Significance
Dysbiosis underlies multiple diseases and can be caused by Paneth cell defects, as observed in obese or IBD patients. Since Paneth cells have microbe regulation properties, it is currently believed that their alterations directly cause dysbiosis. The main conceptual advance here is the demonstration that intestinal tuft cells play an essential role in establishing dysbiosis via a cross talk with altered Paneth cells. We further identify bidirectional interactions between microbiota composition and the host inflammation status, which is controlled by tuft and Paneth epithelial cells. Our findings open perspectives for combinatorial therapies targeting both host and microbiota for a more effective management of dysbiosis-associated chronic diseases.
Keywords: microbiota dysbiosis, gut inflammation, tuft cells, Paneth cells, antimicrobial peptides
Abstract
Gut microbiota imbalance (dysbiosis) is increasingly associated with pathological conditions, both within and outside the gastrointestinal tract. Intestinal Paneth cells are considered to be guardians of the gut microbiota, but the events linking Paneth cell dysfunction with dysbiosis remain unclear. We report a three-step mechanism for dysbiosis initiation. Initial alterations in Paneth cells, as frequently observed in obese and inflammatorybowel diseases patients, cause a mild remodeling of microbiota, with amplification of succinate-producing species. SucnR1-dependent activation of epithelial tuft cells triggers a type 2 immune response that, in turn, aggravates the Paneth cell defaults, promoting dysbiosis and chronic inflammation. We thus reveal a function of tuft cells in promoting dysbiosis following Paneth cell deficiency and an unappreciated essential role of Paneth cells in maintaining a balanced microbiota to prevent inappropriate activation of tuft cells and deleterious dysbiosis. This succinate–tuft cell inflammation circuit may also contribute to the chronic dysbiosis observed in patients.
Alterations in the symbiosis between our organism and the gut microbiota constitute a critical parameter in the occurrence and severity of many diseases. The gut microbiota participates to the intestinal epithelial barrier and protection against pathogens by actively regulating multiple host physiological processes such as metabolism, circadian rhythmicity, digestion, and immune responses (1). Importantly, the gut microbiota, which is established following delivery and breastfeeding, is highly dynamic. Throughout life, its composition varies with environmental and physiological factors such as nutrition, hormonal changes, lifestyle, and aging, among others (1). Perturbation of the microbiota composition and function, a state known as dysbiosis, impacts host homeostasis through altered host–microbiota interactions. Dysbiosis is notably associated with gastrointestinal tract diseases including inflammatory bowel diseases (IBD) and irritable bowel syndrome, as well as with extra-gastrointestinal tract diseases ranging from metabolic syndrome, allergy, and asthma to psychiatric disorders (2–4). In many instances, dysbiosis not only accompanies the pathological state but is sufficient to recapitulate the pathology in previously healthy individuals, as demonstrated by microbiota transfer experiments in preclinical models (2, 5). Importantly, dysbiosis can be caused by both exogeneous factors such as high-fat diet (6) or by endogenous changes such as intestinal epithelial Paneth cell defects (7).
Epithelial Paneth cells were first identified as a critical component of the gut innate immunity involved in the defense against microorganisms (8, 9). Paneth cell differentiation depends on the Wnt signaling pathway (10) and one of its targets, the Sox9 transcription factor (11). Indeed, inactivation of Sox9 in intestinal cells during mouse embryogenesis leads to the absence of Paneth cells and causes hyperplastic crypts with increased cell proliferation (12, 13). Interestingly, Paneth cells are dysfunctional in Crohn’s disease patients and in IBD murine models (14, 15); Paneth cell granules containing antimicrobial peptides display aberrant morphology and reduced secretion, and these alterations are linked to the establishment of dysbiosis (7). Paneth cell function is also altered in obese subjects, with reduced levels of antimicrobial peptides and features of ER stress (16), and rodents fed with a high-fat diet display dysfunctional Paneth cells and associated dysbiosis (17). Deletion of genes encoding defensins (18) or lysozyme (19) further demonstrates the key role of Paneth cells as guardians of the gut microbiota integrity, with defective function serving as a driver of dysbiosis and inflammation. However, despite the tremendous impact of dysbiosis and inflammation on a multitude of diseases, the physiological mechanisms linking these processes to altered Paneth cell function remain to be investigated to potentially provide new therapeutic opportunities to prevent or reverse dysbiosis.
Tuft cells have recently been revealed as key epithelial sentinels that initiate type 2 mucosal immunity following infections with parasites (20–22) or bacteria (23). Upon helminth infections, tuft cells respond by secreting the IL-25 alarmin cytokine, which in turn activates hematopoietic cells in the gut lamina propria, including type 2 innate lymphoid cells (ILC2s). ILC2s produce type 2 cytokines, notably IL-13, which serve several functions, including remodeling of the intestinal epithelium with tuft and goblet cell hyperplasia and eventual parasite expulsion. Mechanistically, tuft cell activation and immunomodulatory action, following parasite infection or artificial dysbiosis caused by polyethylene glycol (PEG) treatment, are dependent on the activation of several receptors including the SucnR1 succinate receptor (24–26), Tas2R taste receptors (27), and the olfactory receptor Vmn2r26 (23). Interestingly, mice with lysozyme-deficient Paneth cells exhibit an altered microbiota concomitant with a type 2 immune response including expansion of tuft cells (19). In addition, tuft cell numbers are strongly decreased in ileal lesions from Crohn’s disease patients and in murine models of IBD (28), and an artificial increase in tuft cell numbers in genetically engineered IBD mouse models improves their inflammatory status (28). Altogether, these results suggest potential cooperation of Paneth and tuft cells to regulate gut microbiota homeostasis and dysbiosis as well as the status of gut mucosa inflammation.
Here, we investigated the hypothesis that a cross talk between Paneth cells and tuft cells drives dysbiosis initiation. Using a murine model with dysfunctional Paneth cells, we show that dysbiosis develops, resulting in a type 2–dominated gut inflammation. Importantly, we found that a cross talk between Paneth cells and chemosensory tuft cells is required to establish dysbiosis. The absence of tuft cells causes a dramatic reduction in inflammation and dysbiosis, even in the context of Paneth cell defects. Mechanistically, we identify a critical role for the SucnR1 tuft cell receptor to link Paneth cell defects with tuft cell activation, a process that occurs independently of the Trpm5 signaling pathway. We thus describe a “Paneth cell–succinate–tuft cell–immune system” circuit that drives dysbiosis, with relevance for IBD and other conditions involving altered Paneth cells.
Results
Maintenance of Intestinal Paneth Cell Function Requires the Sox9 Transcription Factor.
Deletion of the Paneth cell transcription factor Sox9 (11) in the intestinal epithelium during embryonic development (Sox9LoxP/LoxP;Villin-Cre) causes a complete absence of Paneth cells as assessed by expression of lysozyme, a Paneth cell marker (12, 13). We first examined the consequences of Sox9 deletion, induced in the adult intestinal epithelium, on Paneth cell generation. In adult Sox9LoxP/LoxP;Villin-CreERT2 mice, the Sox9 protein became undetectable as early as 3 d after tamoxifen treatment (SI Appendix, Fig. S1A). However, lysozyme-expressing epithelial cells were still detectable at the base of intestinal crypts 2 wk after tamoxifen treatment (Fig. 1A). Since the life span of adult mouse Paneth cells is approximately 2 mo (29), most of these lysozyme-expressing cells were present prior to Sox9 deletion, indicating that Sox9 is not required for the survival of previously differentiated Paneth cells. In addition, lysozyme-expressing Paneth cells were still present several months after Sox9 deletion (SI Appendix, Fig. S1A). BrdU incorporation studies were performed to determine whether these cells were able to differentiate from Sox9-deleted stem/precursor cells. Equivalent percentages of BrdU+/lysozyme+ Paneth cells were detected in Sox9-deficient mice and their littermate controls, accounting for approximately 35% of all Paneth cells after 3 wk of BrdU treatment. Thus, new Paneth cells were generated at similar rates in both mice genotypes (SI Appendix, Fig. S1B). However, there was a significantly higher proportion of mislocalized Paneth cells in the upper crypt and villus compartments in Sox9-deficient mice (15%) as compared to littermate controls (2%), likely explaining the lower number of Paneth cells at the crypt base in the Sox9-deficient mice (SI Appendix, Fig. S1 C and D).
Fig. 1.

