Abstract
The earliest activity-based photoacoustic (PA) probes were developed as diagnostic agents for cancer. Since this seminal work over a decade ago that specifically targeted matrix metalloproteinase-2, PA instrumentation, dye platforms, and probe designs have advanced considerably, allowing for the detection of an impressive list of cancer types. However, beyond imaging for oncology purposes, the ability to selectively visualize a given disease biomarker, which can range from aberrant enzymatic activity to the overproduction of reactive small molecules, is also being exploited to study a myriad of noncancerous disease states. In this review, we have assembled a collection of recent papers to highlight the design principles that enable activity-based sensing via PA imaging with respect to biomarker identification and strategies to trigger probe activation under specific conditions.
Keywords: biomedical imaging, human diseases, activity-based sensing, activatable probes, photoacoustic, biomarkers, in vivo, molecular imaging, optoacoustic, preclinical
1. Introduction
Photoacoustic (PA) imaging is a state-of-the-art imaging technique that allows for detailed in vivo imaging. This modality grants access to previously elusive and inaccessible molecular images with robust depth, precision, and accuracy, revolutionizing the field of molecular imaging. By exploiting the PA effect where optical excitation of a chromophore generates pressure waves which are detected as sound, PA imaging has successfully overcome the limitations of poor resolution in deep tissue that is associated with light-based methods. Initial work in the PA field focused on label-free imaging by employing endogenous light absorbing chromophores such as hemoglobin and melanin to generate contrast.1,2 However, label-free imaging restricts the broad potential of PA imaging due to the inability to detect essential nonlight absorbing biomarkers.
Seminal work by Gambhir and co-workers demonstrated the potential of this modality for cancer diagnostics and monitoring by targeting matrix metalloproteinase-2 (MMP-2) activity utilizing a design that consisted of two PA-active molecules appended together by a peptide substrate.3 MMP-2-mediated cleavage of the probe at the tumor site separates the components, leading to a change in the PA ratio. Since this work over 10 years ago, improvements with respect to instrumentation, dye platforms, and probe designs have been nothing short of revolutionary, lead to major advances in PA cancer imaging. In particular, activity-based sensing (ABS) designs have greatly expanded upon what was possible relative to traditional binding-based sensors (Scheme 1).4−7 Because ABS exploits the chemical reactivity of the analyte, they can be readily tuned to maximize interaction with the biomarker of interest. Furthermore, properties such as the kinetics of the probes can be adjusted to accelerate or attenuate reactivity to target transient or longer lasting (or abundant) molecular events, respectively.
Scheme 1. Schematic Demonstrating Different Probe Design Strategies.
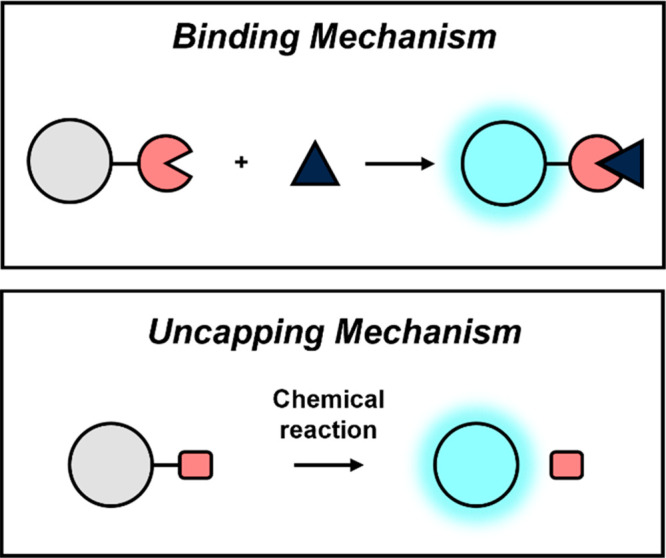
The binding mechanism (top) involves signal enhancement upon interaction between the probe and target. On the other hand, probe activation via an uncapping mechanism (bottom) involves cleavage and separation of the trigger from the PA reporter. Responsive element shown in pink.
In the present review, we highlight ABS probes for PA imaging that focus on noncancer applications. This will include work that target biomarkers found in organ systems such as the brain (section 2), liver (section 3), and stomach (section 4). Additionally, we discuss PA probes that enable the detection of inflammatory markers (section 5), as well as pathogens such as bacteria and viruses that cause infections (section 6). Finally, we will focus on examples that enable the visualization of biomarkers linked to a variety of blood clotting conditions (section 7).
2. Brain Imaging
The brain is one of the most complex organs in the human body, and work is still ongoing to demystify biological processes within this organ.8 Leveraging molecular imaging to study the mechanistic underpinnings of pathophysiological states can expedite the discovery of new treatment options. However, imaging of the brain is not trivial when employing molecular probes owing to low permeability through the blood–brain barrier (BBB), as well as a reliance on invasive procedures (e.g., generation of intracranial windows) to facilitate imaging studies. In this regard, advances in PA imaging and development of new BBB compatible imaging agents have allowed for noninvasive imaging of the brain to detect various biomarkers (e.g., calcium, lithium, ROS) in a manner that was previously not possible.9 We highlight recent work below in this area.
2.1. Emerging Alzheimer’s Disease Biomarkers
Alzheimer’s disease (AD) is a neurological disorder that impacts over 6 million individuals in the United States alone.10 It is characterized by the accumulation of tau proteins and beta amyloid plaques which have been hypothesized to be key culprits in causing mental impairment and dementia.11 Besides these well-recognized hallmarks, AD has also been linked to an increase of reactive oxygen species (ROS) production (e.g., hydrogen peroxide (H2O2)), as well as the accumulation of redox-active metals such as Cu2+.
To detect H2O2 in AD brains, it is critical to employ a PA reporter capable of producing a strong signal upon irradiation. Toward this end, Chan and co-workers remodeled the canonical hemicyanine dye (HD) by substituting the endocyclic oxygen with a sulfur atom, which red shifts the absorbance of the dye by ∼50 nm and concomitantly decreases the fluorescence quantum yield by 85% (to favor ultrasound (US) production).12 Moreover, the authors installed an ortho-chloro group to acidify the phenolic position, which ensures the dye is deprotonated at physiological pH to exhibit a larger extinction coefficient relative to the parent molecule. Capping of the resulting dye (named PA-HD) with an aryl boronate trigger led to the development of PA-HD-H2O2. An ensuing reaction with H2O2 converts the aryl boronate into a phenol, which spontaneously collapses to release PA-HD via quinone-methide elimination chemistry (Figure 1a). In their study, PA-HD-H2O2 was successfully employed to assess oxidative stress (via H2O2 detection) in AD mice (Figure 1b). Relative to age-matched wild-type (WT) animals where the change in the PA signal over time was only 1.02, the corresponding increase was 1.79 in the AD model (Figure 1c). To verify that this was due to probe activation and not dye accumulation, ratiometric analysis was employed. The observed ratiometric PA signal change was 1.23 and 0.85 in AD mice and WT mice, respectively.
Figure 1.
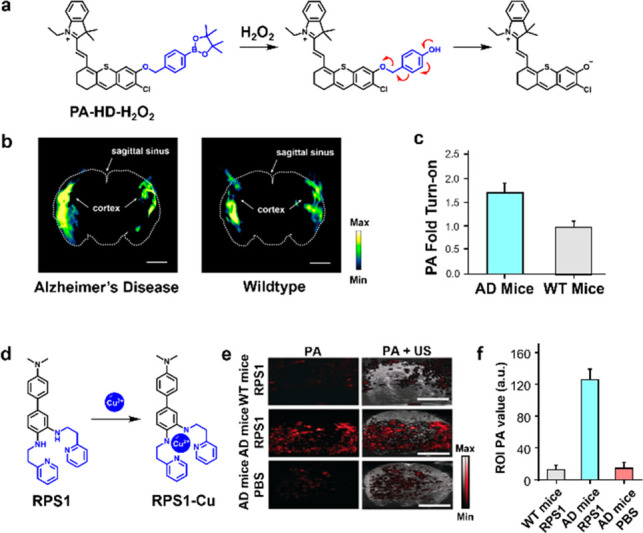
(a) Schematic of the reaction between PA-HD-H2O2 and H2O2 to afford PA-HD. (b) Spectrally unmixed PA images of AD and WT brain. Sagittal sinus and cortex indicated by arrows. (c) Quantified data from (b). (d) Schematic of reaction between RPS1 and Cu(II). (e) PA (left) and PA and ultrasound (right) images of WT mice and AD mice treated with RPS1 (top and middle rows, respectively), and AD mice administered a PBS vehicle control. (f) Quantified data from (e). Adapted with permission from refs (12) and (14). Copyright 2021 and 2019 Wiley.
