Abstract
Objectives:
Gene-environment interactions increase the risk of psychosis. The objective of this study was to investigate gene-gene and gene-environment interactions in psychosis, including single nucleotide variants (SNVs) of dopamine-2 receptor (D2R), N-methyl-d-aspartate receptor (NMDAR), and cannabinoid receptor type 1 (CB1R), lifetime cannabis use, and childhood trauma.
Methods:
Twenty-three SNVs of genes encoding D2R (DRD2: rs1799978, rs7131056, rs6275), NMDAR (GRIN1: rs4880213, rs11146020; GRIN2A: rs1420040, rs11866328; GRIN2B: rs890, rs2098469, rs7298664), and CB1R (CNR1: rs806380, rs806379, rs1049353, rs6454674, rs1535255, rs2023239, rs12720071, rs6928499, rs806374, rs7766029, rs806378, rs10485170, rs9450898) were genotyped in 143 first-episode psychosis patients (FEPp) and 286 community-based controls by Illumina HumanCoreExome-24 BeadChip. Gene-gene and gene-environment associations were assessed using nonparametric Multifactor Dimensionality Reduction software.
Results:
Single-locus analyses among the 23 SNVs for psychosis and gene-gene interactions were not significant (p > 0.05 for all comparisons); however, both environmental risk factors showed an association with psychosis (p < 0.001). Moreover, gene-environment interactions were significant for an SNV in CNR1 and cannabis use. The best-performing model was the combination of CNR1 rs12720071 and lifetime cannabis use (p < 0.001), suggesting an increased risk of psychosis.
Conclusion:
Our study supports the hypothesis of gene-environment interactions for psychosis involving T-allele carriers of CNR1 SNVs, childhood trauma, and cannabis use.
Keywords: Cannabis use, childhood trauma, first-episode psychosis, single nucleotide variants
Introduction
Schizophrenia and other psychoses are complex psychiatric disorders characterized by gene-environment interactions.1 Genome-wide association studies (GWAS) have implicated functional single nucleotide variants (SNVs) in schizophrenia pathogenesis.2-4 Specifically, the largest and most recent GWAS reported that rare and common variants of the GRIN2A gene, which encodes a subunit of the N-methyl-D-aspartate receptor (NMDAR), have a pathogenic role in schizophrenia.5
Environmental factors, including cannabis use and childhood trauma, are also associated with first-episode psychosis and schizophrenia,6,7 and growing evidence supports gene-environment interactions in additive models including both cannabis use and childhood trauma.8 It has also been suggested that gene-environment interactions can be traced to a final common pathway involving the dopaminergic, glutamatergic, and endocannabinoid systems, which underlies psychosis pathogenesis.9 There is a wealth of evidence suggesting that disruptions in NMDAR may lead to dopaminergic impairments (excess in the mesolimbic and reduction in the mesocortical pathways), as already described in schizophrenia.10 Recent evidence has indicated a key role of the endocannabinoid system in cellular and molecular signaling mechanisms involving physiological neurotransmission pathways such as the dopamine11 and glutamate systems.12 However, whether interaction of variants in the dopamine-2 receptor (D2R), NMDAR, and cannabinoid type 1 receptor (CB1R) genes with both cannabis use and childhood trauma increase the risk of psychosis remains unclear.
It is well established that hyperregulation of the dopaminergic system is implicated in the etiology of psychosis.13 One of the longest-standing hypotheses in schizophrenia suggests dopaminergic hyperactivity at the D2R, encoded by the DRD2 gene, which is considered a risk gene for schizophrenia.14
Abnormalities in the glutamatergic system, particularly hypofunction of NMDAR, have also been hypothesized to play a major role in psychosis pathogenesis, since glutamate is the major excitatory neurotransmitter and its dysfunction results in a wide range of impairments.15 NMDAR is composed of several subunits, three of which are NR1, NR2A, and NR2B, encoded by GRIN1, GRIN2A, and GRIN2B, respectively.16 One well-characterized model of glutamatergic hypofunction involves the administration of noncompetitive NMDAR antagonists. This has been shown to induce cognitive- and negative features reminiscent of schizophrenia, including reduction of spatial learning, social withdrawal, and anhedonia,17 in animal models.18 Additionally, exome-sequencing studies have revealed that rare damaging mutations in NMDAR genes are found in brain and blood samples from patients with schizophrenia.19
The glutamatergic system is highly interconnected with the endocannabinoid system.20 The CB1R, encoded by the CNR1 gene, is widely distributed in brain regions (cortex, olfactory bulb, hippocampus, basal ganglia, and cerebellum)21 and peripheral tissues, including skeletal muscle, hepatocytes, and pancreatic beta β-cells.22 Although earlier studies failed to find an association between genetic variants of CNR1 and schizophrenia,23,24 and no GWAS to date has identified common genetic variants within CNR1 associated with psychosis in large population studies,5 there may still be environmental risk factors contributing to psychosis that potentially act through interactions with variants of this gene.
Given that the etiology of psychosis may lie in gene-environment interactions, genetic variants could help define biological subgroups resilient or vulnerable to environmental factors that potentially increase the risk of psychosis. Thus, we hypothesized that SNVs in genes related to dopaminergic, glutamatergic, and endocannabinoid receptors could be involved in the pathogenesis of psychosis through interactions with environmental risk factors. Specifically, we tested for gene-gene interactions and interactions between 23 SNVs of D2R (DRD2), NMDAR (GRIN1, GRIN2A, and GRIN2B), and CB1R (CNR1) and two well-known environmental risk factors for psychosis: childhood trauma and lifetime cannabis use.
Methods
Sample
This study is part of Schizophrenia and Other Psychosis – Translational Research: Environment and Molecular Biology (henceforth, STREAM).25 STREAM is part of the international consortium European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI WP2 Group) (http://www.eu-gei.eu/).26,27 The catchment area selected was the health administrative region of Ribeirão Preto, state of São Paulo, Brazil. The region comprises 26 municipalities corresponding to an area of 9,300 km2 and a population of approximately 1.3 million.
