Abstract
Background:
Bevacizumab-awwb (MVASI®) was the first U.S. Food and Drug Administration-approved biosimilar to Avastin® (reference product [RP]) for the treatment of several different types of cancers, including metastatic colorectal cancer (mCRC), an indication approved based on extrapolation.
Objectives:
Evaluate treatment outcomes in mCRC patients who received first-line (1L) bevacizumab-awwb at treatment initiation or as continuing bevacizumab therapy (switched from RP).
Design:
A retrospective chart review study.
Methods:
Adult patients who had a confirmed diagnosis of mCRC (initial presentation of CRC on or after 01 January 2018) and initiated 1L bevacizumab-awwb between 19 July 2019 and 30 April 2020 were identified from the ConcertAI Oncology Dataset. A chart review was conducted to evaluate patient baseline clinical characteristics and effectiveness and tolerability outcomes during the follow-up. Study measures were reported stratified by prior use of RP: (1) naïve patients and (2) switchers (patients who switched to bevacizumab-awwb from RP without advancing the line of therapy).
Results:
At the end of study period, naïve patients (n = 129) had a median 1L progression-free survival (PFS) of 8.6 months [95% confidence interval (CI), 7.6–9.9] and a 12-month overall survival (OS) probability of 71.4% (95% CI, 61.0–79.5%). Switchers (n = 105) had a median 1L PFS of 14.1 months (95% CI, 12.1–15.8) and a 12-month OS probability of 87.6% (95% CI, 79.1–92.8%). During treatment with bevacizumab-awwb, 20 events of interest (EOIs) were reported in 18 naïve patients (14.0%) and 4 EOIs reported in 4 switchers (3.8%), of which the most commonly reported events were thromboembolic and hemorrhagic events. Most EOIs resulted in emergency department visit and/or treatment hold/discontinuation/switch. None of the EOIs resulted in death.
Conclusion:
In this real-world cohort of mCRC patients who were treated 1L with a bevacizumab biosimilar (bevacizumab-awwb), the clinical effectiveness and tolerability data were as expected and consistent with previously published findings from real-world studies of bevacizumab RP in mCRC patients.
Keywords: bevacizumab-awwb, biosimilar, bevacizumab, metastatic colorectal cancer, real-world outcomes
Introduction
Colorectal cancer (CRC) is a leading cause of tumor-related morbidity and mortality worldwide, and it accounts for 8.6% of all tumor-related mortality in the United States. 1 Approximately 20–25% of patients with CRC present with metastatic disease, 2 which is associated with poor prognosis, especially if left untreated or only treated with supportive care.3,4 Management of metastatic CRC (mCRC) has evolved significantly in the past decades with the introduction of targeted biologic therapies. Bevacizumab (Avastin®, Genentech, Inc., South San Francisco, CA, USA), a monoclonal antibody against vascular endothelial growth factor approved by the U.S. Food and Drug Administration (FDA) in 2004, is among these therapies; and it has been shown to significantly improve the survival of mCRC patients in combination with 5-fluorouracil-based chemotherapy.5–14
Biosimilars are biological products highly similar to the reference product (RP) in terms of safety, purity, and potency,15–17 and they have the potential to expand access to biologic medicines. 18 Biosimilarity between the biosimilar bevacizumab-awwb (MVASI®, Amgen Inc., Thousand Oaks, CA, USA) and bevacizumab RP (Avastin®) in terms of analytic structure and function, pharmacokinetics, and pharmacodynamics has been rigorously evaluated in nonclinical and clinical pharmacologic studies.19,20 A comparative clinical trial (MAPLE trial, ClinicalTrials.gov identifier: NCT01966003) 21 demonstrated similar efficacy, safety, and immunogenicity between biosimilar bevacizumab-awwb and the RP in patients with advanced non-small cell lung cancer (NSCLC). The totality of evidence generated for bevacizumab-awwb19,21 and common mechanism of action across indications provided the scientific justification for applying the principles of extrapolation from NSCLC to other tumor types and resulted in the FDA approval of bevacizumab-awwb for the same indications as bevacizumab RP. 20
Bevacizumab-awwb was first available for use in the United States in July 2019 and has offered more affordable treatment options for patients with certain types of cancers, including mCRC.22–24 Although the accelerated development program for biosimilars improves patient accessibility to biologic therapies, 25 lack of clinical trial data on indications approved on the basis of extrapolation and lack of familiarity with the extrapolation principle may pose challenges to biosimilar adoption among clinicians and patients.26,27 Real-world post-marketing studies can provide useful information to the medical community and patients to increase confidence in biosimilars. 28 We therefore designed this retrospective, observational study to evaluate the real-world experience of bevacizumab-awwb in patients with mCRC.
We previously presented data on a total of 304 patients with mCRC who received bevacizumab-awwb during the first year after its market entry in the United States. The initial descriptive analysis evaluated patient clinical characteristics and utilization patterns of bevacizumab-awwb, reporting that ~80% of patients received bevacizumab-awwb as first-line (1L) therapy either as initiating bevacizumab therapy or as continuing bevacizumab therapy (switched from the RP). 29 In the current analysis, we followed those 1L patients longitudinally and aimed to evaluate clinical outcomes (events of interest [EOIs] and survival outcomes) of patients treated with 1L bevacizumab-awwb.
