Abstract
Objectives: Concerns among susceptible individuals, especially those with vascular malformations, have been raised by reports of thromboembolism following the administration of the SARS-CoV-2 vaccination against coronavirus disease 2019 (COVID-19). This study’s goal was to assess any negative side effects that patients with vascular malformations who received the SARS-CoV-2 vaccine reported after receiving it.
Materials and Methods: Through the three patient groups for vascular malformations in Japan in November 2021, a questionnaire was distributed to patients with vascular malformations who were 12 years of age or older. Multiple regression analysis was used to find relevant variables.
Results: A total of 128 patients responded, representing a response rate of 58.8%. Ninety-six participants (75.0%) had received at least one dose of SARS-CoV-2 vaccine. In total, 84 (87.5%) and 84 (89.4%) subjects experienced at least 1 general adverse response following dose 1 and dose 2, respectively. Adverse reactions related to vascular malformations were reported by 15 participants (16.0%) after the 1st dose and 17 (17.7%) after the 2nd dose. Notably, no case of thromboembolism following vaccination was reported.
Conclusion: The rate of vaccine-related adverse reactions in patients with vascular malformations is not different from that reported in the general population. There is no report of life-threatening responses in the research population.
Keywords: vaccination, adverse reactions, vascular malformations, vascular anomalies, COVID-19
Introduction
Vascular malformations include a broad spectrum of vascular pathology, including proliferating vascular tumors and vascular malformations.1–3) Patients with venous malformations, lymphatic venous malformations, and Klippel–Trenaunay syndrome are at risk of developing life-threatening hematological complications like venous thrombosis.4–6)
As of December 2021, 101 million people in Japan (79.2% of the population) had received the SARS-CoV-2 vaccine 1st dose, and 98 million (77.9%) had received the 2nd dose.7) Several reports of thromboembolic events following SARS-CoV-2 vaccination have been published8,9) with large-scale meta-analyses showing the ChAdOx1 vaccine (Oxford-AstraZeneca) is strongly associated with thromboembolic events.10–12) This has raised concerns about the safety of the vaccines in patients with vascular malformations who are already at risk of such adverse vascular reactions. Furthermore, the Japanese Ministry of Health, Labor and Welfare (MHLW) recently reported the death of a 26-year-old female from intracranial hemorrhage 4 days after receiving her first dose of SARS-CoV-2 vaccination with autopsy reports pointing to intracranial arteriovenous malformations.13) These reports may have further worsened vaccine anxiety in patients with vascular malformations and highlight the need for study of the vaccine safety in this unique population.
Although vascular malformations are strongly associated with thromboembolism,4–6) the association between vascular malformations and side effects of SARS-CoV-2 vaccines particularly thrombotic problems has not previously been studied and remains unclear. This study aims to evaluate the patient-reported adverse reactions and associated factors of SARS-CoV-2 vaccines in patients with vascular malformations and to assess their opinions on the vaccines.
Patients and Methods
Participants and experimental design
A cohort of 249 patients with vascular anomalies in Japan was recruited. Eligibility criteria included (1) diagnosis of vascular malformations, (2) 12 years of age or older, (3) access to the Internet and ability to complete surveys in Japanese, and (4) willingness to participate in the study. Children younger than 12 years old were excluded because the Japanese government had not yet approved SARS-CoV-2 vaccination in this age range at the time of the trial. An electronic self-reported survey was sent via the three patient societies of vascular malformations in Japan: Patients Association of Vascular Anomalies, Kekkan-kikei Network, and Kongo-gata Myakkann-kikei no Kai. Reminders were sent 2 weeks and 1 week prior to survey deadline on November 30, 2021. All study procedures were approved by the local research ethics committee (Mie University Hospital IRBMED Number H2021-212) and were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Data collection and measurements
Data collected included demographic data, age and sex, type of vascular malformations, medical and drug history, details of SARS-CoV-2 vaccination, and adverse reactions. Adverse reactions analyzed were further divided as general adverse reactions such as pain, swelling, induration, itching of the injected site, fever (37.5°C or above), muscle soreness, arthralgia, headache, fatigue, and thromboembolism and adverse reactions related to vascular malformations including pain, redness, swelling, enlargement, and thrombosis of the lesion. Participants who had not been vaccinated were optionally asked for reasons.