A potential cause of elevated oxidative stress in the brain of AD mice may be due to Cu dysregulation. Specifically, it has been hypothesized that Cu2+ reacts with H2O2via Fenton-like chemistry to yield free radicals which is linked to the assembly of amyloid beta fibrils characteristic of AD.13 Since crossing the BBB is a challenge for large contrast agents and activatable imaging probes, Sheng, Zhang, and co-workers designed RPS1, a Cu2+-responsive PA probe that boasts a relatively low molecular weight of 438 Da.14 RPS1 features an aniline-based chelator, which upon binding to Cu2+, leverages the redox chemistry of this metal ion to form an anilinyl radical cation species that exhibits a red-shifted λPA in the NIR region (Figure 1d). Selectivity assays against a panel of divalent metal ions demonstrate excellent selectivity for Cu2+. To examine its ability to pass the BBB for in vivo applications, the authors conducted an experiment to show its permeability through a transwell cell monolayer model that mimics features of the BBB. Additionally, these findings were confirmed in a murine model of AD. Subsequent PA imaging resulted in a 9.57-fold increase in the signal intensity relative to non-AD control mice and an 8.5-fold increase when compared to PBS vehicle-treated AD mice (Figure 1e,f). To further corroborate these results, the authors confirmed plaque deposition and shrunken cell nuclei in AD brain slices, indicative of neurodegeneration.
2.2. Calcium Detection
Calcium ions (Ca2+) plays a key role in cellular messaging, contractility of muscles, immunity, and neuron signaling.15 However, many chemical tools to study Ca2+via molecular imaging are not suited for deep brain imaging and often requires generation of invasive intracranial windows.16 As such, PA probes for this metal ion have the potential to overcome this long-standing challenging by exploiting an ultrasound readout. To this end, Westmeyer and co-workers developed CaSPA, a cell-permeable and calcium-selective PA sensor.17 The design principle behind CaSPA relies on appending BAPTA (a common Ca2+ chelator) onto a semicyanine chromophore (Figure 2a). Upon interaction between Ca2+ by the BAPTA ligand, a photoinduced charge transfer (PCT) event leads to a hypsochromic shift of the probe absorbance profile which has the effect of yielding a turn-off response. CaSPA was applied to an in vivo model to visualize Ca2+ fluxes. Specifically, CaSPA-treated and nontreated control zebra fish were embedded in agar and PA imaging was employed to visualize this metal ion (Figure 2b). Ethanol and pentylenetetrazole (PTZ), two established neuro stimulants that trigger the release of Ca2+ was employed as stimulants. Under both conditions, the authors observed a notable attenuation of the PA signal indicating a binding event had taken place between Ca2+ and CaSPA (Figure 2c).
Figure 2.
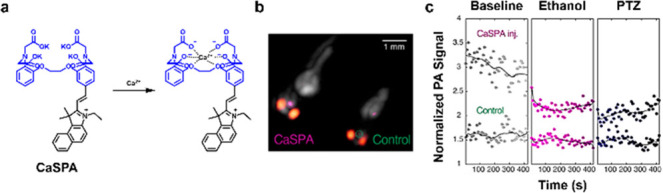
(a) Chemical structure of CaSPA and CasPA–Ca2+ complex. (b) Representative PA images of zebrafish to visualize Ca2+ fluxes in the brain. Purple regions indicate signal from CaSPA, and the orange regions indicate the melanin-containing eyes. (c) Quantified signal before (top curve) and after (bottom curve) treatment with ethanol or PTZ. Adapted from ref (17). Copyright 2018 American Chemical Society.
2.3. Stem Cell Tracking
Stem cell therapies are being explored to treat a myriad of diseases. However, tracking the fate of stem cells once introduced in vivo has been a challenge. To further study the downstream effects of stem cells, Jokerst and co-workers utilized PA imaging to visualize human mesenchymal stem cells (hMSCs) via labeling with Prussian blue nanoparticles (PBNPs) (Figure 3a).18 PBNPs were employed due to its biocompatibility, NIR absorption, stability, and strong PA signal upon irradiation. However, PBNPs have a negative zeta potential, resulting in poor cellular uptake due to charge repulsion with cell membranes. To circumvent this, PBNPs developed in this study were complexed with poly-l-lysine to yield nanocomplexes exhibiting a positive zeta potential. To ensure PBNPs did not alter the stemness properties of hMSCs, flow cytometry was conducted to confirm the presence of three stem cell surface markers: CD73, CD90, and CD105. Further characterization revealed a detection limit of 100 cells/μL and retention of the contrast agent for at least 2 weeks. Labeled and unlabeled hMSCs were injected subcutaneously in mice and imaged (Figure 3b). The authors were able to observe a sustained PA signal when 50,000 cells were introduced. Due to these promising results, hMSCs were then introduced to the brain via intraparenchymal injections to mimic stem cell transplantations in ischemic brain regions characteristic in stroke patients. Labeled hMSCs exhibited a 9.8-fold increase in PA signal increase in the brain, allowing for its detection in vivo (Figure 3c).
Figure 3.
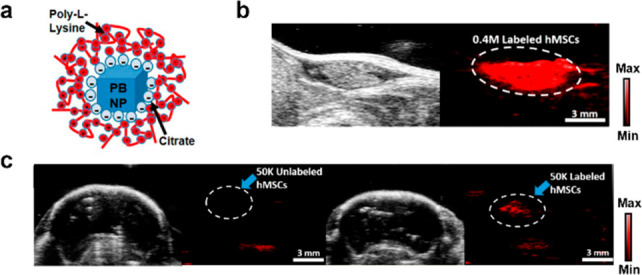
(a) Structure of encapsulated nanoparticle. (b) Representative US (left) and PA (right) image of labeled hMSCs. (c) Representative US and PA images of unlabeled and labeled hMSCs in mouse brain imaging. Adapted from ref (18). Copyright 2017 American Chemical Society.
3. Liver Imaging
As mentioned above, the liver plays a crucial role in human physiology due to its function in detoxifying xenobiotics. Because of the wide range of toxins that the liver encounters, it is susceptible to damage from drugs, ingested foods, and harmful chemicals from the environment. Of note, while inflammation is a common property of liver injury, we will cover this feature in the following section. Beyond the inflammatory response, a wide range of biomarkers ranging from the production of ROS to increased activity of certain liver enzymes has also been associated with liver damage. By taking advantage of these changes, the diversity of activatable probes suitable for molecular imaging of the liver has greatly expanded.
3.1. Elevated Cu in Wilson’s Disease
Wilson’s disease (WD) is a genetic disorder caused by a mutation in the gene encoding ATP7B, a membrane-associated Cu-transporting protein that predominantly impacts the brain and liver. The resulting accumulation of Cu in these organs leads to severe tissue damage which can be fatal if not treated. The levels of Cu in a WD patient are typically assessed through blood tests and invasive liver biopsies.19 Chan and co-workers recently introduced a noninvasive PA imaging alternative based on the application of PACu-1, the first acoustogenic probe for Cu+ visualization.20 PACu-1 features a Cu+-responsive TPA trigger appended to an aza-BODIPY dye through a cleavable ether linkage (Figure 4a). Release of the free dye after an oxidative cleavage event results in a red shift of the λabs by ∼90 nm which is exploited for ratiometric calibration. Of note, the +1-oxidation state was targeted as opposed to Cu2+ because it is the predominate form in the liver owing to the highly reducing environment rich in GSH (10 mM). Upon successfully demonstrating PACu-1 is safe to use in living subjects, it was employed to measure hepatic Cu via biopsy-free assessment (BFA) in a murine model of WD. To remove potential experimental bias, the authors designed and executed two blind studies. First, they successfully identified a single WD mouse out of a group consisting of a total of eight mice that were randomly selected from a larger pool of animals (Figure 4b,c). Second, a group of 12 animals consisting of six WD and six wild-type mice were randomized, and their identities were concealed from the experimenter. Using PACu-1 via PA imaging, the authors were able to correctly stratify each of the 12 animals with a success rate of 100% (Figure 4d). Overall, this work sets the stage to use other biocompatible PA imaging agents to complement invasive biopsies for biomarker detection.