Maintenance of intestinal Paneth cell terminal differentiation requires the Sox9 transcription factor. (A) Representative immunofluorescence costaining of Lyz and β-catenin showing the presence of Lyz+ Paneth cells 2 wk after tamoxifen-induced Sox9 deletion in adult epithelial cells (Sox9LoxP/LoxP;Villin-CreERT2 mice). Delocalized Paneth cells are indicated with a white arrowhead. Images are representative of 3 mice per genotype, 3 independent experiments. (B) Representative immunofluorescence costaining of Sox9, Lyz and β-catenin showing deletion of Sox9 in stem cells, Paneth cells and progenitors in Sox9LoxP/LoxP;Villin-CreERT2 mice (white arrowhead) 2 wk following tamoxifen treatment. Sox9 expression is maintained in Paneth cells (yellow arrowhead) in Sox9LoxP/LoxP;P450a1-Cre mice (3 mice per genotype), 3 independent experiments. (C) Electron micrographs from small intestine highlighting physiological secretory granules in Paneth cells (arrows) in control Sox9LoxP/LoxP mice (n = 4), whereas secretory granules in Sox9-deficient Paneth cells from Sox9LoxP/LoxP;Villin-CreERT2 mice (n = 5, >30 d post tamoxifen treatment) are less electron dense and exhibit heterogeneity in size, shape, and number. In contrast, secretory granules exhibit normal structure in mice with a Sox9 deletion in all epithelial cells except for Paneth cells (Sox9LoxP/LoxP;P450a1-Cre; n = 2, 1 wk post-βNF treatment). (D) Representative immunofluorescence costaining of Muc2, Lyz, and β-catenin in Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice (n = 3 per genotype, 2 wk following tamoxifen treatment). Muc2 expression in Paneth cells was only detected following Sox9 deletion in all epithelial cells (white arrowheads). (E) Expression of Paneth cell markers in Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 small intestine was evaluated by RT-qPCR analysis in Sox9LoxP/LoxP;Villin-CreERT2 and Sox9LoxP/LoxP mice (n = 4 and n = 5, respectively; 2 wk after tamoxifen treatment). Data are shown as a ratio of expression relative to Sox9LoxP/LoxP mice. [Scale bars: 20 µm (A, B, and D), 2 µm (C).] (E) Mann–Whitney U test; *P < 0.05; **P < 0.01. See also SI Appendix, Fig. S1.
The cellular morphology of Sox9-deficient Paneth cells was also altered; lysozyme-containing secretion granules were more diffuse and heterogeneous in Sox9-deficient as compared to wild-type Paneth cells (Fig. 1B). In line with these data, electron microscopy analyses from 3 d to 4 mo following Sox9 deletion revealed a dramatically reduced size of electron-dense secretory granules, embedded within an electron-lucent structure suggestive of mucus, a substance not normally produced by Paneth cells (Fig. 1C). Importantly, these alterations in secretion granule morphology were not detected in Sox9LoxP/LoxP;P450a1-Cre mice (Fig. 1 B and C), in which genetic recombination occurs in all intestinal epithelial cells except Paneth cells (29), 1 wk following treatment with β-naphtoflavone. However, as expected, a proportion of Paneth cells from Sox9LoxP/LoxP;P450a1-Cre mice also displayed abnormal granules 1 mo after Sox9 deletion (SI Appendix, Fig. S1E), due to Paneth cell population turnover and replacement by new, altered, Paneth cells generated from Sox9-deleted epithelial stem cells. These data indicate that maintenance of mature Paneth cells requires Sox9 in a cell-autonomous manner (Fig. 1B).
As intestinal goblet cells express Muc2, the main constituent of the small intestinal mucus, and their differentiation requires the Klf4 transcription factor (30), we next assessed whether Sox9-deficient Paneth cells harbor an intermediate phenotype with mixed features of Paneth and goblet cells (31). Indeed, Sox9-deficient Paneth cells expressed significant levels of Muc2 and Klf4, which were not detected in normal Paneth cells from control mice (Fig. 1D and SI Appendix, Fig. S1F). Moreover, since Paneth cell granules contain antimicrobial peptides, we hypothesized that their altered morphology in Sox9-deficient mice would be associated with altered production of these peptides. Indeed, the presence of altered Paneth cells in Sox9LoxP/LoxP;Villin-CreERT2 mice was associated with a strongly decreased expression of critical antimicrobial peptides such as lysozyme, defensin α29, Reg3β and Reg3γ, as well as the Mmp7 (matrixmetallopeptidase 7) matrix metallopeptidase, which is involved in the activation of defensins (32) (Fig. 1E).
Thus, Sox9 deletion in adult mice does not impact the survival of Paneth cells nor their early differentiation. Notably, though, Sox9-deficient Paneth cells have a strongly modified phenotype, as shown by the altered morphology of lysozyme+ secretion granules as well as Muc2 and Klf4 expression, and moreover, they are frequently mislocalized throughout the crypt and villus compartments. Sox9LoxP/LoxP;Villin-CreERT2 mice therefore constitute a relevant model to study the consequences of Paneth cell alterations in adult intestinal physiopathology.
Abnormal Paneth Cells Are Associated with Intestinal Dysbiosis and Disruption of the Intestinal Mucosal Barrier.
An unbalanced microbiota and defects in intestinal permeability are frequent features of gut diseases. As antimicrobial peptides are essential effectors of host–microbiota interactions (33) and their levels are strongly reduced in Sox9LoxP/LoxP;Villin-CreERT2 mice, we next evaluated whether the presence of Sox9-deficient Paneth cells results in impaired microbiota regulation. Bacterial 16S profiling of feces from Sox9LoxP/LoxP;Villin-CreERT2 mice and Sox9LoxP/LoxP control mice revealed a significant shift in intestinal microbial populations 1 mo following Sox9 deletion. While the global bacterial load was unaffected, the beta diversity of microbial communities differed significantly between Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice (Fig. 2A).
Fig. 2.
Alterations of gut microbiota and intestinal barrier are associated with abnormal Paneth cells. (A) β diversity in microbial communities from Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice (n = 8 mice per genotype). Unweighted principal component analyses (PCA) of UniFrac distance after 16S rRNA sequencing of fecal microbial populations are presented. (B) Intestinal permeability, assessed by oral gavage of FITC-dextran, was evaluated in control mice (Sox9LoxP/LoxP 2 wk post tamoxifen treatment, n = 7), following deletion of Sox9 in all epithelial cells (Sox9LoxP/LoxP;Villin-CreERT2 mice; 2 wk post tamoxifen treatment, n = 7) or under conditions where Sox9 expression was maintained in Paneth cells (Sox9LoxP/LoxP;P450a1-Cre mice; 2 wk post βNF treatment, n = 3). (B) Kruskal–Wallis test with Dunn's post hoc test; NS: not significant; *P < 0.05. The line indicates the median, the box marks the 25th and 75th percentiles, and the whiskers indicate the minimal to maximum values.
We then investigated intestinal epithelial permeability in control and Sox9LoxP/LoxP;Villin-CreERT2 mice. After gavage with fluorescein-5-isothiocyanate (FITC)-dextran, Sox9LoxP/LoxP;Villin-CreERT2 mice exhibited a significantly increased translocation of FITC-dextran from the gastrointestinal tract into the circulation as compared to littermate Sox9LoxP/LoxP controls. In contrast, no increase in epithelial permeability was observed in Sox9LoxP/LoxP;P450a1-Cre mice, indicating that Sox9 deletion in Paneth cells, but not in other epithelial cell lineages, underlies increased paracellular permeability in Sox9LoxP/LoxP;Villin-CreERT2 mice (Fig. 2B). Together, these results strongly suggest that altered Paneth cell morphology and antimicrobial peptide production hamper their function, causing an imbalance in the microbiota, and a disruption of intestinal barrier integrity.
Changes in Mucosal Immunity due to Dysfunctional Paneth Cells Are Dependent on IL-4/IL-13 Signaling and Presence of a Microbiota.
The intestinal dysbiosis-linked increase in epithelial permeability facilitates access of microbes and their products to lamina propria immune cells and downstream inflammation (34–36). We therefore sought to determine the inflammatory state in control and Sox9LoxP/LoxP;Villin-CreERT2 mice. To assess whether altered Paneth cell function and impaired microbiota regulation were associated with changes in intestinal immunity, we profiled expression of key components of type 1 (Tbx21, Ifnγ, and Tnfα), type 2 (Gata3, Il4, Il13, Il25, and Il33), and type 17 (Rorc, Il17a, Il21, and Il22) immune responses. Comparisons of control Sox9LoxP/LoxP mice and Sox9LoxP/LoxP;Villin-CreERT2 mice revealed significant increases in several components of type 2 immune responses including expression of Il4, Il13, and Il25 cytokine genes in the latter mice. In contrast, expression of genes associated with type 1 and type 17 responses was not elevated in Sox9LoxP/LoxP;Villin-CreERT2 mice, with significant decreases in Tnf, Rorc and Il22 genes (Fig. 3A). These data suggest unbalanced immune cell populations in mice with Sox9-deficient Paneth cells, with a shift toward type 2 immune cell populations.
