Abstract

Cobalt bisdicarbollides (COSANs) are inorganic boron-based anions that have been previously reported to permeate by themselves through lipid bilayer membranes, a propensity that is related to their superchaotropic character. We now introduce their use as selective and efficient molecular carriers of otherwise impermeable hydrophilic oligopeptides through both artificial and cellular membranes, without causing membrane lysis or poration at low micromolar carrier concentrations. COSANs transport not only arginine-rich but also lysine-rich peptides, whereas low-molecular-weight analytes such as amino acids as well as neutral and anionic cargos (phalloidin and BSA) are not transported. In addition to the unsubstituted isomers (known as ortho- and meta-COSAN), four derivatives bearing organic substituents or halogen atoms have been evaluated, and all six of them surpass established carriers such as pyrenebutyrate in terms of activity. U-tube experiments and black lipid membrane conductance measurements establish that the transport across model membranes is mediated by a molecular carrier mechanism. Transport experiments in living cells showed that a fluorescent peptide cargo, FITC-Arg8, is delivered into the cytosol.
Introduction
Boron-containing compounds have application potential in pharmaceutical chemistry,1−5 with boron neutron capture therapy being the most prominent example.6 Recently, a new cell-biological as well as medicinal-chemical application line has been documented for icosahedral dodecaborates B12X122– (X = H, Cl, Br, and I; Figure 1a), because their superchaotropic ionic character7−13 enables their use as broadband membrane carriers, that is, they enable cellular uptake of a wide range of otherwise impermeable hydrophilic molecules, including amino acids, neurotransmitters, peptides, and drugs.14,15 This, combined with their chemical inertness,13,16−18 high water solubility,13,17,19 and high biocompatibility,3,5,13,14,20,21 defines a new research area in chaotropic inorganic cluster chemistry. Accordingly, it is of interest to investigate how variations in the boron cluster structure and topology affect their chaotropic character and, thus, their potential membrane carrier activity.
Figure 1.
Chemical structures along with molecular models of (a) dodecaborates, (b) o-carborane, and (c) parent COSAN. Chemical structures of (d) pyrenebutyrate and (e) COSAN derivatives 1–6 evaluated in this work. (f) Schematic representation of the proposed transport mechanism of impermeable oligopeptides facilitated by COSANs as signaled by the carboxyfluorescein (CF) assay. Vesicles are loaded with CF at high concentration, such that the fluorescence is self-quenched (middle). COSAN forms a supramolecular COSAN-cargo complex (i), which interacts with the membrane (ii) and carries the cargo through the membrane (iii). Upon its intravesicular arrival, the complex may dissociate (iv) or form a ternary complex with CF (v), which can also permeate through the membrane (vi). Following escape from the vesicle (vii) the ternary complex may dissociate, resulting in an effective release of diluted, fluorescent CF into the extravesicular space (viii). The time-resolved CF fluorescence intensity increase reports on the kinetics of the transport process. Note that all steps reflect equilibria; the reverse arrows in (iii), (v), and (vi) are omitted to enhance clarity.
Borane compounds that contain in their cluster framework one or more carbon atoms are known as carboranes; most commonly, two carbon atoms are being incorporated (Figure 1b), as one popular synthesis route involves alkynes as building blocks.22 The degradation of carboranes by removal of one BH vertex adjacent to the CH vertices affords polyhedral nido-carborane anions (7,8-C2B9H112– for the ortho- or 7,9-C2B9H112– for the meta-isomer),23 which are long known to serve as so-called dicarbollide ligands for transition metal ions, reminiscent of the organic cyclopentadienyl anion in the formation of ferrocene and related metal complexes. This has led to the highly thermally and chemically stable24,25 family of polyhedral boron clusters named metallacarboranes, with the singly negatively charged cobalt bisdicarbollide, in short COSAN (“CObalt SANdwich complex”) being the most prominent member26 (Figure 1c). Since the double negative charge of the two dicarbollide ligands is compensated by Co3+, a net singly negatively charged anion, with an expectedly higher chaotropicity (lower charge to volume ratio), results. COSANs and their congeners have been extensively tested as boron delivery platforms,24,27−29 HIV protease inhibitors,30,31 carbonic anhydrase IX inhibitors,32 antimicrobial agents,33−36 polymeric materials,37 coordination scaffolds,38 for peptide modification,39 and in conducting polymers40 as well as metal–organic frameworks (MOFs),41 among others.26 However, while their ability to permeate (nondestructively) through artificial42−44 and natural45 membranes, either in their free form or as fluorescent derivatives,46,47 is known, their function as transmembrane carriers of biologically interesting targets (“cargos”) has not been investigated. This potential utilization is being inspired by the recent observation of the same activity for dodecaborates.14,15 The associated applications, ranging from drug delivery and fluorescence staining to therapy, appear particularly attractive, because of promising low (cell) toxicity studies that have already been conducted for COSANs.3,34,36,42,45−51 Note that membrane carrier activity can be studied in both artificial liposomal model membranes and natural cellular systems, which facilitates systematic physicochemical investigations of structure–activity relationships. Toward this goal, we have studied the COSAN derivatives 1–6.52−55 (Figure 1e).
On a continuous scale for aqueous solvation, COSANs fall in the region between superchaotropic and hydrophobic ions.44 The chaotropicity of anions presents an empirical ionic property that can be scaled, among others, by their affinity to macrocycles,7,11,12,56 by cloud-point measurements,8,9,11 and, according to the solvation theory of Marcus,57,58 by their hydrogen-bond breaking propensities and water-structural entropies.56 The chaotropicity of anions increases generally with size and charge delocalization; large cluster anions have been referred to as being superchaotropic,7,8 because they are more chaotropic than SCN–, ClO4–, and PF6– on the classical Hofmeister scale.59−62
It is important to note that COSAN anions adopt some properties reminiscent of amphiphilic ions (stealth amphiphiles or intrinsic amphiphiles),63−65 such as cation-promoted self-assembly to vesicular or micellar structures in water.66−71 Structurally, however, COSANs lack the head-and-tail design required by the IUPAC definition for amphiphiles. Thermochemically important, their self-assembly is enthalpically driven,64 not entropically driven as traditional surfactant micellization. This points to a chaotropic effect rather than a hydrophobic effect as driving force56,72 and to their more appropriate designation as superchaotropic ions. Superchaotropic ions are concomitantly hydrophilic (highly water soluble) as well as lipophilic (high affinity to hydrophobic phases). These criteria are fulfilled for COSANs, which reinforces the investigation of their membrane carrier potential.
Results and Discussion
COSANs as Transmembrane Carriers
Previous studies by microscopy42,45 and electrophysiology29,42,43 techniques as well as fluorescence displacement assays44 have provided evidence for direct membrane permeation of selected COSANs. For example, the permeability of COSANs 1–6 (Figure 1e) has been found to follow the order 6,4 > 2,5 > 3 > 1 by fluorescence44 and 6 > 4 > 1 by electrophysiology.43 This intrinsic membrane affinity can be in part rationalized by their polarity, e.g., the dipole moment of COSAN 1 is significantly larger than that of 2 (6.1 and 2.4 D, calculated values).44
In order to investigate the molecular carrier potential of COSANs, the permeability measurements needed to be first tested for established transport assay formats, namely, the well-established 8-hydroxypyrene-1,3,6-trisulfonate/p-xylene-bis-pyridinium (HPTS/DPX)73 (Scheme S1) and CF74,75 assays (Scheme S2). In the HPTS/DPX assay, a negatively charged dye (HPTS) is quenched by a cationic quencher (DPX) inside the vesicles and efflux of either dye or quencher results in a fluorescence increase. In the CF assay, the dye is self-quenched inside the vesicles and its efflux results in a fluorescence enhancement by dilution. Both assays have been developed for large unilamellar vesicles (LUVs) as zwitterionic phosphocholine model membranes (see Methods in the Supporting Information).
Interestingly, the HPTS/DPX assay showed a fluorescence response to all COSANs 1–6 even in the absence of added cargo (Figures S1 and S2), which demonstrated that the permeation of COSANs through the lipid bilayer leads itself to an efflux of the positively charged quencher DPX. This provided the first experimental indication that the COSANs can act as molecular carriers, e.g., by the formation of a carrier•cargo (COSAN•DPX) complex. COSAN 2 stood out by affording the largest fluorescence increase, and COSAN 4 was active at the lowest concentration (see Table S1); however, whether the activity profiles were due to high intrinsic permeability of the carriers or their transport activity could not be assessed by this preliminary set of experiments. Note that the addition of COSANs did not lead to disruption of the membranes, as evidenced by dynamic light scattering (DLS) experiments (Figures S1 and S2) and model vesicles loaded with CF (vide infra).
