Abstract
Objective
Oral lichen planus (OLP) is a T cell-mediated inflammatory condition in the oral cavity. Mucosal-associated invariant T (MAIT) cells are gaining more relevance in immune diseases because they can be activated by cytokines without T cell receptor stimulation. Herein, we tested the effect of interleukin-23 (IL-23) on the activation status of OLP MAIT cells.
Methods
Peripheral blood mononuclear cells (PBMCs) isolated from OLP patients were stimulated by IL-23 in the absence or presence of phorbol myristate acetate (PMA) and ionomycin. The activation status of MAIT cells was analyzed by flow cytometry after staining for CD3, CD4, CD8, CD161, TCR Vα7.2, and CD69.
Results
The fraction of MAIT cells in OLP peripheral blood was approximately 0.38% to 3.97%, and CD8+ subpopulations overwhelmed CD4+ cells. The mean percentages of OLP MAIT cells in PBMCs and CD8+MAIT cells in MAIT cells were approximately 40%. PMA and ionomycin significantly increased CD69 expression on OLP T cells, MAIT cells, and CD8+MAIT cells. Cells with enhanced activation had different responsiveness to exogenous IL-23, showing increased CD69 expression on OLP T cells, decreased CD69 on OLP CD8+MAIT cells, and no significant change on OLP MAIT cells.
Conclusions
IL-23 showed different effects on the activation status of OLP MAIT cells and CD8+MAIT cells.
Keywords: Mucosal-associated invariant T cell, oral lichen planus, interleukin-23, CD69, phorbol myristate acetate, ionomycin
Introduction
Oral lichen planus (OLP) is a T cell-mediated inflammatory condition that occurs in the oral mucosa. 1 OLP develops as an immunological disease with lymphocyte infiltration of the lamina propria that results in keratinocyte apoptosis and liquefactive destruction of the basal membrane.1,2 OLP lesions can have multiple presentations including reticular, papular, plaque-like, atrophic, erosive, and bullous damages. 3 Although much is still unknown about its etiology, numerous theories consider that immune dysregulation may trigger the chronic inflammatory process in OLP.4,5 CD4+ T helper (Th) cells and CD8+ T cytotoxic cells are strongly involved in the pathogenesis of OLP.4,6 Of note, interferon-γ (IFN-γ)- and interleukin-17 (IL-17)-producing immune cells were found to be present in OLP lesions. 7 Our previous study reported that IFN-γ and IL-17 levels were increased in the serum of OLP patients and within local lesions.8,9 Moreover, we found that IFN-γ contributed to OLP T-cell immune attack towards keratinocytes. 2 Thus, IFN-γ and IL-17 have been implicated as key inflammatory mediators in the immunopathogenesis of OLP.
In humans, mucosal-associated invariant T (MAIT) cells are a type of unconventional innate-like T cells that express the semi-invariant TCRα chain Vα7.2-Jα33/20/12. 10 In human peripheral blood, MAIT cells mostly express CD161, which is a marker of IL-17-producing T cells.1,11 MAIT cells are defined by their high expression of TCR Vα7.2 and CD161 and comprise approximately 1% to 10% of human peripheral blood cells. 1 Our recent study reported the frequency of blood MAIT cells in OLP patients and analyzed the variable functional features of OLP MAIT cells. 12 We found that peripheral MAIT cells were deficient in OLP, but that cellular activation, as detected by CD69, was enhanced in OLP MAIT cells. 12 Activated MAIT cells can induce a rapid innate-like immune response, subsequently releasing cytotoxic products, inflammatory chemokines, and cytokines. 13
Unlike conventional T cells that recognize individual peptides and undergo massive expansion, MAIT cells are restricted by the major histocompatibility complex (MHC)-related protein-1 (MR1) and recognize vitamin B2 (riboflavin) metabolites of microbial origin. 13 Nevertheless, MAIT cells can also be directly activated by inflammatory cytokines in the absence of riboflavin metabolites. 13 Wang et al. reported that exogenous IL-23 could fully reconstitute MAIT cell expansion and activity in IL-23-deficient mice. 14 Additionally, IL-23 stimulation in vitro increased the IL-17 polarization of primary MAIT cells from Sjogren’s Syndrome patients. 15 Studies have demonstrated that IL-23 is overexpressed in OLP serum and can increase IL-17 production in OLP CD4+T cells.9,16,17 Thus, in this study, we examined the effect of IL-23 stimulation in vitro on the activation status of peripheral MAIT cells from OLP patients.