Fig. 3.
Presence of a type 2 immune response in mice with abnormal Paneth cells. (A) Interleukins and transcription factors associated with type 1, 2, and 17 immunity were evaluated in Sox9LoxP/LoxP (n = 4) and Sox9LoxP/LoxP;Villin-CreERT2 (n = 5) small intestine by RT-qPCR analysis. Data are shown as a ratio relative to expression in Sox9LoxP/LoxP mice. (B) Representative immunostainings of Mbp, a marker of eosinophilia, and Gata3, a marker of Th2 lymphocytes and ILC2, in Sox9-deficient lamina propria in the small intestinal epithelium of Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice. n = 3 mice per genotype, killed 2 wk after tamoxifen treatment. (C) Quantification of MBP and Gata3+ cells in Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice. Cells positive for MBP or Gata3 were counted in n = 50 crypt-villus units per mouse (n = 3 mice per genotype). (D) Representative immunostainings illustrating tissue remodeling in the small intestinal epithelium from Sox9LoxP/LoxP;Villin-CreERT2 mice. Dclk1 and PAS stainings, respectively, show tuft cell and goblet hyperplasia in Sox9-deficient small intestine. Retnlβ staining reveals the production of Retnlβ molecule by goblet cells, which are costained with Alcian blue. n = 3 mice per genotype, killed 2 wk after tamoxifen treatment. (E) Quantification of type 2 immune response markers in Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice. Cells positive for Dclk1, PAS, Retnlβ Gata3, or Alcian blue were counted in n = 50 crypt-villus units per mouse (n = 3 mice per genotype). Mice were killed 2 wk after tamoxifen treatment. [Scale bars: 20 µm (B and D).] (A, C, and E) Mann–Whitney U test. *P < 0.05; **P < 0.01; ***P < 0.001 (A, C, and E) Data are shown as mean ± SEM. See also SI Appendix, Fig. S2.
A detailed immunohistochemical analysis of the lamina propria of Sox9LoxP/LoxP;Villin-CreERT2 mice revealed higher densities of major basic protein (MBP)-expressing eosinophils and Gata3+ immune cells (Fig. 3 B and C). The Gata3 transcription factor is involved in the differentiation of both CD3+ Th2 lymphocytes and CD3− type 2 ILC2s (37). Costaining of Gata3 and CD3, used to discriminate Th2 and ILC2 subsets (20), demonstrated a predominantly increased ILC2 population in the lamina propria of Sox9LoxP/LoxP;Villin-CreERT2 mice as compared to Sox9LoxP/LoxP mice (P < 0.0001), with an unchanged Th2 population (SI Appendix, Fig. S2 A and B). Thus, the presence of altered Paneth cell function is linked to the presence of a type 2–dominated inflammation, concomitant with an increased population of Gata3+CD3− immune cells, likely representing ILC2s.
The epithelial compartment also displayed features of a type 2 immune response (20), with significant increases in Dclk1+ tuft cells and periodic acid Schiff (PAS)-stained goblet cells (Fig. 3 D and E). Moreover, expression of the resistin-like beta peptide (Retnlβ), an immune effector peptide produced by small intestinal goblet cells during type 2 immune responses (38), was detected in mice with Sox9-deleted Paneth cells but not in control mice (Fig. 3 D and E). Paneth cell dysfunction was central to these type 2 immune response features as they were not present in Sox9LoxP/LoxP;P450a1-Cre mice (SI Appendix, Fig. S2C). To investigate the link between altered Paneth cells and the type 2 inflammation observed in Sox9LoxP/LoxP;Villin-CreERT2 mice, we crossed these mice with Il-4Rα-deficient mice, in which both the IL-4 and interleukin-13 (IL-13) dimeric receptors are nonfunctional (39). In Sox9LoxP/LoxP;Villin-CreERT2;Il-4Rα−/− mice, a type 2 response was initiated, as shown by the significantly higher number of Gata3+ cells in the lamina propria as compared to Sox9 control mice (SI Appendix, Fig. S2 D and E; P < 0.0001). However, in the absence of IL-4/IL-13 signaling-mediated epithelial remodeling, the Sox9LoxP/LoxP;Villin-CreERT2;Il-4Rα−/− mice did not exhibit hyperplasia of Dclk1+ tuft cells nor goblet cells (Alcian blue staining). Furthermore, Retnlβ expression by goblet cells was not detected (SI Appendix, Fig. S2D), and epithelial paracellular permeability was not elevated in Sox9LoxP/LoxP;Villin-CreERT2;Il-4Rα−/− mice (SI Appendix, Fig. S2F). Thus, the decreased antimicrobial control, dysbiosis, increased transepithelial permeability, and type 2 inflammation observed in mice with Sox9-deficent Paneth cells are dependent on an intact IL-4/IL-13 signaling axis.
We next investigated whether the link between altered Paneth cells and transepithelial permeability and inflammation was directly dependent on the microbiota. Wide-spectrum antibiotic treatment concomitant with Sox9 deletion did not change the differentiation of altered Paneth cells in Sox9LoxP/LoxP;Villin-CreERT2 mice; they maintained small and diffuse secretion granules and exhibited Muc2 and Klf4 expression (SI Appendix, Fig. S3 A and B). However, in sharp contrast with the increased transepithelial permeability observed in non-antibiotic-treated Sox9LoxP/LoxP;Villin-CreERT2 mice (Fig. 2B), permeability was not elevated in antibiotic-treated Sox9LoxP/LoxP;Villin-CreERT2 mice as compared to Sox9LoxP/LoxP mice (SI Appendix, Fig. S3C). Furthermore, no evidence of type 2 inflammation was found in antibiotic-treated Sox9LoxP/LoxP;Villin-CreERT2 mice, with tuft cell, goblet cell, MBP+ eosinophil, Gata3+ Th2 and ILC2 cell numbers, as well as Retnlβ expression indiscernible from those detected in Sox9LoxP/LoxP control animals (SI Appendix, Fig. S3 D and E). These data establish the microbiota milieu as a required link in the altered epithelial permeability and inflammation caused by abnormal Paneth cells.
Tuft Cells Mediate the Effects of Altered Paneth Cells on Type 2 Inflammation and Transepithelial Permeability.
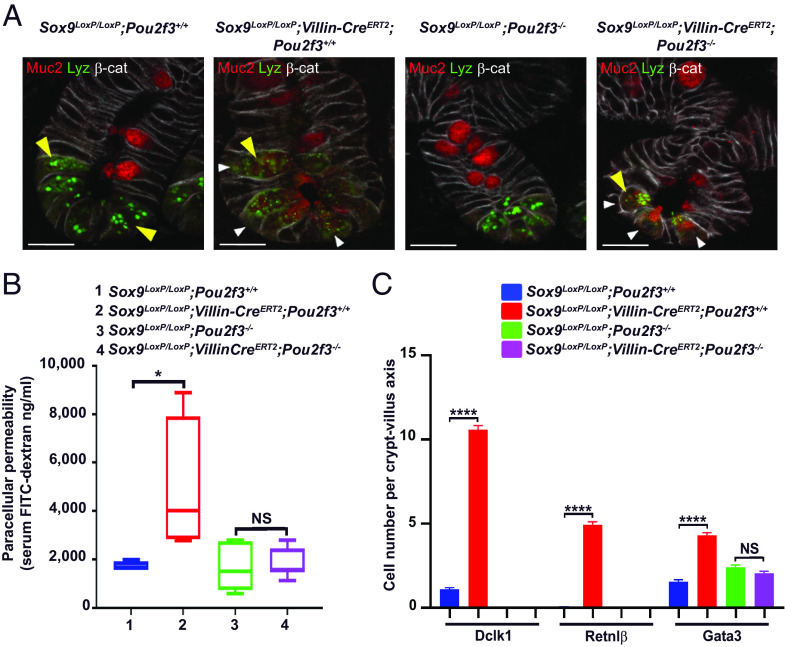
In addition to the microbiota milieu, we and others have shown that intestinal epithelial tuft cells play an integral part in the initiation of a type 2 immune response in response to the presence of parasites in the gut lumen by secreting the IL-25 alarmin cytokine (20–22). However, the role of tuft cells in initiating responses in the context of a physiological dysbiosis remain unknown. To evaluate whether intestinal tuft cells are involved in the Paneth cell–dysbiosis–type 2 inflammation axis, tuft cells were eliminated by knockout of the Pou2f3 transcription factor (20). Specifically, Sox9LoxP/LoxP;Villin-CreERT2 mice were crossed with the Pou2f3−/− line to generate Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− compound mice. Following Sox9 deletion in Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− compound mice, Paneth cells displayed the same abnormal morphology that was detected in Sox9LoxP/LoxP;Villin-CreERT2 mice with diffuse lysozyme staining as well as Muc2 and Klf4 expression (Fig. 4A and SI Appendix, Fig. S4A). Strikingly, though, Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice exhibited no increase in transepithelial permeability (Fig. 4B), and their type 2 inflammation parameters were indiscernible from those of Sox9LoxP/LoxP control mice (Fig. 4C and SI Appendix, Fig. S4B). These data reveal an essential role for tuft cells in linking the dysbiosis resulting from altered Paneth cells with the induction of a type 2 inflammatory response. Thus, our data identify a Paneth cell–microbiota–tuft cell–immune system circuit that regulates gut homeostasis.
