Abstract
Hepatic metabolic clearance is one of the most important factors driving the overall kinetics of chemicals including substances used in various product categories such as pesticides, biocides, pharmaceuticals, and cosmetics. A large number of in vitro systems from purified isozymes and subcellular organelles to hepatocytes in simple cultures and in complex scaffold setups are available for measuring hepatic metabolic clearance for different applications. However, there is currently no approach for systematically characterising and comparing these in vitro methods in terms of their design, applicability and performance. To address this, existing knowledge in the field of in vitro human hepatic metabolic clearance methods was gathered and analysed in order to establish a framework to systematically characterise methods based on a set of relevant components. An analogous framework would be also applicable for non-human in vitro systems. The components are associated with the biological test systems used (e.g. subcellular or cells), the in vitro method (e.g. number of cells, test item solubility), related analytical techniques, data interpretationmethods (based on substrate depletion/metabolite formation), and performance assessments (precision and accuracy of clearance measurements). To facilitate the regulatory acceptance of this class of methods, it is intended that the framework provide the basis of harmonisation work within the OECD.
Keywords: ADME, Chemical risk assessment, In vitro hepatic metabolic clearance, New approach methodology, Toxicokinetics
1. Introduction
Information on hepatic metabolic clearance is indispensable for the safety assessment of xenobiotics. The consideration of hepatic metabolic clearance data, either on their own or in combination with complementary information on absorption, distribution, metabolism and excretion (ADME) can support different aspects of chemical safety assessment including the development of Integrated Approaches to Testing and Assessment (IATA), informing toxicity study design and enhancing the interpretation of toxicological endpoint data (Bessems and Geraets, 2013). Chemical-specific in vitro hepatic metabolic clearance data can also be used to refine the extrapolation of toxicological results from animals to humans (Lipscomb et al., 2004). For example, when extrapolating from rat to human, understanding the qualitative and quantitative differences in clearance between species provides the basis for the derivation of chemical-specific adjustment factors as an informed alternative to using the usual default factor of 4 (the kinetics contribution to the value of 10 used for interspecies variability) (Dorne and Renwick, 2005; Bhat et al., 2017). Clearance data can also be incorporated into Physiologically Based Kinetic (PBK) models to perform In Vitro In Vivo Extrapolation (IVIVE) to support risk assessment using non-animal in vitro approaches (Bessems et al., 2014). As described in the EURL ECVAM strategy for achieving 3Rs impact in the assessment of toxicokinetics and systemic toxicity (Bessems et al., 2015), optimal use of computational and in vitro ADME methods will be essential for successful development of Integrated Approaches to Testing and Assessment (IATA) (Edwards et al., 2016). The importance of such data has been formally recognised at the regulatory level, as reflected for example within the European Union’s Plant Protection Products Regulation (EU No. 283/, 2013) which, since 2015, requires investigation of interspecies differences in metabolism, as follows: “Comparative in vitro metabolism studies shall be performed on animal species to be used in pivotal studies and on human material (microsomes or intact cell systems) in order to determine the relevance of the toxicological animal data and to guide in the interpretation of findings and in further definition of the testing strategy”.
Hepatic metabolic clearance data can be generated using a variety of different in vitro methods. The majority of these methods are based on biological testsystems comprised of subcellular fractions (e.g. liver microsomes or S9) or intact cells, including various systems such as immortalised cell lines or primary hepatocytes. Regarding cellular systems, different approaches can be used ranging from the more traditional ones, such as cell suspensions or adherent cell monolayer cultures, to the more novel test systems, such as three-dimensional spheroid-cultures or microfluidic systems (see Table 1 for more details and references). Although these methods have been designed to produce the same type of information and thus can be considered as belonging to the same class of methods, they typically differ from one another regarding their key technical components, experimental protocols and specific applications (see Table 1 and Section 4 for more details).
Table 1.
Commonly used in vitro hepatic cellular model systems used for clearance estimations: advantages and disadvantages. Modified from Wilk-Zasadna et al 2015 (Wilk-Zasadna et al., 2015)
| Test system | Advantages | Disadvantages | References |
|---|---|---|---|
| Recombinant human enzymes | mainly for drug-drug interaction studies; identification of metabolism pathways, clearance estimate of a single enzyme |
one or few enzymes in isolation in an “artificial” environment, lack of active uptake transport | (Foti et al., 2010), (Buratti et al, 2005); Buratti et al, (2011) |
| Human liver microsomes/Cytosols | major phase I (or phase II) enzymes with proper proportions; metabolic profiles and clearance estimations; inter-individual variability assessable |
insufficient activities for most phase II enzymes; cellular and organ architecture lost, lack of active uptake transport |
(Pelkonen et al., 2009), (Asha and Vidyavathi, 2010) (Buratti et al 2013, 2015) |
| Human liver S9 or homogenate | major phase I and II enzymes; otherwise as microsomes | cellular and organ architecture lost, insufficient proportions of phase II activities (cofactor requirements), lack of active uptake transport, diluted Phase I | (Pelkonen et al., 2009) |
|
Isolated primary human hepatocytes in suspension (also cryopreserved) |
the ‘gold standard’ –all xenobiotic metabolising enzymes present and de novo cofactor production; functional active uptake transport, – testing with multiple batches or pools is recommended to ensure the clearance values are not unduly influenced by variability of individual lots - for inter-individual variation an appropriate number of single donor well characterised samples should be used |
in suspension useful only a limited time period up to a few hours (Relay method for low clearance compounds); |
(McGinnity et al., 2004), (Brown et al., 2007), (Hallifax et al., 2010), (Smith et al., 2012) |
|
Isolated cultured primary human hepatocytes, plated (also cryopreserved) |
contain all drug metabolising enzymes and cofactors; reflect inter-individual variation; viable for extended periods of time, ideal for drug-drug interaction studies (i.e. liver enzyme induction) |
metabolic functions decline over initial ~24 hrs in culture to ~10% of initial suspension levels; when used in sandwich (using scaffold e.g. on matrigel) the maintenance can be longer than 1 day expensive, specialized skills; insufficient studies demonstrating effectiveness for CLint determinations |
(Sahi et al., 2010) (Swift* et al., 2010) (Vinci et al., 2011) (Smith et al., 2012) |
| HepaRG® cell line | maintains metabolic capacities roughly comparable to cultured primary human hepatocytes; robust, good experimental reproducibility |
Lower metabolic function comparable to cultured primary human hepatoctyes, represent one donor only with low inherent CYP2D6 and CYP2C9 activity; transcriptome differs from hepatocytes, significance not elucidated Some CYP activities increasing over time |
(Anthérieu et al., 2010) (Guillouzo et al., 2007), (Turpeinen et al., 2009), (Zanelli et al., 2012) (Pomponio et al,2015) (Truisi et al, 2015) (Bell et al 2017) |
| Human liver slices | basic hepatic architecture preserved, functional metabolic competence at least in earlier phases of incubation |
specialized preparation and incubation techniques; unequal penetration of nutrients depending on slice thickness; gradual deterioration; inflammatory response |
(de Graaf et al., 2010) (Lerche-Langrand and Toutain, 2000), (Olinga et al., 1997; Worboys et al., 1997), (Vickers and Fisher, 2004) |
Having several different in vitro clearance methods to choose from is potentially advantageous, since each one is fit for a particular purpose; on the other hand this can be problematic across the spectrum: for experimentalists during method selection and regulators during data evaluation. Lack of transparency and detail in a clearance method proposed for use in the regulatory arena may ultimately undermine the credibility and acceptance of such an approach as the method appropriateness and reliability cannot be adequately reviewed. To address this issue, we propose the development of a novel framework that can be systematically and routinely applied to characterise the key components of existing and future in vitro clearance methods, to ensure that data derived from these methods are sufficiently understood and trusted for use in a regulatory context.
Development of the framework focuses on identifying the relevant components to be considered when characterising in vitro hepatic metabolic clearance methods to support the assessment of their performance, facilitate their inter-comparison, and to ultimately increase confidence in their use. The components associated with this framework are associated with: i) the biological test systems used (types of test systems employed, subcellular or cells, and their configuration (e.g., suspension or plated, 2D or 3D)), ii) the in vitro method (definition of experimental layout such as number of cells used, test item solubility, cell medium used, total incubation time, etc.), iii) related analytical techniques (description of analytical method/s used to measure parent compound/metabolites in biological media), iv) data interpretation methods (substrate depletion and metabolite formation) and v) performance assessments(accuracy and precision of clearance measurements).
The framework and its components are intended to be more descriptive rather than prescriptive. They are not intended to define how a method should be designed, how it should be used, or what performance it should have. Instead, they provide a tool to facilitate the understanding of how in vitro hepatic metabolic clearance data have been generated and therefore what critical aspects (including uncertainties) need to be taken into account when applying the method for regulatory purposes. It describes key components that usually fit in the materials and methods section of a paper, allowing researchers to repeat the experiment described in the paper. Consequently, the framework is expected to enhance the communication between in vitro method developers, in vitromethod end-users and regulators. This framework builds on the OECDGuidance Document 211 for the reporting of non-guideline in vitro methods (OECD GD 211, 2014). The OECD GD 211 is focused on harmonising the way any in vitro method should be described in order to facilitate the assessment of the quality of the data generated and their adequacy for use in regulatory applications. The aim of this framework has a similar scope but a narrower focus since it addresses only the specific class of in vitro methods that measure/estimate hepatic metabolic clearance, albeit in more detail. Also, given that most of the existing knowledge has been generated using in vitro human hepatic metabolic clearance methods and will be applied in human safety assessment, we have focused on characterisation of a framework for human-derived test methods. However, the framework itself can be applied more broadly to encompass test systems applicable to other (non-) target species.
