Abstract
The epidermis is a stratified squamous epithelium that forms the outermost layer of the skin. Its primary function is to act as a barrier, keeping pathogens and toxins out and moisture in. This physiological role has necessitated major differences in the organization and polarity of the tissue as compared to simple epithelia. We discuss four aspects of polarity in the epidermis – the distinctive polarities of basal progenitor cells as well as differentiated granular cells, the polarity of adhesions and the cytoskeleton across the tissue as keratinocytes differentiate, and the planar cell polarity of the tissue. These distinctive polarities are essential for the morphogenesis and the function of the epidermis and have also been implicated in regulating tumor formation.
Keywords: skin, epidermis, polarity, cancer, asymmetric cell division, epithelia, barrier, basal cell, granular cell, planar cell polarity
1. Introduction to Epidermal Cell Types and Architecture
The epidermis has a multi-layered or stratified architecture that is essential for its function. The entire tissue is attached to an underlying basement membrane which separates it from the dermis below. The cells directly in contact with the basement membrane are basal progenitor cells that are responsible for the rapid turnover of the tissue and for wound-healing (Figure 1). Above these are differentiated cells, collectively referred to as “suprabasal”. There are a number of distinct cell types that the basal progenitors move through on their path through differentiation and eventual death. Spinous cells are the direct progeny of differentiating basal cells, which then progress through a granular cell fate before their eventual death and formation of the cornified layer, the outermost layer of the skin.
Figure 1.
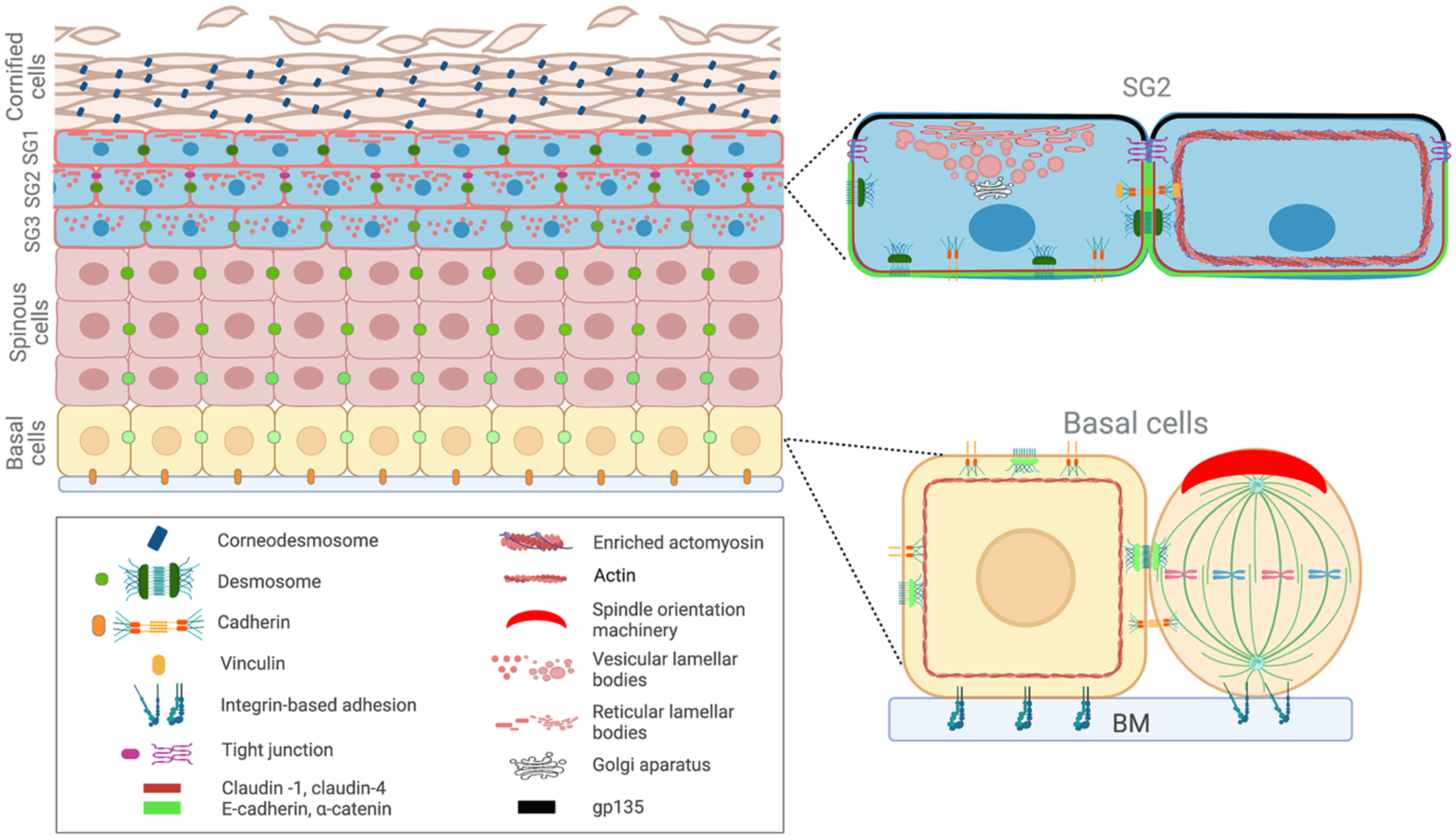
Diagram of epidermal architecture. Polarity in both basal and granular cells are indicated on the right.
2. Development of the Epidermis
The epidermis is born as the polarized ectoderm commits to an epidermal fate. At its earliest stage, epidermal cells are a simple polarized epithelium and have a free apical surface, a hallmark of simple epithelia. However, as the epidermis develops and stratifies, cells make adhesive contacts with other cells on all their surfaces. This poses a problem for the developing epidermis as cell-cell interactions between regions of skin that contact one another must be prevented. Therefore, the surface of the tissue must be non-adhesive to prevent inappropriate cell-cell interactions. In the adult, this is achieved by the dead cornified layer. In contrast, embryos use a specialized and transient cell type, the periderm, to solve this problem. The periderm covers the surface of the epidermis starting shortly after its commitment. The periderm is composed of polarized cells with tight junctions and is replaced as the fully differentiated and dying squames of the cornified envelope are initially produced. Consistently, in the absence of periderm, there are fusions of digits and within the palate, as the periderm provides an antiadhesive coating for the epidermis (Richardson et al., 2014).