Fig. 4.
The presence of tuft cells is required for the development of type 2 immunity and impaired intestinal permeability caused by altered Paneth cells. (A) Representative immunofluorescence costaining of Muc2, Lyz, and β-catenin in Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice. Paneth cells lacking Sox9 in a tuft cell-deficient context display an altered differentiation state since Muc2 is present in Paneth cells in Sox9LoxP/LoxP;Villin-CreERT2 as well as Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice (white arrowhead), and Lyz staining is diffuse in both genotypes compared to control mice (yellow arrowhead). n = 3 mice per genotype, representative of 2 independent experiments. Mice were killed 2 wk after tamoxifen treatment. (B) Intestinal permeability, assessed by FITC-dextran gavage, remains intact when Sox9 is deleted in a tuft cell-deficient mouse model. FITC–dextran was measured in serum from Sox9LoxP/LoxP;Pou2f3+/+ mice (n = 4), Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3+/+mice (n = 4), Sox9LoxP/LoxP;Pou2f3−/− mice (n = 6), and Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice (n = 7). Mice were killed 2 wk after tamoxifen treatment. (C) The type 2 immune response found in Sox9LoxP/LoxP;Villin-CreERT2 mice is abolished in tuft cell-deficient Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice as compared to Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3+/+ mice. Cells positive for Dclk1, Retnlβ or Gata3 were counted in n = 50 crypt-villus units per mouse (n = 5 to 6 mice per genotype). [Scale bars, 20 µm (A).] (B and C) Kruskal–Wallis test with Dunn's post hoc test; NS: not significant; *P < 0.05; ****P < 0.0001. (B) The line indicates the median, the box marks the 25th and 75th percentiles, and the whiskers indicate the minimal to maximum values. (C) Data are shown as mean ± SEM. See also SI Appendix, Fig. S4.
A Paneth–Tuft Cell Cross Talk Underlies the Dysbiosis Occurring following Paneth Cell Dysfunction.
To further investigate the impact of tuft cells on the gut microbial populations in the context of Paneth cell deficiency, we performed shotgun metagenomic sequencing on cecum samples from Sox9LoxP/LoxP, Sox9LoxP/LoxP;Villin-CreERT2, Pou2f3−/−, and Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice. As the cecum microbiota is more relevant to the ileum microbiota than the fecal one, we focused on cecum samples. Bacterial gene richness, which reflects diversity of microbiota bacterial species, was significantly decreased in Sox9LoxP/LoxP;Villin-CreERT2 mice as compared to control Sox9LoxP/LoxP mice (P = 0.007), thus confirming our previous 16S analyses (Fig. 2A). Furthermore, no significant differences were observed between Pou2f3−/− mice as compared to Sox9LoxP/LoxP control mice (P > 0.05). Importantly though, gene richness remained high in Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice that have altered Paneth cells in the absence of tuft cells, at levels comparable to those detected in Sox9LoxP/LoxP mice (Fig. 5A).
Fig. 5.
Increased succinate-producers in the remodeled microbiota of mice with altered Paneth cells lead to tuft cell activation via a succinate–SucnR1 pathway. (A) Gene richness in cecum from Sox9LoxP/LoxP;Pou2f3+/+Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3+/+Sox9LoxP/LoxP;Pou2f3−/−, and Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice. n = 8 mice per genotype. Mice were killed 4 wk after tamoxifen treatment. (B) Schema of the succinate biosynthetic pathway predominant under anaerobic conditions. The two final steps of the succinate production are shown: i) the conversion of malate to fumarate by the fumarate hydratase encoded by the fumABC genes and ii) the transformation of fumarate to succinate by the fumarate reductase encoded by the frdABCD genes. For each enzymatic reaction, all KO (KEGG Ortholog) alternatives (v1 to v3) found in the KEGG database are shown. Color codes refer to their presence (black) or absence (gray) in the mouse BGI 2.6 million genes catalog. (C) The global completeness of the succinate production pathway is significantly increased in Sox9LoxP/LoxP;Villin-CreERT2 mice compared to Sox9LoxP/LoxP mice. The global completeness for succinate production, i.e., the proportion of KOs detected necessary to produce succinate in each microbial species, was computed for each differentially abundant MSP species found in Sox9LoxP/LoxP (blue) and Sox9LoxP/LoxP;Villin-CreERT2 mice (red). (D) Intestinal permeability, assessed by FITC–dextran gavage, remains intact when Sox9 is deleted in SucnR1-deficient mice. FITC-dextran was measured in serum from Sox9LoxP/LoxP;SucnR1+/+ mice (n = 5), Sox9LoxP/LoxP;Villin-CreERT2;SucnR1+/+mice (n = 5), Sox9LoxP/LoxP;SucnR1−/− mice (n = 5), and Sox9LoxP/LoxP;Villin-CreERT2;SucnR1−/− mice (n = 6). Mice were killed 2 wk after tamoxifen treatment. (E) Type 2 immune response is abolished in the absence of SucnR1. Cells positive for Dclk1, Retnlβ or Gata3 were counted in n = 50 crypt-villus units per mouse (n = 5 to 6 mice per genotype). (A) Unpaired Wilcoxon rank-sum test. (C) Unpaired Wilcoxon signed-rank test, P = 0.017. (D and E) Kruskal–Wallis test with Dunn's post hoc test; NS: not significant; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (A, C, and D) The line indicates the median, the box marks the 25th and 75th percentiles, and the whiskers indicate the minimal to maximum values. (E) Data are shown as mean ± SEM. See also SI Appendix, Figs. S5 and S6.
Differential abundance analyses of metagenomic species pangenome (MSP) microbial species between the different mouse groups followed the same trend. After adjustment for multiple testing and using a q-value threshold of 0.05, 40 MSP microbial species were identified as exhibiting differential abundance between Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice. While some of the differentially represented MSP microbial species could not be taxonomically assigned at the species level, we detected an enrichment of MSP microbial species of the Bacteroidales order in Sox9LoxP/LoxP;Villin-CreERT2 mice, while MSP microbial species belonging to the Clostridiales order were preferentially enriched in Sox9LoxP/LoxP mice (SI Appendix, Fig. S5A). Marked alterations in the cecum microbiota were also found upon comparison of Sox9LoxP/LoxP;Villin-CreERT2 and Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice, with 57 differentially abundant MSP species (q value < 0.05); there was a similar trend of enrichment of members of the Bacteroidales in the Sox9LoxP/LoxP;Villin-CreERT2 group (SI Appendix, Fig. S5B). No differences were detected between Sox9LoxP/LoxP and Pou2f3−/− mice, nor between wild-type and Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice, as assessed by the q-value threshold. Taken together, these data indicate that microbiota alterations caused by Paneth cell alterations are markedly reduced in the absence of tuft cells and downstream type 2 inflammation. Thus, tuft cell–dependent type 2 inflammation enhances the initial effects of Paneth cell dysfunction to promote dysbiosis.
Tuft Cell Activation Relies on a Trpm5-Independent Induction of the Succinate–SucnR1 Axis.
