific areas using Population, Intervention/Indicator, Comparison, Outcome and Timeframe (PICOT); carefully chosen keywords were assigned to explore each of them. Using controlled vocabularies and set of operators provided by different databases, strategies were formulated to effectively go through the selected search engines (strategy used for PubMed is shown in Supplementary Table 1). Similar strategies using PICOT framework were used for the other two databases as well.
2.1.2. Internet resources and grey literature
The google search engine was used additionally for identifying articles, which were not captured through the aforementioned search. Grey literature were retrieved from sources with open access such as i) Weekly outbreak reports and media alerts available online from the national Integrated Disease Surveillance Program (IDSP), Directorate General of Health Services under the Ministry of Health and Family Welfare, Government of India [7], and ii) Policy briefs.
2.1.3. Screening of studies
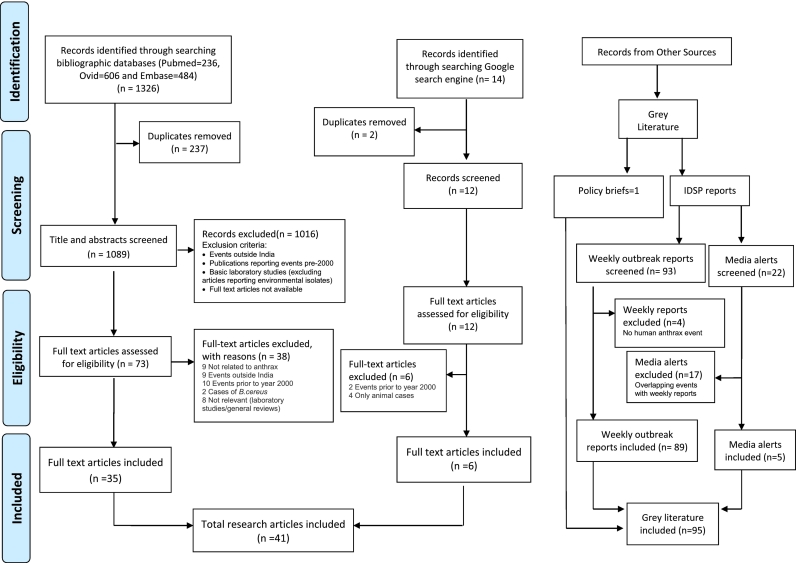
Publications identified through different bibliographic databases were downloaded and screened following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1). These bibliographies were managed using Covidence online tool to facilitate the review process [8]; duplicate articles were removed. The resultant articles were then screened first by viewing title and abstract and then by going through the full text as appropriate. The task of screening was performed independently by two authors (MJ and NC) and the conflicts were resolved by mutual understanding and/or by the intervention of the third author (SP).
Fig. 1.
Search strategy and output data on human anthrax in India.
Bibliographic database-search resulted in inclusion of 35 articles as eligible for data extraction and analyses. Six more journal articles, which were not captured through such effort, could be retrieved through google search and were added to the list of articles. Thus, a total of 41 full text articles were used in final extraction. Grey literature included 89 weekly outbreak reports and 5 media alerts from the IDSP website. Besides, one policy brief was incorporated in the body of grey literature.Screening of IDSP weekly reports resulted in exclusion of four documents, which did not cover human anthrax, while screening of media alerts resulted in exclusion of 17 signals, which were overlapping with IDSP results.
2.1.4. Domains
Five broad domains were considered during the afore-mentioned search:
-
i)
Disease estimates in humans in India (disease burden, incidence, mortality, trend etc)
-
ii)
Geographical and temporal distribution of anthrax in India during January 2000 to December 2021.
-
iii)
Environmental factors (temperature, rainfall, humidity, soil type, water, vegetation, land cover, forest cover, agricultural land etc)
-
iv)
Socio-economic factors (occupation, income, education, culture, behaviour & practices)
-
v)
Policy, environment, biosafety and biosecurity issues
2.2. Data extraction
Articles were categorized as outbreak investigations, qualitative studies, narrative reviews and case reports.A data extraction sheet was prepared comprising attributes, which included demographic details of cases and deaths, clinical features, epidemiological features, occupation, type of animal handling involved, behavioural, socio-economic and environmental factors, other issues and recommendations. Data from qualitative studies and narrative reviews were extracted in the form of a list of issues and collated under similar themes. The data from IDSP available during 2009 to 2021 in weekly reports and media alerts were tabulated separately and used for creation of spot maps, since data prior to 2009 was not available.
2.3. Quality assessment
The articles published in peer reviewed journals were grouped as appropriate (observational, experimental etc) and were assessed for the quality of study design, methodology, analysis and interpretation (Supplementary Table 2). The following quality assessment tools were used:
-
a)
National Institutes of Health (NIH) quality assessment tools for observational studies for cohort and case control studies [9].
-
b)
Joanna Briggs Institute (JBI) critical appraisal checklists for qualitative studies and case reports [10].
-
c)
Public Health Agency of Canada's Infection Prevention and Control Guidelines: Critical Appraisal Tool (CAT) Kit for descriptive studies and narrative reviews [11].
The NIH and JBI critical appraisal tools are recommended by researchers [12] for observational cohort, cross-sectional and case-control studies. For quality assessment of descriptive studies, very few tools are available such as the Critical Appraisal Tool Kit of Public Health Agency of Canada and JBI critical appraisal checklist for studies reporting prevalence data. The JBI tool is the only tool currently available for assessment of case reports. Articles reporting animal cases only, laboratory studies of environmental strains and modelling studies were not included for grading.
2.4. Geographic information system (GIS) maps
All the maps showing district level distribution of cases of anthrax were prepared in Quantum Geographic Information System (QGIS) [13].
2.5. Predictive risk map
Since diseases were recorded at the village level, the same was used for performing predictive risk map analysis. However, analysis of village level data requires matching environmental information. Notably, meteorological stations are limited in number in India and there are almost no stations in villages. Hence, use of remotely sensed variables can be advantageous to fit models and develop predictive risk maps, example of which, in understanding the epidemiology of infectious diseases,is available [14]. Data on human anthrax outbreaks were obtained from IDSP from 2009 to 2021 and geo-referencing of the villages of anthrax cases was carried out; 115 villages reported anthrax cases during this period. Pseudo-absence was generated to fit a Bayesian Additive Regression Trees (BART) model to develop risk map in R [15].