Statistical analysis
A multiple linear regression model was created to determine the variables connected to adverse reactions in individuals with vascular malformations. P<0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS v.27.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 147 participants completed the questionnaire survey, with a survey response rate of 58.8%. Nineteen were disqualified due to being under 12 years of age, leaving a total of n=128 eligible responses (Supplementary Fig. 1).
The demographics of the overall participants are described in Table 1. The most prevalent age group was 12–19 years (n=31, 24.2%) closely followed by 20–29 years (n=27, 21.1%) and 40–49 years (n=27, 21.1%). Female participants accounted for 67 (52.3%) of the total and males 61 (47.7%). The most prevalent vascular malformations included 56 (43.8%) venous malformation (VM), 47 (36.7%) arteriovenous malformation (AVM), and 32 (25.0%) hemangiomas. The most common sites were the lower extremities (n=74, 57.8%) and head and neck (n=32, 25.0%) followed by the upper extremities (n=22, 17.2%) and trunk (n=19, 14.8%). Participants with Klippel–Trenaunay (KT) syndrome and Parkes–Weber (PW) syndrome were investigated together despite having separate etiology, since some participants were unable to discern between the two and referred to their anomaly as KT/PW spectrum.
Table 1 Demographics of the study population.
| Item | Total (N=128) | |
|---|---|---|
| n | Percent | |
| Age | ||
| 12–19 | 31 | 24.2% |
| 20–29 | 27 | 21.1% |
| 30–39 | 17 | 13.3% |
| 40–49 | 27 | 21.1% |
| 50–59 | 23 | 18.0% |
| 60– | 3 | 2.3% |
| Sex | ||
| Female | 67 | 52.3% |
| Male | 61 | 47.7% |
| Type of vascular malformations | ||
| Hemangioma | 32 | 25.0% |
| VM | 56 | 43.8% |
| CM | 8 | 6.3% |
| AVM | 47 | 36.7% |
| LM | 16 | 12.5% |
| KT/PW | 10 | 7.8% |
| BRBNS | 2 | 1.6% |
| Location of vascular malformations | ||
| Head and neck | 32 | 25% |
| Upper extremities | 22 | 17.2% |
| Trunk | 19 | 14.8% |
| Lower extremities | 74 | 57.8% |
| Viscera | 3 | 2.3% |
| Systemic | 2 | 1.6% |
| Medical history | ||
| COVID-19 infection | 2 | 1.6% |
| Hypertension | 7 | 5.5% |
| Diabetes mellitus | 3 | 2.3% |
| Asthma | 19 | 14.8% |
| Seizure | 4 | 3.1% |
| Stroke | 3 | 2.3% |
| Heart disease | 3 | 2.3% |
| Thromboembolism | 12 | 9.4% |
| Coagulation abnormalities | 4 | 3.1% |
| Malignancy | 4 | 3.1% |
| Others | 8 | 6.3% |
| None of the above | 74 | 57.8% |
| Medication | ||
| Antithrombotic drug | 12 | 9.3% |
| Sirolimus | 6 | 4.7% |
| Immunosuppressor | 6 | 4.7% |
| Beta blocker | 3 | 2.3% |
| Others | 2 | 2.3% |
| None of the above | 101 | 78.9% |
| Dose number | ||
| 0 | 32 | 25.0% |
| 1 | 2 | 1.6% |
| 2 | 94 | 73.4% |
| Vaccine company (among vaccinated subjects) | ||
| Pfizer | 75 | 78.1% |
| Moderna | 19 | 19.8% |
| AstraZeneca | 0 | 0% |
| Unknown | 2 | 2.1% |
VM: venous malformation; CM: capillary malformation; AVM: arteriovenous malformation; LM: lymphatic malformation; KT/PW: Klippel–Trenaunay syndrome/Parkes–Weber syndrome; BRBNS: blue rubber bleb nevus syndrome
Regarding the SARS-CoV-2 vaccination, 96 participants (75.0%) had received at least 1 dose of the vaccine, whereas 32 participants (25.0%) were unvaccinated. Vaccine distribution comprised 75 (78.1%) BNT162b2 (Pfizer-BioNTech), 19 (19.8%) mRNA-1273 (NIH-Moderna), and 2 (2.1%) unknown. No participant reported receiving ChAdOx1. Comparison between vaccinated and unvaccinated participants (Supplementary Table S1) showed that the rate of younger vaccinated participants was considerably lower than those of older participants (p=0.005). The percentage of vaccinated participants was 54.8% (n=17) participants aged under 20 years and 81.4% (n=79) in those aged 20 or above. All the patients registered in this study with blue rubber bleb nevus syndrome (BRBNS) (n=2), systemic vascular malformations (n=2), and a history of heart disease (n=3) were all unvaccinated.