Figure 4.
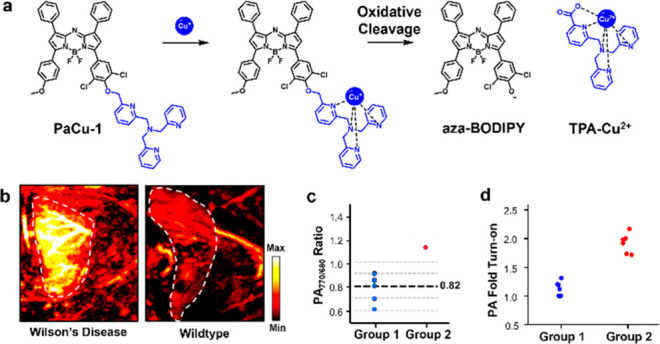
(a) Structure of PaCu-1 and its mechanism of activation after undergoing a redox reaction with Cu+. (b) Representative PA images of WD mice and wild-type mice and (c) its ratiometric signal to identify WD from a cohort of animals in a blind study. (d) PA fold turn-on of mice in a second blind study to stratify a group of WD and wildtype mice via the extent of signal enhancement. Adapted with permission from ref (20). Copyright 2021 National Academy of Sciences.
3.2. Liver Injury Monitoring
As mentioned above, the liver plays a crucial role in human physiology due to its function in detoxifying xenobiotics. Because of the wide range of toxins that the liver encounters, it is susceptible to damage from drugs, ingested foods, and harmful chemicals from the environment. A wide range of biomarkers ranging from the production of ROS to increased activity of certain liver enzymes has been associated with liver damage. By taking advantage of these changes, the diversity of activatable probes suitable for molecular imaging of the liver has greatly expanded.21−29
ROS are known to nonspecifically interact with a multitude of molecules and biomolecules. Additionally, the superoxide anion radical (O2•–) has been found at higher concentrations in damaged livers. To take advantage of the chemical reactivity of this ROS for molecular imaging, Ren, Yuan, and co-workers developed AP, an aza-BODIPY-based ratiometric PA probe for liver imaging (Figure 5a).30 To enhance solubility and introduce a targeting group to the liver, β-galactose was appended to the dye scaffold through a water solubilizing PEG linkage. Furthermore, the dye was capped with a trifluoromethanesulfonate trigger due to its electron-deficient properties as well as its selectivity for O2•–. Upon reaction with O2•–, the trigger is removed to release the latent dye. Of note, this uncapping process also induces a bathochromic shift when compared to the capped probe. To test their lead molecule in cells, the authors confirmed galactose-mediated uptake, as well as probe turnover in an LPS-induced liver injury model in L02 cells. To determine its efficacy for in vivo imaging, the probe was introduced to an isoniazid (INH)-induced liver injury model (Figure 5b). A ratiometric turn-on of 1.5-fold at PA815/PA745 was observed, while the ratiometric signal for the saline control was less than 1 (Figure 5c). Finally, pretreatment with N-acetylcysteine (an ROS scavenger) prior to INH injection decreased the ratiometric turn-on, indicating its antioxidant properties.
Figure 5.
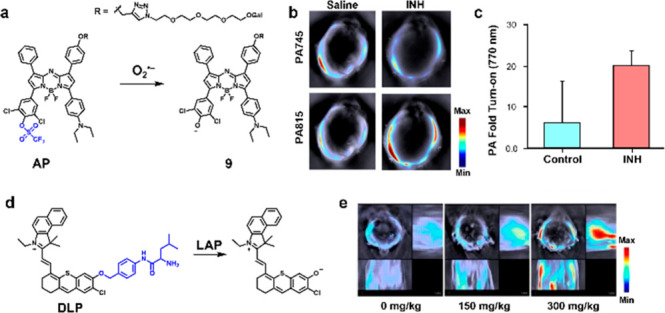
(a) Structure and turnover product of AP. (b) Representative cross-sectional PA images of an INH-induced liver injury model and saline control acquired at t = 40 min. (c) Quantified PA fold turn-on. (d) Structure and turnover product of DLP. (e) Representative cross-sectional and lateral PA images of an acetaminophen-induced liver injury model. Adapted from refs (30) and (31). Copyright 2022 and 2019 American Chemical Society.
Apart from ROS generation, liver injury also leads to various downstream effects, including the overexpression of enzymes such as leucine aminopeptidases (LAP). Zeng, Wu, and co-workers developed a probe (DLP) based on the hemicyanine dye platform to detect this enzymatic activity (Figure 5d).31 A leucyl group was appended on to the chromophore via a self-immolative 4-aminobenzylalcohol linker to mimic an N-terminal leucine. Upon cleavage by LAP, the linker undergoes a quinone methide elimination to release the free dye. The probe was then tested against a panel of metal ions, ROS, biomolecules, and enzymes to show negligible response. Further, the probe was tested in HepG2 human liver cells for LAP activity. Upon pretreatment with bestatin, a LAP inhibitor, the PA signal significantly decreased which supports the mode of action of their probe. To generate a liver injury model, mice were intraperitoneally injected with acetaminophen. Subsequent injection of the probe enabled PA imaging of liver injury via LAP upregulation with a signal that peaked 30 min post dye administration. Additionally, the authors were able to show a dose-dependent signal increase when liver injury was induced with higher dosages of acetaminophen (Figure 5e). Finally, ex vivo imaging corroborated the stronger PA signal within the liver compared to other major organs and H&E staining validated macrophage infiltration.
4. Stomach Imaging
Dysregulation of stomach pH is indicative of many pathologies. For instance, gastric ulcers cause a change of the stomach pH (3.4) compared to healthy subjects (2.9). Similarly, esophageal ulcers and duodenal ulcers also alters the pH (1.9 and 2.1, respectively) in the stomach. To enable PA imaging of stomach pH, Sarma, Sessler, Liu, and co-workers developed a panel of expanded porphyrins.32 Their lead porphyrin, octaphyrin, was encapsulated in DSPE-PEG2000 to yield OctaNPs (Figure 6a). Under acidic redox conditions, OctaNPs undergo a CPET (concerted proton electron transfer) process. Since gastric acid is composed of redox active hydrochloric acid, octaphyrin is reduced from the 32 π-electron nonaromatic state to the 34 π-electron aromatic state, leading to an increased in absorbance at 1200 nm. To test OctaNP in vivo, mice were subjected to intragastric injection, resulting in an immediate increase in PA signal when excited, indicative of a low stomach pH (Figure 6b,c). Additionally, to visualize dynamic changes, mice were pretreated with sodium bicarbonate to neutralize the acidic stomach environment. Upon injection of OctaNP, no PA signal enhancement was observed. However, over a period of 15 min, secretion of gastric acid restores the pH of the stomach, resulting in a gradual PA signal enhancement.
Figure 6.
