Abstract
Introduction
There is sparse knowledge of immediate adverse reactions following COVID-19 vaccination.
Objective
This study aimed to estimate the frequency and number of immediate adverse reactions following COVID-19 vaccination in a Danish population.
Methods
The study used data from the Danish population-based cohort study BiCoVac. The frequencies of 20 self-reported adverse reactions were estimated for each vaccine dose stratified by sex, age, and vaccine type. Also, the distributions of number of adverse reactions following each dose were estimated stratified by sex, age, vaccine type, and prior COVID-19 infection.
Results
A total of 889,503 citizens were invited and 171,008 (19 %) vaccinated individuals were included in the analysis. The most frequently reported adverse reaction following the first dose of COVID-19 vaccine was redness and/or pain at the injection site (20 %) while following the second and third dose, tiredness was the most frequently reported adverse reaction (22 % and 14 %, respectively). Individuals aged 26–35 years, females, and those with a prior COVID-19 infection were more likely to report adverse reactions compared with older individuals, males, and those with no prior COVID-19 infection, respectively. Following the first dose, individuals vaccinated with ChAdOx1-2 (AstraZeneca) reported more adverse reactions compared with individuals vaccinated with other vaccine types. Individuals vaccinated with mRNA-1273 (Moderna) reported more adverse reactions following the second and third dose compared with individuals vaccinated with BNT162b2 (Pfizer-BioNTech).
Conclusion
The frequency of immediate adverse reactions was highest among females and younger persons, however, most of the Danish citizens did not experience immediate adverse reactions following COVID-19 vaccination.
Keywords: COVID-19 vaccination, Adverse reactions
1. Background
In January 2020 WHO declared COVID-19 an international crisis for public health [1]. This led to several political and health-related initiatives [2], [3]. In December 2020, the first COVID-19 vaccine was authorized by the European Medicines Agency (EMA) [4] and within a few months, three other vaccine candidates were approved [5], [6], [7].
Development of a new vaccine is usually a process that takes several years. Due to the urgency of the COVID-19 pandemic, the vaccine development occurred at an accelerated rate. The implementation of COVID-19 vaccines was monitored closely by the authorities with registration of adverse reactions (AR) [8]. Reports of thromboembolic events in individuals vaccinated with the ChAdOx1-2 vaccine led to a rapid assessment of adverse events using the Danish registers [9]. As a reaction to the findings, the Danish authorities suspended the ChAdOx1-2 vaccine [10] and it was later excluded from the Danish vaccination program [11].
Several studies have examined ARs to the different COVID-19 vaccine types [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. However, most of the published studies are small and many were conducted only among healthcare staff [22], [15], [16], [17], [18], [19], [20], thus in selected populations, which are not representative for the general population. The most commonly reported ARs include pain at the injection site [12], [13], [15], [17], [18], [20], [21], [22], headache [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], fatigue [12], [13], [16], [17], [18], [20], [21], [22], joint pain [21], [22], [15], [16], [17], [18], myalgia [15], [16], [17], [19], [20], [21], [22], shiver/chills [16], [17], [20], [21], and fever [14], [16], [21], [22].
In the present study, we report the frequency of immediate ARs following COVID-19 vaccination in Danish citizens and describe the distribution of the individual ARs as well as number of immediate ARs by dose and according to predefined subgroups (sex, age, vaccine type, and prior COVID-19 infection).
2. Methods
2.1. Study setting
The study was conducted in the Danish general population. In Denmark, the COVID-19 vaccines were introduced late December 2020 [23]. Healthcare staff, citizens aged 65 and above, and citizens with increased risk of severe COVID-19 disease (e.g. severe chronic disease [24]) and their relatives were first in line for COVID-19 vaccines. Hereafter the COVID-19 vaccines were gradually made available to all Danish adults, starting with the oldest age groups [25]. Individuals above 18 years were recommended to take the third COVID-19 vaccine dose 140 days after the second dose [26].
2.2. The BiCoVac cohort
BiCoVac is a Danish population-based cohort [27]. The BiCoVac cohort was established to obtain information on immediate and long-term symptoms following COVID-19 vaccination. A random sample of Danish citizens, living in Denmark at the beginning of April 2021, and born between 1956 and 2004 were obtained from the Danish Central Person Register (25 % from each birth year). A total of 911,613 Danes aged 16–65 years were selected for the study. The baseline questionnaires were sent out through a national digital mail system (e-Boks) in May 2021.
