Abstract
Delta-8-tetrahydrocannabinol (Δ8-THC) is a psychotropic cannabinoid produced in low quantities in the cannabis plant. Refinements in production techniques, paired with the availability of inexpensive cannabidiol substrate, have resulted in Δ8-THC being widely marketed as a quasi-legal, purportedly milder alternative to Δ9-THC. Yet, little research has probed the behavioral and physiological effects of repeated Δ8-THC use. The present study aimed to evaluate the effects of acute and repeated exposure to Δ8-THC. We hypothesized that Δ8-THC produces effects similar to Δ9-THC, including signs of drug tolerance and dependence. Adult male and female C57BL/6J mice were treated acutely with Δ8-THC (6.25-100 mg/kg, i.p.) or vehicle and tested in the tetrad battery to quantify cannabimimetic effects (i.e., catalepsy, antinociception, hypothermia, immobility) as compared with a non-selective synthetic cannabinoid (WIN 55,212-2) and Δ9-THC. As previously reported, Δ8-THC (≥12.5 mg/kg) induced cannabimimetic effects. Pretreatment with the CB1 receptor-selective antagonist rimonabant (3 mg/kg, i.p.) blocked each of these effects. In addition, repeated administration of Δ8-THC (50 mg/kg, s.c.) produced tolerance, as well as cross-tolerance to WIN 55,212-2 (10 mg/kg, s.c.) in tetrad, consistent with downregulated CB1 receptor function. Behavioral signs of physical dependence in the somatic signs, tail suspension, and marble burying assays were also observed following rimonabant-precipitated withdrawal from Δ8-THC (≥10 mg/kg BID for 6 days). Lastly, Δ8-THC produced Δ9-THC-like discriminative stimulus effects in both male and female mice. Together, these findings demonstrate that Δ8-THC produces qualitatively similar effects to Δ9-THC, including risk of drug dependence and abuse liability.
Keywords: Cannabis use disorder, substance use disorder, minor phytocannabinoid, drug dependence
1. Introduction
Cannabis products are among the most used psychotropic drugs globally with over 200 million adults, or almost 3% of the world population, reporting marijuana use in 2019 (United Nations, 2021). The United States was the first country to legalize medical marijuana use with the Compassionate Use Act of 1996, followed by Canada (1999), Uruguay (2013), and Australia (2016). Cannabis use continues to rise as more countries and territories allow for non-medical, adult use. In the United States, a total of 18 states permit non-medical use of cannabis products and another 19 have sanctioned comprehensive medical cannabis programs (NCSL, 2022). Due to its well-established behavioral effects, delta-9-tetrahydrocannabinol (Δ9-THC) remains highly regulated federally in the US, but the 2018 “Farm Bill” lifted restrictions on hemp-derived products, broadly defined as having less than 0.3% Δ9-THC by weight (USDA, 2018). As a result, commercial interest in extracting or synthetically producing cannabinoids derived from hemp has increased. While some of these hemp-derived cannabinoids do not share Δ9-THC’s psychoactive effects [e.g., cannabidiol (CBD)], others [e.g., delta-8-tetrahydrocannabinol (Δ8-THC)] may produce adverse physical and/or psychological effects resembling those reported for Δ9-THC. For example, national poison control centers have received an uptick in calls associated with exposure to unregulated products containing Δ8-THC, including reports of vomiting, hallucinations, trouble standing, and loss of consciousness (FDA, 2021).
Phyto-, or plant-derived, cannabinoids produce their effects via modulation of the endogenous cannabinoid receptors (i.e., CB1 and CB2), as well as via other receptor systems (e.g., 5-HT, TRPV1, GPR55). The psychoactive and rewarding properties of Δ9-THC are mediated by CB1 receptors in the brain (Herkenham, 1992; Mackie, 2006). Some full agonists at CB1, such as JWH-018 and other synthetic cannabinoids found in spice mixtures, have been reported to produce dangerous side effects, including seizure, vomiting, psychosis, and death by overdose (Cooper, 2016; Fantegrossi et al., 2018; Klavž et al., 2016; Patton et al., 2013). Partial CB1 agonists, such as Δ9-THC, have anxiolytic (Fokos & Panagis, 2010; Rubino et al., 2007), antiemetic (Badowski, 2017), and analgesic (Lötsch et al., 2018) qualities, as demonstrated in preclinical models and clinical populations; however, prolonged CB1 activation can produce tolerance and physical dependence, thus limiting the clinical utility of CB1 agonists (Aceto et al., 1996; Lichtman & Martin, 1997; Trexler et al., 2019). In addition, chronic cannabis use can lead to the development of Cannabis Use Disorder (CUD), or less commonly, cannabis hyperemesis syndrome, a poorly understood condition of frequent and severe bouts of vomiting often requiring hospitalization (Galli et al., 2011).
Much like Δ9-THC and CBD, lesser-studied compounds produced by the cannabis plant in low quantities (i.e., minor cannabinoids) may have therapeutic benefits but have yet to be clinically tested for safety or efficacy in humans. For example, Δ8-THC is a quasilegal minor cannabinoid with antiemetic effects and structural stability. Early enthusiasm for clinical use of Δ8-THC was dampened by its ability to produce psychotropic effects (Abramahov et al., 1995; Hollister & Gillespie, 1972). When administered acutely, Δ8-THC produces cannabimimetic effects in male ddN, ICR, and Swiss-Webster mice including catalepsy (Watanabe et al., 1983), antinociception, decreased locomotor activity (Compton et al., 1991; Dewey et al., 1970), and hypothermia (Ham & Jong, 1974; Yamamoto et al., 1985). Increased food intake, mobility, and performance in the 8-arm radial maze, as well as decreased hippocampal dopamine and serotonin release, have also been reported in female Sabra mice at a very low dose of Δ8-THC (0.001 mg/kg) (Avraham et al., 2004). Given the recent increase in human consumption of products with high Δ8-THC content, there is a critical need for empirical assessment of acute and repeated Δ8-THC exposure.
The goal of the present study was to test the overarching hypothesis that Δ8-THC is a psychoactive cannabinoid sharing behavioral effects with Δ9-THC, including tolerance, physical dependence, and cannabimimetic discriminative stimulus effects. To this end, male and female mice were administered Δ8-THC and tested using the tetrad battery (i.e., catalepsy, antinociception, hypothermia, immobility) to determine the dose-response and time course of THC-like effects. In addition, separate groups of mice were repeatedly administered Δ8-THC, to assess tolerance and cross-tolerance to the synthetic cannabinoid WIN 55,212-2 using the tetrad battery. Physical dependence was assessed using the rimonabant-precipitated withdrawal model in three separate, established behavioral models of cannabinoid withdrawal: somatic signs, tail suspension, and marble burying (Trexler et al., 2018). Finally, Δ8-THC was evaluated in male and female mice trained to discriminate Δ9-THC from vehicle. Δ9-THC discrimination represents an animal model of the subjective effects of marijuana intoxication (Balster & Prescott, 1992) and is recommended by FDA guidelines as a method for evaluation of cannabinoid abuse potential (FDA, 2017).
2. Methods
2.1. Animals
Adult (approximately 10-20-weeks old) male and female C57BL/6J mice (The Jackson Laboratory; Bar Harbor, ME, USA) were housed in same-sex groups (4-5 per cage) in Polysulfone plastic cages with food and water available ad libitum. Mice were housed in a temperature (21±1°C) and humidity (40±5%) controlled room, on a 12:12 h light-dark cycle (lights on at 0700) in AAALAC accredited facilities. Mice were stratified by sex before random assignment within each cage to treatment group, using a random number generator, such that each cage contained mice from at least two different treatment groups (i.e., no cage contained only Δ8-THC-treated mice). All experiments were carried out during the light phase, starting at approximately 0800 by trained technicians who were blinded to treatment conditions.
