Abstract
Reliable definitions, classifications and prognostic models are the cornerstones of stratified medicine, but none of the current classifications systems in epilepsy address prognostic or outcome issues. Although heterogeneity is widely acknowledged within epilepsy syndromes, the significance of variation in electroclinical features, comorbidities and treatment response, as they relate to diagnostic and prognostic purposes, has not been explored. In this paper, we aim to provide an evidence-based definition of juvenile myoclonic epilepsy showing that with a predefined and limited set of mandatory features, variation in juvenile myoclonic epilepsy phenotype can be exploited for prognostic purposes. Our study is based on clinical data collected by the Biology of Juvenile Myoclonic Epilepsy Consortium augmented by literature data. We review prognosis research on mortality and seizure remission, predictors of antiseizure medication resistance and selected adverse drug events to valproate, levetiracetam and lamotrigine. Based on our analysis, a simplified set of diagnostic criteria for juvenile myoclonic epilepsy includes the following: (i) myoclonic jerks as mandatory seizure type; (ii) a circadian timing for myoclonia not mandatory for the diagnosis of juvenile myoclonic epilepsy; (iii) age of onset ranging from 6 to 40 years; (iv) generalized EEG abnormalities; and (v) intelligence conforming to population distribution. We find sufficient evidence to propose a predictive model of antiseizure medication resistance that emphasises (i) absence seizures as the strongest stratifying factor with regard to antiseizure medication resistance or seizure freedom for both sexes and (ii) sex as a major stratifying factor, revealing elevated odds of antiseizure medication resistance that correlates to self-report of catamenial and stress-related factors including sleep deprivation. In women, there are reduced odds of antiseizure medication resistance associated with EEG-measured or self-reported photosensitivity. In conclusion, by applying a simplified set of criteria to define phenotypic variations of juvenile myoclonic epilepsy, our paper proposes an evidence-based definition and prognostic stratification of juvenile myoclonic epilepsy. Further studies in existing data sets of individual patient data would be helpful to replicate our findings, and prospective studies in inception cohorts will contribute to validate them in real-world practice for juvenile myoclonic epilepsy management.
Keywords: prognosis, classification, definition, juvenile myoclonic epilepsy, drug resistance
Rubboli et al. provide evidence-based diagnostic criteria for juvenile myoclonic epilepsy based on clinical data from the BIOJUME Consortium and literature review. They propose a predictive model showing the prognostic value of absence seizures and female sex in predicting drug resistance and photosensitivity in females in reducing the odds of drug refractoriness.
Graphical Abstract
Graphical Abstract.
Introduction
Few diseases in medicine benefit from a prognostic model, and the epilepsies are no exception. Prognostic models are vital for the mission of stratified medicine, which can be summarized as identifying individuals with a condition who may profit from, or be harmed by, specific interventions.1 Accurate stratification is efficient at an individual and at a group level, permitting both personalization of treatment and smarter clinical trials design. Large-scale primary data sets and secondary meta-analyses of clinical trials and cohort studies offer the possibility of generating such models, testable for their utility in clinical practice (Figure 1).
Figure 1.
Identification of clinical biomarkers by stratified medicine can pave the way to targeted treatments. Stratified medicine adds a step to empirical medicine to associate a patient with a specific therapy that is more likely to be effective and/or safe. Central to the process is the identification of clinical biomarkers that differentiate subgroups of patients with differential treatment response, e.g. response or adverse effects. Based on Trusheim et al.1
Juvenile myoclonic epilepsy (JME), a common and archetypal idiopathic generalized epilepsy (IGE) syndrome, has proven challenging to define, thus rendering prognostic and outcome measures also difficult to delineate. None of the current epilepsy classification systems is focused on prognosis or outcome. A commonly used coding system like Systematized Nomenclature of Medicine–Clinical Terms (SNOMED-CT)2 denotes the syndrome of JME by the single code 62040001. In International Classification of Diseases 10th Revision (ICD-10),3 the JME code G40.B09 is subdivided into intractable versus not intractable (not defined) and then further subdivided by occurrence of status epilepticus (which is so rare as to be atypical in JME). Confusingly, the outcomes (intractability; status epilepticus) are comingled with the disease definitions. This system of coding is clearly inadequate and highlights the need for specificity of subclassification by epilepsy syndromes that goes beyond nosological purposes but that bears also diagnostic and prognostic significance. In 2011, an expert meeting4 nicknamed after its venue in Avignon failed to reach a consensus on the diagnosis and management of JME. Instead two separate classifications were proposed with limited clinical implications and without outlining possible prognostic indicators.4 That paper clearly highlights the variation in how the term JME was used globally, such as whether an abnormal MRI was acceptable and whether generalized abnormalities on the EEG were obligatory. In 2022, the International League Against Epilepsy (ILAE) Task Force on Nosology and Definitions released a position statement on definition of the IGE syndromes, including JME.5 The criteria for the definition of each syndrome relied on literature review until 2019, the most recent edition of the book ‘Epileptic Syndromes of Infancy, Childhood and Adolescence’,6 criteria listed in the ILAE website www.EpilepsyDiagnosis.org,7 and expert opinion from Task Force members. The consensus on the definition of each syndrome was achieved by a modified Delphi process.8 For JME, mandatory diagnostic criteria were myoclonic seizures and 3- to 5.5-Hz generalized spike wave (GSW) or generalized polyspike wave on EEG (even as retrieved historical data). As possible prognostic markers, the paper recognizes that a number of individuals and seizure-specific factors are associated with a tendency towards drug resistance, including the presence of absence seizures, psychiatric comorbidities, a prior history of childhood absence epilepsy (CAE), praxis-induced seizures and younger age at epilepsy onset.5
Progress on prognosis has been made through subclassification of JME.9,10 Although heterogeneity is widely recognized within epilepsy syndromes, confusion remains over the significance of variation in electroclinical features, comorbidities and treatment response.11,12
Here, we aim to show that with a predefined and limited set of mandatory features, variation in JME phenotype can be exploited for prognostic purposes. Our study was based on real clinical data collected by the Biology of Juvenile Myoclonic Epilepsy (BIOJUME) Consortium in concert with data from the published literature. The BIOJUME Consortium is a clinical genetic research project that includes the world’s largest cohort of subjects with JME, encompassing >900 cases.13,14 This cohort provides the possibility of gaining insights from an unprecedented large collection of clinical, EEG, behavioural and treatment data. For our analysis, we selected potential predictor variables that likely underlie clinical heterogeneity, and we focus specifically on easily measured stratifying variables associated with clinically relevant influence on prognosis, treatment outcome or adverse drug effects.