In the initial STREAM dataset, DNA blood samples were collected from 202 first-episode psychosis patients (FEPp) and 293 controls. After quality control (QC) for genotyping, 159 FEPp and 289 community-based controls remained in the STREAM dataset. Of these, 429 subjects (143 FEPp and 286 community-based controls) had complete clinical, biological, and environmental assessments and were included in this study.
The sample was composed of incident cases of affective and non-affective psychosis identified in the mental health services of the Ribeirão Preto catchment area from 1 April 2012 to 31 March 2015. We included patients aged 16 to 64 years, with no gender restrictions, who lived in the catchment area and had sought medical care for psychotic symptoms for the first time.25 We excluded patients with previous contact with mental health services for psychosis, psychotic symptoms due to other medical conditions, or presenting during substance intoxication/withdrawal.
We also included controls aged 16 to 64 years, both sexes, with no history of psychotic symptoms, from the same catchment area. For the recruitment of controls, we considered the total population defined by the latest (2010) census (http://www.ibge.gov.br/), according to place of residence, stratified by sex, in three age groups (16 to 24, 25 to 34, and > 35 years old).
Sociodemographic and clinical assessments
Face-to-face interviews were held to gather sociodemographic and clinical data, as described elsewhere.28
All included patients met diagnostic criteria for FEPp based on a structured clinical interview for DSM-IV-CV axis I disorders (SCID-I).29,30 Moreover, we investigated the history of treatment and the duration of untreated psychosis (DUP) using the Nottingham Onset Schedule (NOS).31 Symptom severity at the time of peripheral blood collection was determined by the Brief Psychiatric Rating Scale (BPRS),32,33 and the use of cannabis and nine other types of psychoactive substances (inhalants, crack, cocaine, stimulants, sedatives, opioids, hallucinogens, ketamine, and other drugs – the latter covering new psychoactive substances, e.g., mephedrone) was assessed by applying the Cannabis Experiences Questionnaire (CEQ).34 This self-report assessment gives information about lifetime cannabis use, age at first use, frequency, duration, and type of cannabis used. We considered the variable lifetime cannabis use as a measure of an individual’s susceptibility, as well as its strong association with genetic variants, consistent with previous studies.35,36 Childhood trauma was assessed using Childhood Trauma Questionnaire (CTQ), which yields a CTQ total score and allows classification of participants into two groups (maltreated or non-maltreated).37,38
Blood samples and genetic analysis
Genotyping and quality control (QC)
Peripheral blood samples were genotyped using a custom Illumina HumanCoreExome-24 BeadChip genotyping array (Cardiff chip) that contains probes for 570,038 genetic variants (Illumina, San Diego, USA). Experiments were performed at the Institute of Psychological Medicine and Clinical Neurology of Cardiff University, as part of a multicenter study for GWAS analysis by DNA microarray techniques. Genotype data were obtained using the GenomeStudio package and transferred into PLINK format for further analysis.
The QC was performed using PLINK v1.0739 or with Perl scripts customized by EU-GEI. Variants and samples with a call rate < 98% and minor allele frequency (MAF) ≤ 0.01 were excluded from the dataset. Variants with Hardy-Weinberg equilibrium (HWE) p < 1e-6 were excluded. We used the sample QC steps for the genotyping as per EU-GEI WP2.27 After QC, 559,505 variants remained, and we extracted from the array only those of interest.
Population stratification
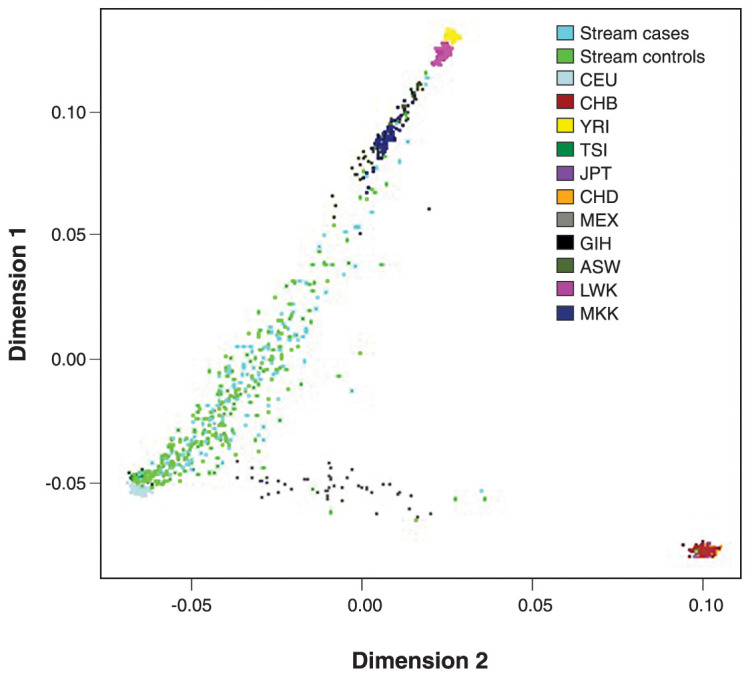
We used the first 10 principal components (PC) from genotyping (using PLINK39 and RStudio40) as covariants to adjust for genetic population stratification. Briefly, as a first step, we merged our sample with the 1000 Genomes Project sample Phase 341 to build ancestry PCs of the overlapping SNVs and, subsequently, we applied k-mean clustering to determine individual populations in our sample based on 1000 Genomes sample information. The principal components analysis (PCA) plot shows the dimensions 1 and 2 that correspond the PC1 and PC2, respectively (explaining the maximum and second greatest variance in the data, respectively) (Figure 1).