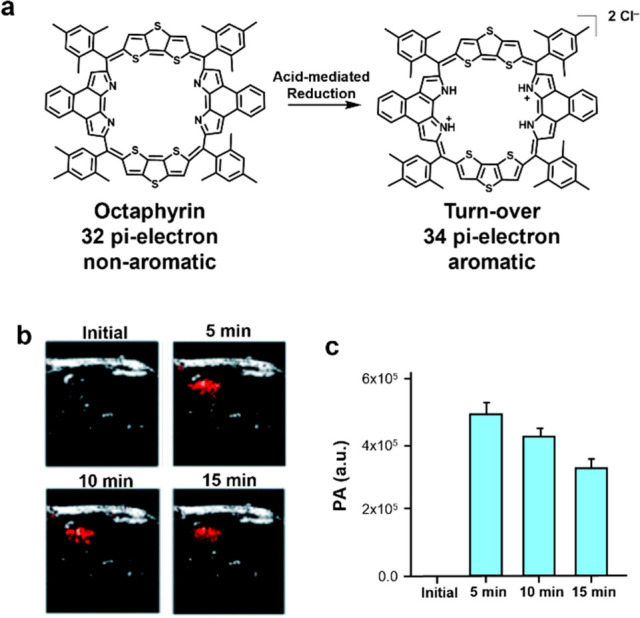
(a) Chemical structure of octaphyrin and its turnover product. (b) Representative PA images of the stomach area of mice before and after injection with octaphyrin. (c) Quantified PA signal. Adapted with permission under CCBY 3.0 license from ref (32). Copyright 2021 Royal Society of Chemistry.
5. Inflammation Imaging
Inflammation is a central process to the body’s ability to illicit an immune response when challenged with a pathogen. However, a chronic inflammatory state is also known to exacerbate various human disease states. What is known in this regard is that inflammation is a complex process involving biomarkers ranging from enzymatic systems to small molecule mediators. Owing to its broad implications in health, significant efforts have been made to develop PA probes to detect inflammatory biomarkers.
5.1. MGL and FAAH Activity Sensing
Monoacylglycerol lipase (MGL) and fatty acid amide hydrolase (FAAH) are two important enzymes of the endocannabinoid system (ECS).33 Both are responsible for producing arachidonic acid (AA), which is a molecule involved in inflammation and energy metabolism. The major difference between MGL and FAAH activity is highlighted by the linkages they hydrolytically cleave. Specifically, MGL processes ester bonds, while FAAH hydrolyzes amides; however, it is noteworthy that there is significant cross-reactivity when considering the substrate scope. For instance, FAAH can hydrolyze both of these linkages with nearly identical rate constants. It is this undesirable cross-reactivity that has precluded the development of enzyme-selective tools to investigate these enzymes in the context of inflammation. To overcome this challenge, ABS-tuning was employed by Chan and co-workers to stabilize the ester bond (via orbital interactions) of a new MGL probe.34 Likewise, the authors prepared a selective substrate for FAAH by linking a PA reporter to AA through an amide bond. The resulting probes, PA-HD-MGL and PA-HD-FAAH (Figure 7a,b), exhibit excellent enzyme selectivity profiles and favorable PA imaging properties. In cellulo studies using LNCaP cells, which is a prostate cancer cell line known to express high levels of both enzymes, allowed the authors to confirm target engagement using small-molecule inhibitors. PA-HD-MGL and PA-HD-FAAH were then employed in a high-fat diet-induced obesity model. Cross-sectional and lateral view PA imaging of the gastrointestinal tract of obese animals revealed a 1.59-fold and 1.74-fold higher signal compared to the control group that was fed a low-fat diet using PA-HD-MGL (Figure 7c,d) or PA-HD-MGL (Figure 7e,f), respectively. These results implicate that upregulation of MGL and FAAH activity may be a driver of inflammation in obese animals.
Figure 7.
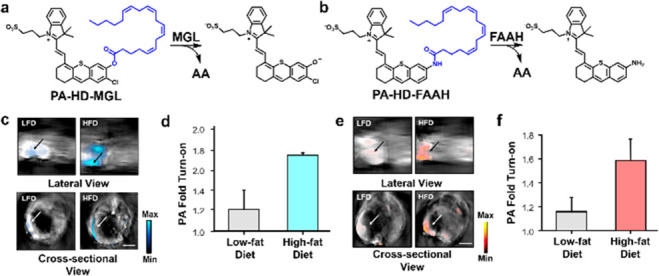
(a) Structure of PA-HD-MGL and (b) PA-HD-FAAH and their turnover products. (c) Representative lateral and cross-sectional PA images and (d) PA fold turn-on between low-fat diet (LFD) and high-fat diet (HFD) mice for PA-HD-MGL. (e) Representative lateral and cross-sectional PA images and (f) PA fold turn-on between LFD and HFD mice for PA-HD-FAAH. Adapted with permission from ref (34). Copyright 2022 Wiley.
5.2. Nitric Oxide Detection
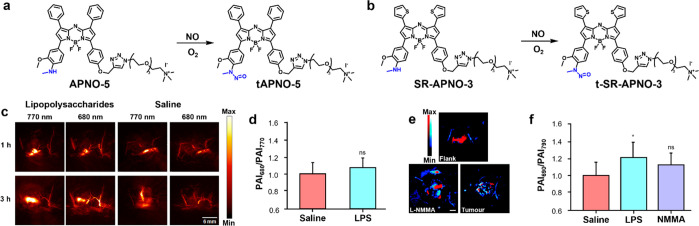
Nitric oxide (NO) is an important gasotransmitter used by the body for signaling, homeostatic maintenance, and the modulation of the inflammatory response.35 However, detecting NO within a living system is an immense challenge owing to its short biological half-life (second range) that results from its high reactivity with an abundance of nucleophilic species. Chan and co-workers addressed this by preparing a panel of five ABS probes featuring different known triggers.36 They found the installation of an N-methylaniline trigger onto an aza-BODIPY dye, not only enabled rapid trapping of NO (via the active species N2O3) to yield a N-nitrosated product, but that this reaction was accompanied by a 91 nm blue shift (764 to 673 nm) (Figure 8a). Of note, molecules such as APNO-5 that can undergo distinct and dramatic changes in their spectral properties upon analyte detection are ideal for ratiometric imaging. To induce an inflammatory response in vivo, the authors treated mice with LPS (major component of Gram-negative bacteria cell walls) prior to the application of APNO-5 or a saline vehicle control. PA imaging was then performed at 770 and 680 nm to detect the probe and product, respectively (Figure 8c). The authors observed a change in the ratiometric signal (PAI680/PAI770) relative to a saline control over time, representing a 1.31-fold ratiometric turn-on (Figure 8d). Despite successfully detecting NO in this context, the authors subsequently prepared a congener (named SR-APNO-3) that was found to be 4.4-fold more sensitive than APNO-5 (Figure 8b).37 This was accomplished by replacing both phenyl groups (on the top hemisphere of APNO-5) with thiophene units which increased the extinction coefficient by facilitating dye planarization through steric relaxation. Moreover, the authors noted a decreased quantum efficiency via the heavy-atom effect. Not only did these improvements enable detection of NO in the LPS-induced inflammation model described above, but the authors were also able for the first time to detect NO in tumors, which is present at levels orders of magnitude lower via PA imaging (Figure 8e,f).
Figure 8.
(a) Structure of APNO-5 and (b) SR-APNO-3. (c) Representative photoacoustic images of APNO-5 in a murine LPS-induced inflammation model and (d) ratiometric PA imaging (PAI680/PAI770) of NO in a LPS-induced inflammation model through intramuscular administration of APNO-5. Adapted from ref (36). Copyright 2018 American Chemical Society. (e) Representative image overlays corresponding to t-SR-APNO-3 (690 nm, blue) and SR-APNO-3 (790 nm, red) after 6 h subcutaneous or intratumoral administration and (f) ratiometric PA imaging (PAI690/PAI790) of NO in a LPS-induced inflammation model through intramuscular administration of SR-APNO-3. Adapted with permission under a CCBY 3.0 license from ref (37). Copyright 2020 Royal Society of Chemistry.
