Abstract
Background
Leptospirosis, which is an easily overlooked zoonotic disease, was once widespread in Guangzhou, China. However, due to the implementation of control measures, the number of cases is decreasing. Based on the characteristics of leptospirosis cases in Guangzhou, China, between 1955 and 2020, we describe the changes and achievements in prevention and control management strategies over that period.
Methods
The development of the leptospirosis control system in Guangzhou occurred over three periods: Period I: 1955–1978; Period II: 1979–2000; and Period III: 2001–2020. Data about leptospirosis cases were obtained from the Guangzhou Center for Disease Control and Prevention (CDC) and national health departments. The demographic characteristics of leptospirosis patients were analyzed using descriptive statistics.
Results
During Period I, only the Guangzhou CDC and medical institutions at every level participated in the leptospirosis control system. During Period II, additional types of organizations, including local CDCs, countryside committees, community committees, and the Patriotic Health Movement Commission, were involved in the control system. Additionally, strong links were established between different organizations. After entering Period III, an increasing number of departments joined the cooperation, and the management of human patients was expanded to include the management of host animals, and thus, the prevalence of leptospirosis was monitored and controlled in various ways. The leptospirosis control system in Guangzhou has been further improved. From 1955 to 2020, a total of 2501 leptospirosis cases were recorded in Guangzhou, and the number of cases decreased significantly over time, from 1608 (Period I) to 744 (Period II) and then to 149 (Period III).
Conclusion
The improvements of the leptospirosis control system in Guangzhou that occurred over decades were associated with a marked decrease in the number of leptospirosis cases. Guangzhou's experience can provide guidance for other countries or cities around the world facing similar challenges.
Keywords: Leptospirosis, Zoonotic disease, Epidemiology, Control strategies, Public health
Highlights
-
•
Leptospirosis cases control and surveillance;and host animals management.
-
•
The establishment of leptospirosis system involves various organizations.
-
•
Various organizations adopt different control strategies.
-
•
There is long-term,close cooperation and communication between these organizations.
1. Introduction
Leptospirosis is a widespread zoonotic disease caused by pathogenic Leptospira bacteria [1]. The host animals of Leptospira may be domestic animals (such as pigs, cattle, and dogs), various rodents (mainly rats), and some wild animals (such as bats) [2,3]. When these animals become infected, Leptospira can be parasitized in the kidneys and excreted intermittently via the urine and thus enter soil and water bodies. Humans are susceptible to leptospirosis when they come into direct contact with the urine of Leptospira-carrying animals or when they come into contact with contaminated soil and water bodies [4,5]. In humans, leptospirosis usually has a biphasic clinical presentation. The first phase is the septicemic phase, which is characterized by nonspecific symptoms such as high fever, headache, weakness, chills, and myalgia. The septicemic phase is followed by an immune phase, during which severe dysfunction of multiple organs, such as the liver, kidney, and lungs, may occur [6,7]. Mild cases of leptospirosis rarely result in death; however, in severe cases (diagnosed in 5–10% of patients), mortality can reach 5–40% [8]. Leptospira survive in warm and humid environments for weeks to years, and tropical and subtropical countries with abundant rainfall and hot climates are areas with high incidence [9,10]. Approximately 1 million cases of human leptospirosis are reported each year worldwide, and approximately 60,000 people die from the infection [11]. The global burden of leptospirosis was estimated to be 2.90 million disability adjusted life years (DALYs) per year [12]. As one of the most neglected zoonotic diseases, leptospirosis causes an enormous disease burden and health challenge worldwide.
In China, the earliest records of leptospirosis date back to the 1930s. Before the 1970s, a dozen outbreaks of leptospirosis occurred in China, causing high morbidity and mortality [13]. Due to improved hygiene, the average annual incidence significantly decreased after the 1970s, from 11.31/100000 population in the 1970s to 5.04/100000 in the 1980s and 2.59/100000 in the 1990s. After entering the new century, the incidence of leptospirosis in China has continued to decrease (0.59/100000) [13]. Guangzhou city also shared the same long-term trend towards decreasing leptospirosis incidence. In the last century, to reduce the prevalence of leptospirosis, relevant departments and organizations in Guangzhou implemented strategies to prevent human infection and control the disease. With advances in prevention and control management, Guangzhou has formed a relatively complete leptospirosis control system, and the number of leptospirosis cases has been greatly reduced.
We divided the development of the leptospirosis control system in Guangzhou into three periods by year: Period I: 1955 (after leptospirosis was listed as a notifiable infectious disease in China) to 1978, Period II: 1979 (after China's reform and opening up) to 2000, and Period III: 2001 (after the 21st century) to 2020. The purpose of this paper is to review the leptospirosis prevention and control strategies implemented in Guangzhou over the three periods and to provide guidance for other countries or cities around the world facing similar challenges.
