Summary
Background
In patients at high risk of thromboembolism who were discharged after hospitalisation due to COVID-19, thromboprophylaxis with rivaroxaban 10 mg/day for 35 days significantly improved clinical outcomes, reducing thrombotic events compared with no post-discharge anticoagulation. The present study aimed to estimate the cost-effectiveness of this anticoagulation strategy.
Methods
Using the database of the MICHELLE trial, we developed a decision tree to estimate the cost-effectiveness of thromboprophylaxis with rivaroxaban 10 mg/day for 35 days versus no thromboprophylaxis in high-risk post-discharge patients for COVID-19 through an incremental cost-effectiveness analysis.
Findings
318 patients in 14 centres in Brazil were enrolled in the primary MICHELLE trial. The mean age was 57.1 years (SD 15.2), 127 (40%) were women, 191 (60%) were men, and the mean body-mass index was 29.7 kg/m2 (SD 5.6). Rivaroxaban 10 mg per day orally for 35 days after discharge decreased the risk of events defined by the primary efficacy outcome by 67% (relative risk 0.33, 95% CI 0.12–0.90; p = 0.03). The mean cost for thromboprophylaxis with rivaroxaban was $53.37/patient, and no prophylaxis was $34.22/patient, with an incremental cost difference of $19.15. The effectiveness means obtained in the intervention group was 0.1457, while in the control group was 0.1421, determining an incremental QALY difference of 0.0036. The estimated incremental cost-effectiveness ratio (ICER) was $5385.52/QALY.
Interpretation
Extended treatment with Rivaroxaban as thromboprophylaxis after hospital discharge for high-risk patients with COVID-19 is a cost-effective treatment option.
Funding
Modest funding was provided by Science Valley Research Institute, São Paulo, Brazil.
Keywords: Thromboprophylaxis, COVID, Anticoagulation, Cost-effectiveness analysis, Direct oral anticoagulants, Thrombosis
Research in context.
Evidence before this study
We searched MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, and Scopus using the terms (“rivaroxaban” OR “apixaban” OR “dabigatran” OR “edoxaban” OR “heparin” OR “enoxaparin”) AND (“extended thromboprophylaxis” OR “out-of-hospital thromboprophylaxis”) AND (“SARS-CoV-2” OR “COVID” OR “coronavirus” OR “COVID-19”) AND (“randomised” OR “clinical trials”), AND (“cost-effectiveness evaluation” OR “costs”) with no date or language restrictions. We did not find published data assessing the cost-effectiveness of thromboprophylaxis after hospitalisation due to COVID-19.
Added value of this study
Using the database of the MICHELLE trial, a multicentre randomised trial led by our group, where a central events committee evaluated all events, we developed a decision tree to estimate the costs and effectiveness of thromboprophylaxis with rivaroxaban 10 mg/day for 35 days versus no thromboprophylaxis in high-risk post-discharge patients for COVID-19 through an incremental cost-effectiveness analysis, for the first time. The MICHELLE trial provided high-quality evidence and combined with this cost-effectiveness analysis, helps guide informed-medical decisions.
Implications of all the available evidence
Extended treatment with rivaroxaban as thromboprophylaxis after hospital discharge for high-risk patients with COVID-19 is a cost-effective treatment option. The findings of this study have important implications for resource prioritization and provide a comprehensive framework to inform policymakers about better decisions in public health.