The baseline questionnaire was timed to obtain information before the COVID-19 vaccines were made available for most Danes. The distribution of the first and second follow-up questionnaires was planned according to the vaccination plan so that most participants would have been offered their first and second dose at the time of the questionnaire. A third follow-up questionnaire was distributed approximately 1 year after baseline. Individuals aged 30–34 years did not receive the third follow-up questionnaire, due to an error in the distribution. Participants were only invited for follow-up questionnaires if they had answered the previous questionnaire. The questionnaires were available in Danish, English, and Arabic. In the present study, participants could be included if they had received one or more COVID-19 vaccines, based on information from the questionnaires.
2.3. Variables
The questionnaires contained information on 20 suspected ARs following COVID-19 vaccination. For each dose of the vaccine, participants were asked if they had experienced any of the following ARs in the week following vaccination: Redness and/or pain at the injection site, skin rash, nausea, vomiting, fever, shivering/chills, tiredness, general malaise, joint pain, muscle pain, headache, diarrhoea, dizziness, swollen lymph nodes, facial swelling, facial paralysis, pain in the arm and legs, allergic reaction, shortness of breath, or bruising/bleeding under the skin. Participants were provided with the following options for each of the ARs 1) No, 2) Yes, mild symptoms, 3) Yes, moderate symptoms, and 4) Yes, severe symptoms. For the analyses, we dichotomized the variables to having experienced the AR following vaccination (Yes, moderate symptoms or Yes, severe symptoms) or not having experienced the AR (No or Yes, mild symptoms). Based on information on the individual ARs, the number of ARs was calculated for each individual and categorized as 0, 1, 2–4, 5–9 or 10 + ARs. In the analyses for each dose, we only included individuals, who had provided information on all 20 ARs.
The questionnaires also contained questions regarding COVID-19 infection and vaccine type (see the Appendix for detailed descriptions of these variables). Age and sex were available from the Central Person Register. Age was categorized into the following age groups 17–25, 26–35, 36–45, 46–55, and 56 +.
2.4. Statistical analyses
Characteristics of the study population were described according to the vaccine doses by numbers and percentages. Further, frequencies of the individual ARs were estimated for each vaccine dose overall and stratified by sex, age group, and vaccine type, respectively.
The distribution of number of ARs was estimated stratified by sex, age, vaccine type, and prior COVID-19 infection, respectively. Also, in sub-analyses, the analysis stratified on prior COVID-19 infection was further stratified on sex, age, and vaccine type, respectively (first and second vaccine dose only, due to small groups when stratifying on the third dose). Differences in distributions of numbers of ARs between groups were tested with a χ2 test.
The frequency and number of ARs for the ChAdOx1-2 vaccine were only estimated for the first dose, because of few individuals having received the ChAdOx1-2 vaccine as their second or third dose.
Since the type of vaccine received varied by age and sex, a multiple logistic regression was used to estimate the relationship between odds of reporting 5 symptoms and above and sex, age, vaccine type, and prior COVID-19 infection (mutually adjusted).
All analyses were made using Stata version 17.
2.5. Ethics
The BiCoVac study was approved by the Danish Data Protection Agency under the Aarhus University comment agreement (journal number 2015-57-0002) and Aarhus University journal number 2016-051-000001, sequential number 2272 (25/3-2021). According to Danish legislation, ethical approval of survey studies based on questionnaires is not required.
2.6. Role of the funding source
TrygFonden had no role in the study design; in the collection, analysis, or interpretation of data; writing the report; or in the decision to submit the article for publication.
2.7. Results
Of the 911,613 individuals selected for the BiCoVac study, 22,110 individuals had no digital mailbox and were therefore not invited. In total, 889,503 individuals were invited to the BiCoVac study and 252,268 (28 %) filled in the baseline questionnaire. Of these, 59 % participated in the first follow-up, 43 % participated in the second follow-up and 25 % participated in the third follow-up. A total of 171,008 individuals responded to all ARs following the first COVID-19 vaccine dose. The corresponding numbers were 130,351 and 54,903 for the second and third dose respectively (Fig. 1 ). The number of individuals included in some of the analyses differs from these totals due to missing information on one or more variables or small groups (<5).