Patients and methods
Study design and data collection
Patient data were collected from the ConcertAI Oncology Dataset, a consolidated electronic medical record (EMR) database of structured and unstructured clinical information, including patient histology, biomarker results, and healthcare provider notes (e.g. performance status and the date and type of disease progression). These data were derived from networks of geographically diverse, primarily community-based oncology practices in the United States that include multiple group purchasing organizations. As a result, practice patterns reflect real-world variability of treatment.29–32
A retrospective medical chart review was conducted to evaluate patient characteristics and treatment outcome data.29–32 Patient baseline characteristics were abstracted from unstructured EMRs by trained clinical research nurses and linked to patient data extracted from standard fields as described previously. 29 Treatment patterns of bevacizumab-awwb were collected, including durations of 1L bevacizumab-awwb use, prior treatment history of RP, and other systemic anticancer therapy (chemotherapy or immunotherapy). The occurrence and date of disease progression were determined from pathology reports, radiological scan notes, and/or statements about disease progression in oncologist progress notes.30,33 EOIs occurring during 1L bevacizumab-awwb containing treatment and their management approaches were also abstracted from patient EMRs.
This study received institutional review board approval from the ethics committee of IntegReview (Austin, TX, USA), now part of Advarra (Columbia, Maryland). Patient confidentiality was maintained throughout the study in accordance with regulations and standards for observational research. Informed consent from the patients for the inclusion of their medical history and treatment outcomes within this work was waived since this retrospective study included only review of existing data without contact with or direct involvement of patients in the study.
Study population
Adult patients (⩾18 years of age) who had a confirmed diagnosis of mCRC (with initial presentation of CRC on or after 1 January 2018) and initiated treatment with bevacizumab-awwb in 1L between 19 July 2019 and 30 April 2020 were included in the analysis. Patients with a known Eastern Cooperative Oncology Group (ECOG) performance status of ⩾3 at mCRC diagnosis were excluded. This analysis included: (1) patients without prior exposure to the RP from diagnosis of mCRC through initiation of 1L bevacizumab-awwb, hereafter referred to as naïve patients; and (2) patients who previously received 1L RP following mCRC diagnosis but switched to bevacizumab-awwb in the same line without disease progression, hereafter referred to as switchers.
Study measures and assessments
Patients were followed from the initiation of 1L therapy (bevacizumab-awwb or RP, systemic anticancer therapy, or combination of bevacizumab-awwb or RP with systemic anticancer therapy) until death, end of the medical record, or end of study period (June 2021), whichever occurred first. Progression-free survival (PFS) and overall survival (OS) were measured from the initiation of 1L therapy, which was defined as the earlier date of either bevacizumab or chemotherapy treatment. Specifically, PFS was defined as the duration from the initiation of 1L therapy to the occurrence of first recorded disease progression, death from any cause, or censoring (end of the medical record or end of study period), whichever occurred first. OS was defined as the duration from the initiation of 1L therapy to death from any cause or censoring (end of the medical record or end of study period), whichever occurred first.
Prespecified EOIs included gastrointestinal perforations, hemorrhages, hypertension, infusion reactions, ovarian failure, proteinuria, thromboembolic events, and wound-healing complications. Actions taken following the occurrence of an EOI were assessed categorically and included dose reduction, treatment hold, treatment discontinuation or switch, emergency department visit, hospitalization, and use of supportive care medications.
Statistical analysis
All results were summarized using descriptive statistics and reported separately for naïve patients and switchers. Patient characteristics and treatment patterns were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Missing data were not imputed and were reported as ‘undocumented’ or ‘unknown.’ 29 PFS and OS probability and time to event outcomes, along with their associated 95% confidence intervals (CIs), were analyzed using Kaplan-Meier curves. The incidence at which each EOI occurred was described at patient level according to the management action(s) for the EOI. More than one EOI could be reported for a patient and each EOI could result in more than one management action.
Results
Patient characteristics
A total of 129 naïve patients and 105 switchers, who were treated primarily at community-based practices in the United States, were included in this analysis. Majority of patients (>65%) were white. The proportion of males was 54% for naïve patients and 64% for switchers, and their mean age was 62 and 65 years, respectively. Overall, about 60% of patients presented with metastatic disease at the initial diagnosis of CRC. Over 70% of patients had colon as the primary affected site of initial disease, and the most common site of metastasis was the liver (65–69%). About 40% of patients were documented to undergo surgical resection of initial disease, and at the initiation of bevacizumab-awwb, most (>70%) had the ECOG performance status score of 0/1 (Table 1).
Table 1.
Demographic and clinical characteristics of patients with mCRC treated with 1L bevacizumab-awwb.
