Abstract
Purpose:
Unpredictable drug efficacy and safety of combined antiepileptic therapy is a major challenge during pharmacotherapy decisions in everyday clinical practice. The aim of this study was to describe the pharmacokinetics of valproic acid (VA), lamotrigine (LTG), and levetiracetam (LEV) in a pediatric population using nonlinear mixed-effect modeling, while machine learning (ML) algorithms were applied to identify any relationships among the plasma levels of the three medications and patients’ characteristics, as well as to develop a predictive model for epileptic seizures.
Methods:
The study included 71 pediatric patients of both genders, aged 2–18 years, on combined antiepileptic therapy. Population pharmacokinetic (PopPK) models were developed separately for VA, LTG, and LEV. Based on the estimated pharmacokinetic parameters and the patients’ characteristics, three ML approaches were applied (principal component analysis, factor analysis of mixed data, and random forest). PopPK models and ML models were developed, allowing for greater insight into the treatment of children on antiepileptic treatment.
Results:
Results from the PopPK model showed that the kinetics of LEV, LTG, and VA were best described by a one compartment model with first-order absorption and elimination kinetics. Reliance on random forest model is a compelling vision that shows high prediction ability for all cases. The main factor that can affect antiepileptic activity is antiepileptic drug levels, followed by body weight, while gender is irrelevant. According to our study, children’s age is positively associated with LTG levels, negatively with LEV and without the influence of VA.
Conclusion:
The application of PopPK and ML models may be useful to improve epilepsy management in vulnerable pediatric population during the period of growth and development.
Keywords: factor analysis of mixed data, lamotrigine, levetiracetam, machine learning, population pharmacokinetics, principal component analysis, random forest, therapy optimization, valproic acid
Plain language summary
Pharmacokinetics and machine learning in epilepsy
Abstract: Nowadays, combined antiepileptic therapy is the best option for a number of pediatric patients. Furthermore, there are no standard procedures in the therapy management of this complex treatment. Besides therapeutic monitoring, the population pharmacokinetic (PopPK) approach and machine learning (ML) are useful sources of information regarding the optimization of therapy. The aim of this study was to describe the pharmacokinetics of valproic acid (VA), lamotrigine (LTG), and levetiracetam (LEV) in a pediatric population using nonlinear mixed-effect modeling, while ML algorithms were applied to identify any relationships among the plasma levels of the three medications and patients’ characteristics. The study included 71 pediatric patients of both genders, aged 2–18 years, on combined antiepileptic therapy. Population pharmacokinetic (PopPK) models were developed separately for VA, LTG, and LEV. Based on the estimated pharmacokinetic parameters and the patients’ characteristics, three ML approaches were applied (principal component analysis, factor analysis of mixed data, and random forest). According to our study, children’s age is positively associated with LTG levels, negatively with LEV and without influence from VA. However, the gender of patients has no influence on drug plasma concentration. Findings demonstrated that the application of PopPK and ML models may be useful to improve epilepsy management in vulnerable pediatric population during the period of growth and development.
Introduction
Successfully managing combined pharmacotherapy in pediatric patients with epilepsy is still a major challenge for clinician because of unpredictable drug efficacy, adverse effects, and a lack of information regarding optimal dosage regimen. Therefore, there is the need for effective and practical decision-making tools to assist clinicians to provide personalized treatment.1,2 Unfortunately, failure in the seizures control with antiepileptic drug (AED) monotherapy is seen in 17–40% of children with epilepsy. Combined antiepileptic therapy is advised when monotherapy remains well tolerated but with poor or suboptimal clinical response, or in the presence of idiosyncratic reaction evidence. 3
Everyday clinical practice includes therapeutic drug monitoring (TDM) as part of personalized epilepsy management regarding obvious clinical benefits, considerable morbidity, mortality, and quality of life. 4 However, it also has limitations as drug concentrations often do not correlate with improved clinical outcome. 1 Pharmacometrics approaches and mathematical simulation tools may be crucial step to improve personalized epilepsy management in vulnerable pediatric population. 2 In addition to population pharmacokinetics (PopPK), a lot of attention has recently been paid to application learning-based algorithms that can be used as a flexible approach to handle complex and high-dimensional data sources. 5
Machine learning (ML) combines patient’s personal information to predict the outcomes of AEDs treatment and, hence, support clinical decision-making. 6 According to the fact that statistical methods may have limited capacity to handle vast quantities of data obtained from the research, and according to this, a growing interest in other techniques such as factor analysis. 7 Compared with conventional modeling methods, simulation has indubitable better accuracy and advantages in dealing with real-world data, because of more complex and deeper insight, and interactive clinical settings.8,9 A combined PopPK and ML approach may have more accurate predictions, facilitate therapy individualization, and improve clinical outcome. 10
The aim of this study was to describe the pharmacokinetics of valproic acid (VA), lamotrigine (LTG), and levetiracetam (LEV) in a pediatric population using nonlinear mixed-effect modeling. Also, ML was applied to identify any relationships among the plasma levels of the three medications and patients’ characteristics, as well as to construct a predictive model for epileptic seizures. The three ML methods utilized in this study refer to principal component analysis (PCA), factor analysis of mixed data (FAMD), and random forest (RF).
Methods
Clinical unit – laboratory analysis
This prospective study was conducted at the Clinic of Pediatric Internal Medicine, Department of Pediatric Neurology (University Clinical Center of Nis, Serbia). Data were collected over a 12-month period, starting in May 2020. The study involved 71 patients with diagnosed epilepsy. Inclusion criteria were as follows: diagnosed epilepsy (G40); patients of both genders aged 2–18 years on dual antiepileptic therapy modalities: VA/LTG, VA/LEV, and LTG/LEV. Poor renal and liver function and other serious disease states were exclusion criteria. The data were collected from medical records and during face-to-face interviews with the patient or his parents or guardians. To protect patient data, each patient was assigned a code at the beginning of the study. The following data were collected: demographic characteristics [gender, age, body weight (BW)]; therapy characteristics (drug formulation, administered dose, dosing interval, plasma drug concentration, dose-adjusted concentration, adverse drug effects); and frequency of seizure control data. All patients have reached the steady-state condition for each of the AEDs included in the therapy. Regarding correspondence with trough levels, all blood samples were collected before the next dose in the morning.
Population pharmacokinetics
A pharmacokinetic analysis of the C-t data collected for LEV, LTG, and VA was performed using nonlinear mixed-effects modeling implemented in Monolix™ 2021R2. The number of compartments describing the LEV, LTG, and VA dispositions was initially clarified. One-, two-, and three-compartment models were investigated. The pharmacokinetic parameters were assumed to have a lognormal distribution, and an exponential model was utilized to represent inter-individual variability. Several error models of residual variability were tested, such as the constant, proportional, and combination constant and proportional models.
Since there was only one measurement available (trough levels) for each patient, it was not feasible to allow the algorithm to search for the maximum likelihood estimates of all parameters. Thus, a stepwise procedure was followed, where initially only one variable was freely estimated while the others were fixed to the values reported in the literature or followed maximum a posteriori estimation. When a robust estimate was found, another pharmacokinetic parameter was allowed to vary. Several combinations for the sequences of estimations were tried, and the one leading to the best fitting results and physiological soundness of the estimates was finally chosen.