Materials and Methods
Study design
OLP patients were recruited from the Department of Oral Medicine, School and Hospital of Stomatology, Wuhan University (China). All patients had been clinically and histopathologically diagnosed with OLP under the WHO criteria. 18 The inclusion and exclusion criteria for this study are listed in Supplemental Table 1. Written informed consent for participation in this research and publication of the related data was obtained from each patient in accordance with the Declaration of Helsinki. Each experiment included blood samples of six donors, who were aged from 42 years to 57 years with a male-to-female ratio of 2:1. Experiments in this study were performed with informed consent under approval by the Ethical Committee Board of the School and Hospital of Stomatology, Wuhan University (approval number 2019A17).
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using lymphocyte separation medium (Catalog #LDS1075, Haoyang Biological Manufacture Co., Ltd., Tianjin, China). Briefly, 1 × 106 PBMCs were cultured per well in 24-well plates and maintained in RPMI 1640 medium with 10% fetal bovine serum (Tianhang Biotechnology Co., Ltd., Zhejiang, China), and then stimulated with or without 10 ng/mL IL-23 (Catalog #CW54, Novoprotein Technology Co., LTD., Shanghai, China) for 72 hours, in the absence or presence of 50 ng/mL phorbol myristate acetate (PMA; Catalog #P6741, Solarbio Science & Technology Co., Ltd., Beijing, China) and 1 μg/mL ionomycin (IM; Catalog #I139530, Aladdin Biochemical Technology Co., Ltd., Shanghai, China). Brefeldin A (Catalog #B1400, APExBIO, Houston, TX, USA) was added at a concentration of 5 μg/mL before flow cytometric analysis according to the manufacturer’s instructions.
Extracellular and intracellular staining for flow cytometry
For extracellular staining, cultured PBMCs were incubated with FITC-anti-CD3 antibody (Catalog #300440, BioLegend, Inc., San Diego, CA, USA), APC-anti-CD4 antibody (Catalog #300514, BioLegend), APC/Cyanine7-anti-CD8 antibody (Catalog #344714, BioLegend), PE-anti-CD161 antibody (Catalog #339904, BioLegend), and PE/Cyanine7-anti-TCR Vα7.2 antibody (Catalog #351712, BioLegend) for 15 minutes at 25°C. To detect intracellular cytokines, cells whose surfaces were stained by markers of MAIT cells were then fixed and permeabilized in Transcription Factor Buffer Set (Catalog #562574, Becton Dickinson and Co., Franklin Lakes, NJ, USA) at 4°C in accordance with the instructions of the manufacturer. Intracellular protein probing was performed by staining with BV421-anti-IL-17A antibody (Catalog #512322, Becton Dickinson and Co.) and BV650-anti-IFN-γ antibody (Catalog #506542, Becton Dickinson and Co.) for 30 minutes at 4°C. Cells were washed and resuspended in phosphate-buffered saline prior to flow cytometric analysis.
Statistical analysis
Data were collected by ACEA NovoCyte flow cytometry (Agilent Technologies Inc., Santa Clara, CA, USA) and analyzed using FlowJo 10 software (BD Life Science Informatics, Ashland, OR, USA). Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as the mean ± standard deviation (SD) when variables were normally distributed or as the median ± interquartile range (IQR) for non-normal variables, which are labeled with “#” in the figures. Statistical differences were analyzed using the paired t test or Wilcoxon matched-pairs signed rank test, respectively. P values <0.05 were defined as statistically significant, referring to two-sided probability. Then, the statistical data from differences or group parameters were then used in the calculation of post hoc statistical power (SP) using G*Power software.
