Abstract
Questions remain regarding the effect of baseline host and exposure factors on vaccine efficacy (VE) across pathogens and vaccine platforms. We report placebo-controlled data from four Phase 3 COVID-19 trials during the early period of the pandemic. This was a cross-protocol analysis of four randomized, placebo-controlled efficacy trials (Moderna/mRNA1273, AstraZeneca/AZD1222, Janssen/Ad26.COV2.S, and Novavax/NVX-CoV2373) using a harmonized design. Trials were conducted in the United States and international sites in adults ≥ 18 years of age. VE was assessed for symptomatic and severe COVID-19. We analyzed 114,480 participants from both placebo and vaccine arms, enrolled July 2020 to February 2021, with follow up through July 2021. VE against symptomatic COVID-19 showed little heterogeneity across baseline socio-demographic, clinical or exposure characteristics, in either univariate or multivariate analysis, regardless of vaccine platform. Similarly, VE against severe COVID-19 in the single trial (Janssen) with sufficient endpoints for analysis showed little evidence of heterogeneity. COVID-19 VE is not influenced by baseline host or exposure characteristics across efficacy trials of different vaccine platforms and countries when well matched to circulating virus strains. This supports use of these vaccines, regardless of platform type, as effective tools in the near term for reducing symptomatic and severe COVID-19, particularly for older individuals and those with common co-morbidities during major variant shifts. Clinical trial registration numbers: NCT04470427, NCT04516746, NCT04505722, and NCT04611802.
Keywords: Comorbidity, Effect modifier, Epidemiologic, Environmental exposure, Occupational exposure, SARS-CoV-2, Vaccine efficacy
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 causing a global pandemic that has resulted in more than 768 million cases and 6·9 million deaths worldwide as of June 2023. Early in the pandemic, the National Institute of Allergy and Infectious Disease (NIAID) at the US National Institutes of Health (NIH) partnered with multiple pharmaceutical companies to expedite the development of an effective vaccine to prevent the spread of SARS-CoV-2. In addition to this unprecedented public–private collaboration, NIAID merged four existing clinical trial networks to form the COVID-19 Prevention Network (CoVPN). The CoVPN, along with the study sites affiliated with contract research organizations (CRO), facilitated the rapid enrollment of the tens of thousands of participants needed for the Phase 3 vaccine safety and efficacy trials [1]. Four SARS-CoV-2 vaccine candidates (mRNA-1273, AZD1222 ChAdOx1 nCoV-19, Ad26.COV2.S, and NVX-CoV2373) have been evaluated through double-blind, randomized, placebo-controlled Phase 3 clinical trials, and were reported to be safe and efficacious in adults ≥ 18 years of age, with early estimates of overall vaccine efficacy (VE) ranging from 56·3 % to 94·1 % [2], [3], [4], [5]. The data from these pivotal Phase 3 VE studies present a unique opportunity to comprehensively evaluate modifiers of COVID-19 VE in preventing symptomatic illness, as well as severe disease, through cross-protocol analysis.
In general, VE is influenced by factors related to the infectious agent (antigenic variants), the vaccine platform, the host (age, sex, genetics, presence of comorbid conditions, and immune function), and the environment (exposure and transmission rate) [6], [7], [8], [9]. For example, VE is lower among men and the elderly for influenza vaccines [7], among the immune suppressed for the hepatitis B vaccine [10], and among children living in low resource settings for rotavirus vaccines [11]. In the individual CoVPN trials, no significant differences in VE based on selected host factors were reported in the subgroup analyses [2], [3], [4], [5]. However, these univariate analyses varied between trials and no multivariate analysis was performed. Here we present a cross-protocol analysis designed with consistent methodology to assess the impact of these modifiers on VE.
2. Materials and methods
2.1. Study design
We performed a participant-level data cross-protocol analysis of the Moderna, AstraZeneca, Janssen, and Novavax trials using [2], [3], [4], [5] data accrued through the blinded, pre-crossover phases from July 2020 through July 2021 (Appendix p. 17, Supplementary Table 1). National populations were excluded if they were enrolled during the circulation of the beta variant or Latin American sites that were not of ancestral/alpha or other lineage due to mismatch with vaccine target (Appendix p. 15, Supplementary Fig. 1).
2.2. Vaccines and adjuvants
The Moderna vaccine was stored between −50 °C an −15 °C and was administered as two doses 28 days apart. The antigen administered was 100 μg mRNA-1273/0.5 mL. The AstraZeneca vaccine was stored between 2 °C and 8 °C and was also administered as two doses 28 days apart. The antigen was AZD1222 (5 × 1010 viral particles)/0.5 mL. The Janssen vaccine was stored between 2 °C and 8 °C and consisted of a single dose of 5 × 1010 viral particles/0.5 mL. The NovaVax vaccine was also stored between 2 °C and 8 °C, was administered as two doses 21 days apart, and the antigen consisted of NVX-CoV2373 (5 μg of SARS-CoV-2 recombinant spike protein adjuvanted with 50 μg of Matrix-M)/0.5 mL. The route of administration was intramuscular in the deltoid muscle of the arm for all of the vaccines. Needle length varied based on the population served with 25–38 mm used for most adults.