We used 12 temporally Fourier- processed MODIS (MODerate resolution Imaging Spectroradiometer) variables with a spatial resolution of 1 km [16]. Temporal Fourier processing is a useful technique for extracting seasonal information from remotely sensed variables. These processed variables are described in terms of their mean, the annual minimum & annual maximum, the amplitudes and phases of the annual (a1 and p1 respectively), bi-annual (a2, p2) and tri-annual (a3, p3) and variance components of the signal. The MODIS channels thus processed are MIR (Middle Infra-Red), daytime Land Surface Temperature (dLST), night time Land Surface Temperature (nLST), Normalized Difference Vegetation Index (NDVI) and the Enhanced Vegetation Index (EVI). Livestock densities of cattle, buffalo, duck, sheep, goat and pigs were obtained from Food and Agricultural organization of the United Nations (FAO) [17]. Human population density was obtained from the Global Rural-Urban Mapping Project (GRUMP) [18].
3. Results
3.1. Data categorization and analyses
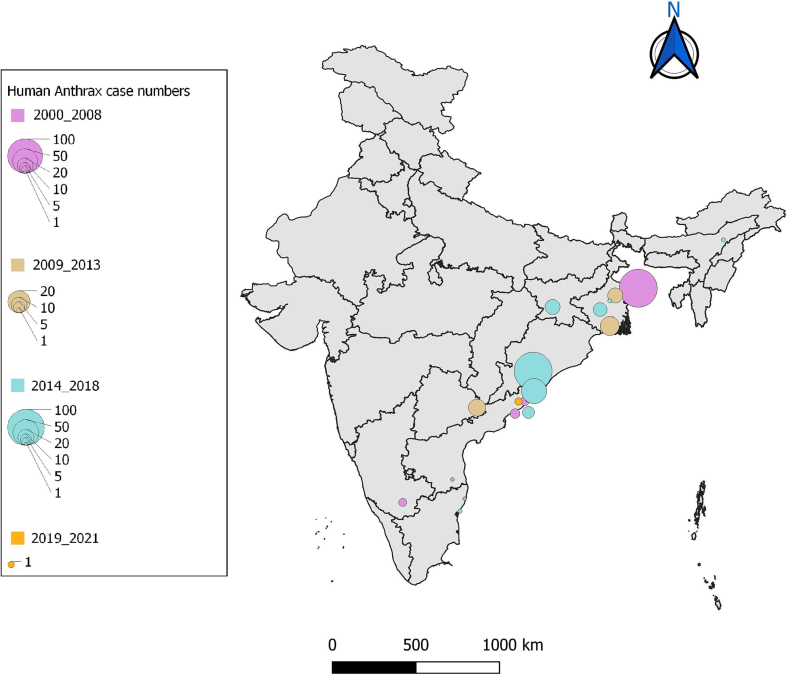
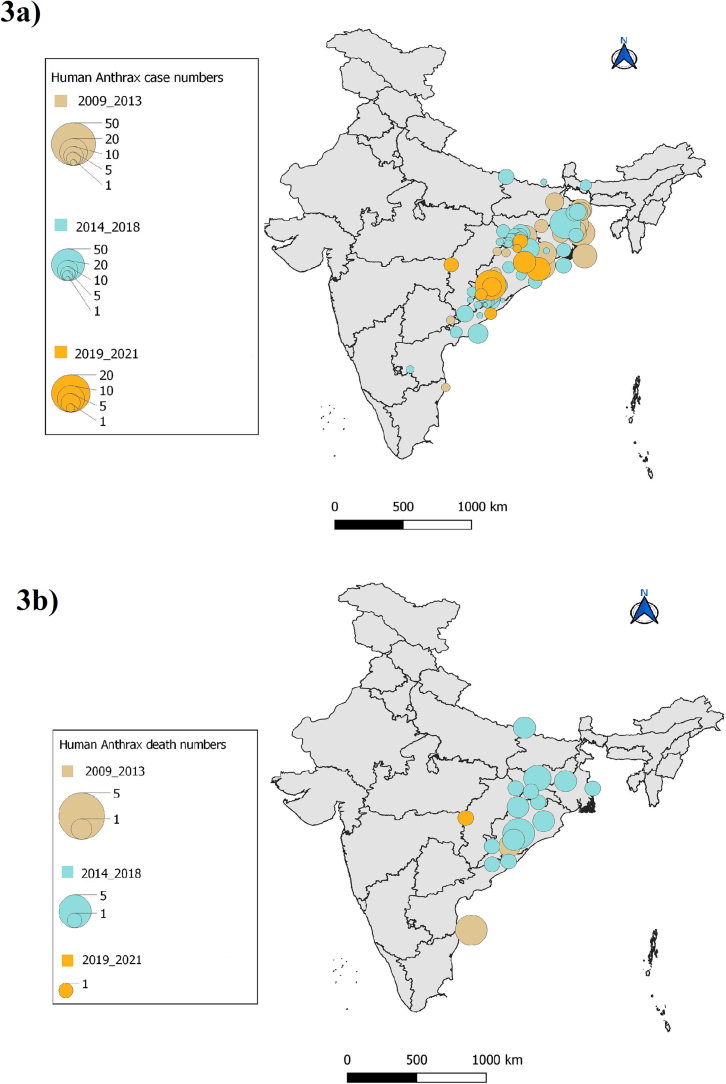
Among the 41 peer-reviewed articles included in final synthesis, ten were outbreak investigations [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] with different study designs (case-control, cross sectional studies etc) and two were qualitative inquiries [29,30]. All outbreak investigations and qualitative studies were rated as medium or high and hence included for data analysis. Out of the six narrative reviews [1,2,[31], [32], [33], [34]], three articles [[32], [33], [34]] with low rating were excluded and out of the 13 [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]] case reports, one case report [38] was excluded following quality assessment (Supplementary Table 2). Most common limitations encountered in these studies were lack of blinding, follow up and measurement of confounding variables. Human anthrax data reported in outbreak investigations and case reports were used for construction of a spatio-temporal distribution map (Fig. 2). Data extracted from qualitative studies, reviews, policy brief and reports were used in identifying risk factors and policy level challenges. The outbreak events recorded in IDSP weekly reports (IDSPWR) were used for construction of spatio-temporal distribution maps of cases and deaths across India for the period 2009 to 2021 (Fig. 3).
Fig. 2.
Map of reported human anthrax outbreaks in peer reviewed articles (India 2000–2021).
Fig. 3.
Spatio-temporal distribution map of a) human anthrax cases b) human anthrax deaths (India IDSP, 2009–2021).