Of the 32 unvaccinated participants, 31 (96.9%) shared their reasons for not receiving the vaccination (Supplementary Table S2). The most common reason was personal concerns regarding the vaccine side effects (n=24, 77.4%) with n=4 (12.9%) explicitly worried about embolism or venous thrombosis following vaccination. The second leading reason was due to concerns raised by others, for instance, family members or their doctors about the vaccine (n=4, 12.9%). Other justifications were mistrust of political authorities and conflicting media messages about vaccinations.
The proportion of participants who reported at least one general adverse reaction following vaccination was 87.5% (n=84) after dose 1 and 89.4% (n=84) after dose 2 (Fig. 1A). The vast majority of the participants reported pain at the injected site, 71.9% (n=69) and 75.5% (n=71); swelling at the injected site, 27.1% (n=26) and 35.1% (n=33); and fever, 21.9% (n=21) and 54.3% (n=51), after dose 1 (Supplementary Table S3) and dose 2, respectively (Supplementary Table S4). Adverse reactions related to the vascular anomaly lesions were reported by 17.7% of the participants (n=17) after dose 1 and 16.0% (n=15) after dose 2 (Fig. 1B). All of the participants who disclosed adverse reactions related to vascular anomaly lesions also reported at least one other negative effect. Notably, none of the participants reported anaphylaxis, thromboembolism, or thrombosis at lesion site after vaccination (Fig. 1).
Fig. 1 Reported adverse reactions of each type of vascular malformations following SARS-CoV-2 vaccine.
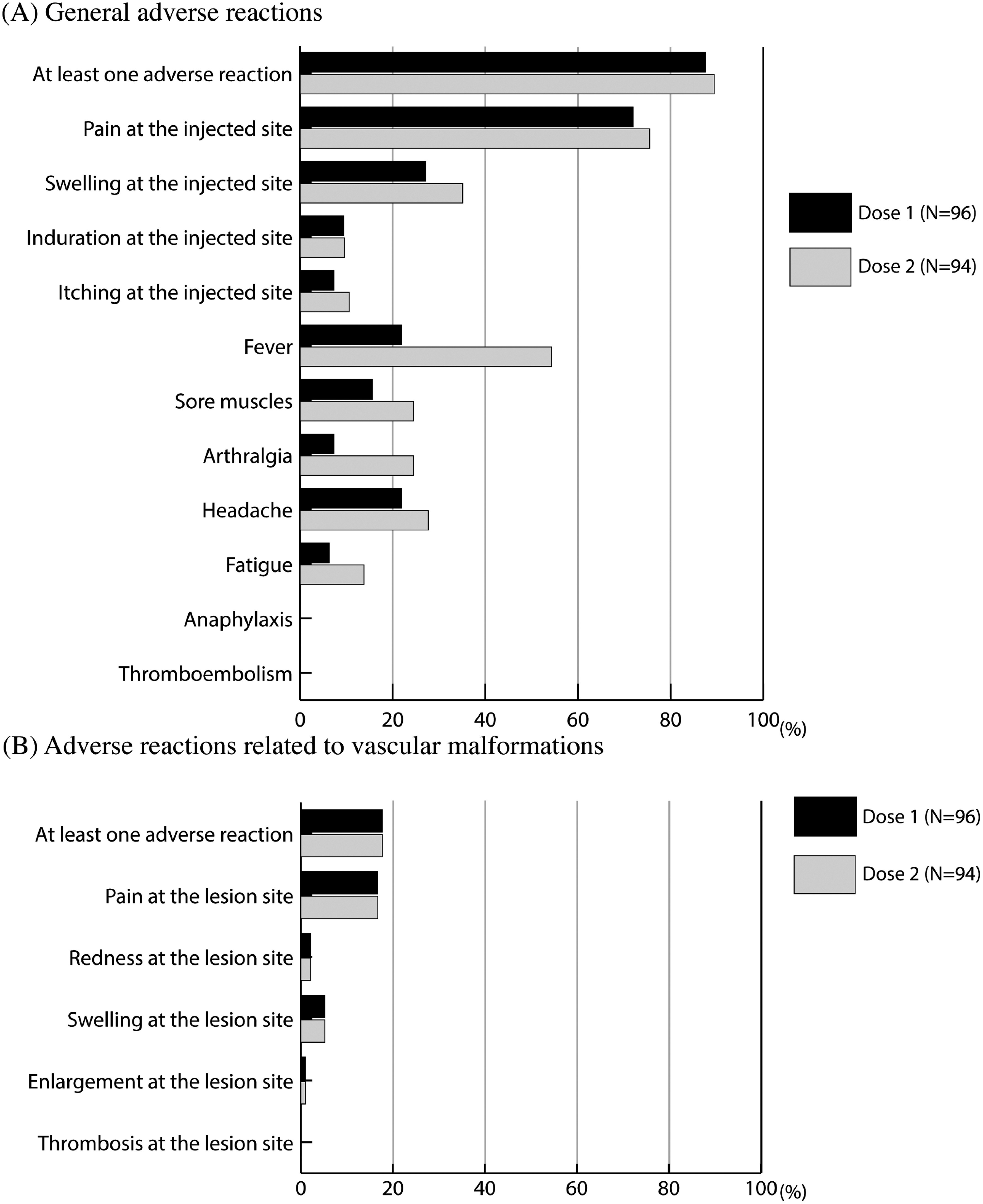
Regression analysis found no factors connected to adverse reactions to dose 1 (Table 2). Older age (p=0.03), a history of COVID-19 infection (p=0.04), asthma (p=0.03), and thromboembolism (p=0.03) were negatively associated with general adverse reactions to dose 2. A higher population of KT/PW 42.9% (n=3) experienced at least one adverse event associated with vascular abnormality lesions to dose 2 (p=0.04).
Table 2 Multiple regression analysis of factors associated with adverse reactions following SARS-CoV-2 vaccination.
| Independent variable | General adverse reactions | Adverse reactions related to lesions of vascular malformations | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | |||||
