Abstract
Rearranged during transfection (RET) is a protooncogene that encodes for receptor tyrosine kinase with downstream effects on multiple cellular pathways. Activating RET alterations can occur and lead to uncontrolled cellular proliferation as a hallmark of cancer development. Oncogenic RET fusions are present in nearly 2% of patients with non-small cell lung cancer (NSCLC), 10–20% of patients with thyroid cancer, and <1% across the pan-cancer spectrum. In addition, RET mutations are drivers in 60% of sporadic medullary thyroid cancers and 99% of hereditary thyroid cancers. The discovery, rapid clinical translation, and trials leading to FDA approvals of selective RET inhibitors, selpercatinib and pralsetinib, have revolutionized the field of RET precision therapy. In this article, we review the current status on the use of the selective RET inhibitor, selpercatinib, in RET fusion-positive tumors: NSCLC, thyroid cancers, and the more recent tissue-agnostic activity leading to FDA approval.
Keywords: LOXO292, precision oncology, RET, selpercatinib, targeted therapy
RET biology
The rearranged during transfection (RET) gene is a protooncogene that is located on chromosome 10. It encodes for a receptor tyrosine kinase that initiates a cellular signaling cascade leading to cell proliferation and growth. The RET receptor is composed of three distinct parts: an extracellular domain, a transmembrane domain, and an intracellular domain. The extracellular domain includes four cadherin-like domains, a calcium-binding site, and a cysteine-rich region. The intracellular domain features a tyrosine kinase enzyme, which can have variable isoforms of the c-terminal tail due to alternative splicing.1–4 Ligand binding to the RET co-receptors leads to the activation of multiple downstream cellular signaling pathways including RAS/MAPK/ERK, PI3K/AKT, and JAK/STAT; all with resulting increase in cellular proliferation and differentiation.5–9
RET fusions
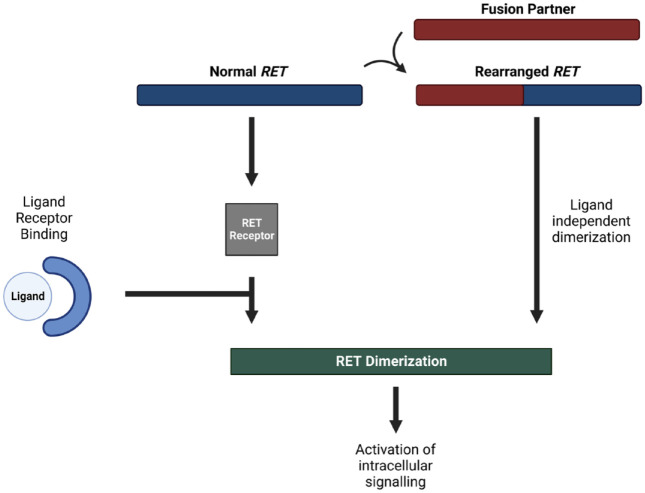
RET can be aberrantly activated by mutations and chromosomal rearrangements (Figure 1); both of which has been linked to the process of oncogenesis in different tumor types. 2 Initial discoveries were made in patients with thyroid cancer who had multiple endocrine neoplasia syndrome, but later evidence suggested a role of RET alterations in other sporadic cancers as well.10–14RET mutations are relatively more frequent, but RET fusion-positive cancers represent a distinct molecular entity that defines a unique clinical subtype.15,16
Figure 1.
RET fusions can lead to ligand-independent activation of the RET pathway, which leads to downstream signaling of multiple other cellular pathways associated with cellular proliferation and survival.
RET, Rearranged during Transfection.