Our results demonstrate that dysbiosis establishment requires a cross talk between Paneth and tuft cells. We were therefore interested in identifying the molecular mechanism underlying this cross talk, and specifically, to understand how the remodeled microbiota, caused by abnormal Paneth cells, activates tuft cells. Metagenomic quantitative analysis of microbiota revealed an enrichment of MSP microbial species of the Bacteroidales order in Sox9LoxP/LoxP;Villin-CreERT2 mice as compared to Sox9LoxP/LoxP mice (SI Appendix, Fig. S5 A and B). Furthermore, analyses of differentially abundant gut metabolic modules (GMM) between Sox9LoxP/LoxP;Villin-CreERT2 and Sox9LoxP/LoxP mice revealed 30 GMM enriched in the Sox9LoxP/LoxP;Villin-CreERT2 mice with a q value < 0.05 (SI Appendix, Table S1). Among the metabolic pathways enriched in Sox9LoxP/LoxP;Villin-CreERT2 mice as compared to Sox9LoxP/LoxP mice, succinate production (P value < 0.001; q value < 0.005) drew our attention. Indeed, succinate produced by parasites has the potential to activate tuft cells via SucnR1, specifically expressed on tuft cells among intestinal epithelial cells (25, 26), thus constituting an attractive candidate to link dysbiosis with tuft cell activation. Succinate–SucnR1 signaling triggers a type 2 immune response (25, 26) which can also be recapitulated by treating mice with PEG and streptomycin, described to increase succinate-producing microbiota (24). Importantly, bacteria from the Bacteroidales order, which includes the Prevotella genus, were enriched in our metagenomic analyses (SI Appendix, Fig. S5A) and produce succinate (40). To investigate the potential role of succinate, we first assessed the potential for succinate production of MSP microbial species with differential representation in Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 mice (SI Appendix, Fig. S5A) by a quantitative analysis of the genes involved in the two final steps performed under anaerobic conditions (malate->fumarate, fumarate->succinate) (Fig. 5B and SI Appendix). Global completeness of the final steps for succinate production, i.e., the proportion of detected kyoto encyclopedia of genes and genomes (KEGG) Orthologs (KOs) that are necessary to produce succinate in each microbial species, was significantly higher in the MSP species enriched in Sox9LoxP/LoxP;Villin-CreERT2 mice (mean ± SD = 39% ± 24%), as compared to the MSP species enriched in Sox9LoxP/LoxP mice (mean ± SD = 22% ± 20%; P = 0.017; Fig. 5C). Thus, microbiota from Sox9LoxP/LoxP;Villin-CreERT2 mice exhibit a higher functional potential for succinate production as compared to that of control mice.
To determine whether succinate plays a functional role in activating tuft cells in mice with Sox9-deficient Paneth cells, we generated compound Sox9LoxP/LoxP;Villin-CreERT2;SucnR1−/− mice. Notably, both transepithelial permeability and markers of type 2 immunity were low in Sox9LoxP/LoxP;SucnR1+/+, Sox9LoxP/LoxP;SucnR1−/−, and Sox9LoxP/LoxP;Villin-CreERT2;SucnR1−/− mice, as compared to Sox9LoxP/LoxP;Villin-CreERT2 mice (Fig. 5 D and E). These data demonstrate the essential role of SucnR1 signaling in the initiation of tuft cell–dependent type 2 inflammation, even in the context of Paneth cell alterations with decreased lysozyme, Mmp7 and Defa29 (defensinalpha 29) expression, and concomitant microbiota changes.
In addition to the importance of SucnR1 in tuft cell activation, several studies have reported a critical role of the transient receptor potential cation channel, subfamily M, member 5 (Trpm5) in mediating tuft cell responses (21, 24, 26, 27). To explore the potential involvement of Trpm5 in tuft cell activation in the context of abnormal Paneth cells, we generated compound Sox9LoxP/LoxP;Villin-CreERT2;Trpm5−/− mice. While Sox9LoxP/LoxP;Trpm5−/− mice did not exhibit elevated transepithelial permeability or expression of type 2 inflammation markers, these parameters were markedly increased in Sox9LoxP/LoxP;Villin-CreERT2;Trpm5−/− mice at levels equivalent to those detected in Sox9LoxP/LoxP;Villin-CreERT2;Trpm5+/+ mice (SI Appendix, Fig. S6 A and B). Thus, tuft cell–dependent type 2 inflammation in response to dysfunctional Paneth cells is mediated by SucnR1 via a Trpm5-independent pathway.
Tuft Cell–Mediated Type 2 Immunity Down-regulates Expression of Antimicrobial RegIII in Paneth Cells.
To more precisely study the mechanisms via which tuft cell–dependent type 2 inflammation mediates dysbiosis in the context of dysfunctional Paneth cells, we first assessed the consequences of Sox9 deletion in organoids derived from Sox9LoxP/LoxP;Villin-CreERT2 and Sox9LoxP/LoxP control mice. In this ex vivo epithelium culture system, the absence of immune cells, microbiota, and other nonepithelial cells allows the direct consequences of altered Paneth cells to be distinguished from the effects due to inflammation. Treatment with 4-OH-tamoxifen rapidly and efficiently caused Sox9 deletion in Sox9LoxP/LoxP;Villin-CreERT2 organoids but not in Sox9LoxP/LoxP controls (SI Appendix, Fig. S7A). Five days after 4-OH-tamoxifen treatment, Paneth cell morphology was altered in Sox9-deficient organoids, with decreased granule size as compared to control Sox9LoxP/LoxP organoids as well as abnormal expression of Klf4 in Paneth cells (SI Appendix, Fig. S7A). Furthermore, analysis of Paneth cell markers revealed strongly reduced lysozyme, Mmp7, and Defa29 expression in Sox9LoxP/LoxP;Villin-CreERT2 organoids as compared to Sox9LoxP/LoxP controls (Fig. 6A), in accord with our in vivo data (Fig. 1E). Notably though, in sharp contrast with in vivo data in Sox9LoxP/LoxP;Villin-CreERT2 mice, expression of regenerating islet-derived 3-beta (RegIIIβ) and –gamma (RegIIIγ) were not altered in Sox9LoxP/LoxP;Villin-CreERT2 organoids (Fig. 6A). RegIIIβ and RegIIIγ belong to the family of C-type lectins, which have direct bactericidal properties by binding the cell wall of gram-positive bacteria (41). These data thus suggest that the attenuated RegIIIβ and RegIIIγ expression observed in Paneth cells in Sox9LoxP/LoxP;Villin-CreERT2 mice is a consequence of the type 2 inflammation caused by the Paneth cell-tuft cell cross talk—and not to Paneth cells alone—promoting dysbiosis in these mice.
Fig. 6.

Dysbiosis is dependent on a cross talk between tuft cells and Paneth cells via the downregulation of the antimicrobial RegIII. (A) RT-qPCR analysis of antimicrobial peptides Lyz1, Mmp7, Defa29, and RegIII in organoids derived from Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 small intestine. Organoids were analyzed 5 d after 4-OH-tamoxifen treatment (n = 2 independent organoid cultures, replicated 3 times). (B) RT-qPCR analysis of antimicrobial peptides Lyz1, Mmp7, Defa29, and RegIII in Sox9LoxP/LoxP;Villin-CreERT2 and Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− small intestine. n = 6 to 8 mice per genotype. Mice were killed 2 wk after tamoxifen treatment. (A) Mann–Whitney U test. (B) Kruskal–Wallis test with Dunn's post hoc test; NS: not significant; *P < 0.05; **P < 0.01; ***P < 0.001. The line indicates the median, the box marks the 25th and 75th percentiles, and the whiskers indicate the minimal to maximum values. See also SI Appendix, Fig. S7.
We then evaluated the potential of IL-13, a key cytokine produced in the context of type 2 immune responses (42), to induce the downregulation of RegIII expression in cultured organoids. Sox9 was deleted as above, and half of the organoids were then treated with recombinant IL-13 (rIL-13) during 72 h before analysis. As we previously showed (Fig. 6A), Sox9 deletion in organoids did not affect significantly RegIIIβ and RegIIIγ gene expression. Notably, exposure to rIL-13 increased expression of the Pou2f3 tuft cell maker and Retnlβ in goblet cells, indicating efficient epithelial remodeling, and significantly reduced expression of RegIIIβ and RegIIIγ (SI Appendix, Fig. S7B). Thus, in the context of Sox9 deficiency, the presence of a single type 2 cytokine is sufficient to down-regulate RegIII in Paneth cells.
In a reciprocal experiment, aimed at evaluating the importance of the inflammatory environment in the reduced RegIIIβ and RegIIIγ expression in mice with altered Paneth cells, we used Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice as a model for Paneth cell dysfunction in the absence of type 2 inflammation. Interestingly, lysozyme, Mmp7, and Defa29 expression were strongly reduced in both Sox9LoxP/LoxP;Villin-CreERT2 and Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− models as compared to control mice, identifying these genes as epithelial cell intrinsic consequences of Sox9 deletion in Paneth cells. In sharp contrast, expression of RegIIIβ and RegIIIγ remained high in Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mice (Fig. 6B), characterized by the absence of type 2 inflammation and an absence of dysbiosis. Indeed, RegIIIβ and RegIIIγ levels were significantly higher than those detected in Sox9LoxP/LoxP;Villin-CreERT2 mice, with an established dysbiosis and active type 2 inflammation (Fig. 6B). Interestingly, similar results were observed in Sox9LoxP/LoxP;Villin-CreERT2;Il-4Rα−/− mice, where RegIIIβ and RegIIIγ levels were similar to those detected in Sox9LoxP/LoxP;Il-4Rα+/+ mice (SI Appendix, Fig. S7C). Thus, the loss of RegIIIβ and RegIIIγ in epithelial cells is dependent on the inflammatory milieu, especially on the type 2 cytokine IL13, and this change likely contributes to the emergence of intestinal dysbiosis of Sox9LoxP/LoxP;Villin-CreERT2 mice.