| Coefficient | p value | Coefficient | p value | Coefficient | p value | Coefficient | p value | |
| Age | −0.09 | 0.58 | −0.30 | 0.03* | 0.12 | 0.41 | −0.03 | 0.84 |
| Sex | ||||||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 0.20 | 0.13 | −0.03 | 0.81 | 0.13 | 0.27 | −0.01 | 0.99 |
| Type of vascular malformations | ||||||||
| Hemangioma | −0.09 | 0.57 | −0.10 | 0.43 | 0.08 | 0.59 | 0.19 | 0.15 |
| VM | −0.24 | 0.91 | −0.17 | 0.35 | 0.14 | 0.47 | 0.18 | 0.33 |
| CM | 0.10 | 0.46 | 0.16 | 0.18 | 0.001 | 1.00 | 0.04 | 0.70 |
| AVM | 0.08 | 0.72 | −0.01 | 0.94 | 0.33 | 0.08 | 0.28 | 0.12 |
| LM | 0.21 | 0.23 | 0.28 | 0.05 | 0.29 | 0.06 | 0.13 | 0.36 |
| KT/PW | 0.11 | 0.57 | 0.18 | 0.25 | 0.18 | 0.27 | 0.34 | 0.04* |
| Location of vascular malformations | ||||||||
| Head and neck | 0.14 | 0.72 | 0.21 | 0.51 | 0.43 | 0.21 | 0.32 | 0.33 |
| Upper extremities | −0.13 | 0.73 | 0.16 | 0.61 | 0.21 | 0.52 | 0.11 | 0.71 |
| Trunk | −0.10 | 0.55 | −0.04 | 0.77 | −0.18 | 0.21 | 0.11 | 0.45 |
| Lower extremities | 0.07 | 0.87 | −0.05 | 0.89 | 0.42 | 0.27 | 0.16 | 0.67 |
| Viscera | −0.01 | 0.95 | 0.07 | 0.59 | −0.12 | 0.38 | −0.03 | 0.81 |
| Medical history | ||||||||
| COVID-19 infection | −0.17 | 0.19 | −0.23 | 0.04* | −0.02 | 0.89 | 0.23 | 0.40 |
| Hypertension | −0.17 | 0.41 | −0.29 | 0.09 | −0.14 | 0.43 | −0.19 | 0.27 |
| Diabetes mellitus | 0.24 | 0.90 | 0.07 | 0.61 | 0.06 | 0.69 | 0.33 | 0.03 |
| Asthma | −0.16 | 0.47 | −0.41 | 0.03* | 0.10 | 0.59 | 0.19 | 0.32 |
| Seizure | −0.01 | 0.95 | −0.14 | 0.33 | 0.15 | 0.36 | −0.06 | 0.68 |
| Stroke | −0.22 | 0.20 | −0.10 | 0.47 | −0.26 | 0.08 | −0.10 | 0.48 |
| Thromboembolism | −0.14 | 0.56 | −0.43 | 0.03* | −0.30 | 0.15 | −0.22 | 0.27 |
| Coagulation abnormalities | −0.02 | 0.91 | −0.08 | 0.55 | 0.04 | 0.77 | −0.02 | NA |
| Malignancy | −0.01 | 0.97 | −0.31 | 0.02* | −0.15 | 0.28 | −0.05 | 0.71 |
| Others | −0.19 | 0.37 | −0.30 | 0.08 | −0.13 | 0.48 | 0.03 | 0.87 |
| Allergy | ||||||||
| Hay fever | −0.19 | 0.47 | −0.03 | 0.09 | 0.33 | 0.14 | 0.25 | 0.26 |
| Contrast agents | −0.15 | 0.54 | 0.07 | 0.74 | 0.09 | 0.69 | −0.11 | 0.61 |
| Others | −0.14 | 0.74 | 0.13 | 0.72 | 0.62 | 0.10 | 0.32 | 0.40 |
| Medication | ||||||||
