Abstract
Objective
To explore the intrinsic mechanism of the mammalian target of rapamycin (mTOR) pathway activation and promotion of neuronal axon growth.
Methods
Human neuroblastoma cells, SH-SY5Y, were induced with all-trans retinoic acid (ATRA; 10 μM for three days) which differentiated the cell line into a neuronal-like state. Immunohistochemical staining was used to detect the differentiation status of the neuronal-like cells. Phosphatase and tensin homolog (PTEN) RNA interference (RNAi) experiments were performed on the differentiated cells; reverse transcription-polymerase chain reaction (RT-PCR) detected transcriptional levels of PTEN following 24 h of interference. After 36 h, western blot assay was used to detect expression levels of ribosomal protein S6 kinase (pS6k) and mTOR. To downregulate the expression of PTEN and cluster of differentiation 44 (CD44), a cell-surface glycoprotein, simultaneously, PTEN siRNA and CD44 siRNA sequences were mixed in equal proportions in co-interference experiments. RT-PCR detected the transcription level of CD44, and the relationship between the CD44 and axonal growth was observed after 48 h of interference.
Results
Microtubule-associated protein 2 (MAP2) expression was enhanced after three days of induction in SH-SY5Y cells. RT-PCR showed the transcription level of PTEN was significantly downregulated after 24 h of PTEN knockdown. mTOR and pS6k protein expression levels were significantly upregulated after 36 h of interference. CD44 transcription levels were upregulated after PTEN gene interference. The neurite length of the cells in the experimental interference group was significantly longer than that in the control group, and the expression level of CD44 was positively correlated with neurite growth. The neurite length of the PTEN-only interference group was significantly greater than that of the co-interference and ATRA groups.
Conclusion
Activation of the mTOR pathway promoted neurite growth through upregulation of CD44 expression, thus promoting neuronal regeneration.
Keywords: mTOR pathway, CD44, Axonal growth, Nerve regeneration
Introduction
The characteristics of nerve injury include death of the nerve cells and dysfunction of axonal signal transduction. The low intrinsic growth activity of neurons is a crucial factor that affects nerve regeneration. Therefore, improving the intrinsic growth ability of neurons and identifying the underlying mechanisms could fundamentally solve the pathogenesis of nerve injury. 1 Several physiological factors affect axon growth and studies have shown that the intrinsic growth capacity of neurons is a major factor affecting axon regeneration. 2 Clarifying the intrinsic regenerative mechanism of neurons is key in the understanding of the basic processes that hamper regeneration in mammalian central nervous system (CNS) and crucial for the development of strategies to promote recovery of circuit connectivity and function. 3
Recent studies have shown that activating the mammalian target of rapamycin (mTOR) pathway can promote the growth of neural axons. 4 In a study that explored the retrograde transport of local axonal injury signals and enhancement of axonal growth capacity in the cell body and the response of Schwann cells that myelinate the damaged axon, investigators found that several classes of signalling pathways that occur in axons and Schwann cells cooperate to generate a robust regenerative response. 5 Another study reported that activating the mTOR signalling pathway increased the expression of some proteins related to axon regeneration. 6 Researchers investigating the effect of the mTOR signalling pathway on nerve myelin regeneration found that the mTOR signalling pathway was activated after nerve injury, and that this activation significantly increased the expression levels of myelin formation-related proteins. 7 While researchers have found that mTOR pathway activation promotes axonal regeneration, the specific mechanisms are unclear.
Cluster of differentiation 44 (CD44) is a cell-surface glycoprotein. 8 The molecule has been shown to have an important role in physiological activities in normal cells and pathological activities in cancer cells, such as cell proliferation, adhesion, and migration. 8 CD44 is activated by binding to hyaluronan (HA) to participate in adhesion and migration. Several studies have shown CD44 has a functional role in axonal growth. For example, in one study investigators found CD44 expression was identified in retinal ganglion cells and initiated their axonal growth. 9 In another study, the relationship between peripheral glial cells and expression of CD44. was investigated and results showed the importance of CD44 and HA in mediating glial cell function during peripheral nerve regeneration. 10 In addition, another study confirmed the influence of CD44 and the HA-mediated motility receptor (RHAMM) on motility in Schwann cells. 11 Although researchers have shown that upregulation of CD44 is related to nerve growth, it is unclear whether this activation promotes the mTOR pathway. Therefore, we decided to explore activation of the mTOR pathway and promotion of neuronal axon growth and investigate the involvement of CD44.