Results
MAIT cell frequencies in PBMCs of OLP patients
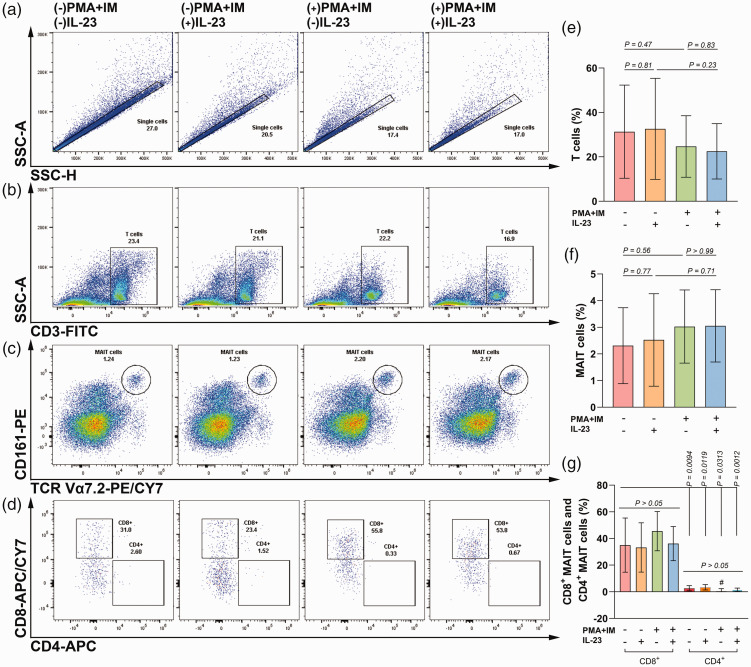
Single cells among isolated PBMCs from OLP patients were gated by SSC-H and SSC-A, in which T cells were gated out by CD3 positive staining. MAIT cells were gated by co-expression of CD161 and TCR Vα7.2 in T cells. Regarding unstimulated OLP MAIT cells, they made up a small fraction of T cells, ranging from 0.38% to 3.97%, in which the CD8+ phenotype was more common than CD4+ cells (SP = 87%). The expansion of MAIT cells seemed to increase after stimulation with PMA/ionomycin, although there was no statistical difference (SP = 24%, Figure 1).
Figure 1.
Effect of IL-23 on the proportion of MAIT cells among PBMCs from OLP patients in the absence or presence of PMA and ionomycin. Briefly, 1 × 106 PBMCs were cultured per well in 24-well plates and stimulated with or without 10 ng/mL IL-23 for 72 hours in the absence or presence of 50 ng/mL PMA and 1 μg/mL ionomycin (IM). (a–d) The gating strategy for T cells, MAIT cells, and MAIT subsets are shown by representative images in different groups. (e–g) Histograms of the proportions of T cells, MAIT cells, and MAIT subsets. Normally distributed variables are presented as mean ± SD, while non-normally distributed data are presented as mean ± IQR and labeled by “#”. P < 0.05 was defined as statistically significant.
MAIT, mucosal-associated invariant T; PBMCs, peripheral blood mononuclear cells; OLP, oral lichen planus; PMA, phorbol myristate acetate; SD, standard deviation; IQR, interquartile range.