| Antithrombotic drug | −0.78 | 0.73 | −0.30 | 0.14 | 0.42 | 0.04 | 0.53 | NA |
| Sirolimus | −0.07 | 0.79 | −0.12 | 0.55 | 0.15 | 0.47 | 0.17 | 0.39 |
| Immunosuppressor | 0.07 | 0.71 | 0.04 | 0.80 | 0.09 | 0.60 | −0.06 | 0.70 |
| Beta blocker | −0.06 | 0.68 | −0.01 | 0.95 | −0.01 | 0.95 | 0.09 | 0.47 |
| Others | 0.08 | 0.79 | 0.03 | 0.91 | 0.39 | 0.16 | 0.51 | 0.09 |
VM: venous malformation; CM: capillary malformation; AVM: arteriovenous malformation; LM: lymphatic malformation; KT/PW: Klippel–Trenaunay syndrome/Parkes–Weber syndrome; NA: not available *p<0.05
This study had too few vaccinated participants with systemic complications like rare systemic vascular malformations and a history of heart disease, coagulation abnormalities, and antithrombotic drug use. Consequently, a detailed analysis of these conditions was not possible for these.
Discussion
The 75.0% SARS-CoV-2 vaccination rate in patients with vascular malformations found in this study was slightly lower than the overall national vaccination rate across Japan which was 77.9% as of December 2021.7) Regarding the general adverse reactions following SARS-CoV-2 vaccines, the frequency of reported reactions was generally similar with the findings observed in clinical trials across the general population with discomfort at the injection site being the most common adverse reaction.14–16) The Ministry of Health, Labor and Welfare (MHLW) of Japan general population study of over 19,000 participants working at hospitals who received BNT162b2 vaccines reported pain at the injected site in 92.5% after the 1st dose and 89.5% following the 2nd dose, while fever was observed in 3.3% after the 1st dose and 38.1% following the 2nd dose.17) With regard to mRNA-1273 vaccine, 84.4% developed the pain at the injection site after the 1st dose and 83.2% after the 2nd, and the incidence of fever was 7.0% after the 1st dose and 76.8% after the 2nd dose in more than 11,000 participants investigated.18) The incidence of pain at the injection site in our study was consistent with the MHLW results for both the BNT162b2 and mRNA-1273 vaccines. This implies that the presence of vascular abnormalities might not be a risk factor for general severe reactions for these vaccinations.