3.2. Spatio-temporal distribution
Data extracted from IDSP weekly reports were analysed with district wise and year wise distribution of outbreaks among the states during 2009 to 2021 (Supplementary Table 3, Fig. 3). Five outbreaks recorded in media alerts during 2016 to 2018 were not reported either in IDSPWR or in peer-reviewed articles and were not included in map construction. Human anthrax outbreaks were predominantly from four states namely, Odisha, Andhra Pradesh, West Bengal and Jharkhand. Although sporadic cases were reported from Assam and Karnataka (Table 2), these did not feature in the IDSPWR.
Table 2.
Risk factor and gap analysis for anthrax in India having policy/programmatic implications.
| S·No | Domains | Risk factors | Recommendations |
|---|---|---|---|
| 1 | Behavioural and cultural factors |
|
|
| 2 | Socio-economic factors |
|
|
| 3 | Environmental factors |
|
|
| 4 | Programmatic gaps |
|
|
Maximum number of cases (n = 439) were reported from Odisha, followed by West Bengal (n = 260), Jharkhand (n = 137) and Andhra Pradesh (n = 81) (Supplementary Table 3). Koraput district, Odisha contributed approximately 46% of the cases (200/439) with an alarmingly high number of 46 outbreaks since 2009. Notably, outbreaks were reported from14 out of 30 districts in Odisha [29]; other endemic districts of the state reporting frequent outbreaks were Sundergarh, Rayagada and Deogarh. The Murshidabad district of West Bengal reported maximum number of outbreaks (17); other districts in the state reporting anthrax were Nadia, Cooch Behar, Burdwan and Bankura. The Vishakapatnam district of Andhra Pradesh recorded outbreaks every year during 2016 to 2019, while sporadic cases were found in Ananthapur and Chittoor districts. During 2015 to 2017, several outbreaks were reported from the districts of Simdega and Gumla in Jharkhand [20,48] (Supplementary Table 3).
3.3. Occurrence of cases and mortality
The case fatality rate ranged from 2% to 38% for cutaneous anthrax in the reported outbreak investigations (Table 1). Most of the human anthrax cases belonged to cutaneous type. One case of inhalational anthrax was reported in a 10 year old boy from Villupuram district, Tamilnadu in 2004, who succumbed to the disease [43]. Two cases of anthrax meningitis were reported; one from Tirupati district, Andhra Pradesh in 2007 [35],who was successfully treated and another from Golaghat district, Assam in 2018, who succumbed [37] (Table 1).
Table 1.
Research articles reporting human anthrax outbreaks during the period 2000 to 2021. (Review articles and qualitative studies were not included).
| State | Year | Districts | Number of cases (deaths) | Type | Attack rate (%) | CFR (%) | Mean/Median Age (Range, yrs) | Gender (M/F) | Study design | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Odisha |
2010–2014 | Koraput district | 325 suspected cases (9 outbreaks) (5 deaths) (12 outbreaks as per IDSP) | Cutaneous | 2% | 36 (4–78) | 82% M | Review of State IDSP data and medical records | Nayak et al., 2019 [19] | |
| Feb – July 2015 | Koraput district | 81 suspected cases in 20 villages (3 deaths) (52 cases, 6 deaths as per IDSP) | Cutaneous | 4% | 38 (5–72) | 89% M | Review of medical records and case-control study | Nayak et al., 2019 [19] | ||
| West Bengal |
June 2007 | Sarkarpara village, Murshidabad, WB (296 from 59 families) | 45 (2 deaths) | Cutaneous | 12% | 4% | Retrospective cohort study | Ray et al., 2009 [22] | ||
| August 2007 | Charbinpara village, Murshidabad, WB (687 from 118 families) | 44 | Cutaneous | 5% | Retrospective cohort study | Ray et al., 2009 [22] | ||||
| 2010 | PaschimMedinipur, West Bengal | 11 (2) | Cutaneous | 7% | 18% | 30 (15–45) | 11 M | Outbreak investigation | Chakraborty et al.,2012 [25] | |
| May 29 to June 8, 2013 | Burdwan district, West Bengal [Oregram and Kathaldanga villages of Bhatar block] | 7 (1) | Cutaneous | 14.3% | 38 (30–60) | 7 M | Cross-sectional study – interviews by house to house visit | Mondalet al., 2015 [27] | ||
| June–July 2014 | PaschimMedinipur, West Bengal (5 remote tribal village) | 11 | Cutaneous | 7.3% | 30 (7–60) | 8 M, 3F | Outbreak investigation | Achar et al., 2018 [23] | ||
| 2015 | Burdwan, West Bengal | 1 | Cutaneous | 34 | M | Case report | Deb and Samanta, 2015 [36] | |||
| Andhra Pradesh |
April 2005 | Araku Valley Mandal, Vishakapatnam, Andhra Pradesh | 5 | Cutaneous | 40 (29–45) | 5 M | Case report | Rao et al., 2005 [39] | ||
| June 2005 | Araku Valley Mandal, Vishakapatnam, Andhra Pradesh | 6 | Cutaneous | 31 (28–54) | 4 M, 2 F | Case report | Rao et al., 2007 [40] | |||
| 2007 | Tirupati | 1 | Anthrax Menigitis | 70 | M | Case report | Bindu et al., 2007 [35] | |||
| Sep 2007 | Araku Valley Mandal, Vishakapatnam, Andhra Pradesh | 4 | Cutaneous | 39 (11–62) | 1 M, 3 F | Case report | Rao et al., 2009 [41] | |||
| July–August 2011 | Musalimadugu village, Chittoor district, Andhra Pradesh | 9 | Cutaneous | 2% | ≥15 yrs | 5 M, 4 F | Outbreak investigation | Reddy et al., 2012 [21] | ||
| 2016 | Vishakapatnam, Andhra Pradesh (tribal hamlets Panasapottu, Goyyagunta, Vennelakota, in Araku valley Mandal 140 km from Viskhapatnanm] | 36 | Cutaneous | 33 M, 1 F, 2 children | Case report | Kumar et al., 2016 [46] | ||||
| 2018 | Kodipunjuvalsa village of Araku valley, Vishakapatnam, Andhra Pradesh | 8 | Cutaneous | 38 (26–67) | 8 M | Case report | Balachandrudu et al., 2018 [45] | |||
| 2020 | Visakhapatnam, Andhra Pradesh, (King George Hospital) | 1 | Cutaneous | 42 | M | Case report | Suggu&Konakanchi, 2020 [42] | |||
| Jharkhand | 2014 | Simdega, Jharkhand | 13 | Cutaneous | 11.1% | 38% | 30 (18–58) | 13 M | Outbreak investigation | Nayak, 2016 [20], Kumar et al. 2019 [48] |
| Assam | 2018 | Golaghat, Assam | 1 (1) | Anthrax Menigitis | 48 | M | Case report | Garg&Panmei, 2018 [37] | ||
| Tamilnadu | 2004 | Villupuram, Tamilnadu | 1 (1) | Inhalational | 10 | M | Case report | Velayudhan et al., 2005 [43] | ||
| Puducherry |
2001 | Puducherry | 1 | Cutaneous | 5 | M | Case report | Vijaikumar, 2001 [44] | ||
| 2015 | Puducherry | 1 (1) | Gastrointestinal | 50 | M | Case report | Iqbal et al., 2015 [47] | |||
| Karnataka | 2004 | Mysore, Karnataka | 4 (4) | Gastrointestinal | Case report | Ichhpujani et al., 2004 [38] | ||||
Gastrointestinal anthrax was reported in four cases from Mysore, Karnataka in 2004 [38], all of whom succumbed within 72 h. One exceptional case of gastrointestinal anthrax with sepsis was reported from Puducherry in 2015 [47]; the 50 year old man died within 18 h of hospital admission (Table 1). Reportedly the patient consumed raw meat under the influence of alcohol and developed fever and gastrointestinal bleeding. Noticeably, the cases were predominantly male. In 2015-outbreak in the state of Odisha, 89% of the 81 cases were men [19]. Men were found to be 35 times more likely to get infected than women [20]. In four outbreak investigations and eight case studies of anthrax, cases occurred in males [20,25,39,45]. Researchers recorded significantly higher risk practices in men compared to women in gender dis-aggregated data [24].