| Characteristic | Naïve patients (n = 129) | Switchers (n = 105) |
|---|---|---|
| Male, n (%) | 69 (53.5%) | 67 (63.8%) |
| Age a in years, mean (SD) | 62.0 (11.3) | 64.8 (10.7) |
| Race, n (%) | ||
| White | 86 (66.7%) | 69 (65.7%) |
| Black or African American | 23 (17.8%) | 17 (16.2%) |
| Other b | 10 (7.8%) | 13 (12.4%) |
| Undocumented | 10 (7.8%) | 6 (5.7%) |
| US region of residence, n (%) | ||
| Midwest | 10 (7.8%) | 13 (12.4%) |
| Northeast | 9 (7.0%) | 3 (2.9%) |
| South | 97 (75.2%) | 80 (76.2%) |
| West | 13 (10.1%) | 9 (8.6%) |
| Practice setting, n (%) | ||
| Academic | 28 (21.7%) | 6 (5.7%) |
| Community | 101 (78.3%) | 99 (94.3%) |
| Tumor stage at initial diagnosis, n (%) | ||
| Stage I/II | 10 (7.8%) | 18 (17.1%) |
| Stage III | 35 (27.1%) | 26 (24.8%) |
| Stage IV | 80 (62.0%) | 60 (57.1%) |
| Undocumented | 4 (3.1%) | 1 (<1%) |
| ECOG performance status, a n (%) | ||
| 0 | 50 (38.8%) | 35 (33.3%) |
| 1 | 44 (34.1%) | 40 (38.1%) |
| 2 | 8 (6.2%) | 7 (6.7%) |
| Undocumented | 27 (20.9%) | 23 (21.9%) |
| Adenocarcinoma at initial diagnosis, n (%) | 123 (95.3%) | 102 (97.1%) |
| Location of primary tumor at initial diagnosis, n (%) | ||
| Left-sided | 73 (56.6%) | 60 (57.1%) |
| Right-sided | 50 (38.8%) | 41 (39.0%) |
| Both-sided | 2 (1.6%) | 0 |
| Undocumented | 4 (3.1%) | 4 (3.8%) |
| Site of initial disease, n (%) | ||
| Colon | 98 (70.5%) | 78 (72.9%) |
| Rectum | 37 (28.7%) | 25 (23.4%) |
| Unknown | 4 (3.1%) | 4 (3.7%) |
| Site of metastatic disease, n (%) | ||
| Distant lymph node | 33 (25.6%) | 27 (25.7%) |
| Liver | 84 (65.1%) | 72 (68.6%) |
| Lung | 36 (27.9%) | 34 (32.4%) |
| Other c | 55 (42.6%) | 44 (41.9%) |
| Surgical resection of initial disease, n (%) | 50 (38.8%) | 44 (41.9%) |
At initiation of bevacizumab-awwb.
Other race included American Indian or Alaska Native, Hispanic, or Latino.
Other sites of metastatic disease included abdominal wall, bone, brain, peritoneum, right abdominal wall, small intestine, spleen, adnexal, adrenal, bladder, lesser curvature of the stomach, mediastinum, omentum, ovaries and fallopian tube and uterine cervix, paracolic fat, pelvic mass, pelvis, pleura, prostate and seminal vesicles, and small bowel.
1L, first-line; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group; mCRC, metastatic colorectal cancer; RP, reference product; SD, standard deviation.
Biomarker status was recorded in the medical chart for a subgroup of patients (Table 2). For both naïve patients and switchers, the majority of tested patients were neuroblastoma rat sarcoma (91.3% and 92.4%, respectively) and B-rapidly accelerated fibrosarcoma (84.4% and 89.2%, respectively) negative, microsatellite stable (92.5% and 91.3%, respectively), and mismatch repair proficient (91.8% and 97.3%, respectively); approximately half (46.7% and 55.6%, respectively) presented with a positive Kirsten rat sarcoma mutation.
Table 2.
Biomarker status of patients with mCRC treated with 1L bevacizumab-awwb.
| Biomarker a | Naïve patients (n = 129) | Switchers (n = 105) |
|---|---|---|
| KRAS test, n (%) | 105 (81.4%) | 81 (77.1%) |
| KRAS status among tested patients, n (%) | ||
| Positive | 49 (46.7%) | 45 (55.6%) |
| Negative | 53 (50.5%) | 36 (44.4%) |
| Other b | 3 (2.9%) | 0 |
| BRAF test, n (%) | 96 (74.4%) | 74 (70.5%) |
| BRAF status among tested patients, n (%) | ||
| Positive | 13 (13.5%) | 8 (10.8%) |
| Negative | 81 (84.4%) | 66 (89.2%) |
| Other b | 2 (2.1%) | 0 |
| NRAS test, n (%) | 92 (71.3%) | 66 (62.9%) |
| NRAS status among tested patients, n (%) | ||
| Positive | 6 (6.5%) | 4 (6.1%) |
| Negative | 84 (91.3%) | 61 (92.4%) |
| Other c | 2 (2.2%) | 1 (1.5%) |
| MSI test, n (%) | 93 (72.1%) | 69 (65.7%) |
| MSI status among tested patients, n (%) | ||
| Microsatellite instability – high (positive) | 3 (3.2%) | 2 (2.9%) |
| Microsatellite instability – stable (negative) | 86 (92.5%) | 63 (91.3%) |
| Other d | 4 (4.3%) | 4 (5.8%) |
| MMR test, n (%) | 96 (75.2%) | 74 (70.5%) |
| MMR status among tested patients, n (%) | ||
| MMR deficient | 7 (7.2%) | 2 (2.7%) |
| MMR proficient | 89 (91.8%) | 72 (97.3%) |
Biomarker results were reported using the medical value that was recorded closest to metastatic CRC diagnosis.
Uninterpretable test results.
Insufficient quality or equivocal test results.
Insufficient quality, equivocal, or undetermined/undocumented test results.
1L, first-line; BRAF, B-rapidly accelerated fibrosarcoma; KRAS, Kirsten rat sarcoma; mCRC, metastatic colorectal cancer; MMR, mismatch repair; MSI, microsatellite instability; NRAS, neuroblastoma rat sarcoma.