According to our results, no enormous increase in thrombotic complications was observed in patients with vascular malformations after vaccination. Several studies have documented vaccine-induced immune thrombotic thrombocytopenia (VIIT) following SARS-CoV-2 vaccination19–26) particularly after ChAdOx1 or Ad26.COV2.S (Janssen/Johnson & Johnson) vaccines.10,21–26) MHLW reported that the incidence rate of VIIT in the general population was 0.1–0.2 cases among 100,000 recipients after BNT162b2 and mRNA-1273, whereas 18–24 cases per 100,000 recipients after ChAdOx1.27) In our group, participants only received BNT162b2 or mRNA-1273 vaccines, which may account for the lack of thrombotic side effects following vaccination. Both of the vaccines are approved for those aged over 12 years old at the research period. In Japan, vaccines of BNT162b2, mRNA-1273, and ChAdOx1 are authorized for use as SARS-CoV-2 vaccines as of December 2020. More careful surveillance is still needed.
Multiple regression analysis in our study revealed that younger age was one of the risk factors of general adverse reactions after second dose of the vaccination (p=0.03) in line with several studies in the general population that reported a higher frequency of adverse reactions following SARS-CoV-2 vaccination in younger individuals than in older.16, 28, 29) In addition, our multiple regression analysis showed that KT/PW was associated with a higher incidence rate of adverse reactions related to vascular anomaly lesions following dose 2 of SARS-CoV-2 vaccine in our study. Symptoms of KT/PW are often severe.6) However, there are few reports that suggest the relationship between these medical conditions and adverse reactions following SARS-CoV-2 vaccine. Furthermore, the number of participants with uncommon abnormalities and concomitant medical problems was relatively low in our cohort. Thus, further studies dealing with a larger number of participants are still required to validate our findings.
The merits of this study are that it is one of the earliest studies on the effects of SARS-CoV-2 vaccination on patients with vascular malformations. The study group was also relatively large with a good response rate and included patients from multiple centers.
Limitations
We admit a number of limitations in this study. Firstly, due to the nature of the retrospective survey design, potential recall bias and self-selection bias may have been present. Secondly, every single participant was Japanese; therefore, regional and ethnical differences could not be evaluated. Future research involving a bigger and more diverse population is necessary. Third, due to the nature of the retrospective patient-reported questionnaire, the recall bias can affect the result, and the evaluation by the patient report can be lack of precision. Lastly, the actual incidence of lethal events in patients with vascular abnormalities cannot be assessed precisely because the severe events are uncommon in the general population. Our cohort included relatively small case number of participants as prevalence of vascular malformations is not so high. Thrombotic events, for example, are also noted in the modest number of vaccine recipients.27) Actually, we also could not assess the effects of SARS-CoV-2 vaccination on patients with these medical conditions with rare systemic vascular malformations and a history of heart disease due to little data. However, our results showed that there is not a noticeably higher rate of fatal consequences following vaccinations among patients with vascular abnormalities.
Conclusion
Based on our results, patients with vascular malformations have a similar SARS-CoV-2 vaccination rate. This study reveals that there is no difference in the rate of patient-reported adverse effects between patients with vascular abnormalities and the general population. Notably, there was no case of thromboembolism in our study group. Our findings overall imply that SARS-CoV-2 vaccination may be equally safe in patients with vascular malformations as in the broader public.
Acknowledgments
We thank all the study participants from 3 patient societies of vascular malformations in Japan: Patients Association of Vascular Anomalies; Kekkan-kikei Network; and Kongo-gata Myakkann-kikei no Kai.
Ethics Statement
The Mie University Hospital Institutional Review Board examined and approved the study’s use of human subjects, and it was carried out in compliance with institutional and national research committee ethical standards (Mie University Hospital IRBMED Number H2021-212). The publication of this original research has informed consent.
Disclosure Statement
Declaration of Conflicting Interests: The authors declared no conflicts of interest. No funding was received for conducting this research.
Author Contributions
Study conception: MS, MN, MK, SY, MO
Data collection: MS, MN, CHB, KM, KD, RI
Analysis: MS, MN, CHB
Investigation: MS, MN, CHB, MK, SY, MO
Writing: MS
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Supplementary Materials
Supplementary materials are available at the online article sites on J-STAGE and PMC.