Materials and methods
We approached this task by separately evaluating diagnostic criteria, prognosis, predictors of drug resistance and adverse events.
Diagnostic criteria for definition
We took the diagnostic criteria of the Avignon meeting as our starting point: these included myoclonic jerks and their timing, age of seizure onset and intelligence.4 We reviewed relevant literature using search terms (‘juvenile myoclonic epilepsy’ OR ‘Janz syndrome’ OR ‘idiopathic generalized epilepsy’ OR ‘genetic generalized epilepsy’) AND (‘late onset’). For intelligence, we performed a systematic search in the PubMed database (search string: ((epilepsy, juvenile myoclonic[MeSH Terms]) OR (juvenile myoclonic epilepsy[Title/Abstract])) AND ((intell*[Title/Abstract]) OR (neuropsych*[Title/Abstract]))). We also analysed primary data in the BIOJUME data set concerning morning predominance of myoclonic seizures. We questioned accepted generalizations eventually leading to restrictive thinking about JME diagnosis. In the field of logic, a ‘fallacy of defective induction’ is a conclusion based on weak (biased or insufficient) premises; e.g. all swans are white, ergo black swans cannot be swans. We also tested some of the prevailing assumptions about phenotype in JME by examining ‘counterfactual’ arguments. A counterfactual argument asks what would be the logical conclusion if the opposite was true; e.g. if black swans are not swans, what are they?
The BIOJUME Consortium recruited >900 participants from 58 sites across nine countries.14 Briefly, BIOJUME eligibility criteria were based on Avignon Class II (Table 1), and all phenotypes were reviewed by a panel of seven expert clinicians (C.P.B., K.H., D.K.P., M.R., G.R., M.S., and RHT). To explore the importance of a circadian entrainment of seizures, we compared participants with predominant morning seizures with those who reported no morning predominance for distribution of sex, age of onset, absence seizure history, history of triggered seizures, antiseizure medication (ASM) resistance, generalized and polyspike wave EEG features, photoparoxysmal response (PPR) and impulsivity score [measured with Barratt Impulsiveness Scale (BIS), versions BIS-Brief and BIS-11].13 We then generated a multivariable model of morning predominance incorporating sex and significant variables from univariate comparison, calculating odds ratios with 95% confidence intervals and exact P-values. Five authors (G.R., C.P.B., K.K.S., M.R.S., and D.K.P.) then summarized the overall evidence on diagnostic criteria and presented conclusions to the wider authorship.
Table 1.
Avignon and ILAE draft criteria versus proposed BIOJUME criteria
| Avignon Class I | Avignon Class II | ILAE Task Force | BIOJUME | |
|---|---|---|---|---|
| Mandatory criteria | Myoclonic jerks without loss of consciousness | Myoclonic jerks without loss of consciousness | Myoclonic seizures | Myoclonic jerks |
| Myoclonic jerks exclusively on or after awakening | Myoclonic jerks predominantly on or after awakening | Myoclonic jerks timing variable | ||
| Generalized EEG abnormalities | Generalized EEG abnormalities | Generalized EEG abnormalities | Generalized EEG abnormalities | |
| Age of onset 10–25 years | Age of onset 6–25 years | Age of onset 8–40 years | Age of onset ≥6 years increases probability of JME | |
| Normal intelligence | No mental retardation or deterioration | Exclude moderate to profound ID | Intelligence conforms to population distribution |
Bold characters highlight distinct features of the BIOJUME diagnostic criteria as compared with previous diagnostic proposals.
Prognosis and predictors of ASM resistance
For prognosis, we evaluated predictors of the specific outcomes of mortality and seizure remission; where JME-specific information was lacking, we imputed findings from the IGEs or epilepsy in general. We reviewed relevant literature, including a recent comprehensive publication of the BIOJUME Consortium14 and a meta-analysis,15 regarding potential predictors of ASM resistance: sex, age of onset, absence seizures, morning predominance of myoclonic seizures, stress-related seizures, PPR and EEG features. We used combinations of MeSH search terms in the PubMed database, unrestricted by year of publication or language: (‘juvenile myoclonic epilepsy’ OR ‘Janz syndrome’) AND humans AND (prognos* OR predict* OR biomarker OR outcome OR mortality OR epidemiology OR remission) AND ((antiseizure OR antiepileptic) AND (drug OR medication)) AND (*resistan* OR refractory OR intractable); sudden unexpected death in epilepsy (SUDEP): (‘generalized epilepsy’) AND (SUDEP) NOT (‘baboon’ OR ‘mouse’). Abstracts were then screened for relevance.