Treatment regimens and effectiveness
All naïve patients and 96.2% of switchers received bevacizumab-awwb in combination with chemotherapies. The most commonly used 1L chemotherapy regimens were fluorouracil, leucovorin, and oxaliplatin (FOLFOX) followed by fluorouracil, leucovorin, and irinotecan (FOLFIRI) (Table 3).
Table 3.
Treatment regimens and effectiveness among patients with mCRC treated with 1L bevacizumab-awwb.
| Treatment Regimens | Naïve patients (n = 129) | Switchers (n = 105) |
|---|---|---|
| Bevacizumab-awwb monotherapy, n (%) | 0 | 3 (2.9%) |
| In combination with chemotherapy, n (%) | 129 (100%) | 101 (96.2%) |
| In combination with immunotherapy, a n (%) | 0 | 1 (<1%) |
| First-line chemotherapy regimens, n (%) | ||
| FOLFOX | 71 (55.0%) | 53 (50.5%) |
| FOLFIRI | 35 (27.1%) | 18 (17.1%) |
| CAPOX | 11 (8.5%) | 6 (5.7%) |
| FOLFOXIRI | 7 (5.4%) | 2 (1.9%) |
| Other | 27 (20.9%) | 34 (32.4%) |
| Duration of 1L RP use prior to switching to bevacizumab-awwb, months, median (range) | NA | 4.4 (0.03–22.7) |
| Duration of 1L bevacizumab-awwb use, months, median (range) | 5.1 (0.03–17.8) | 4.4 (0.03–19.4) |
| Duration of 1L chemotherapy, months, median (range) | 6.2 (0.03–21.2) | 10.2 (2.6–39.2) |
| Duration of total chemotherapy, months, median (range) | 6.6 (0.03–21.2) | 10.7 (2.6–39.2) |
| Effectiveness | ||
| PFS from the initiation of 1L therapy, months, median (95% CI) | 8.6 (7.6–9.9) | 14.1 (12.1–15.8) |
| OS from the initiation of 1L therapy, months, median (95% CI) | 17.3 (14.2–19.3) | 25.9 (19.1–31.6) |
| 12-month OS probability, % (95% CI) | 71.4 (61.0–79.5) | 87.6 (79.1–92.8) |
1L, first-line; CAPOX, capecitabine plus oxaliplatin; CI, confidence interval; FOLFIRI, fluorouracil, leucovorin, and irinotecan; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; FOLFOXIRI, fluorouracil, leucovorin, oxaliplatin, and irinotecan; mCRC, metastatic colorectal cancer; NA, not applicable; OS, overall survival; PFS, progression-free survival; RP, reference product.
Pembrolizumab.
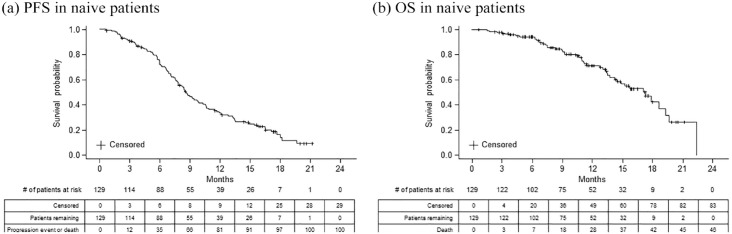
Median duration of available follow-up data was 10.7 (range, 0.7–22.4) months for naïve patients. At the end of study follow-up, naïve patients had been treated with 1L bevacizumab-awwb for a median duration of 5.1 months (range, 0.03–17.8), and the median duration of 1L chemotherapy was 6.2 months (range, 0.03–21.2) (Table 3). During the study period, 100/129 (77.5%) patients had documented disease progression or death, among which 46 patients had a record of death. The median PFS and OS, estimated from the initiation of 1L therapy using Kaplan-Meier curve, were 8.6 (95% CI, 7.6–9.9) months and 17.3 (95% CI, 14.2–19.3) months, respectively (Figure 1). The 12-month OS probability was estimated to be 71.4% (95% CI, 61.0%–79.5%).
Figure 1.
Kaplan-Meier estimate of survival probability for patients with mCRC treated with 1L bevacizumab-awwb who had no prior exposure to the RP.
1L, first-line; mCRC, metastatic colorectal cancer; RP, reference product.
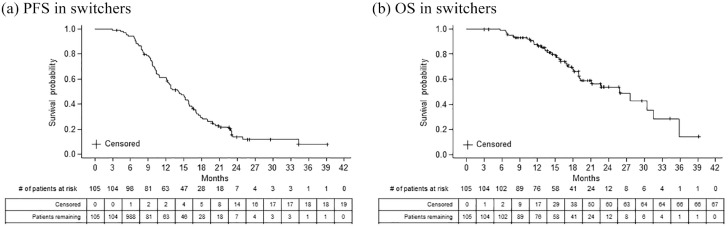
For the switchers, median duration of available follow-up data was 15.8 (range, 2.9–39.2) months. The median duration of 1L chemotherapy was 10.2 months (range, 2.6–39.2). The switcher cohort had been treated with 1L RP for a median of 4.4 months (range, 0.03–22.7) before switching to bevacizumab-awwb within the 1L, and post switch, had been treated with 1L bevacizumab-awwb for a median duration of 4.4 months (range, 0.03–19.4) at the end of study period (Table 3). During the study period, 86/105 (81.9%) switchers had documented disease progression or death, among which 38 patients had a record of death. The median PFS and OS, estimated from the initiation of 1L therapy using Kaplan-Meier curve, were 14.1 (95% CI, 12.1–15.8) months and 25.9 (95% CI, 19.1–31.6) months, respectively. The 12-month OS probability was 87.6% (95% CI, 79.1–92.8%) (Figure 2).