5.3. Osteoarthritis Visualization
Degenerative joint disorders are also characterized by inflammation. Patients afflicted with these conditions experience cartilage degeneration which ultimately leads to osteoarthritis (OA). On a molecular level, glycosaminoglycans (GAGs) are an important component of cartilage that carries negative charges. Taking advantage of this property, Yu, Fan, and co-workers encapsulated water-soluble PA-active melanin nanoparticles (MNPs) with poly-l-lysine to develop PLL-MNPs that have a positive surface charge (+32.5 mV) (Figure 9a).38 Due to charged interactions between GAGs and PLL-MNPs, healthy joints with intact cartilage will exhibit higher PA signal due to higher retention of the contrast agent. An in vitro experiment was performed where cartilage samples were incubated in PLL-MNPs or MNPs to demonstrate that the mechanism involves Coulombic interactions. Following washes with cold PBS, PA imaging revealed higher a signal intensity for PLL-MNP incubation (2580 au) when compared to MNP incubation (1425 au) after 24 h. Furthermore, PLL-MNPs were employed in an OA mouse model by surgically destabilizing the medial meniscus. OA mice exhibited a lower PA signal (1441 au) when compared to the sham-treated control mice (2113 au) (Figure 9b,c). Subsequently, H&E staining revealed higher inflammatory cell infiltration and Safranin-O staining corroborated decreased GAGs in the OA model when compared to sham mice.
Figure 9.
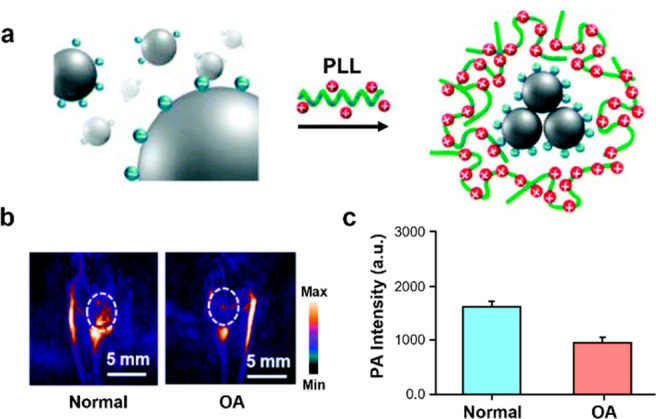
(a) Structure and assembly of PLL-MNPs. (b) Representative PA images of normal mice and osteoarthritis mice. (c) Quantified PA signal. Adapted with permission from ref (38). Copyright 2018 Royal Society of Chemistry.
6. Pathogen Imaging
6.1. Bacteria Detection
Bacterial infections have increasingly become prevalent due to the rise of multi-drug-resistant strains. The ability to visualize bacteria in vivo can lead to advances in diagnosing infections and monitoring treatment. Taking advantage of maltodextrin transporters present in bacterial cells but not mammalian cells, Gambhir and co-workers functionalized a Cy7 derivative with maltotriose or maltohexose to facilitate dye uptake into bacteria (Figure 10a).39 Initial competition experiments were performed to elucidate the mode of uptake using a radiotracer (3H-maltose). After confirming this mechanism, an E. coli-induced myositis model was generated to evaluate performance in vivo. E. coli was injected intramuscularly to the left thigh and heat-inactivated E. coli was introduced to the right. PA imaging confirmed selective accumulation in the left E. coli-infected thigh. While a signal enhancement was observed with both maltotriose and maltohexose-labeled derivates, the authors observed no significant difference between uptake between maltotriose and maltohexose-labeled derivates. Furthermore, since Staphylococcus aureus infections are common in hospital settings, an S. aureus infection mouse model was also generated by subcutaneous inoculation into the back using a bioluminescent Xen 36 strain. One group received vancomycin treatment twice daily, and the other group received no antibiotics. After a week of treatment, bioluminescence and PA imaging both confirmed the decrease in signal from the bacterial infection (Figure 10b,c).
Figure 10.
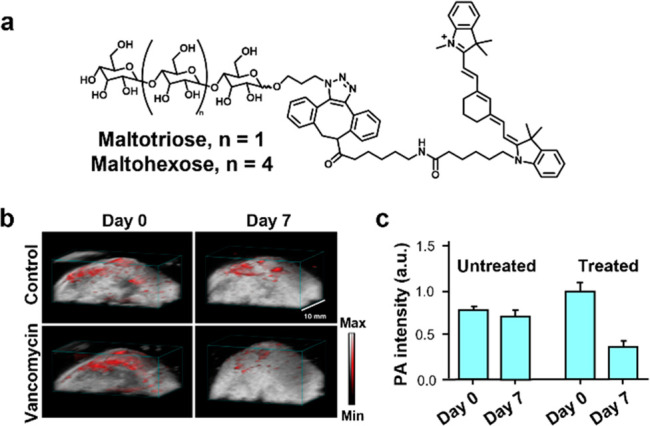
(a) Structure of Cy7–1-maltotriose and Cy7–1-maltohexose. (b) Representative PA images of a bacterial infection thigh model in mice before and after vancomycin treatment or saline treatment. (c) Quantified PA signal. Adapted with permission under a CCBY 4.0 license from ref (39). Copyright 2020 Springer Nature.
In another study, gold nanoparticles were developed to detect bacterial infection. Specifically, Zou, Li, Wang, and co-workers reported AuNP@P1, a P1 peptide surface labeled-gold nanoparticle.40 The design of P1 includes three key elements: the assembly fragment (LVFFAED), enzyme-responsive linker (PLGVRG), and targeting ligand (RVRSAPSS). The latter enables accumulation of the nanoparticle in the bacterial microenvironment, where collagenase IV is overexpressed. Upon cleavage of the enzyme-responsive linker, the self-assembly fragment becomes exposed which allows for the nanoparticles to aggregate at the infection site through hydrogen bonding and π–π and hydrophobic interactions between the residues. This phenomenon increases the retention time via the aggregation/assembly induced retention effect and exhibits increased PA signal enhancement due to enhanced nanoparticle accumulation. A control nanoparticle, AuNP@P2, was also synthesized with a scrambled enzyme-responsive linker so that it would not aggregate in the presence of collagenase IV. Upon incubation with collagenase IV, AuNP@P1 exhibited an increase in absorbance at 710 nm due to aggregation while AuNP@P2 had no change. To examine its utility in vivo, S. aureus was injected into the hindlegs of mice. After 12 h, mice were treated with AuNP@P1 or AuNP@P2. PA imaging experiments concluded that accumulation had peaked 24 h postinjection with a 2-fold signal difference between the probe and control.
6.2. Virus Detection
Viral replication can lead to the expression of unique proteases that can be leveraged to detect viral infections. Jokerst and co-workers took advantage of the propensity for the cyanine dye platform to aggregate to develop a generalizable ABS probe for proteases (Figure 11a).41 J-Aggregation or H-aggregation was promoted by appending two molecules of a cyanine together through a peptide linker, leading to a shift in the maximum absorbance. Upon protease cleavage of the unique peptide sequence, the probe separates into two equivalents of monomeric cyanine. Since the PA signal is directly correlated to the number of photons absorbed, exciting at the maximum absorbance of the monomer (rather than the aggregates) allows for PA imaging of the turned over monomer. An initial proof-of-concept study was performed with a sequence recognized by trypsin in vitro to show a PA signal enhancement in the presence of this biomarker. Further, to show generalizability, the peptide sequence AVLQSGFR, recognized by Mpro from SARS-CoV-2, was inserted between two monomers. Upon introduction of Mpro, an increase in the PA signal was observed, motivating the use of this simple assay for the development of inhibitors for Mpro. Finally, the authors tested the probe in vivo by injecting nude mice with the probe that had been preactivated by trypsin prior to PA imaging (Figure 11b).
Figure 11.
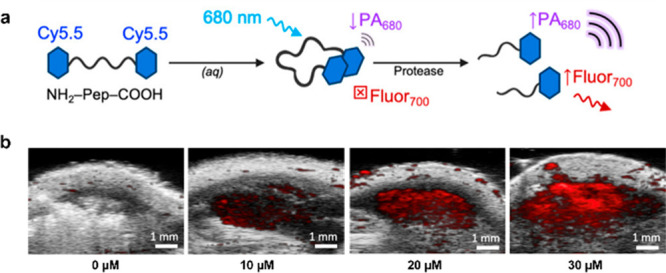
(a) Design principle of linking dyes to promote aggregation. (b) Representative PA images of mice injected with vehicle and trypsin-activated probe. Adapted from ref (41). Copyright 2021 American Chemical Society.
