Synopsis
The unprecedented scope of the coronavirus disease 2019 (COVID-19) pandemic resulted in numerous disruptions to daily life, including for people with multiple sclerosis (PwMS). In this article, we review how disruptions in MS care prompted innovations in delivery of care (e.g., via telemedicine) and mobilized the global MS community to rapidly adopt safe and effective practices. We discuss how our understanding of the risks of COVID-19 in PwMS has evolved along with recommendations pertaining to disease-modifying therapies (DMTs) and vaccines. With lessons learned during the COVID-19 pandemic, we examine potential questions for future research in this new era of MS care.
Key Words: COVID-19, multiple sclerosis, telemedicine, registries, disease-modifying therapy, vaccination
Key Points
-
•
People with MS (PwMS) experienced disruptions in their care and everyday lives during the COVID-19 pandemic.
-
•
Innovations such as telemedicine helped preserve access to clinicians, while its optimal application to future MS care remains a topic of debate.
-
•
Data from large MS registries proved to be informative regarding risks associated with COVID-19 and interactions with MS disease-modifying therapies.
-
•
Many of the risk factors for poor outcomes in COVID-19 for PwMS are similar to those in the general population (e.g., older age, black race); among PwMS, greater disability and B cell depleting therapies are associated with increased risk.
-
•
Vaccines against COVID-19 are safe and effective for PwMS, although humoral responses to vaccination are blunted by certain disease-modifying therapies.
Clinics care points
-
•
Until more data are available, use of telemedicine for MS care should be based on the preferences of people with MS and providers along with local regulations.
-
•
Considering currently available safety data, disease-modifying therapies can be started and sequenced similarly post-pandemic compared to the pre-pandemic era, assuming risks and benefits are discussed in detail with each person with MS.
-
•
Anti-CD20 monoclonal antibody therapies remain first-line options for some, and people on these therapies should be counseled about increased infection risk along with the possibility of impaired vaccine responses. Extended interval dosing requires further investigation, should be considered in select cases, and has relevance beyond the scope of COVID-19.
-
•
COVID-19 vaccines are recommended for people with MS and do not appear to be associated with an increased risk of relapse.
SDN: received consultant fees for scientific advisory boards from Biogen, Genentech, Bristol Myers Squibb, EMD Serono, Greenwich Biosciences, Horizon Therapeutics, Novartis, TG Therapeutics; study lead PI for a Roche clinical trial program; advisor to Autobahn; received research funding (paid directly to institution) from Biogen, Lundbeck, Roche, Genentech, National MS Society, The Stiff Person Syndrome Research Foundation, Department of Defense, and Patient Centered Outcomes Research Institute.
Introduction
As of April 2023, there have been over 760 million confirmed cases and 6.8 million deaths worldwide due to coronavirus disease 2019 (COVID-19).1 In the United States, there have been more than 100 million cases and 1 million deaths.2 The pandemic resulted in profound disruptions to society and healthcare systems globally.
For people with multiple sclerosis (PwMS) and their clinicians, the COVID-19 pandemic presented significant challenges. It not only affected the psychosocial well-being of PwMS but also caused major interruptions in routine MS care.3 For example, missed clinical, laboratory, and imaging appointments related to the pandemic made it more difficult for clinicians to monitor disease activity and quality-of-life issues in PwMS.4 Uncertainty surrounding the safety of MS disease-modifying therapies (DMTs) due to their varied effects on the immune system was also a major concern.
In this review, we will discuss the broad impact of the COVID-19 pandemic on MS care. We will highlight lessons learned by the MS community regarding delivery of care, COVID-19 risks, DMT selection, and strategies to optimize the efficacy of vaccinations against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We will conclude by examining implications for future care as we transition from the COVID-19 global health emergency to a phase of endemic and seasonal infection.5
Disruptions along the continuum of MS care due to COVID-19
The COVID-19 pandemic caused significant disruption for PwMS and the public. Lockdowns and physical distancing measures which were implemented for public safety made it difficult to access routine care for chronic conditions like MS. Naturally, there was uncertainty about which activities outside the home could be done safely and, in some jurisdictions, PwMS may not have been permitted to leave home, with rare exceptions.
Interruptions along the continuum of MS care were common during the pandemic. A cross-sectional survey of more than 1000 PwMS conducted in April 2020 found that 22% cancelled a visit with their neurologist, 11% cancelled an MRI, 21% cancelled a laboratory test, and 10% altered the administration schedule of their DMT.6 Another study of more than 4000 individuals with autoimmune disorders, of whom more than 800 were PwMS showed that nearly half experienced an interruption in healthcare services.7 Delays in infusions and lost rehabilitation visits were frequent sources of disruption.8 Surveys of MS care providers confirmed that postponements in usual care were common and that providers were concerned about the risk COVID-19 posed to PwMS and themselves.9 , 10 Along with consternation about being able to safely monitor PwMS, many providers expressed misgivings about the risk-benefit ratio of using higher efficacy DMTs in the context of the pandemic.9 , 10 Whether disruptions in MS care resulted in any long-lasting consequences at an individual level is currently unknown.
Several studies also showed that changes to daily activities including work and socialization as a result of the pandemic were common among PwMS (i.e., remaining at home, using virtual methods), similar to the general population.8 , 11 Despite the heterogeneity of PwMS regarding health-related quality of life and disability, it was suggested that substantial psychosocial and occupational change might have a greater impact for PwMS, particularly those with pre-existing activity limitations.11 , 12 In one such study, women with MS were more likely than men with MS to experience job termination or furlough during the pandemic and expressed greater concern about the risk posed by COVID-19 to their health.6 Moreover, psychological distress amongst PwMS pertaining to COVID-19 risk adversely affected their well-being, particularly when few effective treatments and no vaccines were available during the early pandemic.13
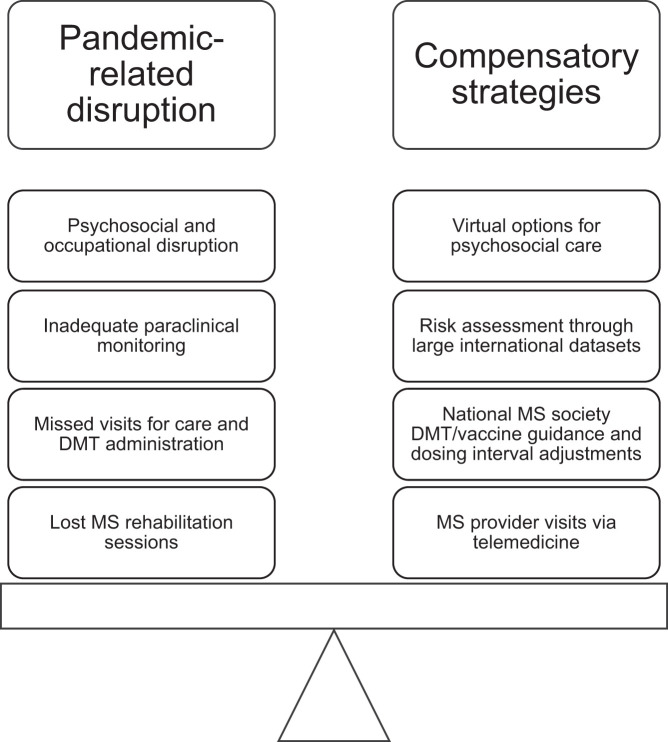
Altogether, public health measures put in place to protect us from COVID-19 no doubt had unintended impacts on MS care and required compensatory strategies to counterbalance them (Figure 1 ). The next section will explore innovative care delivery methods that were accelerated during the pandemic to keep PwMS connected with their providers.
Figure 1.
Disruptions and compensatory strategies in the COVID-19 era of MS care. Examples provided depict how compensatory strategies and innovations by the MS community helped counterbalance pandemic-related disruptions to care.
