Abstract
Cyanobacteria of the genus Trichodesmium provide about 80 Tg of fixed nitrogen to the surface ocean per day and contribute to marine biogeochemistry including the sequestration of carbon dioxide (CO2). Trichodesmium fixes nitrogen in the daylight, despite the incompatibility of the nitrogenase enzyme with oxygen produced during photosynthesis. While the mechanisms protecting nitrogenase remain unclear, all proposed strategies require considerable resource investment. Here we identify a crucial benefit of daytime nitrogen fixation in Trichodesmium spp. that may counteract these costs. We analyzed diel proteomes of cultured and field populations of Trichodesmium in comparison to the marine diazotroph Crocosphaera watsonii sp. WH8501, which fixes nitrogen at night. Trichodesmium’s proteome was extraordinarily dynamic and demonstrated simultaneous photosynthesis and nitrogen fixation, resulting in balanced particulate organic carbon (POC) and particulate organic nitrogen (PON) production. Unlike Crocosphaera, which produces large quantities of glycogen as an energy store for nitrogenase, proteomic evidence is consistent with the idea that Trichodesmium reduces the need to produce glycogen by supplying energy directly to nitrogenase via soluble ferredoxin charged by PsaC. This minimizes ballast associated with glycogen, reducing cell density and decreasing sinking velocity, thus supporting Trichodesmium’s niche as a buoyant, high-light adapted, colony forming cyanobacterium. In order to occupy its niche of simultaneous nitrogen fixation and photosynthesis, Trichodesmium appears to have resigned itself to being a conspicuous consumer of iron, and has therefore developed unique iron acquisition strategies including the use of iron-rich dust. Particle capture by buoyant Trichodesmium colonies may increase the residence time and degradation of mineral iron in the euphotic zone. These findings describe how cellular biochemistry defines and reinforces the ecological and biogeochemical function of these keystone marine diazotrophs.
Biological nitrogen (N2) fixation is a crucial biogeochemical process, being foundational to marine microbial food webs and primary productivity in N-limited ecosystems1–6. A limited number of marine microorganisms can fix nitrogen, and they have distinct yet overlapping niches in the N-limited surface ocean7,8. The ecological drivers differentiating the habitats of diverse marine nitrogen fixers are uncertain but critical for understanding the current and future global N budget.1 As a result our ability to resolve environmental and biochemical controls on nitrogen fixation rates remains limited9–12.
Here, we examine an important yet enigmatic marine nitrogen fixer, Trichodesmium, which contributes significant amounts of fixed nitrogen to the ocean annually, and due to vertical export may disproportionately affect nitrogen and carbon cycling at depth12–15. Trichodesmium performs nitrogen fixation and photosynthesis simultaneously during the photoperiod, and this is perplexing because the nitrogenase enzyme is susceptible to damage by molecular oxygen produced by photosystem II16. It is unclear how Trichodesmium protects the sensitive nitrogenase enzyme from damage by molecular oxygen. In general, photosynthesizing diazotrophs have evolved two main strategies for solving this problem – some, like Crocosphaera, separate the processes temporally, fixing carbon during the day and nitrogen at night17–19. Others, like Anabaena, separate the processes spatially, forming differentiated nitrogen-fixing heterocyst cells that lack the oxygen evolving photosystem II complex20–23. While it has been hypothesized that Trichodesmium forms partially differentiated “diazocyte” cells for the purpose of nitrogen fixation, the evidence for this strategy remains mixed and controversial24–31. Fine-tuned temporal separation of peak nitrogen fixation and photosynthetic activity has also been suggested26,32, though it is also possible for the processes to occur simultaneously33. Regardless of the exact mechanisms, it is clear that daytime nitrogen fixation requires considerable cellular coordination and resource investment in order to protect the vulnerable nitrogenase enzyme.
This study therefore addresses a key question – why does Trichodesmium fix nitrogen during the day? Given that Trichodesmium is ecologically successful and widely distributed in the marine environment, the benefits of daytime nitrogen fixation must outweigh the costs involved in protecting the nitrogenase enzyme from degradation by oxygen. Since cyanobacterial physiology, in particular nitrogen fixation and photosynthesis, is strongly dependent on daily rhythms, we investigated the proteomes of model organism T. erythraeum sp. IMS101 as well as field populations from the Atlantic Ocean during the diel cycle to reveal key biochemical insights on nitrogen and carbon fixation. We then compared the diel proteome of Trichodesmium to the previously published diel proteome of Crocosphaera watsonii WH8501, which fixes nitrogen at night34. Together these high-resolution datasets provide an opportunity to probe the molecular physiology of this unique nitrogen fixer, and to understand the intersection of biochemical activity that allows Trichodesmium species to occupy their important niche in the marine ecosystem.
Whole proteome dynamics of Trichodesmium erythraeum sp. IMS101 vs. Crocosphaera watsonii sp. WH8501
The diel proteome of Trichodesmium erythraeum sp. IMS101 was explored by high-resolution global proteomics analysis of triplicate cultures maintained in a 14:10 light/dark cycle, with ramped dawn and dusk transitions mimicking those in subtropical gyres. Samples were collected every 1 to 3 hours for proteomics, biomass CHN analysis, and nitrogenase activity via acetylene reduction35. The cultures were grown in RMP media supplied with ample phosphorus and trace metal nutrients36. Exponential growth (0.7–0.8 doublings per day) was observed by total protein content (Extended Data Figure 1b). The Trichodesmium filaments appeared healthy, and colonies, which in T. erythraeum are indicative of halted growth, nutrient stress, and/or redox stress35,37–40, were not observed.
The proteomics analysis provided a global look at the molecular physiology of the Trichodesmium cultures. In total, 2492 proteins were identified, representing 56% of the T. erythraeum sp. IMS101 predicted protein encoding genes (Table S1). Protein abundances are reported as Normalized Spectral Abundance Factor (NSAF) normalized spectral counts to control for protein size and small variation in the total protein (~1 μg) injected across the samples. The NSAF normalization therefore allows relative protein abundances to be compared across the experiment. Total spectral counts did not vary systematically across the experiment indicating consistency in proteome depth across the analytical runs (Extended Data Figure 1a). However, based on a colorimetric assay total protein content per volume of culture was greater at night, indicative of night-time protein accumulation and/or higher protein degradation and turnover during the day consistent with the enrichment of protease and peptidase enzymes during the photoperiod (Extended Data Figure 1b, Supplemental Figure S2).
Trichodesmium’s proteome was highly dynamic and key systems such as nitrogen fixation, photosynthesis, central carbohydrate metabolism and ribosomal proteins oscillated four times and as much as 200% within a few hours (Figure 1A and B). Major proteome components included phycobilisome proteins, growth-related proteins (ribosomes and chaperonin GroEL), nitrogenase, and the photosystems (Table S2, Supplementary Figure S3). Nitrogen fixation, light-driven electron transport, photosynthesis and related processes including ATP synthesis (dark blue cluster) clustered separately from most other functional modules (green cluster), indicating temporal separation of nitrogen fixation/photosynthesis from other metabolic activities (Figure 1A). The offsetting of these proteome clusters can be clearly seen in Figure 1B, where the general patterns of protein abundance in the clusters are mirror images of one another. There was no general increase or decrease in protein abundances during the diel cycle that would be indicative of culture effects during the 24-hour experiment (Extended Data Figure 2). The surprisingly rapid dynamics of the proteome were reproduced in three independent proteomic observations: the main experiment described above, a separate replicate experiment conducted one year prior (Extended Data Figure 3, Table S3) and in field populations of Trichodesmium sampled over a diel cycle on March 10, 2018 from the Eastern subtropical Atlantic 65 22.420 °W 17 0.284 °N (Extended Data Figure 4, Table S4). Trichodesmium has a large number of two component regulatory systems, proteases, and peptidases which were abundant, cycled continuously throughout the day and were likely involved in regulating these rapid proteome changes (Table S2 and Supplementary Figure S2).
Figure 1.
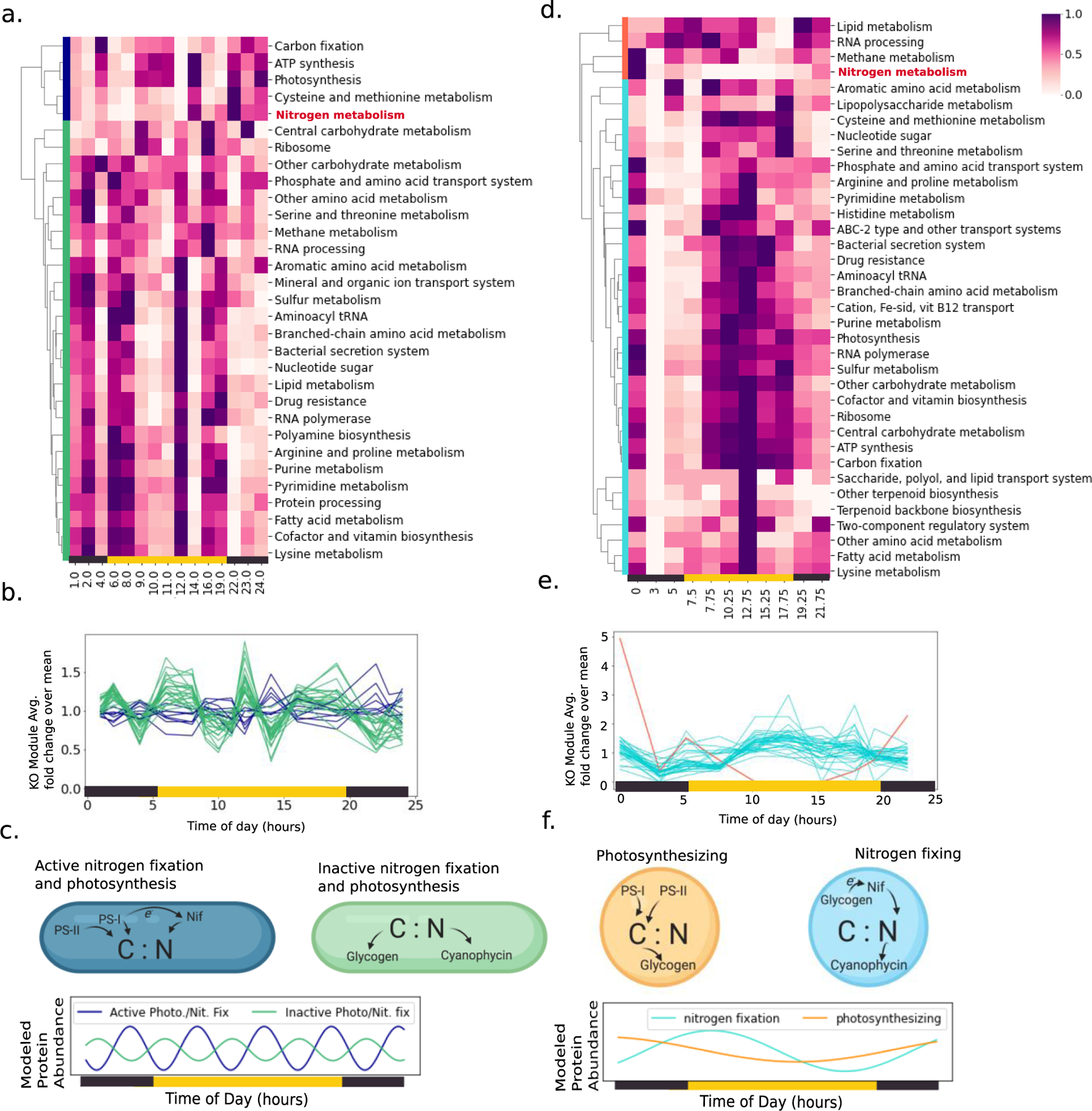
Dynamics of the Trichodesmium proteome in comparison to Crocosphaera. a. Heatmap of the T. erythraeum sp. IMS101 proteome during the diel cycle. Proteins were gathered into KO Modules, summed for each time point, averaged across the three biological replicates, then unit normalized across the row and clustered by an unweighted pair group method with arithmetic mean (UPGMA). Two clusters emerged, indicated by green and dark blue bars on the left hand side. Yellow and black bars at the bottom of the heatmap indicate the light and dark periods, and numbering is hours after midnight. See supplemental figure S1 for a version of this heatmap where the clustering algorithm was also applied to the x axis (time of day). b. The same data with the abundance of each KO module presented as line and colored based on the clustering in panel (a). c. Summary of major physiological modes emergent from the diel proteome of Trichodesmium, with colors consistent with panels (a) and (b) and including active photosynthesis/nitrogen fixation (dark blue) and inactive photosynthesis/nitrogen fixation (green). Modeled protein abundances over the diel cycle were generated by fitting sinusoidal functions to the summed protein abundances in each cluster. Blue model coefficient of determination R2 = −5.7, green model R2 = −2.9. d-f. the same but for Crocosphaera watsonii sp. WH8501, data from ref [35]. Here, the major emergent physiological modes were photosynthesizing (orange), nitrogen fixing (turquoise). Turquoise model R2 = 0.62, orange model R2 = 0.63.