2. Overview of in vitro methods currently used for human hepatic metabolic clearance
In vitro hepatic metabolic clearance methods are designed to estimate the intrinsic clearance (CLint) due to the metabolism of a substance under study. CLint is determined by the activity of enzymes metabolising the substance, and is calculated on the basis of either disappearance/depletion of the parent compound or the appearance/formation of (a) metabolite(s) over time. CLint is converted to the in vivo hepatic metabolic clearance by considering liver weight, liver blood flow and the unbound fraction of the substance in plasma (Rowland and Tozer, 1995).
Regarding the test systems and their configuration used to measure in vitrohepatic metabolic clearance, most of these methods (Table 1) are either specific (e.g. single recombinant enzymes), providing an estimate for a specific single enzyme-catalysed clearance of a compound without the contributions of any other enzymes, or general, such as S9 fraction or cultured hepatocytes, covering a major part or all of the liver metabolic machinery. Most methods use a configuration of isolated human hepatocytes in suspension, which contain the most comprehensive metabolic machinery. Intact hepatocytes contain functional uptake transporters, which may have a significant and chemical-dependent effect on clearance. For example, uptake may be the rate-determining step in the overall hepatic clearance process for both metabolised and non-metabolised compounds (De Bruyn et al., 2016; Menochet et al., 2012; Parker and Houston, 2008). The role of other cell types in the liver is less well understood. However, since hepatocytes constitute about 70–80% of liver cells, as well as the cell type with the highest level of certain metabolic enzymes (e.g. the cytochrome P450 family), they are considered the most important cell population for xenobioticmetabolism.
Regarding the experimental layout to perform an in vitro clearance incubation assay, currently, a test system with isolated and cryopreserved human hepatocytes in suspension is considered as the gold standard for the estimation of in vitro human hepatic metabolic clearance of high to medium clearance ratesubstrates (Dalvie et al., 2009). This cell model includes comprehensive metabolic machinery within a cell membrane with functionally active uptake transporters, reliable cryopreservation, and physiologically relevant enzymatic activity levels for a few hours of incubation. Other assay conditions are dependent on the laboratory and the particular chemical under investigation (e.g.use of one or a range of test item concentrations, choice of carrier solvent, composition of the incubation solution). The incubation assay can be performed with manual preparation but often, especially in cases where several chemicals are analysed, high-throughput robotic systems are employed where multi-well (usually 96- or 364-well) plates are used. For instance an in vitro hepatic metabolic clearance scheme applied in collaboration with the US EPA ToxCast program to screen hundreds of chemicals has employed two test item concentrations (1 μM and 10 μM) and monitored depletion of the parent substance to estimate intrinsic clearance rates and ultimately in vivo hepatic metabolic clearance of the tested compounds (Wetmore et al., 2012, Wetmore et al., 2015). This test system was applied in a high throughput platform to support IVIVE of toxicodynamic information on hundreds of chemicals in hundreds of in vitro toxicity tests. When screening is not the purpose, it is recommended to test >2 concentrations to ensure the substrate concentration is in the linear condition range (i.e. well below the Km).
One of the main advantages of test systems using isolated human hepatocytes in suspension is that they are relatively simple and straightforward to employ. However, depending on the intended application, they do not recapitulate the entire complement of physiological processes that may contribute to in vivoclearance. For instance, the tissue architecture also affects the in vivo clearance of a compound and its metabolites, due to specific physiological features such as the routes and rates of blood flow and biliary flow, the acinar organisation of the tissue, and interactions within and between cells of different types. Flow conditions might be incorporated within specific in vitro methods to more closely mimic the in vivo physiological situation (Table 2). Furthermore, these systems could handle more problematic compounds, such as volatiles and solubility-compromised chemicals, where the compound is introduced into the flow solution in a dedicated compartment to expose the test system under controlled conditions. However, these in vitro methods are very technically complex and not yet well established.
Table 2.
Currently investigated, but not yet established, test systems for the human liver metabolism and clearance estimation.
| Model system | (most important) feature | Reference |
|---|---|---|
| “Simple” 2D or 3D cell cultures | ||
| Primary cell co-cultures | Cell-cell interactions | (Guguen-Guillouzo and Guillouzo, 2010) |
| Embryonic stem cells | Functionality in doubt; Donor variability | (Pal et al., 2012) |
| Induced pluripotent stem cells | Functionality in doubt; donor variability | (Si-Tayeb et al., 2010) |
| Spheroid scaffold-free cultures | improved stability and functionality, simple setup | (Gunness et al., 2013) (Vorrink et al 2017) |
| Scaffold structures without or with flow arrangements | ||
| Micropatterned plated cell cultures (e.g. Hepatopac) | improved stability and functionality | (Chan et al., 2013) |
| HμREL® Biochip (microfluidic flow) | improved stability and functionality, complex setup | (Chao et al., 2009) |
| Hollow-fiber bioreactor | improved stability and functionality, complex setup | (Zeilinger et al., 2011) |
| Perfused multi-well bioreactor | improved stability and functionality, complex setup | (Domansky et al., 2010) |
| Perfused matrix-embedded hepatocyte bioreactor | artificial liver -mimic | (Schmelzer et al., 2010) |
Other more complex test systems have been developed to introduce physiologically significant functions to the in vitro test (see Table 2). Cell-cell interactions that occur in intact liver tissue can be captured by using a 3D configuration (e.g. hanging drops, ultra-low affinity binding plates); alternatively, various scaffolds for cells could be provided, such as matrigel, collagen, fibrin, alginate. Importantly, hepatocyte maintenance, enzyme activities and stability of other processes that may contribute to clearance (e.g., transporters) are much longer lived in these physiologically robust systems, thus mitigating a major shortcoming of the human hepatocyte suspension-based test systems. Examples where these problems are mitigated largely are the co-cultures of human hepatocytes (as HepatoPac®) and HepaRG cells, used for repeated exposure testing up to 14 days (Kratochwil et al., 2017; Pomponio et al., 2015; Bellwon et al., 2015; Truisi et al., 2015). Although still under development, microfluidic systems technology in studying kinetics gives the possibility of integrating individual organs by developing multi-organ models capable of replicating sequential processes that influence the fate (and possibly activity) of a chemical within the body (Imura et al., 2012; Esch et al., 2015).
Once the in vitro intrinsic clearance is derived, independently from the test system used, extrapolation to in vivo hepatic metabolic clearance follows an established scheme: an intrinsic hepatocyte clearance is converted with scaling factors (a number of hepatocytes per gram of liver = ~100–120 million; liver weight = 1500 g or 25.7 g of liver per kilogram body weight) to the hepatic intrinsic clearance (Barter et al., 2007). The intrinsic clearance is then converted to the hepatic clearance using various clearance models, taking into account factors such as the plasma unbound fraction and hepatic blood flow and making various assumptions (e.g. well stirred compartments) (Houston, 1994). From a theoretical point of view, in the calculation of enzyme kinetics, the unbound (“free”) concentration of a chemical at the site of enzyme catalysis should be used, but in its absence, the free plasma concentration generated either experimentally or computationally using quantitative structure–property relationships (QSPR) is often used (Bessems et al., 2014). A less widely established option is to add serum into the incubation mixture and perform no correction for the “free” concentration of the test item (Shibata et al., 2002). Based on the available published data, the predictive performance of the human hepatocyte suspension clearance method has been investigated and found scientifically defensible, although systematic under-prediction seems to be common (Blanchard et al., 2005; Brown et al., 2007; Hallifax et al., 2010). One of the several reasons for this could be the lack of consideration of in vitrobiokinetic parameters (for example non-specific binding); in order to account for such parameters a specific strategy has been proposed whenever an in vitro test system is used (Kramer et al., 2015). Another major confounding factor is our inability to quantify the functionality of the isolated hepatocytes relative to the in vivo situation.
In models where all elements are controlled and a relevant number of compounds have been assessed (i.e. >20), this systematic in vitro to in vivounder-prediction bias can be removed (Sohlenius-Sternbeck et al., 2010; Wood et al., 2017). This procedure improves the estimation of in vivo clearance, allows a clear comparison between models, and provides the basis for analysis of the error and model improvement (Hallifax et al., 2010).
3. Process applied to identify key elements for characterising and describing in vitro hepatic metabolic clearance methods
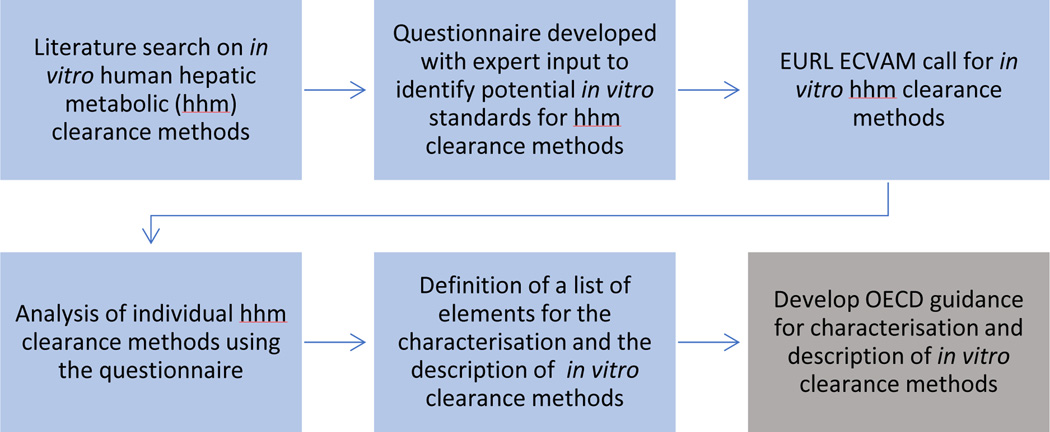
A knowledge-gathering process was designed to map and analyse existing knowledge in the field of in vitro human hepatic metabolic clearance methods. The aim was to identify commonly used experimental attributes of the existing methods as the basis for identifying relevant components to be considered to characterise in vitro hepatic metabolic clearance methods. This approach facilitates assessment of their performance, method comparison and increased confidence in their use. The process of the steps undertaken to establish a systematic framework to characterise in vitro methods for human hepatic metabolic clearance and how this framework will be shared with relevant OECDexpert groups and activities with a view to its further elaboration and utilisation is summarised in Fig. 1.