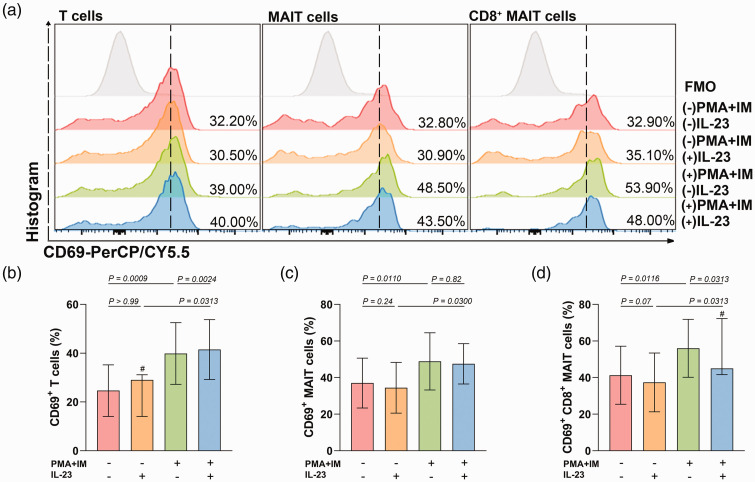
MAIT cell activation in OLP patients
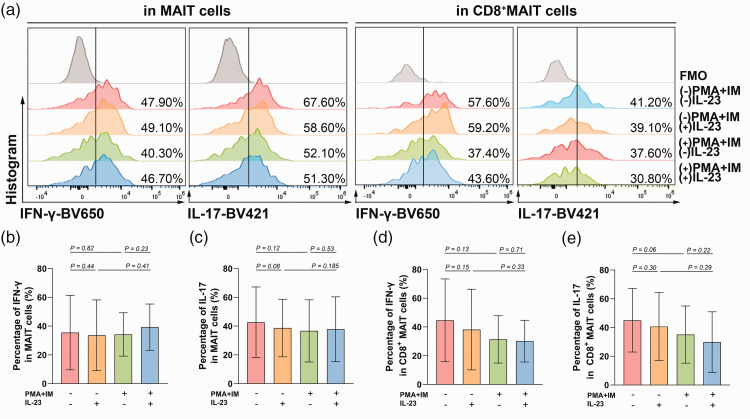
The mean percentages of OLP MAIT cells in PBMCs and CD8+MAIT cells in MAIT cells were approximately 40%. PMA/ionomycin stimulation increased the CD69 expression on T cells, MAIT cells, and CD8+MAIT cells from OLP patients (SP[T] = 99%, SP[MAIT] = 88%, SP[CD8+MAIT] = 87%, Figure 2). IFN-γ and IL-17 production were unchanged in stimulated MAIT cells and CD8+MAIT cells from OLP patients (SP[IFN-γ in MAIT] = 5%, SP[IL-17 in MAIT] = 33%, SP[IFN-γ in CD8+MAIT] = 32%, SP[IL-17 in CD8+MAIT] = 49%, Figure 3).
Figure 2.
The effect of IL-23 on the expression of CD69 in MAIT cells from OLP patients in the absence or presence of PMA and ionomycin. (a) The gating strategy for CD69 staining in T cells, MAIT cells, and CD8+MAIT cells is shown by representative images in different groups and (b–d) Histograms of CD69 expression in T cells, MAIT cells, and CD8+MAIT cells. Normally distributed variables are presented as mean ± SD, while non-normally distributed data are presented as mean ± IQR and labeled by “#”. P < 0.05 was defined as statistically significant.
MAIT, mucosal-associated invariant T; OLP, oral lichen planus; PMA, phorbol myristate acetate; FMO, fluorescence minus one; SD, standard deviation; IQR, interquartile range.
Figure 3.
The effect of IL-23 on the production of IFN-γ and IL-17 by MAIT cells and CD8+MAIT cells from OLP patients in the absence or presence of PMA and ionomycin. (a) The gating strategy for IFN-γ and IL-17 staining in MAIT cells and CD8+MAIT cells is shown by representative images in different groups and (b–e) Histograms of IFN-γ and IL-17 expression in MAIT cells and CD8+MAIT cells. Normally distributed variables are presented as mean ± SD. P < 0.05 was defined as statistically significant.