3. Polarity in the Epidermis
3.1. Polarity in Basal Cells
Basal cells have a clear basal side defined by their attachment to the basement membrane. At this site, there is an enrichment for the hemidesmosomal α6/β4-integrin complex, which is likely stabilized at this site by interactions with the underlying extracellular matrix (ECM). In the absence of α6 or β4-integrin, the epidermal attachment to the underlying basement membrane is lost (van der Neut et al., 1996), resulting in junctional epidermolysis bullosa (Niessen et al., 1996; Ruzzi et al., 1997). However, while there is polarity of this basal membrane marker, it is unclear whether there are any lateral and apical membrane proteins that show polarity in these cells. Common lateral markers of simple epithelia, like E-cadherin, are found at all cell-cell contact sites of these cells, including their “apical” region which makes contact with overlying spinous cells. To our knowledge, there have been no reports showing specific apical enrichment of transmembrane proteins. These cells also lack tight junctions which normally demarcate the borders of apical and lateral membranes. Although it is unknown whether the apical membrane is polarized, there is clear evidence that the apical region of the cell is specified and marked by core polarity machinery like Par3 and Gai3 (Lechler & Fuchs, 2005; Williams et al., 2014). This localization depends on both cell-cell and cell-ECM adhesion as it requires both a-catenin and b1-integrin. Furthermore, the cytoplasm is polarized as both the centrosome and the Golgi Apparatus are consistently apical to the nucleus (Lechler & Fuchs, 2007).
One of the clearest functions of cell polarity in basal progenitors is to allow oriented cell divisions. During early development of the epidermis, basal progenitors divide planar to the underlying basement membrane (Lechler & Fuchs, 2005; Smart, 1970; Williams et al., 2011). These symmetric divisions give rise to two basal daughters. However, concomitant with the stratification of the epidermis, a substantial number of divisions become perpendicular and/or oblique to the underlying basement membrane. A number of lineage tracing and live imaging studies have demonstrated that these are functionally asymmetric – giving rise to one basal cell and one cell that is born into the suprabasal layer of the epidermis (Box et al., 2019; Poulson & Lechler, 2010). The ability to orient mitotic spindles requires both cell-cell and cell-ECM adhesion (Lechler & Fuchs, 2005). Perpendicular alignment of the spindle to produce asymmetric cell divisions is controlled by a core spindle orientation machinery that is polarized to the apical cortex of dividing cells. These divisions are essential for proper stratification and differentiation of the epidermis, as disruption of the core machinery results in randomized or symmetric cell divisions and profound defects in epidermal morphology (Moreci & Lechler, 2021; Seldin et al., 2016; Williams et al., 2011; Zhong et al., 2022).
While a role for spindle orientation in epidermal morphogenesis is evident, the connections to cell fate remain stubbornly murky. In the interfollicular epidermis, activation of Notch signaling is an essential driver of differentiation (Blanpain et al., 2006). Although the molecular connections between spindle orientation and Notch activation in the skin are unknown, it is clear that Notch signaling lies downstream of spindle orientation (Williams et al., 2011). There are likely both intrinsic and extrinsic cues that robustly guide cell fate, complicating this analysis. Notably, a specific population of basal progenitor cells fated to form hair follicles also divides asymmetrically (Ouspenskaia et al., 2016). The molecular machinery required for these asymmetric cell divisions has both overlapping and distinct components with surrounding basal progenitors (Byrd et al., 2016; Moreci & Lechler, 2021). Further, these divisions impact on Wnt signaling to control cell fate (Moreci & Lechler, 2021). Together, these findings show diversity in both the molecular machinery and the downstream signaling pathways of asymmetric cell divisions.
After embryonic stratification, maintenance of tissue architecture does not require precise control of spindle orientation. Rather, cell delamination from the basal layer supports homeostasis (Rompolas et al., 2016). There have been some reports of delamination occurring even in the embryonic epidermis, though neither the rate of this, nor its importance for stratification has been determined (Miroshnikova et al., 2018).
Basal cells give rise to the first differentiated cell type, spinous cells, which have no known polarization at the membrane or subcellular level. That said, spinous cells interact with distinct membranes below (basal cells) and above (other spinous cells), and the mechanics of these layers are distinct, so it is possible that further examination will find aspects of polarity even within these cells.
3.2. Polarity of Granular Cells
Two layers in the epidermis – the granular and cornified layers – compromise distinct components of the epidermal barrier. The outermost barrier is the stratum corneum, which is composed of enucleated cornified keratinocytes immersed in a complex lipid network that provides a watertight environment (Baroni et al., 2012; Blank, 1953; Brandner et al., 2015). The inner barrier is composed of tights junctions (TJs) in the stratum granulosum/granular layer (Furuse et al., 2002; Tellkamp et al., 2014; Tunggal et al., 2005), which seal neighboring cells and are especially important to prevent loss of water from within the body.
The stratum granulosum is composed of three layers in the human skin: the granular layer closest to the spinous cells (SG3), the intermediate granular layer (SG2), and the outermost granular layer adjacent to the stratum corneum (SG1) (Furuse et al., 2002; Kubo et al., 2009; Yoshida et al., 2013). Granular cells do not contact basement membrane and have contacts with other cells on all sides, thus lacking an obvious polarity of simple epithelia. Despite this, we discuss evidence below that these cells are polarized in their organelle localization and their membrane composition.
Granular cells synthesize crucial components for the epidermal barrier that are secreted to the extracellular space through membrane-bound organelles called lamellar bodies (LB) to form part of the stratum corneum (Mahanty & Setty, 2021). Cargo components of LB include precursor lipids, and enzymes necessary for processing them into their final lipid products, as well as proteolytic enzymes important for desquamation, and other proteins like corneodesmosin (Ishida-Yamamoto et al., 2004). Therefore, LB formation is crucial for the establishment of the epidermal barrier, and their defective biogenesis or secretion is linked to diseases with defective barrier function like atopic dermatitis and severe ichthyosis syndromes (Elias & Wakefield, 2014; Feingold, 2012; Ishida-Yamamoto et al., 2018; Montpetit et al., 2008; Sakai et al., 2007).