Bringing care to people with MS using telemedicine
Telemedicine, or telehealth, leverages the ability for individuals and their providers to connect despite not being physically present in the same location. Telemedicine was already being used for chronic and acute medical care prior to the COVID-19 pandemic and can take a variety of forms. These include synchronous contact over an audiovisual platform using the internet, or asynchronous methods such as pre-recorded or other electronic communication.14 Studies prior to the COVID-19 pandemic demonstrated that assessment of PwMS, including disability measures, is feasible in a virtual format.15 , 16 In response to pandemic-related disruptions, many MS centers started conducting most visits using telemedicine.17 , 18
Several studies highlighted benefits of telemedicine for PwMS, including improved access for those who live far from an MS center and those with mobility issues.17 , 19 Some PwMS have greater comfort being in their home environment and having additional carers present virtually who may not otherwise be present at in-person visits.20 Additionally, those with higher disability may benefit from more frequent clinical touchpoints by supplementing in-person visits with telemedicine appointments. Surveys suggest that most neurologists and PwMS who used telemedicine during the COVID-19 pandemic were satisfied with its use, but we don’t yet know whether this translates to better MS disease outcomes.17 , 21 Of great concern is that subtle changes on neurological exam could be missed when using a virtual platform, resulting in inappropriately maintaining therapies that have suboptimal effectiveness.
There are other concerns that telemedicine could make accessing care harder for certain PwMS. For example, persons of lower socioeconomic status (SES), health literacy, and skill with technology may have more difficulty using telemedicine.3 In at least one study, this concern did not bear out given that providers were able to conduct follow-up visits using mainly smartphones (and these are globally available at relatively low cost).15 , 20 It is notable that most surveys examining satisfaction with telemedicine sampled individuals who were of higher SES and may not be generalizable to marginalized populations.3 , 12
Arguably, qualitative evaluation of telemedicine during the exceptional circumstance of the COVID-19 pandemic may have been more focused on its feasibility rather than what might be optimal in the long-term care of PwMS. We can be reassured that most studies viewed the use of telemedicine positively and suggested that no major short-term complications arose as a result of its widespread use.17 , 20 , 22 , 23 It is worth carefully considering how best to integrate this knowledge into future clinical practice. For example, it is unclear whether exclusive use of telemedicine for both new and follow-up visits is ideal as opposed to the use of telemedicine only for follow-ups. One study from Norway suggested that some clinicians were dissatisfied completing new patient visits using telemedicine and looked upon telemedicine more favorably for follow-up visits.19 Furthermore, ensuring universal access remains a concern in jurisdictions with multiple healthcare payers such as the United States.14 Lingering concerns also exist pertaining to cybersecurity and privacy due to use of internet-based platforms.14
Overall, the rapid uptake of telemedicine during the COVID-19 pandemic may result in lasting changes in MS practice. The use of telemedicine is fluid and ever-changing based on regulations around its use and uncertainty on how best to apply it. Future research is needed to elucidate the full range of implications associated with short- and long-term use of telemedicine.
The power of large registries for assessing COVID-19 risk in people with MS
During the early part of the COVID-19 pandemic, MS researchers recognized the need for large studies to answer key questions. The Global Data Sharing Initiative developed a data sharing process to study the effects of COVID-19 in PwMS across small and large efforts globally.24 This provided harmonized data across multiple countries and helped determine potential risk factors using large registries. Registry data was ideal for this purpose because large populations could be studied while adjusting for confounders and examining for rare outcomes. Table 1 summarizes key contributions from several databases.
Table 1.
Selected registries and key contributions to COVID-19 risk assessment in MS
| Registry | Country of origin | Largest MS cohort | Key contributions |
|---|---|---|---|
| Multiple Sclerosis International Federation (MSIF) Global Data Sharing Initiative | Global (HQ in UK) | 5648 | Harmonized data collection through multiple pooled registries24,26 |
| North American Research Committee on Multiple Sclerosis (NARCOMS) | United States | 4955 | Survey showed high rates (84.1%) of SARS-CoV-2 vaccination and determined reasons for vaccine hesitancy in PwMS96 |
| COVID-19 Infections in MS & Related Diseases (COViMS) | United States | 1626 | Risk factors for poor outcome with COVID-19 include increased disability, older age, hypertension, diabetes, obesity, black race, anti-CD20 DMT, and recent corticosteroid treatment25; pregnancy outcomes are no worse in PwMS35 |
| Multiple Sclerosis and COVID-19 (MuSC-19) | Italy | 1362 | Increased risk of poor COVID-19 outcome in PwMS with EDSS >3 or at least 1 comorbidity28 and use of anti-CD20 DMT32 |
| Covisep | France | 347 | Age, EDSS 6 or higher, and obesity were found to be independent risk factors for hospitalization with COVID-1927 |
| German MS Register | Germany | Hundreds | Contributed to the Global Data Sharing Initiative, and provided evidence that SARS-CoV-2 vaccination does not increase risk of relapse83 |
| UK MS Register | UK | 404 | Multiple studies, including those that described common symptoms of self-diagnosed COVID-19 in PwMS38 and determined that PwMS commonly experienced amplification of MS symptoms during COVID-19 infection; an effect that was attenuated by DMT46 |
| Swedish MS Registry | Sweden | 17692 | Corroborated increased risk of hospitalization with COVID-19 in PwMS on anti-CD20 DMT but suggested that this risk may be more modest than the risk associated with older age, increased disability, and medical comorbidities34 |
| MSBase COVID-19 Substudy | Australia | Thousands | Most notable publications relating to COVID-19 were under the auspices of the Global Data Sharing Initiative above |
Abbreviations: HQ – headquartered; PwMS – people with MS; DMT – disease-modifying therapy.
A crucial question was whether PwMS possessed any unique risk factors for poor outcomes with COVID-19. Several registry-based MS studies found that many of the risk factors were similar to individuals in the general population, including older age and the presence of specific medical comorbidities (e.g., diabetes).25, 26, 27 Data from the North American COVID-19 Infections in MS and related diseases (COViMS) registry showed a 30% increase in the risk of hospitalization and intensive care unit (ICU) admission and/or need for artificial ventilation for every 10-year increase in age, with a 76.5% increased risk of death for every 10-year age epoch.25 Hypertension, diabetes, and morbid obesity also increased the risk of poor outcomes. Greater levels of ambulatory disability (e.g., Expanded Disability Status Scale (EDSS) >6 or requiring any assistance to walk) more than double the odds of more severe COVID-19 outcomes.25 , 27 , 28 Being non-ambulatory was associated with a 25-fold increased odds of death compared with fully ambulatory PwMS and black race was associated with >40% increased odds of being hospitalized (but not with an increased risk of death).25
Determining the risk of poor COVID-19 outcomes related to MS DMTs was also an intense focus of investigation. Smaller registries, such as those with hundreds rather than thousands of patients, initially found no effect of DMT exposure.27 , 29 , 30 Revisiting this using larger datasets revealed that anti-CD20 monoclonal antibody therapies were associated with increased risk.25 , 26 , 31 , 32 Rituximab was associated with 4.5-fold increased odds of hospitalization with COVID-19.25 Pooled international data confirmed that ocrelizumab and rituximab (compared to other DMTs) increased the odds of hospitalization and ICU admission but not death.31 This is consistent with data from other patients with immune-mediated disorders included in the Global Rheumatology Alliance registry, which found that rituximab was associated with 4-fold increased odds of death compared to methotrexate-treated individuals.33
DMT-treated status in isolation is not sufficient to risk stratify PwMS. Investigators in Italy found that COVID-19 risk was confined to PwMS in a ‘higher risk’ group, defined as those with EDSS >3 or with at least 1 comorbidity.28 Conversely, PwMS with EDSS 3 or less and no comorbidities had a risk of severe COVID-19 outcome similar to age- and sex-matched controls.28 Of note, untreated PwMS appear to have a higher risk of poor outcome31 that is variably present in different studies following adjustment for factors such as age and MS phenotype.27 , 32 This may reflect that this group is comprised of both individuals who are untreated due to having milder MS and those with more severe disability or progressive course who do not benefit from DMT (and may be at higher baseline risk of severe COVID-19 due to ambulatory status). A study of >17,000 PwMS from Sweden further supported the notion that pre-morbid disability and progressive MS course were likely more predictive of poor COVID-19 outcome compared to DMT type; an increased risk in rituximab-treated PwMS was again seen (albeit to a lesser degree than in smaller studies).34 Pregnant and post-partum PwMS and children with MS do not appear to be at higher risk of poor COVID-19 outcomes, however conclusions are limited by small sample size and under-representation of pregnant/young PwMS with high levels of ambulatory disability/comorbidities.35 , 36 In general, pregnant women who develop COVID-19 may have a higher risk of preterm birth,37 so individualized counseling remains important.