The overall dynamics of Trichodesmium’s proteome differed significantly from those of Crocosphaera, highlighting the unique nitrogen fixation strategy of Trichodesmium. In Trichodesmium, the proteome oscillated four times throughout the diel period, and in this respect the night was just as variable as the day (Figure 1A and B). By contrast, Crocosphaera’s proteome had only one complete oscillation, with most proteins being abundant in the photoperiod, and nitrogenase being most abundant at night consistent with dark nitrogen fixation (Figure 1 C and D). A single proteome oscillation based on light/dark cycling, and in particular the temporal separation of photosynthesis and nitrogenase proteins, is common in unicellular diazotrophs and contrasts starkly with the rapid oscillation of the Trichodesmium proteome (Figure 1E–F)42. We interpret the variation in Trichodesmium’s proteome to reflect the need to accommodate nitrogen fixation, photosynthesis, and/or other processes during the photoperiod, and note that the time scale of the observed changes suggests a crucial role for rapid regulatory processes such as protein post-translational modification, which were not profiled in this experiment and could lead to lower signals at the protein level.
Nitrogen fixation and photosynthesis occur simultaneously during the photoperiod in Trichodesmium
While in Crocosphaera nitrogen fixation was clearly temporally separated from photosynthesis, in Trichodesmium three independent lines of evidence demonstrated that these processes occur simultaneously during the photoperiod: nitrogenase protein concentrations, measured nitrogen fixation rates, and diel changes in organic POC and PON content.
First, photosynthesis and nitrogenase enzymes were highly abundant during the photoperiod and appeared coherently in the same protein cluster, suggesting that these processes are co-regulated (dark blue cluster in Figure 1A and B). The possibility of direct regulatory links between nitrogenase and the photosystem proteins is supported by similar observations of co-regulation in the metaproteomes of field populations sampled across geographic space43.Again, the clustering of the nitrogen fixation and photosynthesis proteins was reproducible in diel proteomes of Trichodesmium field populations and in a separate laboratory replicate conducted one year prior to the main experiment (Extended Data Figures 3 and 4). The diel pattern of nitrogenase protein abundance differed from previously reported patterns in nitrogenase transcripts, which generally increase during the day and decrease at night30. In the proteomes, all major subunits of the nitrogenase enzyme oscillated 3–4 times throughout the diel period, and were most abundant in the early morning and mid-afternoon with a dip at mid-day. Interestingly, we also observed a late-afternoon spike in nitrogenase abundance which was consistent across the replicates and in the field proteomes. These patterns were confirmed by absolute quantitation of the nitrogenase NifH, NifD, and NifK subunits using 15N labeled peptide standards (Figure 2a, Table S5). Unlike Crocosphaera, which completely degrades and resynthesizes its nitrogenase pool each day, in Trichodesmium nitrogenase never fully disappeared and was abundant even when nitrogen fixation rates inferred from acetylene reduction/ethylene production rates were below detection.
Figure 2.

Nitrogenase concentration and activity over the diel cycle. a. Absolute abundance of each of the main nitrogenase enzyme subunit proteins, calibrated using 15N labeled standards b. in vivo specific activity rate for NifH over the diel cycle, calculated by dividing acetylene reduction/ethylene production rates (see Fig 3c) by the measured concentration of the nitrogenase enzyme. Variation in the specific activity implies post-translational control of enzymatic activity30, 45.
The second line of evidence was provided by acetylene reduction/ethylene production rates, an analytical proxy for nitrogen fixation, which demonstrated that nitrogen fixation occurred mainly in the photoperiod, particularly in the early morning just after dawn. This approach was combined with the absolute protein concentration measurements to calculate nitrogenase specific activity rates throughout the diel period. The observed in vivo specific activity varied between 261 and 1934 nmol ethylene mg NifH−1 min−1, and was greatest in the early morning hours, particularly after dawn (Fig 2b). The specific activity is similar to those reported for other nitrogen fixers (900–1200 nmol ethylene NifH−1 min−1for Azotobacter vinelandii, scaling with growth rate)44. The variation in nitrogenase activity contrasted with Crocosphaera which exhibited a clearer on/off signal with nitrogenase only being present and highly active at night (Extended Data Figure 5). Together these observations are consistent with prior evidence that in Trichodesmium the nitrogenase iron protein NifH is regulated by post-translational modification, and/or that energy availability regulates nitrogenase activity30,45. The ability to maintain an intact pool of nitrogenase and modulate its activity by post-translational modification or other means may explain how diel patterns of nitrogen fixation are observed to respond to environmental conditions such as light availability46, pCO247,48, temperature49, and oxygen concentrations33.
The third line of evidence for simultaneous photosynthesis and nitrogen fixation was provided by temporal changes in particulate organic carbon (POC, Figure 3A) and particulate organic nitrogen (PON, Figure 3B), which were balanced throughout the day (Table S6). As neither fixed nitrogen nor carbon were provided to the cultures, changes in POC content can be attributed to carbon fixation and respiration, and changes in PON content attributed to nitrogen fixation and loss. |The relative balance of POC versus PON content can be used to infer excess production: excess carbon fixation occurs when POC and POC:PON increase, excess nitrogen fixation occurs when PON increases and POC:PON decreases, excess carbon respiration occurs when POC and POC:PON decrease, and excess nitrogen loss occurs when PON decreases and POC:PON increases. We also considered situations in which multiple processes occurred i.e. decreased POC:PON ratio associated with carbon respiration and nitrogen production, or increased POC:PON ratio associated with carbon production and nitrogen loss, but this was not observed. Based on this framework, excess nitrogen fixation occurred in the early morning, followed by excess carbon fixation around noon, and then consistency in the POC:PON ratio for much of the afternoon (Figure 3C–E and see also Supplementary Figure S4 and S5 and Table S7 which reports the results of an additional replicate consistent with Figure 3). This indicates simultaneous and generally balanced bulk production of POC and PON through coupled photosynthesis and nitrogen fixation, possibly a key benefit of daytime nitrogenase activity. POC content was only weakly correlated to protein content (r2 = 0.13, Extended Data Figure 6), consistent with previous reports that diel POC content is driven by changes in carbohydrate abundance, and that total protein content is relatively constant50. Relative stability in the POC:PON ratio of Trichodesmium has been observed previously49,51 and an implication is that carbon fixation rates can be used to estimate nitrogen production by Trichodesmium52.
Figure 3.
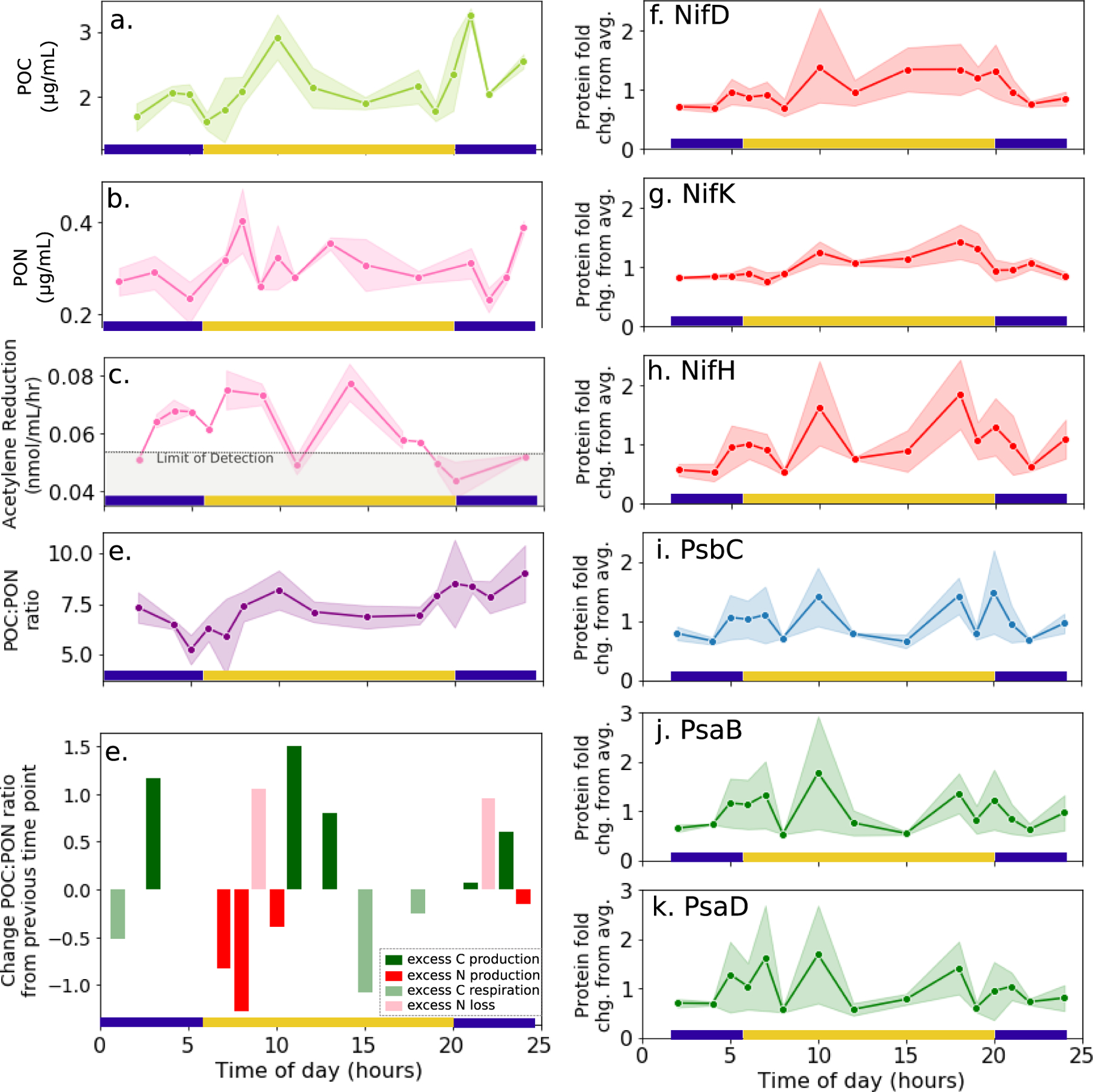
Temporal dynamics of a. POC, b. PON, c. acetylene reduction rates, and d. the POC:PON ratio in cultured T. erythraeum over the diel cycle. e. Temporal changes in the POC:PON ratio relative to previous time point, with bars color coded based on associated changes in either POC or PON content, with the possible processes being excess C production, excess N production, excess C respiration, or excess N loss (see text). On the right, associated temporal changes in the abundance of the three nitrogenase subunits (f-h), photosystem I protein PsbC (i) and photosystem II proteins PsaB and PsaD (j-k). Each scatter point represents the average value across the biological triplicates, and shaded areas represent 90% confidence intervals for the replicates calculated by bootstrapping (n=1000). Protein abundances are normalized to their average value across the entire diel cycle.