Notably, LBs are thought to be largely secreted “apically”, which is a surprising finding for a cell type that lacks many epithelial characteristics. LBs undergo changes in structural conformation and location while they move up through the granular layers. Several studies have proposed different models of LB’s morphology and secretion of their cargo (den Hollander et al., 2016; Elias et al., 1998; Landmann, 1986; Norlen, 2001; Norlen et al., 2003). However, a recent study elucidated the highly detailed 3D structure of LB throughout the different layers of stratum granulosum in human skin (Yamanishi et al., 2019). In the SG3 layer, the LB are vesicular. When they transition to SG2, some LB fuse with the apical plasma membrane and show a reticular structure, while others remain as vesicles. Furthermore, in SG2, the tubular branches of TGN extend underneath the apical reticular network of lamellar bodies and connect to the apical vesicular lamellar bodies. Beneath the cornified layer, in SG1 cells, most LBs are fused to the plasma membrane and form a reticular network in the apical side of the outermost granular layer, in accordance with previous studies (Yamanishi et al., 2019). Thus, cells in the second granular layer show cellular polarization of some of their organelles’ distribution. However, the mechanisms by which these cells define and target components to this “apical” membrane have not been defined.
In addition to SG2 cells being able to direct target secretion apically, they are uniquely characterized by having tight junctions within the epidermis. TJ components, such as occludin and zonula occludens-1, are concentrated at sites between SG2-SG2 junctions (Furuse et al., 2002; Kubo et al., 2009; Yoshida et al., 2013). Additionally, cells in this layer have enriched cortical organization of F-actin compared to spinous layers (Rubsam et al., 2017). While adherens junctions are present in cell-cell contacts throughout all epidermal layers, vinculin, a cytoskeletal protein that couples adherens junctions to the actomyosin complex (le Duc et al., 2010), is enriched in SG2 (Rubsam et al., 2017). SG2 segregate these cell adhesions such that TJs are found between their apical edges, while underneath there is a basolateral network of vinculin-rich adherens junctions (Rubsam et al., 2017) (Figure 1), similar to simple epithelia (St Johnston & Sanson, 2011).
As discussed above, SG2 keratinoyctes have both tight junctions and can secrete LBs in a directional fashion. Additionally, SG2 cells have polarized membrane composition, where TJs demarcate specific membrane domains from each other. Claudin-1, an integral membrane protein component of TJs, and claudin-4 are present only in the basal and lateral sides but are excluded from the apical membrane of SG cells (Yoshida et al., 2013). Adherens junction components such as α-catenin and E-cadherin, have the same pattern as claudin-1. Further, gp135, a protein localized apically in the membrane of TJ-forming simple epithelial cells (Meder et al., 2005), is also confined to the apical side of SG2 cells (Yoshida et al., 2013). This compartmentalization of membrane domains around TJs shows that SG2 cells have polarized cell membranes (Figure 1). However, this apico-basolateral membrane polarity is not present in SG3 and is lost when SG2 cells differentiate into SG1 cells (Yoshida et al., 2013).
3.3. The Cornified Layer
Another intercellular junction that is asymmetrically found in the epidermis are corneodesmosomes (Ishida-Yamamoto & Igawa, 2015). These junctions are modified desmosomes and are only present in between cornified keratinocytes. They play a role as adhesive structures in the cornified layer and in maintaining thickness of the stratum corneum (Jonca et al., 2002). While they originate from desmosomes, their major structural difference is the presence of corneodesmosin (CDSN) in the extracellular region of corneodesmosomes. Desquamation, the loss of the dead squames of the skin, occurs when degradation of their external structure is completed. CDSN is secreted by LBs, as well as proteases, including kallikrein-related proteases and their inhibitors, which control the desquamation process (Ishida-Yamamoto et al., 2005; Ishida-Yamamoto et al., 2004; Jonca et al., 2011; Raymond et al., 2008).
The relative impermeability of the skin is provided by lipid lamellae containing ceramides, cholesterol and free fatty acids, that cement together the cornified envelopes in the stratum corneum (Bouwstra & Ponec, 2006; Feingold, 2012; Mahanty & Setty, 2021). Granular cells synthesize precursor barrier lipids in the Golgi apparatus and endoplasmic reticulum and are then secreted to the extracellular space through LBs. These precursor lipids are processed by enzymes also secreted by LBs and, once in the intercellular space, are arranged into stacked sheets that are observed in the intercellular spaces throughout the cornified layers (reviewed in (Mahanty & Setty, 2021).
To form a functional epidermal barrier, lipids in the intercellular lipid matrix undergo progressive molecular arrangements that result in polarized organization of lipid structures along the stratum corneum. As observed by cryo-EM, the lipid lamellae undergo five different maturation stages in their progress through the cornified layers (Narangifard et al., 2018; Narangifard et al., 2021). In human skin, secreted lipids start as a highly folded, highly hydrated lipid bilayer present in LBs of granular cells. After several steps of reorganization, a mature skin barrier structure is formed at the third to fifth cornified layer, exhibiting stacked lipid bilayers of fully stretched ceramides, with cholesterol associated with ceramide’s shorter sphingoid tail, while free fatty acids are associated with ceramides’ longer fatty acid tail (Narangifard et al., 2021; Norlen et al., 2022).
4. Core Polarity Machinery
Several studies have begun to address the roles of core cell polarity machinery in the epidermis. Below we discuss developmental and homeostatic roles for these proteins in the epidermis, and discuss distinctive roles in tumor formation later in the review.
4.1. Cdc42/Par3/Par6/aPKC
A number of reports have examined the effects of loss of the polarity and cytoskeleton regulator Cdc42 with somewhat contradictory results. In one study, loss of Cdc42 in the epidermis resulted in neonatal lethality with tight junction and barrier defects, as well as decreased levels of other cell-cell junctions (Zhang et al., 2019). In the other, mice were born and developed both hair defects as well as defects in the basement membrane (Wu, Quondamatteo, & Brakebusch, 2006; Wu, Quondamatteo, Lefever, et al., 2006). As tight junctions and transepithelial water loss were not examined in this second case, it is possible that later recombination allowed neonatal mice to escape an essential window of function. However, roles in membrane or organelle polarity or in spindle orientation were not examined in either case.
Similar to loss of Cdc42, Par3 epidermal knockout results in tight junction defects – establishing this as a major target of this polarity machinery (Ali et al., 2016). In addition, there was a defect in spindle orientation noted after Par3 knockdown in the embryonic epidermis (Williams et al., 2014). In contrast, in the adult interfollicular epidermis there was no effect of Par3 loss on spindle orientation, though slight defects were detected in hair follicles (Ali et al., 2016). Rather, Par3 appeared to promote contractility and in its absence the change in mechanics resulted in mitotic defects (both aneuploidy and DNA-damage) (Dias Gomes et al., 2019).