Introduction
COVID-19 was the most important cause of hospitalisation worldwide in 2020 and 2021, surpassing circulatory and other respiratory system diseases.1 Severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection leads to endothelial dysfunction, platelet activation, and stasis.2,3 In addition, the interplay with intense inflammation improves the hypercoagulable state, increasing the risk of thromboembolism, whether venous (venous thromboembolism -VTE) or arterial (arterial thromboembolism - ATE).4 Several studies of post-discharge patients with COVID-19 demonstrated elevated incidences of symptomatic or asymptomatic events, reaching rates between 1.7% and 7.4%.5
Thromboembolic events, a composite of venous (deep-vein thrombosis [DVT], pulmonary embolism [PE]) and arterial (myocardial infarction [MI], stroke and acute limb ischemia) complications, most of the time require hospitalisation, a prolonged treatment, decrease of quality of life and different types of sequelae, depending on the site of thrombosis. The annual costs estimated in the United States range from U$7.5 billion, in the case of DVT,6 to U$ 84 billion in those with myocardial infarction.7 Other possible consequences, such as post-thrombotic syndrome (post-DVT PTS) and heart failure (post-AMI), are responsible for worsening patient quality of life and increasing costs in a broad time horizon.8,9
To mitigate the risk of post-discharge VTE, the MARINER trial published in 2018 evaluated the use of rivaroxaban after hospitalisation in medically ill patients. Patients with a high risk of VTE (defined by the International Medical Prevention Registry on Venous Thromboembolism [IMPROVE] score of ≥4 or 2–3 with elevated D-dimer levels) at hospital discharge were randomly assigned to rivaroxaban 10 mg/day (or 7.5 mg/day if creatinine clearance <50 mL/min) versus placebo for 45 days after hospital discharge. Despite not achieving superiority on its primary outcome (a combination of symptomatic VTE and death due to VTE), this prophylactic anticoagulation strategy led to a 28% relative risk reduction in major and fatal thromboembolic events and a 27% relative risk reduction of symptomatic venous thromboembolism and all-cause death, reducing the global burden of death and disability from VTE.10
The MICHELLE trial published in 2021 was the first study on prophylactic anticoagulation after discharge in patients with COVID-19. In this open-label, multicentre, randomised trial (with blinded adjudication), patients hospitalised with COVID-19 at increased risk for venous thromboembolism with the International Medical Prevention Registry on Venous Thromboembolism [IMPROVE] venous thromboembolism [VTE] score of ≥4 or 2–3 with a D-dimer >500 ng/mL were randomly assigned to receive, at hospital discharge, rivaroxaban 10 mg/day or no anticoagulation for 35 days. The primary efficacy outcome was a composite of symptomatic or fatal venous thromboembolism, asymptomatic venous thromboembolism on bilateral lower-limb venous ultrasound and CT pulmonary angiogram, symptomatic arterial thromboembolism, and cardiovascular death at day 35. The primary safety outcome was major bleeding. The primary and safety analyses were carried out in the intention-to-treat population. This study led by our group demonstrated that oral rivaroxaban 10 mg per day for 35 days after discharge in patients hospitalised by COVID-19 at high risk of thromboembolism decreased the risk of events defined by the primary efficacy outcome by 67% (relative risk 0.33, 95% CI 0.12–0.90; p = 0.03). There was no statistically significant increase in the rate of minor or major bleeding.11
This current study aims to estimate the cost-effectiveness of thromboprophylaxis with rivaroxaban versus no thromboprophylaxis in high-risk patients after hospitalisation for COVID-19 from the perspective of the Brazilian public health system.