Materials and Methods
Cell culture
Human neuroblastoma cells, SH-SY5Y, were obtained from the Chinese Academy of Sciences cell bank. SH-SY5Y cells were cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F12 (DMEM/F12) (1:1) (Gibco; Thermo Fisher Scientific, Inc.) with the addition of 10% foetal bovine serum (FBS) (Gibco), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Thermo Fisher Scientific). The cells were maintained at 37°C in a humidified atmosphere containing 5% carbon dioxide. SH-SY5Y cells were induced with all-trans retinoic acid (ATRA; 10 μM for three days) (Sigma) which differentiated the cell line into a neuronal-like state.
Immunohistochemical staining
The SH-SY5Y cells were seeded in 24-well plates. Small poly-lysine-coated slides were placed in the well plates in advance. After three days induction with ATRA and 24 h incubated in phosphatase and tensin homolog (PTEN) RNA interference (RNAi), immunohistochemical staining for microtubule-associated protein 2 (MAP2) and ribosomal protein S6 kinase (pS6k) was performed.
The 24-well plates were washed three times with phosphate-buffered saline (PBS), fixed with 4% polyformaldehyde solution (PFA) for 30 min, and dried overnight at 37°C. Small sections were removed and placed on slides. Triton (0.3%) was added for 30 min to increase the permeability of cell membranes to antibodies. Hydrogen peroxide (3%, 1/100) was used to repress endogenous peroxidase activity and slides were incubated overnight at 4°C.
On the following day, MaxVisionTM (Maxim Biological Company) ready-to-use rapid immunohistochemistry reagent was added to the slides and they were incubated for 15 min at room temperature (25°C). In addition, 100 µl diaminobenzidine (DAB) (Dingguo Changsheng Biotechnology Co., Ltd.) was added to each slice and they were left for 5 min. The degree of cell staining was assessed under a microscope. The slides were rinsed under flowing water and counterstained with haematoxylin for 10 sec.
RT-PCR to detect transcriptional levels
The three experimental groups were, ATRA, PTEN-RNAi and negative control (NC). The NC group included the negative sequence provided by Gemma Biology Co., Ltd., Changchun, China. When the cells were approximately 90% confluent, the range of cell numbers was 2–4 × 106. Each group of cellular RNA was extracted using Invitrogen TRIzolTM Reagent products (Thermo Fisher), according to the manufacturer’s instructions. The RNA concentration was measured using an ultraviolet transmission reflection detection analyser (Shanghai Institute of Bioengineering), and optical density (OD) measurements were used to quantify the RNA concentration (the mRNA concentration range was 105–345 ng/µl). The mRNA (2 μg) was reverse-transcribed using the reverse transcription kit (Thermo Fisher Scientific). The following polymerase chain reaction (PCR) conditions were used: initial denaturation at 95°C for 3 min, followed by 30 cycles of denaturation at 95°C for 30 sec and annealing and extension at 55°C for 30 sec.
Finally, ethidium bromide (Dingguo Changsheng Biotechnology Co., Ltd) was added to agarose gel and visualized using ultraviolet light. PCR products was detected by agarose gel electrophoresis and the images were captured using a gel imaging system (GE Corporation, USA).
Quantity One software (Bio-Rad Laboratories) was used to analyse the values of several sets of electrophoretic bands, getting grayscale values which represented the expression level of the target gene mRNA. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the control for all RT-PCR results for each gene. The primer sequences for MAP2, PTEN, CD44 and GADPH used in this study are shown in Table 1.
Table 1.
List of primers.
| Name | Primer sequence 5′-3′ forward/reverse | Product length |
|---|---|---|
| MAP2 | Forward: TCAGAGGCAATGACCTTACC | 320 bp |
| Reverse: GTGGTAGGCTCTTGGTCTTT | ||
| PTENHUMAN | Forward: AAGCTTATGACAGCCATCATCAAAGAGAT | 1512 bp |
| Reverse: GGATCCGGAATAAAACGGGAAAGTGCC | ||
| CD44HUMAN | Forward: GCAAGTCTCAGGAAATGGTG | 372 bp |
| Reverse: CAAGTGGGAACTGGAACGAT | ||
| GAPDH | Forward: GTCTTCACCACCATGGAGAA | 266 bp |
| Reverse: ATCCACAGTCTTCTGGGTGG |
MAP2, microtubule-associated protein 2; PTEN, phosphatase and tensin homolog; CD44, cluster of differentiation 44; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Transfections of siRNA
Expression of the corresponding molecules was knocked down using specific small interfering RNA (siRNA). A series of siRNA sequences (Table 2) was designed and synthesized by GenePharma Inc. (Changchun, China). Experimental grouping was performed as follows: PTEN-RNAi group; stable NC group; ATRA group. The transfection was performed using Golden Transfer-SD (Gemma Biology Co., Ltd.).