There are three paralogs of atypical protein kinase C (aPKC) expressed in the epidermis – zeta, iota, and lambda (Helfrich et al., 2007). Expression analysis has shown that zeta is preferentially expressed in basal cells while iota and lambda are more broadly expressed and concentrated in the granular layer. Loss of aPKC activity, through inhibitors or expression of kinase dead forms, resulted in tight junction defects in cultured keratinocytes (Helfrich et al., 2007). Deletion of aPKC lambda in mice, in contrast leads to a pronounced hair follicle stem cell activation phenotype (Niessen et al., 2013; Osada et al., 2015). There is also a small increase in asymmetric cell divisions within both the hair follicle stem cell compartment and the interfollicular epidermis, though it is unclear whether these changes are cause-or-effect, especially as the other isoform members remained.
4.2. Scrib/Dlg/Lgl
Global loss of Scrib in mouse results in both a delay in epidermal development and in neonatal lethality (Pearson et al., 2015). Epidermal-specific deletion had very little effect, though it is not clear whether this is because Scrib is not required autonomously in the epidermis for development, or whether recombination occurred later than its essential period. There has been no thorough characterization of membrane or organelle polarity in these cells. Furthermore, there are 5 Dlg-related genes (Dlg1–5) in mouse and two Lgl genes (Llgl1–2), which has precluded loss of function studies.
4.3. Cell/Cell and Cell/Substratum Adhesion
The classical cadherins E- and P-cadherin are co-expressed in basal cells of the epidermis, while E-cadherin is specifically found throughout the tissue (Tinkle et al., 2004). Loss of E-cadherin alone is sufficient to disrupt both tight junction formation and robust barrier acquisition, consistent with its unique expression in the granular layer (Tunggal et al., 2005). Coincident loss of both E- and P-cadherin in the tissue also resulted in tight junction defects, but also overt cell adhesion defects with separations between cells (Tinkle et al., 2008). Notably, although polarity and spindle orientation were not directly addressed, there was a mis-localization of β4-integrin that may suggest some polarity defects in these cells. Furthermore, loss of a-catenin, an adaptor and cytoskeletal linker in the adherens junction, resulted in spindle orientation defects in the embryonic epidermis, as well as massive hyperproliferation and differentiation defects, due at least in part to activation of Yap signaling (Schlegelmilch et al., 2011; Vasioukhin et al., 2001).
While the classical polarity genes described above were well worked out in fly epithelia, there is one notable exception to their importance for polarity establishment. In the fly gut, many of these regulators are not required for polarity, but integrins are (Chen et al., 2018). This base up rather than top down polarity, may be a more common occurrence in mammals. In support of this, loss of b1-integrin results in clear defects in the mitotic apical localization of Par3 and in spindle orientation in the embryonic skin (Lechler & Fuchs, 2005).
5. Mechanical Polarity Across the Epidermis
The varied tissues that comprise the main layers of the skin (epidermis, dermis and hypodermis) have different mechanical characteristics. The epidermis is mainly composed of keratinocytes, though there are a number of additional resident cell types. The dermis consists of connective tissue, which contains fibrous proteins like collagen (18–30% of dermis volume), and lower amounts of elastin and other components. This is in addition to the cellular components including fibroblasts, vasculature, etc. Lastly, the inner most layer, or hypodermis, contains adipocytes that function as both a cushion and a regulator of other aspects of skin biology (Festa et al., 2011; Joodaki & Panzer, 2018). A recent study reported stiffness levels of the different layers of the human skin in vivo. They measured the Young’s modulus of the forearm of healthy volunteers: epidermis (~4 MPa), dermis (40 kPa), and hypodermis (15 kPa) (Feng et al., 2022). This indicates that there is mechanical polarity across the main layers of the skin. However, there are likely to be differences even within these tissues as different layers of the epidermis are expected to have distinct stiffness levels.
During development, mechanical cues are not evenly distributed across the forming epidermis. Recent studies have shown that anisotropic tissue deformations, like growth rate and morphological changes, induce stretching and crowding in the basal cell layer. These events coordinate planar cell polarity alignment and produce different cell geometries and tension levels along the planar axis of the epidermis (Aw et al., 2016). At E13.5, when the epidermis is a single layer of progenitor cells, neural tube closure induces cell stretching and flattening of basal cells along the midline of the embryo’s back skin, which biases planar cell division and delays stratification. In the contrary, cells in the lateral areas were taller and more crowded, which promoted asymmetric cell divisions to promote stratification (Box et al., 2019).
While adherens junctions are found throughout the epidermis, they are thought to be in a distinct, high-tension state in granular cells. Adherens junctions are mechanosensitive structures that couple intercellular junctions to the cytoskeleton (le Duc et al., 2010). They become engaged under tension, which is thought to involve conformation changes in the adaptor protein a-catenin, allowing increased association with vinculin and potentially other actin-binding proteins (Yonemura et al., 2010). In SG2 cells, there are increased levels of cortical F-actin, suggesting higher contractility. Consistently, vinculin, α−18 (an antibody that recognizes a mechanosensitive conformation of α-catenin) (Yonemura et al., 2010), as well as phosphorylated myosin light chain were high in granular cells, indicating increased actomyosin contractility in these layers (Rubsam et al., 2017). Engagement of these adherens junctions and generation of intercellular tension is mediated by E-cadherin and is required for proper barrier function (Tunggal et al., 2005). E-cadherin also plays a key role in regulating the polarized localization and activation of EGFR, which restricts TJ formation to the SG2 layer, thus allowing the assembly of a functional epidermal barrier (Rubsam et al., 2017). In support of the idea that higher tension in granular layers is required to form the epidermal barrier, depleting MyoIIA and B in mouse epidermis resulted in reduced tension and severe defects in TJ barrier function in vivo (Sumigray et al., 2011). Further, this work demonstrated that microtubules, which become strongly associated with the cell cortex in granular cells (Lechler & Fuchs, 2007), recruit myosinII to the cell cortex resulting in adherens junction engagement, which promotes proper TJ function (Sumigray et al., 2011). Accordingly, loss of Lis1, which is required for microtubule reorganization to the cell cortex in differentiated keratinocytes, resulted in loss of both cortical microtubules and of tight junction barrier activity, leading to perinatal lethality in mice (Lechler & Fuchs, 2007; Sumigray et al., 2011).
The data above suggest that tight control of contractility may be important for many aspects of epidermal biology. In support of this, when contractility was increased in differentiated cells of the epidermis (though genetic mechanisms) there were dramatic consequences. These included both cell autonomous effects on adhesions as well as nonautonomous effects on the proliferation, motility, and cell fates of underlying progenitor cells (Ning et al., 2021). These data highlight the importance of spatially controlled regulation of contractility within a tissue.