Methods
Model assumptions
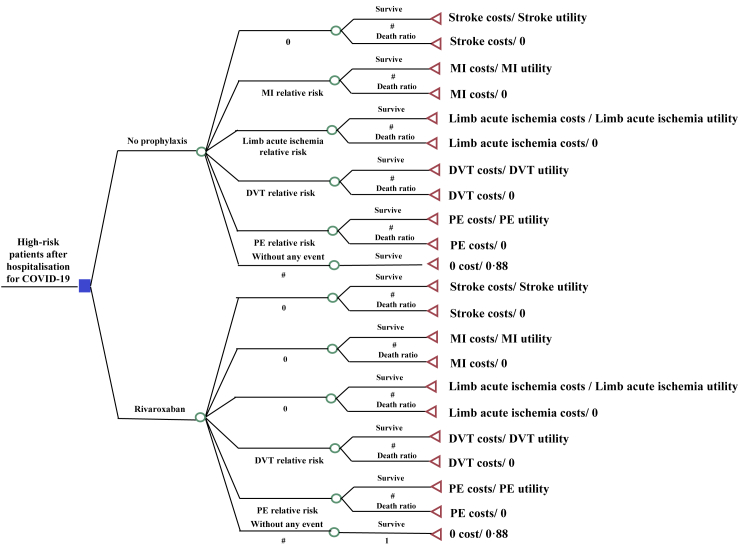
Using the database of the MICHELLE trial, a decision tree was developed to estimate the cost-effectiveness of thromboprophylaxis with oral rivaroxaban 10 mg/day for 35 days versus no thromboprophylaxis in high-risk patients post-discharge for COVID-19 through an incremental cost-effectiveness analysis. It is a unidirectional flow of events followed by different outcomes. It ends with a terminal event in which the patient may have total or partial recovery of health or death.12
At the beginning of the decision tree, patients could receive rivaroxaban or no intervention after hospital discharge. The proportion of all the thromboembolic events, such as DVT, PE, MI, stroke, and limb acute ischemia, was evaluated in the two groups. Each group had three possibilities: no event, thromboembolic event occurrence, or death. Given the low risk of significant clinical events with rivaroxaban, which would result in irrelevant impacts on both costs and effectiveness, the bleeding rate and allergic reactions were not applied in the model. This is a conservative model, with an immediate post-discharge horizon timeline of two months, because we were focused on acute adverse outcome events and their implications. It was the same follow-up time pre-specified for the MICHELLE trial. No discount rate was applied since the model has a horizon timeline of less than one year. Treeage Pro™ software 2022 (www.treeage.com) was used for the analysis. The model is demonstrated in Fig. 1.
Fig. 1.
Decision tree (PE, pulmonary embolism; MI, myocardial infarction; DVT, deep-vein thrombosis).
Efficacy data
DVT, PE, MI, and acute limb ischemia risk ratios were extracted from the raw database of the MICHELLE trial. There was no ischemic stroke in the outcomes of that trial. All relative risks are demonstrated in Table 1.
Table 1.
Thromboembolic events: relative risks.
| Relative Risk (95% CI) | Source | |
|---|---|---|
| DVT symptomatic | 0.14 (0.01–2.69) | Ramacciotti et al.11 |
| DVT asymptomatic | 1.96 (0.18–21.40) | Ramacciotti et al.11 |
| PE symptomatic | 0.49 (0.04–5.35) | Ramacciotti et al.11 |
| PE asymptomatic | 0.25 (0.03–2.17) | Ramacciotti et al.11 |
| Fatal PE | 0.14 (0.01–2.69) | Ramacciotti et al.11 |
| Myocardial infarction | 0.33 (0.01–7.96) | Ramacciotti et al.11 |
| Acute limb ischemia | 0.33 (0.01–7.96) | Ramacciotti et al.11 |
| Stroke | N/A | Ramacciotti et al.11 |
Medical costs
The model was constructed from the Brazilian Public Health System (SUS) perspective. Only direct medical costs associated with treatment during hospitalisation for each event were evaluated. Outpatient costs were not considered in the analysis. Cost studies performed in national hospitals were consulted, and federal databases informed expenses related to each type of event.13 All costs are demonstrated in Table 2.13,16,19
Table 2.
Thromboembolic events: utility, mortality rate, and mean of costs.
The price of rivaroxaban 10 mg was extracted from the List of Maximum Drug Prices from CMED-ANVISA (Health Surveillance National Agency).20 A Brazilian state excise tax (ICMS) of 18% was considered. The thromboprophylaxis with rivaroxaban 10 mg/daily for 35 days represented an expense of U$ 45.92. American Dollar was considered currency and converted in 2022 with a conversion rate of 1 USD = 5.33 Brazilian Real (BRL).