Table 2.
siRNA synthesis sequences.
| Name | Sequences (5′-3′) |
|---|---|
| PTEN-homo | Sense: GCCAGCUAAAGGUGAAGAUTT |
| Antisense: AUCUUCACCUUUAGCUGGCTT | |
| CD44-homo | Sense: GCAGCACUUCAGGAGGUUATT |
| Antisense: UAACCUCCUGAAGUGCUGCTT | |
| Negative Control | Sense: UUCUCCGAACGUGUCACGUTT |
| Antisense: ACGUGACACGUUCGGAGAATT |
siRNA, short interfering RNA; PTEN, phosphatase and tensin homolog; CD44, cluster of differentiation 44.
The assay was performed according to the manufacturer’s instructions. Initially, SH-SY5Y cells were seeded in a six-well plate 9.6 cm2 (1 × 106) for 24 h. Thereafter, cells were incubated in 10 μM ATRA for three days. Transfection reagent (8 µl) and siRNA (4 μg) were used in each experiment. An inverted fluorescence microscope was used to observe the transfection efficiency, and RT-PCR was used to verify transfection efficiency 24 h after transfection.12–14
To downregulate the expression of PTEN and CD44 simultaneously, PTEN siRNA and CD44 siRNA sequences were mixed in equal proportions in co-interference experiments. RT-PCR detected the transcription level of CD44, and the relationship between the CD44 and axonal growth was observed after 48 h of interference.
Western blot assay
Total proteins from the interfered cells were extracted using the radio immunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Scientific, Inc.) supplemented with phosphatase and protease inhibitors (Thermo Fisher Scientific, Inc.). A protein detection kit (Thermo Fisher Scientific Inc.) was used to quantify the protein concentration. Equal amounts of proteins were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes, which were then blocked with 5% non-fat milk for 4 h at room temperature. The membranes were incubated overnight at 4°C with the following primary antibodies: mTOR (dilution 1:100, Cell Signaling Company); phosphorylated ribosomal protein S6 (pS6k) (dilution 1:100, Cell Signaling Technology); -actin (dilution 1:100, Boside Biotechnology Co., Ltd.).
On the following day, the membranes were incubated for 2 h at room temperature with a secondary antibody (Horseradish enzyme labelled goat anti mouse IgG; rabbit anti goat IgG labelled with horseradish enzyme; Beijing Dingguo Changsheng Biotechnology Co., Ltd.) diluted 1:500. The membranes were then placed in PBS with Tween 20 and washed four times for 40 min at room temperature. Enhanced chemiluminescence was used to visualize the blots. Quantity One software (Bio-Rad Laboratories) was used to analyse the values of several sets of blots using grayscale values. -actin was used as the loading control.
Ethical approval and written permissions for this study were deemed unnecessary because the cell line was obtained from the Chinese Academy of Sciences cell bank.
Statistical analysis and measurement of neurite length
Statistical analyses were performed using SPSS Software (version 19 for Windows®; IBM Corp, Armonk, NY, USA). A P-value <0.05 was considered to indicate statistical significance. The data from each experimental group were expressed as mean ± variance and the results were compared and analysed by Student’s t-test (two-tailed distribution, two-sample equal variance).
RT-PCR and western blotting results were measured using Quantity One software (Bio-Rad Laboratories), and five groups of images represented each independent experiment. Cell neurite length was measured using the ImageJ software. From the five experimental groups, 10 cells were selected randomly by a blinded assessor for measurement of neurite length; the process was repeated five times to obtain an average value from 50 cells. The cells selected for each group were independent.