Figure 1. Principal components (PC) analysis plot of the studied Ribeirão Preto catchment area individuals in comparison to 1000 Genomes Project populations. The first two PCs (PC1 and PC2, corresponding to dimensions 1 and 2) are shown, and the colors illustrate the stream sample (143 cases, light blue; 286 controls, neon green) with the 1000 Genome Project sample Phase 3.41 ASW = Americans of African ancestry from Southwest United States; CEU = Utah residents with Northern and Western European ancestry from Utah, United States; CHB = Han Chinese from Beijing, China; CHD = Chinese from metropolitan Denver, Colorado, United States; GIH = Gujarati Indians from Houston, Texas, United States; JPT = Japanese from Tokyo, Japan; LWK = Luhya in Webuye, Kenya; MEX = Americans of Mexican ancestry from Los Angeles, California, United States; MKK = Maasai in Kinyawa, Kenya; TSI = Italians from Tuscany, Italy; YRI = Yoruba in Ibadan, Nigeria.
SNVs selection
We selected D2R and NMDAR genes that were previously significantly associated with schizophrenia and other psychoses,5,14 and the CB1R gene for its functional relevance to this disorder.42 We used three strategies to select SNVs from the five candidate gene regions (covering target sites, upstream, and downstream): i) tagging (Haploview 4.2) at an r2 threshold of 0.6 to capture 98% of the variants most common in HapMap phase II and a MAF > 0.01; ii) SNV functionality according to data published on Ensembl (http://www.ensembl.org); and iii) previous associations with psychosis reported in the literature for DRD2,43-45 GRIN1, GRIN2A, GRIN2B,44,46-48 and CNR1.49
Twenty-three SNVs of the D2R (DRD2: rs1799978, rs7131056, and rs6275), NMDAR (GRIN1: rs4880213, rs11146020; GRIN2A: rs1420040, rs11866328; GRIN2B: rs890, rs2098469, and rs7298664), and CB1R genes (CNR1: rs806380, rs806379, rs1049353, rs6454674, rs1535255, rs2023239, rs12720071, rs6928499, rs806374, rs7766029, rs806378, rs10485170, and rs9450898) were analyzed.
We calculated the power to detect an odds ratio (OR) of 2 in a case-control study with 429 subjects, including 143 FEPp and 286 controls (case rate of 30%), over a range of MAF from 0.09 to 0.50 in order to cover the frequencies of the candidate genes. For all possible combinations of true and test models (additive, dominant, and recessive), assuming an alpha of 0.05, a binary outcome with prevalence of 0.01, and gene-environment interaction, the statistical power reached ∼80% (RStudio Team 2020, CRAN.R-project.org/package=genpwr/).
Statistical analysis
Statistical analyses were conducted in SPSS version 26 and RStudio.40 Data were checked for normality using Shapiro-Wilk’s test. We described the sociodemographic, clinical, and environmental (lifetime cannabis use and childhood trauma) variables between groups (community-based controls and FEPp) using Student’s t test and Fisher’s exact test. HWE was calculated for each selected SNV using an online calculator (Excel-based HWE Test by Michael H. Court [2005-2008], Court lab – HW calculator – important.xls), using a nominal p < 0.05 as the cutoff.
To investigate the effect of SNVs on psychosis, data were analyzed using binary logistic regression models (OR [β], 95%CI), including group as the binary outcome (community-based controls and FEPp) in both unadjusted and adjusted models (sex, age, years of education, tobacco smoking, and the first 10 PCs to correct the possible bias due to an association between SNVs and genetic ancestry in an admixed population), considering the frequencies of the genotypes under the dominant (homozygous ancestral × heterozygous + homozygous variant), additive (homozygous ancestral × heterozygous × homozygous variant), and recessive models (homozygous variant × homozygous ancestral + heterozygous). To determine the significance level for multiple test errors, we performed the Bonferroni’s test and considered an adjusted p < 0.002 (0.05/23) as statistically significant, taking into account the 23 selected SNVs. Moreover, we used Fisher’s exact test for comparisons between the environmental risk factors and psychosis.
Associations between and within gene-gene and between the 23 SNVs and environmental risk factors (lifetime cannabis use [yes or no] and childhood trauma [yes or no]) were tested using the nonparametric Multifactor Dimensionality Reduction (MDR) software (version 3.0.2) (www.epistasis.org), considering 10 data divisions as the same best model for cross-validation consistency (CVC), testing accuracy and an empirical p < 0.05.50 We included a binary outcome (community-based controls and FEPp) to analyze associations between GRIN1, GRIN2A, GRIN2B, DRD2, and CNR1 genotypes in three groups (ancestral homozygous vs. heterozygous vs. minor allele homozygous) in relation to the environmental factors. For models deemed statistically significant in MDR analysis, we subsequently performed the Hosmer-Lemeshow test in the logistic regression to assess goodness of fit. The reference allele was based on population genetics data from African, European, and Native Americans (bank of allele frequency, https://www.ensembl.org/index.html/).
Considering the multifactorial etiology of psychosis (multiple environmental risk factors, polygenicity, and the interactions thereof), MDR has been proposed as a complementary method to linear and logistic regressions to improve the identification of gene-gene and gene-environment interactions given that no genetic mode of inheritance is assumed, and this approach avoids increased type II errors and decreasing the power by reducing the dimensionality of multilocus genotype combinations.51,52 The possible multifactor classes from n combinations between genetic and/or environmental variables are labeled as high-risk or low-risk to disease, and the ratio of the number of patients to the number of controls is calculated within these multifactor classes. MDR analysis calculated the empirical p-value derived from permutation testing (p-values based on 1,000 permutations) at a 0.05 significance level. In our results, we present the mean p-value from the p-value derived from permutation testing. The criteria for the best final interaction models included: i) the minimal prediction error; ii) the CVC 9 or 10-fold cross validation; iii) significance of the p-value (< 0.05); and iv) close values of the training and testing balance accuracy (TBA = 0.55-0.69), as described by Moore.53
We constructed three models (dominant, additive, and recessive) to estimate the average effects of the risk allele for the associations between genes and psychosis. However, an additive genetic model was employed to estimate the effects across each genotype for the gene-gene and gene-environment interactions to show the genetic variant contribution of each gene better.