2.3. Study outcomes
Symptomatic COVID-19 for this analysis was defined as signs or symptoms consistent with COVID-19 and molecularly confirmed by PCR testing, which was harmonized across the studies with minor differences [1]. These were the primary endpoints from each trial except for ENSEMBLE, for which this corresponds to a secondary endpoint of mild, moderate, or severe COVID-19 (a companion paper lists the COVID-19 case definition used in each trial [1]). Severe COVID-19 was defined as additionally having shortness of breath at rest or respiratory distress, respiratory rate ≥30 per minute, heart rate ≥125 per minute, or oxygen saturation ≤93 % on room air, organ failure, ICU admission, or death.
2.4. Predictors of study outcomes
Potential predictors of the study outcomes included demographic characteristics (age, sex, race, ethnicity, and country); comorbid conditions including asthma, cardiovascular disease, hypertension, diabetes, smoking, obesity (BMI ≥ 30 kg/m2), lung disease, liver disease, kidney disease, and well-controlled HIV; SARS-CoV-2 exposure risk (Occupational Safety and Health Administration [OSHA] risk category); and living situation risk score (Appendix p. 14, Supplementary Methods).
2.5. Ethics approval
Institutional review board approval was obtained for the four COVID-19 VE trials [2], [3], [4], [5] (Moderna/mRNA1273: NCT04470427, AstraZeneca/AZD1222: NCT04516746, Janssen/Ad26.COV2.S: NCT04505722, and Novavax/NVX-CoV2373: NCT04611802). Informed consent was obtained after the nature and possible consequences of the study had been fully explained to the subjects.
2.6. Statistical analysis
The objectives of this study are to: 1) determine which baseline characteristics modify COVID-19 VE in each trial; 2) determine if combined baseline characteristics in each trial modify VE; 3) if there is evidence of heterogeneity of efficacy seen within a study based on baseline characteristics, rank the importance of baseline characteristics compared to the combined impact on VE; and 4) evaluate whether VE-modifying characteristics in three of the trials yield improved prediction of VE in the fourth trial.
Analyses were prespecified in a statistical analysis plan (Appendix p. 2–13). Cumulative incidence VE was estimated for each vaccine through a fixed time point after enrollment in the per-protocol cohort. To ensure stable estimation, for each trial we selected the latest time point where the risk set consisted of at least 10 % of participants in both arms (Appendix p. 18–19, Supplementary Tables 2–3 for a summary of the amount of follow-up in each trial). Inverse weighting was used to provide an interpretable and unbiased analysis of how VE varies within covariate subgroups. Weights were fitted via a proportional odds model, logistic regression, and stratified Kaplan-Meier estimator, respectively (details on Appendix p. 14, Supplementary Methods).
Because each trial assessed a separate vaccine platform that may have its own heterogeneity profile, VE was assessed separately for each trial. Univariate analyses of VE were conducted via a nonparametric covariate-adjusted method. Multivariate analyses were conducted via ensemble methods that use cross-validation to combine predictions from a collection of candidate algorithms. To assess heterogeneity, participants within each trial were broken into tertile groups based on their covariate-stratified VE estimates: those predicted to have the lowest VE, those predicted to have the highest, and everyone else. VE within each of these three subgroups was assessed using the observed COVID-19 endpoints.
We assessed the potential benefits of pooling data from the four trials using a leave-one-trial-out procedure. We pooled data from the other three vaccines to estimate their pooled efficacy conditional on baseline covariates. Then, we included this estimated efficacy as an additional putative efficacy modifier in a repetition of the multivariate analysis in the remaining trial. If the four vaccines have similar heterogeneity profiles, pooling the data should increase precision for assessing heterogeneity.
Missing data in the covariates were minimal and were imputed by the median and mode for continuous and categorical variables, respectively. For a given trial, we report both uncorrected and Bonferroni-corrected 95 % confidence intervals for univariate analyses of VE in supplementary tables, and only uncorrected intervals in figures. Analyses were performed in R version 4.2.1.
3. Results
3.1. Study population
In total, 136,096 participants met the inclusion criteria and were randomized within the four trials and, of these, 114,477 were in the per-protocol cohort and not enrolled in South Africa (due to dominant circulating beta variant) or intersex/unknown sex, and therefore part of our analysis cohort (Appendix p. 16, Supplementary Fig. 2). Across the four trials 77,747 (68 %) participants were 18 to 59 years of age and 36,730 (32 %) were 60 or older (Table 1 ). Women represented 52,651 (46 %) of the participants. In terms of ethnicity, 34,878 (30 %) of the participants were Hispanic. The racial composition of participants across the four trials was 6,188 (5 %) American Indian/Alaska Native, 4,891 (4 %) Asian, 10,542 (9 %) Black or African American, and 86,518 (76 %) White. Regarding clinical comorbidities, 42,790 (37 %) of the participants had at least one of the following: diabetes, HIV, or cardiovascular, kidney, liver, or chronic lung disease; 38,761 (34 %) of the participants were obese. Tobacco use was reported by 14,347 (13 %) of participants.
Table 1.