Results
Differentiation of SH-SY5Y cells induced by ATRA
The morphology of SH-SY5Y cells with and without ATRA induction are shown in Figure 1. Following treatment with ATRA the cells became elongated, and neurite outgrowth was obvious. ATRA inhibited neuronal growth by a decrease in N-Myc oncoprotein and an increase in type II protein kinase. 15 Immunohistochemical staining showed that the number of MAP2-positive cells in SH-SY5Y cells induced for three days with ATRA was significantly greater compared with the control group (Figure 2).
Figure 1.
Neurite outgrowth of SH-SY5Y cells.
(a) Control group and (b) Cells following three days of induction with all-trans retinoic acid (ATRA).
Figure 2.
Immunohistochemical staining of microtubule-associated protein 2 (MAP2) proteins.
(a) Control group [con] and (b) Cells following three days of induction with all-trans retinoic acid (ATRA). The expression of MAP2 in the cells in the induction group was significantly enhanced.
RT-PCR was used to determine the expression level of the mRNA that coded for the MAP2 protein in the cells of the induction group. As shown in Figure 3, compared with the control group, the difference was statistically significant. (n = 5, P = 0.0447).
Figure 3.
The transcriptional level of mRNA that coded for the microtubule-associated protein 2 (MAP2) protein of SH-SY5Y cells. (a) After induction with ATRA, the transcription level of MAP2 gene was significantly upregulated compared with the control group and (b) The difference between the two groups was statistically significant. (n = 5, *P < 0.05). con, control; ATRA all-trans retinoic acid; MAP2, microtubule-associated protein 2; GADPH, glyceraldehyde 3-phosphate dehydrogenase;3d, three days.
Figure 4.
The transcriptional level of the mRNA that coded for phosphatase and tensin homolog (PTEN) protein. (a) RT-PCR results showed PTEN transcription levels in each group after 24 h of RNA interference (RNAi) and (b) Statistical analysis of the grayscale bands showed that the differences between groups were statistically significant. (n = 5; *P < 0.05; **P < 0.05). ATRA all-trans retinoic acid; NC, negative control; GADPH, glyceraldehyde 3-phosphate dehydrogenase; PTEN, phosphatase and tensin homolog; RNAi, RNA interference.
RNAi technology was used to downregulate PTEN expression
After the induced SH-SY5Y cells were treated with RNAi technology for 24 h, they were placed under an inverted fluorescence microscope to observe fluorescence interference efficiency of cells in the interference and control groups. RT-PCR was used to detect the transcription level of PTEN in the RNAi and control groups. By comparison with controls, RNAi technology statistically significantly (n = 5, P < 0.05) decreased the transcription level of PTEN in SH-SY5Y cells.
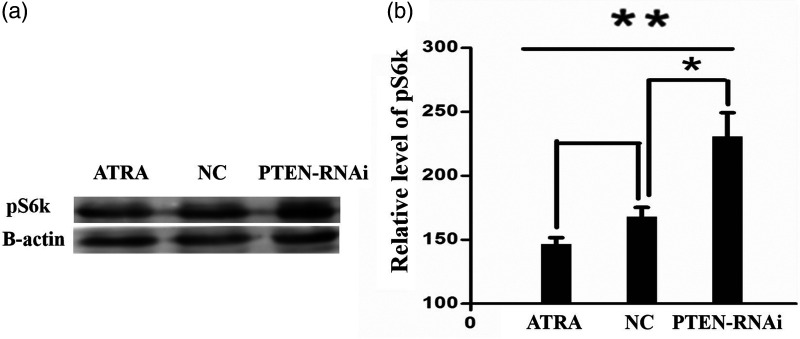
Western blot assay was used to detect expression levels of mTOR and pS6k protein
Following PTEN inactivation, the mTOR signalling pathway was activated. pS6k can show activation of the mTOR signalling pathway. The proteins of experimental cells in the interference and control groups were extracted for the western blot assay and measured. Experimental results confirmed that compared with the control groups, the expression of mTOR and pS6k proteins in the PTEN-RNAi group increased significantly (P < 0.05; (Figures 5 and 6).
Figure 5.
Expression level of mTOR protein detected by western blot assay following phosphatase and tensin homolog (PTEN) gene interference. (a) The expression of mTOR protein in each experimental group by comparison with -actin and (b) Statistical analysis of the grayscale bands showed that the differences between the PTEN-RNAi group and the control groups were statistically significant (n = 5; *P < 0.05; **P < 0.05). ATRA all-trans retinoic acid; NC, negative control; PTEN, phosphatase and tensin homolog; RNAi, RNA interference.