Together the nitrogenase protein concentrations, acetylene reduction rates, and POC/PON content clearly demonstrated daytime nitrogen fixation activity. However, whereas in some studies nitrogen fixation peaks at mid-day concurrent with decreased photosynthetic efficiency14,24,26, here mid-day was associated with a transient decrease in acetylene reduction rates (Figure 3C) consistent with the observed decrease in nitrogenase and photosystem protein abundance, (Figure 2A and Figure 3F–K), nitrogenase specific activity (Figure 2B), and cellular PON content (Figure 3B). The lack of consensus regarding the diel nitrogen fixation patterns of Trichodesmium is not surprising given multiple observations of its dependence environmental conditions. Indeed, diel patterns of nitrogen fixation based on acetylene reduction measurements vary significantly in the literature, and the observed peak occurs at different times ranging from mid-morning to mid-afternoon, indicating that diel nitrogen fixation and its regulation is not well-understood24,33,46,53–55.
Distinct roles for glycogen and nitrogenase in Trichodesmium vs. Crocosphaera
Glycogen production is an integral part of the ecological strategy of cyanobacteria, and particularly serves as a means to regulate stress due to nitrogen limitation or high-light exposure. Glycogen serves two major and related purposes, first as an energy storage compound and second to prevent damage to the photosystems by providing a safe sink for excess electrons when carbon fixation is hindered, for instance by lack of nitrogen56,57. Crocosphaera benefit from these functions by producing large amounts of glycogen during the day and consuming the stored energy to fuel nitrogenase activity at night; therefore in Crocosphaera glycogen content is positively correlated with photosynthesis protein abundance and negatively correlated with nitrogenase abundance34. By contrast, in Trichodesmium glycogen synthesis protein GlcG was strongly and negatively correlated to both the photosynthesis and nitrogen fixation proteins (Spearman correlation coefficient = −0.91, p = 1e−16) (Figure 4 A–E, Table S8), suggesting a fundamentally different role for glycogen production in the physiology of Trichodesmium that is related to its daytime nitrogen fixation patterns.
Figure 4.
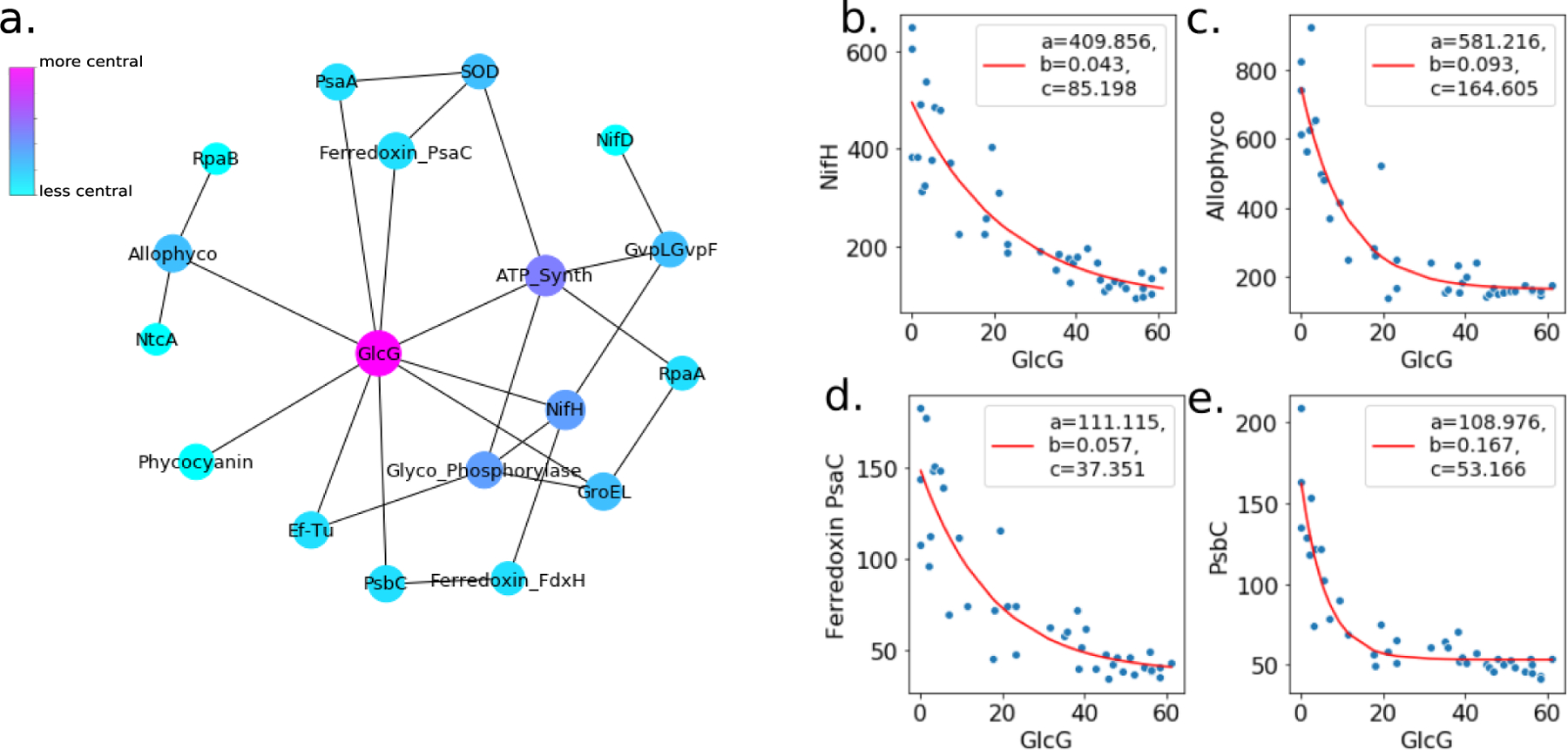
a. Network of negatively correlated photosynthesis and nitrogen fixation related proteins. Edges are drawn for protein pairs with Spearman rank-order correlation coefficients < −0.8 and p values of the correlation < 0.05 . Node size and color designate relative degree of closeness centrality in the network. c-e. Examples of negative protein abundance correlations used to build the network, shown here as a function of the central protein in the network, glycogen synthesis protein GlcG. Each point represents one independent biological observation, i.e. one of the replicates at one of the diel sampling time points. Curves were fit to the general exponential function ae(−bx) + c (red lines) via least-squares regression, and constants for the best fit are provided in the legend.
We suggest that the positive correlation between nitrogenase and the photosystems together with the negative correlation of these enzymes to the glycogen synthesis protein GlcG indicates that Trichodesmium uses the energy-demanding nitrogenase enzyme, which consumes 16 ATP and 8 electrons per reaction turnover58, in lieu of glycogen to modulate energy during the photoperiod. In this way, bursts of photosynthesis at defined intervals during the photoperiod may be linked to bursts of nitrogen fixation that not only relieve N-limitation, ensuring balanced POC and PON production, but also prevent damage to the photosystems by consuming excess light energy. The photosynthesis protein PsaC was positively correlated with the photosystem enzymes and nitrogenase, and may be the conduit for direct energy transfer between photosystem II and nitrogenase (Figure 4D). In this way, Trichodesmium is distinct from Crocosphaera, which uses the non-iron containing flavodoxin to provide electrons to nitrogenase in order to reduce cellular iron demand17. If electrons are supplied to nitrogenase via a soluble ferredoxin charged by PsaC, this would add to the realized iron requirement of the nitrogenase enzyme. However, the mechanism of electron transport to nitrogenase may be flexible depending on iron conditions, since Trichodesmium is known to substitute flavodoxin for ferredoxin during Fe stress and here only high iron conditions were considered59,60.
Daytime nitrogen fixation allows Trichodesmium to occupy a high-light niche
Daytime nitrogen fixation allows the direct shunt of electrons from the photosystems to nitrogenase, and may reduce glycogen production and the density of the cell, therefore helping Trichodesmium to remain neutrally buoyant. Trichodesmium cell density is clearly impacted by the amount of carbohydrate ballast in the cell, and glycogen represents a large fraction of this ballast50,61–63. In order to assess the benefits of daytime nitrogen fixation to Trichodesmium, we introduce a hypothetical night nitrogen fixation scenario in which, like Crocosphaera, the majority of nitrogen fixation is conducted in the dark (Table S9). In this hypothetical night nitrogen fixation scenario, Trichodesmium would have to produce glycogen to provide energy to nitrogenase, and this would increase the POC content of the cell by ~60% (Table 1, Supplemental Box 1). This would most likely cause Trichodesmium cells to sink given the high density of glycogen (0.5–2 g mL−1),64 and the observation that Trichodesmium already invests heavily in buoyancy maintenance by producing gas vesicles, the proteins for which are highly expressed (Table S2), and which occupy up to 70% of the cell volume61. Indeed, the glycogen content of Trichodesmium is much lower than in Crocosphaera indicating reduction of ballast in Trichodesmium cells (Figure 5B). Furthermore, the glycogen content was higher in Trichodesmium cells sampled at depth demonstrating that increased glycogen content is associated with sinking (Extended Data Figure 7).
Table 1.
Calculated benefits and costs of normal daytime nitrogen fixation to Trichodesmium, in comparison to the hypothetical night nitrogen fixation scenario described in the text. Details of the calculations are provided in the supplementary material. POC content is higher in the hypothetical night nitrogen fixation scenario in order to fuel the energy-demanding nitrogenase enzyme in the absence of light energy. Cellular Fe requirements may also differ - nighttime nitrogen fixation allows for “hot-bunking” between the nitrogenase and photosynthesis enzymes since the processes are separated temporally (*)17.
| Maximum POC Content | |
|---|---|
| Actual day nitrogen fixation scenario (μg/mL culture) | 3.976 |
| Hypothetical night nitrogen fixation scenario (μg/mL culture) | 6.362 |
| % increase hypothetical night nitrogen fixation vs. actual day nitrogen fixation | 60% |
| Minimum Fe bound to inactive nitrogenase | |
| Actual day nitrogen fixation scenario (μmol/mol POC) | 10 |
| Hypothetical night nitrogen fixation scenario (μmol/mol POC) | ~ 0* |
Figure 5.