Data from the United Kingdom (UK) MS Register suggested that the likelihood of developing COVID-19 is not influenced by DMT-treated status or premorbid disability; however, these conclusions are limited by patient self-reporting.38
COVID-19 risk also relates to treatments used for MS relapses. Glucocorticoid use in the 2 months preceding infection was associated with a doubling of the odds of hospitalization and quadrupling the risk of death among PwMS with COVID-19.25 Intravenous (IV) methylprednisolone use in the month preceding COVID-19 increases the risk, but it is unknown whether lower doses typically used as premedication interacts with the increased risk observed with anti-CD-20 monoclonal antibodies.32 The reasons for this observation are not well understood, given that dexamethasone is beneficial in severe acute COVID-19 respiratory infection.39 The timing, dose, and duration of corticosteroid administration relative to pathogen exposure may determine the net immunomodulatory and therefore clinical effects.
In summary, pooled data from large registries has proven instrumental for informing the MS community about risk factors for poor outcomes secondary to COVID-19. Harmonized data using variables which were readily collected at the point of care enabled conclusions about clinically relevant risk factors for PwMS that appear consistent across studies. Possible limitations relate to voluntary reporting of data, use of variables only included a priori in the register, and lack of potentially relevant details such as DMT dose/frequency and MS disease activity.
Symptomatic manifestations of COVID-19 in people with MS
Studies based on patient self-report found that COVID-19 symptoms experienced by PwMS (e.g., ageusia, hyposmia, upper respiratory tract symptoms) are no different than individuals in the general population.40, 41, 42 In a study assessing post-acute symptoms in PwMS, nearly 30% of 8000 respondents reported COVID-19 symptoms lasting more than 1 month.43 The risk was higher in those with severe pre-existing neurological disability and mental health comorbidities.43 Many persistent manifestations such as lower respiratory tract symptoms (e.g., cough) and nondescript muscle aches were not consistent with MS, however new or worsened fatigue had a prevalence of nearly 70% among those with post-acute COVID-19 sequelae.43 Since symptoms such as fatigue and cognitive impairment are common to both MS and post-acute COVID-19, any pathogenetic interaction between the two disorders remains speculative currently.44
Evidence is sparse regarding whether COVID-19 produces durable changes in inflammatory disease activity in MS. It is common for PwMS to experience neurological symptoms during acute COVID-19, however in these studies, self-reported data limits the ability to conclusively distinguish pseudoexacerbations from new focal CNS inflammation due to MS.45, 46, 47
Impact of COVID-19 on disease-modifying therapy selection
The expanding landscape of DMTs for PwMS has provided hope in terms of controlling the macroscopic neuroinflammatory component of MS (relapses and new lesions on magnetic resonance imaging). Since many DMTs modulate, suppress, or reconstitute components of the immune system, concerns about safety came up early in the COVID-19 pandemic. Practical recommendations were needed to balance infectious safety concerns with preserving efficacy for people with active MS. We will focus on key lessons about DMT selection in the context of COVID-19. For an in-depth review of SARS-CoV-2 pathogenesis and its relationship to DMT mechanisms, we direct the reader to other published literature.48, 49, 50, 51, 52
So far, no MS DMT has proven to be protective against COVID-19. There was initially optimism that beta interferons could counteract SARS-CoV-2 through antiviral effects and possibly by dampening pro-inflammatory host responses.53 Following one study which showed a non-statistically significant trend towards lower rates of hospitalization for PwMS on beta interferon,25 further studies found no significant beneficial effect.31 , 53 , 54 Hypotheses that other DMTs could attenuate COVID-19 severity through immunomodulatory mechanisms,55 particularly in the case of fingolimod and natalizumab, have not borne out in large datasets.26 , 31 , 48 , 56 , 57
During the pandemic, recommendations for DMT prescribing and risk assessments were largely based on expert consensus or experience with other infectious diseases.51 , 58 Changes in DMT use by PwMS were considered for some patients based on survey data and presumably related to concern about COVID-19.6 , 8, 9, 10 Although, many MS providers remained comfortable with new DMT starts during the pandemic if appropriate based on MS severity, and only a small minority (8%) recommended postponing all DMT administrations.59 This comfort level may have been enhanced by the larger COVID-19 MS registries highlighting that the majority of DMTs did not appear to increase the risk of contracting SARS-CoV-2 or experience more severe COVID-19 outcomes.26 , 31 , 32 Some PwMS may have had new MS disease activity and/or progression due to a change or discontinuation in therapy, however, evidence is lacking in this regard.
Prescribing patterns for DMTs were altered during the COVID-19 pandemic. A study from the UK showed a steady trend to increasing monoclonal antibody DMT prescriptions from 2016–2019 that was reversed in 2020 at the start of the pandemic (except for natalizumab) with a 16.7% reduction in new starts.60 A similar trend was observed in Spain with decreases in prescriptions for anti-CD20 therapies and an increase in new natalizumab prescriptions, ostensibly due to lesser peripheral immunosuppression with natalizumab.61 A study of 670 PwMS prescribed DMT in the United States showed a 10% reduction in intravenous infusion DMT prescriptions with an increase in oral DMT prescriptions (+7%) which persisted from the pre-vaccine to the post-vaccine period of the pandemic.62 Delays in infused DMT (mostly B cell therapies) were more common than switches in DMT class or type. Prescribing patterns of self-injected therapies remained stable, although the study overlapped with a time where ofatumumab (a self-injected anti-CD20 DMT) was becoming more widely prescribed.62
Although the global health emergency has been declared over, endemic COVID-19 risk will continue to factor into therapeutic shared decision-making along with the perceived benefits of treatment. This will require similar ‘risk calculus’ as was used by experienced MS clinicians in the past. Prior to the COVID-19 pandemic, a qualitative study reinforced the idea that providers should engage PwMS in personalized discussions about risk tolerance when prescribing a DMT.63 In this study, many PwMS would accept a risk of non-life-threatening infection in order to better control their MS and preserve function.63
We direct the reader to other excellent resources for a more detailed discussion about starting or sequencing DMTs in the era of COVID-19.52 , 58 , 64, 65, 66 Some general recommendations from the National MS Society apply including: 1) PwMS currently on a DMT should not stop the treatment unless instructed to do so; 2) PwMS with COVID-19 symptoms or with a positive COVID-19 test should speak with their provider(s) (primary care and neurology clinicians); and 3) individualized decisions should be made regarding initiating or switching DMTs.64 Practical recommendations for use of approved DMTs, including when to consider interrupting treatment can be found in Table 2 .
Table 2.