Figure 2.
Kaplan-Meier estimate of survival probability for patients with mCRC treated with 1L bevacizumab-awwb with prior exposure to the RP.
1L, first-line; mCRC, metastatic colorectal cancer; RP, reference product.
Events of interest
During treatment with 1L bevacizumab-awwb, 20 protocol-specified EOIs were reported in 18 naïve patients (14.0%), including eight thromboembolic events, four hemorrhagic events, one wound healing complication, one gastrointestinal perforation, one proteinuria, three hypertension, and two infusion reactions (Table 4). The most frequent actions taken following the occurrence of an EOI in naïve patients were emergency department visit (5.4% of patients), treatment hold (4.7% of patients), and treatment discontinuation/switch (3.1% of patients). Among switchers, four patients (3.8%) had four protocol-specified EOIs, including two thromboembolic events, one proteinuria, and one infusion reaction, during treatment with 1L bevacizumab-awwb. The most frequent actions taken following the occurrence of an EOI in switchers were treatment hold (1.9% of patients) and treatment discontinuation/switch (1.9% of patients) (Table 4). None of the EOIs reported in the study resulted in death.
Table 4.
EOIs (patient level count) reported for naïve patients and switchers during 1L bevacizumab-awwb-containing treatment: overall and according to management.
| EOIs | Management | |||||
|---|---|---|---|---|---|---|
| Overall | Treatment hold | Treatment discontinuation/switch | Serious EOI – Hospitalization | Emergency department visit | Supportive care medication use | |
| Naïve patients (n = 129) | ||||||
| EOIs (patient count), a n (%) | 18 (14.0%) | 6 (4.7%) | 4 (3.1%) | 3 (2.3%) | 7 (5.4%) | 2 (1.6%) |
| GI perforation | 1 (<1%) | 1 (<1%) | 0 | 0 | 1 (<1%) | 0 |
| Hemorrhage | 4 (3.1%) | 1 (<1%) | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0 |
| Hypertension | 3 (2.3%) | 2 (1.6%) | 1 (<1%) | 0 | 1 (<1%) | 1 (<1%) |
| Infusion reaction | 2 (1.6%) | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 |
| Proteinuria | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 |
| Thromboembolic event | 8 (6.2%) | 1 (<1%) | 1 (<1%) | 2 (1.6%) | 3 (2.3%) | 0 |
| Wound healing complication | 1 (<1%) | 0 | 0 | 0 | 1 (<1%) | 1 (<1%) |
| Switchers (n = 105) | ||||||
| EOIs (patient count), a n (%) | 4 (3.8%) | 2 (1.9%) | 2 (1.9%) | 0 | 1 (<1%) | 1 (<1%) |
| Infusion reaction | 1 (<1%) | 0 | 0 | 0 | 1 (<1%) | 0 |
| Proteinuria | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0 | 0 | 1 (<1%) |
| Thromboembolic event | 2 (1.9%) | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 |
The categories of EOIs are not mutually exclusive; >1 EOI can be reported for a patient. Each EOI can result in >1 management.
EOI, event of interest.
Discussion
This retrospective analysis captured the early experience of real-world outcomes with 1L bevacizumab-awwb use in patients with mCRC, an indication approved on the basis of extrapolation. One-year OS probability since the initiation of 1L therapy was estimated to be 71.4% over a period of up to 22 months of available follow-up (median, 10.7 months) for naïve patients and 87.6% over up to 39 months of available follow-up (median, 15.8 months) for switchers. Bevacizumab-awwb appeared to be well tolerated in both naïve patients and switchers, with observed EOIs as expected and similar to those of RP.6–8,11–13,35
Effectiveness outcomes with bevacizumab-awwb in naïve patients were generally consistent with those reported in previously published real-world studies of 1L RP in patients with mCRC.11,12,34–36 In two US registry studies (ARIES and BRiTE), median PFS of ~10 months and median OS of ~23 months were reported in mCRC patients who had a median duration of 5 months of 1L RP use.11,12 However, >80% of patients in these two US registry studies underwent surgical resection of primary tumor,11,12 while only ~40% of patients in our study cohort had prior surgical resection. It has been shown in a meta-analysis of clinical trials that mCRC patients with resected primary tumor (median PFS, 9–10.8 months; median OS, 20.7–29.8 months) had better survival than those without surgery of primary tumor (median PFS, 7–9 months; median OS, 13.4–20 months) when treated with 1L RP. 37 In addition, there was a relatively limited follow-up time in the present study (up to 22 months with a median of 10.7 months for naïve patients), compared to previous reported real-world data of 1L RP (median of ~20 months),11,12 as we aimed to deliver early effectiveness data for the biosimilar bevacizumab-awwb in mCRC. The limited follow-up resulted in 64% of patients censored at the end of study period due to end of record/follow-up in Kaplan-Meier analysis of OS, which may have skewed the survival curve and led to shorter median OS. The 1-year OS probability was a more accurate representation of survival data and among naïve patients treated with bevacizumab-awwb, the 1-year OS probability (71.4%) was within the range of that reported in previous studies of 1L RP in mCRC patients (60–74%).12,34 Our results were also consistent with data from a most recent retrospective, non-inferiority cohort study of patients with mCRC in the United States that compared treatment outcomes of naïve patients initiating bevacizumab-awwb (n = 239) versus historical control group of naïve patients initiating RP (n = 1,206), which showed that one-year OS with bevacizumab-awwb (72.8%) was non-inferior to that with RP (73.1%). 35