Fig. 1.
Flow chart.
The participation rate for the baseline questionnaire was 23 % for males and 34 % for women, and it increased with age ranging from 16 % to 47 %. In the current study there was therefore a larger proportion of females and older individuals than in the invited population (Table 1 ). Most individuals received the BNT162b2 vaccine (79 %, 85 %, and 86 % for the first, second, and third dose, respectively), while few received the ChAdOx1-2 vaccine (10 %, <1 %, and <1 %, respectively). Most individuals did not have a COVID-19 infection prior to receiving their COVID-19 vaccine.
Table 1.
Characteristics of the participants by vaccine dose.
| 1. vaccine N (%) | 2. vaccine N (%) | 3. vaccine N (%) | |
|---|---|---|---|
| Sex | |||
| Men | 64,849 (38) | 47,223 (36) | 20,783 (38) |
| Women | 106,159 (62) | 83,128 (64) | 34,120 (62) |
| Age | |||
| 17–25 | 10,640 (6) | 6,525 (5) | 1,779 (3) |
| 26–35 | 16,039 (9) | 11,711 (9) | 2,027 (4) |
| 36–45 | 24,558 (14) | 18,758 (14) | 6,839 (12) |
| 46–55 | 48,404 (28) | 36,863 (28) | 16,635 (30) |
| 56+ | 71,367 (42) | 56,494 (43) | 27,623 (50) |
| Vaccine type | |||
| BNT162b2 | 134,632 (79) | 110,870 (85) | 47,425 (86) |
| mRNA-1273 | 16,623 (10) | 17,914 (14) | 6,690 (12) |
| ChAdOx1-2 | 16,921 (10) | 463 (<1) | 179 (<1) |
| Other/unknown | 2,832 (2) | 1,104 (1) | 609 (1) |
| COVID-19 infection | |||
| Prior infection | 9,283 (5) | 7,216 (6) | 4,498 (8) |
| No prior infection | 146,898 (86) | 111,101 (85) | 42,220 (77) |
| Unknown | 14,827 (9) | 12,034 (9) | 8,185 (15) |
2.8. Frequencies of adverse reactions
A total of 38 %, 36 %, and 25 % reported one or more ARs following the first, second, and third dose of COVID-19 vaccine, respectively. The proportion reporting severe symptoms was the same after both the first and second vaccine dose (11 %), but it was lower after the third dose (6 %). The most frequently reported ARs were redness and/or pain at the injection site (Dose 1: 20 %, Dose 2: 15 %, Dose 3: 9 %) and tiredness (Dose 1: 19 %, Dose 2: 22 %, Dose 3: 14 %) (Fig. 2 ).
Fig. 2.
Prevalence of reported individual moderate or severe adverse reactions following COVID-19 vaccination by vaccine dose.
The types of the most frequently reported ARs were similar for men and women. However, most ARs were reported more frequently by women than men (Supplementary Fig. 1). Also, the ARs reported were similar across age groups, although most ARs were reported more frequently by individuals younger than 36 years compared with the older age groups (Supplementary Figs. 2-4).
The frequencies of each AR differed between the vaccine types: 53 % vaccinated with ChAdOx1-2 experienced tiredness after the first dose compared with 21 % for mRNA-1273 and 14 % for BNT162b2 (Supplementary Fig. 5).