Figure 6.
Following mTOR pathway activation, western blot assay was used to detect the expression level of the ribosomal protein S6 kinase (pS6k). (a) Expression of pS6k in each experimental group by comparison with -actin. (b) Statistical analysis of the grayscale bands showed that the differences between the PTEN-RNAi group and the control groups were statistically significant (n = 5; *P < 0.05; **P < 0.05).
ATRA all-trans retinoic acid; NC, negative control; PTEN, phosphatase and tensin homolog; RNAi, RNA interference.
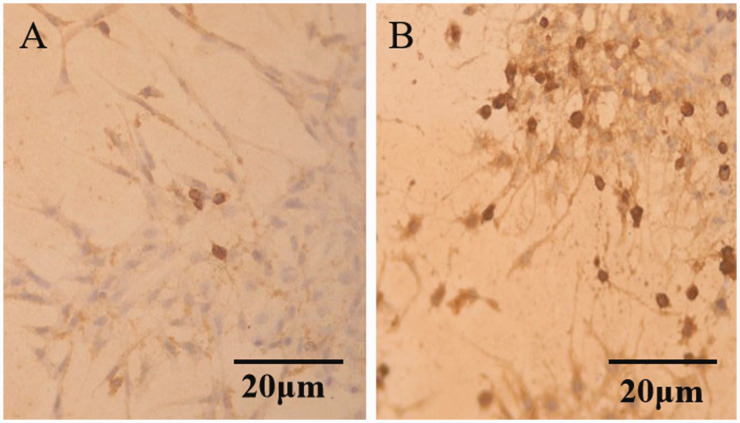
Immunohistochemical staining with the pS6k antibody was performed in the interference and control groups. As shown in Figure 7, the number of pS6k protein-positive cells in the PTEN-RNAi group was significantly greater than that in the control group. Enhanced expression of pS6k protein was a marker of mTOR pathway activation.
Figure 7.
Ribosomal protein S6 kinase (pS6k) expression level following phosphatase and tensin homolog RNA interference (PTEN-RNAi) detected by an immunohistochemical staining method. (a) Control group and (b) The PTEN-RNAi group.
Effect of mTOR pathway activation on neurite length
Lengths of cell neurites after PTEN-RNAi interference (Figure 8c) were statistically significantly greater (P < 0.05) than in the ATRA group (Figure 8b) and the NC group (Figure 8a).
Figure 8.
Length of cell neurites. (a) The negative control (NC) group included the negative sequence provided by Gemma Biology Co. Ltd. (b) The morphology of SH-SY5Y cells with induction by all-trans retinoic acid (ATRA). (c) The morphology of SH-SY5Y cells with induction by phosphatase and tensin homolog RNA interference (PTEN-RNAi) and (d) Statistical comparison of the average length of cell neurites (n = 50; *P < 0.05; **P < 0.05).
Following 36h of experimental interference, the transcriptional levels of CD44 in the interference, NC, and ATRA groups were detected by RT-PCR. The results showed that in the PTEN-RNAi experimental group the transcription level of CD44 was statistically significantly (P < 0.05) greater than that in the NC group and the ATRA group (Figure 9). (P < 0.05).
Figure 9.
Levels of cluster of differentiation 44 (CD44) mRNA detected by RT-PCR following phosphatase and tensin homolog RNA interference (PTEN-RNAi) by comparison with GADPH. (a) The transcriptional level of CD44 mRNA and (b) Statistical comparison between groups (n = 3; *P < 0.05; **P < 0.05).
NC, negative control; ATRA, all-trans retinoic acid; PTEN, phosphatase and tensin homolog; RNAi, RNA interference; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Co-interference
The co-interference effect detected under a fluorescence microscope 24 h after transfection is shown in Figure 10. According to the green fluorescence, the interference of PTEN-RNAi was effective. According to the red fluorescence, the interference of CD44 siRNA was effective, and according to the yellow fluorescence, mutual interference was effective. RT-PCR was used to detect the transcription levels of PTEN and CD44 in the ATRA, PTEN-RNAi, and co-interference groups (Figure 11). The PTEN transcription level in the ATRA group was significantly greater than in the PTEN-RNAi and co-interference groups, indicating that PTEN interference in the PTEN-RNAi and co-interference groups was efficient. The CD44 transcription level in the PTEN-RNAi group was significantly greater than in the ATRA and co-interference groups, which indicated that the CD44 transcription level was significantly enhanced following mTOR pathway activation. As shown in Figure 11c, the transcriptional level of CD44 in the ATRA group was significantly greater than in the co-interference group, indicating that the co-interference group was efficient in CD44 interference.