a. The terminal velocity of phytoplankton particles of various characteristic diameters and specific gravities relative to seawater of density 1025 kg m−3 (see Table S9). Black contours indicate the value of the particle’s terminal velocity in m day−1, with negative values indicating upwards, floating velocity i.e. when the specific gravity is less than 1, and positive values indicating downwards, sinking velocity i.e. when the specific gravity is greater than 1. For a given species, cell density can differ depending on growth conditions (e.g. Prochlorococcus and SAR11, each displayed in exponential (sinking) and stationary (floating) growth). The density of the Trichodesmium particles was modelled using literature values and allowing 25% of the cell volume to be occupied by a gas vesicle, resulting in positive buoyancy. The hypothetical dark nitrogen fixation case increases the density of the Trichodesmium biomass by 60%, resulting in sinking. Arrows are used to indicate the change in terminal velocity for normal light nitrogen fixing and hypothetical dark nitrogen fixing Trichodesmium particles. For large colonies carrying dust particles, the additional mass of iron (1.03 μg Fe per 1 mm diameter colony) further exacerbates the sinking velocity. b. Glycogen content per μg total protein for Trichodesmium and Crocosphera cells over the diel cycle. Crocosphaera glycogen data from Saito et al., 2011 [35].
In contrast to small spheroid Crocosphaera cells, Trichodesmium’s efforts to maintain neutral buoyancy are compounded by its large cell size and tendency to aggregate in filamentous colonies. This is related to the dependence of the drag force on the Reynolds Number, a non-dimensional parameter dependent on speed and shape that exerts a dominant influence on the drag experienced by an object moving through a fluid. We calculated the terminal velocity of spherical marine microbial particles of characteristic diameters and specific gravities selected from the literature including individual Trichodesmium cells and small and large puff-type Trichodesmium colonies (see Table S9). The sinking speed was predicted from a force balance of buoyancy and drag, where the drag coefficient was allowed to vary as a function of Reynolds Number, itself dependent on speed, and the solution found iteratively. The sign of the terminal velocity, dependent on the calculated specific gravity of the particle relative to seawater, indicated whether the particle was expected to sink (downwards velocity) or to float (upwards velocity). Trichodesmium particles were modeled such that 25% of the cell volume was occupied by a low-density gas vesicle, resulting in initial floating velocities for single cells and colonies (Figure 5A). This is consistent with prior observations that a significant fraction of Trichodesmium colonies are positively or at least neutrally buoyant, depending on their carbohydrate content63. The hypothetical dark nitrogen fixation scenario, described above, was modelled by increasing the biomass component of the Trichodesmium specific gravity estimation by 60%, as described in Table 1. In all cases, the added glycogen density resulted in a switch from floating to sinking for the Trichodesmium particles, indicating that use of nitrogenase at night could fundamentally change the buoyant properties of the organism. For large Trichodesmium colonies, the estimated sinking speed for the hypothetical dark nitrogen fixation case exceeded 100 m day−1, meaning that the particles would leave the euphotic zone very rapidly. It should be noted that for tractability we modeled the particles as smooth spheres; surface roughness, particularly “spiky” morphology in puff-type colonies, could increase drag and slow the sinking speed61. These results thus additionally highlight the potentially significant importance of colony morphology on the biogeochemical function of Trichodesmium, in particular their impact on carbon and nitrogen export to depth12. Supporting this, we observed that in field populations the glycogen content of “spiky” Trichodesmium puff colonies is greater than that of Trichodesmium tuft colonies (Extended Data Figure 8).
Thus, whereas in unicellular diazotrophs glycogen provides an essential means to store energy for nighttime nitrogen fixation, in Trichodesmium glycogen ballast presents a significant problem. Shifting nitrogen fixation to the day minimizes glycogen production and provides a means for Trichodesmium colonies to stay afloat. In this way, Trichodesmium’s large size and tendency to form colonies may have driven it to daytime nitrogen fixation or vice versa – by helping Trichodesmium to remain at the surface, the development of daytime nitrogen fixation patterns may have allowed Trichodesmium cells to develop social behaviors including the formation of large colonies. It is unclear whether these behaviors evolved simultaneously or sequentially, and if so, which came first. However, it is clear that daytime nitrogen fixation is crucial to Trichodesmium’s niche as a high-light, neutrally buoyant, large celled, colonial diazotroph.
The iron cost of daytime nitrogen fixation
While clearly beneficial for buoyancy, daytime nitrogen fixation also comes at a significant cost, in particular driving the high iron quota of Trichodesmium cells. As mentioned before, Crocosphaera is able to reduce its daily iron demand by about 40% by completely degrading and re-synthesizing nitrogenase, allowing it to “hot-bunk” iron atoms and repurpose them for photosynthesis during the day17. Assuming that nitrogenase does not exist as an apoenzyme, the rapid cycling of nitrogenase and the fact that it is never fully degraded means that Trichodesmium cannot benefit from this iron sparing strategy as Crocosphaera does. In the quantitative proteomic analysis of the NifH enzyme, the lowest observed concentration of nitrogenase was 27 fmol μg−1 total protein (midnight) and this increased 5.5 fold to a maximum of 116 fmol μg−1 at mid morning (Table S5 and Fig 2). Assuming normal nitrogenase subunit configuration (one NifH dimer + one NifD/NifK heterotetramer) and full loading of the iron-binding sites (15 atoms per enzyme), this is equivalent to a minimum of 187 fmol iron per μg total protein at midnight and a maximum of 877 fmol iron per μg total protein at the height of nitrogen fixation. Using measured ratios of total protein: biomass POC content and the molecular weight of glucose as a conservative stand in for POC, this is equivalent to 10–39 μmol Fe bound to nitrogenase per mol biomass carbon, representing at least 30% of the cellular iron quota, consistent with a prior estimation (Table 1)65,66. Thus the need to maintain simultaneously active pools of nitrogenase and photosynthetic machinery likely drives the high iron requirement of Trichodesmium cells, consistent with the frequent observations of iron stress in natural populations43,59,67.
In order to occupy its niche of simultaneous nitrogen fixation and photosynthesis, Trichodesmium appears to have resigned itself to being a conspicuous consumer of iron, and has developed specific iron acquisition strategies, some of which depend on colony morphology, in order to do so. One particularly relevant iron-acquisition behavior is the ability acquire iron from particulate sources. Particulate iron utilization represents a benefit to colony formation and occurs via specialized mechanisms including biologically mediated reduction of particulate Fe(III) to Fe(II), and possible involvement of siderophores produced by the epibiont community68–71. Daytime nitrogen fixation is a key driver of Trichodesmium’s high iron quota, but it also provides a means for colonies to maintain buoyancy, and may therefore enhance access to atmospheric dust particles that enter at the surface. Given that staying afloat is a significant problem for Trichodesmium colonies, it is interesting that they have adapted to carry dense mineral particles, or in other words are literally carrying small rocks. From previously published synchrotron-based element maps of a Trichodesmium puff, we estimated that a ~1 mm diameter colony carried 1.03 μg of Fe in the form of mineral oxides and silicates71. Assuming the density of the Trichodesmium biomass was similar to that of literature observations, this results in an 56% increase in the density of the colony and a significant increase in the sinking velocity, on the order of 1000 m day−1 in the hypothetical dark nitrogen fixation case (Figure 5, Extended Data Figure 9). Thus, the development of particle-utilization behaviors may reinforce the need for ballast reduction via daytime nitrogen fixation patterns in Trichodesmium, as well as the production of gas vesicles to keep the colony afloat.
By effectively slowing the sinking speed of mineral particles, Trichodesmium colonies may enhance the retention and residence time of particulate minerals in the euphotic zone (Figure 6 inset). This would provide more time for solubilization of iron from these minerals, and thus gas vesicle containing, positively buoyant Trichodesmium colonies may play significant roles in the biogeochemical cycling of particulate metals. In this way, daytime nitrogen fixation and dust utilization reinforce the specific niche of Trichodesmium and underlie its abundance in high-dust surface ocean ecosystems2,70,72,73. Figure 6 summarizes how these multiple aspects of Trichodesmiums’ cellular biochemistry together define its particular ecological niche.
Figure 6.

Summary of physiological properties and behaviors that are reinforced by daytime nitrogen fixation (not to scale). Trichodesmium cells, filaments, and colonies have a tendency to sink due to their large size and tendency to aggregate. Daytime nitrogen fixation minimizes glycogen ballast. Trichodesmium can also to regulate buoyancy through production of gas vesicles which confers neutral and/or positive buoyancy. Thus Trichodesmium is more likely to remain in the upper water column, where they benefit from their high-light adapted pigments, the ability to access iron from dust particles, and interactions with the epibiont community. Additionally, buoyant Trichodesmium colonies may increase the residence time of dust particles in the euphotic zone, providing more time for particle solubilization.
Implications for the diazocyte hypothesis
Trichodesmium is often pointed to as a unique case among nitrogen fixers because it performs nitrogen fixation and photosynthesis simultaneously without the use of differentiated heterocyst cells74,75. Spatial separation of nitrogen fixation into partially differentiated “diazocytes” was proposed in Trichodesmium26,76, however the evidence for this strategy has been inconsistent and unrepeatable27,29,31. Despite lack of further experimental evidence for diazocytes, there has been confusion in the literature resulting in the assumption of spatial separation as a major cost associated with daytime nitrogen fixation58,77 (see Supplemental text for a brief review of the current evidence). Our study suggests that a key benefit of daytime nitrogen fixation is the ability to channel light-derived energy directly to the nitrogenase enzyme, thus reducing glycogen ballast and helping Trichodesmium to remain buoyant. It is unclear how this finding could be reconciled with the diazocyte theory, since this would require large amounts of energy to be transferred from the vegetative to the diazocyte cells. In true heterocyst forming cyanobacteria, reduced carbon is passed to the heterocysts as sucrose, but the Trichodesmium genome lacks the crucial sucrose metabolism proteins.
The molecular mechanisms of the hypothesized diazocyte formation are similarly unclear; the involvement of regulatory protein HetR has been suggested, as this protein is crucial to heterocyst formation in the true-heterocyst forming cyanobacterium Anabaena and the protein is present in Trichodesmium. However, the hetR gene is widely distributed among filamentous cyanobacteria, including non-nitrogen fixing cyanobacteria, evolved prior to heterocysts, and is likely involved in other processes, including filament cooperation more broadly78. Furthermore, we note that T. erythraeum genome lacks other necessary heterocyst formation and maintenance genes79 such as hetN and patS. Thus, though our results do not necessarily preclude the existence of diazocytes, we find also find no evidence for them, instead suggesting that the dynamic cycling of the proteome throughout the diel cycle could support nitrogen fixation without the need to envoke spatial separation.