Symptomatic Covid-19 endpointsa by subgroup and randomization arm in the per-protocol cohort of each trial excluding South African participants and intersex participants (# endpoints / total # participants).
|
Moderna |
AstraZeneca |
Janssen |
Novavax |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Vaccine | Placebo | Vaccine | Placebo | Vaccine | Placebo | Vaccine | |
| Overall | 741/14164 | 55/14287 | 181/8528 | 134/17617 | 538/17113 | 173/17111 | 77/8385 | 17/17272 |
| Socio-demographic | ||||||||
| Age (years) | ||||||||
| 18–29 | 88/1391 | 5/1417 | 29/1072 | 33/2209 | 96/1762 | 32/1774 | 26/1259 | 4/2735 |
| 30–39 | 138/2143 | 11/2169 | 31/1365 | 22/2756 | 71/1970 | 32/1992 | 14/1649 | 5/3338 |
| 40–49 | 180/2665 | 12/2597 | 42/1526 | 41/3281 | 112/3507 | 46/3526 | 14/1676 | 2/3601 |
| 50–59 | 170/2878 | 12/2969 | 39/1762 | 27/3658 | 136/3740 | 25/3680 | 14/1923 | 4/3757 |
| 60–69 | 109/3240 | 10/3339 | 33/1875 | 9/3818 | 90/4437 | 32/4360 | 7/1433 | 1/2946 |
| ≥70 | 56/1847 | 5/1796 | 7/928 | 2/1895 | 33/1697 | 6/1779 | 2/445 | 1/895 |
| Ethnicity | ||||||||
| Hispanic/Latino | 176/2787 | 10/2831 | 59/2064 | 59/4032 | 250/8889 | 93/8767 | 18/1801 | 9/3707 |
| Not Hispanic/ Latino | 565/11377 | 45/11456 | 122/6464 | 75/13585 | 288/8224 | 80/8344 | 59/6584 | 8/13565 |
| Race | ||||||||
| American Indian/Alaska Nativeb | 5/113 | 0/109 | 20/372 | 26/747 | 65/1616 | 27/1641 | 6/522 | 1/1068 |
| Asian | 29/700 | 1/628 | 4/355 | 4/738 | 15/639 | 5/699 | 5/375 | 0/757 |
| Black or African American | 41/1352 | 4/1395 | 15/699 | 6/1401 | 30/1451 | 10/1416 | 8/947 | 1/1881 |
| Other | 30/422 | 1/464 | 3/164 | 2/338 | 24/621 | 6/589 | 1/54 | 0/146 |
| Multiple | 8/304 | 1/300 | 10/203 | 7/421 | 36/951 | 9/928 | 0/137 | 2/296 |
| White | 628/11273 | 48/11391 | 129/6735 | 89/13972 | 368/11835 | 116/11838 | 57/6350 | 13/13124 |
| Sex | ||||||||
| Female | 365/6670 | 25/6848 | 66/3714 | 56/7732 | 239/7629 | 73/7617 | 50/4158 | 10/8283 |
| Male | 376/7494 | 30/7439 | 115/4814 | 78/9885 | 299/9484 | 100/9494 | 27/4227 | 7/8989 |
| Health Characteristics | Placebo | Vaccine | Placebo | Vaccine | Placebo | Vaccine | Placebo | Vaccine |
| Body mass index (kg/m2) | ||||||||
| Healthy weight or Underweight (<25) | 150/3861 | 6/3970 | 41/2482 | 32/5275 | 163/5379 | 58/5501 | 29/2463 | 4/5220 |
| Overweight (≥25, <30) | 267/4938 | 20/4857 | 62/3079 | 44/6406 | 245/7055 | 73/6901 | 14/2719 | 7/5610 |
| Obese (≥30) | 324/5365 | 29/5460 | 78/2967 | 58/5936 | 130/4679 | 42/4709 | 34/3203 | 6/6442 |
| Class 3 Obese (≥40) | 74/995 | 7/1015 | 12/473 | 10/976 | 17/601 | 7/643 | 7/572 | 3/1267 |
| Cardiovascular Disease | 196/4472 | 19/4468 | 49/2392 | 20/5057 | 116/4550 | 24/4422 | 18/2021 | 4/4059 |
| Diabetes | 76/1468 | 5/1484 | 19/873 | 10/1627 | 42/1587 | 17/1608 | 7/858 | 2/1622 |
| HIV | 5/87 | 0/93 | 6/134 | 1/278 | 7/274 | 3/240 | 1/49 | 0/135 |
| Kidney Disease | 4/74 | 0/73 | 2/45 | 0/131 | 3/113 | 0/106 | 1/56 | 0/125 |
| Liver disease | 5/101 | 1/113 | 9/160 | 1/308 | 6/168 | 2/169 | 0/62 | 1/134 |
| Chronic Lung Disease | 37/808 | 5/808 | 13/1055 | 14/2027 | 44/1150 | 16/1114 | 9/1264 | 1/2461 |
| Risk Characteristics | Placebo | Vaccine | Placebo | Vaccine | Placebo | Vaccine | Placebo | Vaccine |