Figure 10.
Transfection effect under a fluorescence microscope. CD44, cluster of differentiation 44; RNAi, RNA interference; PTEN, phosphatase and tensin homolog.
Figure 11.
Level of PTEN mRNA and CD44 mRNA detected by RT-PCR in the ATRA, PTEN-RNAi, and co-interference groups by comparison with GADPH. (a) The transcriptional level of each group. (b) Statistical comparison of PTEN mRNA between groups and (c) Statistical comparison of CD44 mRNA between groups (n = 5; *P < 0.05; **P < 0.05, ***P < 0.01).
ATRA, all-trans retinoic acid; PTEN, phosphatase and tensin homolog; RNAi, RNA interference; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
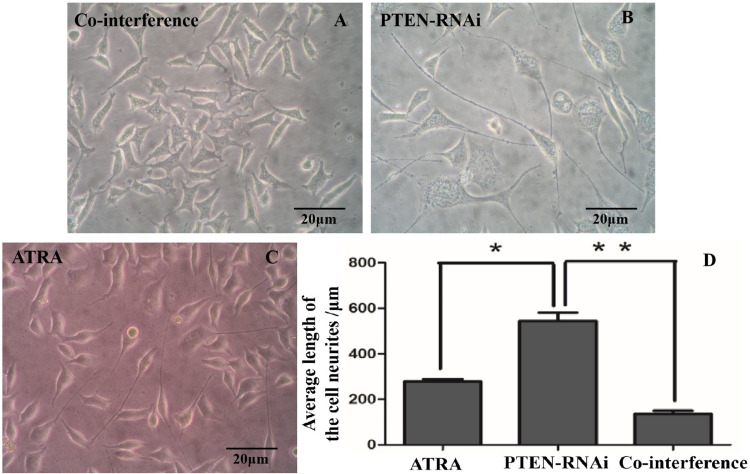
The morphology of the cell neurites in the co-interference, PTEN-RNAi, and ATRA groups is show in Figure 12. The experiment showed that the neurite lengths of cells in the PTEN-RNAi group were significantly greater than in the co-interference and ATRA groups (P < 0.05).
Figure 12.
Morphology of cell neurites. (a) The co-interference group. (b) The PTEN-RNAi group. (c) The ATRA group and (d) Statistical comparison of the lengths of the cell neurites. (n = 50; *P < 0.05; **P < 0.05).
ATRA, all-trans retinoic acid; PTEN, phosphatase and tensin homolog; RNAi, RNA interference.
In summary, experimental results showed that after co-interference with PTEN and CD44, neurite length was significantly shorter than in the PTEN-RNAi group, indicating that CD44 plays a specific role in activating the mTOR pathway to promote neurite elongation.
Discussion
Improving axonal regeneration of damaged nerve cells is vital for treating CNS injuries.16–19 Several studies have shown that the mTOR signalling pathway plays a crucial role in the formation of nerve synapses, neuronal survival, nerve cell development, and axonal regeneration and extension.20–22 Identifying the underlying molecules associated with the mechanisms downstream of the mTOR pathway and neural regeneration may provide useful therapeutic targets for CNS injury. Studies have shown that CD44 is closely related to axonal development.9,24,25 In this current study, we showed that activation of the mTOR pathway promoted neurite outgrowth through upregulation of CD44 expression.
Studies have identified CD44 as a molecule downstream of the mTOR signalling pathway in cancer.25–32 One study determined that the mechanism by which shikonin-induced dental pulp stem cell differentiation occurred is through the mTOR signalling pathway and CD44. 27 Another study in colorectal cancer, found that cancer progression was via the PI3K/AKT/mTOR pathway through fucosylated CD44. 28 In addition, it has been shown that hypoxia-inducible factor (HIF)-2, a down regulator of expression, can inhibit stem cells of human breast cancer, which may be related to the CD44/PI3K/AKT/mTOR signalling pathway. 29 In acute myeloid leukaemia, researchers showed that p-mTOR expression was significantly reduced after anti-CD44 treatment. 30 A study in cholangiocarcinoma, showed that CD44 silencing reduced Akt and mTOR phosphorylation. 31 Other investigators have found a significant correlation between strong CD44 expression and AKT activation in lung cancer patients. 32 The above research suggests that the CD44 molecule is closely related to the mTOR pathway, and regulates several functions through the mTOR pathway.