2.9. Number of adverse reactions
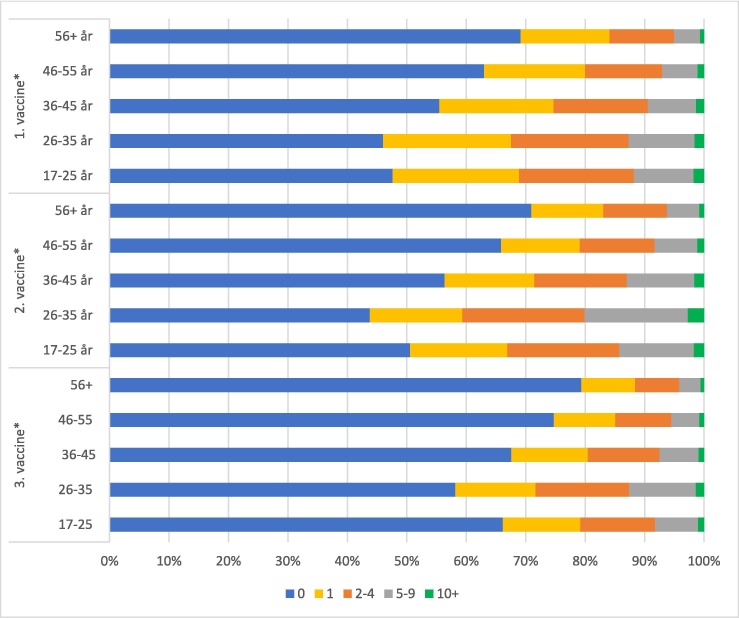
Overall, women reported more ARs than men (Fig. 3 ) and the number of ARs reported was generally lower for the higher age groups. This pattern was present for all vaccine doses (Fig. 4 ).
Fig. 3.
Percentage of respondents reporting 0, 1, 2–4, 5–9 or 10+ moderate or severe immediate adverse reactions following COVID-19 vaccination after first, second and third dose stratified by sex. * p value < 0.001.
Fig. 4.
Percentage of respondents reporting 0, 1, 2–4, 5–9 or 10+ moderate or severe immediate adverse reactions following COVID-19 vaccination after first, second and third dose stratified by age. * p value < 0.001.
Stratified by vaccine type, individuals vaccinated with ChAdOx1-2 reported more ARs following the first dose (71.7 % reported 1 or more and 6.5 % reported 10 or more ARs) compared with individuals vaccinated with BNT162b2 (32.1 % reported 1 or more and less than 0.4 % reported 10 or more ARs) and mRNA-1273 (51.3 % reported 1 or more and 0.8 % reported 10 or more ARs). Following the second and third dose, individuals vaccinated with mRNA-1273 reported more ARs compared with individuals vaccinated with BNT162b2 (Fig. 5 ).
Fig. 5.
Percentage of respondents reporting 0, 1, 2–4, 5–9 or 10+ moderate or severe immediate adverse reactions following COVID-19 vaccination after first, second and third dose stratified by vaccine type. * p value < 0.001.
In general, individuals with prior COVID-19 infection reported more ARs than those with no prior COVID-19 infection (Fig. 6 ). This was also the case when further stratified for sex and vaccine type, respectively (Supplementary figures 6 and 7). The same pattern was observed for the first dose when stratified by age. However, there was no statistically significant difference in the number of ARs between those who had a prior COVID-19 infection and those who did not for the second dose for participants in age groups below 46 years of age (Supplementary figure 8).
Fig. 6.
Percentage of respondents reporting 0, 1, 2–4, 5–9 or 10+ moderate or severe immediate adverse reactions following COVID-19 vaccination after first, second and third dose stratified by prior COVID-19 infection. * p value < 0.001.
When mutually adjusting for sex, age, vaccine type, and prior COVID-19 infection, men and individuals with higher age had lower odds of reporting 5 or more ARs following COVID-19 vaccination compared with women and individuals aged 16–25 years (Table 2 ). This pattern was observed for all vaccine doses. Individuals with a prior COVID-19 infection had higher odds of reporting 5 or more ARs compared with individuals with no prior COVID-19 infection (1. Dose: OR = 2.79 (CI95 % 2.61;2.99) 2. Dose: OR = 1.52 (CI95 % 1.41;1.64) 3. Dose: OR = 1.59 (CI95 % 1.40;1.79)).
Table 2.
Odds ratios for reporting 5 or more immediate adverse reactions for vaccine type, sex, age and prior COVID-19 infection by vaccine dose.