Figure 1:
Blue boxes summarise the process followed to identify relevant elements to be considered to characterise and describe in vitro hepatic metabolic clearance methods. Grey box represents future work that EURL ECVAM intends to perform.
A literature search was initially performed to identify available in vitro human hepatic metabolic clearance methods in the public domain (Fig. 1). Then, an interactive questionnaire was developed in collaboration with a group of international external experts covering different sectors (including academia, industry, governmental institutes). Part of the published clearance questionnaire, together with an example of received replies, is shown in Table 3. It should be noted that most answers came from the pharmaceutical sector, therefore replies can be driven by specific needs (e.g. characterisation of hepatocytes limited to those CYPs which are relevant or drug metabolism, not including other isoforms e.g. those involved primarily in industrial chemical biotransformation). However, the general principles apply to all the chemicals, irrespective of their final use. This interactive questionnaire was then used by the JRC’s EURL ECVAM to publish a call that asked for web-based submission of human hepatic metabolic clearance methods. The aim of this call was to identify: 1) if current users already have criteria, which they apply within their test facility to characterise their methods, and 2) non-publically available methods that can contribute to the definition of relevant components to be considered to characterise in vitroclearance methods. The call for methods also aimed to provide information about essential components, critical protocol parameters that can affect the method’s performance, acceptance criteria used, chemical compounds used as reference, positive and negative controls, the intended application of the method, the measurement endpoint and the number of chemicals that had been tested from the submitted facility using the submitted method.
Table 3:
Part of the published EURL ECVAM interactive questionnaire for detecting standards that users of in vitro human hepatic metabolic clearance method might apply within their laboratories. In the second column examples of received replies are presented
| Question | Example of replies |
|---|---|
|
| |
| For which of the following purpose(s) you apply the submitted method in your test facility? | Screening, development of a new compound, regulatory use, incorporation into a PBPK model, ranking chemicals from low to high clearance, species selection for safety studies etc. |
|
| |
| Which of the following test systems is employed in the in vitro method/ SOP? | Human fresh hepatocytes (single donor or pooled), cryopreserved human hepatocytes (single donor or pooled), S9, microsomes, liver slices, cytosol etc. |
|
| |
| Which is the test system configuration? | Suspension, 2D cultured, 3D cultured, sandwich cultured etc |
|
| |
| Which parameter is measured? | Parent compound depletion rate, metabolite formation rate, both parent compound depletion and metabolite formation rate |
|
| |
| Are there any internal standards or reference compounds, for which human in vivo behaviour is known, included in the in vitro method/SOP? | 27% replied No, 73% replied Yes (e.g. Midazolam, Verapamil, Naloxone, Piroxicam) |
|
| |
| Are acceptance criteria for the biological system (e.g. cell line, tissue model etc) being used and are they specified in the in vitro method/ SOP? An example of acceptance criterion could be a sufficient metabolic activity of CYP3A4, or the transporter competence of the biological system | 55% replied No, 45% replied Yes (e.g. metabolic activities, cell viabilities at the conclusion of the assays intrinsic clearance data relative to historical data, historical data batch wise corrected, the hepatocyte pools were characterized for metabolism (CYP1A2, CYP2C9, CYP2D6, CYP3A4/5, CYP2C19, 7-ethoxycoumarin glucuronidation and 7-ethoxycoumarin sulfation) and viability (Trypan Blue exclusion). The pools were used if they fell within acceptable ranges compared with historical quality control limits, as determined by cell provider, basal activity of CYP 1A2, 3A4, should be greater than the median given by historical data of the provider and viability greater than 75%.) |
|
| |
| Do you correct results for unbound concentrations in the incubation, either by experimental measurement (e.g. Equilibrium dialysis) or by use of a log D prediction? If yes, please describe the approach you follow. | 46% replied No, 27% replied that it is case dependent (e.g. correction is performed sometimes using ultrafiltration, depending on the purpose of the analysis correction might be performed, depending on serum concentration etc) 27% relied YES (e.g. using equilibrium dialysis) |
To reach the wider scientific community, the web call was advertised in relevant scientific journals and it was disseminated through various EURL ECVAM networks (e.g. the EU Network of Laboratories for the Validation of Alternative Methods (EU-NETVAL), DataBase on ALternative Methods (DB-ALM), Preliminary Assessment of Regulatory Relevance (PARERE), ECVAM Stakeholder Forum (ESTAF)), as well as the International Cooperation on Alternative Test Methods (ICATM) and the European Society for In vitroToxicology (ESTIV). In parallel, methods identified through the literature search were analysed using the questionnaire as a tool. Representative methods were selected and used to identify relevant components that characterise in vitrohepatic metabolic clearance methods.
In addition the results from the literature search and the web survey analysis were discussed during an EURL ECVAM expert meeting. External experts involved in the meeting were grouped into experimental experts – mainly scientists that have theoretical and hands-on experience of this class of methods - and a second expert group consisting of risk assessors. The aim of the risk assessors group was to define the proper use of in vitro data within the context of chemical safety assessment. The final outcome of all these efforts was to define a draft set of relevant components that is described in detail below.
Ultimately, these efforts are expected to guide harmonisation at the OECD on the characterisation and description of in vitro human hepatic metabolic clearance methods.
4. List of relevant elements for the characterisation and the description of in vitro hepatic metabolic clearance methods
Based on the outcome of the knowledge-gathering process, the authors suggest a list of relevant components to facilitate the characterisation and comparison of the class of in vitro methods for human hepatic metabolic clearance. The list of components could also be applicable to in vitro systems using non-human test systems. The list, which is intended to serve as a starting point to improve reliability and facilitate an understanding of the data produced by this class of in vitro methods, encompasses components related to five categories: biological test system, in vitro method, chemical analysis, interpretation and performance. Table 4 reports all the components which are described in more detail in the following sections.
Table 4:
List of relevant elements suggested by the authors based on the outcome of the knowledge-gathering process estabilished by EURL-ECVAM.
| 1. Biological test system related elements | Procurement of the test system used (e.g. cells, source, basis for pooling etc). |
| Test system and configuration. | |
| Characterisation of enzyme presence and activity. | |
| Presence of enzyme induction or inhibition pathways. | |
| Transporters present and their activities | |
| 2. In vitro method related elements | Cell density or protein concentration. |
| Cell viability after isolation and/ or thawing. | |
| Test item solubility and stability. | |
| Test item concentration used in the incubation medium. | |
| Solvents used and their concentration in the final incubation mixture. | |
| Protein amount in the incubation mixture and if serum was included. | |
| Non-specific binding of a test item. | |
| The first time point sampled and the schedule of sampling. | |
| The total incubation time. | |
| 3. Chemical Analytics-related elements | Analytical method used to determine the test item and/ or the metabolites formed during the in vitro clearance assay. |
| If the analytical method employed was validated and according to which guideline. | |
| Lower limit of quantification (LLOQ) of the analytical method used. | |
| Acceptance criteria for calibration samples or quality control if used. | |
| Linearity of the method. | |
| 4. Interpretation-related elements | Clearance determination method. |
| Model(s) to derive the in vitro clearance parameter (CLint). | |
| Results and acceptance criteria of the negative/ positive controls. | |
| Percentage of test item consumption at the end of the assay. | |
| 5. Performance-related elements | The lower limit of intrinsic clearance reliably measurable. |
| Within laboratory reproducibility. | |
| Accuracy (Bias) of CLint measurements. | |
| The precision of CLint measurments. |
4.1. Biological test system related components
Procurement of the test system (e.g. cells, source, basis for pooling etc). In addition to more ‘generic’ tests, widely used in both academia and industry (e.g.Bessems et al., 2014), there are a large number of commercial test systems, both subcellular and cellular, often with patented or trade secret configurations. Usually these systems are validated to a certain extent, but it would be advisable to assess them according to the framework described here.
-
Test system and configuration. As shown in Table 1, and described in section 2, several test systems are available. In general, test systems can be categorised into cellular or subcellular and each category presents some advantages. Although subcellular systems, such as microsomes, cytosol and S9 fractions, are experimentally simpler, cellular systems maintain the whole cell integrity and depending on the cell type used (e.g.primary hepatocytes), may express requisite enzymes and transportersunder physiologically-relevant conditions. Subcellular systems only express certain enzymes. Therefore, the choice of the most suitable test system depends on the specific intended use.
Test system configuration will dictate the length of time an in vitro system can maintain its metabolic competence. The most common test system configurations are in suspension or adherent monolayers (i.e. plated). Subcellular systems are always used in suspension while cellular systems can also plated. As explained in Table 1, Table 2, in general, cells when used in a plated configuration can maintain their metabolic competence for longer duration and support the measurement of clearance for low-clearance chemicals (Smith et al., 2012). When used in sandwich configuration they can maintain their features for days (Truisi et al., 2015; Pomponio et al., 2015). However, plated hepatocytes are not typically derived from pooled donors (as in the case of cells in suspension), so there is no consideration of population variability.
-
Characterisation of enzyme(s) presence and activity. Different test systems can have a different set of enzymes participating in chemical metabolism. Therefore, the qualitative characterisation of Phase I and Phase II metabolic competence is an important element to be considered. Characterisation of metabolic competence plays an important role, because lack of enzymes or loss of their activity might contribute to underestimation of clearance. Another level of characterisation is related to the quantitative aspect of metabolic capability and potential variability within the same test system. This, for instance, is the case when primary human hepatocytes are employed. Depending on the pooled-donors source, the isolation, preparation and cryopreservation the level of enzyme/s might vary across different lots.