In a study including 96,324 samples from AACR Project GENIE, 223 RET fusions (0.23%) were identified. Nearly half of RET fusions (54.3%) were identified in patients with non-small cell lung cancer (NSCLC). The second most common tumor type with frequent RET fusions was papillary thyroid cancer (22.8%). Frequently encountered fusion partners were KIF5B, CCDC6, and NCOA4 . 16
In disease-specific analysis, RET fusions are estimated to occur in 2% of NSCLC patients.15,17–22 Such prevalence might be perceived as infrequent, but the fact that lung cancer is estimated to hit nearly a quarter million new patients a year in the United States alone makes the number of patients who might benefit from targeted treatment substantial. 23 RET fusion-positive cancers usually present with distinct clinicopathological characteristics including young age, never smokers, early nodal metastasis, and poorly differentiated histology. 15 A study by Drilon et al. 24 also suggested that RET-rearranged lung cancers commonly present with brain metastasis (present in 25% of patients with stage IV at the time of diagnosis with a lifetime prevalence of 46%) and have suboptimal response to multikinase inhibitor (MKI) therapy. In NSCLC samples with RET fusion, co-occurring alterations were found in KRAS, SETD2, PBRL4, EZH1, and RRAGC genes. 16 In addition to NSCLC, RET fusions have also been implied as part of the molecular profile in various other tumor types. 17
Detection of RET fusions
There are multiple methods that can be used to detect RET fusions which vary in their advantages and disadvantages. 25 For example, immunohistochemistry has been long used as a cheap technology for the detection of RET aberrations but is limited by its low sensitivity and specificity.15,19,20,26,27 Fluorescence in situ hybridization (FISH) can be used to achieve higher sensitivity and specificity, but it cannot identify fusion partners unless the specific fusion partner probe is used.15,26 Polymerase Chain Reaction (PCR) is another alternative that can inform about the exact fusion partner, but it can only evaluate specimens based on known molecular profile which is used to select the used primers and limits its ability to discover new or unknown partners.19,26,28–31
Therefore, next-generation sequencing emerges as the optimum tool for the detection of RET fusion variants, given its high sensitivity and specificity as well as its ability to overcome most of the previously mentioned limits. The cost will remain a challenging concern, especially in low-resource settings but it will hopefully be cheaper with wider applications of genomic testing and more advances in technologies that will characterize the era of personalized cancer medicine.3,31
One promising approach is the use of liquid biopsy for the detection of RET fusions. 25 This has gained lots of interest in the past decade given its minimally invasive nature. In a study by Rich et al., 32 analysis of cell-free DNA (cfDNA) from 32,989 samples collected from patients with diverse cancers revealed the presence of 176 RET alterations (mostly fusions) in 170 patients (0.5%). In NSCLC, this is particularly important given the challenges of obtaining repeated tissue samples. Liquid biopsy in that setting can allow for the detection of originally present RET fusions at baseline samples and emerging fusions during longitudinal monitoring, which offers patients a chance for real-time assessment of therapeutic targetability in an era with the expanded availability of targeted therapy.33,34
Development of RET inhibitors: A historical perspective
Treatment of RET-altered cancers has been quite challenging since response rates to chemotherapy were relatively low. Moreover, limited response and progression-free survival (PFS) benefit has been shown with immunotherapy, possibly due to low levels of Programmed Death Ligand 1 (PDL1) expression and low mutation burden. 35 The first potential for targeting RET alterations came historically from studies that were done on MKIs. 2 Cabozantinib and vandetanib have emerged, among other MKIs, in that regard as key players with evidence of their activity in RET-altered cancers. For example, an objective response rate (ORR) of 28% was observed with cabozantinib in patients with previously treated RET fusion-positive NSCLC. 36 Vandetanib has also demonstrated an ORR of 17% in a similar patient population. Nevertheless, the wide spectrum of toxicities primarily attributed to nonselective inhibition of tyrosine kinases including non-target ones was quite devastating. Moreover, the durability of the response was also another concern.2,37
With that in mind, further efforts have led to the introduction of more selective RET-targeting agents.38,39 So far, two agents, selpercatinib and pralsetinib, have shown promising results in treating RET-driven cancers. Data from clinical trials suggested a potential for both drugs in RET fusion-positive cancers and led to their inclusion in standard of care treatment guidelines 40 (Table 1). This review will primarily focus on selpercatinib and its activity in RET fusion-positive cancers starting with NSCLC and expanding beyond that to tissue-agnostic activity.
Table 1.