All experiments complied with the principles of laboratory animal care (National Research Council, 2011). Further, the Institutional Animal Care and Use Committees at RTI International (drug discrimination) and the University of Connecticut approved all experimental protocols prior to the start of any experimental manipulation.
2.2. Drugs
Δ8-THC used in tetrad, tolerance, and withdrawal studies was synthesized from hemp-derived CBD according to the method patented by 3BC (Farmington, CT, USA) (see supplemental section for detailed methodology) to a concentration of 92%, as determined by HNMR and GC-MS (Arome Science Inc., Farmington, CT, USA). The concentration of Δ9-THC in this solution was < 0.18% Δ9-THC. The Δ8-THC (96.1% purity) used in drug discrimination experiments was generously provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD, USA). The selective CB1 receptor antagonist/inverse agonist rimonabant (SR141716A) and the synthetic cannabinoid receptor agonist WIN 55,212-2 were purchased from Cayman Chemical (Ann Arbor, MI, USA). For the tetrad, tolerance, and withdrawal studies, drugs were dissolved in a vehicle composed of 5% ethanol, 5% Kolliphor EL (Sigma-Aldrich, St. Louis, MO, USA), and 90% normal saline. The vehicle for cannabinoids used in the drug discrimination study was 7.8% Polysorbate 80 (Fisher Scientific, Fair Lawn, NJ, USA) and 92.2% saline (Patterson Veterinary Supply, Columbus, OH, USA). Solutions were protected from light and administered at a volume of 10 μl/g body mass.
2.3. Behavioral Assessments
2.3.1. Tetrad: Evaluation of acute effects
The “tetrad” is a well-characterized battery of four assays used to evaluate the effects of cannabinoid agonists (Lichtman et al., 2001). It consists of assessments of catalepsy, antinociception, core body temperature, and spontaneous locomotor activity. Mice were acclimated to the test room for a minimum of 1 h before testing (Grim et al., 2016; Schlosburg et al., 2009). Catalepsy was assessed first by gently laying the forepaws of each mouse over a metal bar elevated 3 cm above the benchtop. The time immobile on the bar was recorded over up to three immediately consecutive attempts, with a maximum cutoff of 60 s (Long et al., 2009). Antinociception was measured immediately following the catalepsy test by gently placing each mouse headfirst into a small, padded bag and immersing the 1 cm distal tip of the tail into a water bath (Isotemp #2340 2L, Fisher Sciences) heated to 56°C, which elicits a rapid tail withdrawal that is slowed by cannabinoid activation (Falenski et al., 2010; Lichtman et al., 2004). Latency to withdraw the tail from the water was recorded, with a maximum cutoff of 10 s (Falenski et al., 2010). Tail immersion data are represented as a percent of the maximum possible effect (%MPE; [(measured response - baseline response)/(10 s - baseline response)x100]). Hypothermia was assessed by taking rectal temperature using a micro probe thermocouple thermometer designed for use with mice (BAT-12 with RET-3 probe, Physitemp Instruments Inc., Clifton, NJ, USA). Spontaneous locomotor activity was measured last, by placing individual mice into a transparent plastic test chamber (18 cm W x 28 cm L x 12 cm H) inside a sound-attenuating chamber outfitted with a fan, white LED/infrared lighting, and an overhead video camera. Testing lasted 5 min and locomotor activity was scored in real-time using ANY-maze video tracking software (Stoetling, Wool Dale, IL, USA). Time immobile was determined by setting the tracking parameters to a latency of 1200 ms for 90% of the mouse image pixels (Trexler et al., 2019). In cases where mice were repeatedly tested, locomotor activity was not measured, because mice quickly habituate to locomotor testing.
To evaluate the acute effects of Δ8-THC, male and female mice were injected with cumulative doses (6.25, 6.25, 12.5, 25, & 50 mg/kg) of Δ8-THC to create a dose-response curve (final doses: 6.25, 12.5, 25, 50, and 100 mg/kg, i.p.) or vehicle 50 min prior to testing (i.e., 60 min. injection interval). Two separate groups were injected with cumulative doses (1, 9, 20, 20 mg/kg & 1, 2, 7, 20 mg/kg, respectively) of Δ9-THC (final doses: 1, 10, 30, & 50 mg/kg, i.p.) or WIN 55,212-2 (final doses: 1, 3, 10, & 30 mg/kg, i.p.) for potency comparisons. Again, mice were tested 50 min after injection. Locomotor activity was measured once at the highest cumulative dose of Δ8-THC (i.e., 100 mg/kg).
To determine the time course of Δ8-THC-induced behavioral effects, male and female mice were injected with a single dose of Δ8-THC (100 mg/kg, i.p.) or vehicle and tested repeatedly (0, .5, 2, 4, 8, 12, 24, 48 h post injection) in a modified tetrad battery (i.e., no locomotor testing). A different cohort of mice was tested at 0.25 and 1 h (i.e., within the first 2 h) to reduce the potential risk of tissue damage and pain sensitization from rapid repeated exposure to the tail immersion test.
To assess whether the observed tetrad effects are CB1-mediated, a separate group of male and female mice were administered the CB1-selective antagonist rimonabant (3 mg/kg, i.p.) or vehicle 30 min prior to Δ8-THC (100 mg/kg, i.p.) or vehicle treatment. Testing was conducted 50 min after administration of Δ8-THC or vehicle.
2.3.2. Tolerance and cross-tolerance in tetrad
To evaluate the effects of repeated Δ8-THC administration, mice were injected subcutaneously (s.c.) with Δ8-THC (50 mg/kg) or vehicle twice daily for 6 days, as described previously (Falenski et al., 2010; Schlosburg et al., 2009; Trexler et al., 2018, 2020). Mice were tested using a modified tetrad 50 min after each morning injection for the first five days of dosing to monitor for behavioral drug-tolerance. On the seventh day, all mice were injected with WIN 55,212-2 (10 mg/kg, s.c.) instead of Δ8-THC or vehicle and tested in a full tetrad to evaluate cross-tolerance to a synthetic CB1/CB2 agonist.
2.3.3. Precipitated withdrawal paradigm
As with the tolerance and cross tolerance studies, mice were injected (s.c.) repeatedly with Δ8-THC (1-50 mg/kg, twice daily for 5 days). On the sixth day, all mice received a final injection of Δ8-THC or vehicle. After 30 min, mice received an i.p. injection of rimonabant (3 mg/kg) to precipitate withdrawal (Lichtman et al., 2001; Trexler et al., 2020). Subsequently, mice were evaluated in either (1) somatic withdrawal followed immediately by tail suspension test or (2) the marble burying test.
Somatic signs of withdrawal were measured as described previously (Schlosburg et al., 2010; Trexler et al., 2020). Each mouse was placed into an empty, transparent plastic test chamber (20 cm W x 20 cm L x 15 cm H) inside a sound-attenuating chamber outfitted with a fan and white LED/infrared lighting. The apparatus had a mirror facing a video camera to allow for observation of behavior when the mouse faced away from the camera.
After the final Δ8-THC or vehicle injection, mice were habituated to the test apparatus for 30 min. Then, they were briefly removed from the test chamber, injected with rimonabant, and behavior was video recorded for 60 min (Schlosburg et al., 2009). Test chambers were cleaned between subjects using a paper towel moistened with distilled water, and at the end of each test day using Peroxigard (Virox Technologies Inc., Oakville, ON, Canada).
Video files were deidentified and scored by a trained observer who was blinded to treatment conditions. A subset of videos was scored by a second trained observer to ensure inter-rater reliability (r2=0.97). The dependent variables were (1) incidences of paw tremors and (2) head twitches (i.e., an incidence was scored for ‘paw tremor’ when the behavior was observed, not for each individual motion). Incidences were considered separate when either (1) another behavior occurred between the incidences, or (2) there was at least 1 s between incidences (Schlosburg et al., 2009).