Summary of recommendations for commercially available MS disease-modifying therapies in the COVID-19 era
| DMT Class | Brief mechanism of action | Mode of administration | Effect on COVID-19 outcome | Recommendation in SARS-CoV-2 infected PwMS |
|---|---|---|---|---|
| Beta interferon | Inhibits pro-inflammatory cytokines | SQ or IM | No increased risk. Protective effect has not been proven53 | Continue treatment |
| Glatiramer acetate | Modulates T cell cytokine profile to increased Th2; promotes Treg cells | SQ | No increased risk26 | Continue treatment |
| Teriflunomide | Inhibits reactive lymphocyte proliferation | Oral | No increased risk26 | Continue treatment |
| Fumarates | Enhances Nrf2 pathway, improves oxidative stress response, and limits survival/activation of T cell subsets | Oral | No increased risk, except rarely if lymphopenic (ALC <800 cells/mm3)104 | Continue; consider suspending if severe infection or ALC <800 cells/mm3 |
| S1P receptor modulators | Inhibits lymphocyte trafficking out of peripheral lymph nodes | Oral | No evidence of direct increased risk26 but may impair response to vaccines92 | Continue; consider suspending if ALC <200 cells/mm3 |
| Natalizumab | Prevents leukocyte trafficking across the blood-CNS barrier by targeting alpha-4 integrins | IV | No evidence of increased risk.26 Extended interval dosing may reduce risk of hospital exposure71 | Continue; may consider delaying infusion if critically ill |
| Anti-CD20 monoclonal antibodies | Lyses and depletes B lymphocytes by targeting CD20 on their surface | IV (e.g., ocrelizumab) or SQ (ofatumumab) | Increased risk of hospitalization estimated in the range of 2–5-fold.25,26,67 May impair response to vaccines86,94 | Suspend/delay dosing |
| Cladribine | Inhibits DNA synthesis and depletes B > T lymphocytes | Oral – two cycles separated by 1 year | Likely only at increased risk when severely lymphopenic26,51 | Suspend/delay dosing |
| Alemtuzumab | Lyses and depletes mature T and B lymphocytes and several innate immune cells by targeting CD52 on their surface | IV– two cycles separated by 1 year | Likely only at increased risk when severely lymphopenic/leukopenic26,51 | Suspend/delay dosing |
Abbreviations: DMT – disease-modifying therapy; PwMS – people with MS; Th2 – T helper-2 cytokine profile; Treg – T regulatory cells; S1P – sphingosine-1 phosphate; Nrf2 – nuclear factor erythroid 2-related factor 2; IM – intramuscular; SQ – subcutaneous; IV – intravenous; ALC – absolute lymphocyte count.
Most of the COVID-19-specific concern surrounds cell-depleting DMTs such as anti-CD20 monoclonal antibodies. Theoretical concern surrounds the induction phase of alemtuzumab and cladribine treatment due to their respective mechanisms, but they were not shown to be associated with increased risk in studies which included patients in the post-induction phase.27 , 31 , 32 , 67 Interferons, glatiramer acetate, fumarates, teriflunomide, sphingospine-1-phosphate (S1P) receptor modulators and natalizumab were not shown to be associated with an increased risk of COVID-19 severity.27 , 31 , 32 , 67 Lymphopenia with absolute lymphocyte counts (ALC) <800 cells/mm3 can occur rarely with fumarates and with cladribine or alemtuzumab treatment and should be monitored since severe lymphopenia may increase risk.48 , 50 , 51 , 68 Lymphocytes are peripherally sequestered by S1P modulators (e.g., fingolimod), so low lymphocyte counts likely represent a functional lymphopenia and may not increase risk unless the measured ALC is severely reduced (<200 cells/mm3).69 , 70
Even though the anti-CD20 infusions are dosed intermittently, this results in maintenance of a B cell-depleted state and may result in hypogammaglobulinemia.50 Immune reconstitution therapies (alemtuzumab and cladribine) possibly carry a greater peak risk during induction but may be appealing options for some PwMS who prefer their risk to be frontloaded.31 , 48 Although large observational studies did not show statistically significant increases in COVID-19 risk with immune reconstitution therapies, this does not necessarily indicate superior safety, especially since the number of patients on these therapies was small in comparison to the other DMTs.26
Autologous hematopoietic stem cell transplant remains an option for treatment refractory PwMS.64 COVID-19 risk is likely elevated for several months following the immunoablative phase of treatment but this has not been well studied.64 Despite the increased risk of COVID-19 severity with some of the MS DMTs, available data along with expert opinion suggests that some PwMS should continue to use these therapies in the proper clinical context.64 PwMS should also adhere to routine precautions to reduce risk of infection and exposure to COVID-19.64 These therapies remain an effective option for reducing disease activity and undertreating MS may be a greater risk than COVID-19.
During the pandemic, some MS providers preferentially used natalizumab in John Cunningham virus (JCV) seronegative patients who had active disease given lower COVID-19 risks and less impact on vaccination response.30 , 69 The exposure risk of frequent visits to the infusion center can likely be mitigated by extended interval dosing.50 , 71 Extended interval dosing for ocrelizumab and rituximab (i.e., delaying maintenance redosing by four or more weeks or personalized redosing based on CD19 cell count) was found not to be associated with increased MS disease activity in the short-term.72, 73, 74, 75 However, a single cohort study of extended interval ocrelizumab dosing raised concern about increased MRI activity without an increase in confirmed disability progression,76 emphasizing that the long-term safety and efficacy of this off-label approach need to be confirmed with prospective studies.
When PwMS are diagnosed with COVID-19, several Food and Drug Administration (FDA)-approved antiviral treatments may be available to them depending on their baseline risk factors and severity of illness.77 Of note, Evusheld™ and REGEN-COV are not authorized by the FDA anymore since they no longer cover dominant circulating SARS-CoV-2 variants.77
Vaccination against SARS-CoV-2 for people with MS
Since their advent in August 2021, large population-based data shows that SARS-CoV-2 mRNA vaccines are safe and effective in PwMS, as in the general population.78, 79, 80, 81, 82 Adverse effects are typically mild and self-limited (injection site reaction, malaise, headache, fever) and are not associated with increased short-term risk of MS relapse.80 , 81 , 83
Early on, it was not known whether PwMS treated with certain DMT classes (e.g., cell-depleting therapies) would mount adequate immune responses, including memory B cell responses to SARS-CoV-2 vaccination. Multiple studies confirmed that those on cell-depleting therapies (anti-CD20, alemtuzumab) and those on S1P receptor modulators have diminished humoral responses to SARS-CoV-2 vaccination.84, 85, 86, 87, 88 However, T cell-mediated adaptive responses are preserved in many patients on anti-CD20 therapy and may be more robust, suggesting that other immune mechanisms may compensate for a blunted humoral response.86 , 89, 90, 91 These effects persist in the long-term, and affect memory B cells.84 It has become evident that timing of vaccine and DMT administration (see Table 3 ) influences the magnitude of serologic immune response observed.92, 93, 94 However, it is unknown whether these suspected compensatory immune mechanisms or DMT timing result in preventing SARS-CoV-2 infections and/or severe COVID-19 outcomes.
Table 3.