Along with the selection of the base model, the effect of several covariates on the model pharmacokinetic parameters was studied. These included demographic factors such as BW, age (in years), additional antiepileptic medications coadministered (therapeutic regimen), daily dose of the drug, and the presence or absence of epileptic seizures. Covariate analyses were carried out utilizing a combination of stepwise forward and backward selection. The influence of continuous factors was examined using allometric or linear relationships, either untransformed or centered on their ‘mean’ value. Using fixed exponents, allometric scaling was applied to the apparent volumes of distribution (V) and clearance (Cl) parameters (1 for V and 0.75 for Cl). For the continuous and categorical factors, the Pearson correlation test and one-way ANOVA were performed. The Wald test was performed to determine whether the covariates could explain the variation in the final model’s parameters. The significance level was set at 5% in all analyses. The choice of covariates was further made by evaluating the decrease in the −2LL (−2 log likelihood) values, the accuracy of the parameters (in terms of relative standard error), the decrease in inter-individual variability estimates, and each covariate’s physiological soundness on the pharmacokinetic parameter.
The final model was selected using statistical and graphical goodness-of-fit criteria, which allowed potential biases or issues in the structural model to be identified. The precision of parameter estimates was also evaluated using relative standard errors (RSE%). Models were compared using numerical statistical significance criteria, such as log-likelihood, Akaike, and Bayesian information criteria, for selecting non-hierarchical models. Plots of predicted versus observed values, weighted residuals versus concentrations or time, and individual fits were used to evaluate the goodness of fit graphically. Visual predictive check charts, created using 1000 Monte Carlo simulations and 90% confidence intervals, were also utilized to evaluate the model’s predictive performance, stability, and robustness. The complete modeling exercise was carried out using Monolix™ 2021R2 software (Lixoft, Simulation Plus Inc., Lancaster, CA, USA).
Machine learning
ML methods were used to uncover any underlying relationships among the children’s characteristics and pharmacotherapy. The application of ML techniques, a subfield of artificial intelligence, is a powerful tool for detecting correlations between numerous variables.
The phrase ‘machine learning’ refers to the ‘learning’ process, and there are several ways that can be applied. ML algorithms are broadly categorized into four types: supervised, unsupervised, self-supervised, and reinforcement learning. The two most commonly used ML methods are ‘supervised learning’, in which algorithms are trained using labeled input and output data, and ‘unsupervised learning’, in which the algorithm is not given labeled data and must find structure in the input data. In this study, three ML algorithms were used: PCA, FAMD, and RF.
PCA was chosen since it allows identifying relationships among numerical variables, and in our study, we have several variables of this type, like age, BW, and the levels of several drugs. PCA has several advantages since it is easy to compute method, it can handle large amount of data (high-dimensional), and can speed up other ML techniques. Although PCA is a rather powerful technique, it has the limitation of being able to analyze only numerical characteristics. For this reason, FAMD was further used since it allows the concomitant investigation of numerical, ordinal, and categorical data. Thus, the use of FAMD allowed us to further validate the PCA findings and extend them using additional variables like gender, epileptic status, etc. The third approach, RF, was selected because it can be used as a classification technique, using both numerical and dummy variables. Thus, RF allows finding predictive models for a child’s having seizures or not. Also, RF modeling allows quantifying the contribution of all other characteristics (e.g. drug levels, gender, age, BW) on the response variable, which in our case was the existence of seizures or not. Thus, the use of these three ML methods gave us the ability to exploit as much as possible of the information offered by the collected dataset. The entire ML work was done by writing the appropriate codes in Python v. 3.10.8.
PCA
PCA is a statistical process for summarizing the information content of huge data tables using a smaller collection of ‘summary indices’ that may be more easily viewed and examined. PCA seeks lines, planes, and hyperplanes in K-dimensional space that best reflect the data in terms of least squares. A least squares approximation of a set of data points is a line or plane that maximizes the variance of the coordinates on the line or plane. The dataset is ready for computation of the first summary index, the first principal component (PC), after mean-centering and scaling to unit variance. In K-dimensional variable space, the second PC is represented by an orthogonal line to the first PC. This line also crosses through the average point, improving the X-data approximation as much as feasible. The coordinate values of the observations on this plane are referred to as scores, and so visualizing such a projected configuration is referred to as a score plot. The primary component loadings express the orientation of the model plane in the K-dimensional variable space geometrically. The cosine of the angles a1, a2, and a3 gives the direction of PC1 in reference to the original variables. These numbers represent how the initial variables x1, x2, and x3 ‘load’ (or contribute to) PC1. As a result, they are referred to as loadings. The ‘biplot’ is the standard method for examining the loadings and scores together.
The choice of the number of components was based on a scree plot. The primary goal of a scree plot is to illustrate the results of the component analysis and to locate the apparent change in slope (the elbow). In a scatter plot, the eigenvalue is shown against the PCs. The proportion of variance explained by a component is calculated by dividing its eigenvalue by the total of eigenvalues. The first component typically explains a significant percentage of the variability, the successive components explain a moderate portion, and the final components explain only a small portion of the total variability. The adequacy of a PCA model is reflected in the total variability explained by the finally selected principal components as well as from a visual inspection of the biplot.
The PCA analysis in this study was performed with Python v.3.10.8. To create statistical graphs, the package ‘matplotlib’ was utilized, and the PCA analysis was implemented using the libraries ‘seaborn’, ‘sklearn’, and ‘bioinfokit’.
FAMD
FAMD is a technique for analyzing numerical and categorical variables that combines PCA with multiple correspondence analyses (MCA). The utilization of both quantitative and qualitative variables is referred to as ‘mixed’. FAMD functions roughly as a PCA analysis for quantitative variables and as an MCA for qualitative variables. FAMD analyzes data using a combination of PCA and MCA algorithms. FAMD produces a lower-dimensional space by taking into account both continuous and categorical data. The results can be summarized in a biplot, like in PCA, as previously described. The validation of an FAMD model is performed similar to that of PCA models, namely, through biplot observation, the construction of a scree plot, and the assessment of the total variability explained by the selected principal components.
The FAMD code was written in Python v.3.10.8 using, among others, the libraries ‘pandas’, ‘numpy’, ‘sklearn’, ‘matplotlib’, ‘kneed’, and ‘tqdm’.
Random forest
A RF is made up of a huge number of independent decision trees that work together as an ensemble. Each individual tree in the RF produces a class prediction, and the class with the most votes becomes the prediction of our model. It is a usual practice to partition the original dataset into two pieces, ‘train’ and ‘test’, to make the model more robust. The training dataset is used to train the model, and the test dataset is used to assess the model’s performance. The confusion matrix can be used to evaluate the performance of a model in a classification problem (i.e. where the target variable is categorical). When using RF, it is also possible to examine the feature importance to see how each feature contributes to the prediction of the target variable. In this study, the RF analysis was applied to predict epileptic seizures based on the remaining characteristics of the children, such as age, BW, and drug levels. The response variable was set to either 0 (no seizures) or 1 (at least one epileptic seizure during the observation period).