Utilities and mortality rate
The effectiveness of the model was measured in quality-adjusted life-years (QALYs). They are calculated by estimating the years of life for a patient after intervention and weighting each year with a utility score, which varies between 1 (perfect health) and 0 (dead). The average Brazilian utility is 0.88. We consider it for all patients with no events.21 For those who had events, we used utility studies from medical literature focused on national studies based on EQ-5D22 or SF-3623 questionnaires applied to patients who had a thromboembolic event in the last two months, the same time horizon chosen in our study. There is a significant decrease in the quality of life in conditions such as stroke18 and acute limb ischemia.17 All utilities are reported in Table 2.14,15,17,18
The mortality rates were obtained from the information system database on mortality from the Brazil Ministry of Health (Table 2).13
Analyses
Incremental cost-effectiveness ratios (ICER) were calculated as the difference in costs in the rivaroxaban group minus control divided by the difference in health outcomes in both groups. The model's outcome was each comparator's quality-adjusted life-years (QALYs) and the cost per QALY gained. All cost-effectiveness analyses were developed using the Treeage Pro™ software 2022. The reporting in this study follows Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement (Supplementary Table S3).24 This study was approved by the local Institutional Review Board (IRB), CAAE 52371121.7.0000.5485.
Role of funding source
Science Valley Research Institute, São Paulo, Brazil, provided modest funding to access the MICHELLE Trial database. The authors have received no funding to conduct the study. Science Valley Research Institute had no role in study design, data collection, analysis, or interpretation, in the report's writing or in the decision to submit the paper for publication.
Results
Three hundred and eighteen patients in 14 centres in Brazil were enrolled in the primary MICHELLE trial. The mean age was 57.1 years (SD 15.2), 127 (40%) were women, 191 (60%) were men, and the mean body-mass index was 29.7 kg/m2 (SD 5.6). Rivaroxaban 10 mg per day orally for 35 days after discharge decreased the risk of events defined by the primary efficacy outcome by 67% (relative risk 0.33, 95% CI 0.12–0.90; p = 0.03).
For a period of 2-month time frame, the mean cost for thromboprophylaxis with rivaroxaban was $53.37/patient, and no prophylaxis was $34.22/patient, with an incremental cost difference of $19.15. The effectiveness means obtained in the intervention group was 0.1457, while in the control group was 0.1421, determining an incremental QALY difference of 0.0036. The estimated ICER was $5385.52/QALY. Further details on the cost-effectiveness results can be found in Supplementary Table S1.
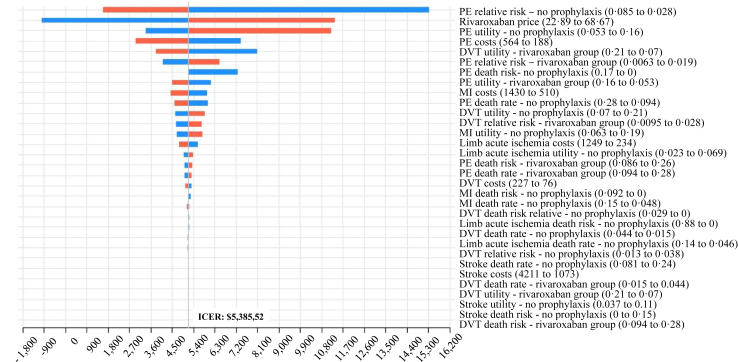
The one-way deterministic sensitivity analysis was performed to assess the parameter variations. The results are disclosed in Tornado Diagram (Fig. 2), which demonstrates how variations in each variable affect the ICER. The bars are distributed in decreasing order of width, showing that those at the top have the most significant effect on the ICER. In contrast, variations in variables near the bottom have relatively little impact on the ICER.
Fig. 2.
One-way sensitivity analysis - Tornado diagram. (Price and cost in USD. PE, pulmonary embolism; MI, myocardial infarction; DVT, deep-vein thrombosis; ICER, incremental cost-effectiveness ratio; CI, confidence interval).
Red bars are the ICER patterns when the parameter values increase. Blue bars are the ICER response when the parameter values decrease. The variable that most impacts directly the model is rivaroxaban's price, followed by hospitalisation costs with PE and stroke. All variables are represented with their respective confidence interval in brackets. No variable at the confidence interval's lower or upper possible values extrapolated $15,965.25. Further details on the values, ranges, and confidence intervals can be found in Supplementary Table S2.