2. Methods
2.1. Study area
Guangzhou, located in southern China (23°N, 113°E), is one of China's megacities(Fig. 1). Covering an area of 7434.4 km2, the city has 11 areas under its jurisdiction, including five urban administrative areas (Tianhe, Yuexiu, Liwan, Haizhu, and Huangpu), four rural administrative areas (Panyu, Nansha, Baiyun and Huadu) and two satellite towns (Zengcheng and Conghua). The population was approximately 3.4 million in 1955 and 18.7 million in 2020. The type of climate in Guangzhou is a marine subtropical monsoon climate, with an annual average temperature of 21.5–22.2 °C. The rainy season lasts from April to August, and the average annual rainfall is approximately 1800 mm. The city is also located in a hilly area, with a developed river system and a vast water area. Due to favorable climate and environmental conditions, most areas in Guangzhou are suitable for agriculture, primarily rice plantation and animal husbandry.
Fig. 1.
Administrative divisions, Guangzhou, China, 2020.
Note: The city of Guangzhou comprises five urban districts (white), four rural districts (light blue) and two satellite towns (deep blue).
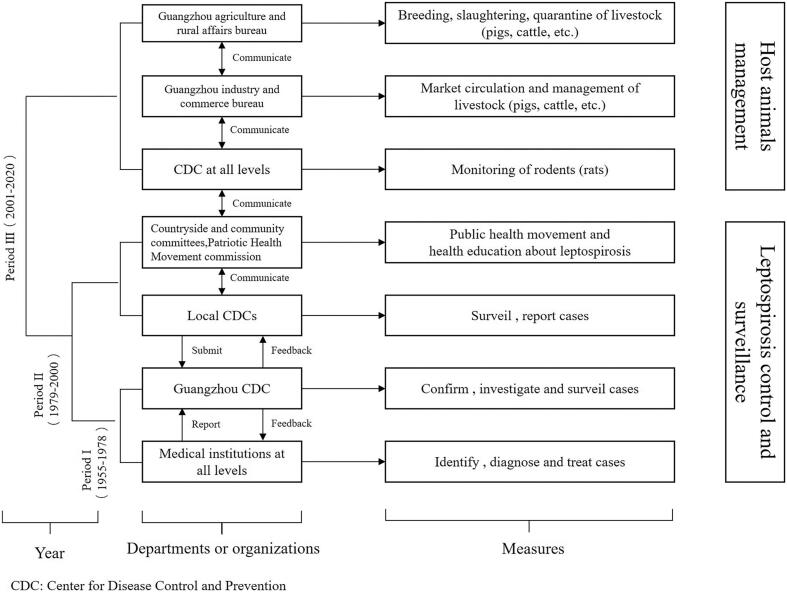
The current leptospirosis control system in Guangzhou was developed and improved over decades and includes two parts: leptospirosis control and surveillance and host animals management (Fig. 2). The Guangzhou Center for Disease Control and Prevention (CDC) is primarily responsible for confirming the diagnosis of leptospirosis in humans and for carrying out epidemiological investigations and surveillance of leptospirosis cases. Medical institutions at all levels are responsible for identifying the types of diseases and diagnosing and treating infected patients. Local centers for disease control and prevention are responsible for case monitoring and reporting in the region and record the details of exposed people when they seek medical help. To improve living conditions and promote public health, local countryside committees, community committees, and the Patriotic Health Movement commission help to educate susceptible populations and conduct public health activities. Considering the variety of host animals, the Guangzhou agriculture and rural affairs bureau is responsible for the breeding, slaughtering and quarantine of livestock (pigs, cattle, etc.), and the Guangzhou industry and commerce bureau is responsible for the circulation and management of livestock in the market. Moreover, CDCs at all levels in Guangzhou monitor the density and Leptospira-carrying rate of rodents (mainly rats) in the corresponding areas. There is long-term, regular, close cooperation and communication between these organizations.
Fig. 2.
Development of leptospirosis control system, by period, Guangzhou, China, 1955–2020.
2.2. Case definition
The definition of leptospirosis follows the standard diagnostic guidelines set by the National Health Commission of the People's Republic of China [14]. Suspected cases are those who have been in contact with contaminated water or the blood or urine of infected animals 1 to 30 days before the onset of the disease and manifested symptoms such as fever, muscle soreness, or asthenia. Clinically diagnosed cases are confirmed when the suspected cases presented with symptoms of lymphadenopathy, gastrocnemius tenderness, or conjunctival hyperemia. Laboratory-confirmed cases are defined when the suspected cases met any of the following conditions: (i) isolation of Leptospira bacteria from patient blood,urine or cerebrospinal fluid; (ii) isolation of Leptospira nucleic acid from patient blood, urine or cerebrospinal fluid; (iii) titers of Leptospira antibodies in convalescent patient serum that are 4 times or more higher than those in early serum samples or a titer of a single serum antibody ≥1:400.