Ethics statement
This study was approved by the ethics committee of Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto (opinion number 12.606/2012). Informed consent was obtained from each participant prior to study enrollment.
Results
Sociodemographic, clinical, and environmental profiles of the participants
FEPp had a relatively higher proportion of males and non-whites, lower education, higher frequency of tobacco smoking, and, as expected, higher BPRS scores than controls (p < 0.001 for all comparisons). Age (p = 0.648) and body mass index (BMI) (p = 0.062) did not differ significantly between groups (Table 1).
Table 1. Sociodemographic and clinical variables of participants (n=429).
| Characteristics | Community-based controls (n=286) | FEPp (n=143) | p-value |
|---|---|---|---|
| Sociodemographic variables | |||
| Sex† | < 0.001 | ||
| Female | 148 (51.7)a | 57 (39.9)a | |
| Male | 138 (48.3)a | 86 (60.1)a | |
| Education in years† | < 0.001 | ||
| ≤ 9 | 64 (22.4)a | 58 (40.6)b | |
| > 9 | 222 (77.6)a | 85 (59.4)b | |
| Skin color† | 0.004 | ||
| White | 196 (68.5)a | 76 (53.1)b | |
| Non-white | 90 (31.5)a | 67 (46.9)b | |
| Clinical variables | |||
| Age,‡ years: mean (SD) | 33.7 (12.6) | 32.8 (12.1) | 0.648 |
| BMI,‡ kg/m2: mean (SD) | 26.4 (6.4) | 25.1 (6.5) | 0.062 |
| BPRS,‡ total score: mean (SD) | 0.8 (1.9)a | 9.0 (7.1)b | < 0.001 |
| Age of onset,‡ years: mean (SD) | 31.3 (11.9) | ||
| DUP, weeks: median (Min-Max) | 10.0 (0.0-1.056.0) | ||
| Duration of disease, weeks: median (Min-Max) | 39.6 (1.6-1.094.4) | ||
| Duration of treatment, weeks: median (Min-Max) | 21.0 (0.0-155.4) | ||
| Tobacco smoking† | < 0.001 | ||
| Yes | 44 (15.4)a | 46 (32.2)b | |
| Lifetime cannabis use† | < 0.001 | ||
| Yes | 55 (19.2)a | 71 (49.7)b | |
| Childhood trauma† | < 0.001 | ||
| Yes | 68 (23.8)a | 74 (51.7)b |
Data presented as n (%), unless otherwise specified.
Significant results are highlighted in bold.
BMI = body mass index; BPRS = Brief Psychiatric Rating Scale; DUP = duration of untreated psychosis; FEPp = first-episode psychosis patients.
Fisher’s exact test.
Student’s t test.
Different superscript letters denote statistically significant differences between means.
The environmental risk factors of lifetime cannabis use and childhood trauma were each significantly associated with FEPp compared to controls (Table 1) (p < 0.001 for both comparisons). However, these two factors were themselves significantly associated in the control group, but not in the FEPp group. The lifetime frequency of either cannabis use or childhood trauma among controls was 7.3% (χ2 = 7.80; p = 0.008), while in the FEPp group it was 25.2% (χ2 = 0.06; p = 0.868) (Table S1 (322.2KB, pdf) , available as online-only supplementary material).
Associations between SNVs and psychosis
Group differences in genotype frequencies and their association with psychosis using the dominant, additive, and recessive models are described in Tables S2, S3, and S4 (322.2KB, pdf) (available as online-only supplementary material), respectively. Single-locus analysis showed no significant association between the 23 SNVs and psychosis (p > 0.05 for all comparisons; adjusted models for sex, age, 10 PCs, years of schooling, and tobacco).
Gene-gene and gene-environment interactions in psychosis
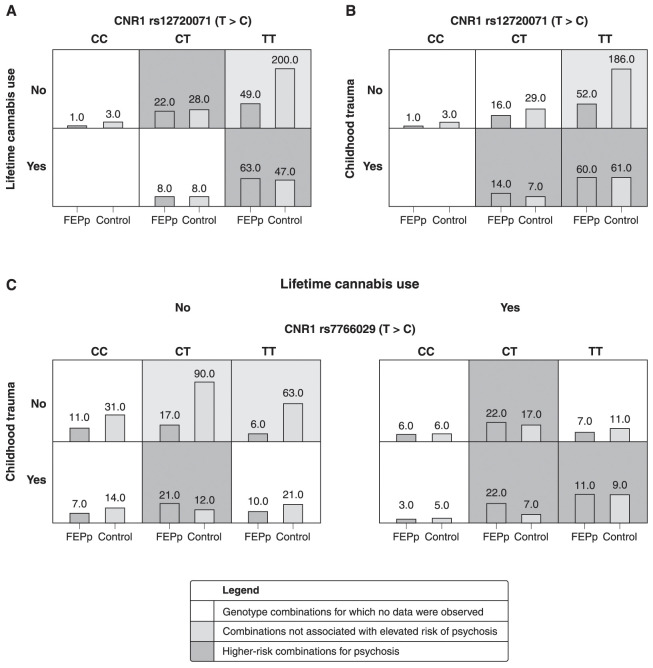
Regarding gene-gene interactions, no significant associations within or between genes were found (p > 0.05 for all comparisons, data not shown). However, MDR analyses demonstrated approximately 60-70% of the ability to classify the individuals included in the analysis and showed significant gene-environment interactions for the polymorphic loci rs12720071 and rs7766029 of the CNR1 gene (Table S5 (322.2KB, pdf) , available as online-only supplementary material). The best association model was represented by the combination of CNR1 rs12720071 with lifetime cannabis use (testing accuracy 66.4%, CVC 10/10, p < 0.001) (Figure 2A). The results suggested a higher risk for psychosis when combining the TT genotype of rs12720071 with lifetime cannabis use (OR = 7.1; 95%CI 2.5-19.8; p < 0.001). The Hosmer-Lemeshow statistic predicts 69.2% of the model.