The metabolic characterisation, both qualitative and quantitative, is frequently done on specific isoforms of cytochromes P450. Extensive knowledge exists on expression and activity levels which facilitate both the characterisation of the qualitative presence of an enzyme as well as the quantitative evaluation of variability that may exist across different lots of the same test system employed. Applications to other critical Phase I (e.g. aldehyde oxidase) and Phase II (e.g. UDP-glucuronosyltransferases (UGTs) or Glutathione-S-Transferases (GST)) enzymes are becoming more frequent. Currently, the enzyme activity is measured by incubating the test system with probe reference chemicals and monitoring the disappearance of the parent or the formation of specific metabolites over time.
For the sake of harmonisation and understanding there is a need to agree on which enzymes need to be characterised, the experimental methodology that should be applied and the probe chemical(s) that should be used for each enzyme.
Enzymatic induction or inhibition pathways. Considering that some substances/test items might induce or inhibit the enzymes that are responsible for their own metabolism, the presence of these pathways is critical for the estimation of hepatic metabolic clearance in vitro. This is particularly relevant during repeated exposure experiments. Regarding inhibition, direct interactions with enzymes are important to account for as both the substrate and end-products that are formed can significantly inhibit the metabolic enzymes, although the relevance of inhibition to the in vivo extrapolation may be uncertain (Jones et al., 2005). Regarding induction, there is are EURL ECVAM validated methods for characterising the cytochrome P450 induction of CYP1A2, CYP2B6 and CYP3A4isoforms, which can be applied for test system characterisation (EC, 2018a and EC 2018b).
Characterisation of transporter(s) presence and activity. In vivo hepatic metabolic clearance initiates when the test item is transported into the hepatocyte either by passive diffusion though the cell membrane and/or by active transport. In hepatocytes, active uptake is mediated by basolateral membrane organic anion transporters including the organic anion transporters OATP1B1, OATP1B3, OATPB2B1, OATP1A2, OAT2, OAT5, the organic cation transporter OCT1 and the Na-taurocholate co-transporting NTCP. Currently, various methodologies for studying hepatocyte uptake and metabolism of test items are available, such as uptake measurements using suspended hepatocytes and various reference chemicals (Soars et al., 2007; Lee et al., 2017). There is a need to standardise these protocols and test their applicability to other cellular test systems. As in the case of metabolic competence, characterisation of transporters and their activity is important since deviations from physiological conditions might lead to underestimation of clearance, particularly if active transport is the rate limiting step over metabolic clearance (Jigorel and Houston, 2012).
4.2. In vitro method related components
Cell density or protein concentration (depending on the biological test system employed). This information facilitates method comparison by normalising clearance data for the cell density or protein concentration, although the former is preferred when enzyme induction occurs. In addition, particularly during the method development phase, considerations should be given to optimise the cell density or protein concentration (together with test item concentration) in order to establish linear conditions prior to running the incubation assays.
Cell viability after isolation and/or thawing. Cell viability can be checked when hepatocytes are isolated as well as after thawing before the incubation assay is initiated in order to know the precise number of viable cells available for the incubation with the test item and to control experimental conditions. A cytotoxicity assay should be performed in advance using exactly the same assay conditions and the same test item to identify experimental conditions which are not toxic to the test system. Indeed, the clearance has to be measured at sublethal concentrations in order to be reliable: kinetics is strictly dependent on the number of cells therefore the change of this parameter during testing would strongly alter the result (Kramer et al., 2015). Alternatively, cell viability can also be measured at the end of the clearance experiment: in case of the absence of metabolism of the test item the investigator can be confident that it is due to the metabolic stability of the test item and not to cytotoxic effects.
Test item solubility and stability. Preliminary work should always be performed to evaluate the test item’s solubility and stability in order to work under controlled experimental conditions. When preparing a test item solution in a specific organic solvent (to make a stock solution) and/or in the incubation medium, it is important to measure the solubility to check that the actual concentration achieved in solution is similar to the nominal one. This to avoid possible underestimation of clearance values due to limited or poor solubility or non-specific binding. Regarding test item stability, it is important to assess whether any disappearance of the test item observed in vitro is due to non-enzymatic processes such as evaporation, photodegradation, plastic binding or general chemical instability of the test item under the assay conditions. These processes can affect the interpretation of clearance results (Kramer et al., 2015). Inclusion of appropriate negative controls (e.g., metabolically inactivated and no-cell controls) throughout the experimental time course will monitor chemical stability throughout the experiment.
Test item concentration in the incubation medium. The optimisation of the test item’s concentration ensures that the metabolic machinery is not saturated and therefore that the assay is conducted under linear kinetic conditions. Three or more concentrations of the test item should be screened to identify concentration(s) near or below Km which can then be tested for the clearance method. Furthermore, as previously explained, it is important that the concentration used does not cause toxicity to the test system.
Solvent concentration in the final incubation mixture. Solvents such as dimethyl sulfoxide (DMSO), acetonitrile and methanol may be used to prepare test item stock solution. Selection of a sufficiently low concentration of these solvents in the incubation media is critical to ensure that artefactual effects (e.g., CYP inhibition, membrane solubilisation) are avoided (Easterbrook et al., 2001; Busby Jr et al., 1999). Generally, the solvent concentration in the incubation mixture ranges between 0.1 and 1% (v/v), but the final experimental conditions should be optimised depending on the test system used.
-
Protein amount in the incubation mixture and inclusion of serum/serum free conditions. By default, any kind of incubation medium is acceptable; however for facilitating the IVIVE extrapolation it is critical to report if serum was included in the incubation medium.
In addition, the test item, depending on its specific physico-chemical properties (e.g. lipophilicity, charge and size), can bind to the proteins in the medium thus affecting the free unbound (bioavailable) concentration and the cell uptake (Pomponio et al., 2015; Armitage et al., 2014). Several methods are commonly used to measure plasma protein bindingincluding equilibrium dialysis, ultrafiltration and ultracentrifugation, solid phase microextraction (SPME), the latter to measure serum constituent binding of drugs in the in vitro exposure medium (Broeders et al., 2011). Alternatively an estimate of the unbound fraction in vitro can be based on an appropriate lipophilic relationship algorithm for either microsomes or hepatocytes (Kilford et al., 2008) The importance of the free fraction in the cultivation medium is higher with high protein binding chemicals (Mielke et al., 2017). Therefore, knowledge of the presence of proteins in the medium could be used by the investigator to evaluate the occurrence of protein binding to further control experimental conditions. This is important since clearance can be underestimated for chemicals that are highly bound to proteins.
Non-specific binding of the test item. Beside protein in the medium, the test item can also bind to labware (e.g sorption to plastic of well-plates, Palmgren et al., 2006) and cell-attachment matrices (e.g. collagen scaffolds) used for the incubation assay (Kramer et al., 2015). The latter situation refers to physical sequestration in the matrix which may limit the amount of the bioavailable test item and lead to an overestimation of the intracellular concentration of the test item (Kramer et al., 2015). Estimating clearance through loss of parent compound as done with the substrate depletion approach may lead to an underestimation of clearance for highly bound chemicals. Irrespective of the chosen approach, it is important to report if the CLint data were corrected for non-specific binding (to both protein and labware) and how this correction was performed. Binding of the studied chemical to a subcellular preparation (e.g. microsomes) or intracellular proteins affect the concentration that reaches and interacts with a biological target (e.g., enzyme), but methods to measure intracellular concentration and binding have not yet been extensively used (Mateus et al., 2013, Mateus et al., 2017).
Number of time points and sampling schedule. The time-dependence of parent disappearance and/or metabolite formation provides necessary information about the kinetics of metabolism for more reliable calculation of clearance.
-
Total incubation time. This depends on the biological test system used and on its configuration, which are critical parameters for maintaining the metabolic competence of the employed test system (refer to “Biological test system related components”).
As shown in Table 1, when hepatocytes in suspension are used, the total incubation time is normally 2–4 h since then loss of enzymatic activity and cell viability occurs. The Relay method (Di and Obach, 2015), which involves transferring the supernatant from hepatocyte incubations to freshly thawed hepatocytes at the end of the incubation time, is used to prolong the total incubation time up to 20 h depending how many transfer cycles are performed. However, results from other studies, demonstrating that for the clearance identification and for in vitro in vivo extrapolation purposes it is necessary to know the time course of the concentration in the cells relevant for toxicity and not only the concentration–time course in the supernatant (Truisi et al., 2015; Mielke et al., 2017) indicates that the “relay method” has some limitations depending on the intended application for which it is used. The “relay method” should be considered as a model to qualitatively evaluate low clearance compounds while for quantitative measurements other cellular-based models may be more suitable. Other test systems, such as plated cells, in different configurations can allow cells to be exposed for even longer periods, up to days or even weeks (see Table 1, Table 2).
The total incubation time is an important consideration to achieve turnover of low clearance chemicals. Incubation time of 2 h is generally sufficient when medium to high clearance chemicals are tested.
4.3. Chemical Analytics-related components
Analytical method used to determine the test item and/or its metabolites formed during the in vitro clearance assay. This is an important method component. Currently, the liquid chromatography-mass spectrometry is the norm, however as discussed above, the choice of the method is always dependent on the chemical behaviour of the analyte (e.g. for volatile analytes, gas chromatography with various detectors can be applied) (Tolonen and Pelkonen, 2015).
Validation of the analytical method. The validation of the analytical method practically demonstrates its appropriateness. Various guidance documents exist for the validation of bioanalytical methods (e.g. US FDA and EMA Guidance on Bioanalytical method validation). Depending on the aim of the in vitro clearance study (e.g. prioritisation of chemicals, in vivo prediction) an analytical method may be validated according to an official guideline or by following an in-house validation as described by Timmerman et al. (2015).