6. Planar cell polarity in the epidermis
In addition to the apical-basal polarity that is characteristic of all epithelia, the epidermis has a notable planer cell polarity (PCP), i.e. polarity within the plane of the tissue. Genetic identification of genes responsible for planar cell polarity in Drosophila have unveiled core conserved machinery that form asymmetric adhesive complexes across epithelial cells (reviewed in (Butler & Wallingford, 2017)).
In the epidermis, disruption of core PCP genes, including Fzd6, Vangl and Celsr results in a loss of the normal stereotypical angling of hair follicles (Devenport & Fuchs, 2008; Guo et al., 2004). Hair follicles are appendages or outgrowths of the epidermis, and in their development on the back of the mouse, they are uniformly pointing toward the anterior, as this angles hairs towards the posterior. In PCP mutant backgrounds, these hair follicles generally grow straight down, and hairs grow in random orientations (Devenport & Fuchs, 2008). Depending on the genetic background, the initial defects in morphogenesis may be corrected during subsequent growth cycles of the hair, thought the mechanisms underlying this remain unknown. Significant insights into the roles of PCP components in the orientation of hair follicles has come from live-imaging of the earliest stages of hair follicle morphogenesis. As the placode is being established, counter-rotational migrations of cells occur within the placode cells, which requires the proper expression of PCP components (Cetera et al., 2018). This converts the radially symmetric placode into one that is polarized along the anterior-posterior axis, allowing angled downgrowth. The migrations that drive this polarization require regulation of acto-myosin contractility and F-actin assembly (Cetera et al., 2018). Consistent with this, mutation of a number of actin regulatory proteins results in planar cell polarity defects in the epidermis (Luxenburg et al., 2015; Padmanabhan et al., 2020).
While PCP is clearly important for hair follicle orientation, the establishment of PCP within the epidermis occurs earlier and throughout the tissue. Elegant work has established that tissue level forces transform an initially isotropic organization of cells and Celsr localization into one that is polarized along the anterior-posterior axis (Aw et al., 2016). Additionally, simply providing tension in the perpendicular direction was sufficient to re-orient the PCP along the lateral axis.
7. Polarity and Skin Cancer
Dysregulation of apicobasal polarity has been implicated in cancers in a number of contexts. Early studies in D. melanogaster larvae suggested a protective role of polarity proteins against neoplastic outgrowth and the presence of tumors when flies were mutant for Lethal Giant Larvae (Lgl), Scribble (Scrib) and Discs large (Dlg) (Bilder, 2004). Like its function in Drosophila, Scrib acts like a tumor suppressor in a mouse model of epidermal carcinogenesis (Pearson et al., 2015). In this case, it appeared to do so by promoting apoptosis. However, some mammalian polarity proteins show both pro-oncogenic and tumor suppressive roles. For example, Par3 and aPKCλ cooperate in tumor formation and progression in a Ras-induced cancer model; however, independently from each other, these proteins exert different functions. While aPKCλ acts as a tumor promoter, Par3 can either serve as a tumor suppressor or promoter (Iden et al., 2012; Vorhagen et al., 2018).
Disruption of spindle orientation, especially in the background of other oncogenes/tumor suppressors leads to tumor-like states in Drosophila (reviewed in (Powell et al., 2010). In the adult epidermis, divisions are largely planar to the basement membrane (Rompolas et al., 2016). Because these cells are not undergoing asymmetric divisions (at least in regard to their apical-basal axis), it was not clear that division orientation played any important role in the adult. However, a number of studies have shown that in squamous cell carcinoma (SCC) there is an increase in non-planar cell divisions of non-tumor initiating cells; while tumor initiating cells, like high-expressing Sox2 SCC cells, bias self-renewal (Boumahdi et al., 2014; Siegle et al., 2014). Furthermore, depletion of proteins associated with promoting cancer stemness in SCC, like VEGFR2, Nrp1 and Sox2, resulted in increased perpendicular cell divisions, thereby inducing tumor regression and reducing the pool of cancer stem cells (Beck et al., 2011; Siegle et al., 2014). Similarly, in a KRASG12D mutant background, there is an increase in non-planar divisions and a lack of overt tumor growth in the short term. In this background, disrupting spindle orientation caused very rapid growth of tumors, again suggesting that asymmetric divisions can be tumor suppressive in the context of a dysregulated epidermis (Morrow et al., 2019). These data suggest that the tissue may compensate for oncogenic challenges by increasing divisions that lead to differentiation.
Oncogenic lesions are also able to induce different polarized mechanical forces in the epidermis. A recent study explored the role of differential tension levels in stratified epithelia in shaping tumor architecture (Fiore et al., 2020). Interestingly, they noticed differences in the polarized tension across the basal-to-apical tissue axis of the epidermis that is tumor-specific. In a BCC mouse model, there was only a slight increase of stiffness from basal to suprabasal layers. However, in SCC and papillomas, the differentiated layers were considerably stiffer than the basal layer. In addition, they discovered that basement membrane stiffness also plays a key role in tumor architecture. Softening of the basement membrane induces budding, like in basal cell carcinoma; while stiffening it promotes tissue folding, like in SCC. In addition, genetically reducing basement membrane stiffness in vivo promoted invasiveness in SCC. Therefore, mechanical forces exerted on basal cells from both above and below are important in shaping the architecture of an initiating skin tumor and its progression (Fiore et al., 2020).
8. Concluding Remarks
It is now clear that there are many aspects of polarity that are essential for the form and function of the epidermis. Further, emerging work hints at important cross-talk between polarity machinery, force-generation and integration of tissue physiology. That said, a detailed molecular understanding of how polarity is established, and how its dysfunction contributes to disease in the skin awaits further investigation.