2.3. Data analysis
All clinically diagnosed and laboratory-confirmed leptospirosis cases were included in this study. All data on leptospirosis cases were obtained from the Guangzhou Municipal Health Statistical Yearbook (Guangzhou Center for Disease Control and Prevention) and the National Notifiable Infectious Disease Reporting System (Chinese Center for Disease Control and Prevention). Demographic data were obtained from the Guangzhou Statistical Yearbook (Guangzhou Municipal Bureau of Statistics). The annual average incidence of leptospirosis was estimated using the annual average population data provided by the Guangzhou Municipal Bureau of Statistics. Age, sex, occupation, area of diagnosis, date of diagnosis, and date of death (if applicable) were extracted for each patient. All personally identifiable data were removed from the dataset prior to analysis. Initial data were entered into the database using EpiData Entry v. 3.1 (EpiData, Odense, Denmark) and then analyzed using Microsoft Excel 2019 and R v. 4.0.1 (R Foundation, Vienna, Austria).
3. Results
3.1. Leptospirosis cases
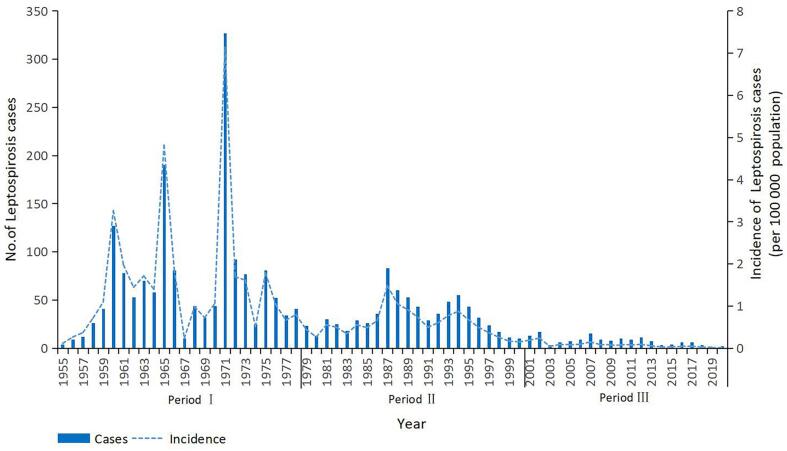
From 1955 to 2020, a total of 2501 cases of leptospirosis were recorded in Guangzhou (Fig. 3). And a total of 1608 cases were recorded during Period I, which accounted for 64.3% (1608/2501) of the cases in the entire study period. The first peak occurred in 1960, with a total of 127 cases. After that, the number of cases declined until a second peak (190 cases) occurred in 1965. The largest outbreak occurred in 1971, with 327 cases, and the annual incidence reached 7.14/100000 population. A total of 744 leptospirosis cases were recorded during Period II. After entering Period II, the number of cases dropped significantly compared with the number before Period II, and the number of cases remained steady at dozens of cases but not >100 cases each year. The annual incidence also showed a downward trend year by year, with occasional small outbreaks (in 1987 and 1994). In 2001, the number of leptospirosis cases decreased to 13, and sporadic cases occurred in Period III. Especially after 2010, the annual number of cases did not exceed 10, and among these years, the number of cases in 2019 was lowest, with only 1 case. A total of 149 cases were recorded during Period III, which was in sharp contrast with Periods I and II. In addition, we also counted the number of deaths in each period, and the result showed 70 deaths during Period I, 77 deaths during Period II, and 5 deaths during Period III.
Fig. 3.
Leptospirosis cases, Guangzhou, China, 1955–2020.