Conclusions
Daytime nitrogen fixation by Trichodesmium requires considerable investment in the regulation, synthesis and degradation of photosystem components, nitrogenase, and other metabolic proteins on short time scales. Here we identify the niche-defining benefits that seem to couteract these costs. In summary, the ability to directly shuttle energy from the photosystems to the nitrogenase enzyme reduces glycogen ballast, allowing Trichodesmium filaments and colonies to remain at the surface. There, Trichodesmium is uniquely poised to access iron from atmospherically-derived dust particles, driving its competitiveness in high-dust environments and underscoring the potential importance of buoyant Trichodesmium colonies in the processing of mineral particles in the euphotic zone9,80. These findings build on previous frameworks relating nutrient acquisition to cell size and shape, where it is generally thought that smaller cells are better competitors in the oligotrophic ocean owing to their larger surface area: volume ratios43,81,82. Specifically, this study highlights that being small is not the only way that a cyanobacterium can maximize its viability in oligotrophic environments, but rather that a large celled, colonial cyanobacterium can become highly competitive in an oligotrophic system by adapting novel biochemical patterns that reinforce each other to define a specialized niche. Importantly, it is not one single aspect of Trichodesmium’s biochemistry and physiology that provide these benefits, but rather a collection of connected cellular processes which together define the ecology and biogeochemical impact of these unique nitrogen fixers.
Methods
Culture conditions
Trichodesmium erythraeum sp. IMS101 was grown in RMP growth medium prepared with oligotrophic Sargasso seawater36. The cultures were not axenic but had been sterile transferred for hundreds of generations; few epibiont proteins were identified suggesting the cultures were strongly dominated by T. erythraeum. The cultures were maintained for months at 26.9°C in a 14:10 day:night light cycle with light ramp up/down at dusk and dawn mimicking conditions at Station ALOHA. Approximately 200 mL of replicate dense, healthy cultures were inoculated into triplicate experimental vessels in a final volume of 1200 mL RMP media with constant, gentle stirring and gentle oxygenation. The cultures were allowed to acclimate and grow for five days before sub-sampling by sterile pipetting every 1–3 hours. Distinct samples (n = 45) were collected onto 0.2 μm filters for proteomics (50 mL), GFF filters for CHN analysis (10 mL) and for acetylene reduction rates (20 mL, 1 hour incubation period). A separate replicate experiment was conducted in the same way one year prior (see Figures S4, Extended Data Figure 3). The doubling time calculated from changes in total protein concentration was 0.66–0.76 per day, and protein content represented approximately 20% of the total biomass POC. CHN analyses were performed by combustion on a Control Equipment Corp Elemental Analyzer model CEC 440HA at the UCSB Marine Science Institute Analytical Laboratory.
Acetylene reduction assay
The acetylene reduction assay was conducted by injecting 2 mL of concentrated acetylene gas into the head-space of a sealed 50 mL nalgene culture bottle containing 20 mL of the sampled culture. The culture was returned to the incubator for one hour, then the reduction product (ethylene) measured by gas chromatography on a Shimadzu GC-8A and calibrated to a 9.1 ppb ethylene standard.
Protein digestion and metaproteomic analysis
Proteins were analyzed by a global proteomics/DDA method. In global proteomics, proteins are broken into small pieces, analyzed, and then reassembled bioinformatically. The protein targets are not selected ahead of time. Proteins were extracted by an SDS detergent-based method and trypsin digested in a polyacrylamide tube gel43,83,84. Protein abundance, which was used to estimate doubling time, was quantified by a colorimetric BCA protein assay (Thermo Fisher). Peptide mixtures were concentrated to 1 μg protein μL−1 solution and 10 μL or 10 μg was injected per analysis. The global proteomes were analyzed by LCamLC-MS involving two orthogonal in-line chromatography steps (PLRP-S (200 µm×150 mm, 3µm bead size, 300Å pore size, NanoLCMS Solutions) followed by C18 column (100m×150 mm, 3µm particle size, 120Å pore size, C18 Reprosil-God, Dr. Maisch GmbH, packed in a New Objective PicoFrit column)) on a Thermo Dionex Ultimate 3000 LC system equipped with two RSLC pumps (McIlvin et al., in prep). The first dimension of chromatography was an eight hour pH=10 gradient (10mM ammonium formate and 10mM ammonium formate in 90% acetonitrile) which was trapped on alternating dual traps and eluted at 30min intervals at 500 nL min−1 onto the C18 column (0.1% formic acid and 0.1% formic acid in 99.9% acetonitrile). The resulting eluent was injected directly onto a Thermo Orbitrap Fusion mass spectrometer with a Thermo Flex ion source (Waltham, MA). MS1 scans were monitored between m/z 380–1580, with a m/z 1.6 MS2 isolation window (CID mode), 50 ms maximum injection time, and 5 s dynamic exclusion time.
Raw spectra were searched using the SequestHT algorithm implemented in Proteome Discoverer 2.2.
Peptide sequences were mapped to a Trichodesmium erythraeum sp. IMS101 genome (RefSeq NC_008312.1) plus non-redundant non-Trichodesmium sequences identified in a recent metatranscriptome analysis of epibiont organisms associated with T. erythraeum sp. IMS101 cultures85. Search parameters were 10ppm parent mass tolerance, 0.8Da fragment mass tolerance, cysteine modification + 57.022 and methionine modification +16. Protein identifications were made via PeptideProphet implemented in Scaffold (Proteome Software) with a stringent 0.01% peptide and 0.3% protein global false discovery rates (FDR). Epibiont protein identifications were very sparse and inconsistent, so only Trichodesmium proteins were considered in the downstream analysis. Relative quantitation was measured by normalized spectral count using the NSAF (normalized spectral count abundance factor) method implemented in Scaffold. The NSAF normalization controls for sample-to-sample variation in amount of material injected into the mass spectrometer as well as variation in protein sequence length 86. Normalized spectral counts can therefore be used to compare protein abundances across samples in the dataset.
Absolute quantitation of the nitrogenase enzyme
Absolute quantitation of the nitrogenase enzyme was conducted by Parallel Reaction Monitoring (PRM)87 using an isotopically labeled standard for each peptide quantified. The heavy-labeled standard peptide was generated by expressing it in a custom designed plasmid in competent tuner(DE3)pLys Escherichia coli cells growing in 15N labeled medium43. The over-expressed standard was isolated in inclusion bodies, trypsin digested, and calibrated using a known amount of commercially available Pierce standard peptide mixture (catalog number 88320), the peptides of which were also included in the custom plasmid. The standard deviation of this calibration was approximately 10%. Linearity of the calibration within the range of expected experimental peptide concentrations was confirmed by a dilution curve based on precursor ion intensity and covering concentrations in range 0.001 to 200 fmol μL−1 (Extended Data Figure 10). Experimental samples were prepared at 0.1 μg μL−1 total experimental protein and contained the heavy labeled peptide standard at a concentration of 10 fmol μL−1; 10 μL of the sample was injected into the mass spectrometer. The liquid chromatography settings were similar to the metaproteomic analysis, but only a single dimension of chromatography (C18 column) and a 2 h chromatography gradient was used. The mass spectrometer was run in Parallel Reaction Monitoring (PRM) mode such that targeted precursor ions (m/z) were isolated for fragmentation and MS2 analysis. Peptide quantitation was performed in Skyline88 using the top 6 most abundant fragment ions for each peptide. The ratio of the heavy (standard) vs. light (experimental) MS2 peak areas were averaged and this was used to calculate the nitrogenase concentration, accounting for protein extraction efficiency, with corrections for measured protein extraction efficiency (Table S6). Absolute nitrogenase concentrations are reported as femtomoles per microgram of total protein.
Proteome analysis and glycogen quantitation of field Trichodesmium populations
Trichodesmium cells were sampled over a diel cycle on March 10, 2018 (Tricolim/AT39–05 expedition) from the Eastern subtropical Atlantic 65 22.420 °W 17 0.284 °N. Samples of mixed morphology were collected in biological triplicate by gentle hand-picking followed by two rinses in 0.2 μm sterile filtered trace metal clean seawater and decanting onto a 0.2 μm supor filter. Samples were flash frozen in liquid nitrogen and stored at −80 °C until analysis. Proteins were digested using the tube gel method described above and analyzed on a Michrom Advance HPLC coupled to a Q-exactive mass spectrometer (Thermo Fisher, Waltham, MA) with a Michrom Advance CaptiveSpray source. A four hour one-dimension chromatography gradient (0.1% formic acid and 0.1% formic acid in 99.9% acetonitrile) was performed using a C18 column (0.1 × 150 mm ID, 3µm particle size, 120Å pore size, C18 Reprosil-Gold, Dr. Maisch GmbH, packed in a New Objective PicoFrit column). MS1 scans were monitored between m/z 380–1280 with 5s dynamic exclusion. Raw spectra were searched using the SequestHT algorithm implemented in Proteome Discoverer 2.2 using a publicly available Trichodesmium metagenome (JGI IMG ID 2821474806). Search parameters were 10ppm parent mass tolerance, 0.6Da fragment mass tolerance, cysteine modification + 57.022 and methionine modification +16. Protein identifications were made via PeptideProphet implemented in Scaffold (Proteome Software) with a 0.06% peptide and 1.5% protein global false discovery rates (FDR). In stations around this region the population was dominated by a Trichodesmium theibautii species89. 1590 proteins were identified.
For glycogen measurements, cells were lysed by boiling the filter split at 95 °C for 10 minutes. Lysate was clarified by centrifugation and glycogen then quantified by the Sigma-Aldrich glycogen assay kit (MAK016) used in colorimetric mode. A separate set of samples collected on August 7, 2017 (JC150 expedition) from the Western Atlantic 31°W 22°N was also analyzed for glycogen content43. These were collected in triplicate from early morning plankton nets deployed to different depths (40, 90, and 160m), dragged for 10 minutes and then returned quickly to the surface, and glycogen content analyzed as before.
Estimation of terminal velocity of phytoplankton particles
The terminal velocity of phytoplankton particles was calculated by solving for a terminal velocity as the solution to a force balance of buoyancy versus drag for representative spherical particles of diameters and specific gravities determined from the literature. The associated Matlab code is provided at https://github.com/naheld/Held2020_TrichoDiel. Particle properties are summarized in Table S9. Trichodesmium specific gravity was calculated by allowing 25% of the cell volume to be occupied by a low density gas vesicle (density = 1.025 kg m3) and the remaining 75% of the cell volume to be occupied by Trichodesmium biomass of medium density as determined from the literature63,90.
The buoyancy force was calculated from the particle’s displacement and the density differential between the particle and surface ocean seawater (1025 kg m-3) via Archimedes’ principle:
| Eq. 1 |
where g denotes gravitational acceleration (9.81 m s−2), r denotes particle radius, and and denote the densities of the phytoplankton and seawater, respectively. Positive indicates a negatively buoyant (sinking) particle. The drag force was calculated according to
| Eq. 2 |
where denotes the drag coefficient for a sphere referenced to cross-sectional area and depends on the Reynolds Number, and denotes the terminal velocity of the particle. Positive values of denote a sinking particle. Terminal velocity was computed as the solution to. To permit a numerical solution we employed an analytical approximation91 to the drag coefficient as a function of Re accurate for smooth spheres to Re<106. The Reynolds Numbers corresponding to the terminal velocities determined for the range of particle sizes and velocities examined span many orders of magnitude, from laminar through turbulent flow regimes (Re range 4.4e−11 – 2.5e3).