References Cited
- Ali NJA, Dias Gomes M, Bauer R, Brodesser S, Niemann C, & Iden S (2016). Essential Role of Polarity Protein Par3 for Epidermal Homeostasis through Regulation of Barrier Function, Keratinocyte Differentiation, and Stem Cell Maintenance. J Invest Dermatol, 136(12), 2406–2416. 10.1016/j.jid.2016.07.011 [DOI] [PubMed] [Google Scholar]
- Aw WY, Heck BW, Joyce B, & Devenport D (2016). Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr Biol, 26(16), 2090–2100. 10.1016/j.cub.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, & Wolf R (2012). Structure and function of the epidermis related to barrier properties. Clin Dermatol, 30(3), 257–262. 10.1016/j.clindermatol.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, Mascre G, Drogat B, Dekoninck S, Haigh JJ, Carmeliet P, & Blanpain C (2011). A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature, 478(7369), 399–403. 10.1038/nature10525 [DOI] [PubMed] [Google Scholar]
- Bilder D (2004). Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev, 18(16), 1909–1925. 10.1101/gad.1211604 [DOI] [PubMed] [Google Scholar]
- Blank IH (1953). Further observations on factors which influence the water content of the stratum corneum. J Invest Dermatol, 21(4), 259–271. 10.1038/jid.1953.100 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, & Fuchs E (2006). Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev, 20(21), 3022–3035. 10.1101/gad.1477606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohee S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, & Blanpain C (2014). SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature, 511(7508), 246–250. 10.1038/nature13305 [DOI] [PubMed] [Google Scholar]
- Bouwstra JA, & Ponec M (2006). The skin barrier in healthy and diseased state. Biochim Biophys Acta, 1758(12), 2080–2095. 10.1016/j.bbamem.2006.06.021 [DOI] [PubMed] [Google Scholar]
- Box K, Joyce BW, & Devenport D (2019). Epithelial geometry regulates spindle orientation and progenitor fate during formation of the mammalian epidermis. Elife, 8. 10.7554/eLife.47102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner JM, Zorn-Kruppa M, Yoshida T, Moll I, Beck LA, & De Benedetto A (2015). Epidermal tight junctions in health and disease. Tissue Barriers, 3(1–2), e974451. 10.4161/21688370.2014.974451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MT, & Wallingford JB (2017). Planar cell polarity in development and disease. Nat Rev Mol Cell Biol, 18(6), 375–388. 10.1038/nrm.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd KM, Lough KJ, Patel JH, Descovich CP, Curtis TA, & Williams SE (2016). LGN plays distinct roles in oral epithelial stratification, filiform papilla morphogenesis and hair follicle development. Development, 143(15), 2803–2817. 10.1242/dev.136010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Leybova L, Joyce B, & Devenport D (2018). Counter-rotational cell flows drive morphological and cell fate asymmetries in mammalian hair follicles. Nat Cell Biol, 20(5), 541–552. 10.1038/s41556-018-0082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sayadian AC, Lowe N, Lovegrove HE, & St Johnston D (2018). An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol, 16(10), e3000041. 10.1371/journal.pbio.3000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander L, Han H, de Winter M, Svensson L, Masich S, Daneholt B, & Norlen L (2016). Skin Lamellar Bodies are not Discrete Vesicles but Part of a Tubuloreticular Network. Acta Derm Venereol, 96(3), 303–308. 10.2340/00015555-2249 [DOI] [PubMed] [Google Scholar]
- Devenport D, & Fuchs E (2008). Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol, 10(11), 1257–1268. 10.1038/ncb1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias Gomes M, Letzian S, Saynisch M, & Iden S (2019). Polarity signaling ensures epidermal homeostasis by coupling cellular mechanics and genomic integrity. Nat Commun, 10(1), 3362. 10.1038/s41467-019-11325-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Cullander C, Mauro T, Rassner U, Komuves L, Brown BE, & Menon GK (1998). The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Investig Dermatol Symp Proc, 3(2), 87–100. 10.1038/jidsymp.1998.20 [DOI] [PubMed] [Google Scholar]
- Elias PM, & Wakefield JS (2014). Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol, 134(4), 781–791 e781. 10.1016/j.jaci.2014.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold KR (2012). Lamellar bodies: the key to cutaneous barrier function. J Invest Dermatol, 132(8), 1951–1953. 10.1038/jid.2012.177 [DOI] [PubMed] [Google Scholar]
- Feng X, Li GY, Ramier A, Eltony AM, & Yun SH (2022). In vivo stiffness measurement of epidermis, dermis, and hypodermis using broadband Rayleigh-wave optical coherence elastography. Acta Biomater. 10.1016/j.actbio.2022.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, & Horsley V (2011). Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell, 146(5), 761–771. 10.1016/j.cell.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore VF, Krajnc M, Quiroz FG, Levorse J, Pasolli HA, Shvartsman SY, & Fuchs E (2020). Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature, 585(7825), 433–439. 10.1038/s41586-020-2695-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, & Tsukita S (2002). Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol, 156(6), 1099–1111. 10.1083/jcb.200110122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Hawkins C, & Nathans J (2004). Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci U S A, 101(25), 9277–9281. 10.1073/pnas.0402802101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich I, Schmitz A, Zigrino P, Michels C, Haase I, le Bivic A, Leitges M, & Niessen CM (2007). Role of aPKC isoforms and their binding partners Par3 and Par6 in epidermal barrier formation. J Invest Dermatol, 127(4), 782–791. 10.1038/sj.jid.5700621 [DOI] [PubMed] [Google Scholar]
- Iden S, van Riel WE, Schafer R, Song JY, Hirose T, Ohno S, & Collard JG (2012). Tumor type-dependent function of the par3 polarity protein in skin tumorigenesis. Cancer Cell, 22(3), 389–403. 10.1016/j.ccr.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Deraison C, Bonnart C, Bitoun E, Robinson R, O’Brien TJ, Wakamatsu K, Ohtsubo S, Takahashi H, Hashimoto Y, Dopping-Hepenstal PJ, McGrath JA, Iizuka H, Richard G, & Hovnanian A (2005). LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol, 124(2), 360–366. 10.1111/j.0022-202X.2004.23583.x [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, & Igawa S (2015). The biology and regulation of corneodesmosomes. Cell Tissue Res, 360(3), 477–482. 10.1007/s00441-014-2037-z [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Igawa S, & Kishibe M (2018). Molecular basis of the skin barrier structures revealed by electron microscopy. Exp Dermatol, 27(8), 841–846. 10.1111/exd.13674 [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Simon M, Kishibe M, Miyauchi Y, Takahashi H, Yoshida S, O’Brien TJ, Serre G, & Iizuka H (2004). Epidermal lamellar granules transport different cargoes as distinct aggregates. J Invest Dermatol, 122(5), 1137–1144. 10.1111/j.0022-202X.2004.22515.x [DOI] [PubMed] [Google Scholar]