Immediately following somatic signs testing, mice were evaluated for struggling in the tail suspension test. The tail suspension test was conducted as previously described (Steru et al., 1985; Trexler et al., 2018). Mice were suspended by their tails using adhesive tape attached to a horizontal bar placed approximately 40 cm above the bench top and video recorded for 6 min. The amount of time the mice actively struggled was hand scored by a trained and blinded observer. A subset of videos was scored by a second observer to ensure inter-rater reliability (r2=0.97). Active struggling was defined as one or more legs repeatedly kicking within one second or arching of the spine. Swinging motions or head movement per se was not scored as struggling.
A second group of mice was assessed in the marble burying assay after the dependence induction regimen. Marble burying was measured as previously described (Broekkamp et al., 1986; Kinsey et al., 2011; Trexler et al., 2018). Plastic test chambers (18 cm W x 28 cm L x 12 cm H) filled with Teklad Aspen Sani-Chip (7090 A; Envigo, Indianapolis, IN, USA) wood bedding (5 cm deep) were placed inside a sound-attenuating chamber outfitted with a fan and white LED/infrared lighting. A 4×5 array of 20 clear glass marbles was laid gently across the top of the leveled bedding in a grid-like fashion. Immediately following injection of rimonabant, each mouse was individually placed facing into the same corner of the chamber and allowed to explore freely for 20 min. At the end of the test, each mouse was quickly and carefully removed, and the number of unburied marbles (≥1/3 of the surface showing) was recorded and subtracted from the 20 total marbles. Locomotor activity was simultaneously recorded for the duration of the test by a camera mounted on the top of the test chamber. Locomotor activity was analyzed in real-time using ANY-maze (Stoelting, Wood Dale, IL, USA) video tracking software. Dependent variables included: percent time immobile and the number of marbles buried. Time immobile was determined by setting the tracking parameters to a latency of 1200 ms for 90% of the mouse image pixels (Trexler et al., 2019).
2.3.4. Drug discrimination
Mice in the drug discrimination procedure were trained and tested in mouse operant chambers (MED Associates, St. Albans, VT, USA) housed within light- and sound-attenuating cubicles. Each chamber was outfitted with a house light, white noise generator, and two nose-poke apertures with a stimulus light over each aperture. A pellet feeder delivered 20 mg food pellets (Bioserv Inc., Frenchtown, NJ, USA) into an illuminated pellet trough centered between the two apertures. Chamber operations (i.e., illumination of lights, generation of white noise, delivery of food pellets, and recording of responses) were controlled by a computer system (MED Associates).
Prior to the start of the present study, male and female C57BL/6J mice had been trained to discriminate between Δ9-THC and vehicle, as described previously (Wiley et al., 2015). Briefly, mice were trained to poke their nose into one of two apertures in the operant chambers 30 min following i.p. injection with 5.6 mg/kg Δ9-THC and to poke their nose in the other aperture 30 min following i.p. vehicle administration according to a fixed ratio 10 (FR10) schedule of food reinforcement, under which 10 consecutive responses on the correct (injection-appropriate) aperture resulted in delivery of a food pellet. A response on the incorrect aperture reset the ratio requirement on the correct aperture. Prior to each 15-min daily training session, animals received a single injection of 5.6 mg/kg Δ9-THC or vehicle in a double alternation schedule (e.g., two sessions with Δ9-THC pre-injection followed by two sessions with vehicle pre-injection).
After the discrimination task was acquired, an initial Δ9-THC dose-effect curve was determined. For this curve, 15-min test sessions were conducted after injection with different doses of Δ9-THC, 10 consecutive responses on either aperture delivered reinforcement. If a mouse responded on the other aperture prior to completing 10 responses on an aperture, the ratio requirement on the original aperture was reset. To be eligible for testing with each dose, a mouse must have completed a training session the previous day in which the criteria for reliable discrimination (i.e., first completed FR10 registered on the injection-appropriate aperture and ≥ 80% of responses on this aperture) had been met. In addition, the mouse must have met these same criteria during the most recent training session with the alternate training compound (i.e., Δ9-THC training dose or vehicle). Baseline discrimination training sessions continued on other weekdays. After completion of the initial Δ9-THC dose-effect curve, mice were tested with other cannabinoids (data reported elsewhere). At the start of the present study, a second Δ9-THC dose-effect curve was determined (as reported herein). Subsequently, a dose-effect curve was determined with Δ8-THC, with each dose injected i.p. 30 min prior to the start of the test session.
2.4. Statistical analyses
Tetrad (i.e., catalepsy, tail immersion, body temperature, locomotor activity) and withdrawal experiment data (i.e., somatic signs, tail suspension) were analyzed using a mixed-design analysis of variance (ANOVA), with dose, timepoint, or days of treatment as the within-subjects independent variable and drug treatment as the between-subjects independent variable. The dose-dependent effects of each compound were analyzed using separate ANOVAs. ED50 values and potency ratios for drug effects in tetrad were calculated using Colquhoun’s method (Ashford & Colquhoun, 1972). Immobility, daily effects in the tolerance experiment, cross-tolerance to WIN 55,212-2, and marble burying were analyzed using an unpaired t-test with drug treatment as the between-subjects independent variable. Main or interaction effects were followed by either Dunnett (e.g., for dose-response curves, comparing to vehicle treatment) or Bonferroni post hoc tests, as appropriate. Sex effects were analyzed using a two-way ANOVA with drug treatment and sex as the between-subjects independent variables. Due to a lack of sex differences in the tetrad and withdrawal experiments, data presented are collapsed across sex.
For each drug discrimination session, responses on the drug-associated aperture as a percentage of responses during the session and response rate (all responses/s) were calculated. ED50 values for percent response on drug aperture were calculated using linear least squares regression analysis. Data from one male and two females treated with 30 mg/kg Δ9-THC (i.p.) were excluded from analysis of percentage of Δ9-THC aperture response selection due to not earning food reinforcement during the session (i.e., fewer than 10 consecutive responses were registered on either aperture/lever), but response rate data were included. Response rate data were analyzed with separate mixed (sex X dose) ANOVAs, followed by Tukey post hoc tests to determine differences between means. Differences were considered statistically significant if p < 0.05.
3. Results
3.1. Acute Δ8-THC produces classic cannabinoid effects via CB1
Consistent with previous literature, all three cannabinoids produced cannabimimetic effects in the tetrad battery. Δ8-THC induced catalepsy at ≥50 mg/kg [ED50=58.47 mg/kg; 95% CI: 44.58-76.71 mg/kg; F(5,90)=31.3, p<0.0001; Fig. 1A], antinociception at 100 mg/kg [F(5,90)=3.9, p=0.0028; Fig. 1B], and hypothermia at ≥12.5 mg/kg [F(5,90)=15.8, p<0.0001; Fig. 1C]. Δ8-THC (100 mg/kg) also increased immobility in the spontaneous locomotor task [t(18)=27.9, p=0.0048; Fig. 1D]. Baseline mean±SEM were: bar test (0 ± 0s), tail immersion test (.99 ± .09s), and body temperature (37.4 ± .12°C).
Fig. 1.