3.4. Risk factors
3.4.1. Behavioural issues and cultural beliefs
Animal handling was involved in all reported cases of human anthrax without exception. The extent of animal handling was directly related to the severity of infection and death. For example, the outbreak investigation by Achar et al. (2018) [23] in Paschim Medinipur, West Bengal reported that all 11 cases with cutaneous anthrax lesions were involved in slaughtering and chopping dead bullock, whereas 43 people involved in washing and cooking were not affected. In another outbreak investigation, two people, who died had sustained deep cut injuries in hands during butchering, while those who consumed cooked meat remained asymptomatic [25].
Handling sick or dead animal or carcasses, consumption of meat, slaughtering and skinning were reported as high risk practices associated with anthrax infection and death [21] (Table 2). Slaughtering, chopping and handling dead bull meat were associated with higher odds of getting infected with anthrax [20]. A qualitative study by Makhija et al. (2020) [24] in the endemic districts of Koraput, Odisha identified risk practices of the local communities such as disposal of carcasses in open air and drying of carcass meat for preservation. Consumption of dead animals was observed even during village get-togethers in endemic districts [49].
Cultural beliefs and practices of the local communities were reported to be contributing to the incidence of anthrax cases. Only 30% people in Koraput, Odisha allowed their animals to be fully vaccinated against anthrax. Makhija et al. (2020) [24] reported specific issues such as choosing traditional treatments and quacks over veterinary healthcare facilities. Celebration of festivals allowing people to hunt and eat bush meat was also underlined.
3.4.2. Socio-economic factors
Ten out of 31 studies reported cases occurring in tribal communities, while three studies recorded cases in farmers or farm laborers. Other occupations included butchering, trading of hide, shepherding and involvement in animal husbandry. A mixed method study in the highly endemic Koraput district, Odisha, revealed that 80% of the population were farmers by occupation, while another 10% were agricultural labourers [24]. Majority of them lived in hilly or forest areas and had strong cultural values and practices [30].
Illiteracy and poor socio-economic status were noted in the communities in endemic regions with frequent anthrax outbreaks. The average monthly income of the tribal communities in Odisha was estimated at INR 1800 (30 US dollars) [19]. A recent cross-sectional survey by Pattnaik et al., [50] highlighted lack of awareness about symptoms and transmission of disease among the livestock owners in the villages of Koraput, Odisha. The district veterinary officials reported that the information, education and communication materials were not effective due to high illiteracy [29].
3.4.3. Environmental factors & isolates
Reported cases were mostly from the eastern coastal regions of India (Fig. 2, Fig. 3). Seasonal trend was observed in the districts of Odisha with predominance of cases during pre-monsoon period of April to June [19,30]. Walsh et al., (2019) [51] modelled the geographical suitability of anthrax across India. The elephant-livestock interface emerged as a dominant feature, while other factors included soil pH, soil-water balance and historical forest loss. Reportedly, anthrax suitability was highest in the regions with a soil pH range of 6–8 and increasing soil–water balance [51].
We located five studies [[52], [53], [54], [55], [56]] reporting isolates of Bacillus anthracis from environmental samples.Two of them were from the state of West Bengal in Panifala hot spring and paper mill sludge. B.anthracis strains were also isolated in samples from Madhya Pradesh,Tamilnadu and Kashmir valley (Supplementary Table 4).
3.4.4. Risk map for anthrax
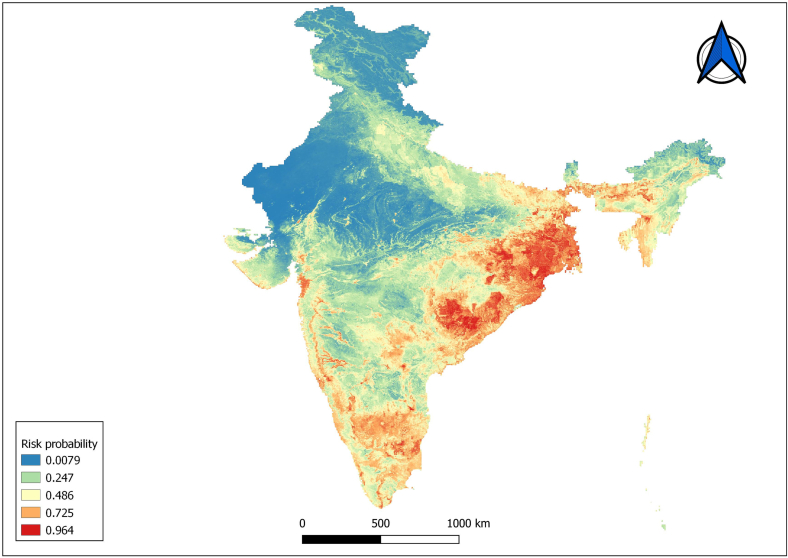
The response curves of selected variables showed optimum conditions for occurrence of human anthrax in India (supplementary Fig. 1) indicating the regions suitable for generation and survival of spores in the environment. The India risk map shows that the risk of human anthrax outbreak is higher in states such as Odisha, southern part of Chattisgarh, southern parts of Jharkhand, most of West Bengal, parts of Sikkim, and western as well as southern parts of Assam (Fig. 4). The other states such as Andhra Pradesh, Telangana, Karnataka, Tamilnadu, Kerala, Goa, Gujarat and Bihar showed a few regions of risk for human anthrax.