MAIT, mucosal-associated invariant T; OLP, oral lichen planus; PMA, phorbol myristate acetate; FMO, fluorescence minus one; SD, standard deviation.
IL-23 played different roles in the activation of OLP T cells, MAIT cells, and CD8+MAIT cells
IL-23 had no influence on the expansion of OLP T cells, MAIT cells, or CD8+MAIT cells (SP[T] = 14%, SP[MAIT] = 15%, SP[CD8+MAIT] = 9%, Figure 1). Single stimulation with IL-23 did not induce changes in CD69 expression on OLP T cells, MAIT cells, or CD8+MAIT cells (SP[T] = 5%, SP[MAIT] = 20%, SP[CD8+MAIT] = 44%). Under stimulation with PMA/ionomycin, IL-23 increased CD69 expression on T cells but decreased CD69 on CD8+MAIT cells, while it had no effect on MAIT cells (SP[T] = 99%, SP[MAIT] = 5%, SP[CD8+MAIT] = 15%, Figure 2). Both in OLP MAIT cells and CD8+MAIT cells stimulated with or without PMA and ionomycin, IL-23 had no effect on IFN-γ and IL-17 production (in MAIT cells without PMA/ionomycin stimulation: SP[IFN-γ] = 11%, SP[IL-17] = 43%; in MAIT cells with PMA/ionomycin stimulation: SP[IFN-γ] = 20%, SP[IL-17] = 9%; in CD8+MAIT cells without PMA/ionomycin stimulation: SP[IFN-γ] = 28%, SP[IL-17] = 16%; in CD8+MAIT cells with PMA/ionomycin stimulation: SP[IFN-γ] = 6%, SP[IL-17] = 21%, Figure 3).
Discussion
In this study, we found that CD3+TCR Vα7.2+CD161+MAIT cells comprised approximately 0.38% to 3.97% of PMBCs in OLP patients. Moreover, the CD8 phenotype was the dominant component of OLP MAIT cells compared with CD4 subsets. The number of peripheral MAIT cells in healthy human has been reported to be approximately 1% to 10%. 1 Our previous study confirmed a decrease in the frequency of peripheral MAIT cells in OLP compared with in healthy individuals. 12 Additionally, OLP MAIT cells primarily showed a CD8 phenotype and strongly granzyme B production, which indicated that these cells might have cytotoxic activities. 12 Granzyme B can mediate the immune attack of T cells towards keratinocytes. 19 Therefore, the above results suggested that MAIT cells might be important in the immunopathogenesis of OLP.
PMA activates protein kinase C, and ionomycin raises intracellular calcium levels and activates cells via bypassing the early activation steps through the TCR, together resulting in NF-κB activation, thus mimicking physiological T cell activation.20,21 Our previous study reported that NF-κB signaling was overactive and CD69 expression was elevated in MAIT cells in OLP;12,22 thus, we speculated that OLP MAIT cells might be activated in a TCR-independent manner. The results of this study showed that PMA/ionomycin stimulation increased CD69 expression on MAIT cells and CD8+MAIT cells from OLP patients.
MAIT cells can also be activated by a cytokine-dependent pathway, which broadens their role in immune inflammatory diseases even in the absence of antigen recognition. 13 The activating cytokine IL-23 was evidenced in OLP serum, 17 suggesting that the OLP peripheral microenvironment might be able to activate MAIT cells. However, in this study, IL-23 alone or in the PMA/ionomycin stimulated state did not increase the activation of MAIT cells. Interestingly, the activation of CD8+MAIT cells under PMA/ionomycin stimulation was decreased by IL-23, while T cell activation was enhanced under the same conditions. We hypothesize that this phenomenon could be attributed to several factors. First, both T cells and MAIT cells are heterogeneous populations, characterized by subsets that differ in phenotype, function, and maturation level. 23 Second, PMA and ionomycin can activate the transcription factors NF-κB and members of the NFAT family, which subsequently regulate downstream gene expression. 24 Although PMA/ionomycin can induce T cell proliferation, it can also either activate T cells or initiate activation-induced cell death (AICD) in lymphocytes under some specific circumstances. 25 NF-κB mediates protection from AICD while NFAT plays a major role in this process.26,27 In OLP, NF-κB signaling was overactive. 22 Tacrolimus, by inhibiting NFAT activation, could significantly reduce the symptoms of OLP. 28 Thus, we speculate that PMA/ionomycin activates MAIT cells and might initiate AICD in these cells. OLP MAIT cells have been presumed to be activated from the IL-23-elevated environment, in which subjecting the cells to PMA/ionomycin could elicit a high proportion of cell death and would not be expected to yield evident responses to greater IL-23 levels. Taken together, the effect of IL-23 on OLP MAIT cells might be related to the cell phenotypes and cells’ original environment and state.