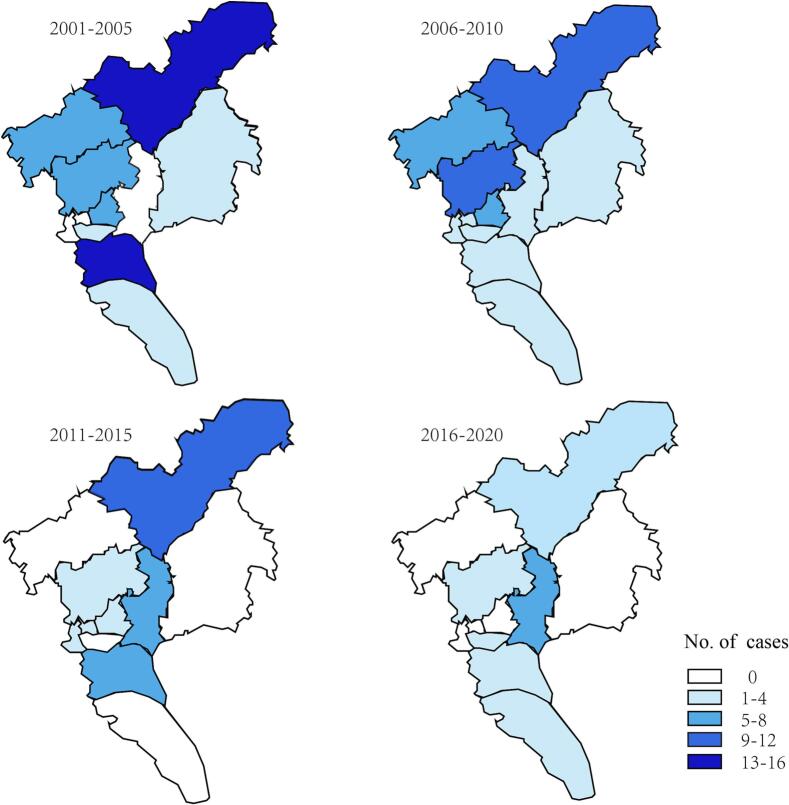
According to the detailed data of leptospirosis cases from the Infectious Disease Reporting System, we analyzed the geographical distribution of leptospirosis cases in Guangzhou during Period III (Fig. 4). Between 2001 and 2020, the proportion of leptospirosis cases in rural areas was 70.5% (105/149), compared with 29.5% (44/149) in urban areas. From 2001 to 2005, the highest number of cases was in the Conghua and Panyu districts, both with 13 cases. From 2006 to 2010, there were sporadic cases in all regions. The regions with the fewest cases were the Haizhu and Nansha districts (both with 1 case), and that with the highest number of cases was the Conghua district (12 cases). Between 2011 and 2015, the number of cases in Guangzhou decreased to 34, but the proportion of cases in rural areas was still higher than that in urban areas, at 61.8% (21/34). Between 2016 and 2020, only 18 cases were reported in Guangzhou, of which the number of cases in several urban areas was 0, and the number of cases in rural areas decreased significantly, with a total of no >9 cases. Over the 20 years, the highest number of leptospirosis cases tended to occur in northern rural areas, especially the Conghua district. However, the number of cases across the city continued to decline over time, with no cases being reported in some rural or urban areas.
Fig. 4.
Geographical distribution of leptospirosis cases, Guangzhou, China, 2001–2020.
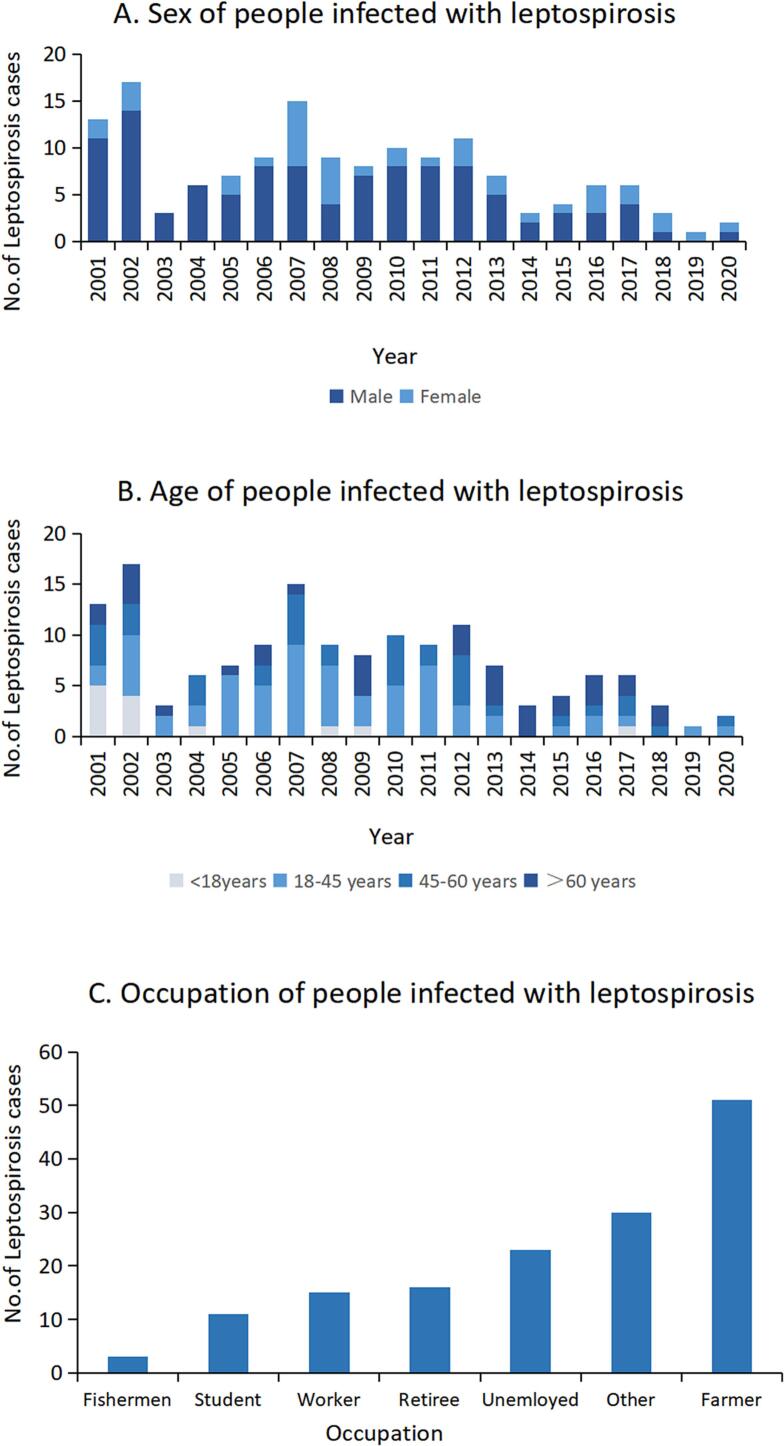
We also analyzed the demographic characteristics of leptospirosis patients during Period III (Fig. 5). There was an obvious gender difference in incidence between males (109 cases) and females (40 cases), with a sex ratio of 2.7:1 (Fig. 5A). Among the 149 leptospirosis patients, the number of infected people in the 18- to 45-year-old age group was greater than that in the other age groups, accounting for 43.0% (64/149). The numbers in the 45- to 60-year-old age group followed, accounting for 25.5% (38/149). The number of infected people in the over 60-year-old age group accounted for 22.8% (34/149), and the number in the age group under 18 was the lowest, accounting for only 8.7% (13/149) (Fig. 5B). The occupational distribution characteristics showed that the highest number of leptospirosis cases occurred in farmers (Fig. 5C). Unemployed people (mainly those who work at home or are waiting for job assignment), retirees, and workers (mainly abattoirs and sewage workers) were also the main occupational groups who were prone to leptospirosis.
Fig. 5.
Demographic characteristics of leptospirosis cases, Guangzhou, China, 2001–2020.
3.2. Development of leptospirosis control system
3.2.1. Period I