Summary of data on FDA and EMA-approved selective RET inhibitors in RET fusion-positive solid tumors.
| Drug | Clinical trial | FDA indication | EMA indication | Data |
|---|---|---|---|---|
| Selpercatinib | LIBRETTO-001, NCT03157128 | Adult patients with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options | ORR = 43.9% 41 | |
| Adult patients with locally advanced or metastatic NSCLC with a RET gene fusion | Advanced RET fusion-positive NSCLC not previously treated with a RET inhibitor | ORR = 84% and 61% in untreated and previously treated patients42,43 | ||
| Adult and pediatric patients 12 years of age and older with advanced or metastatic thyroid cancer with a RET gene fusion who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate) | Advanced RET fusion-positive thyroid cancer who require systemic therapy following prior treatment with sorafenib and/or levatinib | ORR = 79% 44 | ||
| Pralsetinib | ARROW, NCT03037385 | Adult patients with metastatic RET fusion-positive NSCLC | Adult patients with RET fusion-positive advanced NSCLC not previously treated with a RET inhibitor | ORR = 70% and 61% in untreated and previously treated patients 45 |
| Adult and pediatric patients 12 years of age and older with advanced or metastatic RET fusion-positive thyroid cancer who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate) | ORR = 89% 46 |
EMA = European Medicines Agency; FDA = Food and Drug Administration; NSCLC, non-small cell lung cancer; ORR, objective response rate; RET, Rearranged during Transfection.
Selpercatinib
Mechanism of action and preclinical data
Selpercatinib is a selective small molecule inhibitor of RET kinase via ATP competitive mechanism. Preclinical studies have shown that selpercatinib possesses high selective potency against different RET alterations, including fusions and mutations.47,48
Clinical development in NSCLC
Evidence in favor of using selpercatinib in RET fusion-positive NSCLC came from the LIBRETTO-001 trial. LIBRETTO-001 was an open-label phase 1–2 clinical trial including patients with advanced or metastatic solid tumors who harbor RET alterations (fusions and mutations). Patients in the phase 2 portion received 160 mg twice daily and were allowed to continue treatment beyond progression per investigator’s evaluation of clinical benefit.
A total of 247 patients with heavily pretreated and 69 patients with treatment naïve RET fusion-positive NSCLC were included as part of LIBRETTO-001. ORR was 61% (95% Cl: 55–67) in pretreated patients—including 18 patients with complete response, and 84% (95% CI: 73–92) in previously untreated patients – including four patients with complete response. The median PFS was 24.9 months (95% CI: 19.3–not reached) and 22 months (95% CI: 13.8–not reached) in previously treated and previously untreated patients, respectively.42,43 Intracranial activity was quite impressive in 22 patients with measurable central nervous system (CNS) metastasis who showed an ORR of 82% (95% CI: 60–95)—including 23% complete responses. In 80 patients with NSCLC and intracranial disease, the median intracranial PFS was 13.7 months (95% CI: 10.9–not reached). 49 In an updated analysis including 106 patients with baseline intracranial disease, intracranial ORR was 85% (95% CI: 65–96) with a median PFS of 19.4 months (95% CI: 13.8–not reached). The calculated probability of CNS progression in brain metastasis-free patients who received selpercatinib was only 0.7% at 2 years. 43 Based on results from the NSCLC cohort analysis in LIBRETTO-001, selpercatinib received its FDA approval for treatment of metastatic RET fusion-positive NSCLC in 2020. 50
Since LIBRETTO-001 was a single-arm study, an effort to explore the comparative effectiveness of selpercatinib by pooling patient-level data from matched patients in real world, pemetrexed/platinum arm of the KEYNOTE-189 trial, and docetaxel arm of REVEAL trial. PFS was significantly longer for selpercatinib (median not reached) versus pemetrexed and platinum in KEYNOTE-189 (median 12 months) and docetaxel (median 9 months) using targeted maximum likelihood estimation. 51