After activation, MAIT cells can respond with the production of a variety of pro-inflammatory cytokines, whereas their capacity to produce IFN-γ and IL-17 is altered in different immune diseases. 13 Circulating MAIT cells in patients or mouse models with multiple sclerosis showed an activated phenotype and decreased IFN-γ and IL-17 production.29,30 Although MAIT cell numbers were decreased in peripheral circulation in patients with obesity and type 2 diabetes, these cells exhibited a pro-inflammatory phenotype and increased secretion of IFN-γ and IL-17. 30 OLP peripheral MAIT cells were found to be hyperactivated and with a comparable capacity of producing IFN-γ and IL-17 in our previous study, but with no difference from healthy individuals. 12 Additionally, hyperactivated MAIT cells in OLP also expressed higher levels of the exhaustion marker PD-1. 12 In this study, we did not observe any significant changes in IFN-γ or IL-17 production by OLP MAIT cells or CD8+MAIT cells in response to stimulation by PMA/ionomycin or IL-23. We therefore conclude that OLP MAIT cells could have different effector functions from other diseases and that MAIT cell hyperactivation might render the cells exhausted, resulting in loss of response to PMA/ionomycin or IL-23.
Collectively, there was a relatively small quantity of MAIT cells in OLP peripheral blood, and these predominantly showed the CD8 phenotype. Both OLP MAIT cells and CD8+MAIT cells already presented an activated phenotype. Additionally, PMA/ionomycin stimulation significantly increased CD69 expression on OLP T cells, MAIT cells, and CD8+MAIT cells. Cells with enhanced activation had different responsiveness to exogenous IL-23, showing increased expression of CD69 on OLP T cells, decreased expression of CD69 on OLP CD8+MAIT cells, and no significant change of CD69 expression and IFN-γ/IL-17 production on OLP MAIT cells. Given that OLP MAIT cells were presumed to be activated and came from an IL-23-elevated environment, we hypothesize that the effect of IL-23 on OLP MAIT cells might be related to the cell phenotypes and their original environment and state.
This pilot study is the first to investigate the effect of IL-23 on the activation of OLP MAIT cells, and therefore may play an indicative role for future research into the function and mechanism of OLP MAIT cells and treatment targets. The main limitation of this study was the relatively small number of cases. Therefore, further studies are needed that include large patient cohorts as well as longitudinal studies focused on how hyperactivation regulates biological functions in OLP MAIT cells.
Acknowledgements
The authors thank Frontier Science Center for Immunology and Metabolism, Wuhan University, China, for providing the research environment.
Footnotes
Author contributions: Fang Wang and Gang Zhou contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Fang Wang. The first draft of the manuscript was written by Fang Wang. Xiu-Li Zhang, Jing-Ya Yang, and Gang Zhou reviewed and edited the manuscript. All authors read and approved the final manuscript.
The authors declare that there is no conflict of interest.
Funding: This work was supported by grants from the National Natural Science Foundation of China (Nos. 81970949, 82270983, 82201067).