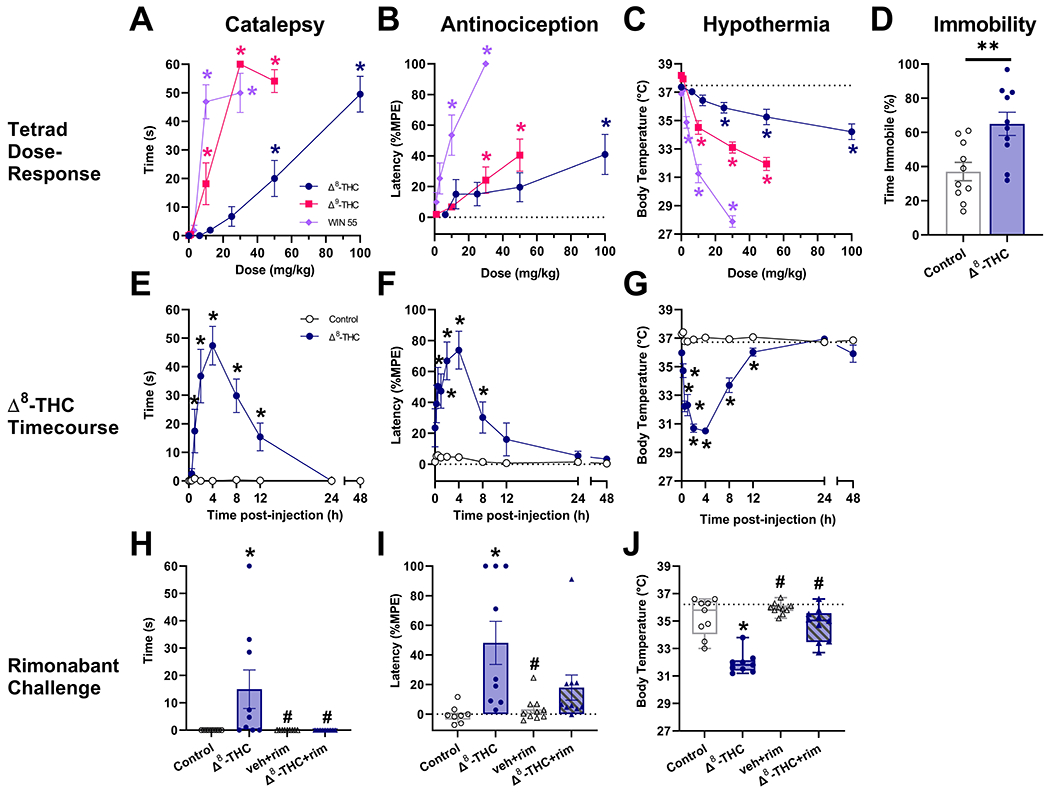
Δ8-THC induces classic cannabinoid effects via CB1 receptors. Male and female mice were treated with Δ8-THC (6.25-100 mg/kg, i.p.), Δ9-THC (1-50 mg/kg, i.p.), or WIN 55,212-2 (1-30 mg/kg, i.p.) 60 min prior to testing in the tetrad battery. Δ8-THC induced catalepsy (A) antinociception (B), hypothermia (C), and hypolocomotion (D), consistent with established cannabinoid effects. The effects of Δ8-THC (100 mg/kg, i.p.) persisted up to 12h post-injection (E-G). Pretreatment with the CB1 receptor-selective antagonist rimonabant (3 mg/kg, i.p.) blocked each of these effects of Δ8-THC (H-J). Data represent mean±SEM (n = 9-10[5m/4-5f]/group);*p<0.05, **p<0.005 v. vehicle; #p<0.05 v. Δ8-THC.
Δ9-THC was about 6 times more potent than Δ8-THC in the catalepsy test [ED50=8.99 mg/kg; 95% CI: 6.44-12.55 mg/kg; F(4,45)=60.2, p<0.0001; Fig. 1A], and produced antinociception [F(4,45)=7.9, p<0.0001; Fig. 1B] and hypothermia [F(4,45)=63.9, p<0.0001; Fig. 1C] at slightly lower doses (≥30 and 10 mg/kg, respectively). WIN 55,212-2 was the most potent, producing catalepsy [ED50=7.41 mg/kg; 95% CI: 5.35-10.27 mg/kg; F(4,68)=40.2, p<0.0001; Fig. 1A] and antinociception [ED50=6.1 mg/kg; 95% CI: 4.3-8.65 mg/kg; F(3,51)=10.4, p<0.0001; Fig. 1B] at ≥10 mg/kg and hypothermia at ≥3 mg/kg [F(4,68)=103.6, p<0.0001; Fig. 1C]. ED50 values for antinociceptive effects of Δ8-THC and Δ9-THC could not be calculated because neither reached 50% efficacy in the tail immersion test.
As previously reported with Δ9-THC (Trexler et al., 2020), Δ8-THC-induced catalepsy [F(9,144)=12.4, p<0.0001; Fig. 1E], antinociception [F(9,144)=6.6, p<0.0001; Fig. 1F], and hypothermia [F(9,144)=32.3, p<0.0001; Fig. 1G] persisted for at least 8-12 h post-injection. Additionally, pretreatment with the CB1 antagonist rimonabant blocked Δ8-THC-induced catalepsy [F(3,34)=4.9, p=0.0062; Fig. 1H], antinociception [F(3,34)=6.9, p=0.0009; Fig. 1I], and hypothermia [F(3,34)=43.833, p<0.0001; Fig. 1J], consistent with a mechanism requiring CB1 activation. Notably, rimonabant per se had no effect in any test.
3.2. Repeated Δ8-THC treatment induces tolerance and cross-tolerance to WIN 55,212-2
After 5-days of repeated administration (50 mg/kg, s.c. twice daily), Δ8-THC-treated mice displayed tolerance to cataleptic [F(4,72)=2.9, p=0.0273; Fig. 2A], antinociceptive [F(4,72)=34.4, p<0.0001; Fig. 2B], and hypothermic [F(4,72)=4.7, p<0.0001; Fig. 2C] effects by day 4, 3, and 2, respectively. Further, cross-tolerance with the non-selective CB1/CB2 agonist WIN 55,212-2 was observed. Acute WIN 55,212-2 had no effect in mice repeatedly treated with Δ8-THC, which did not display catalepsy [t(18)=5.5, p<0.0001; Fig. 2D], antinociception [t(18)=3.5, p=0.0026; Fig. 2E], hypothermia [t(18)=18.1, p<0.0001; Fig. 2F], or increased immobility [t(18)=6, p<0.0001; Fig. 2G].
Fig. 2.
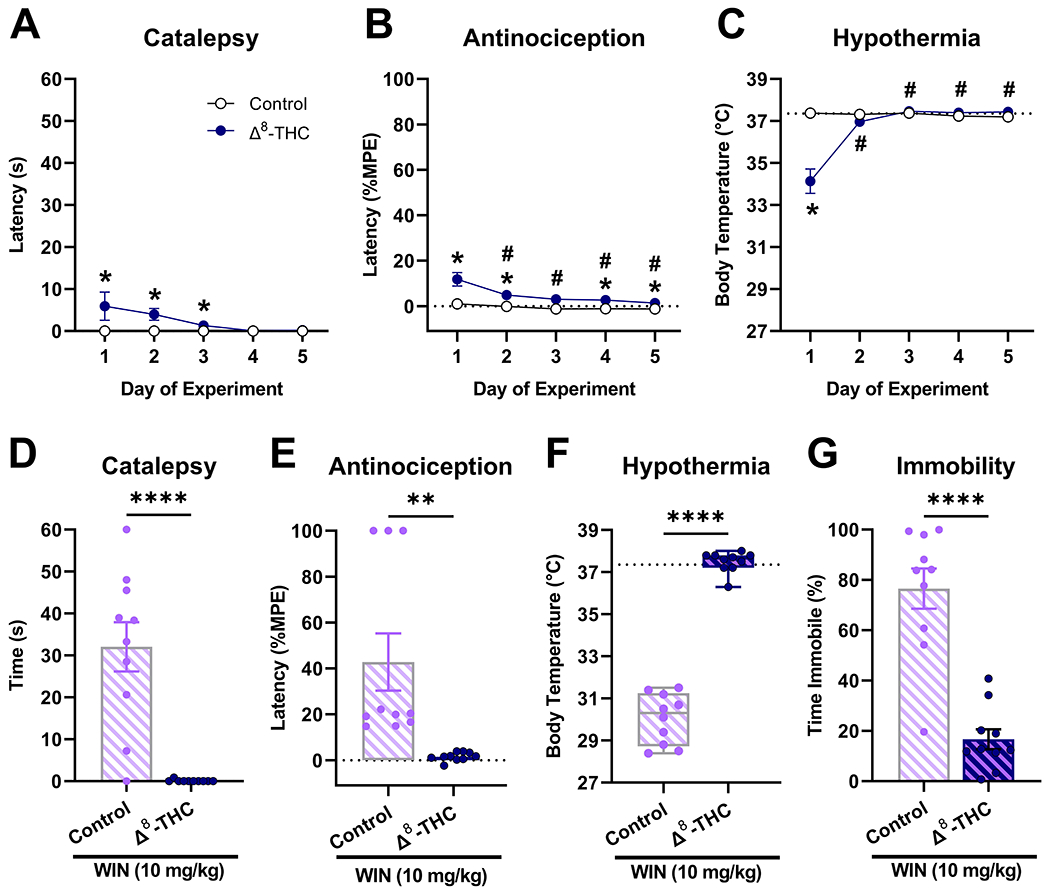
Δ8-THC-treated mice display tolerance and cross-tolerance to WIN 55,212-2. Mice were treated repeatedly with Δ8-THC (50 mg/kg, s.c.) for 5 days and tested daily for catalepsy (A) antinociception (B), and hypothermia (C). On day 7, all mice received WIN 55,212-2 (10 mg/kg, s.c.). Δ8-THC-treated mice displayed cross-tolerance to WIN 55,212-2-induced catalepsy (D) antinociception (E), hypothermia (F), and immobility (G). Dashed line indicates baseline body temperature. Data represent mean±SEM (n = 10[5m/5f]/group); *p<0.05, **p<0.005, ***p<0.0005, ****p<0.00005 v. vehicle; #p<0.05 v. day 1 Δ8-THC.