Selpercatinib maintained its efficacy in NSCLC and tolerable safety profile when tested in different patient populations and different disease settings. For example, in a population with Japanese patients (n = 44 previously treated and 4 previously untreated), the ORR was 55.4%. Another study (LIBRETTO-321; NCT04280081) included Chinese patients with RET-altered cancers. In 47 patients with RET fusion-positive NSCLC, ORR was 69.2% (95% CI: 48.2–85.7). 52 Beyond clinical trials, in a real-world retrospective study, selpercatinib was demonstrated to achieve an ORR of 68% and a disease control rate of 92% in 50 patients with RET fusion-positive NSCLC. This was quite interesting given the inclusion of 14 patients (28%) who had a performance status of ⩾2 who would classically be excluded from clinical trials. 53
Clinical development beyond NSCLC
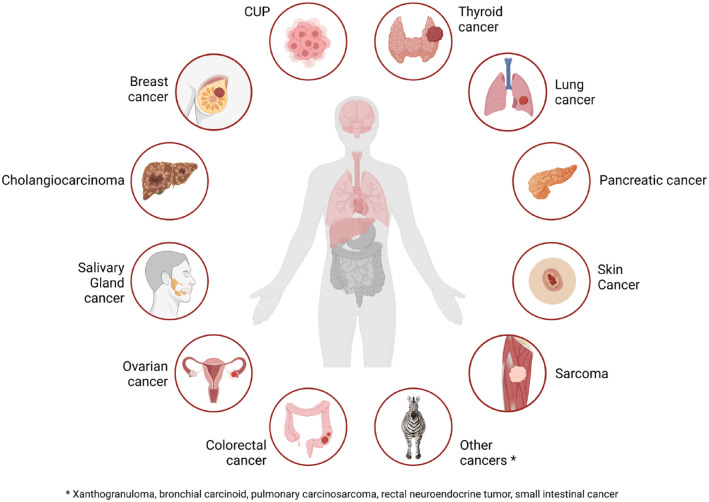
In addition to NSCLC, the initial FDA approval for selpercatinib included patients with advanced or metastatic RET mutant medullary thyroid carcinoma and patients with advanced or metastatic RET fusion-positive thyroid cancer; based on reports with promising results in those other two other cohorts of LIBRETTO-001. 44 For example, the RET fusion-positive thyroid cancer group showed an ORR of 79% (95% CI: 54–94). 44 This cohort had patients with variable thyroid cancer histologies including papillary, poorly differentiated, hurthle cell, and anaplastic carcinomas. 44 Interestingly, selpercatinib use has been demonstrated to enhance radioactive iodine uptake in RET-rearranged thyroid cancer, probably via a drug-induced histological redifferentiation.54,55 An updated report was published for other cohorts of LIBRETTO-001 and was the basis for the tissue-agnostic approval in 2022. 50 In 45 patients with RET fusion-positive non-lung and non-thyroid cancers (12 pancreatic cancer, 10 colon cancer, 4 salivary gland cancer, 3 sarcoma, 3 cancer of unknown primary, 2 breast cancer, 2 skin cancer, 2 cholangiocarcinoma, 2 xanthogranuloma, 1 carcinoid syndrome, 1 ovarian cancer, 1 pulmonary carcinosarcoma, 1 rectal neuroendocrine tumor, and 1 small intestinal cancer), the ORR was 43.9% (95% CI: 28.5–60.3)—including two patients with complete response (Figure 2). The median PFS assessed by independent reviewers was 13.2 months (95% CI: 7.4–26.2). 41
Figure 2.
Pan-cancer efficacy of selpercatinib in RET fusion-positive solid tumors.
RET, Rearranged during Transfection.
Drug-induced toxicities
Despite having a tolerable toxicity profile, the use of selpercatinib has been linked to the occurrence of multiple toxicities that can be quite distinct. For example, chylous effusions have been described in patients treated with selpercatinib. 56 Hypersensitivity reactions have also been reported in selpercatinib-treated patients regardless of prior use of immunotherapy. 57 Other common adverse events include fatigue, hypertension, rash, dry mouth, nausea, abdominal pain, diarrhea, constipation, edema, and headache. 50
Resistance to selpercatinib
Multiple mechanisms of acquired resistance, which commonly limits the durability of response with tyrosine kinase inhibitors, are also being increasingly reported with selpercatinib. While selpercatinib can structurally evade the gatekeeper mutations of RET by wrapping around the tyrosine kinase, 58 resistance to first-generation RET inhibitors, including selpercatinib, has been reported to occur as a result of acquired mutation at the non-gatekeeper sites; namely, solvent front and hinge sites of RET kinase; including RET Y806 and RET G810 mutations.58,59 These form the basis for the design of second-generation RET inhibitors. For example, Solomon et al. demonstrated using cfDNA samples from a patient with CCDC6–RET NSCLC with prior dramatic response to selpercatinib the emergence of RET G810C mutation at the time of progression.59,60 In addition to G810 mutations, other RET-independent resistance mechanisms have also been reported in RET inhibitor-treated patients including amplifications of MET and KRAS genes.61,62NTRK3 fusion as a mechanism of resistance has also been reported in RET fusion-positive lung cancer. 63
Different approaches have been suggested to overcome such resistance including combination with other targeted agents, for example, crizotinib. 61 Moreover, second-generation drugs are currently being explored in early-phase trials and will hopefully delay the emergence of these mutations with a benefit in expanding PFS.