ORCID iD: Fang Wang https://orcid.org/0000-0001-5200-4175
References
- 1.DeAngelis LM, Cirillo N, McCullough MJ.The immunopathogenesis of oral lichen planus-Is there a role for mucosal associated invariant T cells? J Oral Pathol Med 2019; 48: 552–559. 2019/06/07. DOI: 10.1111/jop.12898. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Zhang J, Zhou G.2-Deoxy-D-glucose impedes T cell-induced apoptosis of keratinocytes in oral lichen planus. J Cell Mol Med 2021; 25: 10257–10267. 2021/10/22. DOI: 10.1111/jcmm.16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrozzo M, Porter S, Mercadante V, et al. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontol 2000 2019; 80: 105–125. 2019/05/16. DOI: 10.1111/prd.12260. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Zhang D, Han Q, et al. Role of distinct CD4(+) T helper subset in pathogenesis of oral lichen planus. J Oral Pathol Med 2016; 45: 385–393. 2015/12/24. DOI: 10.1111/jop.12405. [DOI] [PubMed] [Google Scholar]
- 5.Kurago ZB.Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 122: 72–80. 2016/06/05. DOI: 10.1016/j.oooo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Ke Y, Dang E, Shen S, et al. Semaphorin4D Drives CD8(+) T-Cell Lesional Trafficking in Oral Lichen Planus via CXCL9/CXCL10 Upregulations in Oral Keratinocytes. J Invest Dermatol 2017; 137: 2396–2406. 2017/08/02. DOI: 10.1016/j.jid.2017.07.818. [DOI] [PubMed] [Google Scholar]
- 7.Lu R, Zhang J, Sun W, et al. Inflammation-related cytokines in oral lichen planus: an overview. J Oral Pathol Med 2015; 44: 1–14. 2013/12/18. DOI: 10.1111/jop.12142. [DOI] [PubMed] [Google Scholar]
- 8.Hu JY, Zhang J, Ma JZ, et al. MicroRNA-155-IFN-γ Feedback Loop in CD4(+)T Cells of Erosive type Oral Lichen Planus. Sci Rep 2015; 5: 16935. 2015/11/26. DOI: 10.1038/srep16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu R, Zeng X, Han Q, et al. Overexpression and selectively regulatory roles of IL-23/IL-17 axis in the lesions of oral lichen planus. Mediators Inflamm 2014; 2014: 701094. 2014/08/13. DOI: 10.1155/2014/701094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nel I, Bertrand L, Toubal A, et al. MAIT cells, guardians of skin and mucosa? Mucosal Immunol 2021; 14: 803–814. 2021/03/24. DOI: 10.1038/s41385-021-00391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggi L, Santarlasci V, Capone M, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol 2010; 40: 2174–2181. 2010/05/21. DOI: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 12.Yang JY, Wang F, Zhou G.Characterization and function of circulating mucosal-associated invariant T cells and γδT cells in oral lichen planus. J Oral Pathol Med 2021; 51: 74–85. 2021/10/13. DOI: 10.1111/jop.13250. [DOI] [PubMed] [Google Scholar]
- 13.Hinks TSC, Zhang XW.MAIT Cell Activation and Functions. Front Immunol 2020; 11: 1014. 2020/06/17. DOI: 10.3389/fimmu.2020.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Kjer-Nielsen L, Shi M, et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci Immunol 2019; 4: eaaw0402. 2019/11/17. DOI: 10.1126/sciimmunol.aaw0402. [DOI] [PubMed] [Google Scholar]
- 15.Guggino G, Di Liberto D, Lo Pizzo M, et al. IL-17 polarization of MAIT cells is derived from the activation of two different pathways. Eur J Immunol 2017; 47: 2002–2003. 2017/08/18. DOI: 10.1002/eji.201747140. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Luo Z, Lei L, et al. Interaction between oral lichen planus and chronic periodontitis with Th17-associated cytokines in serum. Inflammation 2013; 36: 696–704. 2013/01/23. DOI: 10.1007/s10753-013-9594-2. [DOI] [PubMed] [Google Scholar]