Figure 2. Models assessing gene-environment and gene-gene associations considering: A) Lifetime cannabis use: individuals with lifetime cannabis use who were CNR1 rs12720071 T-allele carriers had a greater risk of psychosis (cross-validation consistency [CVC] = 10/10, testing accuracy = 66.4%, p < 0.001). The bars show the frequency distribution of genotypes for the first-episode psychosis patients (FEPp) and controls (left and right columns, respectively) in relation to lifetime cannabis use; B) Childhood trauma: risk association between individuals with childhood trauma and CNR1 rs12720071-T-allele carriers (CVC = 6/10, testing accuracy = 62.1%, p = 0.005). The bars show the frequency distribution of genotypes for the FEPp and controls (left and right columns, respectively) in relation to childhood trauma; and C) Lifetime cannabis use and childhood trauma: increased risk for developing psychosis in individuals with lifetime cannabis use, childhood trauma, and CNR1 rs7766029 T-allele carrier status (CVC = 8/10, testing accuracy = 69.3%, p < 0.001). The bars show the frequency distribution of genotypes for the FEPp and controls (left and right columns, respectively) in relation to both environmental risk factors.
Moreover, CNR1 rs12720071 (TT genotype) also showed a significant association with childhood trauma as a risk for psychosis (testing accuracy of 62.1%, CVC of 6/10 and p = 0.005) (Figure 2B). Likewise, as happened in the model including cannabis use, the TT genotype was detrimental when childhood trauma was combined (OR = 3.3; 95%CI 1.4-7.9; p = 0.006). Finally, an interaction between CNR1 rs7766029 (T-allele) with both lifetime cannabis use and childhood trauma presented a testing accuracy of 69.3%, CVC of 8/10, and p < 0.001 for association with psychosis (Figure 2C). This model shows that the combination of rs7766029-T homozygosity and the presence of both environmental risk factors increases the risk for psychosis (OR = 3.2; 95%CI 1.5-6.5; p = 0.002). Although models 2(B-C) are significant and clinically relevant, neither reached the considerations described by Moore53 to be classified as a good model. No significant associations between the environmental factors and other SNVs were found.
Discussion
In this case-control study of selected genetic and environmental risk factors for psychoses, we have demonstrated the interaction of SNVs of the CNR1 gene (rs12720071 and rs7766029) with both lifetime cannabis use and childhood exposure to trauma as increasing risk for psychosis based on analyses with the MDR approach. This approach has been applied to many psychiatric diseases, including non-affective54,55 and affective disorders.56,57
Various lines of evidence point to associations of cannabis use and childhood trauma with psychosis.58 We have confirmed both these associations in our sample. A relationship between childhood trauma and cannabis use in the control group indicates that early life stress might be a risk factor for subsequent cannabis use; the loss of this relationship in the FEPp suggests that other factors underlie the greater incidence of cannabis use in this latter group.
A fast-growing field of schizophrenia research has recently suggested the interactive effects of environmental and genetic factors moderating the risk of psychosis.9 We found a significant interaction between CNR1 genotype rs12720071 and cannabis use – an important environmental risk factor – in its association with psychosis. T-allele carriers of CNR1 presented a lower risk of psychosis when cannabis use was absent, while the same allele increased the risk of psychosis when the environmental risk factor was present. Moreover, T-allele carriers of CNR1 (rs12720071) presented an increased risk of psychosis when childhood trauma was present, while the model that included both environmental factors also showed that cannabis use abolished the protective association of rs7766029-T-allele with psychosis in the absence of childhood trauma. However, although we found statistical significance in MDR analysis, neither model met established criteria to be considered “good” in terms of representing the predictive ability of the gene-environment interactions in the case-control study. Thus, our findings may suggest that lifetime cannabis use is moderated by the CNR1 genetic variant for psychosis. Our results demonstrate that CNR1 rs12720071-T homozygosity interacts with lifetime cannabis use, suggesting combined gene-environment influences in mediating phenotypic features of psychosis.
The CB1 receptor, encoded by the CNR1 gene, is the primary brain receptor stimulated by endocannabinoids and exogenously by Δ9-THC.59 Despite conflicting evidence, SNVs of CNR1 gene polymorphic loci (rs12720071 and rs7766029), localized to chromosome 6q14-15, have been associated with cannabis use and psychosis risk.49,60 For instance, cannabis use was reported as a moderating factor between CNR1 rs12720071 genotypes and changes in cognitive performance in schizophrenia.61,62
The two CNR1 SNVs (rs12720071 and rs7766029) are localized in the 3′- untranslated region (3′-UTR) of exon 4, which plays a major role in gene expression regulation.63 Evidence shows that the presence of the variant on rs12720071 could be a binding site of a transcription factor for CCAAT/enhancer-binding protein beta (C/EBPbeta),64 with effects on neurogenesis.65,66 Consequently, the nucleotide switch could potentially change the C/EBPbeta transcription factor binding site, repressing CB1R expression and resulting in cognitive deficits in rs12720071-T-allele carriers who engage in cannabis use.61
Studies have also suggested a functional and direct relationship between NMDA and CB1 receptors that may be dysregulated by cannabinoid agonists, such as Δ9-THC, in precipitating psychosis susceptibility and inducing NMDAR hypofunction,12 which may compromise glutamate signaling and, consequently, essential processes including synaptic plasticity, memory formation, and learning.20
Additionally, although evidence has shown alterations in dopaminergic signaling and NMDAR genes in psychoses,10 inconsistencies remain concerning their associations. Considering our criteria for the MDR analysis, our findings did not show associations among D2R or NMDAR variants with environmental factors as a risk for psychosis, as we did not also reproduce the associations between these variants and psychosis reported previously,5,44,46-48 potentially attributable to the sample size and genetic diversity in different populations.