Clinical trials with selpercatinib in multiple settings and future perspectives
The tissue-agnostic approval of selpercatinib was a landmark in biomarker-driven precision oncology. However, multiple studies are currently ongoing to explore the expanded potential of selpercatinib in RET fusion-positive cancers (Table 2). These are primarily focused on testing in different disease settings and patients’ populations. For example, a phase 3 trial (LIBRETTO-432; NCT04819100) is investigating the use of selpercatinib in the adjuvant setting compared to placebo when given to patients with early-stage NSCLC after curative intent surgery or radiation therapy. 64 In the neoadjuvant setting, NCT04759911 is a phase 2 trial that is evaluating preoperative selpercatinib in patients with thyroid cancer and RET alterations. 65
Table 2.
Examples of ongoing clinical trials for selpercatinib in RET fusion-positive cancers.
| Clinical trial | Phase | Setting | Population |
|---|---|---|---|
| LIBRETTO-432 | 3 | Adjuvant | Patients with early-stage NSCLC after curative intent surgery or radiation therapy |
| NCT04759911 | 2 | Neoadjuvant | Patients with thyroid cancer |
| LIBRETTO-431 | 3 | Advanced | Patients with advanced or metastatic RET fusion-positive nonsquamous NSCLC |
| Lung-MAP | 2 | Advanced | Patients with RET fusion-positive recurrent or metastatic NSCLC |
| ORCHARD | 2 | Advanced | Patients with advanced NSCLC who progressed after treatment with first-line osimertinib |
| FINPROVE | 2 | Advanced | Patients with advanced solid tumors that harbor a RET alteration |
| LIBRETTO-121 | 1/2 | Advanced | Pediatric patients with advanced solid tumors and primary CNS tumors not including lung cancer that harbor a RET alteration |
| Pediatric-MATCH | 2 | Advanced | Pediatric patients with RET-altered cancers |
NSCLC, non-small cell lung cancer; RET, Rearranged during Transfection.
In the advanced and metastatic setting, LIBRETTO-431 (NCT04194944) continues to evaluate the efficacy of selpercatinib in patients with advanced or metastatic RET fusion-positive non-squamous NSCLC. Patients are randomized to receive either selpercatinib or standard platinum-based and pemetrexed-based therapy with or without pembrolizumab as first-line treatment. 60 Selpercatinib is also tested as part of the Lung-MAP lung cancer Master Protocol which is an umbrella trial that includes patients with advanced NSCLC for the purpose of testing various therapeutic regimens including selpercatinib. For example, phase 2 Lung-MAP (NCT05364645) investigates carboplatin and pemetrexed with or without selpercatinib in patients with RET fusion-positive recurrent or metastatic NSCLC. Another arm of Lung-MAP evaluates selpercatinib as a single-agent in the same disease setting. 66 Selpercatinib is also being studied as part of the phase 2 platform study (ORCHARD; NCT03944772) in patients with advanced NSCLC who progressed after treatment with first-line osimertinib. 67 This is also the case in the phase 2 Finnish trial (FINPROVE) which includes patients with advanced solid tumors that harbor a RET alteration. 68
In the pediatric patient population, LIBRETTO-121 (NCT03899792) is a phase 1/2 trial evaluating selpercatinib in patients with advanced solid tumors and primary CNS tumors, not including lung cancer, that harbors a RET alteration. Moreover, the phase 2 pediatric MATCH trial (NCT04320888; NCT03155620) is studying selpercatinib in RET-altered cancers in the pediatric patient population (⩽21 years).
Conclusion
Selpercatinib has led to a paradigm change in the management of RET fusion-positive solid tumors including NSCLC and thyroid cancer. Its current tissue-agnostic approval highlights the potential it has in different tumor types. Multiple studies are ongoing with the aim of exploring selpercatinib use in other disease settings and different patients’ populations.