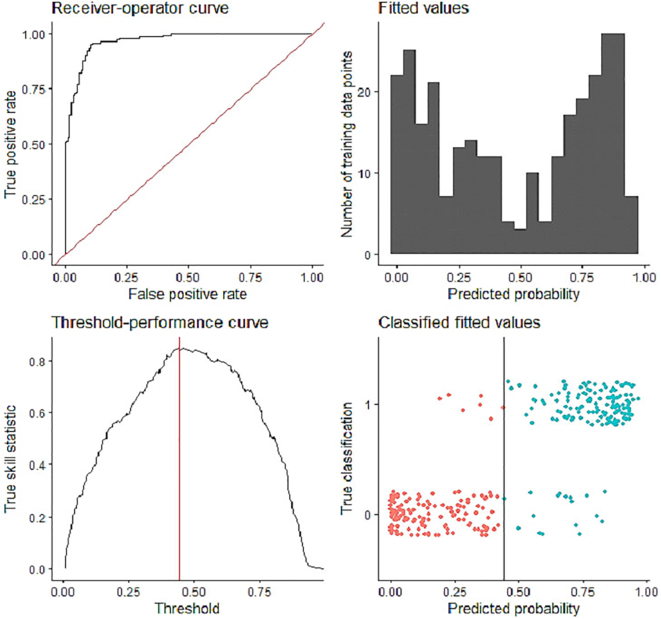
Supplementary Fig. 1.
Model diagnostics to show the model performance.
A high Receiver-Operator Curve, clear split in the predicted probabilities to presence and pseudo-absences shows that model performed adequately.
Fig. 4.
Predictive risk map for human anthrax outbreaks in India depicting the hot spots of anthrax suitability. The colour spectrum of anthrax suitability ranges from blue (low suitability) to red (high suitability). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Programmatic issues
Poor diagnostic facilities at district level and delayed reporting [29,30] were identified as major programmatic challenges (Table 2). Most cases went unreported from hard-to-reach communities since the ground level staff lacked awareness about the disease [29]. Lack of guidance and training among laboratory personnel and sample collectors on safety measures [29], and transportation of samples to far off district laboratories [30] were some of the operational issues.
Lack of appropriate behaviour change communication strategies and awareness activities in the villages were emphasized as gaps by the local communities during focus group discussions in Koraput, Odisha [29]. Although IDSP is an established surveillance system for reporting human anthrax, studies emphasized the need for active surveillance and enhanced reporting [29]. Lack of routine surveillance in livestock was reported by Sahoo et al., 2020 [30], whereas others flagged issues around poor veterinary services with inadequate workforce, delayed reporting and response, and vaccine shortage. Social and cultural barriers to livestock vaccination were other identified gaps [29].
4. Discussion
This systematic review presents clinico-epidemiological, socio-behavioural and programmatic information pertaining to human anthrax in India along with risk mapping. In the process, we synthesised public health issues of relevance, which are integral to discussion around ‘disease elimination science and health’ (DESH) as well as biosafety and biosecurity [57]. Analysis of outbreak investigations and case reports, spanning last two decades, indicated that the occurrences were mostly of cutaneous in nature, while a few sporadic cases of gastrointestinal, inhalational and anthrax meningitis were reported. The gastrointestinal and inhalational type of anthrax had 100% case fatality, while case fatality for cutaneous cases ranged from 2% to 38%. The infection occurred predominantly in men, which could be due to occupational risk-exposure.
Synthesis of evidence on spatial distribution showed that human anthrax outbreaks clustered along the stretch of eastern coastal regions of India. Of particular concern was the state of Odisha from where approximately 62 outbreaks were reported since 2009 with occurrence in almost every year, 4 districts were hotspots (Koraput, Sundergarh, Rayagada, Deogarh); reporting outbreaks regularly. Other states of concern were West Bengal, Andhra Pradesh and Jharkhand. Although several outbreak events of human anthrax were reported from Tamilnadu prior to 2000 [1], no cases featured in IDSP reports except two in 2009 and there was only one case report in 2004 during the past two decades. It would be worthwhile to examine the anthrax control program, reporting mechanism and veterinary services in these states to identify gaps as well as oppurtunities for learning from each other. A time-trend analysis revealed that the number of outbreaks peaked during the years 2015 to 2017. Koraput district in Odisha recorded 33 cases in 2019, indicating continued transmission. Lesser number of cases reported during 2020 to 2021 might reflect either under-reporting or lack of exposure due to restrictions related to the COVID-19 pandemic.
Handling or consumption of sick or dead animals were identified as predominant risk factor reported in all cases. Abbatoir workers, farmers, farm labourers, forest labourers and those dealing with animal products face greater occupational hazard of contracting anthrax disease. Consumption of dead animals was driven by illiteracy, poverty and lack of awareness in many tribal communities living in the hilly and forest areas; traditional beliefs and local practices further added to such risk-exposures. Strategic communication paired with socio-culturally appropriate interventions developed, implemented and monitored through community engagement appear urgent in these settings.
Environmental factors such as soil pH, soil-water balance, organic carbon content, temperature, rainfall and humidity have been identified as important determinants for transmission of anthrax. Noticeably, western India was not affected, which was suggested to be due to low soil pH [37]. The extent of interaction between wildlife and livestock has been suggested as another important contributor to inter-species transmission and subsequent spill-over to humans [51]. Importantly, Krishnamoorthy et al. (2019) [58] suggested conducting periodic regression analysis of time series data of outbreaks to understand the trends, project future outbreaks and to plan appropriate interventions.
In this study, we constructed a risk map for potential anthrax outbreak, which identifed eastern, northeastern and parts of southern regions of the country as high risk zones. This correlated with the spatial distribution data on outbreaks along the eastern and north-eastern states of India, which could be partly explained by the environmental factors. Although occurrence of animal anthrax has been reported from many Indian states [51,58], absence of human anthrax in states where animal anthrax has been prevalent indicates toward differences in socio-behavioural factors, while environmental suitability work as amplifier.
A recent study reported >200 outbreaks of animal anthrax in Karnataka during the study period (1997–2016) [59]. In comparison, we found that there was only one published report (Table 1) of human anthrax in Karnataka (2000 to 2021) and no record in the IDSP database. Pan-India spatial distribution map and construction of risk map for animal anthrax would thus help in understanding the differences between occurrence of human and animal anthrax to inform surveillance planning. Intensive surveillance of animal anthrax and animal vacination in high risk areas are prompted by these findings. Furthermore, screening of environmental samples to detect B. anthracis strains will help map risk zones in endemic regions within India and inform intervention planning.