Summary of recommendations for COVID-19 vaccination in people with MS by treatment status
| MS Treatment | Negative effect on SARS-CoV-2 vaccine response? | Timing of vaccine before starting treatment | Timing of vaccine after treatment started* |
|---|---|---|---|
| No DMT | None: having MS in isolation does not affect ability to mount a humoral response to available COVID-19 vaccines78 | SARS-CoV-2 vaccines are safe and effective for PwMS and recommended for all patients81 | N/A |
| Beta interferon | None78,93 | Do not delay starting for vaccine | No adjustments required; vaccinate when able |
| Glatiramer acetate | None78,93 | Do not delay starting for vaccine | No adjustments required; vaccinate when able |
| Teriflunomide | No data to suggest impaired response78,93 | Do not delay starting for vaccine | No adjustments required; vaccinate when able |
| Fumarates | No reduction in humoral or T cell-dependent responses for dimethyl fumarate; unknown for other fumarate DMTs93,105 | Do not delay starting for vaccine | No adjustments required; vaccinate when able |
| S1P receptor modulators | Impairment in humoral response in PwMS on fingolimod92 | Vaccinate at least 2 weeks before starting | Continue taking the medication as prescribed and vaccinate when able |
| Natalizumab | No data to suggest impaired responses for SARS-CoV-2 vaccines93,105 | Do not delay starting for vaccine | No adjustments required; vaccinate when able |
| Anti-CD20 monoclonal antibodies | Impairment in humoral response was observed in several studies, however certain T cell responses were found to be more robust78,86,87,90,106 | Vaccinate at least 2 weeks before starting | Ideal timing to vaccinate is 4 weeks before next infusion or 4 weeks after last dose of ofatumumab |
| Cladribine | Empiric data suggest no impairment in humoral antibody responses; theoretical concern during lymphodepletive treatment phase84 | Vaccinate at least 2 weeks before starting | No adjustments required; vaccinate when able |
| Alemtuzumab | Empiric data suggest no impairment in humoral antibody responses; theoretical concern during lymphodepletive treatment phase93 | Vaccinate at least 4 weeks before starting | Consider vaccination 24 weeks or more after the last infusion |
| High-dose corticosteroids | Not demonstrated with empirical data but theoretical concern exists given mechanism of action | Vaccinate 3-5 days after last dose | Vaccinate 3-5 days after the last dose |
*Note that ideal timing of therapy with vaccination may not be possible. Abbreviations: DMT – disease-modifying therapy; S1P – sphingosine-1 phosphate; PwMS – people with MS.
It is recommended that all PwMS receive vaccination against SARS-CoV-2 unless there is a contraindication (e.g., allergic reaction) and keep their vaccinations up to date when they are able to receive boosters.82 , 95 Table 3 summarizes recommendations regarding the optimal timing of SARS-CoV-2 vaccinations with various DMTs.
Addressing concerns PwMS may have about vaccination is paramount. Surveys suggest that most PwMS are willing to receive SARS-CoV-2 vaccines (>75-90% depending on the study).96 , 97 The most prevalent concerns relate to long-term safety and efficacy.96 , 98 , 99 In one large patient survey (n=∼5000), factors that increased the likelihood of receiving the SARS-CoV-2 vaccine included obtaining influenza vaccine, older age (≥65 years), higher SES, physical activity, and use of DMT.96 People with MS were less likely to be vaccine hesitant when explicitly counseled by their MS provider about risks and benefits.100 Overall, providers should continue to individualize counseling to promote vaccination wherever possible to reduce long-term COVID-19 risk as the pandemic shifts to a more endemic/seasonal pattern globally.
Discussion
With the end of the COVID-19 global health emergency, it remains unknown whether changes to MS care will persist. A shift to endemic patterns of infection likely means that a degree of normalcy will return. However, the field has demonstrated that MS care can be adapted to meet PwMS in their home environments with telemedicine. Prospective investigation will be required to clarify the extent telemedicine should be used in routine practice based on patient care outcomes.
As efficacious DMTs continue to be developed, the MS community can incorporate lessons learned during the COVID-19 pandemic to develop contingency plans in the event of future viral pandemics. DMT and vaccination guidelines will need to discuss suggested actions in the event of future viral outbreaks. PwMS will require information about risk while on DMTs and how vaccine responses may be impacted.
Finally, the long-term effects of widespread COVID-19 on PwMS are not yet fully understood. The degree to which long-COVID might interact with MS fatigue and other symptomatology has been explored101 , 102 but relationships have not been conclusively demonstrated. It will also take time to study sustained effects of pandemic-related occupational and psychosocial disruption, which may have disproportionately affected certain PwMS,11 , 13 , 68 , 103 particularly those who were already vulnerable socioeconomically. Observational studies should continue to monitor the long-term impact of the COVID-19 pandemic on MS incidence and disease activity; although preliminary data suggests that its short-term impacts are no different than other respiratory viruses.40 , 102 As we enter the post-pandemic COVID-19 era, the future of person-centered MS care looks as promising as ever.
Summary
Despite multiple COVID-19 pandemic-related disruptions along the continuum of MS care, providers and PwMS collaborated to develop innovative solutions. There were unprecedented efforts across the MS community to work together to collect as much information as possible to make informed decisions in the care of PwMS. We reviewed considerations for DMT prescription and vaccination for PwMS in the era of COVID-19 and speculate on questions for future research in this area.
Footnotes
Disclosures
JDK: receives fellowship funding (paid directly to institution) provided by a University of Calgary Medical Group Helios Advanced Training Award.
AS: receives research funding (paid directly to institution) from Multiple Sclerosis Canada, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers and the Department of Defense Congressionally Directed Medical Research Program and is a member of the editorial board for Neurology. She serves as a consultant for Gryphon Bio, LLC. She is a member of the Data and Safety Monitoring Board for Premature Infants Receiving Milking or Delayed Cord Clamping (PREMOD2), Central Vein Sign: A Diagnostic Biomarker in Multiple Sclerosis (CAVS-MS), and Methotrexate treatment of Arthritis caused by Chikungunya virus (MARCH). She is supported (in part) by a Biostatistics/Informatics Junior Faculty Award (BI-2105-37656) from the National Multiple Sclerosis Society. She holds the Kenney Marie Dixon‐Pickens Distinguished Professorship in Multiple Sclerosis Research.
References
- 1.World Health Organization Coronavirus Dashboard [Internet]. Internet. Updated 2023 Feb 8. Accessed 2023 Feb 8, https://covid19.who.int/
- 2.Centers for Disease Control and Prevention, COVID Data Tracker [Internet]. Internet. Accessed 2023 Feb 8, 2023 Feb 8. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 3.Chen M.H., Goverover Y., Botticello A., DeLuca J., Genova H.M. Healthcare Disruptions and Use of Telehealth Services Among People With Multiple Sclerosis During the COVID-19 Pandemic. Arch Phys Med Rehabil. Jul 2022;103(7):1379–1386. doi: 10.1016/j.apmr.2021.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Staker E., Cutter G., Krieger S., Miller A.E. Perceptions of risk and adherence to care in MS patients during the COVID-19 pandemic: A cross-sectional study. Mult Scler Relat Disord. May 2021;50 doi: 10.1016/j.msard.2021.102856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris E. WHO Declares End of COVID-19 Global Health Emergency. JAMA. May 17 2023 doi: 10.1001/jama.2023.8656. [DOI] [PubMed] [Google Scholar]