Predictors of adverse drug events
We evaluated predictors of selected serious adverse drug events associated with the three most commonly prescribed ASMs in JME: valproate, weight gain; lamotrigine, rash; and levetiracetam, psychiatric adverse events. Because these drugs are not limited in their use to epilepsy, we did not specify a disease. We first searched PubMed using MeSH search terms: (levetiracetam [tiab] AND (adverse OR safety OR tolerability) [tiab] AND (psych* OR behav*)) OR f(valpro* [tiab]) AND (‘weight gain’ OR obesity OR overweight OR BMI OR metabolic) OR (lamotrigine AND (rash OR exanthema OR ‘Stevens-Johnson syndrome’ OR toxic epidermal necrolysis OR ‘drug rash with eosinophilia and systemic symptoms’)).
We then analysed data on impulsivity in JME for association with self-reported adverse psychiatric events to levetiracetam,13 calculating the predictive properties of a BIS-Brief cut-off score of ≥21. We also conducted a multiple logistic regression analysis in the same data set of self-reported valproate associated excessive weight gain with the following variables in the model: sex, absence seizure frequency, myoclonic seizure frequency, morning predominant seizures, BIS-Brief score and log of body mass index (BMI).
Statistical analysis
Statistical analysis was carried out on SPSS statistics (version 25). Data on statistical analysis reported in Tables 3 and 4 are reproduced from Shakeshaft et al.13
Table 3.
Demographic and clinical differences in 588 JME patients with and without morning predominant myoclonic seizures in the BIOJUME data set
| Feature comparison in morning entrainment patterns | |||
|---|---|---|---|
| Variable | Morning predominant | Not morning predominant | P-value |
| Sex female | 348/537 (64.8%) | 74/126 (58.7%) | 0.202 |
| Age JME onset years (95% CI) | 16.66 (15.99–17.86) | 16.93 (16.33–17.07) | 0.588 |
| Absence seizures history | 214/525 (40.8%) | 65/124 (52.4%) | 0.018 |
| Triggered seizures | 274/500 (54.8%) | 69/118 (58.5%) | 0.470 |
| ASM resistance | 119/371 (32.1%) | 33/100 (33%) | 0.861 |
| EEG gen spike wave | 254/537 (47.3%) | 82/126 (65.1%) | <0.001 |
| EEG polyspike wave | 358/537 (66.7%) | 72/126 (57.1%) | 0.044 |
| EEG photoparoxysmal response | 165/450 (36.7%) | 40/110 (36.3%) | 0.953 |
| BIS-Brief score (95% CI) | 17.47 (17.02–17.92) | 18.76 (17.77–19.75) | 0.012 |
| Logistic regression of morning predominant seizures | |||
|---|---|---|---|
| Variable | Odds ratio (95% CI) | Z | P-value |
| Absence seizures | 0.65 (0.43–0.99) | −1.99 | 0.047 |
| EEG gen spike wave | 0.42 (0.24–0.72) | −3.12 | 0.002 |
| EEG polyspike wave | 0.88 (0.51–1.51) | −0.48 | 0.634 |
| Sex female | 1.50 (0.97–2.31) | 1.82 | 0.069 |
| Age JME onset | 1.04 (0.98–1.12) | 1.33 | 0.184 |
Values in bold are statistically significant at the 5% level.
Table 4.
Multivariate analysis of excessive weight gain in 134 JME patients ever exposed to valproate
| Logistic regression of excessive weight gain in valproate exposed JME patients | |||
|---|---|---|---|
| Variable | Odds ratio (95% CI) | Z | P-value |
| Absence seizure freq | 1.45 (0.91–2.32) | 1.56 | 0.118 |
| Myoclonic seizure freq | 0.83 (0.52–1.32) | −0.79 | 0.432 |
| Morning predominant seizures | 0.68 (0.28–1.63) | −0.87 | 0.384 |
| Sex female | 1.69 (0.77–3.71) | 1.31 | 0.190 |
| BIS-Brief score ≥21 | 0.74 (0.34–1.62) | −0.75 | 0.454 |
| Log10 BMI | 17.56 (2.96–104.2) | 3.15 | 0.002 |
BMI is measured at outcome and therefore not a predictor.
Results
Diagnostic criteria for definition
Myoclonic jerks
In Dieter Janz’s description of JME in 1957,16 the defining seizure type was myoclonic seizures, which he called ‘impulsive petit mal’, referring to the sudden, impulse-like movement, usually proximally in the upper extremities. The two papers introducing JME to the English-speaking world also included it as part of the definition in 1984 and 1989 as did the 1989 ILAE Proposal.16-18 However, at the 2011 Avignon meeting, two of 14 experts stated that they did not consider myoclonic jerks mandatory for the diagnosis of JME4; the report did not elaborate further.
We could find no evidence in the literature to justify a change from the first definitions specifying myoclonic seizures as a critical feature for diagnosis. We speculate that concerns about misclassification might have been an issue at the Avignon meeting: patients who have poor recall of subtle jerks or who have been treated after a first generalized tonic–clonic seizure (GTCS) may be diagnosed as epilepsy with generalized tonic–clonic seizures alone (EGTCSA). Misclassification might occur also in patients in whom absence seizures predate myoclonus; therefore, in teenaged years, myoclonus is not the main seizure type, or in patients in whom myoclonus responds well to ASMs, and so there is a time where only occasional convulsive seizures, but no myoclonus, are reported. These concerns are not a reason to exclude myoclonic jerks from the definition of JME. Here, we align with the ILAE position statement5 that a history of myoclonic seizures is in fact mandatory for the diagnosis of JME.