We enlisted important programmatic issues through this review, which included lack of active surveillance, non-availability of diagnostic facilities at the periphery and delayed reporting. Strengthening laboratory diagnostic facilities in endemic districts, developing point of care tests, and establishing network with public health institutes and community based organizations, therefore appear urgent as part of the preparedness for future outbreaks. Although some studies indicated [29,30] lack of access of poor tribal communities to diagnostic and treatment facilities, this needs in-depth information for future intervention development.
The challenges reported in veterinary sector included absence of routine livestock surveillance, lack of veterinary services and absence of vaccination of livestock in most of the endemic regions. As regular vaccination of ruminants in endemic districts has been demonstrated to reduce the basic reproduction number (R0) of B.anthracis infection in Karnataka, India [60], active vaccination drive among livestock in the vulnerable geographical areas as highlighted through the present synthesis merits immediate policy and program attention [Table 2].
5. Conclusion
Anthrax, in India with frequent outbreaks, occur in the eastern coastal regions of the country. Anthrax risk map constructed during the present synthesis of evidence can help inform future interventions based on one-health approach (human-animal-environment). The hot spots in vulnerable states will help prioritise such disease containment initiatives and resource allocation plan. Intensive and regular surveillance, and reporting of animal and human anthrax can thus be ensured in outbreak prone regions, through setting up of an early warning system. This study also points toward handling or consumption of dead animals as predominant risk factor, and underlines the importance of strategic communication to be paired with community based intervention. Lastly, we emphasize upon the need for training of health care workers, active community engagement, strategic communication, regular animal vaccination and decentralised, innovative as well as establishment of safe diagnostic facilities along with decentralised biological and behavioural survey.
The following are the supplementary data related to this article.
PICOT framework vis a vis anthrax in India in recent times.
Quality assessment of the included studies.
Distribution of anthrax outbreaks across the districts and states in India (IDSP 2009 to 2021).
Studies reporting environmental isolates of Bacillus anthracis.
Ethical consideration
Not Applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Madhumathi Jayaprakasam: Methodology, Investigation, Formal analysis, Writing – original draft. Nabendu Chatterjee: Writing – original draft, Investigation. Mohammed Mudassar Chanda: Formal analysis, Visualization, Software, Writing – review & editing. Sheikh Mohammed Shahabuddin: Investigation. Monil Singhai: Validation, Writing – review & editing. Simmi Tiwari: Writing – review & editing. Samiran Panda: Conceptualization, Formal analysis, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors acknowledge the Integrated Disease Surveillance Program (IDSP), Directorate General of Health Services, Ministry of Health and Family Welfare, Governmnet of India. Authors would like to thank and acknowledge Prof David J Rogers, Professor of Ecology (Retd), Department of Zoology, Oxford University, UK for providing the Fourier processed MODIS data.
Data availability
Data will be made available on request.
References
- 1.Thappa D.M. Cutaneous anthrax – still a reality in India. Ann. Natl. Acad. Med. Sci. (India). 2019;55:119–123. doi: 10.1055/s-0039-1698494. [DOI] [Google Scholar]
- 2.Goel K. Anthrax: a disease of biowarfare and public health importance. World J. Clin. Cases. 2015;3:20. doi: 10.12998/wjcc.v3.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson J., Kracalik I.T., Ross N., Alexander K.A., Hugh-Jones M.E., Fegan M., Elkin B.T., Epp T., Zhang T.K., Shury W., Bagirova M., Getz W.M., Blackburn J.K. The global distribution of bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat. Microbiol. 2019;4:1337–1343. doi: 10.1038/s41564-019-0435-4. [DOI] [PubMed] [Google Scholar]
- 4.Centres for Diseases Control and Prevention. Emergency Preparedness and Response: Bioterrorism Overview. https://emergency.cdc.gov/agent/agentlist-category.asp.(accessed 06 February 2023).
- 5.Bhattacharya D., Kshatri J.S., Choudhary H.R., Parai D., Shandilya J., Mansingh A., Pattnaik M., Mishra K., Padhi S.P., Padhi A., Pati S. One health approach for elimination of human anthrax in a tribal district of Odisha: study protocol. PLoS One. 2021;27 doi: 10.1371/journal.pone.0251041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panda S., Bhargava B., Gupte M.D. One world one health: widening horizons. Indian J. Med. Res. 2021;153:241–243. doi: 10.4103/ijmr.ijmr_1056_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Integrated Disease Surveillance Program (IDSP). Directorate General of Health Services under the Ministry of Health and Family Welfare, Government of India https://idsp.mohfw.gov.in/index4.php?lang=1&level=0&linkid=406&lid=3689. (accessed 06 February 2023).
- 8.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org. (accessed 06 February 2023).
- 9.National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed 06 February 2023)
- 10.Joanna Briggs Institute (JBI) Critical Appraisal Tools. 2016. https://joannabriggs.org/ (accessed 06 February 2023)
- 11.Moralejo D., Ogunremi T., Dunn K. Critical appraisal tool kit (CAT) for assessing multiple types of evidence. Can. Commun. Dis. Rep. 2017;43:176–181. doi: 10.14745/ccdr.v43i09a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L.L., Wang Y.Y., Yang Z.H., Huang D., Weng H., Zeng X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil. Med. Res. 2020;7:7. doi: 10.4103/ijmm.IJMM_19_111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.QGIS Geographic Information System. QGIS Association. http://www.qgis.org. (accessed 06 February 2023).