| Workplace Risk of Exposurec | ||||||||
| Low | 49/2666 | 36/5204 | 497/16355 | 159/16390 | 39/4951 | 7/9956 | ||
| Medium | 368/6735 | 30/6760 | 93/3765 | 61/7734 | 14/229 | 3/249 | 27/2670 | 6/5607 |
| High | 373/7429 | 25/7527 | 39/2097 | 37/4679 | 27/529 | 11/472 | 11/764 | 4/1709 |
| Risk from Living Conditiond | ||||||||
| Low | 113/2354 | 8/2322 | 78/4365 | 47/9044 | 232/8676 | 66/8739 | 57/6808 | 13/14002 |
| Medium | 564/10229 | 40/10348 | 33/1593 | 25/3227 | 189/4978 | 57/4923 | 16/1117 | 3/2274 |
| High | 40/1179 | 5/1165 | 29/1336 | 25/2744 | 97/2694 | 38/2683 | 2/339 | 0/733 |
| Very High | 24/402 | 2/452 | 41/1234 | 37/2602 | 20/765 | 12/766 | 2/121 | 1/263 |
| Tobacco use | 12/253 | 2/236 | 31/1705 | 22/3471 | 8/369 | 5/374 | 24/2609 | 6/5330 |
| Geographic Location | ||||||||
| USA | 741/14164 | 55/14287 | 145/7423 | 89/15389 | 331/9121 | 95/9156 | 73/7887 | 16/16261 |
| Argentina | 44/1414 | 20/1400 | ||||||
| Brazil | 43/3385 | 12/3394 | ||||||
| Chile | 10/670 | 9/1358 | 6/539 | 3/528 | ||||
| Colombia | 86/1862 | 34/1856 | ||||||
| Mexico | 5/218 | 2/207 | 4/498 | 1/1011 | ||||
| Peru | 26/435 | 36/870 | 23/574 | 7/570 | ||||
Excluded variants were: Beta, Delta, Epsilon, Eta, Gamma, Iota, Lambda, Mu, Kappa, and Zeta.
All participants were right censored at time t0 regardless of whether they were observed to experience event after t0. Separate time points t0 were chosen for each trial such that about 10 % participants are at risk in the vaccine arm.
Category is defined across all clinical sites. Indigenous people from South America were classified together with the American Indian or Alaska Native United States and Mexico demographic according to the FDA definition (American Indian or Alaska Native: A person having origins in any of the original peoples of North and South America (including Central America), and who maintains tribal affiliation or community attachment). In this analysis, the Moderna, AstraZeneca, Janssen and Novavax trials included 222, 1119, 3257, and 1590 participants, respectively, who identified as American Indian or Alaskan Native from North America.
Detailed derivation of exposure risk based on OSHA categories is provided in Supplemental Methods.
Living condition encompasses housing type and household size, detailed derivation provided in Supplemental Methods.
3.2. Baseline covariates have little impact on VE against symptomatic COVID-19 in univariate and multivariate analyses
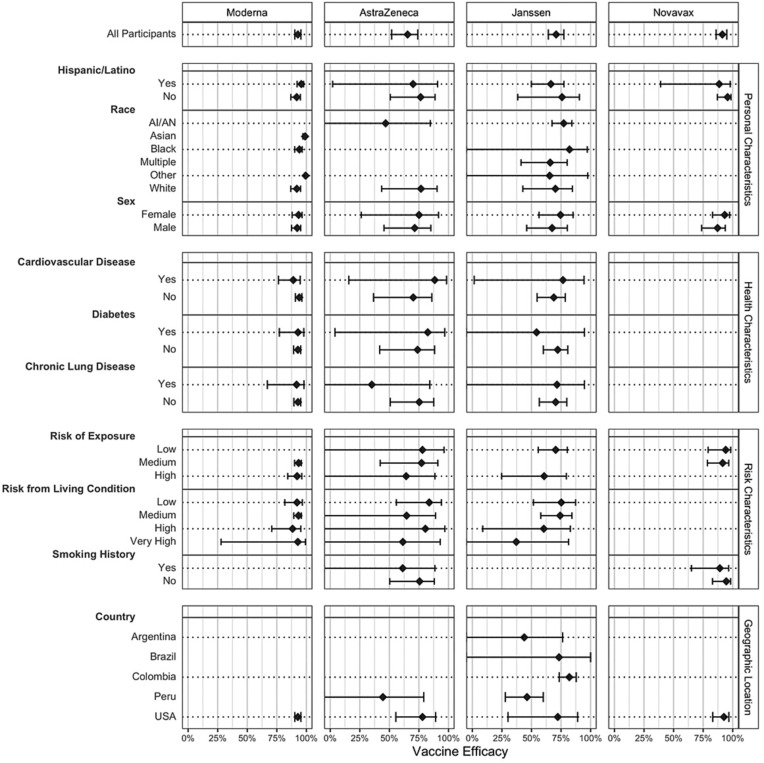
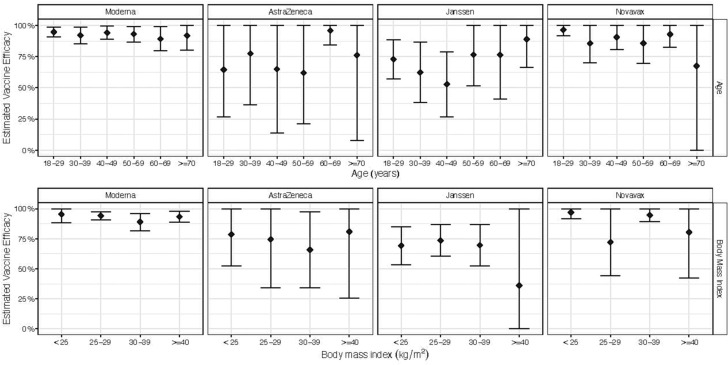
In the analysis using harmonized case definitions, overall VE for preventing symptomatic COVID-19 was 93 % (90–95 %) in Moderna, 65 % (54–76 %) in AstraZeneca, 71 % (64–77 %) in Janssen, and 91 % (87–96 %) in Novavax (Fig. 1 , Appendix p. 20–21, Supplementary Table 4). While VE showed little heterogeneity for participants enrolled at sites within the US, VE ranged from 44 to 82 % for participants in the Janssen trial across different countries (Fig. 1, Appendix p. 20–21, Supplementary Table 4). No difference was observed in VE between older adults and younger populations or based on BMI, race, sex, underlying health conditions, and risk of exposure (see Fig. 2 ).