Before training, the hyper-parameters were established for RF implementation. In general, hyper-parameter tuning was done through trial and error. The number of decision trees in the forest and the number of classes into which the response variable was classified are examples of hyper-parameters. The Gini impurity was the default criterion (loss function) used to assess model classification. It should be noted that numerous settings were tested, but the ones that were ultimately selected are those listed above. The dataset was split into training and test sets, with the latter accounting for 33% of the data (the remaining 67% being from the training set). Following the split, the model was trained on the training set before making predictions on the test set. The RF code was written in Python v.3.10.8 using the libraries ‘sklearn’, ‘matplotlib’, ‘kneed’, ‘tqdm’, ‘math’, and ‘seaborn’, among others.
Results
During the investigation period, we did not record exclusions from the study because there were no signs of serious side effects. Absolute numbers are used to represent descriptive data, demographic information, and pharmacotherapy characteristics of respondents: mean values, standard deviations, center (median) values, and interquartile difference (Table 1). The median age was 10.9 years (interquartile range, 11 years), with girls accounting for 56.3% (N = 40) and boys contributing for the remaining 43.7% (N = 31). The median BW was 37.1 kg (interquartile range: 21 kg). Table 1 also shows the pharmacotherapy characteristics of the children participating in the study. All patients were on dual antiepileptic therapy, which included the following therapeutic regimens and modalities: VA/LTG, VA/LEV, and LTG/LEV. A total of 59.1% of children received the combination of VA and LTG (45.2% boys and 54.8% girls). The therapeutic regimen of VA/LEV was administered to 28.2% of children (40.0% boys and 60.0% girls). Finally, 12.7% of children received the LTG/LEV combination (44.4% boys and 55.6% girls). In the conducted study, it was observed that 93.55% VA, 86.27% LTG, and 68.97% LEV measured concentrations during combined antiepileptic therapy were in the reference range. The highest number of concentrations below the reference range was recorded in the group of patients who used LEV as a part of combined therapy, 27.59% (Supplemental Figure S1). The factors that influence the pharmacokinetic variability of the selected AEDs and are thought to contribute to concentrations outside the therapeutic range were also studied.
Table 1.
Demographic, pharmacotherapy characteristics, and epileptic condition of the children participating in the study.
| A. Demographics | |||||
|---|---|---|---|---|---|
| Characteristic | Value | ||||
| Gender | |||||
| Boys | 31 (43.7%) | ||||
| Girls | 40 (56.3%) | ||||
| Age (years) | |||||
| Mean | 10.9 | ||||
| Median | 11 | ||||
| Interquartile range | 7 | ||||
| Body weight (kg) | |||||
| Mean | 37.1 | ||||
| Median | 35 | ||||
| Interquartile range | 21 | ||||
| B. Pharmacotherapy and epileptic condition | |||||
| Therapeutic regimen | Number (%) | Male (%) | Female (%) | Without epileptic seizure | With epileptic seizure |
| VA/LTG | 42 (59.16) | 19 (45.24) | 23 (54.76) | 37 (88.1) | 5 (11.9) |
| VA/LEV | 20 (28.17) | 8 (40) | 12 (60) | 14 (70) | 6 (30) |
| LTG/LEV | 9 (12.67) | 4 (44.44) | 5 (55.56) | 5 (55.6) | 4 (44.4) |
LEV, levetiracetam; LTG, lamotrigine; VA, valproic acid.
Population pharmacokinetics
The pharmacokinetics of LEV, performed in Monolix™ 2021R2, was best explained by a one-compartment model with first-order oral absorption and elimination. Table 2 displays the pharmacokinetic parameter estimates, as well as their RSE (%) for each parameter in the final best model. Given that the collected LEV steady-state trough plasma concentration data did not provide information about the extent and rate of absorption processes, the absorption rate (Ka) was fixed at 2.6 h−1 according to the values in the existing literature. The constant error model produced the best residual variability performance of any residual error model studied. During stepwise covariate modeling, two statistically significant (p > 0.001) covariates were identified: BW on V and Cl).
Table 2.
Population parameters of the final pharmacokinetic model of the three antiepileptic drugs of the study: (a) levetiracetam, (b) lamotrigine, and (c) valproic acid.
| A. Levetiracetam | ||||
|---|---|---|---|---|
| Parameter | SE | RSE (%) | p Value | |
| Fixed effects | ||||
| Ka | 2.6 | – | – | |
| V | 25.01 | 5.65 | 22.6 | |
| beta_V_logBW | 1 | – | – | <0.001 |
| Cl | 1.51 | 0.27 | 18.1 | |
| beta_Cl_logBW | 0.75 | – | – | <0.001 |
| Between-subject variabilities | ||||
| omega_V | 0.84 | 0.16 | 19.1 | |
| omega_Cl | 0.59 | 0.10 | 17.2 | |
| Correlations | ||||
| corr_V_Cl | 0.86 | 0.18 | 21.4 | |
| Residual error parameters | ||||
| a | 3.82 | 0.96 | 25.0 | |
| B. Lamotrigine | ||||
| Parameter | SE | RSE (%) | p-value | |
| Fixed effects | ||||
| Ka | 1.57 | – | – | |
| V | 5.15 | 1.18 | 22.9 | |
| beta_V_logBW | 2.83 | 0.52 | 18.4 | <0.001 |
| Cl | 0.15 | 0.02 | 13.3 | |
| beta_Cl_DailyDose | 0.0056 | 0.0007 | 13.0 | <0.001 |
| beta_Cl_Regimen | –0.61 | –0.13 | 22.2 | <0.001 |
| Between-subject variabilities | ||||
| omega_V | 0.32 | 0.07 | 23.4 | |
| omega_Cl | 0.28 | 0.07 | 22.7 | |
| Residual error parameters | ||||
| b | 0.15 | 0.03 | 19.8 | |
| C. Valproic acid | ||||
| Parameter | SE | RSE(%) | p-Value | |
| Fixed effects | ||||
| Ka | 1.68 | |||
| V | 15.61 | 4.84 | 31.0 | |
| beta_V_Age | 0.07 | 0.016 | 23.3 | 0.032 |
| beta_V_logBW | 1 | – | – | <0.001 |
| Cl | 0.12 | 0.013 | 10.4 | |
| beta_Cl_VA | 0.0012 | 0.0001 | 9.7 | <0.001 |
| Between-subject variabilities | ||||
| omega_V | 0.33 | 0.081 | 24.5 | |
| omega_Cl | 0.089 | 0.024 | 26.7 | |
| Residual error parameters | ||||
| b | 0.14 | 0.037 | 27.1 | |
a, the constant component of the residual error model; b, the proportional component of the residual error model; beta_Cl_DailyDose, factor for the relationship between Cl and lamotrigine daily dose; beta_Cl_logBW, allometric exponent for the relationship between body weight and Cl; beta_Cl_Regimen, factor for the relationship between Cl and therapeutic regimen (whether existence of valproic acid); beta_V_Age, factor for the relationship between age and volume of distribution; beta_V_logBW, allometric exponent for the relationship between body weight and V; BW, body weight; Cl, clearance; corr_V_Cl, correlation coefficient between V and Cl; F, bioavailability fraction; omega_Cl, between-subject variability for Cl; omega_V, between-subject variability for V; V, volume of distribution.