- 14.Kalluri S., Gilruth P., Rogers D., Szczur M. Surveillance of arthropod vector-borne infectious diseases using remote sensing techniques: a review. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson C.J. Embarcadero: species distribution modelling with Bayesian additive regression trees in R. Methods Ecol. Evol. 2020;11:850–858. doi: 10.1111/2041-210X.13389. [DOI] [Google Scholar]
- 16.Scharlemann J.P.W., Benz D., Hay S.I., Purse B.V., Tatem A.J., Wint G.R.W., Rogers D.J. Global data for ecology and epidemiology: a novel algorithm for temporal Fourier processing MODIS data. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Agricultural Organization of the United Nations https://www.fao.org/livestock-systems/global-distributions/en/ (accessed 06 February 2023)
- 18.Balk D.L., Deichmann U., Yetman G., Pozzi F., Hay S.I., Nelson A. Determining global population distribution: methods, applications and data. AdvParasitol. 2006;62:119–156. doi: 10.1016/S0065-308X(05)62004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayak P., Sodha S.V., Laserson K.F., Padhi A.K., Swain B.K., Hossain S.S., Shrivastava A., Khasnobis P., Venkatesh S.R., Patnaik B., Dash K.C. A cutaneous Anthrax outbreak in Koraput District of Odisha-India 2015. BMC Public Health. 2019;19:470. doi: 10.1186/s12889-019-6787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayak P. European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE) 2015. 2016. Outbreak investigation of anthrax, Simdega, Jharkhand, India – 2014.https://www.escaide.eu/sites/default/files/documents/escaide-2015-abstract-book.pdf (accessed 06 February 2023) [Google Scholar]
- 21.Reddy R., Parasadini G., Rao P., Uthappa C.K., Murhekar M.V. Outbreak of cutaneous anthrax in Musalimadugu village, Chittoor district, Andhra Pradesh, India, July-august 2011. J. Infect. Dev. Ctries. 2012;6:695–699. doi: 10.3855/jidc.2635. [DOI] [PubMed] [Google Scholar]
- 22.Ray T.K., Hutin Y.J., Murhekar M.V. Cutaneous Anthrax, West Bengal, India, 2007. Emerg. Infect. Dis. 2009;15:497–499. doi: 10.3201/eid1503.080972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achar, Satpathi P.S., Mukherjee P., Bera G.C., Satpathi S. Sporadic outbreaks of cutaneous anthrax: a tip of the iceberg. Ann. Trop. Med. Public Health. 2018;11:125. doi: 10.4103/atmph.Atmph_12_17. [DOI] [Google Scholar]
- 24.Makhija S., Kumar S., Hossain S. Risk factor associated with anthrax transmission among the tribal communities of Odisha. Indian J. Public Health Res. Dev. 2020;11:603–608. doi: 10.37506/v11/i1/2020/ijphrd/193889. [DOI] [Google Scholar]
- 25.Chakraborty P.P., Thakurta S.G., Satpathi P.S., Hansda S., Sit S., Achar A., Banerjee D. Outbreak of cutaneous anthrax in a tribal village: a clinico-epidemiological study. J. Assoc. Physicians India. 2012;60:89–93. (PMID: 22715553) [PubMed] [Google Scholar]
- 26.Dandapat P., Chakrabarty A., Dey S., Nanda P.K., Das S.C., Dey S., Kurien A., Chakraborty A., Bandyopadhyay S., Bandyopadhyay S., Singh R.K. Epidemiological and laboratory investigation of a zoonotic anthrax outbreak in West Bengal, India. Asian Pac. J. Trop. Dis. 2017;7:653–658. doi: 10.12980/apjtd.7.2017D7-62. [DOI] [Google Scholar]
- 27.Mondal T.K., Ghosh S., Dasgupta S., Sarkar A.P. Suspected anthrax outbreak: investigation in a rural block of West Bengal and public health response. Indian J. Public Health. 2015;59:302–305. doi: 10.4103/0019-557X.169662. [DOI] [PubMed] [Google Scholar]
- 28.Narayan S.K., Sreelakshmi M., Sujatha S., Dutta T.K. Anthrax meningoencephalitis--declining trends in an uncommon but catastrophic CNS infection in rural Tamil Nadu, South India. J. Neurol. Sci. 2009;281:41–45. doi: 10.1016/j.jns.2009.02.376. [DOI] [PubMed] [Google Scholar]
- 29.Mansingh A., Choudhary H.R., Shandilya J., Bhattacharya D., Kshatri J.S., Parai D., Pattanaik M., Padhi A.K., Jain H.K., Mohanty P., Kanungo S., Pati S. A qualitative exploratory study using one health approach for developing an intervention package for elimination of human anthrax in an endemic district of Odisha, India. Indian J. Med. Res. 2021;153:394–400. doi: 10.4103/ijmr.IJMR_646_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahoo K.C., Negi S., Barla D., Badaik G., Sahoo S., Bal M., Padhi A.K., Pati S., Bhattacharya D. The landscape of Anthrax prevention and control: Stakeholders’ perceptive in Odisha, India. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17093094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thappa D.M., Karthikeyan K. Cutaneous anthrax: an Indian perspective. Indian J. Dermatol. Venereol. Leprol. 2002;68:316–319. (PMID: 17656987) [PubMed] [Google Scholar]
- 32.Dutta T., Sujatha S., Sahoo R.K. Anthrax - update on diagnosis and management. J. Assoc. Physicians India. 2011;59:573–578. (PMID: 22334971) [PubMed] [Google Scholar]
- 33.Datta K.K., Singh J. Anthrax. Indian J. Pediatr. 2002;69:49–56. doi: 10.1007/BF02723777. [DOI] [PubMed] [Google Scholar]
- 34.Patil R.R. Anthrax: public health risk in India and socio-environmental determinants, Indian. J. Commun. Med. 2010;35:189–190. doi: 10.4103/0970-0218.62573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bindu M., Vengamma B., Kumar G. Anthrax meningoencephalitis successfully treated. Eur. J. Neurol. 2007;14 doi: 10.1111/j.1468-1331.2007.01868.x. [DOI] [PubMed] [Google Scholar]
- 36.Deb S., Samanta A.B. A case of cutaneous anthrax presenting with classical lesions. Indian J. Dermatol. 2015;54:e539–e541. doi: 10.1111/ijd.13090. [DOI] [PubMed] [Google Scholar]
- 37.Garg N., Panmei K. First reported case of naturally acquired fatal anthrax from Northeast India. J. Family Med. Prim. Care. 2018;7:632–634. doi: 10.4103/jfmpc.jfmpc_111_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichhpujani R.L., Rajagopal V., Bhattacharya D., Rana U.V.S., Mittal Veena, ArvindRai, Ravishankar A.G., Pasha S.T., JotnaSokhey S. Biswas. An outbreak of human anthrax in Mysore (India) J. Commun. Disord. 2004;36:199–204. (PMID: 16509258) [PubMed] [Google Scholar]