In 1934, Chinese scholars discovered cases of leptospirosis in Guangzhou for the first time, thus confirming the presence of leptospirosis in China. In 1955, leptospirosis was officially listed as one of China's national notifiable infectious diseases, and it was a category B infectious disease [13]. From 1955 to 1978, in accordance with the regulations of the national health departments [15], doctors in medical institutions at every level in Guangzhou had to report to the Guangzhou Center for Disease Control and Prevention within 24 h when they found a suspected case. Subsequently, the suspected patient was given a second and confirmatory diagnosis by the Guangzhou CDC. During Period I, only medical institutions and the Guangzhou CDC were involved in the management of leptospirosis (Fig. 2). Medical institutions at every level, including small health centers in rural areas and large hospitals in urban areas, were responsible for identifying, clinically diagnosing, and treating patients and reporting the cases to the Guangzhou CDC. The Guangzhou CDC took charge of cases verification, investigation of patients, and cases surveillance.
3.2.2. Period II
With the addition of new organizations and the implementation of new measures, the number of leptospirosis cases in this stage decreased significantly compared with the previous stage from 1979 to 2000. In addition to the Guangzhou CDC, the Guangzhou government has also established some local centers for disease control and prevention in various administrative regions to implement the monitoring and reporting of cases to more specific areas (Fig. 2). The local CDCs established close ties with countryside committees, community committees, and the Patriotic Health Movement commission. These civil society groups carried out extensive health education activities on leptospirosis and actively organized the Patriotic Health Movement. The Patriotic Health Movement is a public health movement advocated by the Chinese government with the goal to “prevent and reduce disease, protect people's health”, which reflected the distinctive features of China's health work [16,17]. In this movement, urban areas focused on addressing environmental sanitation problems caused by industrialization to improve the people's living environment. Rural areas focused on water sanitation and toilet reinvention. Ensuring the safety of drinking water and establishing a whole-process supervision system from water source protection and tap water production to a safe water supply could help reduce the chances of exposure to leptospirosis. The construction of safe public toilets could be conducive to scientifically reducing the harm caused by vector organisms. Moreover, the Patriotic Health Movement commission regularly organized the work of eradication of four hazards (flies, mosquitoes, rats, and cockroaches), and the decrease in the number of rodents correspondingly reduced the probability of leptospirosis transmission. Since the launch of the Patriotic Health Movement, the urban and rural environmental sanitation conditions in Guangzhou have been significantly improved. In addition, since China's reform and opening up in 1978, the economic level and living standards have continuously improved, and people's awareness of disease prevention and control has continuously increased [18,19]. Coupled with the effective prevention and control measures taken by some departments or organizations, the annual number of leptospirosis cases in Guangzhou continues to decline.