- 6.Vogel A.C., Schmidt H., Loud S., McBurney R., Mateen F.J. Impact of the COVID-19 pandemic on the health care of >1,000 People living with multiple sclerosis: A cross-sectional study. Mult Scler Relat Disord. Nov. 2020;46 doi: 10.1016/j.msard.2020.102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald KC, Mecoli CA, Douglas M, et al. Risk Factors for Infection and Health Impacts of the Coronavirus Disease 2019 (COVID-19) Pandemic in People With Autoimmune Diseases. Clin Infect Dis. Feb 11 2022;74(3):427-436. doi:10.1093/cid/ciab407 [DOI] [PMC free article] [PubMed]
- 8.Moss B.P., Mahajan K.R., Bermel R.A., et al. Multiple sclerosis management during the COVID-19 pandemic. Mult Scler. Sep 2020;26(10):1163–1171. doi: 10.1177/1352458520948231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateen FJ, Rezaei S, Alakel N, Gazdag B, Kumar AR, Vogel A. Impact of COVID-19 on U.S. and Canadian neurologists' therapeutic approach to multiple sclerosis: a survey of knowledge, attitudes, and practices. J Neurol. Dec 2020;267(12):3467-3475. doi:10.1007/s00415-020-10045-9 [DOI] [PMC free article] [PubMed]
- 10.Morrison E.H., Michtich K., Hersh C.M. How the COVID-19 Pandemic has changed multiple sclerosis clinical practice: Results of a nationwide provider survey. Mult Scler Relat Disord. Jun 2021;51 doi: 10.1016/j.msard.2021.102913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goverover Y, Chen MH, Botticello A, et al. Relationships between changes in daily occupations and health-related quality of life in persons with multiple sclerosis during the COVID-19 pandemic. Mult Scler Relat Disord. Jan 2022;57:103339. doi:10.1016/j.msard.2021.103339 [DOI] [PMC free article] [PubMed]
- 12.Parkinson A, Drew J, Hall Dykgraaf S, et al. 'They're getting a taste of our world': A qualitative study of people with multiple sclerosis' experiences of accessing health care during the COVID-19 pandemic in the Australian Capital Territory. Health Expect. Oct 2021;24(5):1607-1617. doi:10.1111/hex.13284 [DOI] [PMC free article] [PubMed]
- 13.Alschuler K.N., Roberts M.K., Herring T.E., Ehde D.M. Distress and risk perception in people living with multiple sclerosis during the early phase of the COVID-19 pandemic. Mult Scler Relat Disord. Jan 2021;47 doi: 10.1016/j.msard.2020.102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatcher-Martin J.M., Busis N.A., Cohen B.H., et al. American Academy of Neurology Telehealth Position Statement. Neurology. Aug 17 2021;97(7):334–339. doi: 10.1212/WNL.0000000000012185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bove R., Bevan C., Crabtree E., et al. Toward a low-cost, in-home, telemedicine-enabled assessment of disability in multiple sclerosis. Mult Scler. Oct 2019;25(11):1526–1534. doi: 10.1177/1352458518793527. [DOI] [PubMed] [Google Scholar]
- 16.Bove R., Garcha P., Bevan C.J., Crabtree-Hartman E., Green A.J., Gelfand J.M. Clinic to in-home telemedicine reduces barriers to care for patients with MS or other neuroimmunologic conditions. Neurol Neuroimmunol Neuroinflamm. Nov. 2018;5(6):e505. doi: 10.1212/NXI.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbatemarco JR, Hartman J, McGinley M, et al. Providing Person-Centered Care via Telemedicine in the Era of COVID-19 in Multiple Sclerosis. J Patient Exp. 2021;8:2374373520981474. doi:10.1177/2374373520981474 [DOI] [PMC free article] [PubMed]
- 18.McGinley M.P., Ontaneda D., Wang Z., et al. Teleneurology as a Solution for Outpatient Care During the COVID-19 Pandemic. Telemed J E Health. Dec 2020;26(12):1537–1539. doi: 10.1089/tmj.2020.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristoffersen E.S., Sandset E.C., Winsvold B.S., Faiz K.W., Storstein A.M. Experiences of telemedicine in neurological out-patient clinics during the COVID-19 pandemic. Ann Clin Transl Neurol. Feb. 2021;8(2):440–447. doi: 10.1002/acn3.51293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corea F, Ciotti S, Cometa A, et al. Telemedicine during the Coronavirus Disease (COVID-19) Pandemic: A Multiple Sclerosis (MS) Outpatients Service Perspective. Neurol Int. Jan 18 2021;13(1):25-31. doi:10.3390/neurolint13010003 [DOI] [PMC free article] [PubMed]
- 21.McGinley M.P., Gales S., Rowles W., et al. Expanded access to multiple sclerosis teleneurology care following the COVID-19 pandemic. Mult Scler J Exp Transl Clin. Jan-Mar. 2021;7(1) doi: 10.1177/2055217321997467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keszler P., Maloni H., Miles Z., Jin S., Wallin M. Telemedicine and Multiple Sclerosis: A Survey of Health Care Providers Before and During the COVID-19 Pandemic. Int J MS Care. Nov-Dec 2022;24(6):266–270. doi: 10.7224/1537-2073.2021-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li V., Roos I., Monif M., et al. Impact of telehealth on health care in a multiple sclerosis outpatient clinic during the COVID-19 pandemic. Mult Scler Relat Disord. Jul 2022;63 doi: 10.1016/j.msard.2022.103913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters L.M., Parciak T., Walton C., et al. COVID-19 in people with multiple sclerosis: A global data sharing initiative. Mult Scler. Sep. 2020;26(10):1157–1162. doi: 10.1177/1352458520941485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salter A., Fox R.J., Newsome S.D., et al. Outcomes and Risk Factors Associated With SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis. JAMA Neurol. Jun 1 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson-Yap S., Pirmani A., Kalincik T., et al. Updated Results of the COVID-19 in MS Global Data Sharing Initiative: Anti-CD20 and Other Risk Factors Associated With COVID-19 Severity. Neurol Neuroimmunol Neuroinflamm. Nov. 2022;9(6) doi: 10.1212/NXI.0000000000200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louapre C., Collongues N., Stankoff B., et al. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. Sep 1 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sormani M.P., Schiavetti I., Carmisciano L., et al. COVID-19 Severity in Multiple Sclerosis: Putting Data Into Context. Neurol Neuroimmunol Neuroinflamm. Jan 2022;9(1) doi: 10.1212/NXI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bsteh G., Assar H., Hegen H., et al. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: Insights from a nation-wide Austrian registry. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0255316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrambide G., Llaneza-Gonzalez M.A., Costa-Frossard Franca L., et al. SARS-CoV-2 Infection in Multiple Sclerosis: Results of the Spanish Neurology Society Registry. Neurol Neuroimmunol Neuroinflamm. Jul 2021;8(5) doi: 10.1212/NXI.0000000000001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology. Nov 9 2021;97(19):e1870-e1885. doi:10.1212/WNL.0000000000012753 [DOI] [PMC free article] [PubMed]
- 32.Sormani M.P., Salvetti M., Labauge P., et al. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol. Aug 2021;8(8):1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strangfeld A., Schafer M., Gianfrancesco M.A., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. Jul. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longinetti E., Bower H., McKay K.A., et al. COVID-19 clinical outcomes and DMT of MS patients and population-based controls. Ann Clin Transl Neurol. Sep. 2022;9(9):1449–1458. doi: 10.1002/acn3.51646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salter A., Cross A.H., Cutter G.R., et al. COVID-19 in the pregnant or postpartum MS patient: Symptoms and outcomes. Mult Scler Relat Disord. Sep. 2022;65 doi: 10.1016/j.msard.2022.104028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oncel I., Alici N., Solmaz I., Oge D.D., Ozsurekci Y., Anlar B. The Outcome of COVID-19 in Pediatric-Onset Multiple Sclerosis Patients. Pediatr Neurol. Sep 2022;134:7–10. doi: 10.1016/j.pediatrneurol.