3.3. Repeated Δ8-THC treatment induces physical dependence
Mice treated repeatedly with Δ8-THC and then injected with rimonabant exhibited characteristic signs of cannabinoid withdrawal including increased frequency of paw tremors [F(3,36)=33.5, p<0.0001; Fig. 3A], head twitches [F(3,36)=18.5, p<0.0001; Fig. 3B], and time spent struggling in the tail suspension test [F(3,36)=11, p<0.0001; Fig. 3C]. Additionally, rimonabant depressed marble burying [t(38)=2.2, p=0.035; Fig. 3D], but did not induce immobility in the marble burying test in mice that were repeatedly exposed to Δ8-THC [t(38)=1.1, p=0.2882; Fig. 3E].
Fig. 3.
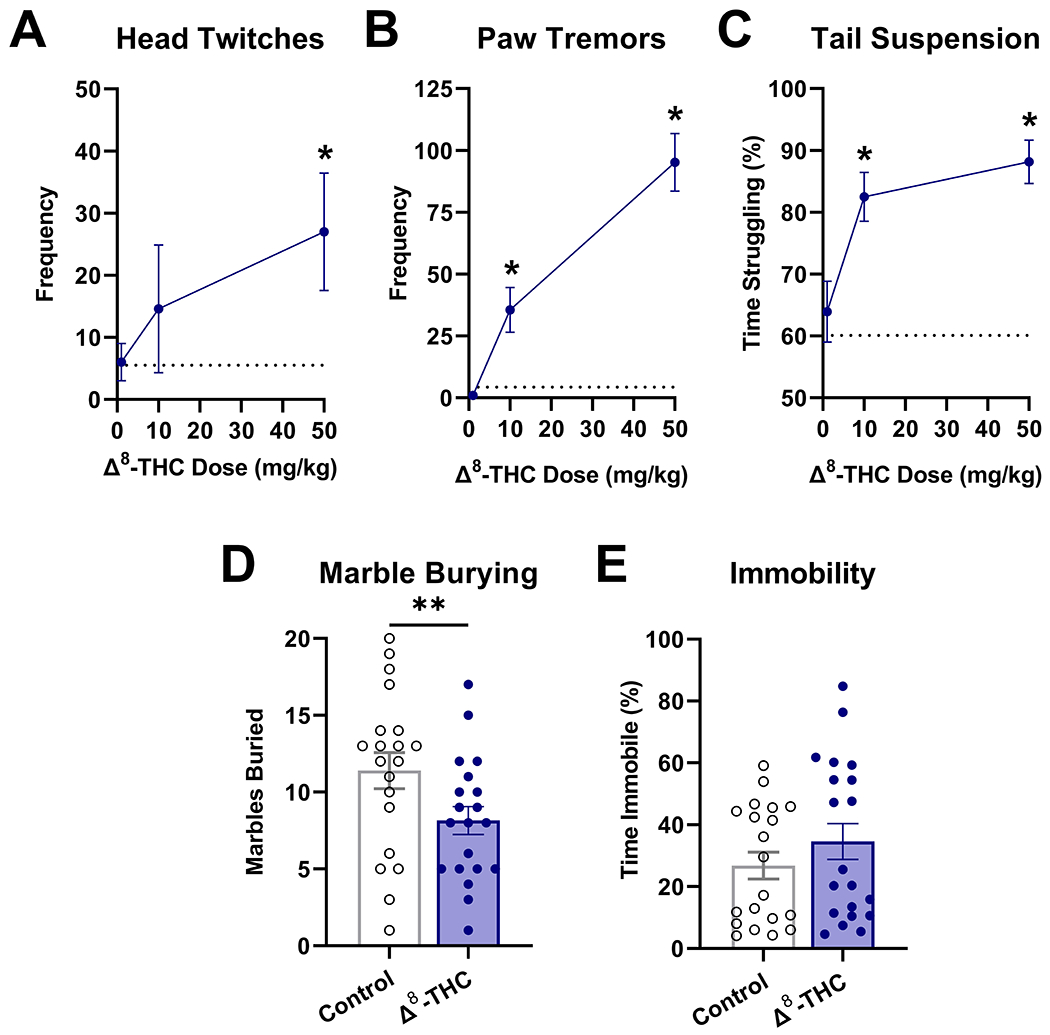
Repeated administration of Δ8-THC induces physical dependence. Mice were treated with Δ8-THC (1-50 mg/kg, s.c.) twice daily for 5 days. On the 6th day, mice received rimonabant (3 mg/kg, i.p.) and were observed for somatic signs of withdrawal: head twitches (A) and paw tremors (B) follow by struggling in the tail suspension test (C). Similarly, mice treated repeatedly with Δ8-THC (50 mg/kg, s.c.) displayed decreased marble burying (D) but no change in mobility (E). Data represent mean±SEM (n = 10[5m/5f]/group);*p<0.05, **p<0.005 v. vehicle.
3.4. Δ8-THC produces Δ9-THC-like psychoactive effects in drug discrimination
Δ9-THC produced dose-dependent increases in responding on the drug-associated aperture in both sexes, with complete substitution (>80% drug-aperture responding) for the 5.6 mg/kg training dose occurring at higher (5.6 and 10 mg/kg) doses (Fig. 4). Potencies were similar across sex, with overlapping confidence intervals (CI): ED50=1.73 mg/kg (95% CI: 1.28-2.34 mg/kg) and 2.31 mg/kg (95% CI: 1.55-3.42 mg/kg) for females and males, respectively. Similarly, Δ8-THC also completely and dose-dependently substituted for Δ9-THC in both sexes; however, it was 2- to 3-fold less potent than Δ9-THC in females [ED50=3.61 mg/kg; 95% CI: 2.60-5.00 mg/kg] and males [ED50=7.79 mg/kg; 95% CI: 5.62-10.80 mg/kg), respectively. In addition, Δ8-THC was approximately 2-fold more potent in females than males. Both drugs decreased response rates at higher doses as compared to their respective vehicles [Δ9-THC: main effect of dose, F(5,55)=5.7, p<0.05; Δ8-THC: main effect of dose, F(5,55)=16.4, p<0.05].
Fig. 4.