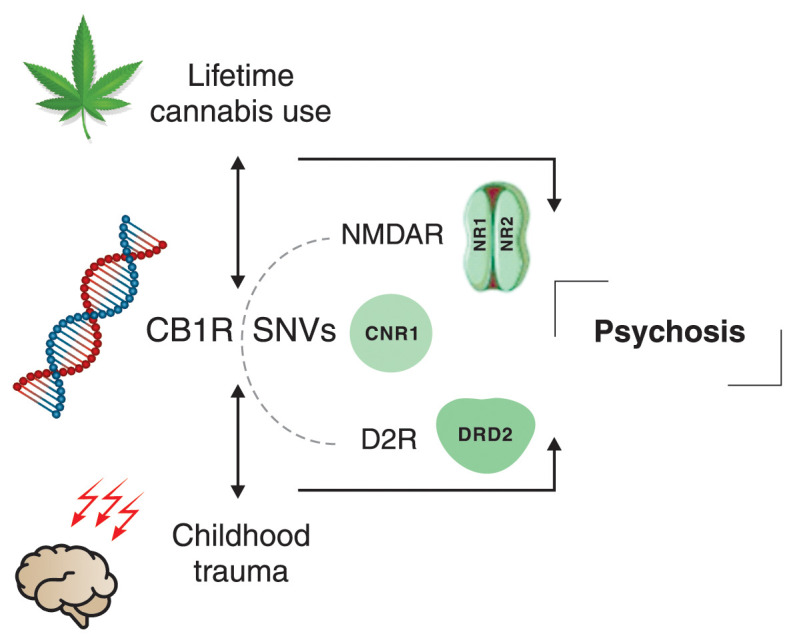
To the best of our knowledge, this is the first study to investigate the potential interactions of D2R, NMDAR, and CB1R SNVs in psychosis using the MDR statistical tool. We also speculate that the interaction between lifetime cannabis use or childhood trauma and CNR1 genotypes may induce neuromodulatory dysfunctions in dopaminergic and glutamatergic pathways, especially in the presence of another environmental risk factor, contributing to the multigene interaction involved in the pathophysiology of psychosis (Figure 3).
Figure 3. Schematic representation of our main findings. In the presence of CNR1 gene variants, lifetime cannabis use and childhood trauma may modulate the N-methyl-D-aspartate receptor (NMDAR) and dopamine-2 receptor (D2R) genes, increasing the risk of psychosis. CB1R = cannabinoid receptor type 1; SNVs = single nucleotide variants.
Despite our small sample size and exploratory nature of the study, our findings demonstrate the risk-increasing effects driven by gene-environment interactions of previously established environmental factors,8 supporting the multifactorial etiology and polygenicity of psychotic disorders. Recent findings have demonstrated that many common variants of risk genes with minor effects have been associated with schizophrenia and other psychosis,5 reinforcing the strong evidence of polygenic inheritance associated with multifactorial risk factors for psychosis. However, our gene-environment interaction findings may be relevant to genetic studies in non-European countries due to the genetic and cultural heterogeneity of the Brazilian population. The results of this study have some limitations. Due to the cross-sectional nature of the analysis presented, we are unable to make comparisons over time. Thus, larger and longitudinal cohorts are required to validate these findings and expand the interpretations about the causal effects of gene-environment interactions. In addition, the higher prevalence of lifetime cannabis use in FEP patients may be skewing the association of gene-environment interaction; however, we do not believe this occurred in our study, since the frequency of the rs12720071 genotype was not significantly different between the FEP group and the controls. Further research should attempt to control the discrepancies in genotype and environmental factor prevalences to understand better their association with psychosis. Finally, we did not include linkage disequilibrium analysis or haplotype-based approaches. Future studies should include haplotypes to identify the cis interaction between selected SNVs in addition to the main effects in relation to psychosis susceptibility.
We stress that the power of our results should be interpreted with caution, due to the small sample size after group comparisons. Our findings merit further investigation in a larger sample to elucidate the minor contributions of each SNV and their relationships with environmental factors.
In conclusion, our study supports the hypothesis of gene-environment interactions for psychosis and uncovers a genetic liability dependent on such interactions, even when the SNVs involved showed no independent effect. Specifically, we suggest a gene-environment interaction involving the SNVs rs12720071 and rs7766029-T-allele of the CNR1 gene, childhood trauma, and lifetime cannabis use in psychosis.
Disclosure
GPR has received honoraria for lectures and/or advisory panel membership from Janssen, Lundbeck, Otsuka, and Sunovion, and a research grant from Sunovion. The other authors report no conflicts of interest.