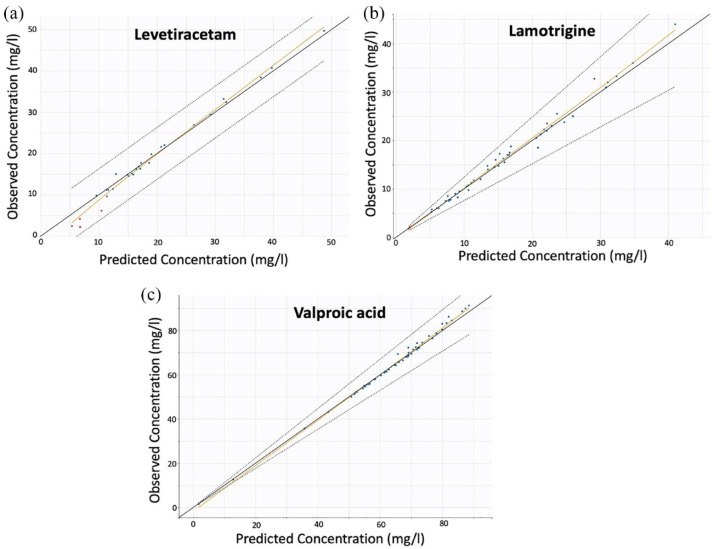
The accuracy and reliability of the parameter estimates were reflected in the RSE (%) values (Table 2). The predictive performance and robustness of the model were confirmed by the relevant predicted versus observed concentration plot (Figure 1(a)) and the individual model predictions overlaid with the observations (Figure 2(a)).
Figure 1.
Individual predicted versus observed concentration data for the models of the three drugs: (a) levetiracetam, (b) lamotrigine, and (c) valproic acid.
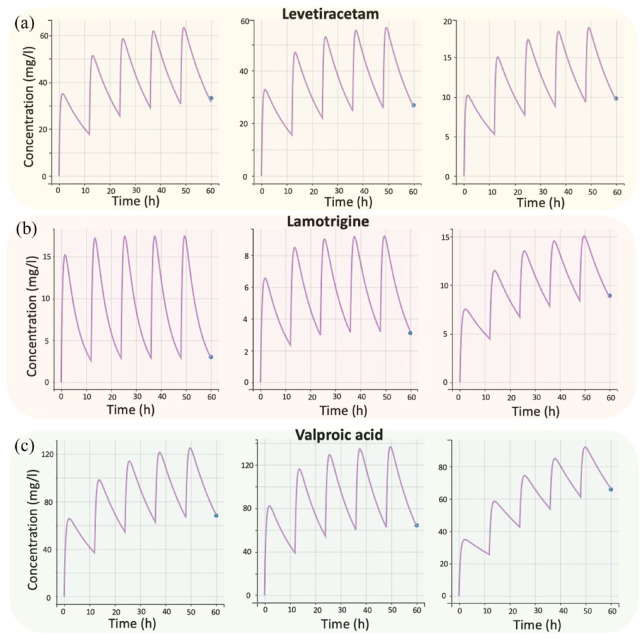
Figure 2.
Individual fittings to the experimental concentration–time data of the three drugs: (a) levetiracetam, (b) lamotrigine, and (c) valproic acid. Due to space limitations, three random subjects were chosen for each drug.
Figure 1 shows the individual predicted versus observed concentration data for the final models of the LEV, LTG, and VA.
In this study, there was only one sampling point for each person’s steady-state trough plasma concentration. This meant that five doses had to be considered before the last dose reached steady state and is shown in the graph. Figure 2 shows the individual fittings to the experimental concentration–time data of the three analyzed AEDs.
A one-compartment model with first-order absorption and elimination kinetics best characterized the kinetics of LTG (Table 2). Given that the gathered LTG steady-state trough plasma concentration data did not give information regarding the extent and rate of absorption processes, the absorption rate was set at 1.57 h−1 based on literature values. The residual variability was estimated using a proportional error model. Three statistically significant (p < 0.001) covariates were identified during the stepwise covariate modeling: BW on apparent V (using a weight-centered individual model), daily dose on apparent Cl, and coadministration of LTG and VA on Cl (denoted as ‘Regimen’ in Table 2). The relevant predicted versus observed concentration plot (Figure 1(b)) and the individual model predictions overlaid with the observations proved the model’s predictive ability and resilience (Figure 2(b)). Figure 1(b) shows that there are no outliers and that the distribution of the observations is relatively symmetrical around the appropriate projected values. The indicative fits (Figure 2(b)) from randomly selected people demonstrate the model’s capacity to effectively anticipate LTG trough levels. As in the case of LEV, since there was only one sampling point for each child’s steady-state trough plasma levels, five LTG doses were simulated before the last dose achieved steady state and is depicted in the graph.
A one-compartment model with first-order absorption and elimination kinetics best characterized the VA kinetics (Table 2). Given that the VA steady-state trough plasma concentration data collected did not provide information about the amount and pace of absorption processes, Ka was set at 1.68 h−1 based on the literature Ka values. The residual variability was estimated using a proportional error model. There were three statistically significant covariates found. BW on apparent V (with an allometric exponent of 0.75; p < 0.001) and daily dose on Cl (p < 0.001) were the most influential factors. Age was also found to have a significant positive impact on distribution V (p-value = 0.032). The predictive performance of the model, as well as its robustness, was validated by the relevant plot of predicted concentration versus observed concentration (Figure 1(c)), as well as by the individual model predictions that were superimposed with the observations (Figure 2(c)).
Machine learning
PCA
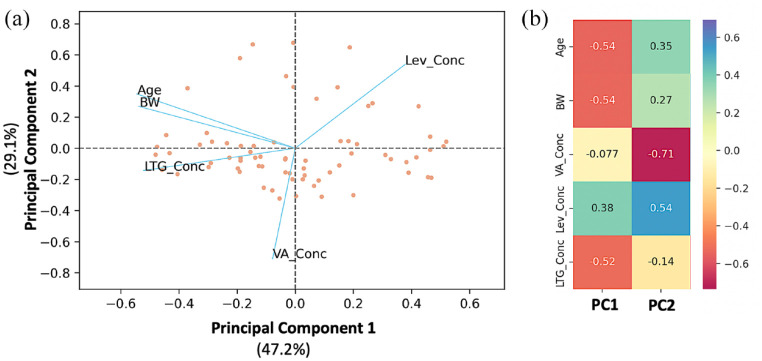
PCA was carried out to extract information about the children (such as their ages and BWs) and investigate how these factors are related to LEV, LTG, and VA levels (Figure 3).
Figure 3.
PCA of the plasma levels of the three antiepileptic drugs, age, and BW. (a) Biplot of the two PC showing the individual scores and the loadings (blue lines) of the pharmacokinetic parameters. (b) Loading values for the two initial PCs.
Lev_Conc, levetiracetam concentration; LTG_Conc, lamotrigine concentration; PC, principal components; PCA, principal component analysis; VA_Conc, valproic acid concentration.