- Jonca N, Guerrin M, Hadjiolova K, Caubet C, Gallinaro H, Simon M, & Serre G (2002). Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J Biol Chem, 277(7), 5024–5029. 10.1074/jbc.M108438200 [DOI] [PubMed] [Google Scholar]
- Jonca N, Leclerc EA, Caubet C, Simon M, Guerrin M, & Serre G (2011). Corneodesmosomes and corneodesmosin: from the stratum corneum cohesion to the pathophysiology of genodermatoses. Eur J Dermatol, 21 Suppl 2, 35–42. 10.1684/ejd.2011.1264 [DOI] [PubMed] [Google Scholar]
- Joodaki H, & Panzer MB (2018). Skin mechanical properties and modeling: A review. Proc Inst Mech Eng H, 232(4), 323–343. 10.1177/0954411918759801 [DOI] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Yokouchi M, Sasaki H, & Amagai M (2009). External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med, 206(13), 2937–2946. 10.1084/jem.20091527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann L (1986). Epidermal permeability barrier: transformation of lamellar granule-disks into intercellular sheets by a membrane-fusion process, a freeze-fracture study. J Invest Dermatol, 87(2), 202–209. 10.1111/1523-1747.ep12695343 [DOI] [PubMed] [Google Scholar]
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, & de Rooij J (2010). Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol, 189(7), 1107–1115. 10.1083/jcb.201001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, & Fuchs E (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature, 437(7056), 275–280. 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, & Fuchs E (2007). Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J Cell Biol, 176(2), 147–154. 10.1083/jcb.200609109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C, Heller E, Pasolli HA, Chai S, Nikolova M, Stokes N, & Fuchs E (2015). Wdr1-mediated cell shape dynamics and cortical tension are essential for epidermal planar cell polarity. Nat Cell Biol, 17(5), 592–604. 10.1038/ncb3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S, & Setty SRG (2021). Epidermal Lamellar Body Biogenesis: Insight Into the Roles of Golgi and Lysosomes. Front Cell Dev Biol, 9, 701950. 10.3389/fcell.2021.701950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder D, Shevchenko A, Simons K, & Fullekrug J (2005). Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol, 168(2), 303–313. 10.1083/jcb.200407072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnikova YA, Le HQ, Schneider D, Thalheim T, Rubsam M, Bremicker N, Polleux J, Kamprad N, Tarantola M, Wang I, Balland M, Niessen CM, Galle J, & Wickstrom SA (2018). Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat Cell Biol, 20(1), 69–80. 10.1038/s41556-017-0005-z [DOI] [PubMed] [Google Scholar]
- Montpetit A, Cote S, Brustein E, Drouin CA, Lapointe L, Boudreau M, Meloche C, Drouin R, Hudson TJ, Drapeau P, & Cossette P (2008). Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet, 4(12), e1000296. 10.1371/journal.pgen.1000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreci RS, & Lechler T (2021). KIF18B is a cell type-specific regulator of spindle orientation in the epidermis. Mol Biol Cell, 32(21), ar29. 10.1091/mbc.E21-06-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow A, Underwood J, Seldin L, Hinnant T, & Lechler T (2019). Regulated spindle orientation buffers tissue growth in the epidermis. Elife, 8. 10.7554/eLife.48482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narangifard A, den Hollander L, Wennberg CL, Lundborg M, Lindahl E, Iwai I, Han H, Masich S, Daneholt B, & Norlen L (2018). Human skin barrier formation takes place via a cubic to lamellar lipid phase transition as analyzed by cryo-electron microscopy and EM-simulation. Exp Cell Res, 366(2), 139–151. 10.1016/j.yexcr.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Narangifard A, Wennberg CL, den Hollander L, Iwai I, Han H, Lundborg M, Masich S, Lindahl E, Daneholt B, & Norlen L (2021). Molecular Reorganization during the Formation of the Human Skin Barrier Studied In Situ. J Invest Dermatol, 141(5), 1243–1253 e1246. 10.1016/j.jid.2020.07.040 [DOI] [PubMed] [Google Scholar]
- Niessen CM, van der Raaij-Helmer MH, Hulsman EH, van der Neut R, Jonkman MF, & Sonnenberg A (1996). Deficiency of the integrin beta 4 subunit in junctional epidermolysis bullosa with pyloric atresia: consequences for hemidesmosome formation and adhesion properties. J Cell Sci, 109 (Pt 7), 1695–1706. 10.1242/jcs.109.7.1695 [DOI] [PubMed] [Google Scholar]
- Niessen MT, Scott J, Zielinski JG, Vorhagen S, Sotiropoulou PA, Blanpain C, Leitges M, & Niessen CM (2013). aPKClambda controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol, 202(6), 887–900. 10.1083/jcb.201307001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning W, Muroyama A, Li H, & Lechler T (2021). Differentiated Daughter Cells Regulate Stem Cell Proliferation and Fate through Intra-tissue Tension. Cell Stem Cell, 28(3), 436–452 e435. 10.1016/j.stem.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlen L (2001). Skin barrier formation: the membrane folding model. J Invest Dermatol, 117(4), 823–829. 10.1046/j.0022-202x.2001.01445.x [DOI] [PubMed] [Google Scholar]
- Norlen L, Al-Amoudi A, & Dubochet J (2003). A cryotransmission electron microscopy study of skin barrier formation. J Invest Dermatol, 120(4), 555–560. 10.1046/j.1523-1747.2003.12102.x [DOI] [PubMed] [Google Scholar]
- Norlen L, Lundborg M, Wennberg C, Narangifard A, & Daneholt B (2022). The Skin’s Barrier: A Cryo-EM Based Overview of its Architecture and Stepwise Formation. J Invest Dermatol, 142(2), 285–292. 10.1016/j.jid.2021.06.037 [DOI] [PubMed] [Google Scholar]
- Osada SI, Minematsu N, Oda F, Akimoto K, Kawana S, & Ohno S (2015). Atypical Protein Kinase C Isoform, aPKClambda, Is Essential for Maintaining Hair Follicle Stem Cell Quiescence. J Invest Dermatol, 135(11), 2584–2592. 10.1038/jid.2015.222 [DOI] [PubMed] [Google Scholar]