Fig. 1.
Estimates of vaccine efficacy against symptomatic Covid-19 within subgroups defined by categorical baseline covariates, with corresponding 95 % confidence intervals. In the AstraZeneca an Novavax studies, the number of events among Black/African-American participants was too low to estimate VE.
Fig. 2.
Estimated vaccine efficacy by age and body mass index (BMI) across the four trials, with corresponding 95 % confidence intervals. Estimates and intervals are derived according to a working model that enforced that the relative risk of a Covid-19 endpoint on vaccine versus on placebo must be log-linear.
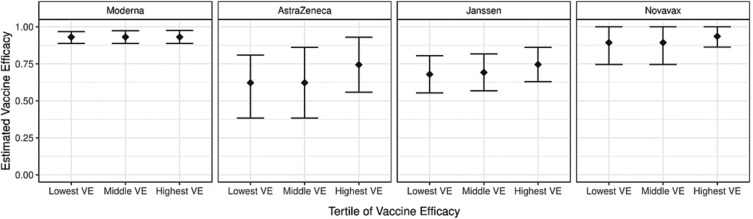
When participants with the lowest predicted VE were compared to participants with the highest predicted VE for each trial, there was little variability in estimates of VE across the subgroups defined by baseline covariates (Fig. 3 ). When tertiles of VE were pooled from three trials, and then used to predict VE of the fourth, and no heterogeneity was found in the subgroups formed by baseline covariates (Appendix p. 22, Supplementary Table 5).
Fig. 3.
Estimated tertiles of vaccine efficacy (VE) against Covid-19 endpoint as defined by all baseline covariates, with corresponding 95 % confidence intervals (CIs). For each vaccine, vaccine efficacy was first predicted using all baseline covariates, and then participants were broken into three subgroups as defined by this prediction: the 33·33 % with lowest predicted VE (Lowest VE), the 33·33 % with the next lowest predicted VE (Middle VE), and the 33·33 % with the highest predicted VE (Highest VE). Vaccine efficacy was then estimated in these subgroups using a cross-validation method. If needed, estimates were projected to satisfy the population-level constraint that efficacy should be nondecreasing when moving from the first to the third tertile of participants.
3.3. VE against severe COVID-19 remained high across all demographic subsets
VE for severe COVID-19 was restricted to the Janssen trial due to very few endpoints in the vaccine arm of the other three trials (Appendix p. 23, Supplementary Table 6). VE without modeling the effect of baseline covariates was 85 % (78–91 %) and did not vary substantially by demographics, obesity, living conditions, or geographic region (Appendix p. 26, Supplementary Table 7). In multivariate analysis, there was also no evidence of heterogeneity across tertiles of VE (Appendix p. 27, Supplementary Table 8).
4. Discussion
Understanding the factors that impact VE is crucial to ensure that vaccines are effective in a wide variety of hosts and settings. The ability to evaluate host and environmental factors that may modify VE is frequently limited by trial design, which often excludes vulnerable populations or may be too small to allow for subgroup analysis. Further, the lack of harmonization across different efficacy studies often prevents larger scale, cross-protocol analysis. The COVID-19 pandemic and subsequent coordinated worldwide engagement in the rapid development of vaccines created an opportunity to evaluate host characteristics and environmental factors in a novel cross-protocol analysis of pivotal studies conducted early in the pandemic. In these trials, populations known to be at high risk for acquisition of the disease or for developing severe disease were enrolled. Thorough baseline information was recorded for all participants, allowing a more nuanced view of VE in different subgroups during an extraordinarily well-characterized period of global risk. This multi-national harmonized collaboration offers an unprecedented chance to compare COVID-19 VE in a large, diverse adult sample using a consistent definition of disease, exposure risk, and statistical methodology across vaccine platforms.
The COVID-19 pandemic highlighted the significant impact host and demographic factors can have on health outcomes. The dramatic disparities in COVID-19-related morbidity and mortality seen in elderly patients, populations of color, and those with various comorbidities further underscores the need to understand the potential impact of these factors on responses to available COVID-19 vaccines [12], [13], [14], [15], [16]. Our study revealed that these individual characteristics that raised the risk of COVID-morbidity and mortality did not impact VE against symptomatic and severe COVID-19. Our findings confirm those of real-world studies [17], which have shown similar VE across populations and age groups.