Discussion
There was no consensus on anticoagulation after discharge at the beginning of the COVID-19 pandemic, and the costs of unnecessary anticoagulant prescriptions may have been very high, or the absence of that may have led to several thromboembolic preventable events. The MICHELLE trial demonstrated that rivaroxaban is effective and safe as thromboprophylaxis in high-risk patients after hospitalisation for COVID-19 and became the standard to guide thromboprophylaxis in those patients.11 A meta-analysis published in November 2022, including more than 10,000 patients, demonstrated that extended thromboprophylaxis was associated with a statistically significant reduced composite endpoint of thrombosis and all-cause mortality in patients with COVID-19 after discharge (OR 0.52; 95% CI: 0.41–0.67, p = 0.0001). Extended anticoagulant therapy was not associated with a significant increase in serious bleeding events (OR: 1.64; 95% CI: 0.95–2.82, p = 0.07), supporting the clinical benefit of post-hospitalisation thromboprophylaxis in selected COVID-19 patients.25
To evaluate the cost-effectiveness of this therapy, the incremental cost-effectiveness analysis technique was chosen because we analysed only acute thromboembolic events after hospitalisation. The incremental cost-effectiveness ratio of $5385.52/QALY demonstrated its cost-effectiveness. It means a gain of one year with the quality of life per $5385.52 expense.
There are multiple cost-effectiveness analyses related to COVID-19 vaccination and treatments with remdesivir or monoclonal antibodies in the literature.26 Still, we found no studies addressing anticoagulation cost-effectiveness during or after hospitalisation. There is also a lack of DVT, PE, and limb acute ischemia costs data worldwide, including Brazil, where the MICHELLE trial was conducted. However, all data related to direct medical costs and government expenses in Brazil are published monthly on a public government website (DATASUS),13 becoming a reliable data source. Only acute events were assessed in a deterministic analysis because the horizon time was determined for two months post-discharge.
Cost-effectiveness analysis takes into consideration a balance. The Tornado diagram (Fig. 2) discloses costs for the variables, and at the top of the chart, pulmonary embolism risk was the most relevant variable, followed by rivaroxaban cost. A higher relative risk of PE in the no prophylaxis group decreases the ICER. If PE were rare, it would increase the cost of PE prophylaxis, increasing ICER. The same rationale can apply to all thrombotic outcomes. In addition, the more expensive rivaroxaban, the higher the ICER.
The ICER limit in Brazil is considered BRL 40,000.00/QALY (USD 7504.69/QALY), but in a pandemic scenario, the limit increases to BRL 120,000.00/QALY (USD 22,514.00/QALY). In the United States, the cost threshold is $50,000 to $150,000; in the UK, the most used threshold is £30,000 by QALY.27 In our model analysis, based on the Brazilian perspective, we met an ICER of $5385.52/QALY, which means using rivaroxaban post-discharge in patients hospitalised by COVID-19 is highly cost-effective, considering the increased limit of ICER due to pandemic.
Rivaroxaban price is a variable that significantly affects the model. If the price of rivaroxaban were $68.67 (the highest variation), the ICER would be $11,822.81/QALY. Rivaroxaban's price in Brazil was extracted from the ANVISA list and cost U$1.30 for each pill, comparatively cheaper than other countries, such as the USA. Furthermore, the price may be even lower in clinical practice, lowering the ICER. If the treatment price were $22.89, with complete exemption from taxes, for example, using rivaroxaban would be cost-saving. No upper bound of all variables exceeded $15,965.10/QALY on ICER, showing that the model remains cost-effective even in the worst-case scenario.
Choosing a conservative model with a short time horizon generates some limitations inherent to the model, such as only the analysis of acute complications. Significant sequelae with high cost, i.e., heart failure after MI and stroke sequelae, were not analysed. If the horizon were more prolonged, it would impact the quality of life lost and costs due to chronic effects such as post-PE illness, heart failure, and post-thrombotic syndrome. The model would be more cost-effective and may become cost-saving. Furthermore, different events could happen in the same patient (i.e., DVT plus MI), but we did not model the impact of this possible occurrence.