Age of seizure onset
One major operational challenge remains the definition of the ‘age of onset’. It could be defined as the time of diagnosis, which is usually after the first GTCS. Given that myoclonic seizures precede GTCS by an average of 1–3.3 years,13,19 the age at first motor seizure may be more appropriate20,21 but then does not account for the subgroup of patients with CAE later developing JME.22 Sometimes the ‘age of onset’ of epilepsy might be difficult to determine in older patients with a single GTCS who do not recall myoclonus in adolescence.23 On the assumption that JME is a neurodevelopmental disorder, an upper maximal age of onset appears plausible.24 Conversely, as a polygenic disease25 with variable penetrance and a high incidence of generalized EEG changes amongst asymptomatic relatives,26 one may assume a variability in both genetic liability and environmental (seizure-provoking) factors allowing for the possibility of late (>25 years) first-time seizures.
The Avignon meeting proposed an age of onset between 10 and 25 years as Class I criterion or between 6 and 25 years as Class II criterion for the diagnosis of JME.4 The ILAE position statement permits an onset between 8 and 40 years, with a warning that an age of onset before 8–9 years or between 25 and 40 years should prompt one to reconsider the diagnosis (Table 1).5 There is broad consensus in the literature about the typical age of first GTCS in JME, which lies between 12 and 18 years in ∼75% of patients.13,19,27-29 However, evidence for exclusionary upper and lower age limits is sparse, and many cohorts included patients with an age of myoclonus onset below 6 or above 25.27-29
The existence of late-onset IGE and IGE with phantom absences as distinct entities remains controversial.21,23,30,31 Suffice to say, late-onset JME is extremely rare. Reichsoellner et al.31 showed that 28/429 IGE patients (5.7%) had late seizure onset, including two with JME (0.5%). JME mimics, including progressive myoclonus epilepsies, in adults are usually accompanied by additional neurological symptoms like dementia or ataxia12,32 (Supplementary Table 1), and true misdiagnosis is uncommon. The same applies to patients with debut before the age of 6. Although there is a broad range of genetic conditions associated with myoclonic epilepsy in children <6 years, progression of neurological/cognitive deficits and seizure severity33 usually exclude the diagnosis of JME. In the BIOJUME data set, the lower age of onset was constrained at 6 years, but the phenotyping committee diagnosed JME in participants with age of onset 6–37 years in females and 9–40 years in males.13
Intelligence
The observation of an impaired cognition and intelligence was not explicitly included in the early definitions, nor as a diagnostic criterion for JME in early papers or the ILAE definition of 1989.17 In the Avignon paper,4 intelligence was included as a criterion in both the proposed classes of diagnostic criteria (Table 1): in Class I, the criterion was ‘normal intelligence’ and in Class II ‘no mental retardation or deterioration’. Although not specifically discussed in the paper, the basis for proposing these criteria was that eight out of the 14 experts believed that ‘abnormal cognition’ was not allowed as part of the JME diagnosis. The recent ILAE position statement admits that ‘mild intellectual disability’ is observed in some patients; however, this clinical feature is considered an alert that should lead to consider alternative diagnoses.5
The question of whether intelligence needs to be normal in JME, and the scientific basis for having normal intelligence as an inclusion criterion, is not well addressed in the existing literature, and it is not further elaborated in the recent ILAE Position Paper.5 A review by Ratcliffe et al.32 addressing cognitive function in IGEs finds that the intelligence quotient (IQ) in JME is consistently reported to be within normal range and only slightly lower than IQ in healthy controls. None of the identified 124 papers in our literature search included patients below an IQ of 70. Thus, these results exemplify the inductive fallacy: namely, most people with JME have normal intelligence ergo normal intelligence defines JME. If we examine the counterfactual argument, then we have to ask first, whether not only those below the normal (95%) range of intelligence are excluded from the diagnosis but also those above the normal range; and second, if individuals with the typical features of JME in the context of intellectual disability do not have JME, then what do they have? This fallacy is likely to have been amplified by studies that focus on the initial diagnosis and concerns about missing progressive myoclonus epilepsies, without a commensurate attention on mature longitudinal studies. Intellectual disability, impaired neurocognitive functions, affected behavioural phenotypes and psychiatric symptoms are often reported and discussed as overlapping features; however, they might represent endophenotypes with shared aetiological factors. Several of the identified genetic susceptibility variants of JME are located in genes or regions inferring susceptibility also to other neurodevelopmental disorders (e.g. GABRA1, EFHC1, and BRD2),34 suggesting common pathological mechanisms. Recently, copy number variants contributing to both intellectual impairment and IGE have been identified (such as microdeletions at 15q13.3, 15q11.2, and 16p13.11),35 supporting the concept that it is possible to diagnose an IGE syndrome in patients with intellectual disability. In addition, recent studies applied advanced MRI technology to reveal subtle brain developmental abnormalities in JME, leading to pathophysiological hypotheses potentially explaining a range of clinical symptoms of JME, including impaired cognitive functions.25,36 As such, the view of JME as a disorder where inherent neurodevelopmental factors may also contribute to the phenotype and where JME is part of a much wider disease spectrum than the traditional inclusion criteria embrace may uncover key pathological mechanisms of JME. Such an expanded phenotypic view would certainly bring some challenges to both clinical and research classifications and perhaps prompt a subclassification of endophenotypes to enable clinical studies of well-defined and homogeneous entities. However, it may also enable the revelation of other hitherto unknown aspects of JME, which have remained concealed due to the limitations of the existing diagnostic criteria.