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yam C., Jokubaitis V., Hellwig K., Dobson R. MS, pregnancy and COVID-19. Mult Scler. Sep 2020;26(10):1137–1146. doi: 10.1177/1352458520949152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evangelou N., Garjani A., dasNair R., et al. Self-diagnosed COVID-19 in people with multiple sclerosis: a community-based cohort of the UK MS Register. J Neurol Neurosurg Psychiatry. Aug 27 2020;92(1):107–109. doi: 10.1136/jnnp-2020-324449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. Feb 25 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sepulveda M., Llufriu S., Martinez-Hernandez E., et al. Incidence and Impact of COVID-19 in MS: A Survey From a Barcelona MS Unit. Neurol Neuroimmunol Neuroinflamm. Mar 4 2021;(2):8. doi: 10.1212/NXI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiavetti I., Carmisciano L., Ponzano M., et al. Signs and symptoms of COVID-19 in patients with multiple sclerosis. Eur J Neurol. Dec 2022;29(12):3728–3736. doi: 10.1111/ene.15554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parrotta E., Kister I., Charvet L., et al. COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm. Sep. 2020;7(5) doi: 10.1212/NXI.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garjani A., Middleton R.M., Nicholas R., Evangelou N. Recovery From COVID-19 in Multiple Sclerosis: A Prospective and Longitudinal Cohort Study of the United Kingdom Multiple Sclerosis Register. Neurol Neuroimmunol Neuroinflamm. Jan. 2022;9(1) doi: 10.1212/NXI.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellucci G., Rinaldi V., Buscarinu M.C., et al. Multiple Sclerosis and SARS-CoV-2: Has the Interplay Started? Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.755333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etemadifar M, Sedaghat N, Aghababaee A, et al. COVID-19 and the Risk of Relapse in Multiple Sclerosis Patients: A Fight with No Bystander Effect? Mult Scler Relat Disord. Jun 2021;51:102915. doi:10.1016/j.msard.2021.102915 [DOI] [PMC free article] [PubMed]
- 46.Garjani A., Middleton R.M., Hunter R., et al. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult Scler Relat Disord. Jul 2021;52 doi: 10.1016/j.msard.2021.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kataria S., Tandon M., Melnic V., Sriwastava S. A case series and literature review of multiple sclerosis and COVID-19: Clinical characteristics, outcomes and a brief review of immunotherapies. eNeurologicalSci. Dec. 2020;21 doi: 10.1016/j.ensci.2020.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabreira V., Abreu P., Soares-Dos-Reis R., Guimaraes J., Sa M.J. Multiple Sclerosis, Disease-Modifying Therapies and COVID-19: A Systematic Review on Immune Response and Vaccination Recommendations. Vaccines (Basel) Jul 11 2021;9(7) doi: 10.3390/vaccines9070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhry F., Jageka C., Levy P.D., Cerghet M., Lisak R.P. Review of the COVID-19 Risk in Multiple Sclerosis. J Cell Immunol. 2021;3(2):68–77. doi: 10.33696/immunology.3.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng C., Kar I., Chen C.K., et al. Multiple Sclerosis Disease-Modifying Therapy and the COVID-19 Pandemic: Implications on the Risk of Infection and Future Vaccination. CNS Drugs. Sep. 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giovannoni G., Hawkes C., Lechner-Scott J., Levy M., Waubant E., Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. Apr. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hollen C., Bernard J. Multiple Sclerosis Management During the COVID-19 Pandemic. Curr Neurol Neurosci Rep. Aug. 2022;22(8):537–543. doi: 10.1007/s11910-022-01211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson-Yap S., Pirmani A., De Brouwer E., et al. Severity of COVID19 infection among patients with multiple sclerosis treated with interferon-beta. Mult Scler Relat Disord. Oct 2022;66 doi: 10.1016/j.msard.2022.104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedman M.S., Jack D., Murgasova Z., Todorovic M., Seitzinger A. Outcomes of COVID-19 among patients treated with subcutaneous interferon beta-1a for multiple sclerosis. Mult Scler Relat Disord. Nov. 2021;56 doi: 10.1016/j.msard.2021.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohn N., Konen F.F., Pul R., et al. Experience in Multiple Sclerosis Patients with COVID-19 and Disease-Modifying Therapies: A Review of 873 Published Cases. J Clin Med. Dec 16 2020;(12):9. doi: 10.3390/jcm9124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tasat D.R., Yakisich J.S. Rationale for the use of sphingosine analogues in COVID-19 patients. Clin Med (Lond). Jan. 2021;21(1):e84–e87. doi: 10.7861/clinmed.2020-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teymouri S., Pourbayram Kaleybar S., Hejazian S.S., et al. The effect of Fingolimod on patients with moderate to severe COVID-19. Pharmacol Res Perspect. Feb. 2023;11(1) doi: 10.1002/prp2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciotti J.R., Grebenciucova E., Moss B.P., Newsome S.D. Multiple Sclerosis Disease-Modifying Therapies in the COVID-19 Era. Ann Neurol. Dec 2020;88(6):1062–1064. doi: 10.1002/ana.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Portaccio E., Fonderico M., Hemmer B., et al. Impact of COVID-19 on multiple sclerosis care and management: Results from the European Committee for Treatment and Research in Multiple Sclerosis survey. Mult Scler. Jan. 2022;28(1):132–138. doi: 10.1177/13524585211005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams T, Mishra R, Bharkhada B, et al. Impact of the COVID-19 pandemic on the prescription of disease-modifying therapy for multiple sclerosis in England: a nationwide study. J Neurol Neurosurg Psychiatry. Mar 10 2022;93(11):1229-1230. doi:10.1136/jnnp-2021-328340 [DOI] [PMC free article] [PubMed]
- 61.Cobo-Calvo A., Zabalza A., Rio J., et al. Impact of COVID-19 pandemic on frequency of clinical visits, performance of MRI studies, and therapeutic choices in a multiple sclerosis referral centre. J Neurol. Apr. 2022;269(4):1764–1772. doi: 10.1007/s00415-021-10958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaheer R., Amin R., Riddick L., et al. Impact of COVID-19 on prescribing patterns and treatment selection of disease modifying therapies in multiple sclerosis. Mult Scler Relat Disord. Mar. 2023;71 doi: 10.1016/j.msard.2023.104575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox R.J., Cosenza C., Cripps L., et al. A survey of risk tolerance to multiple sclerosis therapies. Neurology. Apr 2 2019;92(14):e1634–e1642. doi: 10.1212/WNL.0000000000007245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National MS Society, Disease Modifying Therapy Guidance During COVID-19 [Internet]. Updated 2023. https://www.nationalmssociety.org/coronavirus-covid-19-information/ms-treatment-guidelines-during-coronavirus
- 65.MS International Federation, MS, COVID-19 and Vaccines - Updated Global Advice [Internet]. Updated May 24, 2022. https://www.msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/
- 66.Pugliatti M., Berger T., Hartung H.P., Oreja-Guevara C., Bar-Or A. Multiple sclerosis in the era of COVID-19: disease course, DMTs and SARS-CoV2 vaccinations. Curr Opin Neurol. Jun 1 2022;35(3):319–327. doi: 10.1097/WCO.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 67.Sormani M.P., De Rossi N., Schiavetti I., et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol. Apr 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abbadessa G., Lavorgna L., Trojsi F., Coppola C., Bonavita S. Understanding and managing the impact of the Covid-19 pandemic and lockdown on patients with multiple sclerosis. Expert Rev Neurother. Jul. 2021;21(7):731–743. doi: 10.1080/14737175.2021.1957673. [DOI] [PubMed] [Google Scholar]
- 69.Luna G, Alping P, Burman J, et al. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. Feb 1 2020;77(2):184-191. doi:10.1001/jamaneurol.2019.3365 [DOI] [PMC free article] [PubMed]
- 70.Sullivan R., Kilaru A., Hemmer B., et al. COVID-19 Infection in Fingolimod- or Siponimod-Treated Patients: Case Series. Neurol Neuroimmunol Neuroinflamm. Nov. 2021;9(1) doi: 10.1212/NXI.0000000000001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foley J.F., Defer G., Ryerson L.Z., et al. Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing-remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial. Lancet Neurol. Jul. 2022;21(7):608–619. doi: 10.1016/S1474-4422(22)00143-0. [DOI] [PubMed] [Google Scholar]