According to Goldin et al., that validated the IMPROVE inpatients with COVID-19, approximately 45.7% of patients are classified as high-risk.28 Considering that the number of hospitalisation caused by COVID-19 in Brazil was 1,564,842 since the beginning of the pandemic until September/2022,29 around 715,133 patients would be classified as high-risk and considering the risk relative of 0.33, observed on the MICHELLE trial, almost 480,000 would have benefited from rivaroxaban use, saving expenses with thromboembolic complications and improving the quality of life.
In conclusion, the extended treatment with rivaroxaban as thromboprophylaxis after hospital discharge for high-risk patients with COVID-19 is a cost-effective treatment option. The findings of this study have important implications for resource prioritization and provide a comprehensive framework to inform policymakers about better decisions in public health.
Contributors
CCCO, ER, and LBA conceived the trial and wrote the initial proposal. Literature search, figures, data analysis, data interpretation, and writing were performed by CCCO, MSS and RRAF. CMR, ER, VCRA, RAC, CASM, AT, ACS, JF, and RDL contributed to data interpretation, manuscript writing, review, and editing. All authors had direct access to all the data, significantly contributed to the manuscript and agreed to submit it for publication. CCCO and ER directly accessed and verified the underlying data reported in the manuscript.
Data sharing statement
All data used in this study are openly accessible and available through the sources listed in Table 1, Table 2.
Declaration of interests
ER reports grants and consulting fees from Bayer and Pfizer; grants from the Brazilian Ministry of Science and Technology; and personal fees from Aspen Pharma, Biomm Pharma, and Daiichi-Sankyo, outside the submitted work. LBA reports grants from Bayer, Pfizer and the Brazilian Ministry of Science and Technology. ACS reports consulting fees from Janssen Research & Development LLC, Bayer, Portola, Boehringer Ingelheim, Bristol-Meyers Squibb, ATLAS group and grants from Janssen and Boehringer Ingelheim. AT reports personal fees from Janssen and Recovery Force and grants from Bio Tap, Idorsia, Bristol-Myers Squibb, Novo Nordisk, Janssen, and Doasense. RDL reports grants and personal fees from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and personal fees from Amgen, Bayer, and Boehringer Ingelheim outside the submitted work.
No grants from pharmaceutical companies developing or manufacturing rivaroxaban were involved in this study.
Acknowledgements
We would like to thank Science Valley Research Institute São Paulo, Brazil, for its contribution with modest funding to help access and review The MICHELLE trial database. No other funding was provided.
Footnotes
This article has not been presented anywhere. This article has not been published.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100543.
Appendix A. Supplementary data
References
- 1.Zimmermann I.R., Sanchez M.N., Alves L.C., et al. COVID-19 as the leading cause of hospital deaths in the Brazilian public health system in 2020. Int J Infect Dis. 2021;113:162–165. doi: 10.1016/j.ijid.2021.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P., Zhao W., Kaatz S., Latack K., Schultz L., Poisson L. Factors associated with risk of postdischarge thrombosis in patients with COVID-19. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.35397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannis D., Allen S.L., Tsang J., et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137(20):2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahan C.E., Holdsworth M.T., Welch S.M., Borrego M., Spyropoulos A.C. Deep-vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemostasis. 2011;106(3):405–415. doi: 10.1160/TH11-02-0132. [DOI] [PubMed] [Google Scholar]
- 7.Bishu K.G., Lekoubou A., Kirkland E., et al. Estimating the economic burden of acute myocardial infarction in the US: 12 Year national data. Am J Med Sci. 2020;359(5):257–265. doi: 10.1016/j.amjms.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Ashrani A.A., Heit J.A. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28(4):465–476. doi: 10.1007/s11239-009-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbich M., Globe G., Pantiri K., et al. A systematic review of medical costs associated with heart failure in the USA (2014-2020) Pharmacoeconomics. 2020;38(11):1219–1236. doi: 10.1007/s40273-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spyropoulos A.C., Ageno W., Albers G.W., et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379(12):1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