For Trichodesmium colonies with hypothetical night nitrogen fixation, the density of the particle was increased by 60%. The mass of dust particles was estimated from a previously published synchrotron-based X-ray fluorescence element map of a natural Trichodesmium puff-type colony of diameter ~1mm that contained dust particles (Extended Data Figure 10)89. The integrated iron mass was calculated by isolating particles from the element map using a concentration mask and using pixel density to estimate the integrated iron concentration of all particles in the colony. The estimated iron mass was 1.03 μg or 18.4 nmol Fe.
Statistical analyses
Downstream analyses including data preparation, plotting and statistical analyses were conducted in Python 3.0 using the matplotlib 3.3.4 (http://matplotlib.org/)92, seaborn 0.11.1 (https://seaborn.pydata.org DOI: 10.5281/zenodo.592845), and scipy 1.6.1 (https://docs.scipy.org/)93 libraries. Hierarchical clustering as in Fig 1a and c was performed by the unweighted pair group method with arithmetic mean (UPGMA) implemented in scipy and plotted in seaborn. Sinusoidal modelling for Fig 1e–f was performed by optimization of a basic sinusoidal function via least squares regression implemented in scipy. Protein expression networks were generated using the Python networkX 2.4 library (https://networkx.github.io)94 using pre-calculated Spearman correlation statistics (Table S8), where a positive correlation between two proteins was defined as Spearman correlation coefficient > 0.8, p < 0.05 (two sided student’s t-test), and a negative correlation between two proteins defined as Spearman correlation coefficient < −0.8, p < 0.05 (two sided student’s t-test).
Extended Data
Extended Data Fig. 1.

A) Average total spectral counts (peptide to spectrum matches) with error bar representing +/− one standard deviation, at each tie point for triplicate biological replicates. Each data point is also shown individually as black scatter points. Yellow and indigo bars indicate the light and dark periods, respectively. Total spectral counts were relatively uniform and do not vary systematically throughout the diel cycle, implying consistency in the proteome analyses. B) Total protein content in the culture shown with error bar representing +/− one standard deviation, for biological duplicates after protein precipitation and purification, measured by a colorimetric BSA assay. Higher protein abundances at night may suggest nighttime cell growth. Again, each data point is also shown individually as black scatter points. Yellow and indigo bars indicate the light and dark periods, respectively.
Extended Data Fig. 2.

Dynamics of the entire proteome of Trichodesmium erythraeum sp. IMS101 over the diel cycle. The dynamic range of the normalized spectral count data can be observed, as well as fluctuations in protein abundance occurring throughout the experiment.
Extended Data Fig. 3.
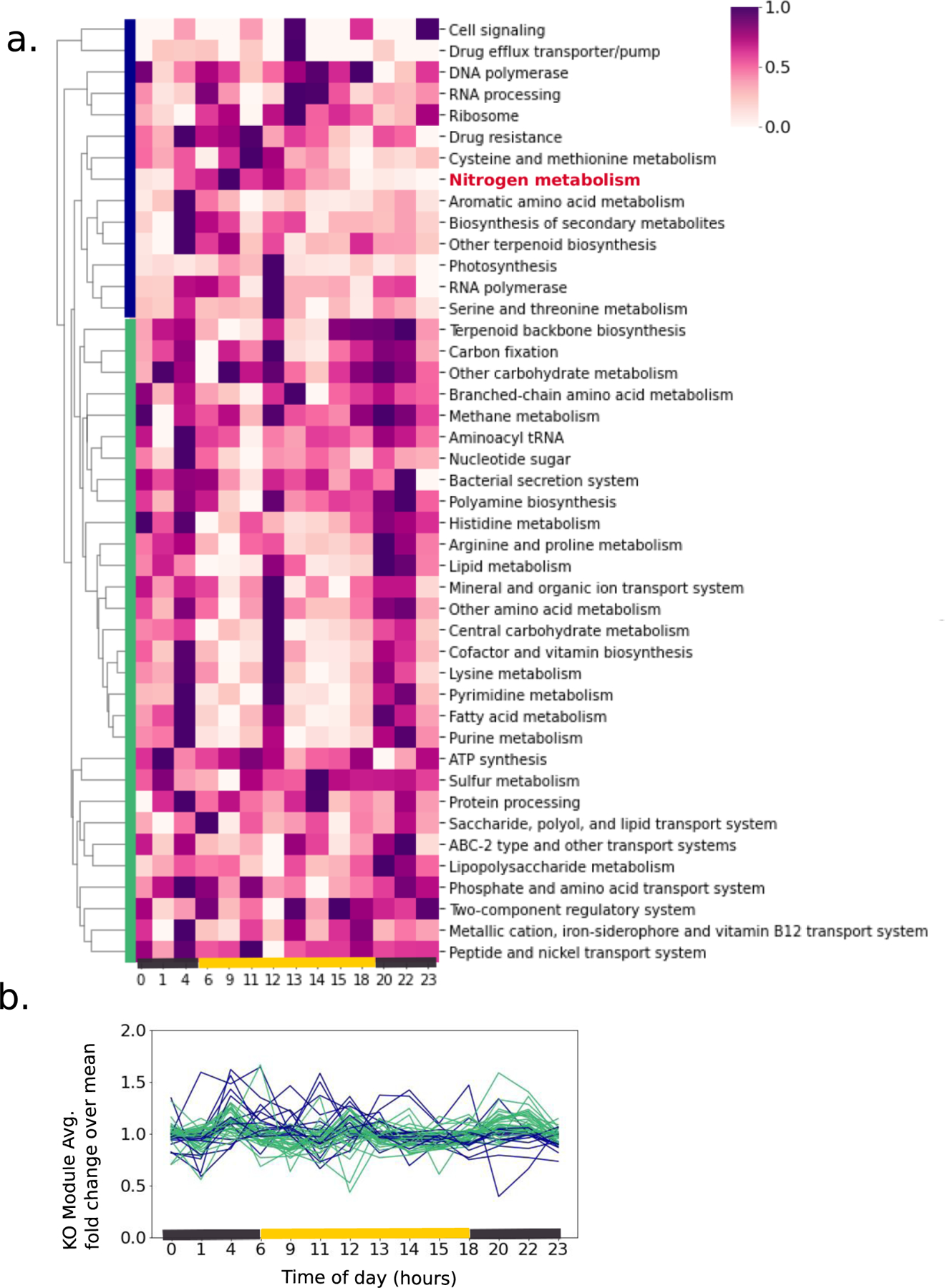
A) Clustered heatmap of a singlicate replicate diel experiment conducted one year prior to the main experiment, with the same set up and experimental conditions. Protein abundances were summed for each KO module and normalized across each row. B) Dynamics of the proteome clusters over the diel cycle, with each KO module represented as a line and colored based on the clustering in panel (A). Rapid oscillations of the proteome and clustering of the nitrogenase/nitrogen metabolism proteins with the photosystems are similar in the main experiment.
Extended Data Fig. 4.
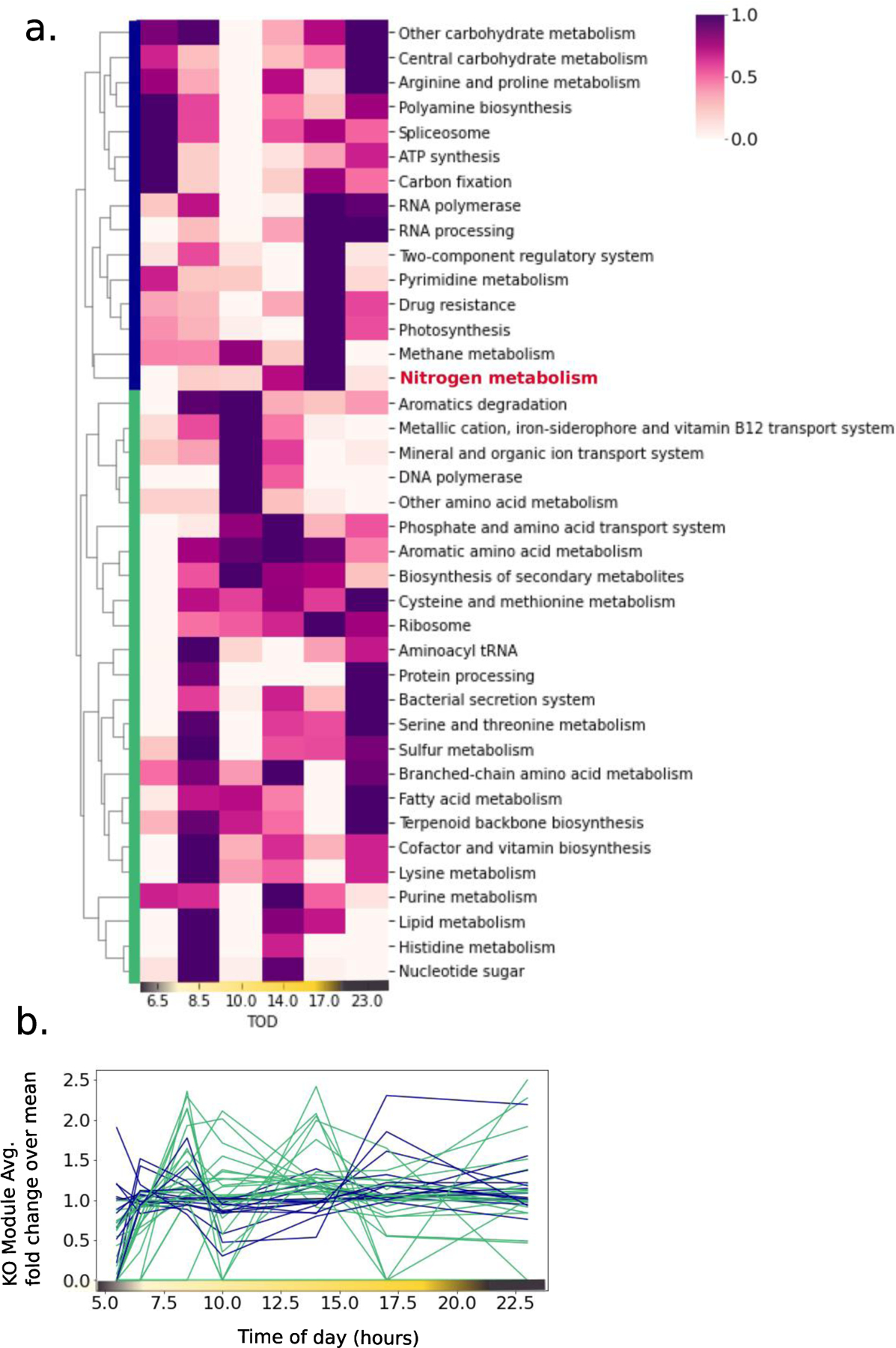
A) Clustered heatmap of the proteome of a field Trichodesmium population sampled over the diel cycle. Protein abundances were summed for each KO module and normalized across each row. B) Dynamics of the proteome clusters over the diel cycle, with each KO module represented as a line and colored based on the clustering in panel (A). Though the sampling was lower resolution than in the laboratory experiments, the rapid oscillations of the proteome are reproduced.