- Ouspenskaia T, Matos I, Mertz AF, Fiore VF, & Fuchs E (2016). WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell, 164(1–2), 156–169. 10.1016/j.cell.2015.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan K, Grobe H, Cohen J, Soffer A, Mahly A, Adir O, Zaidel-Bar R, & Luxenburg C (2020). Thymosin beta4 is essential for adherens junction stability and epidermal planar cell polarity. Development, 147(23). 10.1242/dev.193425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HB, McGlinn E, Phesse TJ, Schluter H, Srikumar A, Godde NJ, Woelwer CB, Ryan A, Phillips WA, Ernst M, Kaur P, & Humbert P (2015). The polarity protein Scrib mediates epidermal development and exerts a tumor suppressive function during skin carcinogenesis. Mol Cancer, 14, 169. 10.1186/s12943-015-0440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, & Lechler T (2010). Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol, 191(5), 915–922. 10.1083/jcb.201008001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AE, Shung CY, Saylor KW, Mullendorff KA, Weiss JB, & Wong MH (2010). Lessons from development: A role for asymmetric stem cell division in cancer. Stem Cell Res, 4(1), 3–9. 10.1016/j.scr.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AA, Gonzalez de Peredo A, Stella A, Ishida-Yamamoto A, Bouyssie D, Serre G, Monsarrat B, & Simon M (2008). Lamellar bodies of human epidermis: proteomics characterization by high throughput mass spectrometry and possible involvement of CLIP-170 in their trafficking/secretion. Mol Cell Proteomics, 7(11), 2151–2175. 10.1074/mcp.M700334-MCP200 [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Hammond NL, Coulombe PA, Saloranta C, Nousiainen HO, Salonen R, Berry A, Hanley N, Headon D, Karikoski R, & Dixon MJ (2014). Periderm prevents pathological epithelial adhesions during embryogenesis. J Clin Invest, 124(9), 3891–3900. 10.1172/JCI71946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Kawaguchi K, Park S, Gonzalez D, Brown S, Boucher J, Klein AM, & Greco V (2016). Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science, 352(6292), 1471–1474. 10.1126/science.aaf7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubsam M, Mertz AF, Kubo A, Marg S, Jungst C, Goranci-Buzhala G, Schauss AC, Horsley V, Dufresne ER, Moser M, Ziegler W, Amagai M, Wickstrom SA, & Niessen CM (2017). E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat Commun, 8(1), 1250. 10.1038/s41467-017-01170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzi L, Gagnoux-Palacios L, Pinola M, Belli S, Meneguzzi G, D’Alessio M, & Zambruno G (1997). A homozygous mutation in the integrin alpha6 gene in junctional epidermolysis bullosa with pyloric atresia. J Clin Invest, 99(12), 2826–2831. 10.1172/JCI119474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Akiyama M, Sugiyama-Nakagiri Y, McMillan JR, Sawamura D, & Shimizu H (2007). Localization of ABCA12 from Golgi apparatus to lamellar granules in human upper epidermal keratinocytes. Exp Dermatol, 16(11), 920–926. 10.1111/j.1600-0625.2007.00614.x [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, & Camargo FD (2011). Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell, 144(5), 782–795. 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin L, Muroyama A, & Lechler T (2016). NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. Elife, 5. 10.7554/eLife.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, & Schober M (2014). SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun, 5, 4511. 10.1038/ncomms5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH (1970). Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br J Dermatol, 82(3), 276–282. 10.1111/j.1365-2133.1970.tb12437.x [DOI] [PubMed] [Google Scholar]
- St Johnston D, & Sanson B (2011). Epithelial polarity and morphogenesis. Curr Opin Cell Biol, 23(5), 540–546. 10.1016/j.ceb.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Sumigray KD, Chen H, & Lechler T (2011). Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J Cell Biol, 194(4), 631–642. 10.1083/jcb.201104009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellkamp F, Vorhagen S, & Niessen CM (2014). Epidermal polarity genes in health and disease. Cold Spring Harb Perspect Med, 4(12), a015255. 10.1101/cshperspect.a015255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle CL, Lechler T, Pasolli HA, & Fuchs E (2004). Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci U S A, 101(2), 552–557. 10.1073/pnas.0307437100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle CL, Pasolli HA, Stokes N, & Fuchs E (2008). New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci U S A, 105(40), 15405–15410. 10.1073/pnas.0807374105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, & Niessen CM (2005). E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J, 24(6), 1146–1156. 10.1038/sj.emboj.7600605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, & Sonnenberg A (1996). Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet, 13(3), 366–369. 10.1038/ng0796-366 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, & Fuchs E (2001). Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell, 104(4), 605–617. 10.1016/s0092-8674(01)00246-x [DOI] [PubMed] [Google Scholar]
- Vorhagen S, Kleefisch D, Persa OD, Graband A, Schwickert A, Saynisch M, Leitges M, Niessen CM, & Iden S (2018). Shared and independent functions of aPKClambda and Par3 in skin tumorigenesis. Oncogene, 37(37), 5136–5146. 10.1038/s41388-018-0313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, & Fuchs E (2011). Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature, 470(7334), 353–358. 10.1038/nature09793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Ratliff LA, Postiglione MP, Knoblich JA, & Fuchs E (2014). Par3-mInsc and Galphai3 cooperate to promote oriented epidermal cell divisions through LGN. Nat Cell Biol, 16(8), 758–769. 10.1038/ncb3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Quondamatteo F, & Brakebusch C (2006). Cdc42 expression in keratinocytes is required for the maintenance of the basement membrane in skin. Matrix Biol, 25(8), 466–474. 10.1016/j.matbio.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Wu X, Quondamatteo F, Lefever T, Czuchra A, Meyer H, Chrostek A, Paus R, Langbein L, & Brakebusch C (2006). Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev, 20(5), 571–585. 10.1101/gad.361406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi H, Soma T, Kishimoto J, Hibino T, & Ishida-Yamamoto A (2019). Marked Changes in Lamellar Granule and Trans-Golgi Network Structure Occur during Epidermal Keratinocyte Differentiation. J Invest Dermatol, 139(2), 352–359. 10.1016/j.jid.2018.07.043 [DOI] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, & Shibata M (2010). alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol, 12(6), 533–542. 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Yokouchi M, Nagao K, Ishii K, Amagai M, & Kubo A (2013). Functional tight junction barrier localizes in the second layer of the stratum granulosum of human epidermis. J Dermatol Sci, 71(2), 89–99. 10.1016/j.jdermsci.2013.04.021 [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang X, Guo F, Jia Q, Liu N, Chen Y, Yan Y, Huang M, Tang H, Deng Y, Huang S, Zhou Z, Zhang L, & Zhang L (2019). Cdc42 Deficiency Leads To Epidermal Barrier Dysfunction by Regulating Intercellular Junctions and Keratinization of Epidermal Cells during Mouse Skin Development. Theranostics, 9(17), 5065–5084. 10.7150/thno.34014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong T, Wu X, Xie W, Luo X, Song T, Sun S, Luo Y, Li D, Liu M, Xie S, & Zhou J (2022). ENKD1 promotes epidermal stratification by regulating spindle orientation in basal keratinocytes. Cell Death Differ. 10.1038/s41418-022-00958-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