Lower limit of quantification (LLOQ) of the analytical method. For defining the LLOQ there are established procedures described by EMA and FDA guidelines (EMA, 2011; FDA, 2018) for the validation of bioanalytical methods. According to these guidelines, the analyte signal of the LLOQ sample should be at least five times the signal of a blank sample. Since the LLOQ is chemical dependent, a good practice is to compare LLOQ with the initial incubation concentration used in the in vitro clearance method. In general, the LLOQ should be <10% of the initial incubation concentration when clearance is measured by disappearance of the parent chemical.
Acceptability criteria. Commonly established criteria as defined by EMA and FDA (EMA, 2011; FDA, 2018) guidelines for the validation of bioanalytical methods could be used for calibration samples or quality control (if used).
Linearity of the method. Considering that during an in vitro hepatic metabolic clearance method either disappearance/depletion of the parent compound or the combined appearance/formation of metabolites is monitored with time, it is important to know the linearity of the analytical method employed to measure the samples. Guidance on how to establish linearity is given in the EMA and FDA guidelines (EMA, 2011; FDA, 2018).
4.4. Interpretation-related components
Clearance determination method. As briefly mentioned above (section 2), two experimental approaches are used to measure in vitro hepatic metabolic clearance: disappearance/depletion of the parent compound and the combined appearance/formation of metabolites. The most appropriate approach will mostly depend on the chemical being tested and on the intended application. In general the disappearance approach is used when there is a high turnover of the chemical within the in vitrosystem. Metabolite formation method is used when there is relatively slow turnover by monitoring the time-dependent metabolite(s) formation. Obviously the metabolite formation approach requires that the metabolites to be monitored are known in advance, since an analytical standard is needed for their exact quantification.
Model(s) to derive the in vitro intrinsic clearance (CLint). When monitoring the disappearance of the test item it is common practice to use first-order elimination kinetics (Smith et al., 2012). However, if other models are used, the choice of model should be thoroughly justified and reported. In cases of product inhibition or enzyme degradation, there is a need to switch to two-phase decay functions (Jones et al., 2005). Ideally the output from an in vitro experiment should be the in vitro intrinsic clearance, which refers to the free fraction (bioavailable) test item, and it should be expressed as μL per minute per million cells (Houston et al., 2012). This would enable the end user of the data (e.g. a PBK modeller) to choose appropriate scaling factors for in vitro to in vivo extrapolation purposes.
Results and acceptability criteria of the negative/positive controls. Inclusion of assay negative controls consisting of test item only (Smith et al., 2012) or of test item and inactivated test system (Wetmore et al., 2012) can give valuable information related to possible non-enzymatic losses of the test item as previously explained for “test item solubility and stability”. Currently, there is no practical guidance on how to run the negative controls in such in vitro methods and also how to use the negative control information during the in vitro data treatment. For the positive control, it is common practice to include in the incubation set at least one chemical that is metabolised within the time frame of the in vitroassay and for which the in vitro CLint is known based on historical data.
Percentage of test item consumption at the end of the assay. An achievement of at least 20% consumption of the test item has been suggested, providing the analytical method sensitivity allows sufficient discrimination(Smith et al., 2012).
4.5. Performance-related components
-
Lower limit of intrinsic clearance that can be reliably measured. As previously explained, the lower CLint limit is directly related to the stability of the test system with regard to cell viability, Phase I and Phase II enzyme stability and the intrinsic physicochemical properties of the relevant test items. Although physico-chemical considerations will be compound-specific, the other two factors are system-dependent; and the longer these components can be maintained the more sensitive the system will be to measuring lower Clint values. Although cell viability assessments have been well characterised, there is no established procedure for defining the time course of hepatic enzyme stability despite earlier efforts to define CYP3A4 and CYP1A2 stabilities in cryopreserved human hepatocytes suspensions and in matched sandwich cultures (Smith et al., 2012). Thus, there is a need to develop a standard methodology for estimating these stabilities. Such efforts can then be incorporated into an approach to define acceptable lower limits of clearance measurements across different test systems.
It has been proposed (Di and Obach, 2015) that the low limits of CLintmeasurements in liver microsomes is about 12 μL/min/mg protein [assuming a limit of 120 min for a measurable in vitro t1/2 (half-life)] when using 0.5 mg/mL microsomal protein and 1 h incubation time. For a 4 h human hepatocyte suspension incubation containing 0.5 million cells/mL, a lower limit of CLint that can be measured is about 2.5 μL/min/million cells, which scales to 6.3 mL/min/kg body weight. Based on these considerations, one possible approach is to use a clearance methodology suitable for low clearance determination when the tested chemical shows <20% depletion. However this approach can be waived based on the intended purpose of the study. For instance, for screening and prioritisation applications the use of a non-optimised clearance methodology can still be fit for purpose to test low-clearance chemicals since the main aim is to rank chemicals based on their clearance rates. On the contrary, when the application is focused on predicting in vivoclearance values, it becomes more important to employ a methodology which is suitable to quantitative clearance for lower-turnover compounds.
Within laboratory reproducibility This is an important information related to the quantitative utility of the method. There is a need to define appropriate reference chemicals for measuring the within laboratory reproducibility.
Accuracy (Bias) of CLint measurements. Common practice in the literature is to define the accuracy based on the ratio of in vitro CLint (measured) and in vivo CLint (observed) for a set of reference chemicals with known human in vivo clearance values. According to Obach et al. (1997), a prediction with an average-fold (geometric mean) error ≤ 2 was considered successful. This approach was also used (Zanelli et al., 2012) to compare the ability of cryopreserved human hepatocytes in suspension with that of cryopreserved HepaRG cells in suspension for measuring the in vitro CLint. However, there is a need to provide guidance on the number and selection of the reference chemicals used. Underprediction of in vivoCLint is a common finding and this may be greater than the 2-fold bias indicated above, indeed it has been documented that bias is clearance dependent; low clearance is often predicted with minimal bias whereas high clearance predictions may show bias >10-fold (Wood et al., 2017).
Precision of CLint measurements. The precision can be assessed using the root mean square error (RMSE) as defined and proposed (Sheiner and Beal, 1981) for a set of measurements of in vitro CLint values for different reference chemicals with known human in vivo clearance (observed). There is a need to provide guidance on the number and selection of the reference chemicals to be used. Special attention should be given to identifying and using reference chemicals where hepatic metabolic clearance is the main route of elimination. Ideally reference chemicals should be fully characterised kinetically to cover a wide range of clearance values observed in vivo, various physicochemical properties and various mechanistic properties e.g. transport mechanism/enzyme involvement (Houston et al., 2012).
5. Discussion
Historically, data on ADME properties and the predicted kinetics have been extensively used for the safety assessment of pharmaceuticals. They have been used as well in the pesticide domain but less so in other chemical frameworks as in the EU REACH Regulation and Cosmetic Products Regulation (Regulation (EC) No 1907/2006 and Regulation (EC) No 1223/2009). There is an increasing demand (from researchers, test method developers, industrial and regulatory risk assessors) to generate and integrate toxicokinetic information in chemical risk assessment. This is based on the fact that modern toxicology aims to understand the underlying mechanisms of toxicity, rather than rely only on phenomenological evidence based on in vivo toxicity studies in test species (Berg et al., 2011; SCHER, 2013; EFSA, 2014; EPA, 2014). This new vision implies a shift in chemical risk assessment toward a focus on internal dose/concentration and early molecular and cellular effects. External exposure to a chemical does not automatically mean that all of the dose will be bioavailableas an internal dose and therefore able to trigger molecular/cellular changes that may ultimately cause a specific toxicological or adverse effect. Hence knowledge of a chemical’s toxicokinetics can assist in the interpretation of in vitro/in vivotoxicological findings, by relating the chemical dose/concentration with the observed toxicity effects (Adler et al., 2011; Coecke et al., 2013). In addition, toxicokinetic data can be used to improve the design of toxicity tests (both in vivoand in vitro).
Regulatory agencies recognise the need to integrate toxicokinetics data together with toxicodynamics information to improve chemical risk assessment (ECHA, 2014; EFSA, 2014). Still, focusing at the EU level, toxicokinetic data are not consistently required across the pieces of legislation covering the different chemical sectors (Bessems et al., 2015).
Overall, considering the regulatory need for toxicokinetic information coupled with the increasing use in modern toxicology of New Approach Methodologies (NAMs), several in vitro ADME methods have been developed with many focused on providing hepatic metabolic clearance data (Adler et al., 2011; Bessems et al., 2014). This is because hepatic metabolic clearance together with renal clearance often represents the main driving process of kinetics to determine the dose/concentration-time profile of a chemical in a biological system (Wilk-Zasadna et al., 2015). Therefore, hepatic metabolic clearance data can provide sound mechanistic support to the chemical risk assessment process.
Considering that several in vitro hepatic metabolic clearance methods exist which may significantly vary in their experimental design and in their intended application, the knowledge-gathering process based on: (1) the literature search on in vitro human hepatic metabolic clearance methods, (2) a questionnaire developed with expert input to identify potential components for characterising such methods, (3) a call for outline procedures and/or standard operating procedures; and (4) an expert workshop) which has served to map their technical diversity. The outcome of this work has been used to outline the framework and its components described in this publication.