3.2.3. Period III
The leptospirosis control system has further improved after entering the 21st century. Owing to the reduction in the number of cases, the epidemic intensity has become sporadic. Period III mainly focused on leptospirosis monitoring. The Guangzhou CDC was responsible for the organization, implementation, training and guidance of the monitoring work. Through regular collection, analysis and feedback of monitoring data, Guangzhou CDC conducted supervision, inspection and quality assessment once a year and generated an annual summary of the monitoring work. The local CDCs were responsible for investigating leptospirosis cases and fulfilling various monitoring tasks. Furthermore, local CDCs received technical training and guidance from the superior CDC and collected, organized, summarized and reported monitoring data regularly. Medical institutions at every level cooperated with all CDCs to collect specimens from outpatient and inpatient cases. At the same time, Period III added management of host animals on the basis of Period II and Period I (Fig. 2). Considering that rodents are the main host animals of Leptospira [20,21], the national health department issued some relevant monitoring plans, requiring CDC at all levels to investigate and monitor the species, distribution, density and Leptospira-carrying rate of rats during the epidemic season. Since some livestock (such as pigs and cattle) were proven to be carriers of Leptospira [22], Guangzhou has also strengthened the production and management of such livestock and their meat products to prevent mutual infection between livestock or transmission to humans. According to management plans proposed by the Guangzhou agriculture and rural affairs bureau, livestock farms were required to fodder livestock with feeds containing Leptospira-killing antibiotics and immunize livestock by vaccination at regular intervals. In addition, the Guangzhou agriculture and rural affairs bureau was also responsible for supervising the slaughter and quarantine of livestock. And the Guangzhou industry and commerce bureau took charge of managing the market circulation of livestock and their meat products. To date, multiple departments or organizations in Guangzhou have jointly established a complete leptospirosis control system.
4. Discussion
Over the years, the establishment of this leptospirosis control system has involved the participation of an increasing number of organizations, illustrating the importance of multisectoral collaboration. Additionally, prevention and control management reflects the concept of “one health” to a certain extent. Advocated by the Food and Agriculture Organization of the United Nations (FAO), the World Organization for Animal Health (OIE) and the World Health Organization (WHO), One Health is an integrated health strategy focused on human health, environmental health and animal health [23,24]. In this leptospirosis control system, the management of patients by CDCs and medical institutions at all levels and a series of health education activities show care for human health. Other organizations conduct patriotic health movements and strengthen host animal management while safeguarding environmental health and animal health. Multidisciplinary and multisectoral collaborations help to solve complex problems at the human-animal-environment interface [25].
Although the epidemic intensity of leptospirosis in Guangzhou is mainly sporadic at this stage, the following challenges for completely eliminating the threat of leptospirosis remain: (i) As a zoonotic natural foci disease, leptospirosis is widely distributed around the world, with various types of Leptospira species and a wide variety of reservoirs. The Leptospira-carrying rate of host animals and the numbers of people with silent infections cannot be ignored [26,27]. (ii) Leptospirosis often presents as an acute undifferentiated fever disease, and its presentation is similar to that of other zoonotic diseases, such as malaria and dengue fever [28,29]. It could be easily missed or misdiagnosed when doctors have limited diagnostic ability or lack awareness of these zoonotic diseases. (iii) Summer and autumn are the rice harvesting seasons in Guanghou, as well as the peak epidemic periods of leptospirosis [30]. Because leptospirosis has an obvious seasonal high incidence, once the epidemic conditions are met, such as suitable temperature, frequent rainfall, flood disasters, and lack of protection for susceptible people, outbreaks may occur [31,32]. (iv) There is a lack of awareness of the disease and limited preventive measures in the population. In particular, high-risk groups (such as farmers and fishermen) are prone to infection after exposure to suspected pathogenic factors without proper protection.Vaccination may be the most effective measure to prevent and control the epidemic of leptospirosis, but the duration of protection against leptospirosis by vaccines at this stage is short, and the number of vaccinated people is low [33,34]. In addition to vaccine prophylaxis, penicillin and doxycycline can also be used as drug prophylaxis. However, it has been reported that the use of antibiotics does not prevent leptospirosis infection but may have a significant protective effect in reducing morbidity and mortality [2].
One limitation of this study was that only the recorded leptospirosis cases were analyzed. The number of people infected with leptospirosis was probably underestimated because many of them may manifest subclinical symptoms or very mild symptoms and do not seek medical help. Consequently, promoting knowledge of leptospirosis among the general population is still quite necessary.
Although the leptospirosis control system in Guangzhou has achieved good results in reducing the prevalence of leptospirosis, these departments or organizations should improve disease prevention and control strategies on the basis of the original ones in the future. The diagnostic capabilities of medical staff should be strengthened, and health education for high-risk populations should be further developed. A long-term program for monitoring and managing host animals should be conducted and implemented. Furthermore, environmental departments should be incorporated into the leptospirosis control system to jointly protect environmental health.