- 17.Mardani M, Mofidi H, Dastgheib L, et al. Elevated Serum Interleukin-23 Levels in Patients with Oral and Cutaneous Lichen Planus. Mediators Inflamm 2021; 2021: 5578568. 2021/08/03. DOI: 10.1155/2021/5578568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng YS, Gould A, Kurago Z, et al. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 122: 332–354. 2016/07/13. DOI: 10.1016/j.oooo.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Roopashree MR, Gondhalekar RV, Shashikanth MC, et al. Pathogenesis of oral lichen planus–a review. J Oral Pathol Med 2010; 39: 729–734. 2010/10/07. DOI: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsiogkas SG, Mavropoulos Α, Skyvalidas DN, et al. Delphinidin diminishes in vitro interferon-γ and interleukin-17 producing cells in patients with psoriatic disease. Immunol Res 2021; 70: 161–173. 2021/11/27. DOI: 10.1007/s12026-021-09251-y. [DOI] [PubMed] [Google Scholar]
- 21.Subrahmanyam PB, Maecker HT.Mass Cytometry Analysis of T-Helper Cells. Methods Mol Biol 2021; 2285: 49–63. 2021/05/01. DOI: 10.1007/978-1-0716-1311-5_4. [DOI] [PubMed] [Google Scholar]
- 22.Zhou G, Xia K, Du GF, et al. Activation of nuclear factor-kappa B correlates with tumor necrosis factor-alpha in oral lichen planus: a clinicopathologic study in atrophic-erosive and reticular form. J Oral Pathol Med 2009; 38: 559–564. 2009/05/21. DOI: 10.1111/j.1600-0714.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 23.Tao H, Pan Y, Chu S, et al. Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals. Nat Commun 2021; 12: 2029. 2021/04/03. DOI: 10.1038/s41467-021-22162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stankov K, Bogdanovic G, Stankov S, et al. Expression analysis of genes involved in apoptosis, proliferation and endoplasmic reticulum stress in ionomycin/PMA treated Jurkat cells. J Buon 2012; 17: 369–376. 2012/06/29. [PubMed] [Google Scholar]
- 25.Catalá-Rabasa A, Ndagire D, Sabio JM, et al. High ACSL5 transcript levels associate with systemic lupus erythematosus and apoptosis in Jurkat T lymphocytes and peripheral blood cells. PloS one 2011; 6: e28591. 2011/12/14. DOI: 10.1371/journal.pone.0028591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera-Walsh I, Cvijic ME, Xiao G, et al. The NF-kappa B signaling pathway is not required for Fas ligand gene induction but mediates protection from activation-induced cell death. J Biol Chem 2000; 275: 25222–25230. 2011/12/14. DOI: 10.1074/jbc.M000444200. [DOI] [PubMed] [Google Scholar]
- 27.Macian F.NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 2005; 5: 472–484. 2005/06/02. DOI: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Cerdeira C, Sanchez-Blanco E, Molares-Vila A.Clinical application of development of nonantibiotic macrolides that correct inflammation-driven immune dysfunction in inflammatory skin diseases. Mediators Inflamm 2012; 2012: 563709. 2012/12/22. DOI: 10.1155/2012/563709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Seth E, Zimmer CL, Reuterwall-Hansson M, et al. Primary sclerosing cholangitis leads to dysfunction and loss of MAIT cells. Eur J Immunol 2018; 48: 1997–2004. 2018/09/27. DOI: 10.1002/eji.201847608. [DOI] [PubMed] [Google Scholar]
- 30.Kumar V, Ahmad A.Role of MAIT cells in the immunopathogenesis of inflammatory diseases: New players in old game. Int Rev Immunol 2018; 37: 90–110. 2017/11/07. DOI: 10.1080/08830185.2017.1380199. [DOI] [PubMed] [Google Scholar]