- 11.Ramacciotti E., Barile Agati L., Calderaro D., et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet (London, England) 2022;399(10319):50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta N., Verma R., Dhiman R.K., Rajsekhar K., Prinja S. Cost-effectiveness analysis and decision modelling: a tutorial for clinicians. J Clin Exp Hepatol. 2020;10(2):177–184. doi: 10.1016/j.jceh.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DATASUS. http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/niuf.def [In pt_BR)]
- 14.Locadia M., Bossuyt P.M.M., Stalmeier P.F.M., et al. Treatment of venous thromboembolism with vitamin K antagonists: patients' health state valuations and treatment preferences. Thromb Haemostasis. 2004;92(6):1336–1341. doi: 10.1160/TH04-02-0075. [DOI] [PubMed] [Google Scholar]
- 15.Brandão S.M.G., Rezende P.C., Rocca H.-P.B.-L., et al. Comparative cost-effectiveness of surgery, angioplasty, or medical therapy in patients with multivessel coronary artery disease: MASS II trial. Cost Eff Resour Allocation. 2018;16(1):55. doi: 10.1186/s12962-018-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teich V., Piha T., Fahham L., et al. Acute coronary syndrome treatment costs from the perspective of the supplementary health system. Arq Bras Cardiol. 2015;105(4):339–344. doi: 10.5935/abc.20150129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam D.J., Beard J.D., Cleveland T., et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet (London, England) 2005;366(9501):1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 18.Marinho C., Monteiro M., Santos L., Oliveira-Filho J., Pinto E.B. Gait performance and quality of life in stroke survivors: a cross-sectional study. Revista Pesquisa em Fisioterapia. 2018;8(1):79–87. [Google Scholar]
- 19.Safanelli J., Vieira L.G.D.R., de Araujo T., et al. The cost of stroke in a public hospital in Brazil: a one-year prospective study. Arq Neuro Psiquiatr. 2019;77(6):404–411. doi: 10.1590/0004-282X20190059. [DOI] [PubMed] [Google Scholar]
- 20.ANVISA . Agência Nacional de Vigilância Sanitária - Anvisa; 2020. Medicines market regulation chamber-ANVISA CMED; p. 787.https://wwwgovbr/anvisa/pt-br/pagina-inicial [Google Scholar]
- 21.Santos M., Cintra M.A., Monteiro A.L., et al. Brazilian valuation of EQ-5D-3L health states: results from a saturation study. Med Decis Making. 2016;36(2):253–263. doi: 10.1177/0272989X15613521. [DOI] [PubMed] [Google Scholar]
- 22.Balestroni G., Bertolotti G. EuroQol-5D (EQ-5D): an instrument for measuring quality of life. Monaldi Archives for Chest Disease = Archivio Monaldi Per Le Malattie Del Torace. 2012;78(3):155–159. doi: 10.4081/monaldi.2012.121. [DOI] [PubMed] [Google Scholar]
- 23.Ware J., Ma K., Keller S.D. Vol. 8. Health Assessment Lab; Boston, MA: 1993. SF-36 physical and mental health summary scales: a user’s manual; pp. 23–28. [Google Scholar]
- 24.Husereau D., Drummond M., Augustovski F., et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG. 2022;129(3):336–344. doi: 10.1111/1471-0528.17012. [DOI] [PubMed] [Google Scholar]
- 25.Dai M.F., Xin W.X., Kong S., Ding H.Y., Fang L. Effectiveness and safety of extended thromboprophylaxis in post-discharge patients with COVID-19: a systematic review and meta-analysis. Thromb Res. 2023;221:105–112. doi: 10.1016/j.thromres.2022.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiolet T., Kherabi Y., MacDonald C.-J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann P.J., Cohen J.T., Weinstein M.C. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 28.Goldin M., Lin S.K., Kohn N., et al. External validation of the IMPROVE-DD risk assessment model for venous thromboembolism among inpatients with COVID-19. J Thromb Thrombolysis. 2021;52(4):1032–1035. doi: 10.1007/s11239-021-02504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenakin T. New lives for seven transmembrane receptors as Drug targets. Trends Pharmacol Sci. 2015;36(11):705–706. doi: 10.1016/j.tips.2015.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.