5. Conclusions
For all these years, the incidence of leptospirosis in Guangzhou has been maintained at a low level, which is inseparable from the active application of preventive measures and the concerted cooperation of various departments. The application of leptospirosis prevention and control management in Guangzhou can also provide experience and guidance for countries or regions around the world where leptospirosis is endemic.
Funding source
This work was supported by the Key Project of Medicine Discipline of Guangzhou [grant number 2021–2023-12]; the Science and Technology Plan Grant of Guangzhou [grant number 202102080035]; and the Basic Research Project of Key Laboratory of Guangzhou [grant number 202102100001].
Ethical statement
All the data of leptospirosis cases were part of continuing public health surveillance data of a notifiable infectious disease determined by the National Health Commission of China and ethical approval was not required.
CRediT authorship contribution statement
Ziyi Zeng: Methodology, Visualization, Writing – original draft, Writing – review & editing. Haiyan Chen: Methodology, Visualization, Writing – original draft, Writing – review & editing. Jianmin Xu: Formal analysis, Methodology, Writing – review & editing. Hao Zhang: Formal analysis, Methodology, Writing – review & editing. Conghui Xu: Investigation, Resources, Writing – review & editing. Lirui Fan: Investigation, Resources, Writing – review & editing. Shouyi Chen: Investigation, Resources, Writing – review & editing. Kuncai Chen: Conceptualization, Project administration, Writing – review & editing. Zhicong Yang: Conceptualization, Supervision, Writing – review & editing. Yuehong Wei: Conceptualization, Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
We declare that we have no competing financial interests.
Acknowledgements
We thank all the participants in the study. We would also like to appreciate the efforts of medical and health institutions in Guangzhou City for disease reporting and data collection.
Data availability
Data will be made available on request.
References
- 1.Levett P.N. Leptospirosis. Clin. Microbiol. Rev. 2001;14(2):296–326. doi: 10.1128/cmr.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharti A.R., Nally J.E., Ricaldi J.N., Matthias M.A., Diaz M.M., Lovett M.A., et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 2003;3(12):757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.Yalin W., Lingbing Z., Hongliang Y., Jianmin X., Xiangyan Z., Xiaokui G., et al. High prevalence of pathogenic Leptospira in wild and domesticated animals in an endemic area of China. Asian Pac J Trop Med. 2011;4(11):841–845. doi: 10.1016/s1995-7645(11)60205-8. [DOI] [PubMed] [Google Scholar]
- 4.Adler B. History of leptospirosis and leptospira. Curr. Top. Microbiol. Immunol. 2015;387:1–9. doi: 10.1007/978-3-662-45059-8_1. [DOI] [PubMed] [Google Scholar]
- 5.Casanovas-Massana A., Pedra G.G., Wunder E.A., Jr., Diggle P.J., Begon M., Ko A.I. Quantification of Leptospira interrogans survival in soil and water microcosms. Appl. Environ. Microbiol. 2018;84(13) doi: 10.1128/aem.00507-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Brito T., Silva A., Abreu P.A.E. Pathology and pathogenesis of human leptospirosis: a commented review. Rev. Inst. Med. Trop. Sao Paulo. 2018;60 doi: 10.1590/s1678-9946201860023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajapakse S. Leptospirosis: clinical aspects. Clin. Med. (London, England). 2022;22(1):14–17. doi: 10.7861/clinmed.2021-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karpagam K.B., Ganesh B. Leptospirosis: a neglected tropical zoonotic infection of public health importance-an updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(5):835–846. doi: 10.1007/s10096-019-03797-4. [DOI] [PubMed] [Google Scholar]
- 9.Vijayachari P., Sugunan A.P., Shriram A.N. Leptospirosis: an emerging global public health problem. J. Biosci. 2008;33(4):557–569. doi: 10.1007/s12038-008-0074-z. [DOI] [PubMed] [Google Scholar]
- 10.Goarant C. Leptospirosis: risk factors and management challenges in developing countries. Res. Reports Trop. Med. 2016;7:49–62. doi: 10.2147/rrtm.S102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa F., Hagan J.E., Calcagno J., Kane M., Torgerson P., Martinez-Silveira M.S., et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl. Trop. Dis. 2015;9(9) doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torgerson P.R., Hagan J.E., Costa F., Calcagno J., Kane M., Martinez-Silveira M.S., et al. Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Negl. Trop. Dis. 2015;9(10) doi: 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu W., Lin X., Yan J. Leptospira and leptospirosis in China. Curr. Opin. Infect. Dis. 2014;27(5):432–436. doi: 10.1097/qco.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Communicable Disease Control and Prevention . People’s Medical Publishing House (PMPH); Beijing: 2008. Diagnostic Criteria for Leptospirosis (WS290–2008) [Google Scholar]