The addition of COSANs 1–6 to CF-loaded liposomes did not afford a direct fluorescence response, which rendered the CF assay the method of choice to investigate the activity of this type of carriers. The absence of a response in the CF assays, due to the anionic character of the CF probe, confirmed also the membrane integrity in the presence of COSANs, a conclusion which could also be independently drawn from the unchanged band profiles in DLS experiments (Figures S1–S5). Note that any membrane disruption or pore formation would have led to a strong fluorescence increase of CF, an effect which can always be artificially induced by addition of detergent (e.g., Triton 100-X = TX-100, also used for calibration, Figures S1 and S2).
Heptaarginine Transport across Model Membranes
Once we had identified the CF assay as a robust transport assay, which is unaffected by the intrinsic permeation of COSANs, we studied the transport of impermeable cargos into the model membranes.74,75 Oligoarginines are representative cationic peptides which do not translocate by themselves into zwitterionic phosphocholine vesicles.74,76,77 Their uptake requires additives, conventionally amphiphilic counterion activators74,77−79 or, as found recently, superchaotropic carriers14 such as B12X122–. Accordingly, we used the heptaarginine peptide Trp-Arg7 as impermeable cargo to assess the transport abilities of COSANs. In a typical experiment, CF fluorescence intensity was monitored upon addition of COSAN as the carrier (t = 50 s) and the impermeable peptide cargo (t = 100 s), see Figure 2a. The full release of encapsulated CF was achieved by adding detergent (TX-100, t = 600 s), which allows also for a normalization of the fluorescence intensity data and permits quantification of the carrier activity. Kinetic experiments were conducted at different COSAN concentrations, and the resulting normalized fluorescence traces were converted into dose–response curves (Figure 2b). Hill analysis provided the characteristic membrane carrier parameters, namely, the maximal activity, Ymax, the carrier concentration needed to reach 50% of maximal activity, EC50, and the activator efficiency,Ea,14,75 (Table 1). The latter presents a measure of both Ymax and EC50 and therefore presents a cumulative “goodness” parameter to compare different carriers.
Figure 2.
(a) Changes in CF emission (λex = 492 nm, λem = 517 nm) in EYPC⊃CF liposomes (4 μM phospholipids) as a function of time upon addition of COSAN 2 (3 μM) at t = 50 s, Trp-Arg7 (1 μM, red) at t = 100 s, and TX-100 at t = 600 s. (b) Dose–response curves for Trp-Arg7 transport of the different COSANs, and Hill curve fits in comparison with the reference carriers B12Br122– (gray trace) and pyrenebutyrate (blue trace). (c) Transport efficiency of 2 (4 μM) in liposomes of different lipid composition: DMPE/DPPG/CHOL (black trace), EYPC/CHOL (red trace), EYPC (blue trace), and DPPC/DOPS (green trace); DMPE: 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine; DPPG: 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol); CHOL: cholesterol; EYPC: egg yolk phosphatidylcholine; DPPC: 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DOPS: 1,2-dioleoyl-sn-glycero-3-phospho-L-serine. (d) Transport kinetics for the “conventional” (first carrier, then cargo, solid line) vs reverse addition (first cargo, then carrier, dashed line).
Table 1. Characteristic Transmembrane Transport Parameters for COSANsa as Molecular Carriers of Heptaarginine,b Determined by CF Assay in EYPC Vesicles.
| carrier | Ymax (%)c | EC50 (μM)d | Eae |
|---|---|---|---|
| PyBu | 100 | 103 | 4.8 |
| B12Br122–f | 95 | 48 ± 4 | 6.1 |
| 1 | 100 | 3.2 | 12.1 |
| 2 | 100 | 2.3 | 12.8 |
| 3 | 100 | 0.9 | 14.7 |
| 4 | 100 | 1.9 | 13.2 |
| 5 | 98 | 2.8 | 12.1 |
| 6 | 100 | 0.9 | 14.6 |
COSANs used as cesium salts, analogous experiments with the sodium salt of 1 confirmed that the counter cation does not affect significantly the transport activity (Figure S6 and Table S2).
1 μM Trp-Arg7 for experiments with COSANs and PyBu; note that experiments at higher peptide concentration (20 μM), performed for COSAN 2, afforded comparable activity (Figure S7 and Table S3).
Maximal activity; ±5% error (SD).
Effective concentration to reach 50% activity of Ymax; 5% error (SD), unless stated differently.
Activator efficiency, Ea = Ymax (pEC50)/f, where pEC50 is the negative logarithm of EC50 in mM, and f is a scaling factor, which is set as 20.6 to calibrate the highest (previously known) activator efficiency as 10;75,76 10% error (SD, calculated by considering error propagation with respect to both, Ymax and EC50).
Transport experiments with B12Br122– were conducted with the HPTS/DPX assay and 20 μM Trp-Arg7.14
All COSAN derivatives 1–6 showed exceptionally efficient transport (Ea > 10) of heptaarginine (Trp-Arg7) into the vesicles (Figures S4–S5) with Ea values far superior to pyrenebutyrate (PyBu, Figure 1d and S3), the prototype of an anionic amphiphilic counterion activator.75 COSANs also outperformed B12Br122– (Figures 1a and S3), which serves as the reference point for superchaotropic carriers.14 We included both benchmark compounds throughout our study. COSANs showed very small EC50 values, which approach the nanomolar range (Table 1). The values are one order of magnitude lower than that for B12Br122– and up to two orders of magnitude lower than that for pyrenebutyrate, which translates into much smaller carrier concentrations to induce transport of Trp-Arg7. The efficient transport for COSANs corroborates the conceptual observation made for B12Br122–, namely, that amphiphilicity (head-to-tail design) of the carrier is not required to activate transport. At the same time, a globular structure of the superchaotropic cluster ions as for B12Br122– is no prerequisite either, because COSANs are prolate ellipoids (Figure 1c).
Since all COSANs 1–6 showed very high but similar activity (Ea = 13.5 ± 1.5), we employed COSAN 2 in follow-up experiments as a representative COSAN carrier, as it showed a good water solubility and suitable cell viability (vide infra). First, to better mimic natural membranes as well as pharmaceutically relevant liposome formulations,80,81 which commonly include DPPC lipids, cholesterol, and anionic components,82,83 complementary experiments were conducted in four different vesicle types of largely varying lipid composition84 (Figures 2c and S8). In all tested liposomes, COSAN 2 shows excellent transport abilities, with low EC50 values of 0.2–1.1 μM, while preserving membrane integrity.
Transport Mechanism
Molecular transport through the membrane can be achieved by a range of mechanisms. In one prototypal case, it is assisted by membrane carriers or ionophores,85,86 which usually bind to the cargo and translocate the lipid bilayer. Alternatively, there could be the formation of channels or (larger) pores,86,87 which typically traverse the bilayer membrane, forming a stable open passage between the intra- and extravesicular environments and allowing the analytes to diffuse freely. Although the COSAN derivatives are only ca. ∼13 Å in length, too short to traverse the entire bilayer (∼40 Å thickness for the studied vesicles),88,89 their known self-assembly ability68,69 could potentially lead to channel or pore formation of the “barrel-rosette type”,90,91 which needed to be excluded. To elucidate the transport mechanism, an extended set of experiments was performed: dye leakage tests, black lipid membrane conductance studies, and U-tube experiments.
First, we found no fluorescence change when only one of the components, namely, COSAN or heptaarginine, was present (Figure 2a). Second, DLS experiments of the liposomes were conducted before and after the addition of carrier and cargo, and these confirmed that the membrane remained intact (Figures S4 and S5). Accordingly, the increase in fluorescence was due to actual transport, and it could be ruled out that dye leakage produced (large) pore formation. While the formation of such pores (sufficiently large to allow the low-molecular weight dye to flow out) can be readily excluded, the formation of smaller channels is more difficult to evaluate.