Morning predominance of myoclonic seizures
Diurnal variability in cortical excitability in individuals with IGE, with cyclic increases in the morning, has been elegantly demonstrated using transcranial magnetic stimulation–electroencephalography (TMS-EEG) methods.37,38 The broader amplitude of the paroxysms in the frontal regions in JME39 suggests a role of the frontal cortex in leading generalized discharges.40 Interestingly, some thalamic nuclei known to modulate functional states such as sleep and wakefulness (e.g. intralaminar nuclei and the reticular nucleus) are directly connected to the frontal cortex. Since the time of awakening is a critical condition for the activation of the epileptic discharges, the evidence that these nuclei play a role in spike-wave generation in experimental models of generalized epilepsies41 may suggest their involvement also in provoking myoclonus in JME. In addition, animal studies suggest that feedback loops of Clock proteins, amongst several other factors, influence this circadian variability as, in their absence, fluctuations in cortical excitability are diminished.42 In the other direction, epilepsy (and many other neuropsychiatric disorders), stress, inflammation, xenobiotics and genetic/epigenetic factors may alter the temporal expression and amplitude of Clock genes, leading to disrupted circadian rhythms in sleep and cortisol,42 possibly explaining the failure to exhibit morning peaks in cortical excitability in some patients with IGE.
The Avignon paper4 emphasizes the exclusive (narrow classification) or predominant (broad) timing of myoclonic seizures on awakening. It is difficult to find data on true circadian seizure distribution in JME because most studies since 1989 use this feature as an inclusion criterion, possibly leading to a circular reinforcement of the definition. This may be an example of the ‘inductive fallacy’, assuming that because the majority of individuals show awakening seizures, and ‘ignoring’ those who do not, that it is an invariant feature and therefore a mandatory diagnostic criterion. However, amongst the earliest descriptions of JME, Janz20 cites two studies in which an awakening predominance varied between 52% and 74%, and his own work acknowledges that such a pattern is not always seen. Subsequent studies report >80% awakening myoclonic seizures, and slightly less for tonic–clonic seizures, even when explicitly using ‘awakening’ seizures as an inclusion criterion (Table 1). In the recent ILAE position statement, myoclonic seizures are reported to occur ‘most commonly’ within the first hour after awakening.5 One study suggested that the lack of the awakening pattern was associated with praxis induction43; and it has also been suggested that awakening myoclonic and ‘grand-mal’ seizures may be differently inherited from those occurring at random times of day.44 When we look at the counterfactual argument, then clearly when myoclonic seizures are the predominant seizure type but not predominantly on awakening and other features (age of onset and EEG) are typical, there is no other diagnostic possibility than JME (Tables 2 and 3).
Table 2.
Previous observations of awakening predominant seizures in JME
| Authors | Inclusion criteria | Awakening MCJ | Awakening GTC |
|---|---|---|---|
| Simonsen 1975, cited in Janz20 | Not stated | 52% | NA |
| Van Heycop ten Ham 1982, cited in Janz20 | Not stated | 74% | NA |
| Janz20 | Not stated | 95% | NA |
| Clement et al. 45 | Not stated | 8/10 (80%) | 6/9 (67%) |
| Panayiotopoulos46 | ‘Unequivocal clinical evidence of generalized seizures with myoclonic jerks mainly on awakening’ | 56/66 (88%) | 11/16 (69%) |
| Murthy47 | ‘Clinical evidence of generalized seizures with myoclonic jerks mainly on waking’ | 112/120 (93.3%) | 87/120 (78.4%) |
| Dhanuka48 | ‘Unequivocal clinical evidence of generalized seizures with myoclonic jerks on awakening’ | 24/24 (100%) | NA |
| Uchida43 | All had unequivocal diagnosis of JME based on ‘electroclinical characteristics, including normal physical and neurological examinations, brain imaging and generalized 4- to 6-Hz spike or polyspike-wave complexes.’7,42 | No Photic Induction 23/25 (92%) Photic induction 12/20 (60%) |
Bold characters highlight seizures occurrence in relation to the sleep-wake cycle.
MCJ, myoclonic jerks; GTC, generalized tonic–clinic seizures.
In the BIOJUME data set, 537/663 (81%) exhibited morning predominant myoclonic jerks. We investigated whether individuals with and without this feature differed significantly in demographic, clinical or prognostic features, i.e. whether awakening versus non-awakening predominant seizures represented a disease different to JME. We found no difference in sex or age distribution, but those lacking morning predominance were more likely to experience absence seizures; there was no difference in photosensitivity (Table 3), no association with triggers and importantly no difference in ASM resistance. In a multiple logistic regression model including sex, age of onset, absence seizures and EEG patterns, we confirmed that morning predominance is independently negatively associated with absence seizures [odds ratio (OR) 0.65; 95% confidence interval (CI) 0.43–0.99] and lack of GSW on EEG (Table 3). We found that individuals lacking morning predominance reported higher trait impulsivity, which may have been confounded by absence seizure frequency.13
The best evidence from these data is that absence seizures and EEG patterns are the most important influences on awakening patterns of seizures in JME. What we do not know is whether patients change in their circadian seizure patterns over time or whether a pattern of morning predominance is established from the onset—this would require a prospective longitudinal study.
Conclusion
We confirm myoclonic seizures as a mandatory diagnostic criterion for JME because of the lack of contrary evidence (Table 1). It is difficult to set age of onset criteria. However, definite cases with onset between the ages of 6 and 40 years have been agreed upon in our BIOJUME data set, thus slightly deviating from the ILAE position statement that admits a lower age limit not inferior to 8 years.5 We do not specify an intelligence range in our proposed definition, admitting an intelligence range conforming to population distribution. Future studies addressing the true IQ distribution of JME may reveal whether the degree of cognitive affection in JME harbours a prognostic value, also in terms of response to treatment, which should be included in a prognostic classification. Last, taking neurobiological, historical and empirical evidence together, we propose that morning predominance be considered a variable, not mandatory, feature in the definition of JME that is irrelevant to prognostic classification.