| 1. vaccine |
2. vaccine |
3. vaccine |
||||
|---|---|---|---|---|---|---|
| n (%) | OR (95 % CI) | n (%) | OR (95 % CI) | n (%) | OR (95 % CI) | |
| Vaccine type | ||||||
| BNT162b2 | 4033 (3) | 1.00 | 6365 (6) | 1.00 | 1633 (4) | 1.00 |
| mRNA-1273 | 1052 (7) | 1.95 (1.81–2.10) | 4551 (28) | 5.12 (4.89–5.35) | 695 (12) | 3.08 (2.80–3.40) |
| ChAdOx1-2 | 5830 (38) | 15.51 (14.80–16.25) | ||||
| Sex | ||||||
| Women | 9054 (9) | 1.00 | 8749 (12) | 1.00 | 1886 (7) | 1.00 |
| Men | 1861 (3) | 0.51 (0.48–0.54) | 2167 (5) | 0.44 (0.42–0.46) | 442 (3) | 0.40 (0.36–0.44) |
| Age | ||||||
| 16–25 | 1050 (11) | 1.00 | 799 (14) | 1.00 | 116 (8) | 1.00 |
| 26–35 | 1674 (12) | 0.83 (0.76–0.92) | 2153 (20) | 0.97 (0.89–1.07) | 171 (12) | 1.06 (0.82–1.36) |
| 36–45 | 2012 (9) | 0.65 (0.59–0.71) | 2151 (13) | 0.79 (0.72–0.87) | 380 (7) | 0.78 (0.63–0.97) |
| 46–55 | 3002 (7) | 0.55 (0.50–0.59) | 2691 (8) | 0.58 (0.53–0.64) | 719 (5) | 0.70 (0.57–0.86) |
| 56+ | 3177 (5) | 0.43 (0.39–0.46) | 3122 (6) | 0.43 (0.39–0.47) | 942 (4) | 0.55 (0.45–0.67) |
| COVID-19 infection | ||||||
| No prior infection | 9486 (7) | 1.00 | 9964 (9) | 1.00 | 1983 (5) | 1.00 |
| Prior infection | 1429 (16) | 2.79 (2.61–2.99) | 952 (13) | 1.52 (1.41–1.64) | 345 (8) | 1.59 (1.40–1.79) |
Individuals vaccinated with ChAdOx1-2 and mRNA-1273 had higher odds (OR = 15.51 (CI95 % 14.80;16.25) and OR = 1.95 (CI95 % 1.81;2.10), respectively) of reporting 5 or more ARs following the first vaccine dose compared with individuals vaccinated with BNT162b2. After the second and third dose individuals vaccinated with mRNA-1273 had higher odds than individuals vaccinated with BNT162b2.
3. Discussion
The present study is one of the first large population-based studies in Denmark to investigate immediate ARs following the first, second, and third COVID-19 vaccine dose, respectively. The most frequently reported ARs following COVID-19 vaccination were redness and/or pain at the injection site after the first dose, and tiredness after the second and third dose. In general, women reported more ARs than men and younger participants reported more ARs than older participants. These patterns were observed for all vaccine doses. Individuals with prior COVID-19 infection reported more ARs following COVID-19 vaccination than individuals with no prior COVID-19 infection. For the first dose, individuals who received ChAdOx1-2 reported the most ARs compared to individuals receiving mRNA-1273 and BNT162b2.
3.1. Strengths and limitations
A strength of the study is the population-based design and large sample size of the BiCoVac cohort. Further, it is a strength that data was obtained during the pandemic and timed according to the implementation of the COVID-19 vaccine schedule in the general Danish population. However, this was also a limitation as vaccine schedules and recommendations were continuously adapted. Thus, despite efforts to distribute the questionnaires according to the vaccination schedule, questionnaires were not distributed according to the vaccination date for the specific individual, which could potentially increase the risk of recall bias.
The BiCoVac cohort had a low response rate at baseline varying with age and sex of the invited individuals, with lowest participation among men and younger individuals. The study can therefore be subject to selection issues, which could affect both the overall prevalence of ARs in the population and in the comparison of subgroups. It can be speculated that individuals, who experience ARs are more likely to participate. This could lead to an overestimation of ARs. However, it could also be speculated that the low participation of particularly younger individuals may have caused an underestimation of the prevalence of ARs in the population, as we found more ARs in younger individuals. When comparing the prevalence of ARs after the third dose with that of the first and second dose, it is also important to bear in mind, that only few individuals aged 30–34 years were included in the analyses for the third dose. Also, the time from vaccination to answering the questionnaire on symptoms was longer for the third vaccine dose compared with the first and second dose. Hence participants may have underestimated the prevalence of immediate symptoms after the third dose.
The study aimed to describe the distributions of each AR and the number of ARs according to each dose with descriptive analyses. In addition, an adjusted analysis supported the pattern observed in the descriptive analyses.