- 72.Maarouf A., Rico A., Boutiere C., et al. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflamm. Sep 2020;7(5) doi: 10.1212/NXI.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rolfes L., Pawlitzki M., Pfeuffer S., et al. Ocrelizumab Extended Interval Dosing in Multiple Sclerosis in Times of COVID-19. Neurol Neuroimmunol Neuroinflamm. Sep 2021;8(5) doi: 10.1212/NXI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahi N.K., Abidi S.M.A., Salim O., Abraham R., Kalra S., Al-Araji A. Clinical impact of Ocrelizumab extended interval dosing during the COVID-19 pandemic and associations with CD19(+)B-cell repopulation. Mult Scler Relat Disord. Nov. 2021;56 doi: 10.1016/j.msard.2021.103287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Lierop Z.Y., Toorop A.A., van Ballegoij W.J., et al. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult Scler. Jun. 2022;28(7):1121–1125. doi: 10.1177/13524585211028833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanghi A., Avolio C., Signoriello E., et al. Is It Time for Ocrelizumab Extended Interval Dosing in Relapsing Remitting MS? Evidence from An Italian Multicenter Experience During the COVID-19 Pandemic. Neurotherapeutics. Sep. 2022;19(5):1535–1545. doi: 10.1007/s13311-022-01289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.National MS Society, Medicines to Prevent and Treat COVID-19 [Internet]. Updated January 27, 2023. https://www.nationalmssociety.org/coronavirus-covid-19-information/suspected-covid-19-and-ms
- 78.Achiron A., Dolev M., Menascu S., et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler. May 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garjani A., Patel S., Bharkhada D., et al. Impact of mass vaccination on SARS-CoV-2 infections among multiple sclerosis patients taking immunomodulatory disease-modifying therapies in England. Mult Scler Relat Disord. Jan. 2022;57 doi: 10.1016/j.msard.2021.103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lotan I., Wilf-Yarkoni A., Friedman Y., Stiebel-Kalish H., Steiner I., Hellmann M.A. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): Early experience from a tertiary MS center in Israel. Eur J Neurol. Nov. 2021;28(11):3742–3748. doi: 10.1111/ene.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stefanou M.I., Palaiodimou L., Theodorou A., et al. Safety of COVID-19 vaccines in multiple sclerosis: A systematic review and meta-analysis. Mult Scler. Apr. 2023;29(4-5):585–594. doi: 10.1177/13524585221150881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coyle P.K., Gocke A., Vignos M., Newsome S.D. Vaccine Considerations for Multiple Sclerosis in the COVID-19 Era. Adv Ther. Jul. 2021;38(7):3550–3588. doi: 10.1007/s12325-021-01761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frahm N, Fneish F, Ellenberger D, et al. Frequency and Predictors of Relapses following SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis: Interim Results from a Longitudinal Observational Study. J Clin Med. May 24 2023;12(11)doi:10.3390/jcm12113640 [DOI] [PMC free article] [PubMed]
- 84.Disanto G., Galante A., Cantu M., et al. Longitudinal Postvaccine SARS-CoV-2 Immunoglobulin G Titers, Memory B-Cell Responses, and Risk of COVID-19 in Multiple Sclerosis Over 1 Year. Neurol Neuroimmunol Neuroinflamm. Jan 2023;(1):10. doi: 10.1212/NXI.0000000000200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu X., Wang L., Shen L., Tang K. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: A meta-analysis. EBioMedicine. Jul. 2022;81 doi: 10.1016/j.ebiom.2022.104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brill L., Rechtman A., Zveik O., et al. Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Treated With Ocrelizumab. JAMA Neurol. Dec 1 2021;78(12):1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kornek B., Leutmezer F., Rommer P.S., et al. B Cell Depletion and SARS-CoV-2 Vaccine Responses in Neuroimmunologic Patients. Ann Neurol. Mar. 2022;91(3):342–352. doi: 10.1002/ana.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen J.A., Bermel R.A., Grossman C.I., et al. Immunoglobulin G immune response to SARS-CoV-2 vaccination in people living with multiple sclerosis within Multiple Sclerosis Partners Advancing Technology and Health Solutions. Mult Scler. Jun. 2022;28(7):1131–1137. doi: 10.1177/13524585211061343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ali A., Dwyer D., Wu Q., et al. Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine. Oct 1 2021;39(41):6111–6116. doi: 10.1016/j.vaccine.2021.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Apostolidis S.A., Kakara M., Painter M.M., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. Nov. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gadani S.P., Reyes-Mantilla M., Jank L., et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. Nov. 2021;73 doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Achiron A., Mandel M., Gurevich M., et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J Neurol. May. 2022;269(5):2286–2292. doi: 10.1007/s00415-022-11030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tallantyre E.C., Vickaryous N., Anderson V., et al. COVID-19 Vaccine Response in People with Multiple Sclerosis. Ann Neurol. Jan. 2022;91(1):89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Disanto G., Sacco R., Bernasconi E., et al. Association of Disease-Modifying Treatment and Anti-CD20 Infusion Timing With Humoral Response to 2 SARS-CoV-2 Vaccines in Patients With Multiple Sclerosis. JAMA Neurol. Dec 1 2021;78(12):1529–1531. doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.National MS Society, COVID-19 Vaccine Guidance for People Living with MS [Internet]. Updated February 23, 2023. https://www.nationalmssociety.org/coronavirus-covid-19-information/covid-19-vaccine-guidance
- 96.Marrie RA, Dolovich C, Cutter GR, Fox RJ, Salter A. Attitudes toward coronavirus disease 2019 vaccination in people with multiple sclerosis. Mult Scler J Exp Transl Clin. Apr-Jun 2022;8(2):20552173221102067. doi:10.1177/20552173221102067 [DOI] [PMC free article] [PubMed]
- 97.Ciotti JR, Perantie DC, Moss BP, et al. Perspectives and experiences with COVID-19 vaccines in people with MS. Mult Scler J Exp Transl Clin. Jan-Mar 2022;8(1):20552173221085242. doi:10.1177/20552173221085242 [DOI] [PMC free article] [PubMed]
- 98.Ehde D.M., Roberts M.K., Humbert A.T., Herring T.E., Alschuler K.N. COVID-19 vaccine hesitancy in adults with multiple sclerosis in the United States: A follow up survey during the initial vaccine rollout in 2021. Mult Scler Relat Disord. Sep 2021;54 doi: 10.1016/j.msard.2021.103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uhr L., Mateen F.J. COVID-19 vaccine hesitancy in multiple sclerosis: A cross-sectional survey. Mult Scler. Jun. 2022;28(7):1072–1080. doi: 10.1177/13524585211030647. [DOI] [PubMed] [Google Scholar]
- 100.Yap S.M., Al Hinai M., Gaughan M., et al. Vaccine hesitancy among people with multiple sclerosis. Mult Scler Relat Disord. Nov. 2021;56 doi: 10.1016/j.msard.2021.103236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barzegar M., Mirmosayyeb O., Gajarzadeh M., et al. COVID-19 Among Patients With Multiple Sclerosis: A Systematic Review. Neurol Neuroimmunol Neuroinflamm. Jul. 2021;8(4) doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bsteh G., Assar H., Gradl C., et al. Long-term outcome after COVID-19 infection in multiple sclerosis: a nation-wide multicenter matched-control study. Eur J Neurol. Jun 25 2022 doi: 10.1111/ene.15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chiaravalloti N.D., Amato M.P., Brichetto G., et al. The emotional impact of the COVID-19 pandemic on individuals with progressive multiple sclerosis. J Neurol. May 2021;268(5):1598–1607. doi: 10.1007/s00415-020-10160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehta D., Miller C., Arnold D.L., et al. Effect of dimethyl fumarate on lymphocytes in RRMS: Implications for clinical practice. Neurology. Apr 9 2019;92(15):e1724–e1738. doi: 10.1212/WNL.0000000000007262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levit E., Longbrake E.E., Stoll S.S. Seroconversion after COVID-19 vaccination for multiple sclerosis patients on high efficacy disease modifying medications. Mult Scler Relat Disord. Apr. 2022;60 doi: 10.1016/j.msard.2022.103719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bar-Or A., Calkwood J.C., Chognot C., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology. Oct 6 2020;95(14):e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]