Conductance experiments were further performed across a black lipid bilayer membrane (consisting of 1,2-diphytanoyl-sn-glycero-3-phosphocholine, DPhPC) using KCl as an electrolyte (see Methods in the Supporting Information). The current–voltage relationship was found to be linearly dependent on COSAN concentration (Figure 3b, black symbols and lines) but insignificantly dependent on KCl concentrations, even at the highest COSAN concentration (Figure 3b, red symbols and lines, Figure S9). Accordingly, the concentration of COSAN is rate-determining which is characteristic of a carrier-mediated transport (of K+). For self-assembled channels and pores, a strong dependence of the I–V relationship on cargo concentration would be expected, which was not observed.93
Figure 3.

(a) Schematic representation of the conductance experiment; BLM: black lipid membrane. (b) Conductance-voltage relationship from synthetic BLM experiments by varying the COSAN 5 concentration at fixed salt content (1 mM KCl, black symbols and lines), or by varying the salt concentrations at fixed COSAN content (100 μM 5, red symbols and lines). (c) Schematic representation of the U-tube experiment. (d) Photographs of an aqueous (yellow) donor solution containing 2, Trp-Arg7, and CF (cis) at the beginning and end of the U-tube experiment; note that the trans solution on the right had become yellow. Carrier, cargo, and dye concentrations were fixed as 100, 50, and 200 μM. (e) Fluorescence response of CF in the trans chamber. Black star trace: 1, Trp-Arg7, and CF; solid red circle trace: 2, Trp-Arg7, and CF; open blue star trace: 3, Trp-Arg7, and CF; green cross trace: 4, Trp-Arg7, and CF; half purple circle: 5, Trp-Arg7, and CF; solid orange triangle trace: 6, Trp-Arg7, and CF; open light blue triangle trace: Trp-Arg7 and CF; open black circle trace: 2 and CF; open gray square trace: CF. (f, g) Microcalorimetric titrations in water: Thermograms (top) for sequential injections of COSAN 2 into Trp-Arg7 (f) as well as protamine (g) solutions, after subtraction of the heats of dilution obtained by titration of 2 into water, and corresponding reaction heats (bottom) from integration of the calorimetric traces. Concentrations in mM were 2/cargo: (f) 1/0.020 and (g) 2/0.008.92
U-tube experiments were also designed, because this methodology is thought of as providing unambiguous evidence for “real” carrier activity.73,74 Shortly, CHCl3 (as the representative hydrophobic phase) was added at the bottom of the U-tube, and two aqueous phases (cis and trans) were placed in both arms of the U-tube (Figure 3c). The trans phase (also known as acceptor) initially contained just buffer, while the cis phase (also known as donor) was charged with cargo (Trp-Arg7), carrier (COSAN), and CF as dye to monitor transport; the CHCl3 layer was stirred and aliquots of the trans phase were collected at different times. Initially, the trans phase was nonfluorescent (Figure 3d, left), but carrier-mediated transport of CF (as the ternary complex, see step (vi) in Figure 1f) from the cis phase across the CHCl3 phase led to a marked increase in fluorescence (Figure 3d right, and Figure S10). It transpires that COSANs display phase-transfer activity. As controls, Figure 3e shows that there is no significant increase in fluorescence in the presence of only one component (COSAN or cargo). The combined experiments (dye leakage, black lipid membrane conductance, and U-tube) led us to propose the carrier-mediated translocation mechanism in Figure 1f.
Transmembrane transport by boron cluster-based carriers is facilitated by the chaotropic effect.14,56 This provides not only a conceptual difference to the conventional amphiphilic transport pathway, but it allows also for a more rigorous mechanistic treatment of the transport process, because the interactions between clusters and cargo (step (i) in Figure 1f) are strongly enthalpically driven. This thermochemical detail presents a unique experimental handle since it allows the identification of the process by isothermal titration calorimetry (ITC). In essence, carrier-cargo affinities can be directly measured and used as a powerful predictor of their transport potential. Indeed, the ITC experiments reveal a very strong (binding constants >106 M–1) supramolecular interaction between COSANs 1–6 and Trp-Arg7 (Figures 3f, S11 and S12). COSAN 2 also interacts strongly with the longer peptide protamine (Figure 3g) and even with the amino acid arginine (Figure S13). The thermochemical ITC fingerprint for this dynamic interaction (enthalpically driven, Table S4) is consistent with the chaotropic effect56 and the superchaotropic nature of boron cluster anions,7 which is also responsible for the concomitant affinity of COSANs to lipid bilayer membranes, step (ii) in Figure 1f.
The thermochemical data demonstrate additionally that the interactions are reversible, that is, the carrier is in a dynamic equilibrium with a water-soluble carrier•cargo complex. In line with this superchaotropic carrier design,14 the transport efficiency of Trp-Arg7 was found to be independent of the sequence of COSAN/peptide carrier/cargo addition (Figure 2d). In contrast, when (less water-soluble) amphiphilic counterion activators are employed, they need to be added first to allow their insertion into the membrane.94 Otherwise, if cargo is added first and amphiphilic carrier thereafter, cargo-carrier aggregation and precipitation generally occur, which drastically reduces the uptake efficiency.14
Since it is already known that COSANs permeate through the lipid bilayer on their own, we assume that the COSAN•cargo complexes permeate through the lipid bilayer membrane as well (step (iii) in Figure 1f). Regardless of the atomistic details of the actual shuttling process (which may involve lipid flip-flop95,96 or other processes), transport occurs without sacrificing the integrity of the lipid bilayer membrane at the selected concentrations. It should also be noted here that the low micromolar concentrations at which transport is achieved (EC50 values in Table 1) fall far below the high millimolar aggregation concentrations42,66,68,70,71 at which cation-induced aggregation effects of COSANs would interfere. The absence of detectable DLS signals excluded formation of nanoscale assemblies of the COSANs, in the absence and presence of heptaarginine, at the pertinent transport concentrations.
Cargo Scope and Selectivity
First, focusing on peptides as cargo molecules, we tested the dependence of the transport activity of COSAN 2 on the amino acid type. Most amphiphilic activators, and prominently pyrenebutyrate, activate the transport of arginine-rich peptides74−76 but fail to activate the transport of lysine-rich analogues.97 Although this has been referred to as “arginine magic”,74,75,77 similar tricks have long been missing for arginine-deficient peptides, even if they are also positively charged. Indeed, efficient transport of lysine-containing peptides has only recently been achieved by using encapsulating (macrocyclic) amphiphilic carriers such as calixarenes98 or, according to our recent findings,14 dodecaborates. Importantly, COSAN 2 was also found to activate the efficient transport of Trp-Lys7 into the vesicles, again independent on the sequence of carrier•cargo addition (Figure 2d).
We further explored the influence of the peptide length in both Arg as well as Lys derivatives and tested other impermeable molecules of biological interest. COSAN 2 was found to be selective in transporting only arginine and lysine oligo- and polypeptides, while the same assay provided no response for negative, neutral, and low-molecular weight compounds. The oligopeptides Trp-Gly6 and Tyr-Thr-Leu-Thr-Val-Lys as well as the cyclic oligopeptide phalloidin, which contain no or only one positively charged residue, are not transported, neither is Trp-Lys3 which is apparently too short or insufficiently positively charged (Table 2, Figures S14–S16). Seven amino acid residues, with at least two arginine or four lysine residues, appear to define a typical cut-off at the lower end. This provides an interesting contrast to B12Br122–, which has been found to transport a much broader range of cargo molecules, including neutral and cationic amino acids.14 Accordingly, in comparison to boron clusters, COSAN 2 acts as a more active but at the same time more selective carrier, only for cationic peptides. Nevertheless, it retains a broader cargo spectrum than PyBu and shows higher activity. Accordingly, COSANs expand the toolbox of molecular carriers substantially, as summarized in Table 3.
Table 2. Transmembrane Transport Parameters for COSAN 2a as Molecular Carrier of Different Cargo Compounds,b Determined with the CF Assay in EYPC Vesicles.
| cargo | Ymax (%)c | EC50 (μM)d | Ete |
|---|---|---|---|
| polylysine | 82 | 0.0080 | 10.0 |
| protamine | 83 | 0.030 | 9.0 |
| Trp-Arg7 | 100 | 0.30 | 8.6 |
| Trp-Lys6 | 99 | 1.2 | 6.9 |
| Trp-Leu-Arg-Thr-Leu-(Arg)2-Leu | 100 | 1.5 | 6.7 |
| Trp-Lys7 | 89 | 1.4 | 6.1 |
| Leu-(Arg)2-Trp-Ser-Leu-Gly | 60 | 19 | 2.5 |
| Trp-(Lys)3 | n.af | n.a | n.a |
| Tyr-Thr-Leu-Thr-Val-Lys | n.a | n.a | n.a |
| Trp-Gly6 | n.a | n.a | n.a |
| BSA | n.a | n.a | n.a |
| Other inactive analytesg | n.a | n.a | n.a |
All experiments conducted with 4 μM COSAN 2.