Extended Data Fig. 5.

In vivo specific activity of the nitrogenase NifH protein (nmol ethyelene produced per min per mg NifH) over the diel cycle for Crocosphaera watsonii. Unlike in Trichodesmium which exhibits significant variability in nitrogenase activity throughout the diel cycle, in Crocosphaera nitrogenase is either not present or highly present and very active.
Extended Data Fig. 6.

POC content versus total protein spectral counts in the main laboratory experiment. These are weakly correlated suggesting that POC content is driven mainly by carbohydrate content, not protein abundance.
Extended Data Fig. 7.

Glycogen content of Trichodesmium populations sampled in situ by depth. The populations were sampled on August 7, 2017 at 31°W 22°N in the early morning. Error bars are standard deviations of the mean value of the biological triplicates, and corresponding data points are plotted in grey circles. For each depth, n = 3 samples collected from replicate phytoplankton net sampling events, n = 2 samples for depth = 160 m.
Extended Data Fig. 8.

Glycogen content of Trichodesmium colonies sampled in situ and separated by morphology. The populations were sampled from the surface on March 10, 2018 at 65 22.420 °W 17 0.284 °N and separated by morphology at the time of picking.
Extended Data Fig. 9.

Synchrotron-based element maps used to determine mass of particulate iron associated with a puff-type colony, data originally collected as in Held et al., 202020. The left image is the X-ray fluorescence-based concentration, the middle image represents pixels with sufficiently high Fe to be considered a particle, and the right image is the product of the left and middle images. The total particulate Fe was determined as the area integrated Fe of the right image. The scale bar represents 180 microns. As detailed in Held et al., 202020, five Trichodesmium colonies of differing morphologies and degrees of particle association were examined in this way. These images are representative of a Trichodesmium colony with average-to-high particle loading.
Extended Data Fig. 10.

Calibration curves for 15N labeled standard peptides used for absolute quantitation of the nitrogenase proteins. Precursor ion intensities were linearly correlated with analyzed peptide concentrations between 0–10 fmol μL−1.
Supplementary Material
Acknowledgements
This work was supported by NSF Graduate Research Fellowship grant 1122274 (NAH), Gordon and Betty Moore Foundation grant GBMF-3782 (MAS), National Science Foundation grants OCE-1657766, OCE-1850719, and OCE-1924554 (MAS), National Institute of Health grant GM135709–02 (MAS), the Woods Hole Oceanographic Institution Ocean Ventures Fund (NAH). NAH was additionally supported by Principles of Microbial Ecosystems collaboration of the Simons Foundation (grant ID 542379). We acknowledge the scientific staff and crew of the AT39–05/Tricolim research expedition particularly chief scientist David Hutchins, and the JC150/Ziploc expedition, particularly chief scientist Claire Mahaffey. Special thanks to Barbara White. We gratefully acknowledge the contributions of Marc Strous and three anonymous reviewers.
Footnotes
Code availability
Fully reproducible code for sinking calculations, statistics and plotting is available at https://github.com/naheld/Held2020_TrichoDiel.
Competing interests
The authors declare no competing interests.
Data availability
The mass spectrometry proteomics data has been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD016332 and 10.6019/PXD016332 (laboratory experiments) and identifier PXD027796 and 10.6019/PXD027796 (field data). The processed proteomic data are also available at the Biological and Chemical Oceanography Data Management Office (BCO-DMO) (https://www.bco-dmo.org/dataset/783873). Source data are provided for main text Figures 1–5 and Extended Data Figures 1–10.
References
- 1.Zehr JP Capone DG Changing perspectives in marine nitrogen fixation. Science 9514, 729 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Karl D et al. Dinitrogen fixation in the world’s oceans. Biogeochemistry 57–58, 47–98 (2002). [Google Scholar]
- 3.Dugdale R & Wilkerson F Nutrient Limitation of New Production in the Sea. in Primary Productivity and Biogeochemical Cycles in the Sea (eds. Falkowski PG, Woodhead AD & Vivirito K) 107–122 (Springer; US, 1992). doi: 10.1007/978-1-4899-0762-2_7. [DOI] [Google Scholar]
- 4.Carpenter EJ & Capone DG Nitrogen Fixation in the Marine Environment. in Nitrogen in the Marine Environment (Elsevier, 2008). doi: 10.1016/B978-0-12-372522-6.00004-9. [DOI] [Google Scholar]
- 5.Gruber N, Sarmiento JL Global patterns of marine nitrogen fixation and denitrification. Global Biogeochem. Cycles 11, 23–266 (1997). [Google Scholar]
- 6.Buchanan PJ, Chase Z, Matear RJ, Phipps SJ, Bindoff NL Marine nitrogen fixers mediate a low latitude pathway for atmospheric CO2 drawdown. Nat. Commun 1–10 (2019) doi: 10.1038/s41467-019-12549-z. [DOI] [PMC free article] [PubMed]
- 7.Monteiro FM, Follows MJ & Dutkiewicz S Distribution of diverse nitrogen fixers in the global ocean. Global Biogeochem. Cycles 24, 1–16 (2010). [Google Scholar]
- 8.Church MJ, Björkman KM, Karl DM, Saito MA & Zehr JP Regional distributions of nitrogen-fixing bacteria in the Pacific Ocean. Limnol. Oceanogr 53, 63–77 (2008). [Google Scholar]
- 9.Monteiro FM, Dutkiewicz S & Follows MJ Biogeographical controls on the marine nitrogen fixers. Global Biogeochem. Cycles 25, 1–8 (2011). [Google Scholar]
- 10.Dutkiewicz S, Ward BA, Monteiro F & Follows MJ Interconnection of nitrogen fixers and iron in the Pacific Ocean: Theory and numerical simulations. Global Biogeochem. Cycles 26, 1–16 (2012). [Google Scholar]
- 11.Walworth NG et al. Nutrient-colimited Trichodesmium as a nitrogen source or sink in a future ocean. Appl. Environ. Microbiol 84, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGillicuddy DJ Jr. Do Trichodesmium spp. populations in the North Atlantic export most of the nitrogen they fix? Global Biogeochem. Cycles 28, 103–114 (2014). [Google Scholar]
- 13.Carpenter EJ & Romans K Major Role of the Cyanobacterium Trichodesmium in Nutrient Cycling in the North Atlantic Ocean. Science (80-. ) 254, 1989–1992 (1991). [DOI] [PubMed] [Google Scholar]
- 14.Bergman B, Sandh G, Lin S, Larsson J & Carpenter EJ Trichodesmium--a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol. Rev 37, 286–302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capone DG Trichodesmium, a Globally Significant Marine Cyanobacterium. Science (80-. ) 276, 1221–1229 (1997). [Google Scholar]
- 16.Gallon JR The oxygen sensitivity of nitrogenase: a problem for biochemists and micro-organisms. Trends Biochem. Sci 6, 19–23 (1981). [Google Scholar]
- 17.Saito MA et al. Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc. Natl. Acad. Sci. U. S. A 108, 2184–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dron A et al. Light-dark (12:12) cycle of carbon and nitrogen metabolism in Crocosphaera watsonii WH8501: Relation to the cell cycle. Environ. Microbiol 14, 967–981 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Mohr W, Intermaggio MP & LaRoche J Diel rhythm of nitrogen and carbon metabolism in the unicellular, diazotrophic cyanobacterium Crocosphaera watsonii WH8501. Environ. Microbiol 12, 412–421 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Flores E & Herrero A Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol 8, 39–50 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Burnat M, Herrero A & Flores E Compartmentalized cyanophycin metabolism in the diazotrophic filaments of a heterocyst-forming cyanobacterium. Proc. Natl. Acad. Sci. U. S. A 111, 3823–3828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman DM, Tucker D & Sherman LA Heterocyst development and localization of cyanophycin in N2-fixing cultures of Anabaena sp. PCC 7120 (Cyanobacteria). J. Phycol 941, 932–941 (2000). [Google Scholar]
- 23.Lamont HC, Silvester WB & Torrey JG Nile red fluorescence demonstrates lipid in the envelope of vesicles from N 2 -fixing cultures of Frankia . Can. J. Microbiol 34, 656–660 (1988). [Google Scholar]
- 24.Saino T Diel variation in nitrogen fixation by a marine blue-green alga , Trichodesmium thiebautii 25, 1259–1263 (1978). [Google Scholar]
- 25.Saino T & Hattori A Aerobic nitrogen fixation by the marine non-heterocystous cyanobacterium Trichodesmium (Oscillatoria) spp.: Its protective mechanism against oxygen. Mar. Biol 70, 251–254 (1982). [Google Scholar]
- 26.Berman-Frank I et al. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science (80-. ) 294, 1534–1537 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Ohki K & Taniuchi Y Detection of nitrogenase in individual cells of a natural population of Trichodesmium using immunocytochemical methods for fluorescent cells. J. Oceanogr 65, 427–432 (2009). [Google Scholar]
- 28.Eichner M et al. N2 fixation in free-floating filaments of Trichodesmium is higher than in transiently suboxic colony microenvironments. New Phytol 222, 852–863 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohki K Intercellular localization of nitrogenase in a non-heterocystous cyanobacterium (cyanophyte), Trichodesmium sp. NIBB1067. J. Oceanogr 64, 211–216 (2008). [Google Scholar]
- 30.Ohki Zehr, F. Regulation of Nitrogenase activity in relation to the light-dark regime in Trichodesmium. J. Gen. Microbiol 2679–2685 (1992).