In our study, the survival durations with bevacizumab-awwb were longer in switchers than in naïve patients. However, the inclusion of immortal time during which disease progression or death could not occur until patients switched to 1L bevacizumab-awwb may have biased the survival analysis in switchers. To account for this bias introduced naturally to switchers, we performed a landmark analysis using the methods described by Anderson et al., 38 with a similar analysis conducted in naïve patients for data interpretation. Landmark analyses have been shown previously to account for the potential impact of immortal time bias (ITB) on time-dependent events in survival analyses by excluding patients who have experienced the EOI or are censored before the landmark time and only including patients who have reached the landmark without events.39–44 Our 6-month landmark analysis of PFS included 88 naïve patients and 49 switchers who remained alive and progression-free at 6 months after initiation of 1L therapy. Kaplan-Meier analysis showed that median PFS was numerically comparable between the two cohorts of patients: 11.5 (95% CI, 9.4–13.5) months for naïve patients and 10.3 (9.4–13.9) months for switchers (Supplemental Figure A). The 6-month landmark analysis of OS included 102 naïve patients and 53 switchers who remained alive at 6 months after start of 1L treatment. The median OS was also numerically comparable between naïve patients (17.9 [95% CI, 14.5–19.7] months) and switchers (17.2 [15.3–not available] months) (Supplemental Figure B). Results of our landmark analyses suggested that treatment outcomes with 1L bevacizumab-awwb were comparable in patients with or without prior exposure to the RP once ITB was accounted for.
EOIs reported in naïve patients and switchers during bevacizumab-awwb–containing treatment were also generally consistent with previously published data for mCRC patients treated with 1L RP6–8,11–13,35 and consistent with adverse reactions described in bevacizumab-awwb labeling. 24 The aforementioned non-inferiority cohort study in mCRC patients confirmed that no significant differences in serious adverse events were detected between bevacizumab-awwb and RP treatment groups. 35 In our study, the proportion of patients with protocol-specified EOIs appeared to be higher in naïve patients than that in switchers. However, the switchers were previously treated with bevacizumab RP and the patients who had EOIs while on RP could have discontinued the treatment and therefore, were not included in the present analysis (i.e. selection bias).
Biosimilars are an alternative, potential cost-saving treatment option, and clinical data of a biosimilar in indications that were extrapolated are of great interest to physicians and patients.26,27 American Society of Clinical Oncology issued a position statement on the use of biosimilars underscoring the need for post-marketing evidence development. 28 Effectiveness and safety data of bevacizumab-awwb in mCRC remain limited. This real-world study provides an early experience of 1L bevacizumab-awwb use longitudinally in a tumor type where a clinical trial was not conducted. Our data, as well as the recent results from the non-inferiority cohort study, 35 showed that 1L bevacizumab-awwb appeared to be well tolerated and effective for the treatment of patients with mCRC in real-world setting and the effectiveness outcomes with 1L bevacizumab-awwb seemed comparable to those previously reported real-world data of 1L RP.11,12,34,36
Our study has several limitations. First, compared to 1L RP in mCRC real-world studies,11,12 this retrospective analysis of 1L bevacizumab-awwb had a relatively short follow-up time, which needs to be taken into consideration when interpreting the OS data, especially for naïve patients. With the limited follow-up data, the 12-month OS probability may more accurately reflect the survival outcome for our study population and the results show that it is in line with previous publications. Second, most patients in this study were treated in a community practice setting. Generalization of data from this analysis to academic treatment settings may not be appropriate. However, the ConcertAI Oncology Dataset includes a consolidated EMR database derived from networks of geographically diverse oncology practices that are members of multiple group purchasing organizations. As a result, patient data from the dataset were representative in terms of mCRC patient population coverage in the United States. Another important limitation is the presence of ITB for switchers. Patients who switched from the RP to bevacizumab-awwb had to survive to the time point of switching, which introduced the ITB for estimates of OS and PFS. When interpreting the survival outcome data for the group of switchers, such selection bias needs to be considered. As discussed above, the ITB and its potential impact on OS and PFS in switchers have been accounted for in the landmark analysis (Supplemental figure).
Conclusions
This real-world study in US community-based oncology practices showed that 1L bevacizumab-awwb treatment was effective and well tolerated in patients with mCRC who either initiated therapy with the biosimilar bevacizumab-awwb or switched from RP to bevacizumab-awwb. Treatment outcomes observed with 1L bevacizumab-awwb were generally consistent with previously published real-world data for mCRC patients treated with 1L RP.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231182386 for Real-world outcomes among patients with metastatic colorectal cancer treated first line with a bevacizumab biosimilar (bevacizumab-awwb) by Ran Jin, Adesuwa S. Ogbomo, Neil A. Accortt, Lincy S. Lal, Geetanjali Bishi, Darcie Sandschafer and Jerome H. Goldschmidt in Therapeutic Advances in Medical Oncology
Acknowledgments
Jinling Wu, MD, PhD of BioScience Communications, New York, NY, provided medical writing support (funded by Amgen Inc.).