7. Blood Clot Imaging
7.1. Thrombosis Monitoring
Thrombin is an important enzyme involved in blood coagulation to close wounds. However, thrombosis occurs when a clot obstructs blood flow with potentially lethal consequences. On the other hand, if thrombosis can be located and resolved early using biomarkers such as thrombin and heparin, treatment can be lifesaving.42 Using thrombin as a biomarker for thrombosis, Lu, Chan, and co-workers developed the first activatable aptamer-based PA probe (Figure 12a).43 The design principle relies on a fluorophore (IRDye 800CW) and a quencher (IRDye QC-1) pair which are each bound to a separate DNA aptamer. Both aptamers are hybridized to third aptamer containing a thrombin binding sequence. In the absence of thrombin, the fluorophore is in close proximity to the quencher and thus, exhibits a small PA780/PA725 ratio. However, in the presence of thrombin, the reduction of contact quenching results in a large PA780/PA725 ratio. To determine its utility in biological systems, the probe was tested in human serum spiked with varying amounts of thrombin, revealing a LOD of 344 nM. Furthermore, the probe was applied to an in vivo thrombosis model where mice were injected with thrombin in the left flank and PBS in the right flank. PA imaging revealed a larger ratiometric turn-on response on the thrombin flank, demonstrating probe activation had taken place (Figure 12b,c).
Figure 12.
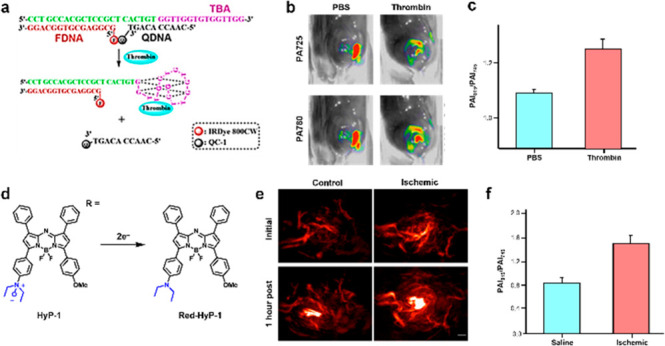
(a) Design principle of activatable DNA aptamers to detect thrombin in blood clotting. (b) Representative PA images at 725 and 780 nm. (c) PA fold turn-on between thrombin-treated mice and PBS-treated mice. Adapted from ref (43). Copyright 2017 American Chemical Society. (d) Structure of HyP-1 and its turnover product. (e) Representative PA images of an ischemia mouse model and nonischemia control. (f) Quantified PA fold turn-on. Adapted with permission under a CCBY 4.0 license from ref (44). Copyright 2017 Springer Nature.
7.2. Ischemic Hypoxia Sensing
Hypoxia is an important feature of peripheral artery diseases, liver injury, and gastrointestinal inflammation. It is defined as a situation where the demand for oxygen by tissue is greater than what is available. Notably, Chan and co-workers have developed HyP-1, a hypoxia-responsive PA probe based on the aza-BODIPY scaffold which features a N-oxide-based trigger (Figure 12d).44 Under hypoxic conditions, CYP450 enzymes react with the N-oxide group, undergoing two-electron reduction to yield the uncapped dye (Red-HyP-1) which results in a red-shifted emission. HyP-1 has an absorbance and emission maxima at 670 and 697 nm, respectively, while red-Hyp-1 (the turned over product) has an absorbance and emission maxima at 760 and 798 nm, respectively. Due to the large Stokes shift, this platform was employed for multimodal (fluorescence and PA) ratiometric imaging. In their study, the authors generated a murine hindlimb ischemia model via ligation of the femoral artery to block blood flow. An hour after the procedure, mice were injected intramuscularly with HyP-1 and imaged using both modalities 1 h postinjection. Ratiometric fluorescence imaging exhibited a 3-fold increase in signal and PA imaging displayed a 3.1-fold turn-on response (Figure 12e,f). It is noteworthy that the N-oxide trigger developed in this study can detect acute hypoxia, whereas probes based on the traditional aryl nitro moiety can only detect chronic hypoxia due to the requirement for nitroreductase overexpression.
8. Conclusion
With the advent of activatable PA probes that employ an ABS design strategy, the detection and visualization of a wide range of biomarkers is now readily possible. Although the most common application of such probes is to study cancer-associated properties in vivo, the field has experienced tremendous growth in recent years to now include the molecular imaging of various other human disease states as we have highlighted in this review. A summary of the probes covered can be found in Table 1. The unique ability to reliably detect a molecular feature with high resolution and in deep tissue, sets PA imaging apart from other approaches. Indeed, this modality has already been used in humans to visualize inflammatory arthritis and scleroderma to name a few.45,46 However, it is notable that these representative clinical studies were performed in a label-free manner, and therefore, one would expect the ability to accurately diagnose these conditions will improve substantially when augmented with PA probes. In some instances, we anticipate that the application of PA probes can potentially replace invasive biopsies such as for Wilson’s disease (section 3.1). In order for PA probes to make a successful transition into the healthcare setting and become a mainstay, it is critical for probe markers to demonstrate utility in models beyond small laboratory animals. Indeed, the use of large animals (e.g., canine) exhibiting naturally occurring pathologies can expedite use in humans. Along these lines, probe developers should interface with bioengineers to actively develop PA imaging instrumentation for use in humans. On the probe development front, new PA-active dye platforms are highly desirable and should be actively pursued since the stronger the signal output, as predicted by a large PA brightness factor, can enable greater diagnostic accuracy.47 Additionally, a move to the shortwave infrared window (section 4) can allow for greater tissue penetration as interference from endogenous absorbers like blood is minimized.
Table 1. Summary of Small Molecule Probes Highlighted in This Review Article.
| name | λabs | biomarker | disease model | mode of activation | delivery method | Author(s); year |
|---|---|---|---|---|---|---|
| PA-HD-H2O2 | 645 | H2O2 | mouse Alzheimer’s disease | uncapping | intravenous | Chan; 2021 |
| turnover | 745 | |||||
| RPS1 | 710 | Cu2+ | mouse Alzheimer’s disease | binding | intravenous | Sheng, Zhang; 2019 |
| CaSPA | 550 | Ca2+ | zebrafish neurostimulation | binding | intraventricular | Westmeyer; 2018 |
| PA-HD-MGL | 600 | MGL | mouse inflammation | uncapping | intraperitoneal | Chan; 2022 |
| turnover | 740 | |||||
| PA-HD-FAAH | 658 | FAAH | mouse inflammation | uncapping | intraperitoneal | Chan; 2022 |
| turnover | 730 | |||||
| APNO-5 | 764 | NO | mouse inflammation | uncapping | subcutaneous | Chan; 2018 |
| turnover | 673 | |||||
| SR-APNO-3 | 790 | NO | mouse flank tumor | uncapping | intratumoral | Chan; 2020 |
| turnover | 690 | |||||
| PACu-1 | 678 | Cu+ | mouse Wilson’s disease | uncapping | intraperitoneal | Chan; 2021 |
| turnover | 767 | |||||
| AP | 745 | O2•– | mouse liver injury | uncapping | intravenous | Ren, Yuan; 2022 |
| turnover | 835 | |||||
| DLP | 666 | DLP | mouse liver injury | uncapping | intravenous | Zeng, Wu; 2019 |
| turnover | 705 | |||||
| HyP-1 | 670 | hypoxia | mouse ischemic hypoxia | uncapping | intramuscular | Chan; 2017 |
| Red-Hyp-1 | 760 | |||||
| octaphyrin | 1200 | pH | mouse stomach pH | N/A | intragastric | Sarma, Sessler, Liu; 2021 |
| Cy7–1-maltotriose/hexose | 750 | maltodextrin transporter | mouse bacterial infections | N/A | intravenous | Gambhir; 2020 |
| Cy5.5 dimers | 680 | protease activity | mouse viral infection | N/A | subcutaneous | Jokerst; 2021 |
Acknowledgments
K.L. thanks the Summer Predoctoral Institute (SPI), California Predoctoral Program, NIH Chemistry-Biology Interface Training Grant (T32-GM136629). J.C. acknowledges the Camille and Henry Dreyfus Foundation for support. The authors thank the National Science Foundation (1752879) and the National Institutes of Health (R35GM133581) for supporting this work. The authors thank Mr. Oliver D Pichardo Peguero for drafting an early version of sections 5.1 and 5.2.