Prognosis
Mortality
Premature mortality is recognized across epilepsy in general and varies by aetiology and between high- and low-income countries.49 A systematic review of 56 population and hospital-based epilepsy cohorts show that intractable epilepsy, symptomatic epilepsy, generalized seizures, brain tumours and ischaemic heart disease as causal factors and later studies accord with these findings.50,51 Comorbidity, especially neurological, male sex and race/ethnicity were associated with premature mortality amongst US Medicare patients52; demographic and clinical codes also accurately predicted risk of death in epilepsy patients from US insurance data.53 UK data confirm the importance of social deprivation as a risk factor not only for premature mortality in epilepsy but also for intellectual disability.54 The North American SUDEP Registry (NASR) suggested a risk higher than expected in patients with IGE (IGE accounted for one fourth of SUDEP cases in NASR), an association that awaits independent confirmation given the low number of events.55 Premature death or SUDEP seems to be rare in JME. Out of a series of 170 consecutive JME, only two patients with possible or probable SUDEP were reported, both cases suffering from severe mental disorders.56 Few other cases who died probably of SUDEP have been described, either in subjects not taking antiepileptic treatment or with well-controlled epilepsy.57,58
Seizure remission
IGEs in general have a better treatment outcome than symptomatic epilepsies,15,59 but the difference is not stark. The 1-year seizure freedom rate for people aged 9–33 years with IGE was 68.1%, compared with 62.5% for focal epilepsies,60 with some authors reporting much lower rates.61 It is often repeated that ‘80% of people with IGE respond to ASM’,5 but is that still the case when women of child-bearing potential are not to be prescribed valproate?59 There are very few studies either of people with untreated IGE or with a prospective design. Retrospective studies in limited patients series show that seizure remission is possible for many.62,63 Febrile seizures and epileptiform runs >3 s are suggested predictors of poorer long-term outcome in JME,60,64 whilst treatment with valproate and longer seizure freedom prior to ASM withdrawal are associated with higher chance of remission in treated cohorts.65-67 In the JME subclassification of Martinez-Juarez et al.,9 58% of ‘classic JME’ (‘patients with adolescent onset of myoclonic, tonic–clonic and clonic–tonic–clonic seizures with or without rare-to-infrequent absences’) achieve seizure freedom on ASM therapy and 5% off medication, whilst in the group of CAE evolving to JME, only 7% were seizure free. Baykan et al.57 reported a benign course in about two-thirds of a cohort of 48 JME patients, with remission or marked alleviation of myoclonia for >8 years after patients achieved the age of ∼33 years, suggesting that in a significant proportion of patients, myoclonic seizures have a tendency towards remitting or becoming milder with age. Similar results were observed in a retrospective study on 61 JME patients who were followed for a mean duration of ∼29 years; 65% of patients had a 5-year remission with a mean age at the last seizure of 27.4 years.68 One-third of the seizure-free patients attempted ASM withdrawal and ∼50% of them relapsed. In contrast with these findings, another study61 reported a 2-year remission rate only in 22% of subjects in a cohort of 145 JME patients, with twice as high relapse rates in patients attempting drug withdrawal as compared with those who continued their ASM regimen. Finally, a recent study69 investigated in a JME cohort the 4-year remission for all seizure types starting within 2 years (early sustained remission) or after 2 years (delayed sustained remission) since the initiation of ASM intake. Four-year seizure remission was observed in 67.3% subjects, in line with previous studies.57,68 Early sustained remission was achieved by 59.2% patients. Spontaneous seizure relapse after 4-year remission occurred in 15.7% of patients with early sustained remission and in 35.5% of those with delayed sustained remission. Catamenial seizures and earlier age at epilepsy onset significantly predicted delayed sustained remission. These findings suggest that a positive response to the first ASM that leads to an early seizure remission can predict a favourable long-term seizure outcome.
Predictors of drug response
ASM resistance
The literature search yielded 923 studies (search date 11 February 2022), but only two relevant recent papers had not been included in the 2019 meta-analysis of Stevelink et al.15
Age of onset
Data from the BIOJUME consortium show that myoclonus onset before the age of 12 years was associated with drug resistance only in females; the age stratification accords with other studies.15
Sex
In the BIOJUME data set, there are significant treatment outcome differences between males and females with JME, necessitating sex stratification in management.14 Absence seizures strongly predict ASM resistance in both sexes, but, in females only, stress-precipitated seizures and catamenial seizures are associated with ASM resistance and PPR with seizure freedom. Females with both absence seizures and ‘stress-related’ precipitants (defined as physiological states that influence neurobiological stress circuits, including stress itself, sleep deprivation, menstrual cycle and concentration efforts, discussed in Shakeshaft et al.14) constitute the prognostic subgroup in JME with highest prevalence of ASM resistance (49%) compared with females with neither (15%); see also below. A proposed prognostic classification based on these findings is illustrated in Fig. 2.
Figure 2.
Proposed predictive model of ASM resistance in JME based on Shakeshaft et al. 14 At each stratum, blue denotes better prognosis, orange worse prognosis, and grey denotes neutral effect on outcome.