In this present study, the induced differentiation of SH-SY5Y cells in vitro was selected as the neural cell model. The mTOR pathway was activated by PTEN-RNAi technology and the transcriptional level of CD44 following mTOR pathway activation was detected using RT-PCR. The results showed that the transcriptional level of CD44 was significantly upregulated. and neurite growth was significantly prolonged.
Our study had several limitations. For example, our results were from an in vitro study using a cell line from a malignant tumour. Therefore, our in vitro model using SH-SY5Y cells, will not represent the all characteristics of neurons. Moreover, mechanisms of the body are complex with many interactions which cannot be replicated by in vitro work. In addition, we did not identify the type of neurites and our sample sizes were small. In the future, we would consider the effect of glial cells on the mTOR signalling pathway and how they interplay in the PTEN-driven activation of CD44. In summary, we showed that activating the mTOR pathway promoted neurite outgrowth via upregulation of CD44 expression which promoted neuronal regeneration. Further research is needed to confirm our results.
Footnotes
Funding: This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jiwei Zhang https://orcid.org/0000-0002-1658-7390
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
References
- 1.Yang P.New strategy to promote adult spinal cord regeneration: enhance adult neurons’ intrinsic growth capability. Sheng Li Ke Xue Jin Zhan 2009; 40: 14–18. [PubMed] [Google Scholar]
- 2.Yang P, Yang Z.Enhancing intrinsic growth capacity promotes adult CNS regeneration. J Neurol Sci 2012; 312: 1–6. [DOI] [PubMed] [Google Scholar]
- 3.Enes J.Electrical activity suppresses intrinsic growth competence in adult primary sensory neurons. J. Ludwig Maximilians Universitat munchen 2009. Available from: https://edoc.ub.uni-muenchen.de/10070/1/Enes_Joana.pdf [Google Scholar]
- 4.Ding Y, Chen Q.mTOR pathway: a potential therapeutic target for spinal cord injury. Biomed Pharmacother 2022; 145: 112430. [DOI] [PubMed] [Google Scholar]
- 5.Abe N.Regulation of peripheral nerve regeneration by the mTOR pathway. 2010. Available from: https://openscholarship.wustl.edu/etd/4/
- 6.Yin H, Shen L, Xu C, et al. Lentivirus-mediated overexpression of miR-29a promotes axonal regeneration and functional recovery in experimental spinal cord injury via PI3K/Akt/mTOR pathway. Neurochem Res 2018; 43: 2038–2046. [DOI] [PubMed] [Google Scholar]
- 7.Ge C, Liu D, Sun Y.The promotive effect of activation of the Akt/mTOR/p70S6K signaling pathway in oligodendrocytes on nerve myelin regeneration in rats with spinal cord injury. Br J Neurosurg 2020; Dec 21: 1–9. [DOI] [PubMed] [Google Scholar]
- 8.Weng X, Maxwell-Warburton S, Hasib A, et al. The membrane receptor CD44: novel insights into metabolism. Trends Endocrinol Metab 2022; 33: 318–332. [DOI] [PubMed] [Google Scholar]
- 9.Ries A, Goldberg JL, Grimpe B.A novel biological function for CD44 in axon growth of retinal ganglion cells identified by a bioinformatics approach. J Neurochem 2007; 103: 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouasti S. Hyaluronic acid biomaterials for perspective peripheral nerve regeneration. The University of Manchester (United Kingdom). 2012. Available from: https://research.manchester.ac.uk/en/studentTheses/hyaluronic-acid-biomaterials-for-perspective-peripheral-nerve-reg
- 11.Ouasti S, Faroni A, Kingham PJ, et al. Hyaluronic acid (HA) receptors and the motility of Schwann cell (-like) phenotypes. Cells 2020; 9: 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park K, Kang TW, Kim MK, et al. Effects of Intracavernosal IGF-1 Gene Delivery on Erectile Function in the Aging Rat. Korean Journal of Urology 2005; 46: 406–413. Available from: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-209446 [Google Scholar]