Glossary
Abbreviations
- TPA
tris[(2-pyridyl)methyl]amine
- GSH
glutathione
- PEG
polyethylene glycol
- DPSE-PEG2000
distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol)
- LOD
limit of detection
- NP
nanoparticle
The authors declare no competing financial interest.
References
- Tsang V. T. C.; Li X.; Wong T. T. W. A Review of Endogenous and Exogenous Contrast Agents Used in Photoacoustic Tomography with Different Sensing Configurations. Sensors (Switzerland) 2020, 20 (19), 5598. 10.3390/s20195595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Tang Y.; Yao J. Photoacoustic Tomography of Blood Oxygenation: A Mini Review. Photoacoustics 2018, 10, 65–73. 10.1016/j.pacs.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi J.; Kothapalli S. R.; Bohndiek S.; Yoon J. K.; Dragulescu-Andrasi A.; Nielsen C.; Tisma A.; Bodapati S.; Gowrishankar G.; Yan X.; Chan C.; Starcevic D.; Gambhir S. S. Molecular Photoacoustic Imaging of Follicular Thyroid Carcinoma. Clin. Cancer Res. 2013, 19 (6), 1494–1502. 10.1158/1078-0432.CCR-12-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. H.; Reinhardt C. J.; Chan J. Advances in Activity-Based Sensing Probes for Isoform-Selective Imaging of Enzymatic Activity. Angew. Chemie - Int. Ed. 2021, 60 (10), 5000–5009. 10.1002/anie.202003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East A. K.; Lucero M. Y.; Chan J. New Directions of Activity-Based Sensing for in Vivo NIR Imaging. Chem. Sci. 2021, 12 (10), 3393–3405. 10.1039/D0SC03096A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.; Dodani S. C.; Chang C. J. Reaction-Based Small-Molecule Fluorescent Probes for Chemoselective Bioimaging. Nat. Chem. 2012, 4 (12), 973–984. 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruemmer K. J.; Crossley S. W. M.; Chang C. J. Activity-Based Sensing: A Synthetic Methods Approach for Selective Molecular Imaging and Beyond. Angew. Chemie - Int. Ed. 2020, 59 (33), 13734–13762. 10.1002/anie.201909690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J.; Wang L. V. Photoacoustic Brain Imaging: From Microscopic to Macroscopic Scales. Neurophotonics 2014, 1 (1), 011003. 10.1117/1.NPh.1.1.011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash K. J.; Li C.; Xia J.; Wang L. V.; Clark H. A. Optical Drug Monitoring: Photoacoustic Imaging of Nanosensors to Monitor Therapeutic Lithium in Vivo. ACS Nano 2015, 9 (2), 1692–1698. 10.1021/nn5064858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2021 Alzheimer’s disease facts and figures. Alzheimer's & Dementia 2021, 17 (3), 327–406. 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- Bloom G. S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71 (4), 505–508. 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- Gardner S. H.; Brady C. J.; Keeton C.; Yadav A. K.; Mallojjala S. C.; Lucero M. Y.; Su S.; Yu Z.; Hirschi J. S.; Mirica L. M.; Chan J. A General Approach to Convert Hemicyanine Dyes into Highly Optimized Photoacoustic Scaffolds for Analyte Sensing**. Angew. Chem., Int. Ed. 2021, 60 (34), 18860–18866. 10.1002/anie.202105905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. J.; Zhang X.; Chen W. W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Reports 2016, 4 (5), 519–522. 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Sheng Z.; Yang Z.; Hu D.; Long X.; Feng G.; Liu Y.; Yuan Z.; Zhang J.; Zheng H.; Zhang X. Activatable Small-Molecule Photoacoustic Probes That Cross the Blood–Brain Barrier for Visualization of Copper(II) in Mice with Alzheimer’s Disease. Angew. Chem., Int. Ed. 2019, 58 (36), 12415–12419. 10.1002/anie.201904047. [DOI] [PubMed] [Google Scholar]
- Brini M.; Calì T.; Ottolini D.; Carafoli E. Neuronal Calcium Signaling: Function and Dysfunction. Cell. Mol. Life Sci. 2014, 71 (15), 2787–2814. 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. T. Imaging Calcium Signals in Vivo: A Powerful Tool in Physiology and Pharmacology. Br. J. Pharmacol. 2011, 163 (8), 1605–1625. 10.1111/j.1476-5381.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.; Seeger M.; Jiang Y.; Mishra A.; Sigmund F.; Stelzl A.; Lauri A.; Symvoulidis P.; Rolbieski H.; Preller M.; Deán-Ben X. L.; Razansky D.; Orschmann T.; Desbordes S. C.; Vetschera P.; Bach T.; Ntziachristos V.; Westmeyer G. G. Calcium Sensor for Photoacoustic Imaging. J. Am. Chem. Soc. 2018, 140 (8), 2718–2721. 10.1021/jacs.7b03064. [DOI] [PubMed] [Google Scholar]
- Kim T.; Lemaster J. E.; Chen F.; Li J.; Jokerst J. V. Photoacoustic Imaging of Human Mesenchymal Stem Cells Labeled with Prussian Blue-Poly(l -Lysine) Nanocomplexes. ACS Nano 2017, 11 (9), 9022–9032. 10.1021/acsnano.7b03519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J.; Moyer T. P.; Rakela J. The Liver Biopsy Diagnosis of Wilson’s Disease: Methods in Pathology. Am. J. Clin. Pathol. 1994, 102 (4), 443–446. 10.1093/ajcp/102.4.443. [DOI] [PubMed] [Google Scholar]
- Lucero M. Y.; Tang Y.; Zhang C. J.; Su S.; Forzano J. A.; Garcia V.; Huang X.; Moreno D.; Chan J. Activity-Based Photoacoustic Probe for Biopsy-Free Assessment of Copper in Murine Models of Wilson’s Disease and Liver Metastasis. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (36), e2106943118. 10.1073/pnas.2106943118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Huang S.; Wang J.; Sun L.; Zeng F.; Wu S. Activatable Probes for Diagnosing and Positioning Liver Injury and Metastatic Tumors by Multispectral Optoacoustic Tomography. Nat. Commun. 2018, 9 (1), 3983. 10.1038/s41467-018-06499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai X.; Wang Z.; Cheong H.; Wang Y.; Zhang R.; Lin J.; Zheng Y.; Gao M.; Xing B. Multispectral Optoacoustic Imaging of Dynamic Redox Correlation and Pathophysiological Progression Utilizing Upconversion Nanoprobes. Nat. Commun. 2019, 10 (1), 1–11. 10.1038/s41467-019-09001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Wu Y.; Chen J.; Zhong J.; Zeng F.; Wu S. A Turn-on Optoacoustic Probe for Imaging Metformin-Induced Upregulation of Hepatic Hydrogen Sulfide and Subsequent Liver Injury. Theranostics 2019, 9 (1), 77–89. 10.7150/thno.30080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H.; Li B.; Zhao M.; Wei P.; Yuan W.; Zhang M.; Han X.; Chen Y.; Yi T. Real-Time Monitoring and Accurate Diagnosis of Drug-Induced Hepatotoxicity in Vivo by Ratio-Fluorescence and Photoacoustic Imaging of Peroxynitrite. Nanoscale 2020, 12 (18), 10216–10225. 10.1039/D0NR00963F. [DOI] [PubMed] [Google Scholar]