3.2. Comparison with other studies
Other studies found pain at the injection site [17], [18], [20], [21], [22], fatigue [16], [17], [18], [20], [21], [22], and headache [16], [17], [18], [19], [20], [21], [22] to be the most common ARs following COVID-19 vaccination. These studies mainly included health professionals and other selected populations and are thus potentially not directly comparable with our study in a general population. However, the types of ARs reported in these studies were similar to those identified in the present study. However, the proportions of the different ARs varied considerably in the literature: Previous studies report proportions of headache varying between 10 % and 45 % [22], [16], [17], [18], [19], [20], while the present study found proportions in headache between 7 % and 12 % depending on dose. These differences might be explained by different definitions of ARs (the present study only count moderate and severe symptoms), differences in sample sizes, and different study populations (all the studies have healthcare workers as their population) [22], [16], [17], [18], [19], [20].
In correspondence with the present study, most previous studies found a higher frequency of symptoms in women compared with men [18], [20], [22] which could be due to women generally reporting more somatic symptoms than men [28]. Also, in line with the present study, previous studies found that younger individuals reported more ARs than older individuals [22], [18], [19], [20]. A study by Beatty et al. found that individuals vaccinated with mRNA-1273 experienced more ARs than individuals vaccinated with BNT162b2 [29], which corresponds with the finding that more individuals vaccinated with BNT162b2 presented with serological vaccine hyporesponsiveness at day 90 compared with individuals vaccinated with mRNA-1273 [30]. In the present study, a similar pattern was observed with a larger proportion reporting ARs following mRNA-1273 than following BNT162b2. A study in UK found that for the first dose, individuals vaccinated with ChAdOx1-2 reported systemic adverse effects more frequently than individuals vaccinated with BNT162b2. However, the opposite was found for local adverse effects [31]. Similarly, another study found that individuals vaccinated with ChAdOx1-2 reported systemic adverse events more frequently after the first dose than individuals vaccinated with mRNA-1273 and BNT162b2. After the second dose individuals vaccinated with mRNA-1273 reported systemic adverse events more frequent than individuals vaccinated with the two other types [32]. The present study also found more frequent ARs following COVID-19 vaccination with ChAdOx1-2 at the first dose compared to mRNA-1273 and BNT162b2. We however did not have enough participants receiving ChAdOx1-2 to investigate immediate symptoms following the second and third dose of ChAdOx1-2.
We found that individuals with prior COVID-19 infection reported more ARs compared with individuals with no prior COVID-19 infection, which could reflect immunological recall [33]. Similar findings were observed in other studies [19], [29], [34], but not all [18]. A study among hospital workers found that individuals with prior COVID-19 infection were more likely to report symptoms after the first COVID-19 vaccine dose (but not the second dose) compared with individuals with no prior COVID-19 infection [35]. A study among individuals in nursing homes did not find higher rates of more severe ARs in individuals with prior COVID-19 infection than without prior COVID-19 infection [36], potentially due to a weaker immunological response in the elderly.
The prevalence of COVID-19 infection reported in the study and in the Danish population at the time was comparable. Hence the prevalence in the current study was between 5 and 8 %, varying with vaccine dose, compared with a prevalence between 5 and 13 % in the general Danish population at the time most Danish citizens got vaccinated (May 2021–December 2021) [37].
4. Conclusion
In this large population-based study, we found that the majority of individuals vaccinated did not experience the investigated immediate ARs following COVID-19 vaccination. Redness and/or pain at the injection site were the most frequently reported ARs following the first dose of COVID-19 vaccine. Tiredness was the most frequently reported AR following the second and third dose. Females, younger individuals, and those with a prior COVID-19 infection reported ARs more frequently. Individuals vaccinated with ChAdOx1-2 reported the most ARs and individuals vaccinated with BNT162b2 reported the least ARs.
5. Authors’ roles
KTH, FKP, BHB, SMT, and DR participated in the conception of the study and design, writing of the article, and interpretation of the results. KTH, FKP, and DR performed the analysis. KTH and FKP wrote the first draft of the manuscript. All authors critically revised, commented on, and approved the final manuscript.