Acknowledgments
Figures were created using tools from Biorender.com.
Footnotes
ORCID iD: Vivek Subbiah  https://orcid.org/0000-0002-6064-6837
https://orcid.org/0000-0002-6064-6837
Contributor Information
Mohamed A. Gouda, Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center. Houston, TX, USA
Vivek Subbiah, Sarah Cannon Research Institute, 1100 Dr. Martin L. King Jr. Blvd. Suite 800. Nashville, TN 37203, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Mohamed A. Gouda: Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Vivek Subbiah: Conceptualization; Formal analysis; Funding acquisition; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: V. Subbiah was at UT MD Anderson Cancer Center, Houston, TX, USA when this article was submitted and is currently affiliated to Sarah Cannon Research Institute, Nashville, TN, USA when this article was published. V. Subbiah is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. V. Subbiah acknowledges the support of The Jacquelyn A. Brady Fund. V. Subbiah is supported by NIH grants (R01CA242845 and R01CA273168); the MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention & Research Institute of Texas (RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (1U01 CA180964), a National Center for Advancing Translational Sciences Clinical and Translational Science Award (UL1 TR000371), and an MD Anderson Cancer Center Support Grant (P30 CA016672).
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vivek Subbiah reports research funding/grant support for clinical trials from AbbVie, Agensys, Inc., Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines Corporation, Boston Biomedical, Inc., Boston Pharmaceuticals, Celgene Corporation, D3 Bio, Inc., Dragonfly Therapeutics, Inc., Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Inc., Incyte Corporation, Inhibrx, Loxo Oncology, MedImmune, MultiVir, Inc., NanoCarrier, Co., National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center, and Vegenics Pty Ltd.; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte Corporation, Novartis, and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Jazz Pharmaceuticals, Incyte Corporation, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo, and R-Pharm US; and other relationship with Medscape. Mohamed Gouda has no conflicts of interest to disclose.
Availability of data and materials: Not applicable.
References
- 1.Takahashi M, Ritz J, Cooper GM.Activation of a novel human transforming gene, RET, by DNA rearrangement. Cell 1985; 42: 581–588. [DOI] [PubMed] [Google Scholar]
- 2.Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018; 15: 150–167. [DOI] [PubMed] [Google Scholar]
- 3.Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev 2019; 81: 101911. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaka Y, Itoh F, Tahira T, et al. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989; 4: 1519–1521. [PubMed] [Google Scholar]
- 5.Gainor JF, Shaw AT.Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 2013; 18: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faivre S, Djelloul S, Raymond E.New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol 2006; 33: 407–420. [DOI] [PubMed] [Google Scholar]
- 7.Qian Y, Chai S, Liang Z, et al. KIF5B-RET fusion kinase promotes cell growth by multilevel activation of STAT3 in lung cancer. Mol Cancer 2014; 13: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phay JE, Shah MH.Targeting RET receptor tyrosine kinase activation in cancer. Clin Cancer Res 2010; 16: 5936–5941. [DOI] [PubMed] [Google Scholar]
- 9.Alberti L, Carniti C, Miranda C, et al. RET and NTRK1 proto-oncogenes in human diseases. J Cell Physiol 2003; 195: 168–186. [DOI] [PubMed] [Google Scholar]
- 10.Arighi E, Borrello MG, Sariola H.RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 2005; 16: 441–467. [DOI] [PubMed] [Google Scholar]
- 11.Jhiang SM.The RET proto-oncogene in human cancers. Oncogene 2000; 19: 5590–5597. [DOI] [PubMed] [Google Scholar]
- 12.Donis-Keller H, Dou S, Chi D, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 1993; 2: 851–856. [DOI] [PubMed] [Google Scholar]
- 13.Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994; 367: 375–376. [DOI] [PubMed] [Google Scholar]
- 14.Mulligan LM, Kwok JB, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993; 363: 458–460. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012; 30: 4352–4359. [DOI] [PubMed] [Google Scholar]