All analytes were used as aqueous solutions.
Maximal activity; ±5% error (SD).
Effective concentration to reach 50% activity of Ymax; 5% error (SD).
Transport efficiency, Et = Ymax (pEC50)/f, where pEC50 is the negative logarithm of EC50 in mM and f is a scaling factor which we set as 41.8 to calibrate the highest analyte transport efficiency in this series to an arbitrary value of 10; 10% error (SD, calculated by considering error propagation with respect to both, Ymax and EC50).
n.a. = no detectable activity.
The following small-molecule cargos were also tested but displayed no activity: acetylcholine; glutamic acid; phenylalanine; tryptophan; Vitamin B1; ranitidine; ampicillin; Kanamycin A; vecuronium; pancuronium; phalloidin.
Table 3. Selectivity of Molecular Carriersa.
| carrier | cationic peptides (>1000 Da) | low-molecular-weight analytes (<1000 Da) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arg7 | PolyArg | Prot. | Lys7 | PolyLys | ACh | Glu | Phe | Trp | Vit. B1 | Ranit. | Amp. | Kan. A | Vecur. | Pancur. | Phall. | |
| PyBu | + | + | + | |||||||||||||
| B12Br122– | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| COSAN 2 | + | + | + | + | + | |||||||||||
Abbreviations. Arg7: heptaarginine; PolyArg: polyarginine; Prot.: protamine; Lys7: heptalysine; PolyLys: polylysine; ACh: acetylcholine; Glu: glutamic acid; Phe: phenylalanine; Trp: tryptophan; Vit. B1: vitamin B1; Ranit: ranitidine; Amp: ampicillin; Kan. A: Kanamycin A; Vecur: vecuronium; Pancur: pancuronium; Phall: phalloidin.
Delivery in Live Cells
The peptide transport through artificial vesicles encouraged us to investigate the transport capabilities of COSANs in live CHO-K1 cells (Chinese hamster ovary cells), by using an octaarginine labeled with fluorescein isothiocyanate, FITC (FITC-Arg8). At low peptide concentration (6 μM), in the absence of carrier, confocal fluorescence microscopy showed insignificant peptide uptake (Figure 4a left panels); however, in the presence of a fixed concentration of the carriers (13 μM), either PyBu (as amphiphilic carrier), B12Br122– (as standard chaotropic carrier), or COSAN 2, strong diffuse fluorescence in both the cytosol and nucleus was only detected for COSAN 2 (Figure 4a). In addition, colocalization experiments (with LysoTracker) showed a predominant cytosolic transport and a minor endosomal localization of the FITC-labeled peptide cargo (Figures S17 and S18).
Figure 4.
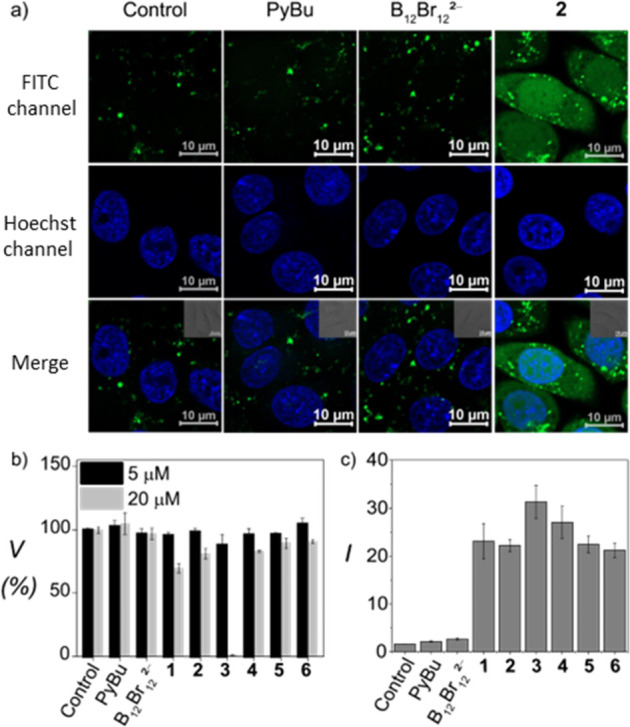
(a) FITC-Arg8 uptake (6 μM) alone and promoted by B12Br122–, pyrenebutyrate, and COSAN 2, (13 μM, from left to right) in CHO-K1 cells. Representative images of three biological replicates. (b) Viability of CHO-K1 cells (resazurin assay) incubated with 5 and 20 μM activator for 24 h. (c) Normalized fluorescence intensity of CHO-K1 cells, incubated with FITC-Arg8 (6 μM), in the absence and presence of carriers (13 μM), measured by flow cytometry. Error bars refer to standard deviation (n = 3).
Similar diffuse fluorescence was observed for all tested derivatives 1–6 (Figure S19). This signaled carrier-mediated peptide transport into the cells by the COSANs and validated their carrier activity not only for artificial but also cellular membranes. Flow cytometry experiments corroborated the more effective peptide uptake of FITC-Arg8 in the presence of COSANs 1–6 than that for B12Br122– and pyrenebutyrate (Figures 4c and S20). This agrees with the trend observed in the liposomal experiments. Interestingly, when the same peptide sequence was labeled with carboxytetramethylrhodamine (TAMRA-Arg8), uptake into CHO-K1 cells was also observed, but in this case the confocal micrographs for COSAN 2 and the brominated cluster B12Br122– were comparable (Figure S21). This observation is consistent with the selectivity findings from Table 3, namely that different carriers excel in transporting different cargos, and that even variations in the attached chromophore may display pronounced effects.
With regard to cytotoxicity, resazurin assays showed that CHO-K1 cells tolerated all investigated COSANs well after 24 h at the concentration and incubation conditions required for transport (2–20 μM), except for COSAN 3 above 5 μM (Figures 4b and S22). In regard to cell-line transferability, peptide uptake facilitated by 2 was also observed for living HeLa cells, human lung carcinoma epithelial cells A549, and mouse hypothalamic GnRH neuronal GT1-7 cells (Figure S23).
Conclusions
We have investigated the use of six COSAN derivatives as molecular carriers for hydrophilic oligopeptides through model and cellular membranes. All COSANs were highly active in transporting the prototypal cationic peptide heptaarginine into model liposomes, independent of their lipid composition, membrane surface charge, and lipid phase. In liposomal assays, COSANs show lower EC50 values than pyrenebutyrate, the amphiphilic reference in the field. Combined mechanistic studies (dye leakage, U-tube, and black lipid membrane conductance) revealed that the cargo transport by COSANs is achieved via a carrier mechanism, and not by channel or pore formation. The enthalpy-driven complexation (established by ITC experiments) and the preserved transport independent of carrier-cargo sequence of addition establish COSANs as highly bioactive members of the new class of superchaotropic carriers. This expands the range of previously reported amphiphilic carriers such as pyrenebutyrate. Cellular experiments corroborated efficient carrier-mediated cytosolic uptake of an arginine peptide which indicated translocation across the plasma membrane and internal membranes. At low micromolar concentrations and with short incubation times, no obvious cellular damage is observed.
COSANs do not display the concentration window and the broadband carrier activity of dodecaborates (B12Br122–). In this sense, they are more selective, but less specific than the amphiphilic pyrenebutyrate, which transports mainly arginine-based peptides. Their carrier activity appears to be more robust toward substitution than that of dodecaborates (where increasing the polarizability (Cl < Br < I) strongly increases chaotropicity and membrane affinity). Specifically, COSANs tolerate chemical variations such as those in derivatives 1–6, which potentially allows for a fine tuning of their activity through subtle structural variations.36,99,100
Acknowledgments
Y.C., A.B.-B., and W.M.N. thank the DFG for financial support through projects NA-868/14, NA-868/15, and NA-868/17. Y.C. thanks the International Cooperation Training Program for Innovative Talents of the China Scholarship Council and the China University of Petroleum (East China) for a doctoral fellowship. B.G. acknowledges financial support to Czech Science Foundation, project No. 2114409S. We thank S. Springer, Constructor University, for his assistance with flow cytometry and M. Rehders for experimental support in cell biology and confocal microscopy. J.M. thanks the Spanish AEI (SAF2017-89890-R, PCI2019-103400, PID2020-117143RB-I00), the Xunta de Galicia (ED431G 2019/03, ED431C 2017/25, 2016-AD031, the Oportunius Program (Gain)), the ERC-Stg (DYNAP-677786), the ERC-PoC (TraffikGene, 838002), the HFSP (RGY0066/2017), and the ERDF. G.S. and Y.F.-C. thank AEI for their predoctoral fellowships (PRE2018-085973; FPU21/04747). This work was adapted from the PhD thesis of Y.C.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c01623.