Acknowledgements
This study was supported by the research grant from the clinical research fund of Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grants 2012/05178-0 and 2013/08216-2) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001). CML, FC-Z, HAF, and RS are recipients of fellowships from FAPESP (grants 2020/15752-1; 2021/07448-3, and 2019/13229-2; 2017/00624-5 and 2015/02948-7; 2013/11167-3, respectively). AMO receives grant from CAPES – Finance Code 001. PRM, PL-J, SIB, RL, and CMD-B are recipients of fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
How to cite this article: Loureiro CM, Corsi-Zuelli F, Fachim HA, Shuhama R, de Oliveira AM, Menezes PR, et al. Lifetime cannabis use and childhood trauma increase risk of psychosis in carriers of CNR1 genetic variants: findings from the STREAM study. Braz J Psychiatry. 2023;45:226-235. http://doi.org/10.47626/1516-4446-2022-2882
References
- 1.McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia – an overview. JAMA Psychiatry. 2020;77:201. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 2.Huo Y, Li S, Liu J, Li X, Luo XJ. Functional genomics reveal gene regulatory mechanisms underlying schizophrenia risk. Nat Commun. 2019;10:670. doi: 10.1038/s41467-019-08666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang X, Wang S, Liu L, Du Y, Cheng B, Wen Y, et al. Integrating genome-wide association study with regulatory SNP annotation information identified candidate genes and pathways for schizophrenia. Aging. 2019;11:3704–15. doi: 10.18632/aging.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niu H-M, Yang P, Chen H-H, Hao R-H, Dong S-S, Yao S, et al. Comprehensive functional annotation of susceptibility SNPs prioritized 10 genes for schizophrenia. Transl Psychiatry. 2019;9:56. doi: 10.1038/s41398-019-0398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8. doi: 10.1038/s41586-022-04434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popovic D, Schmitt A, Kaurani L, Senner F, Papiol S, Malchow B, et al. Childhood trauma in schizophrenia: current findings and research perspectives. Front Neurosci. 2019;13:274. doi: 10.3389/fnins.2019.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Forti M, Quattrone D, Freeman TP, Tripoli G, Gayer-Anderson C, Quigley H, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6:427–36. doi: 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guloksuz S, Pries LK, Delespaul P, Kenis G, Luykx JJ, Lin BD, et al. Examining the independent and joint effects of molecular genetic liability and environmental exposures in schizophrenia: results from the EUGEI study. World Psychiatry. 2019;18:173–82. doi: 10.1002/wps.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahbeh MH, Avramopoulos D. Gene-environment interactions in schizophrenia: a literature review. Genes (Basel) 2021;12:1850. doi: 10.3390/genes12121850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33. doi: 10.1002/wps.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laksmidewi AAAP, Soejitno A. Endocannabinoid and dopaminergic system: the pas de deux underlying human motivation and behaviors. J Neural Transm (Vienna) 2021;128:615–30. doi: 10.1007/s00702-021-02326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Muñoz M, Sánchez-Blázquez P, Merlos M, Garzón-Niño J. Endocannabinoid control of glutamate NMDA receptors: the therapeutic potential and consequences of dysfunction. Oncotarget. 2016;7:55840–62. doi: 10.18632/oncotarget.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martel JC, McArthur SG. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003. doi: 10.3389/fphar.2020.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 16.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 17.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–5. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 18.Lee G, Zhou Y. NMDAR hypofunction animal models of schizophrenia. Front Mol Neurosci. 2019;12:185. doi: 10.3389/fnmol.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–16. doi: 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Blázquez P, Rodríguez-Muñoz M, Garzón J. The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: implications in psychosis and schizophrenia. Front Pharmacol. 2014;4:169–169. doi: 10.3389/fphar.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- 22.González-Mariscal I, Krzysik-Walker SM, Doyle ME, Liu Q-R, Cimbro R, Calvo S-CS, et al. Human CB1 receptor isoforms, present in hepatocytes and β-cells, are involved in regulating metabolism. Sci Rep. 2016;6:33302. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seifert J, Ossege S, Emrich HM, Schneider U, Stuhrmann M. No association of CNR1 gene variations with susceptibility to schizophrenia. Neurosci Lett. 2007;426:29–33. doi: 10.1016/j.neulet.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Tsai SJ, Wang YC, Hong CJ. Association study of a cannabinoid receptor gene (CNR1) polymorphism and schizophrenia. Psychiatr Genet. 2000;10:149–51. doi: 10.1097/00041444-200010030-00008. [DOI] [PubMed] [Google Scholar]
- 25.Del-Ben CM, Shuhama R, Loureiro CM, Ragazzi TCC, Zanatta DP, Tenan SHG, et al. Urbanicity and risk of first-episode psychosis: incidence study in Brazil. Br J Psychiatry. 2019;215:726–9. doi: 10.1192/bjp.2019.110. [DOI] [PubMed] [Google Scholar]
- 26.Jongsma HE, Gayer-Anderson C, Lasalvia A, Quattrone D, Mulè A, Szöke A, et al. Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry. 2018;75:36–46. doi: 10.1001/jamapsychiatry.2017.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gayer-Anderson C, Jongsma HE, Di Forti M, Quattrone D, Velthorst E, de Haan L, et al. The EUropean Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI): Incidence and First-Episode Case-Control Programme. Soc Psychiatry Psychiatr Epidemiol. 2020;55:645–57. doi: 10.1007/s00127-020-01831-x. [DOI] [PubMed] [Google Scholar]
- 28.Loureiro CM, Shuhama R, Fachim HA, Menezes PR, Del-Ben CM, Louzada-Junior P. Low plasma concentrations of N-methyl-d-aspartate receptor subunits as a possible biomarker for psychosis. Schizophr Res. 2018;202:55–63. doi: 10.1016/j.schres.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; 1997. Structured clinical interview for DSM-IV-TR axis I disorders, clinical version (SCID-CV) [Google Scholar]
- 30.Del-Ben CM, Vilela JAA, Crippa JAS, Hallak JEC, Labate CM, Zuardi AW. Confiabilidade da “Entrevista Clínica Estruturada para o DSM-IV – Versão Clínica” traduzida para o português. Braz J Psychiatry. 2001;23:156–9. [Google Scholar]