Historically, methods for the in vitro measurement of hepatic metabolic clearance have been developed for pharmaceuticals which often have high bioavailability and occupy a well-defined chemical space (characterised by a log Pow between 2 and 5 and molecular masses between 150 and below 500 Da). Limited experience exists regarding the applicability of these methods outside this chemical space to include other groups of chemicals, e.g. industrial chemicals, food contaminants, plant protection products and cosmetic ingredients. However, Rendic and Guengerich (2015) did not find very large differences in oxidative metabolism (CYPs, aldo-keto reductases, AKRs, FMOs etc) of almost 2000 chemicals and > 8000 reactions in the metabolism of the chemicals divided into subgroups such as anthropogenic chemicals used in commerce, naturally occurring and endogenous compounds, and drugs (as divided into marketed drugs and new chemical entities or drug candidates). Furthermore, CYP3A4seemed to be the most frequently involved enzyme in metabolising both pharmaceuticals (Zanger et al., 2008) and pesticides (Abass et al., 2012) although at the concentrations relevant for human exposure other CYPs can be much more active, with CYP3A4 becoming predominant when the others are saturated (Buratti et al., 2005). In general, it seems reasonable to conclude that bioactive chemicals such as pesticides and biocides might have a similar metabolic behaviour to pharmaceuticals when sharing similar physicochemical properties. The situation is more complex for industrial chemicals and environmental contaminants, covering a broader chemical space, for which the metabolic behaviour can be very diverse. Furthermore, industrial/environmental chemicals can be very lipophilic and volatile and this can be a challenge when performing an in vitro metabolic clearance method since they can highly bind to the plastic labware equipment used (e.g. the well-plate used for the incubation assay) (Tirelli et al., 2007).
Different considerations should be given for application of in vitro clearance assays to complex chemical mixtures. In this case, there is the inherent problem that one has to be able to measure the clearance parameters for each substance and for each component of a mixture, which requires preferably metabolomics approaches (Pelkonen et al., 2012).
The results described here show that a variety of in vitro human hepatic metabolic clearance methods exist and are used for different applications across chemical sectors. This is an advantage as appropriate in vitro models might be suitable for various domains of chemical space. But this also causes a considerable challenge when it comes to characterisation and definition of the components as the framework needs to serve most if not all available methods. The current paper describes the approach taken to start the development of such a framework and a number of crucial components.
This framework should not be interpreted as prescribed list of components that always have to be considered to fully characterise a certain method; nor does it claim to be complete. Depending on the specific application, only some components might be considered when characterising a certain in vitro method. The framework and its components should be fit for purpose and used in a flexible manner. In this respect, this framework incorporates aspects of the modular approach for the validation of in vitro methods (Hartung et al., 2004).
However, the list of components described in this publication is just a first step toward establishing a fully operational framework to characterise this class of in vitro methods but it cannot be considered exhaustive. In fact, this publication presents a list of relevant components that the authors suggest based on the outcome of the knowledge-gathering process. Further work is needed to evaluate if other relevant components also have to be taken on board.
Regarding reference chemicals for characterising the mechanistic basis of a method and its reliability, they should ideally cover a wide range of chemicals with high, medium and low clearance values in vivo, various physicochemical properties and various mechanisms, e.g. transport mechanism/enzyme involvement (Houston et al., 2012). The main limitation of the list proposed by Houston et al. (2012) is that it contains only CYP-substrate chemicals. Thus, the authors propose to extend this list of substrates with other Phase I enzymes and Phase II enzymes. Substrates for transporters should be included as well. The inclusion of these reference chemicals will enable data comparisons across different methods and also indicate the relevance (in vivo prediction) of the method.
Characterisation of metabolic competence might be less critical when primary cellular systems are used as a main tool to predict CLint. The reason is that live cells contain the complete tissue-specific metabolic machinery (containing the whole suit of both Phase I and II enzymes) within a proper cellular regulatory environment. Nevertheless, as explained in section 4, it should be always recommended to check the maintenance of the metabolic capability over time (including storage and culturing conditions, beside the time duration of the experimental phase).
The analytical method employed to measure the concentration in the produced in vitro samples is another critical attribute to be considered while characterising in vitro human hepatic metabolic clearance methods. Hence, developing specific and sensitive analytical methods is a prerequisite for performing such studies. In the drug area specific and sensitive analytical methods have to be developed because they are needed to enable measuring concentration-time profiles in regulatory requested pharmacokinetic studies. This investment will also become necessary in other areas if clearance methods are to be used for improved risk assessment.
Overall, in vitro hepatic metabolic clearance methods represent a broad and diverse class of methods where different technical aspects need to be considered to fully explore their potential to support chemical risk assessment.
The framework described represents the first attempt to outline the relevant components to be considered to characterise these methods and speed up their use, especially when employed for regulatory decision making.
Further work is needed to evaluate if other components should be considered and to further elaborate those outlined here by providing guidance, and when needed recommendations, on how to evaluate and assess their contribution to method characterisation.
Therefore, with a view to international uptake and harmonisation, this framework will be shared with relevant OECD expert groups and activities with a view to its further elaboration and utilisation.
Acknowledgments
This paper is dedicated to our former collaborator and friend Dr. Edward W. Carney, who passed away on 12 January 2015.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency or the European Commission.
References
- Abass K, Turpeinen M, Rautio A, Hakkola J, Pelkonen O, 2012. Metabolism of Pesticides by Human Cytochrome P450 Enzymes In Vitro – A Survey, In: Perveen F (Ed.), Insecticides - Advances in Integrated Pest Management. InTech, Rijeka, Croatia, pp. 165–194. [Google Scholar]
- Adler S, Basketter D, Creton S, Pelkonen O, van Benthem J, Zuang V, Testai E, et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects – 2010. 2011. Arch. Toxicol 85, 367–485. [DOI] [PubMed] [Google Scholar]
- Anthérieu S, Chesné C, Li R, Camus S, Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J, Guguen-Guillouzo C, Guillouzo A, 2010. Stable Expression, Activity, and Inducibility of Cytochromes P450 in Differentiated HepaRG Cells. Drug Metabolism and Disposition 38, 516–525. [DOI] [PubMed] [Google Scholar]
- Armitage JM, Wania F, Arnot JA, 2014. Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment. Environ. Sci. Technol 48 (16), 9770–9779. [DOI] [PubMed] [Google Scholar]
- Asha S, Vidyavathi M, 2010. Role of Human Liver Microsomes in In Vitro Metabolism of Drugs—A Review. Applied Biochemistry and Biotechnology 160, 1699–1722. [DOI] [PubMed] [Google Scholar]
- Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, Houston JB, Lake BG, Lipscomb JC, Pelkonen OR, Tucker GT, Rostami-Hodjegan A; 2007. Scaling Factors for the Extrapolation of In Vivo Metabolic Drug Clearance From In Vitro Data: Reaching a Consensus on Values of Human Microsomal Protein and Hepatocellularity Per Gram of Liver. Current Drug Metabolism 8, 33–45. [DOI] [PubMed] [Google Scholar]
- Bell CC, Lauschke VM, Vorrink SU, Palmgren H, Duffin R, Andersson TB, Ingelman-Sundberg M;2017. Transcriptional, Functional, and Mechanistic Comparisons of Stem Cell-Derived Hepatocytes, HepaRG Cells, and Three-Dimensional Human Hepatocyte Spheroids as Predictive In Vitro Systems for Drug-Induced Liver Injury. Drug Metab Dispos. 45, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellwon P, Truisi GL, Bois FY, Wilmes A, Schmidt T, Savary CC, Parmentier C, Hewitt PG, Schmal O, Josse R, Richert L, Guillouzo A, Mueller SO, Jennings J, Testai E, Dekant W; 2015. Kinetics and dynamics of cyclosporine A in three hepatic cell culture systems Toxicol. In Vitro 30, 62–78. [DOI] [PubMed] [Google Scholar]
- Berg N, De Wever B, Fuchs HW, Gaca M, Krul C, Roggen EL, 2011. Toxicology in the 21st century – Working our way towards a visionary reality. Toxicology in Vitro 25, 874–881. [DOI] [PubMed] [Google Scholar]
- Bessems JG, Coecke S, Gouliarmou V, Whelan M, Worth A 2015. EURL ECVAM strategy for achieving 3Rs impact in the assessment of toxicokinetics and systemic toxicity EUR - Scientific and Technical Research Reports, Institute for Health and Consumer Protection. [Google Scholar]