Vesicle preparation, transport experiments, black lipid bilayer conductance measurements, U-tube and ITC experiments, and cellular studies (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sivaev I. B.; Bregadze V. V. Polyhedral boranes for medical applications: current status and perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. 10.1002/ejic.200900003. [DOI] [Google Scholar]
- Gabel D. Boron clusters in medicinal chemistry: perspectives and problems. Pure Appl. Chem. 2015, 87, 173–179. 10.1515/pac-2014-1007. [DOI] [Google Scholar]
- Hey-Hawkins E.; Viñas C.. Boron-Based Compounds: Potential and Emerging Applications in Medicine; John Wiley & Sons, 2018. [Google Scholar]
- Axtell J. C.; Saleh L. M. A.; Qian E. A.; Wixtrom A. I.; Spokoyny A. M. Synthesis and Applications of Perfunctionalized Boron Clusters. Inorg. Chem. 2018, 57, 2333–2350. 10.1021/acs.inorgchem.7b02912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier N. A.; Teh J.; Reichel D.; Zahorsky-Reeves J. L.; Perez J. M.; Spokoyny A. M. Ex Vivo and In Vivo Evaluation of Dodecaborate-Based Clusters Encapsulated in Ferumoxytol Nanoparticles. Langmuir 2021, 37, 14500–14508. 10.1021/acs.langmuir.1c02506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway A. H.; Tjarks W.; Barnum B. A.; Rong F.-G.; Barth R. F.; Codogni I. M.; Wilson J. G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998, 98, 1515–1562. 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- Assaf K. I.; Ural M. S.; Pan F.; Georgiev T.; Simova S.; Rissanen K.; Gabel D.; Nau W. M. Water Structure Recovery in Chaotropic Anion Recognition: High-Affinity Binding of Dodecaborate Clusters to γ-Cyclodextrin. Angew. Chem., Int. Ed. 2015, 54, 6852–6856. 10.1002/anie.201412485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskar B.; Diat O.; Nardello-Rataj V.; Bauduin P. Nanometer-Size Polyoxometalate Anions Adsorb Strongly on Neutral Soft Surfaces. J. Phys. Chem. C 2015, 119, 20985–20992. 10.1021/acs.jpcc.5b06273. [DOI] [Google Scholar]
- Buchecker T.; Schmid P.; Renaudineau S.; Diat O.; Proust A.; Pfitzner A.; Bauduin P. Polyoxometalates in the Hofmeister series. Chem. Commun. 2018, 54, 1833–1836. 10.1039/C7CC09113C. [DOI] [PubMed] [Google Scholar]
- Ivanov A. A.; Falaise C.; Landy D.; Haouas M.; Mironov Y. V.; Shestopalov M. A.; Cadot E. Tuning the chaotropic effect as an assembly motif through one-electron transfer in a rhenium cluster. Chem. Commun. 2019, 55, 9951–9954. 10.1039/C9CC05136H. [DOI] [PubMed] [Google Scholar]
- Yao S.; Falaise C.; Ivanov A. A.; Leclerc N.; Hohenschutz M.; Haouas M.; Landy D.; Shestopalov M. A.; Bauduin P.; Cadot E. Hofmeister effect in the Keggin-type polyoxotungstate series. Inorg. Chem. Front. 2021, 8, 12–25. 10.1039/D0QI00902D. [DOI] [Google Scholar]
- Khlifi S.; Marrot J.; Haouas M.; Shepard W. E.; Falaise C.; Cadot E. Chaotropic Effect as an Assembly Motif to Construct Supramolecular Cyclodextrin–Polyoxometalate-Based Frameworks. J. Am. Chem. Soc. 2022, 144, 4469–4477. 10.1021/jacs.1c12049. [DOI] [PubMed] [Google Scholar]
- Hollow S. E.; Johnstone T. C. Encapsulation of closo-dodecaiodododecaborate in 2-hydroxypropyl-γ-cyclodextrin prevents hemolysis. Chem. Commun. 2022, 58, 2375–2378. 10.1039/D1CC06348K. [DOI] [PubMed] [Google Scholar]
- Barba-Bon A.; Salluce G.; Lostalé-Seijo I.; Assaf K. I.; Hennig A.; Montenegro J.; Nau W. M. Boron clusters as broadband membrane carriers. Nature 2022, 603, 637–642. 10.1038/s41586-022-04413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.-Y.; Guo D.-S. Superchaotropic Boron Clusters as Membrane Carriers for the Transport of Hydrophilic Cargos. Angew. Chem., Int. Ed. 2022, 61, e202204979 10.1002/anie.202204979. [DOI] [PubMed] [Google Scholar]
- Muetterties E. L.; Balthis J. H.; Chia Y. T.; Knoth W. H.; Miller H. C. Chemistry of Boranes. VIII. Salts and Acids of B10H10-2 and B1212-2. Inorg. Chem. 1964, 3, 444–451. 10.1021/ic50013a030. [DOI] [Google Scholar]
- Plesek J. Potential applications of the boron cluster compounds. Chem. Rev. 1992, 92, 269–278. 10.1021/cr00010a005. [DOI] [Google Scholar]
- Barton J. L.; Wixtrom A. I.; Kowalski J. A.; Qian E. A.; Jung D.; Brushett F. R.; Spokoyny A. M. Perfunctionalized Dodecaborate Clusters as Stable Metal-Free Active Materials for Charge Storage. ACS Appl. Energy Mater. 2019, 2, 4907–4913. 10.1021/acsaem.9b00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki K.; Gabel D.; Roccatano D. Structure and Dynamics of Dodecaborate Clusters in Water. Inorg. Chem. 2012, 51, 4894–4896. 10.1021/ic300223z. [DOI] [PubMed] [Google Scholar]
- Larsen D.; Beeren S. R. Enzyme-mediated dynamic combinatorial chemistry allows out-of-equilibrium template-directed synthesis of macrocyclic oligosaccharides. Chem. Sci. 2019, 10, 9981–9987. 10.1039/C9SC03983J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebula J.; Fink K.; Boratyński J.; Goszczyński T. M. Supramolecular chemistry of anionic boron clusters and its applications in biology. Coord. Chem. Rev. 2023, 477, 214940 10.1016/j.ccr.2022.214940. [DOI] [Google Scholar]
- Bregadze V. I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Chem. Rev. 1992, 92, 209–223. 10.1021/cr00010a002. [DOI] [Google Scholar]
- Beckett M. A.; Brellochs B.; Chizhevsky I. T.; Damhus T.; Hellwich K.-H.; Kennedy J. D.; Laitinen R.; Powell W. H.; Rabinovich D.; Viñas C.; Yerin A. Nomenclature for boranes and related species (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 355–381. 10.1515/pac-2018-0205. [DOI] [Google Scholar]
- Sivaev I. B.; Bregadze V. I. Chemistry of Cobalt Bis(dicarbollides). A Review. Collect. Czech. Chem. Commun. 1999, 64, 783–805. 10.1135/cccc19990783. [DOI] [Google Scholar]
- Cabrera-González J.; Sánchez-Arderiu V.; Viñas C.; Parella T.; Teixidor F.; Núñez R. Redox-Active Metallacarborane-Decorated Octasilsesquioxanes Electrochemical and Thermal Properties. Inorg. Chem. 2016, 55, 11630–11634. 10.1021/acs.inorgchem.6b02394. [DOI] [PubMed] [Google Scholar]
- Dash B. P.; Satapathy R.; Swain B. R.; Mahanta C. S.; Jena B. B.; Hosmane N. S. Cobalt bis(dicarbollide) anion and its derivatives. J. Organomet. Chem. 2017, 849–850, 170–194. 10.1016/j.jorganchem.2017.04.006. [DOI] [Google Scholar]