The aging process is accompanied by senescence of the immune system often characterized by dysregulated inflammatory responses and impairments in the processes of B and T cell differentiation [18]. The immune response to COVID-19 is a salient example. Early in the pandemic, age ≥ 65 years was recognized as a risk factor for severe health outcomes associated with COVID-19 [19]. Mechanisms hypothesized to explain differences in the clinical course of COVID-19 by age include lower levels of interferon gene expression and T cell diversity in older adults [20]. It therefore became important to evaluate VE in the elderly and to prioritize vaccinating them once an approved vaccine became available. Older adults are still the age group with the highest number of incident COVID-19 cases, and the highest rates of hospitalization and death and clinical treatment guidelines characterize age as the most important risk factor for progression to severe COVID-19. In this analysis of randomized controlled trials conducted early in the pandemic, we did not observe a difference in VE between older adults and younger populations. Similarly, observational studies conducted early in the pandemic, reported that the Moderna and Pfizer/BNT162b2 (Pfizer/BNT) vaccines were found to be highly effective (94 %) in preventing hospitalization in fully vaccinated individuals ≥65 years of age [21] and a single dose of the Janssen vaccine was >84 % effective at preventing severe disease in older adults [22]. Thus, these highly effective vaccines using the same dose as younger persons resulted in similar short-term protection. Whether response to boosting, alteration in the circulating virus transmission kinetics, or immune escape characteristics alter our observations is unknown and worthy of continued monitoring and evaluation. In addition, observational studies of the real-world effectiveness of the primary series of the Pfizer/BNT vaccine reported no significant differences in vaccine effectiveness based on individual characteristics like elevated body mass index, hypertension, or type 2 diabetes; however, the presence of three or more comorbidities was associated with slightly lower vaccine effectiveness [16].
A significant concern early in the COVID-19 pandemic was the risk to individuals being regularly exposed to the virus. Our findings showing that risk of exposure had little impact on VE in the short term is supported by multiple reviews of workers in high-risk occupations who were among the first to receive COVID-19 vaccinations. Several observational studies have explored the relationship between occupation and COVID-19 VE in health care personnel [23], [24], [25], [26], first responders, and other essential frontline workers [5], [27]. Prior to the emergence of variants, vaccine effectiveness against COVID-19 in fully vaccinated health care workers ranged from 85 % for Pfizer/BNT in Israel to 100 % for Moderna in France [23], [24]. All existing data confirm that personal or environmental variables do little to blunt the short-term effectiveness of a variety of formulations of COVID-19 vaccines in comparison to the VE observed in the trials, and that effectiveness is comparable over a range of vaccine recipients [1].
The number of severe endpoints across both arms was sufficient to evaluate modifiers of VE only for the ENSEMBLE trial. In this trial, which also was the only trial that evaluated a single dose vaccine regimen, VE against severe disease was 74·6 % ≥28 days after vaccination [3], and did not vary substantially by age or comorbidities [28]. A recent review and network meta-analysis comparing the VE of COVID-19 vaccines found no statistical difference in risk of severe disease among the eight COVID-19 vaccine Phase 3 randomized controlled trials, suggesting that there is limited heterogeneity across vaccine platforms [29]. Overall, vaccines against COVID-19 are incredibly successful at preventing the most severe outcomes of disease, which is encouraging as new platforms and updated vaccines are rolled out and the virus continues to mutate at a rapid pace.
Interrogation of VE within subgroups is a significant challenge for individual studies let alone across large, multinational trials. Our analysis offers several improvements over previous studies of the heterogeneity of COVID-19 VE [2], [3], [4], [5]. First, the trials analyzed here had a harmonized design utilizing very similar endpoints and sample sizes, and all were placebo-controlled. We also used a consistent method to analyze randomized trial datasets across the different vaccine regimens, ensuring that the endpoint and covariate definitions were harmonized. Additionally, our analysis made use of all available blinded, placebo-controlled follow-up data. To provide unbiased and generalizable findings, we used inverse probability weighting in our analysis. Furthermore, we conducted a multivariate analysis using a state-of-the-art machine learning method to evaluate the extent to which multiple baseline covariates can predict VE.
Our study had several limitations. First, while we found that VE after the primary vaccination series was not influenced by baseline host or risk characteristics in the four Phase 3 vaccine efficacy trials, we have limited insight into whether the variables examined have a long-term effect on VE due to the relatively short follow up in the blinded/pre-crossover periods of these trials. Due to the high efficacy of vaccines, the period for accumulating cases for determination of VE was brief, limited to the interval in each study prior to crossover to active vaccine and termination of the placebo-controlled design and/or emergency use authorization and subsequent vaccine availability across populations. Second, the vaccines were each designed with the same target on SARS-CoV-2, the Spike protein, and had a good match with circulating strains at the time. This protein, while important for establishing an immune response in each of these vaccine platforms, has also been subject to rapid, persistent mutation, and VE of these vaccines against less-matched variants may decrease as virus variants emerge. Therefore, our findings may not apply to future data where longer follow up is observed in the context of emerging variants. Third, the Janssen vaccine trial data referenced here was a single dose series, while the other three studies used two-dose regimens, and none included a booster dose, which is the current standard of care, in the blinded/pre-crossover phase of the trials. Fourth, the trials enrolled in a variety of locations worldwide; while this enhanced the racial and ethnic diversity of the population within each trial, the spread of viral variants was occurring at different times in different locales, likely affecting enrollment of trial participants, and variability in VE estimates. Fifth, while we were able to analyze most factors commonly cited as affecting VE, because of small participant numbers we could not assess the effect of immunocompromising conditions like HIV or organ transplantation with suboptimal responses to COVID-19 vaccines. Finally, as the four trials had different start and end dates, differences in timing could have introduced uncontrolled confounders. We attempted to address this by controlling for the effect of variants in the statistical analysis.