- Bessems JG, Geraets L; 2013. Proper knowledge on toxicokinetics improves human hazard testing and subsequent health risk characterisation. A case study approach. Regulatory Toxicology and Pharmacology 67,325–334. [DOI] [PubMed] [Google Scholar]
- Bessems JG, Loizou G, Krishnan K, Clewell HJ, Bernasconi C, Bois F, Coecke S, Collnot EM, Diembeck W, Farcal LR, Geraets L, Gundert-Remy U, Kramer N, Kuesters G, Leite SB, Pelkonen OR, Schröder K, Testai E, Wilk-Zasadna I, Zaldivar-Comenges JM, 2014. PBTK modelling platforms and parameter estimation tools to enable animal-free risk assessment: recommendations from a joint EPAA--EURL ECVAM ADME workshop. Regul Toxicol Pharmacol. 68:119–139. [DOI] [PubMed] [Google Scholar]
- Blanchard N, Alexandre E, Abadie C, Lavé T, Heyd B, Mantion G, Jaeck D, Richert L, Coassolo P, 2005. Comparison of clearance predictions using primary cultures and suspensions of human hepatocytes. Xenobiotica 35, 1–15. [DOI] [PubMed] [Google Scholar]
- Broeders JJW, Blaauboer BJ, Hermens JLM, 2011. Development of a negligible depletion-solid phase microextraction method to determine the free concentration of chlorpromazine in aqueous samples containing albumin. J. Chromatogr. A 1218, 8529–8535. [DOI] [PubMed] [Google Scholar]
- Brown HS, Griffin M, Houston JB, 2007. Evaluation of cryopreserved human hepatocytes as an alternative in vitro system to microsomes for the prediction of metabolic clearance. Drug Metab Dispos 35, 293–301. [DOI] [PubMed] [Google Scholar]
- Buratti FM, D’Aniello A, Volpe MT, Meneguz A, Testai E; 2005. Malathion bioactivation in the human liver: the contribution of different P450 isoforms. Drug Metab. Dispos, 33, 295–302. [DOI] [PubMed] [Google Scholar]
- Buratti FM, Scardala S, Funari E, Testai E; 2011. Human Glutathione Transferases catalyzing the conjugation of the hepatoxin Microcystin-LR. Chem. Res. Toxicol 24, 926–933. [DOI] [PubMed] [Google Scholar]
- Buratti FM, Scardala S, Funari E, Testai E; 2013. The conjugation of Microcystin-RR by human recombinant GSTs and hepatic cytosol. Toxicol. Letters 219, 231– 238. [DOI] [PubMed] [Google Scholar]
- Buratti FM, Testai E; 2015. Species- and congener-differences in Microcystin-LR and –RR GSH conjugation in human, rat and mouse hepatic cytosol Toxicol. Lett. 232, 133–140. [DOI] [PubMed] [Google Scholar]
- Busby WF Jr, Ackermann JM, Crespi CL, 1999. Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug Metab Dispos. 27, 246–9. [PubMed] [Google Scholar]
- Chan TS, Yu H, Moore A, Khetani SR, Tweedie D, 2013. Meeting the Challenge of Predicting Hepatic Clearance of Compounds Slowly Metabolized by Cytochrome P450 Using a Novel Hepatocyte Model, HepatoPac. Drug Metabolism and Disposition 41, 2024–2032. [DOI] [PubMed] [Google Scholar]
- Chao P, Maguire T, Novik E, Cheng KC, Yarmush ML, 2009. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem Pharmacol 78, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coecke S, Pelkonen O, Batista Leite S, Bernauer U, Bessems JGM, Bois FY, Gundert-Remy U, Loizou G, Testai E, Zaldívar J-M. 2013. Toxicokinetics as a key to the integrated toxicity risk assessment based primarily on non-animal approaches. Toxicol. In Vitro 27, 1570–1577. [DOI] [PubMed] [Google Scholar]
- Dalvie D, Obach RS, Kang P, Prakash C, Loi C-M, Hurst S, Nedderman A, Goulet L, Smith E, Bu H-Z, Smith DA, 2009. Assessment of Three Human in Vitro Systems in the Generation of Major Human Excretory and Circulating Metabolites. Chemical Research in Toxicology 22, 357–368. [DOI] [PubMed] [Google Scholar]
- De Bruyn T, Stieger B, Augustijns PF, Annaert PP, 2015. Clearance Prediction of HIV Protease Inhibitors in Man: Role of Hepatic Uptake. Journal of Pharmaceutical Sciences, n/a-n/a. [DOI] [PubMed] [Google Scholar]
- de Graaf IAM, Olinga P, de Jager MH, Merema MT, de Kanter R, van de Kerkhof EG, Groothuis GMM, 2010. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protocols 5, 1540–1551. [DOI] [PubMed] [Google Scholar]
- Di L, Obach RS, 2015. Addressing the challenges of low clearance in drug research. Aaps J 17, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG, 2010. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorne JL, Renwick AG, 2005. The refinement of uncertainty/safety factors in risk assessment by the incorporation of data on toxicokinetic variability in humans. Toxicol Sci 86,20–26. [DOI] [PubMed] [Google Scholar]
- Easterbrook J, Lu C, Sakai Y, Li AP, 2001. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab Dispos 29, 141–4. [PubMed] [Google Scholar]
- Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA, 2016. Adverse Outcome Pathways-Organizing Toxicological Information to Improve Decision Making. J Pharmacol Exp Ther. 356, 170–81. [DOI] [PubMed] [Google Scholar]
- ECHA, 2014. Guidance on Information Requirements and Chemical Safety Assessment-Chapter R.7C: Endpoint specific guidance. version 2 [Google Scholar]
- EFSA, 2014. Modern methodologies and tools for human hazard assessment of chemicals. EFSA Journal 12, 3638. [Google Scholar]
- EMA, 2011. Guideline on bioanalytical method validation. [Google Scholar]
- EPA, 2014. Next Generation Risk Assessment: Incorporation of Recent Advances in Molecular, Computational, and Systems Biology. EPA/600/R-14/004. [Google Scholar]
- Esch EW, Bahinski A, Huh D 2015. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14, 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti RS, Wienkers LC, Wahlstrom JL, 2010. Application of cytochrome P450 drug interaction screening in drug discovery. Combinatorial Chemistry and High Throughput Screening 13, 145–158. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Guillouzo A, 2010. General review on in vitro hepatocyte models and their applications. Methods Mol Biol 640, 1–40. [DOI] [PubMed] [Google Scholar]
- Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C, 2007. The human hepatoma HepaRG cells: A highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chemico-Biological Interactions 168, 66–73. [DOI] [PubMed] [Google Scholar]
- Gunness P, Mueller D, Shevchenko V, Heinzle E, Ingelman-Sundberg M, Noor F, 2013. 3D organotypic cultures of human HepaRG cells: a tool for in vitro toxicity studies. Toxicol Sci 133, 67–78. [DOI] [PubMed] [Google Scholar]
- Hallifax D, Foster J, Houston JB, 2010. Prediction of Human Metabolic Clearance from In Vitro Systems: Retrospective Analysis and Prospective View. Pharmaceutical Research 27, 2150–2161. [DOI] [PubMed] [Google Scholar]
- Hartung T, Bremer S, Casati S, Coecke S, Corvi R, Fortaner S, Gribaldo L, Halder M, Hoffmann S, Roi AJ, Prieto P, Sabbioni E, Scott L, Worth A, Zuang V, 2004. A modular approach to the ECVAM principles on test validity. Altern Lab Anim 32, 467–472. [DOI] [PubMed] [Google Scholar]
- Houston JB, 1994. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol 47, 1469–1479. [DOI] [PubMed] [Google Scholar]
- Houston JB, Rowland-Yeo K, Zanelli U, 2012. Evaluation of the novel in vitro systems for hepatic drug clearance and assessment of their predictive utility. Toxicology in Vitro 26, 1265–1271. [DOI] [PubMed] [Google Scholar]
- Imura Y, Yoshimura E, Sato K 2012. Micro total bioassay system for oral drugs: evaluation of gastrointestinal degradation, intestinal absorption, hepatic metabolism, and bioactivity. Anal Sci 28, 197–199. [DOI] [PubMed] [Google Scholar]
- Jigorel E, Houston JB, 2012. Utility of drug depletion-time profiles in isolated hepatocytes for accessing hepatic uptake clearance: identifying rate-limiting steps and role of passive processes. Drug Metab Dispos. 40, 1596–1602. [DOI] [PubMed] [Google Scholar]
- Kemper RA, Nabb DL, Gannon SA, Snow TA, Api AM, 2006. Comparative metabolism of geranyl nitrile and citronellyl nitrile in mouse, rat and human hepatocytes. Drug Metabolism and Disposition 34, 1019–1029. [DOI] [PubMed] [Google Scholar]
- Kilford PJ, Gertz M, Houston JB, Galetin A, 2008. Hepatocellular binding of drugs: correction for unbound fraction in hepatocyte incubations using microsomal binding or drug lipophilicity data. Drug Metab Dispos 36, 1194–1197. [DOI] [PubMed] [Google Scholar]
- Kramer NI, Di Consiglio E, Blaauboer BJ, Testai E, 2015. Biokinetics in repeated-dosing in vitro drug toxicity studies. Toxicology in Vitro 30, 217–224. [DOI] [PubMed] [Google Scholar]
- Kratochwil NA, Meille C, Fowler S, Klammers F, Ekiciler A, Molitor B, Simon S, Walter I, McGinnis C, Walther J, Leonard B, Triyatni M, Javanbakht H, Funk C, Schuler F, Lavé T, Parrott NJ 2017. Metabolic Profiling of Human Long-Term Liver Models and Hepatic Clearance Predictions from In Vitro Data Using Nonlinear Mixed-Effects Modeling. The AAPS Journal 19, 534–550. [DOI] [PubMed] [Google Scholar]
- Lee SC, Arya V, Yang X, Volpe DA, Zhang L; 2017. Evaluation of transporters in drug development: Current status and contemporary issues. Adv Drug Deliv Rev. 1, 116:100–118. [DOI] [PubMed] [Google Scholar]
- Lerche-Langrand C, Toutain HJ, 2000. Precision-cut liver slices: Characteristics and use for in vitro pharmaco-toxicology. Toxicology 153, 221–253. [DOI] [PubMed] [Google Scholar]
- Lipscomb JC, Meek ME, Krishnan K, Kedderis GL, Clewell H, Haber L, 2004. Incorporation of Pharmacokinetic and Pharmacodynamic Data into Risk Assessments. Toxicol Mech Methods. 14,145–58. [DOI] [PubMed] [Google Scholar]
- McGinnity DF, Soars MG, Urbanowicz RA, Riley RJ, 2004. Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab Dispos 32, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Menochet K, Kenworthy KE, Houston JB, Galetin A, 2012. Simultaneous assessment of uptake and metabolism in rat hepatocytes: a comprehensive mechanistic model. J Pharmacol Exp Ther 341, 2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke H, Di Consiglio E, Kreutz R, Partosch F, Testai E, Gundert-Remy U; 2017. The importance of protein binding for the in vitro-in vivo extrapolation (IVIVE) – example of ibuprofen, a highly protein bound substance. Archives of Toxicology 91, 1663–1670. [DOI] [PubMed] [Google Scholar]
- Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, Macintyre F, Rance DJ, Wastall P, 1997. The Prediction of Human Pharmacokinetic Parameters from Preclinical and In Vitro Metabolism Data. Journal of Pharmacology and Experimental Therapeutics 283, 46–58. [PubMed] [Google Scholar]
- OECD, 2014. Guidance Document for describing non-guideline in vitro test methods. [Google Scholar]
- Olinga P, Meijer DKF, Slooff MJH, Groothuis GMM, 1997. Liver slices in in vitro pharmacotoxicology with special reference to the use of human liver tissue. Toxicology in Vitro 12, 77–100. [DOI] [PubMed] [Google Scholar]
- Pal R, Mamidi MK, Das AK, Gupta PK, Bhonde R, 2012. A simple and economical route to generate functional hepatocyte-like cells from hESCs and their application in evaluating alcohol induced liver damage. J Cell Biochem 113, 19–30. [DOI] [PubMed] [Google Scholar]
- Palmgren JJ, Mönkkönen J, Korjamo T, Hassinen A, Auriola S 2006. Drug adsorption to plastic containers and retention of drugs in cultured cells under in vitro conditions. Eur J Pharm Biopharm. 64, 369–78. [DOI] [PubMed] [Google Scholar]
- Parker AJ, Houston JB, 2008. Rate-limiting steps in hepatic drug clearance: comparison of hepatocellular uptake and metabolism with microsomal metabolism of saquinavir, nelfinavir, and ritonavir. Drug Metab Dispos 36, 1375–1384. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Pasanen M, Lindon JC, Chan K, Zhao L, Deal G, Xu Q, Fan TP, 2012. Omics and its potential impact on R&D and regulation of complex herbal products. J Ethnopharmacol 140, 587–593. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Tolonen A, Rousu T, Tursas L, Turpeinen M, Hokkanen J, Uusitalo J, D’Yvoire MB, Coecke S, 2009. Comparison of metabolic stability and metabolite identification of 55 ECVAM/ICCVAM validation compounds between human and rat liver homogenates and microsomes - A preliminary analysis. Altex 26, 214–222. [DOI] [PubMed] [Google Scholar]
- Pomponio G, Savary CC, Parmentier C, Bois F, Guillouzo A, Romanelli L, Richert L, Di Consiglio E, Testai E; 2015. In vitro kinetics of amiodarone and its major metabolite in two human liver cell models after acute and repeated treatments Toxicol. In Vitro 30, 36–51. [DOI] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP, 2015. Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals. Chemical Research in Toxicology 28, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahi J, Grepper S, Smith C, 2010. Hepatocytes as a tool in drug metabolism, transport and safety evaluations in drug discovery. Current Drug Discovery Technologies 7, 188–198. [DOI] [PubMed] [Google Scholar]
- SCHER, Opinion on Addressing the New Challenges for Risk Assessment, Adopted by the SCHER by written procedure on March 2013. [Google Scholar]
- Schmelzer E, Triolo F, Turner ME, Thompson RL, Zeilinger K, Reid LM, Gridelli B, Gerlach JC, 2010. Three-dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng Part A 16, 2007–2016. [DOI] [PubMed] [Google Scholar]
- Schug M, Stöber R, Heise T, Mielke H, Gundert-Remy U, Godoy P, Reif R, Blaszkewicz M, Ellinger-Ziegelbauer H, Ahr HJ, Selinski S, Günther G, Marchan R, Blaszkewicz M, Sachinidis A, Nüssler A, Oberemm A, Hengstler JG; 2013. Pharmacokinetics explain in vivo/in vitro discrepancies of carcinogen-induced gene expression alterations in rat liver and cultivated hepatocytes. Arch Toxicol. 87, 337–45. [DOI] [PubMed] [Google Scholar]
- Sheiner LB, Beal SL, 1981. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9, 503–512. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Takahashi H, Chiba M, Ishii Y, 2002. Prediction of hepatic clearance and availability by cryopreserved human hepatocytes: an application of serum incubation method. Drug Metab Dispos 30, 892–896. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA, 2010. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Nolan CK, Edwards MA, Hatfield JB, Stewart TW, Ferguson SS, Lecluyse EL, Sahi J, 2012. A comprehensive evaluation of metabolic activity and intrinsic clearance in suspensions and monolayer cultures of cryopreserved primary human hepatocytes. J Pharm Sci 101, 3989–4002. [DOI] [PubMed] [Google Scholar]
- Soars MG, Grime K, Sproston JL, Webborn PJH, Riley RJ, 2007. Use of Hepatocytes to Assess the Contribution of Hepatic Uptake to Clearance in Vivo. Drug Metab Dispos 35, 859–865. [DOI] [PubMed] [Google Scholar]
- Sohlenius-Sternbeck AK., Afzelius L, Prusis P, Neelissen J, Hoogstraate J, Johansson J, Floby E, Bengtsson A, Gissberg O, Sternbeck J, Petersson C, 2010. Evaluation of the human prediction of clearance from hepatocyte and microsome intrinsic clearance for 52 drug compounds. Xenobiotica 40, 637–49. [DOI] [PubMed] [Google Scholar]
- Swift B, Pfeifer ND, Brouwer KLR, 2010. Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metabolism Reviews 42, 446–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman P, Lowes S, McDougall S, Colligon I, Miksic J, Chowdhury S, White S, Aubry AF, Jenkins R, Kemper C, 2015. Scientific or regulated validation: a tiered approach? Meeting report from a joint EBF/DVDMDG workshop. Bioanalysis 7, 1703–1710. [DOI] [PubMed] [Google Scholar]
- Tirelli V, Catone T, Turco L, Di Consiglio E, Testai E, De Angelis I 2007. Effects of the pesticide chlorpyriphos on an in vitro model of intestinal barrier, Toxicol. In Vitro 21, 308–313. [DOI] [PubMed] [Google Scholar]
- Truisi GL, Di Consiglio E, Parmentier C, Savary CC, Pomponio G, Bois F, Lauer B, Jossé R, Hewitt PG, Mueller SO, Richert L, Guillouzo A, Testai E; 2015. Understanding the biokinetics of ibuprofen after single and repeated treatments in rat and human in vitro liver cell systems Toxicol. Lett 233, 172–186. [DOI] [PubMed] [Google Scholar]
- Tolonen A, Pelkonen O, 2015. Analytical challenges for conducting rapid metabolism characterization for QIVIVE. Toxicology 332, 20–29. [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Tolonen A, Chesne C, Guillouzo A, Uusitalo J, Pelkonen O, 2009. Functional expression, inhibition and induction of CYP enzymes in HepaRG cells. Toxicology in Vitro 23, 748–753. [DOI] [PubMed] [Google Scholar]
- USFDA, 2013. Draft guidance for industry Bioanalytical Method Validation. [Google Scholar]
- Vickers AEM, Fisher RL, 2004. Organ slices for the evaluation of human drug toxicity. Chemico-Biological Interactions 150, 87–96. [DOI] [PubMed] [Google Scholar]
- Vinci B, Duret C, Klieber S, Gerbal-Chaloin S, Sa-Cunha A, Laporte S, Suc B, Maurel P, Ahluwalia A, Daujat-Chavanieu M, 2011. Modular bioreactor for primary human hepatocyte culture: Medium flow stimulates expression and activity of detoxification genes. Biotechnology Journal 6, 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorrink SU, Ullah S, Schmidt S, Nandania J, Velagapudi V, Beck O, Ingelman-Sundberg M, Lauschke VM; 2017. Endogenous and xenobiotic metabolic stability of primary human hepatocytes in long-term 3D spheroid cultures revealed by a combination of targeted and untargeted metabolomics. FASEB J. 31, 2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS, 2012. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci 125, 157–174. [DOI] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW, Houck KA, Strope CL, Cantwell K, Judson RS, LeCluyse E, Clewell HJ, Thomas RS, Andersen ME 2015. Incorporating High-Throughput Exposure Predictions With Dosimetry-Adjusted In Vitro Bioactivity to Inform Chemical Toxicity Testing. Toxicol. Sci 148, :121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk-Zasadna I, Bernasconi C, Pelkonen O, Coecke S, 2015. Biotransformation in vitro: An essential consideration in the quantitative in vitro-to-in vivo extrapolation (QIVIVE) of toxicity data. Toxicology 332, 8–19. [DOI] [PubMed] [Google Scholar]
- Wood FL, Houston JB, Hallifax D 2017. Clearance Prediction Methodology Needs Fundamental Improvement: Trends Common to Rat and Human Hepatocytes/Microsomes and Implications for Experimental Methodology. Drug Metab Dispos 45, 1178–1188. [DOI] [PubMed] [Google Scholar]
- Worboys PD, Bradbury A, Houston JB, 1997. Kinetics of drug metabolism in rat liver slices. III. Relationship between metabolic clearance and slice uptake rate. Drug Metab Dispos 25, 460–467. [PubMed] [Google Scholar]
- Yamagata T, Zanelli U, Gallemann D, Perrin D, Dolgos H, Petersson C, 2017. Comparison of methods for the prediction of human clearance from hepatocyte intrinsic clearance for a set of reference compounds and an external evaluation set. Xenobiotica 47, 741–751. [DOI] [PubMed] [Google Scholar]
- Zanelli U, Caradonna NP, Hallifax D, Turlizzi E, Houston JB, 2012. Comparison of cryopreserved HepaRG cells with cryopreserved human hepatocytes for prediction of clearance for 26 drugs. Drug Metab Dispos 40, 104–110. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Turpeinen M, Klein K, Schwab M, 2008. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem 392, 1093–1108. [DOI] [PubMed] [Google Scholar]
- Zeilinger K, Schreiter T, Darnell M, Soderdahl T, Lubberstedt M, Dillner B, Knobeloch D, Nussler AK, Gerlach JC, Andersson TB, 2011. Scaling down of a clinical three-dimensional perfusion multicompartment hollow fiber liver bioreactor developed for extracorporeal liver support to an analytical scale device useful for hepatic pharmacological in vitro studies. Tissue Eng Part C Methods 17, 549–556. [DOI] [PubMed] [Google Scholar]