- 31.Finzi-Hart JA et al. Fixation and fate of C and N in the cyanobacterium Trichodesmium using nanometer-scale secondary ion mass spectrometry. Proc. Natl. Acad. Sci. U. S. A 106, 9931 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandh G, El-Shehawy R, Díez B & Bergman B Temporal separation of cell division and diazotrophy in the marine diazotrophic cyanobacterium Trichodesmium erythraeum IMS101. FEMS Microbiol. Lett 295, 281–288 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Küpper H et al. Traffic Lights in Trichodesmium . Regulation of Photosynthesis for Nitrogen Fixation Studied by Chlorophyll Fluorescence Kinetic Microscopy. Plant Physiol 135, 2120–2133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito MA et al. Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc. Natl. Acad. Sci. U. S. A 108, 2184–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohki K & Fujita Y Aerobic nitrogenase activity measured as acetylene reduction in the marine non-heterocystous cyanobacterium Trichodesmium spp. grown under artificial conditions. Mar. Biol 98, 111–114 (1988). [Google Scholar]
- 36.Waterbury JB & Willey JM Isolation and Growth of Marine Planktonic Cyanobacteria. Methods Enzymol 167, 100–105 (1988). [Google Scholar]
- 37.Chen YB, Zehr JP & Mellon M Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: Evidence for a circadian rhythm. J. Phycol 32, 916–923 (1996). [Google Scholar]
- 38.Berman-Frank I, Bidle KD, Haramaty L & Falkowski PG The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol. Oceanogr 49, 997–1005 (2004). [Google Scholar]
- 39.Bell PRF et al. Laboratory culture studies of Trichodesmium isolated from the Great Barrier Reef lagoon, Australia. Hydrobiologia 532, 9–21 (2005). [Google Scholar]
- 40.Tzubari Y, Magnezi L, Be’Er A & Berman-Frank I Iron and phosphorus deprivation induce sociality in the marine bloom-forming cyanobacterium Trichodesmium. ISME J 12, 1682–1693 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Held NA, McIlvin MR, Moran DM, Laub MT & Saito MA Unique Patterns and Biogeochemical Relevance of Two-Component Sensing in Marine Bacteria. mSystems 4, 1–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aryal UK & Sherman LA Transcriptomic and Proteomic Analysis to Understand Systems-level Properties of Diurnal Cycles in Nitrogen-fixing Cyanobacteria. Cyanobacteria Omi. Manip (2017) doi: 10.21775/9781910190555.06. [DOI]
- 43.Held NA et al. Co-occurrence of Fe and P stress in natural populations of the marine diazotroph Trichodesmium. Biogeosciences 17, 2537–2551 (2020). [Google Scholar]
- 44.Klugkist J, Haaker H, Wassink H & Veeger C The catalytic activity of nitrogenase in intact Azotobacter vinelandii cells. Eur. J. Biochem 146, 509–515 (1985). [DOI] [PubMed] [Google Scholar]
- 45.Zehr JP, Wyman M, Miller V, Capone DG & Duguay L Modification of the Fe Protein of Nitrogenase in Natural Populations of Trichodesmium thiebautii Modification of the Fe Protein of Nitrogenase in Natural Populations of Trichodesmium thiebautii. Appl. Environ. Microbiol 59, 669–676 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez IB & Ho T-Y Diel nitrogen fixation pattern of Trichodesmium: the interactive control of light and Ni. Sci. Rep 4, 4445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eichner M, Kranz SA & Rost B Combined effects of different CO2 levels and N sources on the diazotrophic cyanobacterium Trichodesmium. Physiol. Plant 152, 316–330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchins DA et al. Irreversibly increased nitrogen fixation in Trichodesmium experimentally adapted to elevated carbon dioxide. Nat. Commun 6, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levitan O et al. Combined effects of CO2 and light on the N2-fixing cyanobacterium Trichodesmium IMS101: a mechanistic view. Plant Physiol 154, 346–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villareal TA & Carpenter EJ Buoyancy regulation and the potential for vertical migration in the oceanic cyanobacterium Trichodesmium. Microb. Ecol 45, 1–10 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Rabouille S, Staal M, Stal LJ & Soetaert K Modeling the dynamic regulation of nitrogen fixation in the cyanobacterium Trichodesmium sp. Appl. Environ. Microbiol 72, 3217–3227 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breitbarth E, Wohlers J, Kläs J, LaRoche J & Peeken I Nitrogen fixation and growth rates of Trichodesmium IMS-101 as a function of light intensity. Mar. Ecol. Prog. Ser 359, 25–36 (2008). [Google Scholar]
- 53.Chen YB et al. Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp strain IMS101. J. Bacteriol 180, 3598–3605 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabouille S, Staal M, Stal LJ & Soetaert K Modeling the Dynamic Regulation of Nitrogen Fixation in the Cyanobacterium. Society 72, 3217–3227 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capone DG, O’Neill JM, Zehr J & Carpenter EJ Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautti. Appl. Environ. Microbiol 56, 3532–3536 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gründel M, Scheunemann R, Lockau W & Zilliges Y Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiol. (United Kingdom) 158, 3032–3043 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Jackson SA, Eaton-Rye JJ, Bryant DA, Posewitz MC & Davies FK Dynamics of photosynthesis in a glycogen-deficient glgC mutant of Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol 81, 6210–6222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boatman TG, Davey PA, Lawson T & Geider RJ The physiological cost of diazotrophy for Trichodesmium erythraeum IMS101. PLoS One 13, 1–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chappell PD, Moffett JW, Hynes AM & Webb EA Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium. ISME J 6, 1728–1739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chappell PD & Webb EA A molecular assessment of the iron stress response in the two phylogenetic clades of Trichodesmium. Environ. Microbiol 12, 13–27 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Walsby AE The properties and buoyancy providing role of gas vacuoles in trichodesmium ehrenberg. Br. Phycol. J 13, 103–116 (1978). [Google Scholar]
- 62.Villareal TA & Carpenter EJ Diel buoyancy regulation in the marine diazotrophic cyanobacterium Trichodesmium thiebautii. Limnol. Oceanogr 35, 1832–1837 (1990). [Google Scholar]
- 63.Romans KM, Carpenter EJ & Bergman B Buoyancy Regulation in the Colonial Diazotrophic Cyanobacterium Trichodesmium Tenue: Ultrastructure and Storage of Carbohydrate, Polyphosphate, and Nitrogen. J. Phycol 30, 935–942 (1994). [Google Scholar]
- 64.Wang L et al. Molecular Structure of Glycogen in Escherichia coli. Biomacromolecules 20, 2821–2829 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Berman-Frank I, Cullen JT, Shaked Y, Sherrell RM & Falkowski PG Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol. Oceanogr 46, 1249–1260 (2001). [Google Scholar]
- 66.Kustka AB et al. Iron requirements for dinitrogen- and ammonium-supported growth in cultures of Trichodesmium (IMS 101): Comparison with nitrogen fixation rates and iron:carbon ratios of field populations. Limnol. Oceanogr 49, 1224 (2004). [Google Scholar]
- 67.Paerl HW, Prufert-Bebout ILE, Guo C & Carolina N Iron-Stimulated N2 Fixation and Growth in Natural and Cultured Populations of the Planktonic Marine Cyanobacteria Trichodesmium spp 1044–1047 (1994). [DOI] [PMC free article] [PubMed]
- 68.Rubin M, Berman-Frank I & Shaked Y Dust-and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nat. Geosci 4, 529–534 (2011). [Google Scholar]
- 69.Polyviou D et al. Desert dust as a source of iron to the globally important diazotroph Trichodesmium. Front. Microbiol 8, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basu S & Shaked Y Mineral iron utilization by natural and cultured Trichodesmium and associated bacteria. Limnol. Oceanogr 63, 2307–2320 (2018). [Google Scholar]
- 71.Held NA, Sutherland KM, Webb EA, Mcilvin MR & Natalie R Mechanisms and heterogeneity of mineral use by natural colonies of the cyanobacterium Trichodesmium. bioRxiv 1–13 (2020) doi: 10.1101/2020.09.24.295147. [DOI]
- 72.Basu S, Gledhill M, de Beer D, Prabhu Matondkar SG & Shaked Y Colonies of marine cyanobacteria Trichodesmium interact with associated bacteria to acquire iron from dust. Commun. Biol 2, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyrrell T et al. Large-scale latitudinal distribution of Trichodesmium spp. in the Atlantic Ocean. J. Plankton Res 25, 405–416 (2003). [Google Scholar]
- 74.Robson RL & Postgate JR Oxygen and Hydrogen in Biological Nitrogen Fixation. Ann. Rev. Mircrobiol 183–207 (1980). [DOI] [PubMed]
- 75.Zehr JP Nitrogen fixation by marine cyanobacteria. Trends Microbiol 19, 162–173 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Bergman B & Carpenter EJ Nitrogenase Confined To Randomly Distributed Trichomes in the Marine Cyanobacterium Trichodesmium-Thiebautii. Journal of Phycology vol. 27 158–165 (1991). [Google Scholar]
- 77.Inomura K, Wilson ST & Deutsch C Mechanistic Model for the Coexistence of Nitrogen Fixation and Photosynthesis in Marine Trichodesmium. mSystems 4, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janson S, Matveyev A & Bergman B The presence and expression of hetR in the non-heterocystous cyanobacterium Symploca PCC 8002. FEMS Microbiol. Lett 168, 173–179 (1998). [DOI] [PubMed] [Google Scholar]
- 79.Zhang JY, Chen WL & Zhang CC hetR and patS, two genes necessary for heterocyst pattern formation, are widespread in filamentous nonheterocyst-forming cyanobacteria. Microbiology 155, 1418–1426 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Moore JK, Doney SC, Glover DM & Fung IY Iron cycling and nutrient-limitation patterns in surface waters of the world ocean. Deep. Res. Part II Top. Stud. Oceanogr 49, 463–507 (2001). [Google Scholar]
- 81.Chisholm SW Phytoplankton Size. in Primary Productivity and Biogeochemical Cycles in the Sea (eds. Falkowski PG, Woodhead AD & Vivirito K) 213–237 (Springer; US, 1992). doi: 10.1007/978-1-4899-0762-2_12. [DOI] [Google Scholar]
- 82.Young KD The Selective Value of Bacterial Shape. Microbiol. Mol. Biol. Rev 70, 660–703 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu X & Zhu H Tube-Gel Digestion: A Novel Proteomic Approach for High Throughput Analysis of Membrane Proteins. Mol Cell Proteomics 4, 1948–1958 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saito MA et al. Multiple nutrient stresses at intersecting Pacific Ocean biomes detected by protein biomarkers. Science 345, 1173–7 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Lee MD et al. Transcriptional activities of the microbial consortium living with the marine nitrogen-fixing cyanobacterium Trichodesmium reveal potential roles in community-level nitrogen cycling. Appl. Environ. Microbiol 84, AEM.02026–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Wen Z, Washburn MP & Florens L Refinements to label free proteome quantitation: How to deal with peptides shared by multiple proteins. Anal. Chem 82, 2272–2281 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Gallien S, Bourmaud A, Kim SY & Domon B Technical considerations for large-scale parallel reaction monitoring analysis. J. Proteomics 100, 147–159 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Pino LK, Searle BC, Bollinger JG, Nunn B, MacLean B, MacCoss MJ The Skyline Ecosystem: Informatics for Quantitative Mass Spectrometry Proteomics. Mass Spectrom Rev 176, 139–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Held NA et al. Mechanisms and heterogeneity of in situ mineral processing by the marine nitrogen fi xer Trichodesmium revealed by single-colony metaproteomics. ISME Commun 1–9 (2021) doi: 10.1038/s43705-021-00034-y. [DOI] [PMC free article] [PubMed]
- 90.White AE, Spitz YH & Letelier RM Modeling carbohydrate ballasting by Trichodesmium spp. Mar. Ecol. Prog. Ser 323, 35–45 (2006). [Google Scholar]
- 91.Morrison FA An Introduction to Fluid Mechanics (Cambridge University Press, 2013). [Google Scholar]
- 92.Hunter JD Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng 9, 90–95 (2007). [Google Scholar]
- 93.Virtanen P et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagberg AA, Schult DA & Swart PJ Exploring network structure, dynamics, and function using NetworkX. 7th Python Sci. Conf. (SciPy 2008) 11–15 (2008).
- 95.Held NA et al. Mechanisms and heterogeneity of in situ mineral processing by the marine nitrogen fixer Trichodesmium revealed by single-colony metaproteomics. ISME Commun 1–9 (2021) doi: 10.1038/s43705-021-00034-y. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data has been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD016332 and 10.6019/PXD016332 (laboratory experiments) and identifier PXD027796 and 10.6019/PXD027796 (field data). The processed proteomic data are also available at the Biological and Chemical Oceanography Data Management Office (BCO-DMO) (https://www.bco-dmo.org/dataset/783873). Source data are provided for main text Figures 1–5 and Extended Data Figures 1–10.