- 16.Adashek JJ, Desai AP, Andreev-Drakhlin AY, et al. Hallmarks of RET and co-occuring genomic alterations in RET-aberrant cancers. Mol Cancer Ther 2021; 20: 1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato S, Subbiah V, Marchlik E, et al. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res 2017; 23: 1988–1997. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012; 18: 378–381. [DOI] [PubMed] [Google Scholar]
- 19.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012; 18: 382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt A, Morten J, Ji Q, et al. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized phase III studies. BMC Cancer 2015; 15: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohno T, Tsuta K, Tsuchihara K, et al. RET fusion gene: translation to personalized lung cancer therapy. Cancer Sci 2013; 104: 1396–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michels S, Scheel AH, Scheffler M, et al. Clinicopathological characteristics of RET rearranged lung cancer in European patients. J Thorac Oncol 2016; 11: 122–127. [DOI] [PubMed] [Google Scholar]
- 23.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7–33. [DOI] [PubMed] [Google Scholar]
- 24.Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol 2018; 13: 1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belli C, Penault-Llorca F, Ladanyi M, et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol 2021; 32: 337–350. [DOI] [PubMed] [Google Scholar]
- 26.Tsuta K, Kohno T, Yoshida A, et al. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer 2014; 110: 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Go H, Jung YJ, Kang HW, et al. Diagnostic method for the detection of KIF5B-RET transformation in lung adenocarcinoma. Lung Cancer 2013; 82: 44–50. [DOI] [PubMed] [Google Scholar]
- 28.Cheng TY, Cramb SM, Baade PD, et al. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol 2016; 11: 1653–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikiforov YE.Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol 2008; 21: S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizukami T, Shiraishi K, Shimada Y, et al. Molecular mechanisms underlying oncogenic RET fusion in lung adenocarcinoma. J Thorac Oncol 2014; 9: 622–630. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara R, Auger N, Auclin E, et al. Clinical and translational implications of RET rearrangements in non-small cell lung cancer. J Thorac Oncol 2018; 13: 27–45. [DOI] [PubMed] [Google Scholar]
- 32.Rich TA, Reckamp KL, Chae YK, et al. Analysis of cell-free DNA from 32,989 advanced cancers reveals novel co-occurring activating RET alterations and oncogenic signaling pathway aberrations. Clin Cancer Res 2019; 25: 5832–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolfo C, Mack P, Scagliotti GV, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of lung cancer. J Thorac Oncol 2021; 16: 1647–1662. [DOI] [PubMed] [Google Scholar]
- 34.Krebs MG, Malapelle U, André F, et al. Practical considerations for the use of circulating tumor DNA in the treatment of patients with cancer: a narrative review. JAMA Oncol 2022; 8: 1830. [DOI] [PubMed] [Google Scholar]
- 35.Thein KZ, Velcheti V, Mooers BHM, et al. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer 2021; 7: 1074–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016; 17: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol 2017; 35: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahal R, Evans EK, Hu W, et al. Abstract 2641: the development of potent, selective RET inhibitors that target both wild-type RET and prospectively identified resistance mutations to multi-kinase inhibitors. Cancer Res 2016; 76: 2641–2641. [Google Scholar]
- 39.Brandhuber BJ, Nanda N, Haas J, et al. Abstract B192: identification and characterization of highly potent and selective RET kinase inhibitors for the treatment of RET-driven cancers. Mol Cancer Ther 2015; 14: B192–B192. [Google Scholar]
- 40.Singh N, Temin S, Baker S, Jr, et al. Therapy for Stage IV non-small-cell lung cancer without driver alterations: ASCO Living Guideline. J Clin Oncol 2022; 40: 3323–3343. [DOI] [PubMed] [Google Scholar]
- 41.Subbiah V, Wolf J, Konda B, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol 2022; 23: 1261–1273. [DOI] [PubMed] [Google Scholar]
- 42.Drilon A, Oxnard GR, Tan DSW. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. New Engl J Med 2020; 383: 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drilon A, Subbiah V, Gautschi O, et al. Selpercatinib in patients with RET fusion–positive non–small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol 2022; 41: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. New Engl J Med 2020; 383: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 2021; 22: 959–969. [DOI] [PubMed] [Google Scholar]