In the plane formed by the two PCs, the observations (study participants) are represented as dots, while the lines represent the vectors of the variables. These variables include age, BW, levetiracetam concentration (Lev_Conc), lamotrigine concentration (LTG_Conc), and valproic acid concentration (VA_Conc). Scree plots were developed in order to determine the most appropriate number of primary components (plots are not shown due to space limitations). The first two PCs account for 76.3% of the total variance in the data, with the first component explaining 47.2% and the second component accounting for 29.1%, respectively. An examination of Figure 3 reveals that age and BW are located in close proximity to one another on the left side of the plot, not far from the second PC. While the vector representing LEV concentration is located in the upper right-hand corner of the plane, the vector representing VA concentration is somewhat near the negative side of the second PC. The only distinction is that the vector of LTG concentration.
These findings indicate that LEV concentration is negatively affected by age and BW, a finding that is in agreement with those from the PopPK. It is observed in Table 2 that the apparent V and Cl increase with BW. Thus, increases in BW would lead to higher V and Cl values, which, in turn, result in lower LEV values. This conclusion also derives from the PCA analysis (Figure 3), where the BW and LEV concentrations lie on opposite sides of the plot in terms of the first component. The loadings for BW and Lev_Conc are −0.54 and 0.38, respectively. Since age is very closely related to BW, the same effect on LEV levels is also expected with age.
For LTG, the PCA findings are in line with those derived from the PopPK. BW and LTG_Conc were found to lie on different parts of the scale with respect to the second PC (Figure 3), implying a negative contribution of BW to LTG levels. The finding that therapeutic regimen influences LTG Cl can also be depicted from the PCA, since a slight positive relationship can be seen between VA and LTG levels, while the LEV concentrations seem to negatively affect LTG levels. In the case of VA, the negative value of ‘beta_V_Age’ (i.e. equal to −0.07, Table 2) can be observed as the placement of the VA concentration and age vectors in opposite directions in terms of the second PC.
It should be noted that PCA was also applied to a dataset where the daily doses of the three drugs were included as variables. The results of this PCA analysis are shown in Supplemental Figure S2. Other characteristics, like the daily dose of each drug, could not be assessed using ML techniques due to the limited sample size.
FAMD
Figure 4 presents the results after applying FAMD which allowed us to verify the findings outlined above in the case of Figure 3 and allowed us to also explore the impact of other characteristics, like the occurrence of an epileptic seizure and gender.
Figure 4.
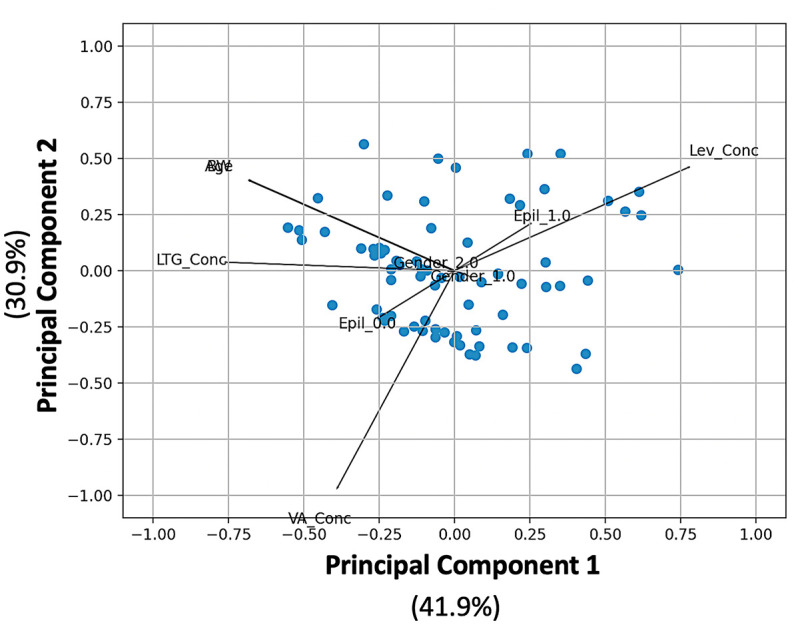
FAMD for the plasma levels of the three antiepileptic drugs, age, BW, existence of epileptic seizures, and gender.
BW, body weight; Epil_0.0, no seizures; Epil_1.0, seizures; FAMD, factor analysis of mixed data; Gender_1.0, boy; Gender_2.0, girl; Lev_Conc, levetiracetam concentration; LTG_Conc, lamotrigine concentration; VA_Conc, valproic acid concentration.
Figure 4 depicts the completed FAMD model’s biplot, score, and loading plot. The total percentage of the variability described by the first two main components was 72.8% (41.9% for the first and 30.9% for the second). A visual examination of the FAMD data reveals that the common features are compatible with PCA (see loading matrix results in Supplemental Table S1). Age and BW, for example, are directly opposite each other on the left side of the figure (Supplemental Table S1). The arrangement of the LEV, LTG, and VA vectors is very similar to that observed in the PCA. The loading scores for the two gender values (i.e. either boy or girl) are both very close to zero (0.053 for boys and −0.053 for girls), implying that gender has minimal or no influence on the blood levels of any of the three drugs. In the same context, the FAMD plot (Figure 4) reveals that there is no association between gender and epileptic activity. Besides, the fact that the LEV concentration and the epileptic activity (Epil_1.0) vectors are heading in the same direction may imply that higher LEV concentrations are associated with epileptic activity. Also, the FAMD plot shows that high levels of LTG and VA are more likely to be linked to low epileptic activity.
Random forest
The RF technique was used to identify the predictors of epileptic seizures in children on antiepileptic therapy. To prevent bias during the creation of the RF model, it was important to apply RF to datasets that were balanced in terms of the number of children who had and did not have epileptic seizures. Thus, a randomly chosen subset of 30 children was selected, consisting of 15 children with epileptic seizures and 15 without. The mean age of the participants in this dataset was 10.1 years, and the mean BW was 33.7 kg. The ratio of girls to boys was 9:6 for both groups.
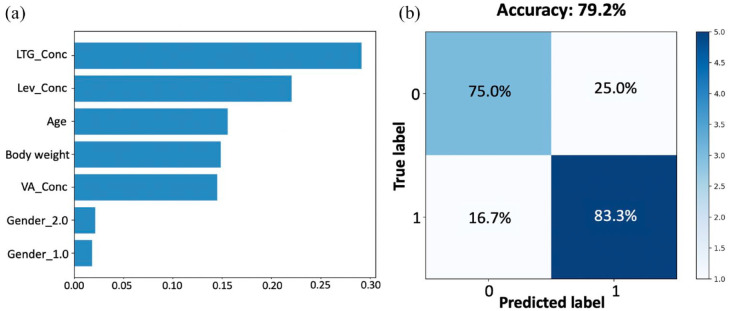
Figure 5(a) shows the importance of the variables included in the analysis, ordered from highest to lowest contribution to the occurrence of an epileptic seizure. The highest contribution comes from LTG levels (29.1%), followed by LEV concentration (22.0%). Age, BW, and VA levels all played a smaller role in the epileptic condition, accounting for 15.5%, 14.8%, and 14.5%, respectively. Gender appeared to have no effect on the response since the contribution of either being a girl (Gender_2.0) or boy (Gender_1.0) was in the range of 1–2%. A confusion matrix was designed to express how many of a RF classifier’s predictions were correct and when they were incorrect (i.e. when the RF classifier became ‘confused’; Figure 5(b)). The overall accuracy of the RF model was 80.0% (75.0% for true negatives and 83.3% for true positives).