- 39.Rao R.R., Padmaja J., Lalitha M.K., Rao P.V.K., Gopal K.V.T., Kumar H.K.Y., Mohanraj P. An outbreak of cutaneous anthrax in a non-endemic district--Visakhapatnam in Andhra Pradesh. Indian J. Dermatol. Venereol. Leprol. 2005;71:102–105. doi: 10.4103/0378-6323.13994. [DOI] [PubMed] [Google Scholar]
- 40.Rao R., Padmaja J., Lalitha M.K., Rao P.V., Kumar H.K., Gopal K.V., Jaideep M., Mohanraj P. Cutaneous anthrax in a remote tribal area--Araku Valley, Visakhapatnam district, Andhra Pradesh, southern India. Int. J. Dermatol. 2007;46:55–58. doi: 10.1111/j.1365-4632.2006.03043.x. [DOI] [PubMed] [Google Scholar]
- 41.Rao T.N., Venkatachalam K., Ahmed K., Padmaja I.J., Bharthi M., Rao P.A. A mini-outbreak of cutaneous anthrax in Vizianagaram District, Andhra Pradesh, India. Indian J. Dermatol. Venereol. Leprol. 2009;75:416–418. doi: 10.4103/0378-6323.53158. [DOI] [PubMed] [Google Scholar]
- 42.Suggu S., Konakanchi V.C. Cutaneous anthrax in a tribal man: a case report. Postgrad. Med. J. 2021;97:744–745. doi: 10.1136/postgradmedj-2020-138686. [DOI] [PubMed] [Google Scholar]
- 43.Velayudhan M.N., Nalini P., Kanungo R., Shashikala T.A., Cherian S. Srinivasan. Inhalational anthrax with acute respiratory distress syndrome. Ann. Trop. Paediatr. 2009;25:49–52. doi: 10.1179/146532805X23362. [DOI] [PubMed] [Google Scholar]
- 44.Vijaikumar M., Thappa D.M., Jeevankumar B. Cutaneous anthrax: still a reality in India. Pediatr. Dermatol. 2001;18:456–457. doi: 10.1046/j.1525-1470.2001.1983d.x. [DOI] [PubMed] [Google Scholar]
- 45.Balachandrudu B., Bindu S.S.A., Kumar C.N., Malakondaiah P. An outbreak of cutaneous anthrax in a tribal area of Visakhapatnam district, Andhra Pradesh. J. Dr. NTR Univ. Health Sci. 2018;7:49–53. doi: 10.4103/jdrntruhs.Jdrntruhs_81_17. [DOI] [Google Scholar]
- 46.Kumar A., Raju B.T.V.N., Vardhan K.R.H., Prasad P.G. An outbreak of cutaneous anthrax in tribal areas of Visakhapatnam. J. Evol. Med. Dent. Sci. 2016;5:4378–4381. doi: 10.14260/jemds/2016/999. [DOI] [Google Scholar]
- 47.Iqbal N., Basheer A., Ramesh A.N., Vimal J., Mookkappan S., Kanungo R., Anandhalakshmi I. Princess. Gastrointestinal anthrax in coastal South India: a critical alert on a fatal masquerader. JMM Case Rep. 2015;2 doi: 10.1099/jmmcr.0.000013. [DOI] [Google Scholar]
- 48.Kumar M., Seema K., Prasad A., Sharma A.K., Sherwal B.L. Molecular confirmation of the circulating bacillus anthracis during outbreak of anthrax in different villages of Simdega District, Jharkhand. Indian J. Med. Microbiol. 2019;37:116–119. doi: 10.4103/ijmm.IJMM_19_111. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharya D., Barla D., Pati S. ICMR-Regional Medical Research Centre; Bhubanewar: 2019. How Can Anthrax Outbreaks Be Prevented in Odisha? [Google Scholar]
- 50.Pattnaik M., Kshatri J.S., Choudhary H.R., Parai D., Shandilya J., Mansingh A., Padhi A.K., Pati S., Bhattachary D. Assessment of socio-behavioural correlates and risk perceptions regarding anthrax disease in tribal communities of Odisha, Eastern India. BMC Infect. Dis. 2022;22:53. doi: 10.1186/s12879-022-07035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh M.G., Mor S.M., Hossain S. The elephant–livestock interface modulates anthrax suitability in India. Proc. Biol. Sci. 2019;286:20190179. doi: 10.1098/rspb.2019.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aarti C., Khusro A., Agastian P., Darwish N.M., Farraj D.A.A. Molecular diversity and hydrolytic enzymes production abilities of soil bacteria. Saudi J. Biol. Sci. 2020;27:3235–3248. doi: 10.1016/j.sjbs.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee A., Halder U., Chaudhry V., Varshney R.K., Mantri S., Bandopadhyay R. Draft genome sequence of the nonpathogenic, Thermotolerant, and exopolysaccharide-producing bacillus anthracis strain PFAB2 from Panifala hot water spring in West Bengal, India. Genome Announc. 2016;4 doi: 10.1128/genomeA.01346-16. e01346–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dixit S.J., Kutan A.K.K., Shrivastava R.M. Genetic characterization of Sulphur and iron oxidizing bacteria in manganese mining area of Balaghat and Chhindwara, Madhya Pradesh, India. Indian. J. Biotechnol. 2018;17:595–601. [Google Scholar]
- 55.Ganguly R.K., Midya S., Chakraborty S.K. Antioxidant and anticancer roles of a novel strain of bacillus anthracis isolated from vermicompost prepared from paper mill sludge. Biomed. Res. Int. 2018;2018:1073687. doi: 10.1155/2018/1073687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shafi S., Kamili A.N., Shah M.A., Bandh S.A., Dar R. Dynamics of bacterial class Bacilli in the deepest valley lake of Kashmir-the Manasbal Lake. Microb. Pathog. 2017;104:78–83. doi: 10.1016/j.micpath.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Panda S. Looking back to move forward: a travel rule underlined by the current pandemic. Int. J. Public Health. 2022;66:403–406. doi: 10.4103/ijph.ijph_1513_22. [DOI] [PubMed] [Google Scholar]
- 58.Krishnamoorthy P., Kurli R., Patil S.S., Roy P., Suresh K.P. Trends and future prediction of livestock diseases outbreaks by periodic regression analysis. Indian J. Anim. Sci. 2019;89:369–376. [Google Scholar]
- 59.Chanda M.M., Prajapati A., Yogisharadhya R., L U., Palegar M.S., Hemadri D., Shome B.R., Shivachandra S.B. Elevation determines the spatial risk of Anthrax outbreaks in Karnataka, India. Acta Trop. 2023;240 doi: 10.1016/j.actatropica.2023.106848. [DOI] [PubMed] [Google Scholar]
- 60.Krishnamoorthy P., Suresh K.P., Dheeraj R., Roy P. Basic reproduction number (R0), an epidemiological tool for prioritizing livestock diseases—an example of Karnataka. Indian J. Anim. Sci. 2020;90:510–514. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PICOT framework vis a vis anthrax in India in recent times.
Quality assessment of the included studies.
Distribution of anthrax outbreaks across the districts and states in India (IDSP 2009 to 2021).
Studies reporting environmental isolates of Bacillus anthracis.
Data Availability Statement
Data will be made available on request.