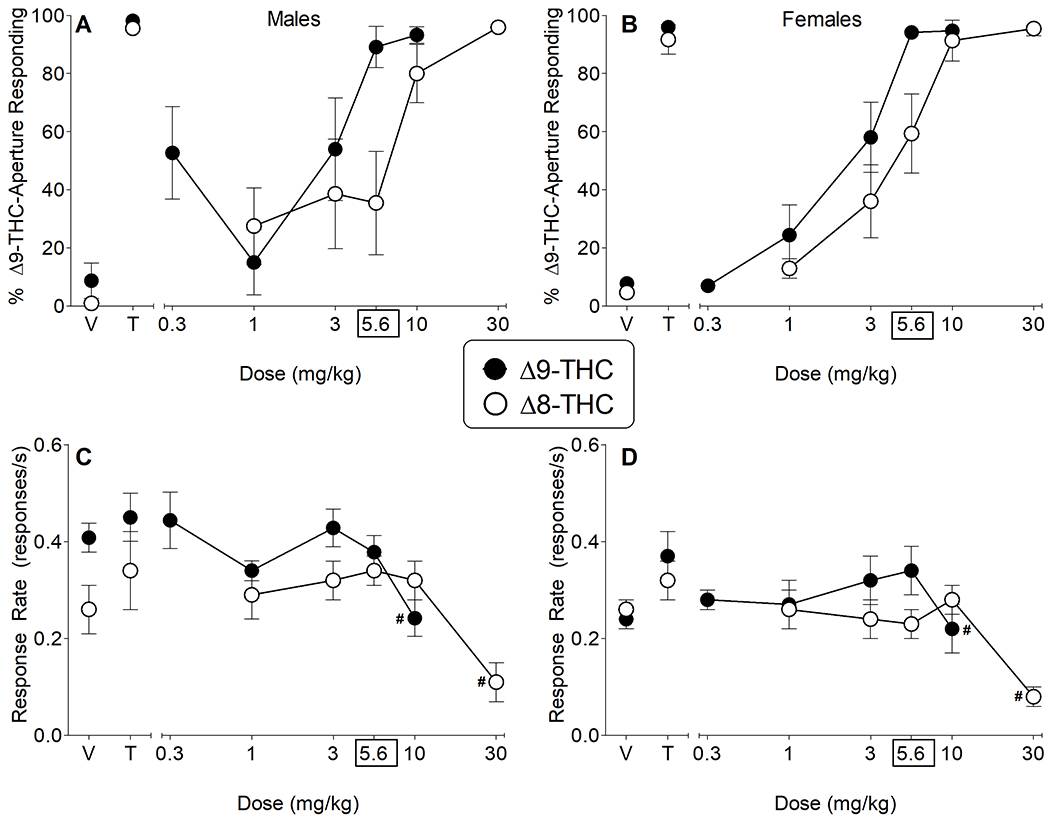
Δ8-THC substitutes for Δ9-THC. Effects of vehicle, Δ9-THC and Δ8-THC on percentage of responses that occurred on the Δ9-THC-associated aperture (A-B) and response rate (C-D) in male (left panels) and female (right panels) mice trained to discriminate 5.6 mg/kg Δ9-THC from vehicle. Control tests with vehicle (V) and 5.6 mg/kg Δ9-THC (T) were conducted prior to each dose-effect curve, with results shown at the left side of the panels. Data represent mean±SEM (n = 13[5m/8f]/group except for % responding on the Δ9-THC-associated aperture for 30 mg/kg Δ8-THC where n = 10[4m/6f]). # indicate significant main effect for dose (as compared to vehicle) across both sexes (p<0.05).
4. Discussion
The current project evaluated the acute and repeated effects of Δ8-THC in both male and female mice. As per previous work using male rodents, Δ8-THC produced acute cannabimimetic effects, as quantified here using the tetrad battery. Acute Δ8-THC effects peaked at 2-4 h and were blocked by the CB1 selective antagonist rimonabant, indicating a CB1 receptor-dependent mechanism. The observed tetrad effects decreased with repeated exposure, an effect consistent with drug tolerance that was further supported by cross-tolerance to the synthetic cannabinoid agonist WIN 55,212-2. Moreover, when treated with rimonabant, mice repeatedly administered Δ8-THC showed classic somatic signs of withdrawal, as well as increased struggling in the tail suspension test and decreased marble burying, consistent with physical cannabinoid dependence. Δ8-THC also produced dose-dependent Δ9-THC-like discriminative stimulus effects in mice trained to discriminate Δ9-THC from vehicle.
In accordance with previous reports (Compton et al., 1991; Ham & Jong, 1974; Hollister & Gillespie, 1972), we found that acute Δ8-THC was similar to, but slightly less potent than Δ9-THC (a partial CB1 agonist), and both Δ8-THC and Δ9-THC were less potent than WIN 55,212-2 (a full CB1 agonist). Species differences may account for discrepancies in previously reported Δ8-THC vs. Δ9-THC potency comparisons. For instance, Δ9-THC was about 1.2-3 times more potent than Δ8-THC in rats (Compton et al., 1991, 1993; Jarbe & Henriksson, 1974) as compared with 1.1-1.5 times more potent in mice (Compton et al., 1991; Dewey et al., 1970; Ham & Jong, 1974) indicating that the potency shift could be more pronounced in rats than mice. Furthermore, Δ8-THC substituted for Δ9-THC in a 2-choice drug discrimination task at 1.4 times higher doses in pigeons (Jarbe et al., 1977). Thus, the majority of current studies, regardless of species, use a 2:1 (Δ8-THC:Δ9-THC) dose ratio with no direct dose-effect comparisons. Depending on the behavior assessed, we found Δ9-THC to be 2-6 times more potent than Δ8-THC, suggesting that the potency shift varies based on the species and test used.
The drug discrimination paradigm in this experiment compares the subjective effects of cannabinoid compounds in mice. We found that Δ8-THC completely and dose-dependently substituted for Δ9-THC, indicating that Δ8-THC produces psychoactive effects qualitatively similar to those of Δ9-THC. In humans, the onset and duration of subjective effects, including mood elevation and intensity of ‘high’, are reported to be delayed and shorter-lasting with orally ingested Δ8-THC as compared to Δ9-THC (Hollister & Gillespie, 1972). Similarly, two recent online surveys indicate that consumers perceive the effects of Δ9-THC to be more intense and longer lasting than Δ8-THC (Kruger & Kruger, 2022). Conversely, vaporized Δ8-THC concentrate compared to high Δ9-THC content cannabis produced similar levels of self-reported and physical impairment in a small sample of male frequent cannabis smokers (Wurz et al., 2022). In the present study, the time course for the tetrad effects of Δ8-THC was nearly identical to that previously observed of Δ9-THC, with peak effects at 2-4 h that returned to baseline by 24 h (Trexler et al., 2020), again underscoring methodological and potential species differences across the extant Δ8-THC literature.
We also found that mice treated subcutaneously with Δ8-THC developed tolerance to its effects in the tetrad battery by day 5 of repeated dosing, paralleling previous work in male ddN mice that developed tolerance to intravenous Δ8-THC-induced catalepsy (Watanabe et al., 1983), hypothermia (Yamamoto et al., 1985), and prolongation of barbiturate-induced sleep (Watanabe et al., 1987). The present study extends these findings to quantify, in both males and females, antinociception and cross-tolerance to WIN 55,212-2 tetrad effects, including depressed spontaneous locomotor activity. Despite the high dose of WIN 55,212-2 (10 mg/kg) used on the cross-tolerance test day, mice treated with Δ8-THC were completely tolerant, consistent with severely downregulated CB1 receptor function (Falenski et al., 2010; Schlosburg et al., 2010).