Figure 5.
Variable importance scores (a) and confusion matrix (b), from the RF analysis, predicting the existence or not of epileptic activity. The features parameters were levetiracetam concentration (Lev_Conc), valproic acid concentration (VA_Conc), lamotrigine concentration (LTG_Conc), gender (1.0 for boys, 2.0 or girls), and BW.
The classification performance of the RF model was also assessed. Based on the classification values shown in Figure 5, the performance criteria were as follows: sensitivity (75.0%), specificity (83.3%), precision (81.8%), negative predictive value (76.9%), and model accuracy (79.2%).
Discussion
From a clinical standpoint, establishing an adequate therapeutic regimen capable of achieving the fundamental goals of epilepsy treatment, as reflected in optimal control and reduction of the number of seizures, with the least risk of adverse effects,11–13 is a real challenge. While there are no specific rules or criteria for selecting AEDs for combination therapy, there is a need for a tool to assist physicians in clinical practice.
The choice of combined therapy applied in the conducted research is in accordance with the literature data and presented by the following therapeutic modalities: VA/LTG (59.16%), VA/LEV (28.17%), and LTG/LEV (12.67%; Table 1).13,14
The TDM of AEDs is an important step in the cascade of decisions related to the optimization of epilepsy therapy, relying on the significant pharmacokinetic variability of these drugs, which is particularly pronounced in the pediatric population. 15 We found that 93.55% of the VA, 86.27% of the LTG, and 68.97% of the LEV measured concentrations were within the reference range (administered doses of VA, LTG, and LEV were in accordance with leading recommendations, with no seizure recorded during the study) (Supplemental Figure S1). 16 More than 30% of LEV concentrations were outside the therapeutic range (12–46 mg/L), indicating the importance of clinical pharmacokinetic considerations. 17 The most prominent result, in terms of concentrations that are within the reference range, was recorded for VA (93.55%), which was the most commonly used antiepileptic drug, and TDM performed routinely, while clinical practice did not recommend routine monitoring for LTG and LEV.
Results from the PopPK model for LEV obtained during stepwise covariate modeling showed BW as statistically significant on V and Cl (Table 2). Previously published PopPK modeling results for LEV in the pediatric population emphasized BW as an important covariate, which is in accordance with our findings (Supplemental Table S2).18–20 According to this, our results support the use of a weight-based LEV dosing regimen and provide a basis for a recommended pediatric dosage regimen. Toublanc et al. findings confirmed BW, dosage regimen, and AED inductors, but they also highlighted age. 19 They showed that children under 10 years have a 30–40% higher Cl, therefore requiring higher doses to achieve optimal concentrations. Their results indicated a lower effect of BW on CL than on V, resulting in a longer half-life among patients with greater BW. 19 This can probably be explained by the natural maturation of the renal function during childhood. 20 Johannessen Landmark et al. found that age and comedication had an effect on AEDs. 21 The impact of VA and LTG coadministration on LEV levels was further investigated; however, no statistically significant findings were observed.
For LTG published PopPK models in the pediatric population confirmed the influence of VA on LTG pharmacokinetic variability (Supplemental Table S3).22–25 In our model, the covariates for Cl were BW and VA that emphasize an individual approach to patients on combined AEDs therapy. The pharmacokinetics of LTG is complex and varies significantly among individuals, and they can be affected by various factors, including age, body BW, concomitant medication, or genetic variations. 26 Lamotrigine pharmacokinetics in children is different from adults due to the lower expression and activity of UDP-glucuronosyl transferase (UGT) and developmental growth. 23 Furthermore, coadministration of AEDs influences LTG pharmacokinetics variability in children also (VA inhibits UGT). Our results are consistent with results that showed covariates related to LTG Cl were BW and coadministration of inhibitors.25,27 A significant effect of VA on lamotrigine clearance was identified (see Table 2). It was found that the coadministration of VA, which is an inhibitor, decreases LTG clearance. The latter is in line with literature findings showing that VA inhibits LTG clearance. 27 Patients who have undergone LTG/VA treatment with LTG concentrations above the therapeutic range (according to guidelines, the reference range is 3–15 mg/ml) require additional clinical monitoring with a focus on the occurrence of side effects and subsequent reduction of LTG dose. 28
Similarly, we found BW and age as covariates in the population V model for VA (Table 2). Any influence from the coadministration of the other two drugs, LEV and LTG, was not found to be statistically significant. Gender, carbamazepine dosage, BW, UGT, and cytochrome P450 genotype are all factors that have been studied. 22 Considering the large number of data available for model development, special attention needs to be paid to pharmacokinetic changes according to age-related physiological maturation in pediatric patients.29–31 In fact, the parameter estimates for the first-order absorption rate constant, apparent Cl, and V were very close to those reported in other PopPK studies, such as those performed for LEV, LTG, VA (Supplemental Tables S2–S4). The BW has been well known as an important covariate in drug dosing regimens for pediatric patients. Ding et al. imply that both age and BW determine drug management simultaneously in children younger than 2 years, whereas BW is the most important factor in older children. 29
It should be mentioned that all population pharmacokinetic models (for LEV, LTG, and VA) were found to adequately describe the experimental data (Supplemental Figures 1 and 2). Additional validation plots are shown in Supplemental Figures S3–S5. Also, the combination use of all three drugs (LEV, LTG, and VA) was explored in this study by incorporating as a covariate the type of regimen (e.g. monotherapy, two-drug therapy, or three-drug therapy). However, only in the case of LTG, a significant effect of VA on clearance was observed.
In epilepsy, ML has been used for seizure detection, detection of epileptogenic lesions, differentiating epileptic seizures, and outcome prediction for medical and surgical management.32–36 In this study, three ML algorithms (PCA, FAMD, and RF) have been carefully evaluated to uncover any underlying relationships among the children characteristics and pharmacotherapy.