Our study supports that VE was high early in the pandemic independent of vaccine platform, host factors, and settings. The recruitment of racially and ethnically diverse participants, and the availability of placebo-controlled data enabled us to conclude that the vaccines were effective for all, including those most at risk for COVID-19. The responses to COVID-19 vaccines currently vary based on interval since last exposure to the virus, the immunologic background of the host, and the continued emergence of variants of concern such as omicron sublineages predominating in China, Europe, and the United States as the pandemic enters its third year. This analysis provides strong support for development and use of vaccines that are well-matched to circulating virus strains regardless of vaccine platform type, as effective tools in the near term for reducing symptomatic and severe COVID-19 infections, particularly for high-risk individuals during major variant shifts in the circulating SARS-CoV-2 strain [30]. This has broad implications for future COVID-19 vaccination strategy development and provides strong support that vaccination remains the best tool to prevent the most severe and debilitating forms of COVID-19.
5. Data sharing
Access to data underlying findings described in this manuscript may be allowed in accordance with the individual data sharing policies of the pharmaceutical companies contributing data to this analysis. As each of the clinical trials included in this meta-analysis are ongoing, data availability will begin after publication of the final study results in 2023 and 2024.
Funding
This work was supported by the Biomedical Advanced Research and Development Authority (BARDA) and National Institute of Allergy and Infectious Diseases (NIAID) (grants UM1 AI068614 and UM1 AI068635). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
CRediT authorship contribution statement
Christine B. Turley: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft. LaKesha Tables: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft. Trevon Fuller: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization. Lisa J. Sanders: Conceptualization, Methodology, Writing – original draft. Hyman Scott: Conceptualization, Methodology. Amaran Moodley: Conceptualization, Methodology, Writing – original draft. Amanda Woodward Davis: Methodology, Project administration. Sijia Li: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization. Lars van der Laan: Methodology, Software. Peter B. Gilbert: Conceptualization, Funding acquisition, Methodology. Lindsey R. Baden: Investigation. Paul Goepfert: Investigation. Karen Kotloff: Investigation. Cynthia L. Gay: Investigation. Ann R. Falsey: Investigation. Hana M. El Sahly: Investigation. Magdalena E. Sobieszczyk: Investigation. Yunda Huang: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation. Kathleen M. Neuzil: . Lawrence Corey: Funding acquisition. Beatriz Grinsztejn: Conceptualization, Investigation, Project administration. Glenda Gray: Conceptualization, Investigation, Project administration. Nadine Rouphael: Conceptualization, Project administration, Supervision. Alex Luedtke: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Visualization, Writing – original draft.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Brett Leav reports a relationship with Moderna Inc that includes: employment. Jacqueline Miller reports a relationship with Moderna Inc that includes: employment. Kathryn Shoemaker reports a relationship with AstraZeneca R&D Gaithersburg that includes: employment and equity or stocks. An Vandenbosch reports a relationship with Janssen Pharmaceuticals Inc that includes: employment. Jerald Sadoff reports a relationship with Janssen Pharmaceuticals Inc that includes: employment. Wayne Woo reports a relationship with Novavax Inc that includes: employment. Iksung Cho reports a relationship with Novavax Inc that includes: employment. Lisa M. Dunkle reports a relationship with Novavax Inc that includes: employment. Peter Gilbert reports a relationship with Janssen Pharmaceuticals Inc that includes: consulting or advisory. Hana M. El Sahly reports a relationship with Gilead Sciences Inc that includes: funding grants. Hana M. El Sahly reports a relationship with Janssen Global Services LLC that includes: funding grants. Hana M. El Sahly reports a relationship with Merck & Co Inc that includes: funding grants. Hana M. El Sahly reports a relationship with Sanofi Pasteur Inc that includes: funding grants. Kathleen M. Neuzil reports a relationship with Pfizer Inc that includes: funding grants. Ann R. Falsey reports a relationship with Biofire Diagnostics Inc that includes: funding grants. Ann R. Falsey reports a relationship with Janssen Biotech Inc that includes: funding grants. Ann R. Falsey reports a relationship with Merck & Co Inc that includes: funding grants. Ann R. Falsey reports a relationship with Pfizer Inc that includes: funding grants. Ann R. Falsey reports a relationship with Novavax Inc that includes: consulting or advisory.