Funding
This work was supported by TrygFonden (id-number: 153678).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.06.069.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.SSI. Tidslinje for covid-19 2022 [Available from: https://covid19.ssi.dk/-/media/arkiv/subsites/covid19/presse/tidslinje-over-covid-19/covid-19-tidslinje-lang-for-2020-2022-version-2---december-2022.pdf. [Last accessed: 14/2 2023].
- 2.Andersen TM. Effekterne af ikke-farmaceutisk intervention under Covid-19 pandemien - En oversigt Finansministeriet2021 [Available from: https://fm.dk/media/25165/5-baggrundspapir-effekterne-af-ikke-farmaceutisk-intervention-under-covid-19-pandemien-en-oversigt.pdf. [Last accessed:
- 3.Perra N. Non-pharmaceutical interventions during the COVID-19 pandemic: A review. Phys Rep. 2021;913:1–52. doi: 10.1016/j.physrep.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EMA recommends first COVID-19 vaccine for authorisation in the EU: European Medicines Agency; 2020 [Available from: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu. [Last accessed: 08-02 2022].
- 5.EMA recommends COVID-19 Vaccine Moderna for authorisation in the EU: European Medicines Agency; 2021 [Available from: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu. [Last accessed: 14/2 2023].
- 6.EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU European Medicines Agency2021 [Available from: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu. [Last accessed: 9/3 2023].
- 7.EMA recommends COVID-19 Vaccine Janssen for authorisation in the EU European Medicines Agency2021 [Available from: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-janssen-authorisation-eu. [Last accessed: 14/2 2023].
- 8.Generelt om vacciner mod COVID-19: Lægemiddelstyrelsen [Available from: https://laegemiddelstyrelsen.dk/da/nyheder/temaer/generelt-om-vacciner-mod-covid-19/. [Last accessed: 03-03-2022 2022].
- 9.Pottegård AL, L.C.; Karlstad, Ø.; Dahl, Jesper; Andersen, Morten; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021(373). [DOI] [PMC free article] [PubMed]
- 10.Sundhedsstyrelsen. Pausering af brug af COVID-19 Vaccine AstraZeneca i det danske COVID 19 vaccinationsprogram 2021 [Available from: https://www.sst.dk/-/media/Udgivelser/2021/Corona/Vaccination/Notater/Notat-om-pausering-af-COVID-19-Vaccine-AstraZeneca-i-det-danske-COVID-19-vaccinationsprogram.ashx. [Last accessed: 04.11. 2022].
- 11.Sundhedsstyrelsen. Danmark fortsætter vaccineudrulning uden AstraZeneca-vaccinen 2021 [Available from: https://www.sst.dk/da/Nyheder/2021/Danmark-fortsaetter-vaccineudrulning-uden-AstraZeneca-vaccinen. [Last accessed: 14.04.21.
- 12.Auster O., Finkel U., Dagan N., Barda N., Laufer A., Balicer R.D., et al. Short-term adverse events after the third dose of the BNT162b2 mRNA COVID-19 vaccine in adults 60 years or older. JAMA Netw Open. 2022;5(4) doi: 10.1001/jamanetworkopen.2022.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menni C., May A., Polidori L., Louca P., Wolf J., Capdevila J., et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022;22(7):1002–1010. doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen P.-Y., Wu B.-J., Su M.-C., Lin Y.-H., Chiang S.-C., Wu J.-C., et al. Risk factors and incidence rates of self-reported short-term adverse events of COVID-19 vaccine booster dose. Vaccines (Basel) 2022;10(7) doi: 10.3390/vaccines10071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali M.D., Almadan L.Z., Alghamdi R.A., Alghamdi A.S., Almarhoon S.A., Hassan Y.AM., et al. Evaluation of prevalence of side-effects associated with booster dose of mRNA-Based COVID-19 vaccine among healthcare workers in eastern province, Saudi Arabia: A descriptive cross-sectional study. Infect Drug Resist. 2022;15:4335–4346. doi: 10.2147/IDR.S374265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadali R.A.K., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Im J.H., Kim E., Lee E., Seo Y., Lee Y., Jang Y., et al. Adverse events with the pfizer-BioNTech COVID-19 vaccine among korean healthcare workers. Yonsei Med J. 2021;62(12) doi: 10.3349/ymj.2021.62.12.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosun S., Ozkan Ozdemir H., Erdogan E., Akcay S., Aysin M., Eskut N., et al. Adverse events report of inactivated COVID-19 vaccine from 4040 healthcare workers. Postgrad Med. 2022;134(1):104–110. doi: 10.1080/00325481.2021.1999708. [DOI] [PubMed] [Google Scholar]