PCA was applied to identify possible associations among the model parameters.9,37 PCA results revealed that LTG levels were unaffected by VA concentration. A small negative association was observed between LEV and LTG or VA concentration values. Also, an increase in LTG concentrations was observed with age (Figure 3). Besides, there appears to be no association between VA levels and age. The opposite performance is observed for LEV, where an increase in age is accompanied by a decrease in LEV levels. Published results by Zhang et al. have proven that the efficacy of LEV on newly diagnosed patients with epilepsy could be predicted using a support vector machine model. 38
Aimed to possible influence of gender, concomitant medications, and epileptic activity, a factor analysis of mixed data was performed in this study. 39 Results of FAMD showed that gender has no influence on the levels of any of the three drugs and is not associated with epileptic activity (Figure 4). The epileptic activity vectors indicated that high LEV levels increase the chance of epileptic activity (3.45% of LEV concentrations were above the therapeutic range). This finding can seem contradictory at first, but it may refer to the situation where higher LEV doses are needed to control patients with non-responding epileptic seizures. Also, FAMD showed that high LTG and/or VA are more associated with low epileptic activity. There are a small number of studies that have used ML for LTG or AEDs combinations; yet, the results of our FAMD analysis may confirm the potential importance of this analytical approach to pediatric patients’ dose adjustment. 40
RF made it possible to rank the importance of the characteristics and made a ‘decision tree’ stronger to help with clinical procedures.37,41 The main factor affecting antiepileptic activity was high AEDs levels, followed by age, BW, and VA levels. It appears that LTG concentrations are independent of VA levels. Our results showed a small negative association between LEV levels and LTG or VA concentrations (Figure 5). According to our findings, therapeutic outcomes were not influenced by gender. Delen et al. 42 demonstrated promising results in a study aimed at predicting at-risk patients using ML techniques. Besides, future research is necessary and should include a larger number of patients, external validation, standardization of reporting, and a prospective evaluation of the ML model on patient outcomes.
This study has several limitations. Initially, the sample size is relatively small to allow identifying relationships among patients’ characteristics and the pharmacokinetic parameters. Possibly, additional influence from covariates could be identified if the sample size was larger. In addition, there was only a single measurement (trough levels) of the drug levels for each patient. As a result, there may be ambiguity about the nature of the observed variability, namely, whether it corresponds to inter-individual or intra-individual variability. If more measurement points were available, it would allow to describe more precisely and more easily the pharmacokinetic parameter estimates. Also, more sampling points and a larger sample size would possibly allow identifying the impact of more covariates on the pharmacokinetic estimates. However, in regular antiepileptic TDM, it is commonly assumed that the antiepileptic drug concentrations measured will be those at minimal steady-state, that is, trough concentrations. The latter becomes even more important in the case of children and adolescents, as in our study, where there are additional ethical and practical reasons prohibiting the research from having more rich sampling schedules.
In conclusion, the application of popPK and ML models may be useful to improve seizure control and risk management. Reliance on the RF model is a compelling vision that shows high prediction ability for all cases. The main factor that can affect antiepileptic activity in children is AED levels, followed by BW, while gender appears to be of minor importance. According to our study, children’s age is positively associated with LTG levels, negatively with LEV and without influence from VA. More prospective, multicenter studies with large sample sizes should be conducted to develop certain predictive models, that can be widely accepted in the field of combined AEDs treatment to improve epilepsy management in vulnerable pediatric populations.
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986231181337 for Joint use of population pharmacokinetics and machine learning for optimizing antiepileptic treatment in pediatric population by Ivana Damnjanović, Nastia Tsyplakova, Nikola Stefanović, Tatjana Tošić, Aleksandra Catić-Đorđević and Vangelis Karalis in Therapeutic Advances in Drug Safety
Acknowledgments
I.D., N.S., and A.C.D. were thankful to Ministry of Education, Science and Technological Development of Republic of Serbia (Grant No: 451-03-47/2023-01/200113), while T.T., N.T. and V.K. are not members of the mentioned project.
Footnotes
ORCID iD: Ivana Damnjanović  https://orcid.org/0000-0003-0079-8457
https://orcid.org/0000-0003-0079-8457
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ivana Damnjanović, Department of Pharmacy, Faculty of Medicine, University of Nis, Boulevard Dr Zoran Djindjic 81, Nis 18000, Serbia.
Nastia Tsyplakova, Department of Pharmacy, School of Health Sciences, National and Kapodistrian University of Athens, Athens, Greece.
Nikola Stefanović, Department of Pharmacy, Faculty of Medicine, University of Nis, Nis, Serbia.
Tatjana Tošić, Clinic of Pediatric Internal Medicine, Department of Pediatric Neurology, University Clinical Center of Nis, Nis, Serbia.
Aleksandra Catić-Đorđević, Department of Pharmacy, Faculty of Medicine, University of Nis, Nis, Serbia.
Vangelis Karalis, Department of Pharmacy, School of Health Sciences, National and Kapodistrian University of Athens, Athens, Greece.
Declarations
Ethics approval and consent to participate: All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocols were approved by the Ethics Committee of the Faculty of Medicine, University of Nis (Decision No. 12-3782/4). Data were collected after parents or guardians provided written informed consent. None of the data could be traced back to an identifiable patient.
Consent for publication: Not applicable.
Author contributions: Ivana Damnjanović: Conceptualization; Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Nastia Tsyplakova: Formal analysis; Methodology; Software; Writing – original draft, Writing – review & editing.
Nikola Stefanović: Formal analysis; Writing – review & editing.
Tatjana Tošić: Data curation; Investigation.
Aleksandra Catić-Đorđević: Conceptualization; Writing – original draft.
Vangelis Karalis: Conceptualization; Formal analysis; Methodology; Software; Writing – original draft, Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
References
- 1.Singh KD, Osswald M, Ziesenitz VC, et al. Personalised therapeutic management of epileptic patients guided by pathway-driven breath metabolomics. Commun Med 2021; 1: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebedev G, Fartushnyi E, Fartushnyi I, et al. Technology of supporting medical decision-making using evidence-based medicine and artificial intelligence. Procedia Comput Sci 2020; 176: 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atugonza R, Kakooza-Mwesige A, Lhatoo S, et al. Multiple anti-epileptic drug use in children with epilepsy in Mulago hospital, Uganda: a cross sectional study. BMC Pediatr 2016; 16: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliva CF, Gangi G, Marino S, et al. Single and in combination antiepileptic drug therapy in children with epilepsy: how to use it. AIMS Med Sci 2021; 8: 138–146. [Google Scholar]
- 5.Janssen A, Bennis FC, Mathôt RAA. Adoption of machine learning in pharmacometrics: an overview of recent implementations and their considerations. Pharmaceutics 2022; 14: 1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao L, Cai M, Chen Y, et al. Prediction of antiepileptic drug treatment outcomes of patients with newly diagnosed epilepsy by machine learning. Epilepsy behav 2019; 96: 92–97. [DOI] [PubMed] [Google Scholar]