- 13.Pu X, Guo QX, Long HA, et al. Effects of mTOR-STAT3 on the migra=tion and invasion abilities of hepatoma cell and mTOR-STAT3 expression in liver cancer. Asian Pac J Trop Med 2014; 7: 368–372. [DOI] [PubMed] [Google Scholar]
- 14.Sharma J. Optimization of siRNA Transfection for Breast and Glioa Cancer Cell Cultures. 2012. Available from: https://celprogen.com/content/uploads/file/Optimization%20of%20siRNA%20Transfection%20for%20Breast%20and%20Glioa%20Cancer%20Cell%20Cultures.pdf
- 15.Kim SN, Kim SG, Park SD, et al. Participation of type II protein kinase A in the retinoic acid-induced growth inhibition of SH-SY5Y human neuroblastoma cells. J Cell Physiol 2000; 182: 421–428. [DOI] [PubMed] [Google Scholar]
- 16.Fournier AE, Takizawa BT, Strittmatter SM.Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci 2003; 23: 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly DJ, Popovich PG.Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 2008; 209: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannila SS, Filbin MT.The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol 2008; 209: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ba M, Bonhoeffer F.Perspectives on axonal regeneration in the mammalian CNS. Trends Neurosci 1994; 17: 473–479. [DOI] [PubMed] [Google Scholar]
- 20.Tang BL.Promoting axonal regeneration through exosomes: an update of recent findings on exosomal PTEN and mTOR modifiers. Brain Res Bull 2018; 143: 123–131. [DOI] [PubMed] [Google Scholar]
- 21.Xiao H, Zhang Q, Zhong P, et al. Securinine promotes neuronal development and exhibits antidepressant-like effects via mTOR activation. ACS Chem Neurosci 2021; 12: 3650–3661. [DOI] [PubMed] [Google Scholar]
- 22.Gong R, Park CS, Abbassi NR, et al. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem 2006; 281: 18802–18815. [DOI] [PubMed] [Google Scholar]
- 23.Glezer I, Bittencourt JC, Rivest S.Neuronal expression of Cd36, Cd44, and Cd83 antigen transcripts maps to distinct and specific murine brain circuits. J Comp Neurol 2009; 517: 906–924. [DOI] [PubMed] [Google Scholar]
- 24.Lin L, Chan SO.Perturbation of CD44 function affects chiasmatic routing of retinal axons in brain slice preparations of the mouse retinofugal pathway. Eur J Neurosci 2003; 17: 2299–2312. [DOI] [PubMed] [Google Scholar]
- 25.Clohessy JG, Reschke M, Pandolfi PP.Found in translation of mTOR signaling. Cell Res 2012; 22: 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh AC, Liu Y, Edlind MP, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. J Nature 2012; 485: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kajiura K, Umemura N, Ohkoshi E, et al. Shikonin induces odontoblastic differentiation of dental pulp stem cells via AKT–mTOR signaling in the presence of CD44. Connect Tissue Res 2021; 62: 689–697. [DOI] [PubMed] [Google Scholar]
- 28.Pan S, Liu Y, Liu Q, et al. HOTAIR/miR-326/FUT6 axis facilitates colorectal cancer progression through regulating fucosylation of CD44 via PI3K/AKT/mTOR pathway. Biochim Biophys Acta Mol Cell Res 2019; 1866: 750–760. [DOI] [PubMed] [Google Scholar]
- 29.Bai J, Chen WB, Zhang XY, et al. HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling. World J Stem Cells 2020; 12: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadhoum SZ, Madhoun NY, Abuelela AF, et al. Anti-CD44 antibodies inhibit both mTORC1 and mTORC2: a new rationale supporting CD44-induced AML differentiation therapy. Leukemia 2016; 30: 2397–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thanee M, Dokduang H, Kittirat Y, et al. CD44 modulates metabolic pathways and altered ROS-mediated Akt signal promoting cholangiocarcinoma progression. PloS one 2021; 16: e0245871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elakad O, Häupl B, Labitzky V, et al. Activation of CD44/PAK1/AKT signaling promotes resistance to FGFR1 inhibition in squamous-cell lung cancer. NPJ Precis Oncol 2022; 6: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]