Lack of sex stratification has perhaps contributed to inconsistent evidence for differential prognosis in females in previous studies.70 The relationship between sex and prognosis is nuanced however, suggesting a hierarchy of factors: stress precipitants only increase the odds of ASM resistance in females ‘without’ absence seizures, whilst photosensitivity decreases the odds of ASM resistance in females ‘regardless’ of absence seizures.14
Absence seizures
The strong effect of absence seizures on ASM resistance reported multiple times71 is strongly associated with trait impulsivity in JME.13 Absence seizures, visual sensitivity and stress-related precipitants in JME may not just be clinical features, but the instantiation of separate seizure susceptibility networks with their own distinct effect on seizures and behavioural outcomes,72 which may suggest completely new therapeutic approaches such as circuit-specific therapy.73,74
Triggered seizures
In the BIOJUME data set, around half of all individuals with JME report triggered seizures, with a slight female excess (59% versus 50%).14 Moreover, this subgroup reports a large proportion (median 70%) of their seizures to be triggered, with one in five estimating that ‘all’ their seizures are precipitated. In previous analysis, we saw that just five triggers accounted for >80% of the total: sleep disturbance, stress, alcohol, visual/lights stimuli and menstrual cycle, which are well-known in JME75,76 and other epilepsies.77-79 We found no association of any trigger with seizure control in males but a marked difference in trigger/seizure control associations, depending on whether females experience absence seizures.
Photoparoxysmal response
PPR recorded in the EEG lab can be seen in up to 90% of untreated subjects with JME according to the ILAE position statement.5 However, precise estimates can be marred by the fact that photosensitivity can fluctuate across days or weeks, can be lost with age, can be modified by treatment, and can depend on laboratory stimulation procedures.80 PPR and self-reported photosensitivity are also more common in females, consistent with previous studies showing a female excess of 1.5–2.81-83 BIOJUME data analysis shows a strong relationship between self-reported triggered seizures and PPR, with 71% of those with PPR reporting triggered seizures.14 More specifically, 63% of those who report light/visual patterns as a trigger also had PPR, and 24% of those with PPR reported light/visual patterns as a trigger.14 A lesser degree of association was observed between the presence of PPR and other precipitants: stress, sleep disturbance, praxis and concentration in females, and with alcohol in males.14 This finding suggests that failure to conduct a sex-stratified analysis may explain the lack of overall association in a recent meta-analysis that found a protective effect of PPR on seizure freedom in four out of five studies.70 One possibility is that the component of seizure susceptibility mediated via visual pathway hypersensitivity76,84,85 is effectively treated by current ASMs, a hypothesis we are unable to test in the BIOJUME data but that merits further investigation.
Psychiatric comorbidity
Coexistent psychiatric or personality disorders are associated with ASM resistance10,15,27,57,86-88 although in the absence of prospective studies, the direction of this association is uncertain.89
EEG biomarkers
Focal EEG features have been variably defined in the literature (e.g. lateralized GSW, asymmetric amplitude of GSW, focal spikes or focal slowing),87 but prognostic analyses have not included known predictors in multivariable analyses.90,91 Hence, although focal EEG features have been proposed as a predictor of ASM resistance and refuted by others,60,92 confounding by other variables is possible, and hence their utility is unproven. However, other EEG features93 such as generalized polyspike train during sleep have been validated in multivariable analyses and replicated in IGE cohorts, including JME.93,94 Differences in functional network topology computed from EEG may also provide biomarkers of drug resistance.95 However, the assessment of specific EEG features, or prolonged recording, is not a routine part of the neurophysiological practice and therefore may be impractical for a simple classification scheme.
ASM adverse events
Our literature search identified 470 articles related to levetiracetam (n = 20 relevant after reviewing abstracts), 522 for valproate (n = 19) and 381 for lamotrigine (n = 34). Adverse effects to the three most common ASMs used in IGE appear to correlate with efficacy: valproate, levetiracetam and lamotrigine in declining order.59,96-98 Since the probability of failure with the first ASM is so high,59 due to either lack of efficacy or intolerable adverse effects, there is an urgent need for predictive tools to avoid iatrogenic morbidity. Some adverse effects are shared across ASMs, for example somnolence and dizziness, whilst others are more specific such as paradoxical seizure exacerbation, or skin rash with lamotrigine; psychiatric disturbance with levetiracetam99; and weight gain or polycystic ovary syndrome with valproate.100
Levetiracetam
Attempts have been made to develop predictors of psychiatric adverse effects to levetiracetam.13,101,102 The most recent of these showed that people who had levetiracetam-induced psychosis had an increased polygenic risk score for schizophrenia than those who did not.103 HLA-A*1101 is enriched amongst Korean epilepsy patients with psychiatric adverse events to levetiracetam.104 Genetic variation in dopaminergic activity has also been suggested as an association.102
Trait impulsivity is independently associated with the risk of an adverse psychiatric event on levetiracetam, as shown by a score on the BIS-Brief (8 items) of ≥21.13 This association is independent of sex and seizure frequency and replicates similar findings using the extended BIS (BIS-11).102 However, the predictive value of BIS ≥ 21 is poor, with a positive predictive value (PPV) 50% and negative predictive value 69% in the discovery data set.13 Higher cut points of BIS increase PPV marginally at the cost of sensitivity. Female sex, social deprivation, a past history of depression, anxiety, personality disorder or recreational drug use (all associated with impulsivity) and status epilepticus have also been suggested as risk factors for psychiatric adverse events to levetiracetam101,104,105 and topiramate106,107; these factors need prospective validation.