- 7.Beam AL, Kohane IS. Big data and machine learning in health care. JAMA 2018; 319: 1317–1318. [DOI] [PubMed] [Google Scholar]
- 8.Zheng P, Yu Z, Mo L, et al. An individualized medication model of sodium valproate for patients with bipolar disorder based on machine learning and deep learning techniques. Front Pharmacol 2022; 13: 890221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karatza E, Markantonis SL, Savvidou A, et al. Pharmacokinetic and pharmacodynamic modeling of levetiracetam: investigation of factors affecting the clinical outcome. Xenobiotica 2020; 50: 1090–1100. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Zhang M, Wen Y, et al. Machine learning advances the integration of covariates in population pharmacokinetic models: valproic acid as an example. Front Pharmacol 2022; 13: 994665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Málaga I, Sánchez-Carpintero R, Roldán S, et al. New anti-epileptic drugs in paediatrics. An Pediatr 2019; 91: 415.e1–415.e10. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Kim DW, Lee ST, et al. Antiepileptic drug selection according to seizure type in adult patients with epilepsy. J Clin Neurol 2020; 16: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pipek LZ, Pipek HZ, Castro LHM. Seizure control in mono- and combination therapy in a cohort of patients with idiopathic generalized epilepsy. Sci Rep 2022; 12: 12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verrotti A, Tambucci R, Di Francesco L, et al. The role of polytherapy in the management of epilepsy: suggestions for rational antiepileptic drug selection. Expert Rev Neurother 2020; 20: 167–173. [DOI] [PubMed] [Google Scholar]
- 15.Johannessen Landmark C, Johannessen SI, Patsalos PN. Therapeutic drug monitoring of antiepileptic drugs: current status and future prospects. Expert Opin Drug MetabToxicol2020 2020; 16: 227–238. [DOI] [PubMed] [Google Scholar]
- 16.Patsalos PN, Spencer EP, Berry DJ. Therapeutic drug monitoring of antiepileptic drugs in Epilepsy: a 2018 Update. Ther Drug Monit 2018; 40: 526–548. [DOI] [PubMed] [Google Scholar]
- 17.Sinha J, Karatza E, Gonzalez D. Physiologically-based pharmacokinetic modeling of oxcarbazepine and levetiracetam during adjunctive antiepileptic therapy in children and adolescents. CPT Pharmacometr Syst Pharmacol 2021; 11: 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito S, Yano I, Hashi S, et al. Population pharmacokinetic modeling of levetiracetam in pediatric and adult patients with epilepsy by using routinely monitored data. Ther Drug Monit 2016; 38: 371–378. [DOI] [PubMed] [Google Scholar]
- 19.Toublanc N, Sargentini-Maier ML, Lacroix B, et al. Retrospective population pharmacokinetic analysis of levetiracetam in children and adolescents with epilepsy. Clin Pharmacokinet 2008; 47: 333–341. [DOI] [PubMed] [Google Scholar]
- 20.Chhun S, Jullien V, Rey E, et al. Population pharmacokinetics of levetiracetam and dosing recommendation in children with epilepsy. Epilepsia 2009; 50: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 21.Johannessen Landmark C, Baftiu A, Tysse I, et al. Pharmacokinetic variability of four newer antiepileptic drugs, lamotrigine, levetiracetam, oxcarbazepine, and topiramate: a comparison of the impact of age and comedication. Ther Drug Monit 2012; 34: 440–445. [DOI] [PubMed] [Google Scholar]
- 22.Tauzin M, Tréluyer JM, Nabbout R, et al. Dosing recommendations for lamotrigine in children: evaluation based on previous and new population pharmacokinetic models. J Clin Pharmacol 2021; 61: 677–687. [DOI] [PubMed] [Google Scholar]
- 23.Xu S, Liu L, Chen Y, et al. Population pharmacokinetics of lamotrigine co-administered with valproic acid in Chinese epileptic children using nonlinear mixed effects modeling. Eur J Clin Pharmacol 2018; 74: 583–591. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZB, Ji SM, Han Y, et al. Population pharmacokinetic models of lamotrigine in different age groups of Chinese children with epilepsy. Eur J Clin Pharmacol 2017; 73: 445–453. [DOI] [PubMed] [Google Scholar]
- 25.Brzaković B, Vućićević K, Kovaćević SV, et al. Pharmacokinetics of lamotrigine in paediatric and young adult epileptic patients—nonlinear mixed effects modelling approach. Eur J Clin Pharmacol 2014; 70: 179–185. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Xu S, Wang Z, et al. A population pharmacokinetic-pharmacogenetic model of lamotrigine in Chinese children with epilepsy. Ther Drug Monit 2018; 40: 730–737. [DOI] [PubMed] [Google Scholar]
- 27.Inoue K, Yamamoto Y, Suzuki E, et al. Factors that influence the pharmacokinetics of lamotrigine in Japanese patients with epilepsy. Eur J Clin Pharmacol 2016; 72: 555–562. [DOI] [PubMed] [Google Scholar]
- 28.Reimers A, Berg JA, Larsen Burns M, et al. Reference ranges for antiepileptic drugs revisited: a practical approach to establish national guidelines. Drug Des Dev Ther 2018; 12: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding J, Wang Y, Lin W, et al. A population pharmacokinetic model of valproic acid in pediatric patients with epilepsy: a non-linear pharmacokinetic model based on protein-binding saturation. Clin Pharmacokinet 2015; 54: 305–317. [DOI] [PubMed] [Google Scholar]
- 30.Mei S, Feng W, Zhu L, et al. Effect of CYP2C19, UGT1A8, and UGT2B7 on valproic acid clearance in children with epilepsy: a population pharmacokinetic model. Eur J Clin Pharmacol 2018; 74: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 31.Williams JH, Jayaraman B, Swoboda KJ, et al. Population pharmacokinetics of valproic acid in pediatric patients with epilepsy: considerations for dosing spinal muscular atrophy patients. J Clin Pharmacol 2012; 52: 1676–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smolyansky ED, Hakeem H, Ge Z, et al. Machine learning models for decision support in epilepsy management: a critical review. Epilepsy Behav 2021; 123: 108273. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui MK, Morales-Menendez R, Huang X, et al. A review of epileptic seizure detection using machine learning classifiers. Brain Inform 2020; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin B, Krishnan B, Adler S, et al. Automated detection of focal cortical dysplasia type II with surface-based magnetic resonance imaging postprocessing and machine learning. Epilepsia 2018; 59: 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zsom A, LaFrance W, Jr, Blum A, et al. Ictal autonomic activity recorded via wearable-sensors plus machine learning can discriminate epileptic and psychogenic nonepileptic seizures. Annu Int Conf IEEE Eng Med Biol Soc 2019; 3502–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbasi B, Goldenholz DM. Machine learning applications in epilepsy. Epilepsia 2019; 60: 2037–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karalis VD. Machine learning in Bioequivalence: towards identifying an appropriate measure of absorption rate. Appl Sci 2022; 13: 418. [Google Scholar]
- 38.Zhang J, Han X, Zhao H, et al. Personalized prediction model for seizure-free epilepsy with levetiracetam therapy: a retrospective data analysis using support vector machine. Br J Clin Pharmacol 2018; 84: 2615–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James G, Hastie T, Tibshirani R, et al. An Introduction to statistical learning with applications in R. 7th ed.Berlin/Heidelberg, Germany: Springer, 2017. [Google Scholar]
- 40.Zhu X, Huang W, Lu H, et al. A machine learning approach to personalized dose adjustment of lamotrigine using noninvasive clinical parameters. Sci Rep 2021; 11: 5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashton JJ, Young A, Johnson MJ, et al. Using machine learning to impact on long-term clinical care: principles, challenges, and practicalities. Pediatr Res 2023; 93: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delen D, Davazdahemami B, Eryarsoy E, et al. Using predictive analytics to identify drug-resistant epilepsy patients. Health Inform J 2020; 26: 449–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986231181337 for Joint use of population pharmacokinetics and machine learning for optimizing antiepileptic treatment in pediatric population by Ivana Damnjanović, Nastia Tsyplakova, Nikola Stefanović, Tatjana Tošić, Aleksandra Catić-Đorđević and Vangelis Karalis in Therapeutic Advances in Drug Safety