In the present study, the effects of rimonabant-precipitated Δ8-THC withdrawal were virtually identical to those of Δ9-THC withdrawal (Trexler et al., 2018), including increased somatic signs and struggling in the tail suspension test at ≥10 mg/kg. These behaviors were evident in both withdrawal-induced (i.e., somatic signs, struggling in tail suspension test) and withdrawal-depressed (i.e., marble burying) models. Despite face validity limitations of antagonist-precipitated withdrawal paradigms, that is, withdrawal in humans occurs during drug abstinence, rimonabant administration increases the frequency of spontaneous cannabinoid withdrawal related behaviors in mice (Trexler et al., 2018), increasing signal strength while having no overall effect on valence. Moreover, although not commonly associated with cannabinoid withdrawal, female Wistar rats administered Δ8-THC for 14 days showed increased grooming during abstinence (Sjödén et al., 1973), endorsing the ability of Δ8-THC withdrawal to alter behavior.
Cannabis use disorder (CUD) is a diagnosable human disease characterized by both physiological and psychological pathology. The prevalence of CUD is increasing, as a function of the increase in recreational marijuana use. The Δ9-THC-like effects produced by Δ8-THC in the present study suggest abuse potential and likelihood for the development of CUD with repeated use. Cannabis withdrawal syndrome occurs in a subset of CUD patients resulting in symptoms including shakiness, abdominal pain, anxiety, irritability, and insomnia (Patel & Marwaha, 2020). In mice, the stress hormone corticosterone increases during Δ9-THC withdrawal (Kesner et al., 2021; Trexler et al., 2018), further supporting the idea that cannabinoid withdrawal is a physiological stressor. In addition, sex differences have been reported previously for Δ9-THC withdrawal including greater increases in striatal dopamine release, sleep disturbances, reward seeking, locomotor activity, and plasma corticosterone in male vs. female C57BL/6J mice (Kesner et al., 2021). Although this study was neither designed nor intended to probe sex effects, our analyses did not reveal biologically relevant sex differences.
5. Conclusions
The present studies revealed that Δ8-THC produces cannabinoid effects in vivo that are mediated by CB1 receptors and are roughly equivalent to those of the major phytocannabinoid Δ9-THC. In line with previous research, Δ8-THC was found to have slightly lower potency than Δ9-THC. Repeated administration of Δ8-THC resulted in tolerance, cross-tolerance to the potent cannabinoid WIN 55,212-2, and both withdrawal-induced and -depressed behaviors. Additionally, Δ8-THC substituted for Δ9-THC in a drug discrimination task, indicating similar subjective effects of either drug. These data support the idea that Δ8-THC is a psychoactive cannabinoid that produces effects similar to those of Δ9-THC, including evidence of dependence and abuse potential.
Supplementary Material
Acknowledgements
We thank the UConn Animal Care Services for their technical support. This project was supported financially by the National Institutes of Health [AT010773, DA039335, DA048153, and DA052690].
Abbreviations:
- CB1
Cannabinoid receptor subtype 1
- CB2
Cannabinoid receptor subtype 2
- Rimonabant
SR141716A; selective CB1 receptor antagonist/inverse agonist
- Δ8-THC
Δ8-tetrahydrocannabinol
- Δ9-THC
Δ9-tetrahydrocannabinol
References
- Abramahov A, Abrahamov A, & Mechoulam R (1995). An efficient new cannabinoid antiemetic in pediatric oncology. Life Sciences, 56(23–24), 2097–2102. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Scates SM, Lowe JA, & Martin BR (1996). Dependence on Δ9-tetrahydrocannabinol: Studies on precipitated and abrupt withdrawal. The Journal of Pharmacology and Experimental Therapeutics, 278(3), 1290–1295. [PubMed] [Google Scholar]
- Ashford JR, & Colquhoun D (1972). Lectures on Biostatistics: An Introduction to Statistics with Applications in Biology and Medicine. Journal of the Royal Statistical Society. Series A (General), 135(4). 10.2307/2344687 [DOI] [Google Scholar]
- Avraham Y, Ben-Shushan D, Breuer A, Zolotarev O, Okon A, Fink N, Katz V, & Berry EM (2004). Very low doses of Δ8-THC increase food consumption and alter neurotransmitter levels following weight loss. Pharmacology Biochemistry and Behavior, 77(4), 675–684. 10.1016/j.pbb.2004.01.015 [DOI] [PubMed] [Google Scholar]
- Badowski ME (2017). A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemotherapy and Pharmacology, 80(3), 441–449. 10.1007/s00280-017-3387-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-Gelouin D, & Lloyd KL (1986). Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. European Journal of Pharmacology, 126(3), 223–229. 10.1016/0014-2999(86)90051-8 [DOI] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, & Schuster CR (1988). Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology, 94(2), 206–212. 10.1007/BF00176846 [DOI] [PubMed] [Google Scholar]
- Compton DR, Prescott WR, Martin BR, Siegal C, Gordon PM, & Razdan RK (1991). Synthesis and Pharmacological Evaluation of Ether and Related Analogues of delta-8-, delta-9-, and delta-9,11-tetrahydrocannabinol. Journal of Medical Chemistry, 34(11), 3310–3316. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, & Martin BR (1993). Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. Journal of Pharmacology and Experimental Therapeutics, 265(1), 218–226. [PubMed] [Google Scholar]
- Cooper ZD (2016). Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. In Current Psychiatry Reports (Vol. 18, Issue 5). Current Medicine Group LLC; 1. 10.1007/s11920-016-0694-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey WL, Harris LS, Howes JF, Kennedy JS, Granchelli FE, Pars HG, & Razdan RK (1970). Pharmacology of some Marijuana Constituents and Two Heterocyclic Analogues. Nature, 226(2), 1265–1267. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, & Sim-Selley LJ (2010). FAAH−/− Mice Display Differential Tolerance, Dependence, and Cannabinoid Receptor Adaptation After Δ9-Tetrahydrocannabinol and Anandamide Administration. Neuropsychopharmacology, 35(8), 1775–1787. 10.1038/npp.2010.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Wilson CD, & Berquist MD (2018). Pro-psychotic effects of synthetic cannabinoids: interactions with central dopamine, serotonin, and glutamate systems. In Drug Metabolism Reviews (Vol. 50, Issue 1, pp. 65–73). Taylor and Francis Ltd. 10.1080/03602532.2018.1428343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokos S, & Panagis G (2010). Effects of δ9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. Journal of Psychopharmacology, 24(5), 767–777. 10.1177/0269881109104904 [DOI] [PubMed] [Google Scholar]
- Galli JA, Sawaya RA, & Friedenberg FK (2011). Cannabinoid Hyperemesis Syndrome. Current Drug Abuse Reviews, 4(4), 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wise LE, Chen Y, Gujjar R, Mahadevan A, Cravatt BF, & Lichtman AH (2013). The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sciences, 92(8–9), 498–505. 10.1016/j.lfs.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Samano KL, Ignatowska-Jankowska B, Tao Q, Sim-Selly LJ, Selley DE, Wise LE, Poklis A, & Lichtman AH (2016). Pharmacological characterization of repeated administration of the first generation abused synthetic cannabinoid CP47,497. Journal of Basic and Clinical Physiology and Pharmacology, 27(3), 217–228. 10.1515/jbcpp-2015-0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham MTEN, & Jong YDE (1974). Tolerance to the hypothermic and aggression-attenuating effect of Δ8- AND Δ9-tetrahydrocannabinol in mice. 28, 144–148. [DOI] [PubMed] [Google Scholar]
- Harris HM, Sufka KJ, Gul W, & Elsohly MA (2016). Effects of Delta-9-Tetrahydrocannabinol and Cannabidiol on Cisplatin-Induced Neuropathy in Mice. Planta Medica, 82(13), 1169–1172. 10.1055/s-0042-106303 [DOI] [PubMed] [Google Scholar]