Altogether, these data support the hypothesis of a step-wise model of dysbiosis in the context of abnormal Paneth cells: 1) dysfunctional Paneth cells first induce mild changes in microbiota composition which activate tuft cells; 2) tuft cell activation then triggers gut inflammation; and 3) the inflammatory environment promotes further microbiota remodeling via RegIIIβ and RegIIIγ downregulation in epithelial cells, eventually leading to dysbiosis.
To test this hypothesis, we performed a kinetic analysis of the main epithelial and immune system parameters occurring 3, 5, and 7 d following Sox9 deletion. Nearly complete suppression of Sox9 expression was already observed by 3 d, concomitant with altered expression of lysozyme, Muc2, and Klf4 in Paneth cells (SI Appendix, Fig. S8A). In contrast, no evidence of type 2 immune responses (increased numbers of lamina propria Gata3+ cells; SI Appendix, Fig. S8B) or IL13-dependent epithelial remodeling (Retnlβ expression, increased frequency of Dclk1+ tuft cells, altered RegIII expression; SI Appendix, Fig. S8 B and C) was observed at this time point. However, by 5 d after initiation of tamoxifen treatment, Gata3+ immune cell numbers were increased, but no evidence of epithelial remodeling was detected (SI Appendix, Fig. S8B). Finally, after 7 d, the elevated numbers of Gata3+ immune cells in the lamina propria were associated with epithelial remodeling, as assessed by changes in Retnlβ, Dclk1 and RegIII expression (SI Appendix, Fig. S8 B and C).
Altogether, our study demonstrates that Sox9-deficient Paneth cells cause chronic type 2 inflammation and the establishment of dysbiosis via a three-step pathway requiring a cross talk between Paneth and tuft cells (Fig. 7 and SI Appendix, Fig. S9). Moreover, our findings imply a yet unidentified but critical homeostatic function of Paneth cells in controlling succinate-producing microbiota species to prevent the inappropriate activation of tuft cells in the absence of a parasite infection and subsequent deleterious dysbiosis. Finally, our findings that inhibition of either SucnR1 signaling on tuft cells or IL4R/IL13R signaling on target epithelial cells disrupts the feedback to Paneth cells with a subsequent attenuation of dysbiosis identify pathways that may be therapeutically targeted in patients with dysbiosis.
Fig. 7.
A three-step mechanism for establishment of dysbiosis and inflammation due to the cross Talk between altered Paneth cells and tuft cells through succinate–SucnR1 signaling and type 2 cytokines. In homeostatic conditions, Paneth cells regulate microbiota composition, and the presence of tuft cells is rare. Step 1: In the absence of Sox9, Paneth cells are altered and express normal levels of RegIII but lower levels of Lyz1, leading to mild alterations in the microbiota and increased succinate production potential. Step 2: Sensing of increased succinate levels by tuft cells, via the SucnR1, results in the initiation of a type 2 immune response. Step 3: Type 2 cytokines cause IL4rα-dependent remodeling of epithelial cells, including ectopic Retnlβ expression in goblet cells, tuft cell lineage amplification, and reduction of RegIII levels. This remodeling accentuates the deficiency in microbiota regulation by epithelial cells, eventually causing dysbiosis. As tuft cells cooperate with altered Paneth cells to drive dysbiosis, pharmacological inhibition of tuft cell activity may represent a strategy for controlling inflammation and dysbiosis in predisposed patients with altered Paneth cells. See also SI Appendix, Figs. S8 and S9.
Discussion
Many genetic and environmental factors can impair Paneth cell function, resulting in dysbiosis and increased susceptibility to chronic diseases (7, 14–17, 19). To assess the impact of dysfunctional Paneth cells in adult intestinal physiopathology, we generated an inducible allele for Sox9 deletion. Although we and others previously reported an absence of Paneth cells in mice in which Sox9 deletion in the gut epithelium occurred during embryo development (12, 13), induction of its deletion in adult mice did not impair Paneth cell lineage specification, but these cells had an altered phenotype. This suggests additional Sox9 roles during embryonic development that specify the Paneth cell lineage. In our adult model, morphological defects in Paneth cells are reminiscent of those present in Paneth cells from IBD patients (14, 15) and obese subjects (16), two conditions strongly linked to dysbiosis. Our data confirm the central role of Sox9 specifically in Paneth cells since, 1) defects in Paneth cells, as well as type 2 immune responses, are absent in Sox9LoxP/LoxP;P450a1-Cre mice, where Sox9 is deleted in all intestinal epithelial cells except Paneth cells, and, 2) the specific deletion of lysozyme in Paneth cells in the gut is sufficient to cause a type 2 immune response (19). Taking advantage of this model to assess the underlying mechanisms responsible for dysbiosis initiation and inflammation induced by Paneth cell defects, we investigated the potential involvement of tuft cells, sentinel cells in the intestinal epithelium that are capable of integrating host–microorganism interactions to regulate mucosal immunity. The findings reported here now establish sentinel tuft cells as central contributors to dysbiosis and chronic inflammation following primary Paneth cell defects. Notably, on its own, Paneth cell dysfunction has only limited pathological consequences. We thus identify a Paneth-tuft cell cross talk that is critical to promote gut dysbiosis.
Tuft cells can be activated by various metabolites. In the context of parasite infection, where tuft cells are indispensable in the initiation of antihelminthic and antiprotozoan immunity (20–22), the Tritrichomonas protist–derived metabolite succinate is able to activate tuft cells and trigger a type 2 immune response as well as adaptive small intestinal remodeling (25, 26). Moreover, extracts of the parasitic helminth Trichinella spiralis can activate tuft cells through Tas2r bitter taste receptors to initiate type 2 immunity (27). Recently, binding of the N-undecanoylglycine bacterial metabolite to the vomeronasal Vmn2r26 receptor on the tuft-2 tuft cell subset was shown to facilitate bacterial eradication through prostaglandin-D2 signaling and increased mucus secretion (23). However, potential tuft cell function in the context of endogenous microbiota had not been evaluated. Our data indicate that tuft cells activated following Paneth cell dysfunction play a critical role in promoting microbiota remodeling and subsequent inflammation. Mechanistically, this is likely mediated by downregulation of Paneth cell–expressed antibacterial lectins RegIIIβ and RegIIIγ previously recognized for their roles in regulating microbial populations (43) and their involvement in dysbiosis (44, 45). Thus, initial Paneth cell defects, with limited impact on microbiota composition, activate tuft cells. Activated tuft cells, in turn, trigger type 2 inflammation, which causes additional Paneth cell alterations and increased transepithelial permeability, possibly through alteration of Claudin adhesion proteins expression as already reported in the context of exposure to different inflammatory cytokines (46). The mechanisms underlying the latter remain unclear but likely involve the microbiota and/or the effects of type 2 cytokines on epithelial cells as increased permeability is absent in both antibiotic-treated and Il4rα-deficient mice. Based on these data, it is interesting to speculate that tuft cells may play a role in the pathogenesis and pathophysiology of numerous diseases associated with Paneth cell defects, caused by either environmental stressors and/ or genetic predisposition.
Our study highlights a central role for microbe-derived succinate in the activation of tuft cells following Paneth cell alterations. Luminal succinate was previously reported to activate tuft cells in the context of parasitic infection by Tritrichomonas (25, 26) or after artificial perturbations in the microbiota composition (24). Such activation requires the Sucnr1 succinate receptor, which, in the intestinal epithelium, is specifically expressed by tuft cells. Interestingly, obese and Crohn’s disease patients, both with altered Paneth cells, have a dysbiotic microbiota with increased capacity to produce succinate (47, 48). In the mammalian gut, succinate is mainly produced by bacteria belonging to the Bacteroidetes phylum, and monocolonization of germ-free mice with Bacteroides vulgatus (recently reclassified as Phocaeicola vulgatus), a species known to be associated with intestinal inflammation when present in high concentration (49), is sufficient to increase cecal succinate levels (50). Importantly, we found that Bacteroides vulgatus species were enriched in the cecum of dysbiotic Sox9LoxP/LoxP;Villin-CreERT2 mice, in which metagenomic analyses revealed increased succinate production potential. Furthermore, using compound Sox9LoxP/LoxP;Villin-CreERT2;Sucnr1−/− mice with altered Paneth cells, we demonstrated a central role of succinate in activating tuft cells. While increased luminal succinate concentrations are essential for the induction of tuft cell–triggered immune responses against parasite infections, our results suggest that excessive representation of succinate-producing endogenous bacterial species can have a detrimental impact. Consequently, our data highlight a key role of Paneth cells in controlling endogenous succinate-producing bacteria to avoid illegitimate tuft cell activation. We have thus identified a previously unsuspected “Paneth cell–tuft cell” cross talk, regulated by the luminal microorganism composition, to control gut mucosal immunity. While this cross talk is critical in preventing tuft cell activation in the absence of parasite infections, it may also be involved in pathophysiological situations. For instance, Paneth cell alterations have been observed following mouse infection with the Nippostrongylus brasiliensis helminth, with concomitant downregulation of RegIIIγ and lysozymes 1 and 2, and these alterations were dependent on IL13 and Stat6 signaling (51). In addition, helminth infections modulate bacterial microbiota composition, and, conversely, microbiota impact parasitic colonization (52). Therefore, it is plausible that following parasitic infection, microbiota changes and subsequent Paneth cell alterations may lead to an increase in succinate-producing bacterial species to reinforce type 2 immune responses, promoting helminth expulsion.