Acknowledgments
Acknowledgments
The COVID-19 Prevention Network (CoVPN) was formed through a partnership of multiple existing NIAID-funded clinical trial networks, including the HIV Vaccine Trials Network (HVTN), Vaccine Treatment and Evaluation Units (VTEUs), the HIV Prevention Trials Network (HPTN), the AIDS Clinical Trials Group (ACTG), and the Infectious Diseases Clinical Research Consortium (IDCRC). We would also like to acknowledge and thank the trial participants, caregivers, investigators, health care providers, and research staff who contributed to the four trials.
All authors attest they meet the ICMJE criteria for authorship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.06.066.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Mena Lora A.J., Long J.E., Huang Y., Baden L.R., El Sahly H.M., Follmann D., et al. Rapid development of an integrated network infrastructure to conduct Phase 3 COVID-19 vaccine trials. JAMA Network Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.51974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunkle L.M., Kotloff K.L., Gay C.L., Áñez G., Adelglass J.M., Barrat Hernández A.Q., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2021;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falahi S., Kenarkoohi A. Host factors and vaccine efficacy: Implications for COVID-19 vaccines. J Med Virol. 2022;94:1330–1335. doi: 10.1002/jmv.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhakal S., Klein S.L. Host factors impact vaccine efficacy: implications for seasonal and universal influenza vaccine programs. J Virol. 2019;93:e00797–e819. doi: 10.1128/JVI.00797-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaslow D.C. Force of infection: a determinant of vaccine efficacy? NPJ Vaccines. 2021;6:51. doi: 10.1038/s41541-021-00316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langwig K.E., Gomes M.G.M., Clark M.D., Kwitny M., Yamada S., Wargo A.R., et al. Limited available evidence supports theoretical predictions of reduced vaccine efficacy at higher exposure dose. Sci Rep. 2019;9:3203. doi: 10.1038/s41598-019-39698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee G.-H., Lim S.-G. CpG-adjuvanted Hepatitis B vaccine (HEPLISAV-B®) update. Expert Rev Vaccines. 2021;20:487–495. doi: 10.1080/14760584.2021.1908133. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho M.F., Gill D. Rotavirus vaccine efficacy: current status and areas for improvement. Hum Vaccin Immunother. 2019;15:1237–1250. doi: 10.1080/21645515.2018.1520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magesh S., John D., Li W.T., Li Y., Mattingly-app A., Jain S., et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic review and meta-analysis. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butt A.A., Talisa V.B., Yan P., Shaikh O.S., Omer S.B., Mayr F.B. Real-world effectiveness of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) mRNA vaccines in preventing confirmed infection in patients on chronic hemodialysis. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chodick G., Tene L., Rotem R.S., Patalon T., Gazit S., Ben-Tov A., et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74:472–478. doi: 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas S.J., Perez J.L., Lockhart S.P., Hariharan S., Kitchin N., Bailey R., et al. Efficacy and safety of the BNT162b2 mRNA COVID-19 vaccine in participants with a history of cancer: subgroup analysis of a global phase 3 randomized clinical trial. Vaccine. 2022;40:1483–1492. doi: 10.1016/j.vaccine.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinti M., Appay V., Campisi J., Frasca D., Fülöp T., Sauce D., et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Klein S.L., Garibaldi B.T., Li H., Wu C., Osevala N.M., et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65 doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida M., Worlock K.B., Huang N., Lindeboom R.G.H., Butler C.R., Kumasaka N., et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature. 2022;602:321–327. doi: 10.1038/s41586-021-04345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenforde M.W., Olson S.M., Self W.H., Talbot H.K., Lindsell C.J., Steingrub J.S., et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 Years - United States, January-March 2021. Morb Mortal Wkly Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moline H.L., Whitaker M., Deng L., Rhodes J.C., Milucky J., Pham H., et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥65 Years - COVID-NET, 13 states, February-April 2021. Morb Mortal Wkly Rep. 2021;70:1088–1093. doi: 10.15585/mmwr.mm7032e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angel Y., Spitzer A., Henig O., Saiag E., Sprecher E., Padova H., et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325:2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paris C., Perrin S., Hamonic S., Bourget B., Roué C., Brassard O., et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect. 2021;27 doi: 10.1016/j.cmi.2021.06.043. 1699.e5-.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., et al. Effectiveness of mRNA Covid-19 vaccine among U.S. health care personnel. New Engl J Med. 2021;385:e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallal P.C., Hartwig F.P., Horta B.L., Silveira M.F., Struchiner C.J., Vidaletti L.P., et al. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho R.J.Y. Warp-Speed Covid-19 vaccine development: beneficiaries of maturation in biopharmaceutical technologies and public-private partnerships. J Pharm Sci. 2021;110:615–618. doi: 10.1016/j.xphs.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardt K., Vandebosch A., Sadoff J., Le Gars M., Truyers C., Lowson D., et al. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2022;22:1703–1715. doi: 10.1016/S1473-3099(22)00506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotshild V., Hirsh-Raccah B., Miskin I., Muszkat M., Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acevedo M.L., Gaete-Argel A., Alonso-Palomares L., de Oca M.M., Bustamante A., Gaggero A., et al. Differential neutralizing antibody responses elicited by CoronaVac and BNT162b2 against SARS-CoV-2 Lambda in Chile. Nat Microbiol. 2022;7:524–529. doi: 10.1038/s41564-022-01092-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.