- Fan X.; Ren T.; Yang W.; Zhang X.; Yuan L. Activatable Photoacoustic/Fluorescent Dual-Modal Probe for Monitoring of Drug-Induced Liver Hypoxia: In Vivo. Chem. Commun. 2021, 57 (69), 8644–8647. 10.1039/D1CC02999A. [DOI] [PubMed] [Google Scholar]
- Roberts S.; Khera E.; Choi C.; Navaratna T.; Grimm J.; Thurber G. M.; Reiner T. Optoacoustic Imaging of Glucagon-like Peptide-1 Receptor with a near-Infrared Exendin-4 Analog. J. Nucl. Med. 2021, 62 (6), 839–848. 10.2967/jnumed.120.252262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East A. K.; Lee M. C.; Smaga L. P.; Jiang C.; Mallojjala S. C.; Hirschi J. S.; Chan J. Synthesis of Silicon-Substituted Hemicyanines for Multimodal SWIR Imaging. Org. Lett. 2022, 24, 8509. 10.1021/acs.orglett.2c03382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z.; Zhang C.; Zhang L.; Wang S.; Hu S.; Zhao S. Precise in Vivo Inflammation Imaging in the NIR-II Window Using 1065 Nm Photoacoustic Probe for in Situ Visual Monitoring of Pathological Processes Related to Hepatitis. ACS Sensors 2022, 7 (2), 641–648. 10.1021/acssensors.1c02632. [DOI] [PubMed] [Google Scholar]
- Wu H.; Xia F.; Zhang L.; Fang C.; Lee J.; Gong L.; Gao J.; Ling D.; Li F. A ROS-Sensitive Nanozyme-Augmented Photoacoustic Nanoprobe for Early Diagnosis and Therapy of Acute Liver Failure. Adv. Mater. 2022, 34 (7), e2108348. 10.1002/adma.202108348. [DOI] [PubMed] [Google Scholar]
- Fan X. P.; Yang W.; Ren T. B.; Xu S.; Gong X. Y.; Zhang X. B.; Yuan L. Engineering a Ratiometric Photoacoustic Probe with a Hepatocyte-Specific Targeting Ability for Liver Injury Imaging. Anal. Chem. 2022, 94 (2), 1474–1481. 10.1021/acs.analchem.1c05026. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Qi Y.; Zhan C.; Zeng F.; Wu S. Diagnosing Drug-Induced Liver Injury by Multispectral Optoacoustic Tomography and Fluorescence Imaging Using a Leucine-Aminopeptidase-Activated Probe. Anal. Chem. 2019, 91 (13), 8085–8092. 10.1021/acs.analchem.9b00107. [DOI] [PubMed] [Google Scholar]
- Chen J.; Sedgwick A. C.; Sen S.; Ren Y.; Sun Q.; Chau C.; Arambula J. F.; Sarma T.; Song L.; Sessler J. L.; Liu C. Expanded Porphyrins: Functional Photoacoustic Imaging Agents That Operate in the NIR-II Region. Chem. Sci. 2021, 12 (29), 9916–9921. 10.1039/D1SC01591E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Egmond N.; Straub V. M.; Van Der Stelt M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 441–463. 10.1146/annurev-pharmtox-030220-112741. [DOI] [PubMed] [Google Scholar]
- Lucero M. Y.; Gardner S. H.; Yadav A. K.; Borri A.; Zhao Z.; Chan J. Activity-Based Photoacoustic Probes Reveal Elevated Intestinal MGL and FAAH Activity in a Murine Model of Obesity. Angew. Chem., Int. Ed. 2022, 61 (44), e202211774. 10.1002/anie.202211774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Pittman R. N.; Popel A. S. Nitric Oxide in the Vasculature: Where Does It Come from and Where Does It Go? A Quantitative Perspective. Antioxidants Redox Signal. 2008, 10 (7), 1185–1198. 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt C. J.; Zhou E. Y.; Jorgensen M. D.; Partipilo G.; Chan J. A Ratiometric Acoustogenic Probe for in Vivo Imaging of Endogenous Nitric Oxide. J. Am. Chem. Soc. 2018, 140 (3), 1011–1018. 10.1021/jacs.7b10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt C. J.; Xu R.; Chan J. Nitric Oxide Imaging in Cancer Enabled by Steric Relaxation of a Photoacoustic Probe Platform. Chem. Sci. 2020, 11 (6), 1587–1592. 10.1039/C9SC05600A. [DOI] [Google Scholar]
- Chen L.; Ji Y.; Hu X.; Cui C.; Liu H.; Tang Y.; Qi B.; Niu Y.; Hu X.; Yu A.; Fan Q. Cationic Poly-l-Lysine-Encapsulated Melanin Nanoparticles as Efficient Photoacoustic Agents Targeting to Glycosaminoglycans for the Early Diagnosis of Articular Cartilage Degeneration in Osteoarthritis. Nanoscale 2018, 10 (28), 13471–13484. 10.1039/C8NR03791D. [DOI] [PubMed] [Google Scholar]
- Zlitni A.; Gowrishankar G.; Steinberg I.; Haywood T.; Sam Gambhir S. Maltotriose-Based Probes for Fluorescence and Photoacoustic Imaging of Bacterial Infections. Nat. Commun. 2020, 11 (1), 1–13. 10.1038/s41467-020-14985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. Z.; Guo X. Y.; Zou M. S.; Zheng Z. Q.; Li Y. C.; Li X. D.; Li L. L.; Wang H. Bacteria-Instructed In Situ Aggregation of AuNPs with Enhanced Photoacoustic Signal for Bacterial Infection Bioimaging. Adv. Healthc. Mater. 2020, 9 (1), 1901229. 10.1002/adhm.201901229. [DOI] [PubMed] [Google Scholar]
- Moore C.; Borum R. M.; Mantri Y.; Xu M.; Fajtová P.; O’Donoghue A. J.; Jokerst J. V. Activatable Carbocyanine Dimers for Photoacoustic and Fluorescent Detection of Protease Activity. ACS Sensors 2021, 6 (6), 2356–2365. 10.1021/acssensors.1c00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim W.; Takemura K.; Zhou J.; Zhou J.; Jin Z.; Borum R. M.; Xu M.; Cheng Y.; He T.; Penny W.; Miller B. R.; Jokerst J. V. Enhanced Photoacoustic Detection of Heparin in Whole Blood via Melanin Nanocapsules Carrying Molecular Agents. ACS Nano 2022, 16 (1), 683–693. 10.1021/acsnano.1c08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Smaga L. P.; Satyavolu N. S. R.; Chan J.; Lu Y. DNA Aptamer-Based Activatable Probes for Photoacoustic Imaging in Living Mice. J. Am. Chem. Soc. 2017, 139 (48), 17225–17228. 10.1021/jacs.7b07913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox H. J.; Hedhli J.; Kim T. W.; Khalili K.; Dobrucki L. W.; Chan J. A Bioreducible N-Oxide-Based Probe for Photoacoustic Imaging of Hypoxia. Nat. Commun. 2017, 8 (1), 1794. 10.1038/s41467-017-01951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J.; Xu G.; Cao M.; Marquardt A.; Francis S.; Gandikota G.; Wang X. A Functional Study of Human Inflammatory Arthritis Using Photoacoustic Imaging. Sci. Rep. 2017, 7 (1), 1–9. 10.1038/s41598-017-15147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Zhang L.; Li S.; Han X.; Yuan Z. Imaging Molecular Signatures for Clinical Detection of Scleroderma in the Hand by Multispectral Photoacoustic Elastic Tomography. J. Biophotonics 2018, 11 (6), e201700267. 10.1002/jbio.201700267. [DOI] [PubMed] [Google Scholar]
- Zhou E. Y.; Knox H. J.; Liu C.; Zhao W.; Chan J. A Conformationally Restricted Aza-BODIPY Platform for Stimulus-Responsive Probes with Enhanced Photoacoustic Properties. J. Am. Chem. Soc. 2019, 141 (44), 17601–17609. 10.1021/jacs.9b06694. [DOI] [PMC free article] [PubMed] [Google Scholar]