Tuft cell signaling downstream of SucnR1 activation has, thus far, been found to depend on Trpm5, following parasite infections or treatment with succinate (24, 25), although some elements of the type 2 immune response were still present in Trpm5-deficient mice treated with succinate in drinking water (26). In our study, it is striking that Trpm5 was dispensable for the SucnR1-dependent tuft cell activation in response to endogenous microbiota, suggesting that specific triggers may activate distinct tuft cell signaling pathways, potentially resulting in differential impacts on mucosal immunity.
In conclusion, our data support the host+microbiota ecosystem model, also called a holobiont (1), wherein there is a bidirectional interaction between microbial composition and the host inflammation status. Our study reveals a Paneth cell-tuft cell cross talk, opening perspectives for combinatorial therapies targeting both host and microbiota for a more effective management of dysbiosis-associated chronic diseases.
Materials and Methods
Animal Strains.
All animal studies were approved by the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation (APAFIS#10413-2017062817274261 v2). Animals were housed in an SPF animal facility, and male and female mice were analyzed between 10 and 16 wk of age. All mice were on a C57BL/6 genetic background (except those containing the IL4Rα−/− allele, which are on a mixed genetic background). The Sox9LoxP/LoxP;Villin-CreERT2 conditional mouse model was generated by crossing Sox9LoxP/LoxP mice (53) with Villin-CreERT2 mice (54). The Pou2f3-deficient model has been described previously (55). The Pou2f3−/− mice have been crossed with Sox9LoxP/LoxP;Villin-CreERT2 mice to generate the Sox9LoxP/LoxP;Villin-CreERT2;Pou2f3−/− mouse model. Sox9LoxP/LoxP;Villin-CreERT2 mice were also crossed with IL4Rα−/− mice kindly provided by J. Allen or Trpm5−/− mice from Jackson Laboratory (56) to establish Sox9LoxP/LoxP;Villin-CreERT2;IL4Rα−/− and Sox9LoxP/LoxP;Villin-CreERT2;Trpm5−/− models. SucnR1−/− mice were from the Cyagen company and were crossed with Sox9LoxP/LoxP;Villin-CreERT2 mice to generate the Sox9LoxP/LoxP;Villin-CreERT2;SucnR1−/− mouse model. All mouse genotypes were obtained by breeding double heterozygous mice, and analysis was performed using littermates. Cre recombinase activity was induced by tamoxifen (Sigma-Aldrich), administrated by oral gavage at a daily single dose of 1 mg during 5 d. The Sox9 LoxP/LoxP;P450a1-Cre mouse model was established by crossing Sox9LoxP/LoxP mice with P450a1-Cre mice provided by Douglas Winton (57). Beta-naphthoflavone (βNF, MP Biomedicals) was administered by oral gavage at a daily single dose of 2 mg during 5 d as described in ref. 29 to induce p450 responsive element-controlled Cre expression.
RNA Extraction and qPCR.
Total RNA was extracted from snap-frozen intestinal tissues using TRIzol reagent (Life Technologies) and qPCR was performed using LightCycler 480 SYBR Green I Master (Roche Diagnostics) as previously described (58). Primer sets used for each gene analyzed are presented in SI Appendix, Table S2. Detailed protocols and information are available in SI Appendix.
Transmission Electron Microscopy.
Sample preparation was performed as previously described (59). Detailed protocols and information are available in SI Appendix.
Intestinal Permeability Measurement Using FITC-Dextran.
Paracellular permeability was assessed by measuring the passage of 4-kDa FITC–dextran from the intestinal lumen into systemic circulation, as previously described (60). Detailed protocols and information are available in SI Appendix.
Fluorescent and Bright-Field Immunohistochemistry on Paraffin-Embedded Tissue.
Immunohistochemistry analyses were performed as previously reported (20). Detailed protocols and information are available in SI Appendix.
Fecal Bacterial DNA Isolation and 16S rRNA Sequencing.
Microbial communities profiling was performed by GenoScreen (Lille, France). 16S rDNA amplicon library was prepared using a 16S primer pair that encompasses a 463 pb targeting the V3-V4 region, using a protocol developed by GenoScreen and named Metabiote®. Detailed protocols and information are available in SI Appendix.
Cecal DNA Extraction and High-Throughput Metagenomic Sequencing.
DNA extraction was performed following IHMS SOP 07 V2 for each sample, as previously described (61). Briefly, samples underwent thermal, chemical, and mechanical lysis and operations to eliminate cell debris, proteins, aromatic compounds, and RNA. Alcoholic precipitation of DNA salts was performed before cleaning, and the DNA pellet was reconstituted in TE buffer. Fluorometric quantitation using Qubit (ThermoFisher Scientific) and FilterMax (Molecular Devices) were used to assess the quantity of DNA, and Fragment Analyzer 1.0 (Agilent Technologies) was used to assess DNA quality. Three micrograms of high-molecular-weight DNA (>10 kbp) was sheared into fragments of approximately 150 bp using an ultrasonicator (Covaris, Woburn, US), and DNA fragment library construction was performed using the Ion Plus Fragment Library and Ion Xpress Barcode Adapters Kits (ThermoFisher Scientific). Purified and amplified DNA fragment libraries were sequenced using the Ion Proton Sequencer (ThermoFisher Scientific), with a minimum of 20 million high-quality reads generated per library. Detailed protocols and information are available in SI Appendix.
Organoid Culture.
Organoid cultures were established from Sox9LoxP/LoxP and Sox9LoxP/LoxP;Villin-CreERT2 intestinal crypts as previously described (62). Detailed protocols and information are available in SI Appendix.
Statistical Analysis.
Statistical analysis was performed using the software GraphPad Prism. For gene expression and intestinal permeability measurements, sample (n) was defined as the number of mice used in the experiments, and the exact Mann–Whitney U test or Kruskal–Wallis test was used to calculate the P value. For histological data quantification, sample (n) was defined as the number of cells per crypt-villus unit, and 50 crypt-villus axes were counted per tissue section from at least 3 mice from each genotype. Since normal distribution was tested and was very rarely reached, the exact Mann–Whitney U test or Kruskal–Wallis test was used to calculate the P value. Results are shown as histograms representing means and SEMs for each genotype or as box plot depicting numerical data through their medians and the variability through the minimal to maximal values. No statistical methods were used to predetermine sample size, the experiments were not randomized, and the investigators were not blinded during experiments and data assessment. Experiments were replicated at least twice (qPCR, immunohistochemistry, and electron microscopy) except for ethical reasons (intestinal permeability measurements) or due to the cost of the experiments (microbiota sequencing).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We acknowledge M. Belkahla, A. Deborgher, and C. Joséphine for technical inputs, J. Allen for sharing the IL4r KO mouse line, the RAM-iExplore, RAM-PCEA animal facilities, and Luc Forichon for maintenance of mouse colonies, Muriel Asari for iconography, Daniel Fisher and the Jay team for proofreading their critical reading of the manuscript, and the Réseau d’Histologie Expérimentale de Montpellier and the Montpellier RIO Imaging facilities. This work was supported by Agence Nationale de la Recherche (ANR-14-CE14-0025-01 and ANR-17-CE15-0016-01 to N.T. and P.J.), Institut National du Cancer (INCa 2014-174 to P.J. and INCA_2018-158 to N.T. and P.J.), and SIRIC Montpellier Cancer (grant INCa_Inserm_DGOS_12553 to P.J.); the P.J. team is “Equipe Labellisée Ligue contre le Cancer”; S.S. was supported by an Inserm-Région Languedoc-Roussillon Fellowship and Ligue Nationale contre le Cancer, J.N. was supported by the Labex EpiGenMed (an “Investissements d’avenir” program ANR-10-LABX-12-01) and Fondation pour la Recherche Médicale (FDT201805005851). Additional funding was from the Metagenopolis grant ANR-11-DPBS-0001.