Supplementary Data
References
- 1).Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982; 69: 412-20. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 2).Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol 1997; 24: 701-10. doi: 10.1111/j.1346-8138.1997.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 3).Mulligan PR, Prajapati HJ, Martin LG, et al. Vascular anomalies: classification, imaging characteristics and implications for interventional radiology treatment approaches. Br J Radiol 2014; 87: 20130392. doi: 10.1259/bjr.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Mazereeuw-Hautier J, Syed S, Leisner RI, et al. Extensive venous/lymphatic malformations causing life-threatening haematological complications. Br J Dermatol 2007; 157: 558-63. doi: 10.1111/j.1365-2133.2007.08003.x. [DOI] [PubMed] [Google Scholar]
- 5).Ndzengue A, Rafal RB, Balmir S, et al. Klippel-Trenaunay syndrome: an often overlooked risk factor for venous thromboembolic disease. Int J Angiol 2012; 21: 233-6. doi: 10.1055/s-0032-1328969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Nelson KJ, Bennett R, Lam A, et al. Clinical presentation and outcomes after endovascular management in a mixed pediatric and adult Klippel-Trenaunay syndrome population. J Vasc Surg Venous Lymphat Disord 2021; 9: 1495-503.e1. doi: 10.1016/j.jvsv.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 7).Prime Minister’s Office of Japan. COVID-19 vaccine coverage. (in Japanese) Available from: https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html [Retrieved on December 31, 2021].
- 8).Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021; 372: n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 9).Østergaard SD, Schmidt M, Horváth-Puhó E, et al. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet 2021; 397: 1441-3. doi: 10.1016/S0140-6736(21)00762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Mani A, Ojha V. Thromboembolism after Covid-19 vaccination: a systematic review of such events in 286 patients. Ann Vasc Surg 2022; 84: 12-20.e1. doi: 10.1016/j.avsg.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Cheruiyot I, Kipkorir V, Ngure B, et al. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann Vasc Surg 2021; 70: 273-81. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Matar RH, Than CA, Nakanishi H, et al. Outcomes of patients with thromboembolic events following coronavirus disease 2019 AstraZeneca vaccination: a systematic review and meta-analysis. Blood Coagul Fibrinolysis 2022; 33: 90-112. doi: 10.1097/MBC.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Ministry of Health, Labour and Welfare of Japan. Reported cases of death after SARS-CoV-2 vaccination (BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (NIH-Moderna)). (in Japanese) Available from: https://www.mhlw.go.jp/content/10906000/000772690.pdf [Retrieved on January 12, 2022].
- 14).Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603-15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403-16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA 2021; 325: 2201-2. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 17).Ministry of Health, Labour and Welfare of Japan. A midterm report of adverse reactions after BNT162b2 (Pfizer-BioNTech). (in Japanese) Available from: https://www.mhlw.go.jp/content/10601000/000830659.pdf [Retrieved on December 31, 2021].
- 18).Ministry of Health, Labour and Welfare of Japan. A midterm report of adverse reactions after mRNA-1273 (NIH-Moderna). (in Japanese) Available from: https://www.mhlw.go.jp/content/10601000/000862143.pdf [Retrieved on December 31, 2021].
- 19).Merchant HA. CoViD vaccines and thrombotic events: EMA issued warning to patients and healthcare professionals. J Pharm Policy Pract 2021; 14: 32. doi: 10.1186/s40545-021-00315-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Gabarin N, Patterson S, Pai M, et al. Venous thromboembolism and mild thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Thromb Haemost 2021; 121: 1677-80. doi: 10.1055/a-1585-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021; 384: 2092-101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med 2021; 385: 1680-9. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384: 2124-30. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384: 2202-11. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021; 325: 2448-56. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Tefera L, Cameron SJ. SVM Communications: vaccine-induced immune thrombotic thrombocytopenia (VITT)—what the vascular medicine physician should know. Vasc Med 2021; 26: 579-81. doi: 10.1177/1358863X211030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Ministry of Health, Labour and Welfare of Japan. The current situation of adverse reactions after SARS-CoV2 vaccination. (in Japanese) Available from: https://www.mhlw.go.jp/content/10601000/000846559.pdf [Retrieved on January 16, 2023]. [Google Scholar]
- 28).Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21: 939-49. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021; 27: 981-4. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