- Kaniowski D.; Kulik K.; Suwara J.; Ebenryter-Olbińska K.; Nawrot B. Boron Clusters as Enhancers of RNase H Activity in the Smart Strategy of Gene Silencing by Antisense Oligonucleotides. Int. J. Mol. Sci. 2022, 23, 12190. 10.3390/ijms232012190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniowski D.; Suwara J.; Ebenryter-Olbińska K.; Jakóbik-Kolon A.; Nawrot B. EGFR-Targeted Cellular Delivery of Therapeutic Nucleic Acids Mediated by Boron Clusters. Int. J. Mol. Sci. 2022, 23, 14793. 10.3390/ijms232314793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuez-Martínez M.; Queralt-Martín M.; Muñoz-Juan A.; Aguilella V. M.; Laromaine A.; Teixidor F.; Viñas C.; Pinto C. G.; Pinheiro T.; Guerreiro J. F.; Mendes F.; Roma-Rodrigues C.; Baptista P. V.; Fernandes A. R.; Valic S.; Marques F. Boron clusters (ferrabisdicarbollides) shaping the future as radiosensitizers for multimodal (chemo/radio/PBFR) therapy of glioblastoma. J. Mater. Chem. B 2022, 10, 9794–9815. 10.1039/D2TB01818G. [DOI] [PubMed] [Google Scholar]
- Kožíšek M.; Cígler P.; Lepšík M.; Fanfrlík J.; Řezáčová P.; Brynda J.; Pokorná J.; Plešek J.; Grüner B.; Grantz Šašková K.; Václavíková J.; Král V.; Konvalinka J. Inorganic Polyhedral Metallacarborane Inhibitors of HIV Protease: A New Approach to Overcoming Antiviral Resistance. J. Med. Chem. 2008, 51, 4839–4843. 10.1021/jm8002334. [DOI] [PubMed] [Google Scholar]
- Řezáčová P.; Pokorná J.; Brynda J.; Kožíšek M.; Cígler P.; Lepšík M.; Fanfrlík J.; Řezáč J.; Grantz Šašková K.; Sieglová I.; Plešek J.; Šícha V.; Grüner B.; Oberwinkler H.; Sedláček J.; Kräusslich H.-G.; Hobza P.; Král V.; Konvalinka J. Design of HIV Protease Inhibitors Based on Inorganic Polyhedral Metallacarboranes. J. Med. Chem. 2009, 52, 7132–7141. 10.1021/jm9011388. [DOI] [PubMed] [Google Scholar]
- Grüner B.; Brynda J.; Das V.; Šícha V.; Štěpánková J.; Nekvinda J.; Holub J.; Pospíšilová K.; Fábry M.; Pachl P.; Král V.; Kugler M.; Mašek V.; Medvedíková M.; Matějková S.; Nová A.; Lišková B.; Gurská S.; Džubák P.; Hajdúch M.; Řezáčová P. Metallacarborane Sulfamides: Unconventional, Specific, and Highly Selective Inhibitors of Carbonic Anhydrase IX. J. Med. Chem. 2019, 62, 9560–9575. 10.1021/acs.jmedchem.9b00945. [DOI] [PubMed] [Google Scholar]
- Kvasničková E.; Masák J.; Čejka J.; Mat’átková O.; Šícha V. Preparation, characterization, and the selective antimicrobial activity of N-alkylammonium 8-diethyleneglycol cobalt bis-dicarbollide derivatives. J. Organomet. Chem. 2017, 827, 23–31. 10.1016/j.jorganchem.2016.10.037. [DOI] [Google Scholar]
- Vaňková E.; Lokočová K.; Kašparová P.; Hadravová R.; Křížová I.; Mat’átková O.; Masák J.; Šícha V. Cobalt Bis-Dicarbollide Enhances Antibiotics Action towards Staphylococcus epidermidis Planktonic Growth Due to Cell Envelopes Disruption. Pharmaceuticals 2022, 15, 534. 10.3390/ph15050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennour I.; Ramos M. N.; Nuez-Martínez M.; Xavier J. A. M.; Buades A. B.; Sillanpä R.; Teixidor F.; Choquesillo-Lazarte D.; Romero I.; Martinez-Medina M.; Viñas C. Water soluble organometallic small molecules as promising antibacterial agents: synthesis, physical–chemical properties and biological evaluation to tackle bacterial infections. Dalton Trans. 2022, 51, 7188–7209. 10.1039/D2DT01015A. [DOI] [PubMed] [Google Scholar]
- Kubiński K.; Masłyk M.; Janeczko M.; Goldeman W.; Nasulewicz-Goldeman A.; Psurski M.; Martyna A.; Boguszewska-Czubara A.; Cebula J.; Goszczyński T. M. Metallacarborane Derivatives as Innovative Anti-Candida albicans Agents. J. Med. Chem. 2022, 65, 13935–13945. 10.1021/acs.jmedchem.2c01167. [DOI] [PubMed] [Google Scholar]
- Núñez R.; Romero I.; Teixidor F.; Viñas C. Icosahedral boron clusters: a perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 2016, 45, 5147–5173. 10.1039/C6CS00159A. [DOI] [PubMed] [Google Scholar]
- Hardie M. J. The use of carborane anions in coordination polymers and extended solids. J. Chem. Crystallogr. 2007, 37, 69–80. 10.1007/S10870-006-9153-X. [DOI] [Google Scholar]
- Fink K.; Boratynski J.; Paprocka M.; Goszczynski T. M. Metallacarboranes as a tool for enhancing the activity of therapeutic peptides. Ann. N. Y. Acad. Sci. 2019, 1457, 128–141. 10.1111/nyas.14201. [DOI] [PubMed] [Google Scholar]
- Masalles C.; Borrós S.; Viñas C.; Teixidor F. Are Low-Coordinating Anions of Interest as Doping Agents in Organic Conducting Polymers?. Adv. Mater. 2000, 12, 1199–1202. . [DOI] [Google Scholar]
- Brus J.; Czernek J.; Urbanova M.; Rohlíček J.; Plecháček T. Transferring Lithium Ions in the Nanochannels of Flexible Metal–Organic Frameworks Featuring Superchaotropic Metallacarborane Guests: Mechanism of Ionic Conductivity at Atomic Resolution. ACS Appl. Mater. Interfaces 2020, 12, 47447–47456. 10.1021/acsami.0c12293. [DOI] [PubMed] [Google Scholar]
- Verdiá-Báguena C.; Alcaraz A.; Aguilella V. M.; Cioran A. M.; Tachikawa S.; Nakamura H.; Teixidor F.; Viñas C. Amphiphilic COSAN and I2-COSAN crossing synthetic lipid membranes: planar bilayers and liposomes. Chem. Commun. 2014, 50, 6700–6703. 10.1039/c4cc01283f. [DOI] [PubMed] [Google Scholar]
- Rokitskaya T. I.; Kosenko I. D.; Sivaev I. B.; Antonenko Y. N.; Bregadze V. I. Fast flip–flop of halogenated cobalt bis(dicarbollide) anion in a lipid bilayer membrane. Phys. Chem. Chem. Phys. 2017, 19, 25122–25128. 10.1039/C7CP04207H. [DOI] [PubMed] [Google Scholar]
- Assaf K. I.; Begaj B.; Frank A.; Nilam M.; Mougharbel A. S.; Kortz U.; Nekvinda J.; Grüner B.; Gabel D.; Nau W. M. High-Affinity Binding of Metallacarborane Cobalt Bis(dicarbollide) Anions to Cyclodextrins and Application to Membrane Translocation. J. Org. Chem. 2019, 84, 11790–11798. 10.1021/acs.joc.9b01688. [DOI] [PubMed] [Google Scholar]
- Tarrés M.; Canetta E.; Paul E.; Forbes J.; Azzouni K.; Viñas C.; Teixidor F.; Harwood A. J. Biological interaction of living cells with COSAN-based synthetic vesicles. Sci. Rep. 2015, 5, 7804. 10.1038/srep07804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaari M.; Gaztelumendi N.; Cabrera-Gonzalez J.; Peixoto-Moledo P.; Vinas C.; Xochitiotzi-Flores E.; Farfan N.; Ben Salah A.; Nogues C.; Nunez R. Fluorescent BODIPY-Anionic Boron Cluster Conjugates as Potential Agents for Cell Tracking. Bioconjugate Chem. 2018, 29, 1763–1773. 10.1021/acs.bioconjchem.8b00204. [DOI] [PubMed] [Google Scholar]
- Muñoz-Flores B. M.; Cabrera-González J.; Viñas C.; Chávez-Reyes A.; Dias H. V. R.; Jiménez-Pérez V. M.; Núñez R. Organotin Dyes Bearing Anionic Boron Clusters as Cell-Staining Fluorescent Probes. Chem. – Eur. J. 2018, 24, 5601–5612. 10.1002/chem.201705804. [DOI] [PubMed] [Google Scholar]