Author contributions
N.C., S.S., H.M.B., N.T., and P.J. designed research; N.C., J.N., S.S., V.M., V.D., M.A., B.Q., F.H., I.G., A.L., A.G., P.C., L.G., S.T., D.G., C.C., and C.B. performed research; D.W. and I.M. contributed new reagents/analytic tools; N.C., J.N., S.S., F.G., V.M., V.D., M.A., F.T., F.H., I.G., A.L., A.G., C.C., F.B., C.B., H.M.B., and P.J. analyzed data; and N.C. and P.J. wrote the paper with inputs from F.G., V.M., V.D., H.M.B. and N.T.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Nathalie Coutry, Email: Nathalie.coutry@igf.cnrs.fr.
Philippe Jay, Email: philippe.jay@igf.cnrs.fr.
Data, Materials, and Software Availability
Shotgun metagenomic data are fully available under BioProject ID: PRJEB40719 (63) for shotgun metagenomic data and PRJNA944985 for 16S rRNA sequencing data (http://www.ncbi.nlm.nih.gov/bioproject/944985) (64). All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Kundu P., Blacher E., Elinav E., Pettersson S., Our gut microbiome: The evolving inner self. Cell 171, 1481–1493 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Turnbaugh P. J., et al. , An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Ni J., Wu G. D., Albenberg L., Tomov V. T., Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Järbrink-Sehgal E., Andreasson A., The gut microbiota and mental health in adults. Curr. Opin. Neurobiol. 62, 102–114 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Settanni C. R., Ianiro G., Bibbò S., Cammarota G., Gasbarrini A., Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Progress in Neuro-Psychopharmacol. Biol. Psychiatry 109, 110258 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Carmody R. N., et al. , Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 17, 72–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu, et al. , Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight 1, e86907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salzman N. H., Ghosh D., Huttner K. M., Paterson Y., Bevins C. L., Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422, 522–526 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Vaishnava S., Behrendt C. L., Ismail A. S., Eckmann L., Hooper L. V., Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. U.S.A. 105, 20858–20863 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Es J. H., et al. , Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 7, 381–386 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Blache P., et al. , SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J. Cell Biol. 166, 37–47 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastide P., et al. , Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 178, 635–648 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori-Akiyama Y., Akiyama H., Rowitch D. H., De Crombrugghe B., Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. U.S.A. 100, 9360–9365 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadwell K., et al. , A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanDussen K. L., et al. , Genetic variants synthesize to produce paneth cell phenotypes that define subtypes of Crohn’s disease. Gastroenterology 146, 200–209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodin C. M., et al. , Reduced Paneth cell antimicrobial protein levels correlate with activation of the unfolded protein response in the gut of obese individuals. J. Pathol. 225, 276–284 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Guo X., et al. , High fat diet alters gut microbiota and the expression of paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediators Inflamm. 2017, 9474896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehkamp J., Stange E. F., Paneth’s disease. J. Crohn’s Colitis 4, 523–531 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Yu S., et al. , Paneth cell-derived lysozyme defines the composition of mucolytic microbiota and the inflammatory tone of the intestine. Immunity 53, 398–416.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerbe F., et al. , Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howitt M. R., et al. , Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Moltke J., Ji M., Liang H.-E., Locksley R. M., Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature 529, 221–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong Z., et al. , Intestinal Tuft-2 cells exert antimicrobial immunity via sensing bacterial metabolite N-undecanoylglycine. Immunity 55, 686–700.e7 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Lei W., et al. , Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. U.S.A. 115, 5552–5557 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadjsombati M. S., et al. , Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49, 33–41.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider C., et al. , A metabolite-triggered tuft cell-ILC2 Circuit drives small intestinal remodeling. Cell 174, 271–284.e14 (2018), 10.1016/j.cell.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo X.-C., et al. , Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc. Natl. Acad. Sci. U.S.A. 116, 5564–5569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee A., et al. , Succinate produced by intestinal microbes promotes specification of tuft cells to suppress ileal inflammation. Gastroenterology 159, 2101–2115.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ireland H., Houghton C., Howard L., Winton D. J., Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev. Dyn 233, 1332–1336 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Katz J. P., et al. , The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 129, 2619–2628 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troughton W. D., Trier J. S., Paneth and goblet cell renewal in mouse duodenal crypts. J. Cell Biol. 41, 251–268 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson C. L., et al. , Regulation of intestinal α-Defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286, 113–117 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Sankaran-Walters S., Hart R., Dills C., Guardians of the gut: Enteric defensins. Front. Microbiol. 8, 647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira A. P. B., Texeira T. F. S., Ferreira A. B., M. do C. G. Peluzio, R. de C. G. Alfenas, Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 108, 801–809 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Bischoff S. C., et al. , Intestinal permeability–A new target for disease prevention and therapy. BMC Gastroenterol. 14, 189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thevaranjan N., et al. , Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 21, 455–466.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoyler T., et al. , The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Artis D., et al. , RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. U.S.A. 101, 13596–13600 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban J. F. Jr., et al. , IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 (1998). [DOI] [PubMed] [Google Scholar]
- 40.De Vadder F., et al. , Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 24, 151–157 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Gassler N., Paneth cells in intestinal physiology and pathophysiology. World J. Gastrointest. Pathophysiol. 8, 150–160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barner M., Mohrs M., Brombacher F., Kopf M., Differences between IL-4Ra-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr. Biol. 8, 669–672 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Salzman N. H., Bevins C. L., Dysbiosis—A consequence of Paneth cell dysfunction. Semin. Immunol. 25, 334–341 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Vaishnava S., et al. , The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loonen L. M., et al. , REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal. Immunol. 7, 939–947 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Prasad S., et al. , Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Invest. 85, 1139–1162 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Connors J., Dawe N., Van Limbergen J., The role of succinate in the regulation of intestinal inflammation. Nutrients 11, 25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serena C., et al. , Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 12, 1642–1657 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bloom S. M., et al. , Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory Bowel disease. Cell Host Microbe. 9, 390–403 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Setoyama H., Imaoka A., Ishikawa H., Umesaki Y., Prevention of gut inflammation by Bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with Bacteroides strains isolated from ulcerative colitis patients. Microbes Infect. 5, 115–122 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Fricke W. F., et al. , Type 2 immunity-dependent reduction of segmented filamentous bacteria in mice infected with the helminthic parasite Nippostrongylus brasiliensis. Microbiome 3, 40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds L. A., Finlay B. B., Maizels R. M., Cohabitation in the Intestine: Interactions among helminth parasites, bacterial microbiota, and host immunity. J. Immunol. 195, 4059–4066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kist R., Schrewe H., Balling R., Scherer G., Conditional inactivation ofSox9: A mouse model for campomelic dysplasia. genesis 32, 121–123 (2002). [DOI] [PubMed] [Google Scholar]
- 54.El Marjou F., et al. , Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto I., Ohmoto M., Narukawa M., Yoshihara Y., Abe K., Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat. Neurosci. 14, 685–687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., et al. , Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell 112, 293–301 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Ireland H., et al. , Inducible cre-mediated control of gene expression in the murine gastrointestinal tract: Effect of loss of β-catenin. Gastroenterology 126, 1236–1246 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Bruschi M., et al. , Loss of Apc rapidly impairs DNA methylation programs and cell fate decisions in Lgr5+ intestinal stem cells. Cancer Res. 80, 2101–2113 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Chauvet N., et al. , Complementary actions of dopamine D2 receptor agonist and anti-vegf therapy on tumoral vessel normalization in a transgenic mouse model. Int. J. Cancer 140, 2150–2161 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Karhausen J., et al. , Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meslier V., et al. , Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69, 1258–1268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato T., et al. , Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Coutry N., et al. , Shotgun Metagenomic sequences for dysbiosis of gut mucosa in the crosstalk between Paneth and tuft cells. ENA EBI Database. https://www.ebi.ac.uk/ena/browser/view/PRJEB40719. Deposited 15 November 2022.
- 64.Coutry N., Nguyen J., Jay P., 16S rRNA sequencing data of Sox9-deficient mice as compared to controls. NCBI BioProject. http://www.ncbi.nlm.nih.gov/bioproject/944985. Accessed 15 March 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Shotgun metagenomic data are fully available under BioProject ID: PRJEB40719 (63) for shotgun metagenomic data and PRJNA944985 for 16S rRNA sequencing data (http://www.ncbi.nlm.nih.gov/bioproject/944985) (64). All study data are included in the article and/or SI Appendix.