Footnotes
ORCID iD: Darcie Sandschafer  https://orcid.org/0009-0009-8531-9726
https://orcid.org/0009-0009-8531-9726
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ran Jin, Amgen Inc., 1 Amgen Center Dr, Thousand Oaks, CA 91320, USA.
Adesuwa S. Ogbomo, ConcertAI, Cambridge, MA, USA
Neil A. Accortt, Amgen Inc., Thousand Oaks, CA, USA
Lincy S. Lal, ConcertAI, Cambridge, MA, USA
Geetanjali Bishi, ConcertAI, Cambridge, MA, USA.
Darcie Sandschafer, Amgen Inc., Thousand Oaks, CA, USA.
Jerome H. Goldschmidt, Blue Ridge Cancer Care, Blacksburg, VA, USA
Declarations
Ethics approval and consent to participate: This study received institutional review board approval from the Ethics Committee of IntegReview (Austin, Texas), now part of Advarra (Columbia, Maryland). Informed consent from the patients for the inclusion of their medical history and treatment outcomes within this work was waived since this retrospective study included only review of existing data without contact with or direct involvement of patients in the study.
Consent for publication: Not applicable.
Author contributions: Ran Jin: Conceptualization; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Adesuwa Ogbomo: Data curation; Formal analysis; Investigation; Methodology; Writing – review & editing.
Neil A. Accortt: Conceptualization; Investigation; Methodology; Writing – review & editing.
Lincy S. Lal: Formal analysis; Investigation; Methodology; Writing – review & editing.
Geetanjali Bishi: Formal analysis; Investigation; Writing – review & editing.
Darcie Sandschafer: Conceptualization; Investigation; Methodology; Writing – review & editing.
Jerome H. Goldschmidt: Conceptualization; Investigation; Methodology; Writing – review & editing.
Funding: This study was funded by Amgen Inc., Thousand Oaks, CA.
RJ, NA, and DS are employees and shareholders of Amgen Inc. ASO, LSL, and GB are employees of ConcertAI, which received funding from Amgen Inc. for the conduct of this study. JHG is a consultant for Amgen and is a speaker for G1 Therapeutics and BMS.
Availability of data and materials: Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://www.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
References
- 1.SEER. Cancer stat facts: colorectal cancer. https://seer.cancer.gov/statfacts/html/colorect.html (2022, accessed 25 August 2022).
- 2.Hu CY, Bailey CE, You YN, et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg 2015; 150: 245–251. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Liang L, Yu Y, et al. Primary tumour resection could improve the survival of unresectable metastatic colorectal cancer patients receiving bevacizumab-containing chemotherapy. Cell Physiol Biochem 2016; 39: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 4.Kim TW, Elme A, Kusic Z, et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer 2016; 115: 1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobrero A.A tribute to biologics in advanced colorectal cancer treatment. Ann Oncol 2016; 27: 1372–1374. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342. [DOI] [PubMed] [Google Scholar]
- 7.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of Bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005; 23: 3706–3712. [DOI] [PubMed] [Google Scholar]
- 8.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26: 2013–2019. [DOI] [PubMed] [Google Scholar]
- 9.McCahill LE, Yothers G, Sharif S, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol 2012; 30: 3223–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrelli F, Borgonovo K, Cabiddu M, et al. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: a pooled analysis of 29 published trials. Clin Colorectal Cancer 2013; 12: 145–151. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz HI, Bekaii-Saab TS, Bendell JC, et al. Safety and effectiveness of bevacizumab treatment for metastatic colorectal cancer: final results from the Avastin® Registry – investigation of effectiveness and safety (ARIES) observational cohort study. Clin Oncol (R Coll Radiol) 2014; 26: 323–332. [DOI] [PubMed] [Google Scholar]
- 12.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist 2009; 14: 862–870. [DOI] [PubMed] [Google Scholar]
- 13.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014; 371: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 14.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015; 16: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry; (2015). https://www.fda.gov/media/82647/download [Google Scholar]
- 16.Nabhan C, Parsad S, Mato AR, et al. Biosimilars in oncology in the United States: a review. JAMA Oncol 2018; 4: 241–247. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Chen B, Hermanson T, et al. Regulatory and clinical considerations for biosimilar oncology drugs. Lancet Oncol 2014; 15: e594–e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IQVIA. The Impact of Biosimilar Competition in Europe. https://www.medicinesforeurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf (2018, accessed 25 August 2022).