- 46.Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 2021; 9: 491–501. [DOI] [PubMed] [Google Scholar]
- 47.Brandhuber B, Haas J, Tuch B, et al. The development of a potent, KDR/VEGFR2-sparing RET kinase inhibitor for treating patients with RET-dependent cancers. Eur J Cancer 2016; 69: S144–S144. [Google Scholar]
- 48.Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 2018; 29: 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res 2021; 27: 4160–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.FDA. RETEVMO™ (selpercatinib) capsules, for oral use: FDA Packaging Insert, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213246s008lbl.pdf
- 51.Rolfo C, Hess LM, Jen MH, et al. External control cohorts for the single-arm LIBRETTO-001 trial of selpercatinib in RET+ non-small-cell lung cancer. ESMO Open 2022; 7: 100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu S, Cheng Y, Huang D, et al. Efficacy and safety of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer: a phase II clinical trial (LIBRETTO-321). Ther Adv Med Oncol 2022; 14: 17588359221105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Illini O, Hochmair MJ, Fabikan H, et al. Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): a retrospective analysis of patients treated through an access program. Ther Adv Med Oncol 2021; 13: 17588359211019675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groussin L, Bessiene L, Arrondeau J, et al. Selpercatinib-enhanced radioiodine uptake in RET-rearranged thyroid cancer. Thyroid 2021; 31: 1603–1604. [DOI] [PubMed] [Google Scholar]
- 55.Lee YA, Lee H, Im SW, et al. NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J Clin Investig 2021; 131: e144847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prete A, Gambale C, Cappagli V, et al. Chylous effusions in advanced medullary thyroid cancer patients treated with selpercatinib. Eur J Endocrinol 2022; 187: 905–915. [DOI] [PubMed] [Google Scholar]
- 57.McCoach CE, Rolfo C, Drilon A, et al. Hypersensitivity reactions to selpercatinib treatment with or without prior immune checkpoint inhibitor therapy in patients with NSCLC in LIBRETTO-001. J Thorac Oncol 2022; 17: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subbiah V, Shen T, Terzyan SS, et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol 2021; 32: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solomon BJ, Tan L, Lin JJ, et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J Thorac Oncol 2020; 15: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solomon B, Zhou CC, Drilon A, et al. Phase III study of selpercatinib versus chemotherapy +/− pembrolizumab in untreated RET positive non-small-cell lung cancer. Future Oncol 2021; 17: 763–773. [DOI] [PubMed] [Google Scholar]
- 61.Rosen EY, Johnson ML, Clifford SE, et al. Overcoming MET-dependent resistance to selective RET inhibition in patients with RET fusion-positive lung cancer by combining selpercatinib with crizotinib. Clin Cancer Res 2021; 27: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin JJ, Liu SV, McCoach CE, et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann Oncol 2020; 31: 1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Subbiah V, Shen T, Tetzlaff M, et al. Patient-driven discovery and post-clinical validation of NTRK3 fusion as an acquired resistance mechanism to selpercatinib in RET fusion-positive lung cancer. Ann Oncol 2021; 32: 817–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuboi M, Goldman JW, Wu YL, et al. LIBRETTO-432, a phase III study of adjuvant selpercatinib or placebo in stage IB-IIIA RET fusion-positive non-small-cell lung cancer. Future Oncol 2022; 18: 3133–3141. [DOI] [PubMed] [Google Scholar]
- 65.Selpercatinib Before Surgery for the Treatment of RET-Altered Thyroid Cancer, https://clinicaltrials.gov/ct2/show/NCT04759911?term=Selpercatinib&draw=3&rank=10 (2021, accessed 12 July 2022). [DOI] [PubMed]
- 66.Steuer CE, Papadimitrakopoulou V, Herbst RS, et al. Innovative clinical trials: the LUNG-MAP Study. Clin Pharmacol Ther 2015; 97: 488–491. [DOI] [PubMed] [Google Scholar]
- 67.Yu HA, Goldberg SB, Le X, et al. Biomarker-directed phase II platform study in patients with EGFR sensitizing mutation-positive advanced/metastatic non-small cell lung cancer whose disease has progressed on first-line osimertinib therapy (ORCHARD). Clin Lung Cancer 2021; 22: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The Finnish National Study to Facilitate Patient Access to Targeted Anti-cancer Drugs (FINPROVE), https://clinicaltrials.gov/ct2/show/NCT05159245?term=Selpercatinib&draw=3&rank=27 (2021, accessed 12 July 2022).