- Gona K. B.; Zaulet A.; Gómez-Vallejo V.; Teixidor F.; Llop J.; Viñas C. COSAN as a molecular imaging platform: synthesis and “in vivo” imaging. Chem. Commun. 2014, 50, 11415–11417. 10.1039/C4CC05058D. [DOI] [PubMed] [Google Scholar]
- Fuentes I.; García-Mendiola T.; Sato S.; Pita M.; Nakamura H.; Lorenzo E.; Teixidor F.; Marques F.; Viñas C. Metallacarboranes on the Road to Anticancer Therapies: Cellular Uptake, DNA Interaction, and Biological Evaluation of Cobaltabisdicarbollide [COSAN]–. Chem. – Eur. J. 2018, 24, 17239–17254. 10.1002/chem.201803178. [DOI] [PubMed] [Google Scholar]
- Tarrés M.; Canetta E.; Viñas C.; Teixidor F.; Harwood A. J. Imaging in living cells using νB–H Raman spectroscopy: monitoring COSAN uptake. Chem. Commun. 2014, 50, 3370–3372. 10.1039/C3CC49658A. [DOI] [PubMed] [Google Scholar]
- Nuez-Martinez M.; Pinto C. I. G.; Guerreiro J. F.; Mendes F.; Marques F.; Muñoz-Juan A.; Xavier J. A. M.; Laromaine A.; Bitonto V.; Protti N.; Crich S. G.; Teixidor F.; Viñas C. Cobaltabis(dicarbollide) ([o-COSAN]−) as Multifunctional Chemotherapeutics: A Prospective Application in Boron Neutron Capture Therapy (BNCT) for Glioblastoma. Cancers 2021, 13, 6367. 10.3390/cancers13246367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo I.; Teixidor F.; Kivekäs R.; Sillanpä R.; Viñas C. Methylation and Demethylation in Cobaltabis(dicarbollide) Derivatives. Organometallics 2003, 22, 4642–4646. 10.1021/om030412r. [DOI] [Google Scholar]
- Hurlburt P. K.; Miller R. L.; Abney K. D.; Foreman T. M.; Butcher R. J.; Kinkhead S. A. New synthetic routes to B-halogenated derivatives of cobalt dicarbollide. Inorg. Chem. 1995, 34, 5215–5219. 10.1021/ic00125a021. [DOI] [Google Scholar]
- Mátel Ĺ.; Macášek F.; Rajec P.; Heřmánek S.; Plešek J. B-Halogen derivatives of the bis (1, 2-dicarbollyl) cobalt (III) anion. Polyhedron 1982, 1, 511–519. 10.1016/S0277-5387(00)81604-6. [DOI] [Google Scholar]
- Hawthorne M. F.; Young D. C.; Andrews T. D.; Howe D. V.; Pilling R. L.; Pitts A. D.; Reintjes M.; Warren L. F.; Wegner P. A. pi.-Dicarbollyl derivatives of the transition metals. Metallocene analogs. J. Am. Chem. Soc. 1968, 90, 879–896. 10.1021/ja01006a008. [DOI] [Google Scholar]
- Assaf K. I.; Nau W. M. The Chaotropic Effect as an Assembly Motif in Chemistry. Angew. Chem., Int. Ed. 2018, 57, 13968–13981. 10.1002/anie.201804597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y. Effect of Ions on the Structure of Water: Structure Making and Breaking. Chem. Rev. 2009, 109, 1346–1370. 10.1021/cr8003828. [DOI] [PubMed] [Google Scholar]
- Marcus Y. ViscosityB-coefficients, structural entropies and heat capacities, and the effects of ions on the structure of water. J. Solution Chem. 1994, 23, 831–848. 10.1007/BF00972677. [DOI] [Google Scholar]
- Okur H. I.; Hladílková J.; Rembert K. B.; Cho Y.; Heyda J.; Dzubiella J.; Cremer P. S.; Jungwirth P. Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions. J. Phys. Chem. B 2017, 121, 1997–2014. 10.1021/acs.jpcb.6b10797. [DOI] [PubMed] [Google Scholar]
- Kunz W.; Lo Nostro P.; Ninham B. W. The present state of affairs with Hofmeister effects. Curr. Opin. Colloid Interface Sci. 2004, 9, 1–18. 10.1016/j.cocis.2004.05.004. [DOI] [Google Scholar]
- Collins K. D.; Washabaugh M. W. The Hofmeister effect and the behaviour of water at interfaces. Q. Rev. Biophys. 1985, 18, 323–422. 10.1017/S0033583500005369. [DOI] [PubMed] [Google Scholar]
- Hofmeister F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. 10.1007/BF01918191. [DOI] [Google Scholar]
- Ďord’ovič V.; Tošner Z.; Uchman M.; Zhigunov A.; Reza M.; Ruokolainen J.; Pramanik G.; Cígler P.; Kalíková K.; Gradzielski M.; Matějíček P. Stealth Amphiphiles: Self-Assembly of Polyhedral Boron Clusters. Langmuir 2016, 32, 6713–6722. 10.1021/acs.langmuir.6b01995. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alvarez R.; Ďord’ovič V.; Uchman M.; Matějíček P. Amphiphiles without Head-and-Tail Design: Nanostructures Based on the Self-Assembly of Anionic Boron Cluster Compounds. Langmuir 2018, 34, 3541–3554. 10.1021/acs.langmuir.7b03306. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alvarez R.; Medos Z.; Tosner Z.; Zhigunov A.; Uchman M.; Hervo-Hansen S.; Lund M.; Bester-Rogac M.; Matějíček P. Total Description of Intrinsic Amphiphile Aggregation: Calorimetry Study and Molecular Probing. Langmuir 2018, 34, 14448–14457. 10.1021/acs.langmuir.8b03462. [DOI] [PubMed] [Google Scholar]
- Matějíček P.; Cígler P.; Procházka K.; Král V. Molecular Assembly of Metallacarboranes in Water: Light Scattering and Microscopy Study. Langmuir 2006, 22, 575–581. 10.1021/la052201s. [DOI] [PubMed] [Google Scholar]
- Chevrot G.; Schurhammer R.; Wipff G. Surfactant Behavior of “Ellipsoidal” Dicarbollide Anions: A Molecular Dynamics Study. J. Phys. Chem. B 2006, 110, 9488–9498. 10.1021/jp060930q. [DOI] [PubMed] [Google Scholar]
- Bauduin P.; Prevost S.; Farras P.; Teixidor F.; Diat O.; Zemb T. A theta-shaped amphiphilic cobaltabisdicarbollide anion: transition from monolayer vesicles to micelles. Angew. Chem., Int. Ed. 2011, 50, 5298–5300. 10.1002/anie.201100410. [DOI] [PubMed] [Google Scholar]
- Uchman M.; Ďord’ovič V.; Tošner Z.; Matějíček P. Classical Amphiphilic Behavior of Nonclassical Amphiphiles: A Comparison of Metallacarborane Self-Assembly with SDS Micellization. Angew. Chem., Int. Ed. 2015, 54, 14113–14117. 10.1002/anie.201506545. [DOI] [PubMed] [Google Scholar]
- Malaspina D. C.; Viñas C.; Teixidor F.; Faraudo J. Atomistic Simulations of COSAN: Amphiphiles without a Head-and-Tail Design Display “Head and Tail” Surfactant Behavior. Angew. Chem., Int. Ed. 2020, 59, 3088–3092. 10.1002/anie.201913257. [DOI] [PubMed] [Google Scholar]
- Medoš Ž.; Hleli B.; Tošner Z.; Ogrin P.; Urbič T.; Kogej K.; Bešter-Rogač M.; Matějíček P. Counterion-Induced Aggregation of Metallacarboranes. J. Phys. Chem. C 2022, 126, 5735–5742. 10.1021/acs.jpcc.2c00107. [DOI] [Google Scholar]
- Wennerström H.; Lindman B.; Micelles Physical chemistry of surfactant association. Phys. Rep. 1979, 52, 1–86. 10.1016/0370-1573(79)90087-5. [DOI] [Google Scholar]
- Takeuchi T.; Bagnacani V.; Sansone F.; Matile S. Amphiphilic Counterion Activators for DNA: Stimuli-Responsive Cation Transporters and Biosensors in Bulk and Lipid Bilayer Membranes. ChemBioChem 2009, 10, 2793–2799. 10.1002/cbic.200900512. [DOI] [PubMed] [Google Scholar]