- 19.Seo N, Polozova A, Zhang M, et al. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. MAbs 2018; 10: 678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas M, Thatcher N, Goldschmidt J, et al. Totality of evidence in the development of ABP 215, an approved bevacizumab biosimilar. Immunotherapy 2019; 11: 1337–1351. [DOI] [PubMed] [Google Scholar]
- 21.Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and safety of the biosimilar ABP 215 compared with bevacizumab in patients with advanced nonsquamous non-small cell lung cancer (MAPLE): a randomized, double-blind, phase III study. Clin Cancer Res 2019; 25: 2088–2095. [DOI] [PubMed] [Google Scholar]
- 22.de Lartigue J. Bevacizumab-awwb becomes first biosimilar approved for cancer treatment. JCSO 2018; 16: e60–e62. [Google Scholar]
- 23.FDA approves first biosimilar for cancer treatment (2017). https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-first-biosimilar-cancer-treatment
- 24.MVASI (bevacizumab-awwb) (package insert). South San Francisco, CA, USA: Amgen Inc.; revised June 2019. [Google Scholar]
- 25.Kabir ER, Moreino SS, Sharif Siam MK.The breakthrough of biosimilars: a twist in the narrative of biological therapy. Biomolecules 2019; 9: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther 2017; 33: 2160–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabhan C, Jeune-Smith Y, Valley A, et al. Community oncologists’ perception and acceptance of biosimilars in oncology. J Clin Pathw 2018; 4: 43–47. [Google Scholar]
- 28.Lyman GH, Balaban E, Diaz M, et al. American Society of Clinical Oncology statement: biosimilars in oncology. J Clin Oncol 2018; 36: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes W, DeClue RW, Accortt NA, et al. Real-world use of bevacizumab-awwb, a bevacizumab biosimilar, in US patients with metastatic colorectal cancer. Future Oncol 2021; 17: 5119–5127. [DOI] [PubMed] [Google Scholar]
- 30.Houts AC, Ogale S, Sommer N, et al. Treatment patterns and outcomes in patients with KRAS wild-type metastatic colorectal cancer treated in first line with bevacizumab- or cetuximab-containing regimens. J Gastrointest Cancer 2019; 50: 69–77. [DOI] [PubMed] [Google Scholar]
- 31.Houts AC, Ogale S, Zafar Y, et al. Progression-free survival in patients receiving chemotherapy alone (C) or chemotherapy with bevacizumab (CB) for first-line treatment of KRAS mutant metastatic colorectal cancer in community oncology settings. J Gastrointest Cancer 2019; 50: 16–22. [DOI] [PubMed] [Google Scholar]
- 32.Ivanova JI, Saverno KR, Sung J, et al. Real-world treatment patterns and effectiveness among patients with metastatic colorectal cancer treated with ziv-aflibercept in community oncology practices in the USA. Med Oncol 2017; 34: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan KJ, Skinner KE, Fernandes AW, et al. Real-world treatment patterns among patients with unresected stage III non-small-cell lung cancer. Future Oncol 2019; 15: 2943–2953. [DOI] [PubMed] [Google Scholar]
- 34.Oppelt KA, Kuiper JG, Ingrasciotta Y, et al. Characteristics and absolute survival of metastatic colorectal cancer patients treated with biologics: a real-world data analysis from three European countries. Front Oncol 2021; 11: 630456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham C, Niu F, Delate T, et al. Real-world outcomes of biosimilar bevacizumab-awwb versus reference bevacizumab in patients with metastatic colorectal cancer. J Clin Oncol 2022; 40(suppl): 3552. [DOI] [PubMed] [Google Scholar]
- 36.Khakoo S, Chau I, Pedley I, et al. ACORN: observational study of bevacizumab in combination with first-line chemotherapy for treatment of metastatic colorectal cancer in the UK. Clin Colorectal Cancer 2019; 18: 280–291.e5. [DOI] [PubMed] [Google Scholar]
- 37.Cao D, Zheng Y, Xu H, et al. Bevacizumab improves survival in metastatic colorectal cancer patients with primary tumor resection: a meta-analysis. Sci Rep 2019; 9: 20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson JR, Cain KC, Gelber RD.Analysis of survival by tumor response. J Clin Oncol 1983; 1: 710–719. [DOI] [PubMed] [Google Scholar]
- 39.Gleiss A, Oberbauer R, Heinze G.An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int 2018; 31: 125–130. [DOI] [PubMed] [Google Scholar]
- 40.van Uden DJP, van Maaren MC, Strobbe LJA, et al. Better survival after surgery of the primary tumor in stage IV inflammatory breast cancer. Surg Oncol 2020; 33: 43–50. [DOI] [PubMed] [Google Scholar]
- 41.Kimpel O, Bedrose S, Megerle F, et al. Adjuvant platinum-based chemotherapy in radically resected adrenocortical carcinoma: a cohort study. Br J Cancer 2021; 125: 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanitis T, Proskorovsky I, Ambavane A, et al. Survival analysis in patients with metastatic merkel cell carcinoma treated with avelumab. Adv Ther 2019; 36: 2327–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dekkers OM, Groenwold RHH. When observational studies can give wrong answers: the potential of immortal time bias. Eur J Endocrinol 2021; 184: E1–E4. [DOI] [PubMed] [Google Scholar]
- 44.Mi X, Hammilla BG, Curtis LH, et al. Impact of immortal person-time and time scale in comparative effectiveness research for medical devices: a case for implantable cardioverter-defibrillators. J Clin Epidemiol 2013; 66: S138–S144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231182386 for Real-world outcomes among patients with metastatic colorectal cancer treated first line with a bevacizumab biosimilar (bevacizumab-awwb) by Ran Jin, Adesuwa S. Ogbomo, Neil A. Accortt, Lincy S. Lal, Geetanjali Bishi, Darcie Sandschafer and Jerome H. Goldschmidt in Therapeutic Advances in Medical Oncology