- 31.Singh SP, Cooper JE, Fisher HL, Tarrant CJ, Lloyd T, Banjo J, et al. Determining the chronology and components of psychosis onset: The Nottingham Onset Schedule (NOS) Schizophr Res. 2005;80:117–30. doi: 10.1016/j.schres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Crippa JA, Sanches RF, Hallak JE, Loureiro SR, Zuardi AW. A structured interview guide increases Brief Psychiatric Rating Scale reliability in raters with low clinical experience. Acta Psychiatr Scand. 2001;103:465–70. doi: 10.1034/j.1600-0447.2001.00185.x. [DOI] [PubMed] [Google Scholar]
- 33.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 34.Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–91. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stringer S, Minică CC, Verweij KJ, Mbarek H, Bernard M, Derringer J, et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry. 2016;6:e769. doi: 10.1038/tp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasman JA, Verweij KJH, Gerring Z, Stringer S, Sanchez-Roige S, Treur JL, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci. 2018;21:1161–70. doi: 10.1038/s41593-018-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassi-Oliveira R, Stein LM, Pezzi JC. Tradução e validação de conteúdo da versão em português do Childhood Trauma Questionnaire. Rev Saude Publica. 2006;40:249–55. doi: 10.1590/s0034-89102006000200010. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 39.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RStudio Team RStudio: Integrated Development for R. http://www.studio.com/
- 41.1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garani R, Watts JJ, Mizrahi R. Endocannabinoid system in psychotic and mood disorders, a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110096. doi: 10.1016/j.pnpbp.2020.110096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nkam I, Ramoz N, Breton F, Mallet J, Gorwood P, Dubertret C. Impact of DRD2/ANKK1 and COMT polymorphisms on attention and cognitive functions in schizophrenia. PLoS One. 2017;12:e0170147. doi: 10.1371/journal.pone.0170147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He M, He H, Yang L, Zhang J, Chen K, Duan Z. Functional tag SNPs inside the DRD2 gene as a genetic risk factor for major depressive disorder in the Chinese Han population. Int J Clin Exp Pathol. 2019;12:628–39. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang JP, Malhotra AK. Pharmacogenetics and antipsychotics: therapeutic efficacy and side effects prediction. Expert Opin Drug Metab Toxicol. 2011;7:9–37. doi: 10.1517/17425255.2011.532787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu YP, Ding M, Zhang XC, Liu Y, Xuan JF, Xing JX, et al. Association between polymorphisms in the GRIN1 gene 5’ regulatory region and schizophrenia in a northern Han Chinese population and haplotype effects on protein expression in vitro. BMC Med Genet. 2019;20:26. doi: 10.1186/s12881-019-0757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Li W, Zhang H, Yang G, Wang X, Ding M, et al. Association study of N-Methyl-D-Aspartate Receptor Subunit 2B (GRIN2B) polymorphisms and schizophrenia symptoms in the Han Chinese population. PLoS One. 2015;10:e0125925. doi: 10.1371/journal.pone.0125925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Z, Niu W, Bi Y, Zhang R, Ren D, Hu J, et al. A study of single nucleotide polymorphisms of GRIN2B in schizophrenia from Chinese Han population. Neurosci Lett. 2016;630:132–5. doi: 10.1016/j.neulet.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 49.Ferretjans R, Souza RP, Panizzutti B, Ferrari P, Mantovani L, Campos-Carli SM, et al. Cannabinoid receptor gene polymorphisms and cognitive performance in patients with schizophrenia and controls. Braz J Psychiatry. 2022;44:26–34. doi: 10.1590/1516-4446-2020-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–82. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- 51.Gilbert-Diamond D, Moore JH. Analysis of gene-gene interactions. Curr Protoc Hum Genet. 2011;Chapter 1:Unit1.14. doi: 10.1002/0471142905.hg0114s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritchie MD, Hahn LW, Moore JH. Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genet Epidemiol. 2003;24:150–7. doi: 10.1002/gepi.10218. [DOI] [PubMed] [Google Scholar]
- 53.Moore JH, Andrews PC. Epistasis analysis using multifactor dimensionality reduction. Methods Mol Biol. 2015:1253. doi: 10.1007/978-1-4939-2155-3_16. [DOI] [PubMed] [Google Scholar]
- 54.Prats C, Arias B, Moya-Higueras J, Pomarol-Clotet E, Parellada M, González-Pinto A, et al. Evidence of an epistatic effect between Dysbindin-1 and Neuritin-1 genes on the risk for schizophrenia spectrum disorders. European Psychiatry. 2017;40:60–4. doi: 10.1016/j.eurpsy.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Fan M, Wang Q, He G, Fu Y, Li H, et al. Polymorphisms in MicroRNA genes and genes involving in NMDAR signaling and schizophrenia: a case-control study in Chinese Han population. Sci Rep. 2015;5:12984. doi: 10.1038/srep12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y, et al. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2009;114:224–31. doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Oh S, Lee J, Kwon M-S, Weir B, Ha K, Park T. A novel method to identify high order gene-gene interactions in genome-wide association studies: gene-based MDR. BMC Bioinform. 2012;13:S5. doi: 10.1186/1471-2105-13-S9-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21:100. doi: 10.1007/s11920-019-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onaivi ES. Cannabinoid receptors in brain: pharmacogenetics, neuropharmacology, neurotoxicology, and potential therapeutic applications. Int Rev Neurobiol. 2009;88:335–69. doi: 10.1016/S0074-7742(09)88012-4. [DOI] [PubMed] [Google Scholar]
- 60.Gouvêa ES, Santos Filho AF, Ota VK, Mrad V, Gadelha A, Bressan RA, et al. The role of the CNR1 gene in schizophrenia: a systematic review including unpublished data. Braz J Psychiatry. 2017;39:160–71. doi: 10.1590/1516-4446-2016-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho BC, Wassink TH, Ziebell S, Andreasen NC. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr Res. 2011;128:66–75. doi: 10.1016/j.schres.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuzman MR, Kuharic DB, Ganoci L, Makaric P, Kekin I, Gajsak LR, et al. Association of CNR1 genotypes with changes in neurocognitive performance after eighteen-month treatment in patients with first-episode psychosis. Eur Psychiatry. 2019;61:88–96. doi: 10.1016/j.eurpsy.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel O V, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26:362–7. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ménard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, et al. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- 66.Pulido-Salgado M, Vidal-Taboada JM, Saura J. C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS. Prog Neurobiol. 2015;132:1–33. doi: 10.1016/j.pneurobio.2015.06.003. [DOI] [PubMed] [Google Scholar]