- Sakai N.; Matile S. Anion-Mediated Transfer of Polyarginine across Liquid and Bilayer Membranes. J. Am. Chem. Soc. 2003, 125, 14348–14356. 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]
- Nishihara M.; Perret F.; Takeuchi T.; Futaki S.; Lazar A. N.; Coleman A. W.; Sakai N.; Matile S. Arginine magic with new counterions up the sleeve. Org. Biomol. Chem. 2005, 3, 1659–1669. 10.1039/b501472g. [DOI] [PubMed] [Google Scholar]
- Perret F.; Nishihara M.; Takeuchi T.; Futaki S.; Lazar A. N.; Coleman A. W.; Sakai N.; Matile S. Anionic Fullerenes, Calixarenes, Coronenes, and Pyrenes as Activators of Oligo/Polyarginines in Model Membranes and Live Cells. J. Am. Chem. Soc. 2005, 127, 1114–1115. 10.1021/ja043633c. [DOI] [PubMed] [Google Scholar]
- Gasparini G.; Bang E.-K.; Montenegro J.; Matile S. Cellular uptake: lessons from supramolecular organic chemistry. Chem. Commun. 2015, 51, 10389–10402. 10.1039/C5CC03472H. [DOI] [PubMed] [Google Scholar]
- Chuard N.; Fujisawa K.; Morelli P.; Saarbach J.; Winssinger N.; Metrangolo P.; Resnati G.; Sakai N.; Matile S. Activation of Cell-Penetrating Peptides with Ionpair−π Interactions and Fluorophiles. J. Am. Chem. Soc. 2016, 138, 11264–11271. 10.1021/jacs.6b06253. [DOI] [PubMed] [Google Scholar]
- Peng S.; Barba-Bon A.; Pan Y.-C.; Nau W. M.; Guo D.-S.; Hennig A. Phosphorylation-Responsive Membrane Transport of Peptides. Angew. Chem., Int. Ed. 2017, 56, 15742–15745. 10.1002/anie.201707979. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Zhang Q. Development of liposomal formulations: From concept to clinical investigations. Asian J. Pharm. Sci. 2013, 8, 81–87. 10.1016/j.ajps.2013.07.010. [DOI] [Google Scholar]
- Bulbake U.; Doppalapudi S.; Kommineni N.; Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. K.; Sandén C.; Selegård R.; Liedberg B.; Aili D. Tuning Liposome Membrane Permeability by Competitive Peptide Dimerization and Partitioning-Folding Interactions Regulated by Proteolytic Activity. Sci. Rep. 2016, 6, 21123. 10.1038/srep21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mady M. M.; Ghannam M. M. Stability of anionic liposomes in serum and plasma. Afr. J. Pharm. Pharmacol. 2011, 5, 898–1905. 10.5897/AJPP11.462. [DOI] [Google Scholar]
- For the different anionic formulations previously reported in the literature (refs (82),83), we found that the combination DMPE/DPPG/CHOL, in a molar ratio of 1/2/1, is the most stable under the conditions of the study; vesicles retained stability and ensured reproducible measurements for 1 month.
- Davis J. T.; Okunola O.; Quesada R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 2010, 39, 3843–3862. 10.1039/b926164h. [DOI] [PubMed] [Google Scholar]
- Gale P. A.; Davis J. T.; Quesada R. Anion transport and supramolecular medicinal chemistry. Chem. Soc. Rev. 2017, 46, 2497–2519. 10.1039/C7CS00159B. [DOI] [PubMed] [Google Scholar]
- Matile S.; Vargas Jentzsch A.; Montenegro J.; Fin A. Recent synthetic transport systems. Chem. Soc. Rev. 2011, 40, 2453–2474. 10.1039/c0cs00209g. [DOI] [PubMed] [Google Scholar]
- Tahara Y.; Fujiyoshi Y. A new method to measure bilayer thickness: Cryo-electron microscopy of frozen hydrated liposomes and image simulation. Micron 1994, 25, 141–149. 10.1016/0968-4328(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Kučerka N.; Nieh M.-P.; Katsaras J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta, Biomembr. 2011, 1808, 2761–2771. 10.1016/j.bbamem.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Saha T.; Gautam A.; Mukherjee A.; Lahiri M.; Talukdar P. Chloride Transport through Supramolecular Barrel-Rosette Ion Channels: Lipophilic Control and Apoptosis-Inducing Activity. J. Am. Chem. Soc. 2016, 138, 16443–16451. 10.1021/jacs.6b10379. [DOI] [PubMed] [Google Scholar]
- Malla J. A.; Umesh R. M.; Vijay A.; Mukherjee A.; Lahiri M.; Talukdar P. Apoptosis-inducing activity of a fluorescent barrel-rosette M+/Cl– channel. Chem. Sci. 2020, 11, 2420–2428. 10.1039/C9SC06520B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- We introduce here (top panels f and g) the “arrow-notation” for ITC titrations, where the compound on the top corresponds to the compound in the syringe, which is being added (arrow) to the compound contained in the cell (bottom); the additional advantage of this notation is that the top and bottom compounds correspond directly, written as a fraction with nominator and denominator, to the stoichiometric n value obtained from the ITC experiment, e.g., 2/protamine = 17.
- Hanke W.; Schlue W. R.. Planar Lipid Bilayer; Academic press: London, 1993. [Google Scholar]
- Takeuchi T.; Kosuge M.; Tadokoro A.; Sugiura Y.; Nishi M.; Kawata M.; Sakai N.; Matile S.; Futaki S. Direct and Rapid Cytosolic Delivery Using Cell-Penetrating Peptides Mediated by Pyrenebutyrate. ACS Chem. Biol. 2006, 1, 299–303. 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- Smith B. D.; Lambert T. N. Molecular ferries: membrane carriers that promote phospholipid flip-flop and chloride transport. Chem. Commun. 2003, 2261–2268. 10.1039/b303359g. [DOI] [PubMed] [Google Scholar]
- Song B.; Yuan H.; Pham S. V.; Jameson C. J.; Murad S. Nanoparticle permeation induces water penetration, ion transport, and lipid flip-flop. Langmuir 2012, 28, 16989–17000. 10.1021/la302879r. [DOI] [PubMed] [Google Scholar]
- Robison A. D.; Sun S.; Poyton M. F.; Johnson G. A.; Pellois J.-P.; Jungwirth P.; Vazdar M.; Cremer P. S. Polyarginine Interacts More Strongly and Cooperatively than Polylysine with Phospholipid Bilayers. J. Phys. Chem. B 2016, 120, 9287–9296. 10.1021/acs.jpcb.6b05604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y.-C.; Barba-Bon A.; Tian H.-W.; Ding F.; Hennig A.; Nau W. M.; Guo D.-S. An Amphiphilic Sulfonatocalix[5]arene as an Activator for Membrane Transport of Lysine-rich Peptides and Proteins. Angew. Chem., Int. Ed. 2021, 60, 1875–1882. 10.1002/anie.202011185. [DOI] [PubMed] [Google Scholar]
- Cebula J.; Fink K.; Goldeman W.; Szermer-Olearnik B.; Nasulewicz-Goldeman A.; Psurski M.; Cuprych M.; Kędziora A.; Dudek B.; Bugla-Płoskońska G.; Goszczyński T. M. Structural patterns enhancing the antibacterial activity of metallacarborane-based antibiotics. ChemRxiv 2023, 10.26434/chemrxiv-2022-t2g39-v2. [DOI] [PubMed] [Google Scholar]; https://chemrxiv.org/engage/chemrxiv/article-details/63f623e232cd591f12511faa (accessed May 16, 2023).
- Wei W. Hofmeister Effects Shine in Nanoscience. Adv. Sci. 2023, 2302057. 10.1002/advs.202302057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