- 15.Liang S., Yang C., Zhong B., Guo J., Li H., Carlton E.J., et al. Surveillance systems for neglected tropical diseases: global lessons from China’s evolving schistosomiasis reporting systems, 1949-2014. Emerg. Themes Epidemiol. 2014;11:19. doi: 10.1186/1742-7622-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S.N., Liu Z.B., Gu Z.W. Disease control and prevention in China in the 20(th) century and prospects for the new millennium. Environ. Health Prev. Med. 2002;7(3):132–137. doi: 10.1265/ehpm.2002.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X., Yuan B., Yu Y., Jian W. Governance function analysis of the patriotic health movement in China. Glob. Health Res. Policy. 2019;4:34. doi: 10.1186/s41256-019-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y., Zhang Y., Cao X., Wang C., Wang Y., Zhang M., et al. Forty years of reform and opening up: China’s progress toward a sustainable path. Sci. Adv. 2019;5(8) doi: 10.1126/sciadv.aau9413. eaau9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai K.C., Li Q., Bao X.L., Zhu J., He X.X. An empirical study of economic cycle, air quality, and National Health since Reform and opening up. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.706955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosson J.F., Picardeau M., Mielcarek M., Tatard C., Chaval Y., Suputtamongkol Y., et al. Epidemiology of leptospira transmitted by rodents in Southeast Asia. PLoS Negl. Trop. Dis. 2014;8(6) doi: 10.1371/journal.pntd.0002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boey K., Shiokawa K., Rajeev S. Leptospira infection in rats: a literature review of global prevalence and distribution. PLoS Negl. Trop. Dis. 2019;13(8) doi: 10.1371/journal.pntd.0007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler B., de la Peña Moctezuma A. Leptospira and leptospirosis. Vet. Microbiol. 2010;140(3–4):287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Cleaveland S., Sharp J., Abela-Ridder B., Allan K.J., Buza J., Crump J.A., et al. One health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017;372(1725) doi: 10.1098/rstb.2016.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overgaauw P.A.M., Vinke C.M., Hagen M., Lipman L.J.A. A one health perspective on the human-companion animal relationship with emphasis on zoonotic aspects. Int. J. Environ. Res. Public Health. 2020;17(11) doi: 10.3390/ijerph17113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalerie L., Wardeh M., Lebrasseur O., Nanyingi M., McIntyre K.M., Kaba M., et al. One hundred years of zoonoses research in the horn of Africa: a scoping review. PLoS Negl. Trop. Dis. 2021;15(7) doi: 10.1371/journal.pntd.0009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride A.J., Athanazio D.A., Reis M.G., Ko A.I. Leptospirosis. Curr. Opin. Infect. Dis. 2005;18(5):376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 27.Fouts D.E., Matthias M.A., Adhikarla H., Adler B., Amorim-Santos L., Berg D.E., et al. What Makes a Bacterial Species Pathogenic?:Comparative Genomic Analysis of the Genus Leptospira. PLoS Negl. Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susilawati T.N., McBride W.J. Acute undifferentiated fever in Asia: a review of the literature. South. Asian J. Trop. Med. Pub. Health. 2014;45(3):719–726. [PubMed] [Google Scholar]
- 29.Cachay E.R., Vinetz J.M. A global research agenda for leptospirosis. J. Postgrad. Med. 2005;51(3):174–178. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Wang H., Yan J. Leptospirosis prevalence in Chinese populations in the last two decades. Microbes Infect. 2012;14(4):317–323. doi: 10.1016/j.micinf.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Chaiwattanarungruengpaisan S., Suwanpakdee S., Sangkachai N., Chamsai T., Taruyanon K., Thongdee M. Potentially pathogenic Leptospira species isolated from a waterfall in Thailand. Jpn. J. Infect. Dis. 2018;71(1):65–67. doi: 10.7883/yoken.JJID.2017.363. [DOI] [PubMed] [Google Scholar]
- 32.Dhewantara P.W., Hu W., Zhang W., Yin W.W., Ding F., Mamun A.A., et al. Climate variability, satellite-derived physical environmental data and human leptospirosis: a retrospective ecological study in China. Environ. Res. 2019;176 doi: 10.1016/j.envres.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Luo D., Xue F., Ojcius D.M., Zhao J., Mao Y., Li L., et al. Protein typing of major outer membrane lipoproteins from Chinese pathogenic Leptospira spp. and characterization of their immunogenicity. Vaccine. 2009;28(1):243–255. doi: 10.1016/j.vaccine.2009.09.089. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y., Ye Q. Human leptospirosis vaccines in China. Human Vacc. & Immunotherap. 2018;14(4):984–993. doi: 10.1080/21645515.2017.1405884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.