- Herkenham M (1992). Cannabinoid Receptor Localization in Brain: Relationship to Motor and Reward Systems. Annals of the New York Academy of Sciences, 654(1), 19–32. 10.1111/j.1749-6632.1992.tb25953.x [DOI] [PubMed] [Google Scholar]
- Hollister LE, & Gillespie HK (1972). Delta-8- and delta-9-tetrahydrocannabinol: Comparison in man by oral and intravenous administration Delta-8-tetrahydrocannabinol. Clinical Pharmacology and Therapeutics, 14(3), 353–357. [DOI] [PubMed] [Google Scholar]
- Jarbe T, & Henriksson B (1974). Discriminative Response Control Produced with Hashish, Tetrahydrocannabinols (Δ8-THC and Δ9-THC), and Other Drugs. Psychopharmacologia, 40(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Jarbe T, Henriksson B, & Ohlin G (1977). Δ9-THC as a discriminative cue in pigeons: effects of Δ8-THC, CBD, and CBN. Archives Internationales De Pharmacodynamie Et De Therapie, 228(1), 68–72. [PubMed] [Google Scholar]
- Kesner A, Mateo Y, Abrahao K, Ramos-Maciel S, Pava M, Gracias A, Paulsen R, Carlson H, & Lovinger D (2021). Sex-dependent changes in murine striatal dopamine release, sleep, and behavior during spontaneous Δ−9-tetrahydrocannabinol abstinence. https://europepmc.org/article/ppr/ppr427868 [DOI] [PMC free article] [PubMed]
- Khasabova IA, Gielissen J, Chandiramani A, Harding-Rose C, Odeh DA, Simone DA, & Seybold VS (2011). CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behavioral Pharmacology, 22(5–6), 607–616. 10.1097/FBP.0b013e3283474a6d.CB1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, & Cole EC (2013). Acute Δ9-tetrahydrocannabinol blocks gastric hemorrhages induced by the nonsteroidal anti-inflammatory drug diclofenac sodium in mice. European Journal of Pharmacology, 715(1–3). 10.1016/j.ejphar.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, Selley DE, Imad Damaj M, & Lichtman AH (2011). The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology, 60(2–3), 244–251. 10.1016/j.neuropharm.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavž J, Gorenjak M, & Marinšek M (2016). Suicide attempt with a mix of synthetic cannabinoids and synthetic cathinones: Case report of non-fatal intoxication with AB-CHMINACA, AB-FUBINACA, alpha-PHP, alpha-PVP and 4-CMC. Forensic Science International, 265, 121–124. 10.1016/j.forsciint.2016.01.018 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, & Martin BR (2001). Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacology, Biochemistry, and Behavior, 69(1–2), 181–188. https://doi.org/S0091-3057(01)00514-7 [DOI] [PubMed] [Google Scholar]
- Lichtman Aron H., & Martin BR (1997). The selective cannabinoid antagonist SR 141716A blocks cannabinoid- induced antinociception in rats. Pharmacology Biochemistry and Behavior, 57(1–2), 7–12. 10.1016/S0091-3057(96)00121-9 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, & Cravatt BF (2004). Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain, 109(3):319–327. [DOI] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, & Nagarkatti P (2007). CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clinical Immunology, 122(3), 259–270. 10.1016/j.clim.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, & Cravatt BF (2009). Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nature Chemical Biology, 5(1), 37–44. 10.1038/nchembio.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Weyer-Menkhoff I, & Tegeder I (2018). Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. In European Journal of Pain (United Kingdom) (Vol. 22, Issue 3, pp. 471–484). Blackwell Publishing Ltd. 10.1002/ejp.1148 [DOI] [PubMed] [Google Scholar]
- Mackie K (2006). Mechanisms of CB1 receptor signaling: Endocannabinoid modulation of synaptic strength. In International Journal of Obesity (Vol. 30, pp. S19–S23). 10.1038/sj.ijo.0803273 [DOI] [PubMed] [Google Scholar]
- National Conference of State Legislatures. (2022). State Medical Cannabis Laws. [Google Scholar]
- Oliver MB, Woolley JK, & Limperos AM (2013). The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Theories and Models of Communication, 60(804), 411–424. 10.1515/9783110240450.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, & Marwaha R (2020). Cannabis Use Disorder. StatPearls Publishing. 10.4135/9781483365817.n232 [DOI] [PubMed] [Google Scholar]
- Patton AL, Chimalakonda KC, Moran CL, Mccain KR, Radominska-Pandya A, James LP, Kokes C, & Moran JH (2013). K2 Toxicity: Fatal case of psychiatric complications following AM2201 exposure. Journal of Forensic Sciences, 58(6), 1676–1680. 10.1111/1556-4029.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Sala M, Viganò D, Braida D, Castiglioni C, Limonta V, Guidali C, Realini N, & Parolaro D (2007). Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Δ9-tetrahydrocannabinol in rats. Neuropsychopharmacology, 32(9), 2036–2045. 10.1038/sj.npp.1301330 [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, & Cravatt BF (2010). Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature Neuroscience, 13(9), 1113–1119. 10.1038/nn.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BLA, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, & Lichtman AH (2009). Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS Journal, 11(2), 342–352. 10.1208/s12248-009-9110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödén PO, Järbe TUC, & Henriksson BG (1973). Effects of long-term administration and withdrawal of tetrahydrocannabinols (Δ8-THC and Δ9-THC) on open-field behavior in rats. Pharmacology, Biochemistry and Behavior, 1(3), 243–249. 10.1016/0091-3057(73)90111-1 [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, & Simon P (1985). The tail suspension test: A new method for screening antidepressants in mice. In Psychopharmacology (Vol. 85). [DOI] [PubMed] [Google Scholar]
- Trexler KR, Eckard ML, & Kinsey SG (2019). CB1 positive allosteric modulation attenuates Δ9-THC withdrawal and NSAID-induced gastric inflammation. Pharmacology Biochemistry and Behavior, 177, 27–33. 10.1016/j.pbb.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler KR, Nass SR, Crowe MS, Gross JD, Jones MS, McKitrick AW, Siderovski DP, & Kinsey SG (2018). Novel behavioral assays of spontaneous and precipitated THC withdrawal in mice. Drug and Alcohol Dependence, 191, 14–24. 10.1016/j.drugalcdep.2018.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler KR, Vanegas SO, Poklis JL, & Kinsey SG (2020). The short-acting synthetic cannabinoid AB-FUBINACA induces physical dependence in mice. Drug and Alcohol Dependence, 214(May), 108179. 10.1016/j.drugalcdep.2020.108179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. (2018). Farm Bill. https://www.usda.gov/farmbill
- U.S. Food and Drug Administration. (2021). 5 Things to Know about Delta-8 Tetrahydrocannabinol – Delta-8 THC. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahydrocannabinol-delta-8-thc [PMC free article] [PubMed]
- United Nations. (2021). World Drug Report 2021.
- Watanabe K, Narimatsu S, Yamamoto I, & Yoshimura H (1987). Cross-tolerance development to the prolongation of pentobarbitone-induced sleep by Δ8-tetrahydrocannabinol and 11-hydroxy-Δ8-tetrahydrocannabinol in mice. Journal of Pharmacy and Pharmacology, 39(11), 945–947. 10.1111/j.2042-7158.1987.tb03136.x [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamamoto I, & Yoshimura H (1983). Development of tolerance and cross-tolerance to the cataleptogenic effects of delta-8-tetrahydrocannabinol and 11-hydroxy-delta-8-tetrahydrocannabinol in mice. European Journal of Pharmacology, 94, 349–351. [DOI] [PubMed] [Google Scholar]
- Yamamoto I, Watanabe K, Narimatsu S, Hamajima K, & Yoshimura H (1985). Cross-tolerance to the hypothermic effect of Δ8-tetrahydrocannabinol 11-hydroxy-Δ8-tetrahydrocannabinol and chlorpromazine in the mouse. European Journal of Pharmacology, 111(2), 159–166. 10.1016/0014-2999(85)90752-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