- 19.Quiroga B., Sánchez-Álvarez E., Goicoechea M., de Sequera P. COVID-19 vaccination among Spanish nephrologists: Acceptance and side effects. J Healthc Qual Res. 2021;36(6):363–369. doi: 10.1016/j.jhqr.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riad A., Hocková B., Kantorová L., Slávik R., Spurná L., Stebel A., et al. Side effects of mRNA-based COVID-19 vaccine: nationwide phase IV study among healthcare workers in slovakia. Pharmaceuticals (Basel) 2021;14(9) doi: 10.3390/ph14090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed R.A., Garout R.M., Wahid S., Ayub F., Firas ZinAlddin L.M., Sultan I. A survey on the side effects of pfizer/BioNTech COVID-19 vaccine among vaccinated adults in Saudi Arabia. Cureus. 2021;13(11) doi: 10.7759/cureus.19222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borroni E., Consonni D., Cugno M., Lombardi A., Mangioni D., Bono P., et al. Side effects among healthcare workers from a large Milan university hospital after second dose of BNT162b2 mRNA COVID-19 vaccine. Med Lav. 2021;112(6):477–485. doi: 10.23749/mdl.v112i6.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hvor mange danskere bliver vaccineret mod covid-19? Statens Serum Institut2020 [Available from: https://www.ssi.dk/aktuelt/nyheder/2020/12_hvor-mange-danskere-bliver-vaccineret-mod-covid-19_29122020. [Last accessed: 20/02 2023].
- 24.Sundhedsstyrelsen. Personer med øget risiko ved COVID-19 - Fagligt grundlag; 2021.
- 25.Sundhedsstyrelsen. Vaccinationskalender 2021 [Available from: https://www.sst.dk/-/media/Udgivelser/2021/Corona/Vaccination/Kalender/Vaccinationskalender-01072021.ashx?sc_lang=da&hash=2F626F7E6433723A88DE3B36228F8C03. [Last accessed: 03-04 2023].
- 26.Sundhedsstyrelsen. Tilbud om 3. stik mod covid-19.
- 27.Bicovac [Available from: https://ph.au.dk/bicovac. [Last accessed: 03-04 2023].
- 28.Barsky A.J., Peekna H.M., Borus J.F. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16(4):266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Søgaard O.S., Reekie J., Johansen I.S., Nielsen H., Benfield T., Wiese L., et al. Characteristics associated with serological COVID-19 vaccine response and durability in an older population with significant comorbidity: the Danish Nationwide ENFORCE Study. Clin Microbiol Infect. 2022;28(8):1126–1133. doi: 10.1016/j.cmi.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kant A., Jansen J., van Balveren L., van Hunsel F. Description of frequencies of reported adverse events following immunization among four different COVID-19 vaccine brands. Drug Saf. 2022;45(4):319–331. doi: 10.1007/s40264-022-01151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Tong P., Whiteman N., Sanjari Moghaddam A., Zarghami M., Zuiani A., et al. Immune recall improves antibody durability and breadth to SARS-CoV-2 variants. Sci Immunol. 2022;7(78) doi: 10.1126/sciimmunol.abp8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debes A.K., Xiao S., Colantuoni E., Egbert E.R., Caturegli P., Gadala A., et al. Association of vaccine type and prior SARS-CoV-2 infection with symptoms and antibody measurements following vaccination among health care workers. JAMA Intern Med. 2021;181(12) doi: 10.1001/jamainternmed.2021.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bardenheier B.H., Gravenstein S., Blackman C., Gutman R., Sarkar I.N., Feifer R.A., et al. Adverse events following one dose of mRNA COVID-19 vaccination among US nursing home residents with and without a previous SARS-CoV-2 infection. J Am Med Dir Assoc. 2021;22(11):2228–2232. doi: 10.1016/j.jamda.2021.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coronavirus (COVID-19) Cases Our World in Data2023 [Available from: https://ourworldindata.org/covid-cases. [Last accessed: 08-06 2023].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.