Valproate
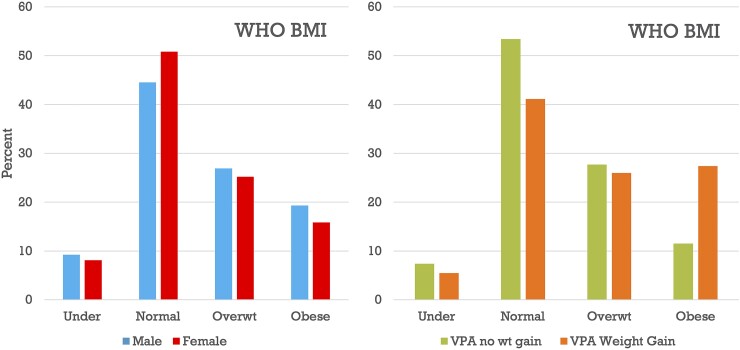
Valproate exposure is associated with obesity in the BIOJUME data set (Fig. 3). We found no relevant univariate or multivariate associations with valproate weight gain in the BIOJUME data set including BIS-Brief score, a measure of trait impulsivity (Table 4). Candidate gene studies suggest that valproate-associated weight gain is associated with (i) variation in satiety and energy homeostasis genes leptin and ankyrin101 and (ii) polymorphisms in Cytochrome P450, CYP2C19 and in CD36, PPARγ, GNB3 in Han Chinese.108-113 (Fig. 3 and Table 4).
Figure 3.
Association of valproate with obesity in the BIOJUME data set. WHO categorization of weight in 695 JME patients in the BIOJUME data set by sex (left) and by lifetime exposure to valproate (right). Legend: BMI, body mass index; Overwt, overweight; VPA, valproate; WHO, World Health Organization; wt, weight.
Lamotrigine
There is a known HLA predisposition to severe cutaneous reaction to aromatic ASMs (phenytoin, carbamazepine, oxcarbazepine, and lamotrigine),114 and these HLA types are more common in certain ancestries such as HLAB*15:02 in Chinese and Southeast Asian; HLAB*24:02 in Han Chinese and possibly other Asian, European and American populations115,116; HLAB*31:01 in Japanese; and HLAB*44:03 in Koreans.114 Several other candidate loci have been reported in different populations.117-119 Independent risk factors such as history of previous cutaneous reactions with another ASM, age < 13 years and polytherapy were identified in retrospective data.120,121
Discussion
We propose a simplified set of mandatory criteria for defining JME that refine and expand those proposed recently by the ILAE Position Paper on definition of IGEs.5 However, differently from this position statement, our proposal, besides literature data, is based on previous analysis performed by our group in the large BIOJUME cohort, thus advancing from current definitions resulting from expert consensus to criteria based on ‘ground truth’ clinical data. The criteria that we propose include (i) myoclonic jerks as mandatory seizure type and (ii) generalized EEG abnormalities, in line with the ILAE statement; in addition, we provide as additional criteria that deviate from the ILAE proposal: (iii) absence of a circadian timing for myoclonia not exclusionary for JME; (iv) age of onset ranging from 6 to 40 years consistent with the diagnosis of JME; and (v) intelligence conforming to population distribution. We show the prognostic value, when appropriately analysed, of some variables (such as absence seizures and female sex with regard to ASM resistance), whilst we find that other features (e.g. morning predominance of myoclonia) had no value and may be discarded. When considering prognosis, a simple model is not sufficient to understand the outcome of interest, such as drug response or adverse events. Evidence suggests that, for ASM resistance in JME, female sex is a key stratifying variable overlooked in previous research or unmasked by the avoidance of valproate in females of child-bearing age. A sex-stratified analysis brings into perspective the prognostic roles and therapeutic potential of taking in consideration and making recommendations manipulating stress response, catamenial seizures and photosensitivity in women. Future research should focus on refining, replicating and validating proposed predictive models122 using large-scale data sets and meta-analyses and in mapping stratifying factors onto evidence-based interventions and clinical guidelines (Table 5).
Table 5.
Robust and potential stratifiers in JME
| Robust criteria | Requires validation | |
|---|---|---|
| Variable features for treatment outcome prediction | Absence seizures Female sex Catamenial seizures Photosensitivity Age of first motor seizure onset <12 years |
Stress-triggered seizures in females |
| Variable features for prediction of selected adverse event to ASM | Lamotrigine rash
|
Levetiracetam psychiatric symptoms
|
Existing taxonomy structures do not focus on outcomes; thus, their usefulness for stratified medicine or for clinical trials is limited.123,124 When we focus on prognosis, the relevance of definition and classification becomes obvious. In this paper, we propose a set of simplified criteria to define JME aiming to avoid the inductive fallacies characterized by earlier attempts, and our scheme is robust to counterfactual arguments. The debate around the morning occurrence of myoclonic seizures seems moot in light of the finding that lack of circadian pattern probably reflects the co-occurrence of absence seizures and EEG GSWs. An interesting hypothesis that should be tested in patients featuring absence seizures is whether morning predominance could be, at least partially, restored by complete control of this seizure type.
There are enough markers of differential treatment outcome to make a case for a stratified treatment approach in JME. The idea of stratified treatment is novel in epilepsy but commonplace, for example, in cardiology and oncology. In this example, it would entail selecting treatment according to the various prognostic subgroups that each patient falls into guided by a combination of demographic, clinical, neurophysiological, molecular or other biomarker indications. The change from current practice is to consider the management of each prognostic factor separately or in combination as appropriate and according to the relative influence of each factor on the outcome of interest. An analogy might be made with managing cardiovascular disease, simultaneously attending to blood pressure, lipids, exercise and diet. In JME, absence seizures are the strongest stratifying factor with regard to ASM resistance or seizure freedom for both sexes, and this finding has been replicated multiple times.15 After absence seizures, it is evident that sex is the major stratifying factor, revealing very strongly elevated odds of ASM resistance conferred by self-report of catamenial and stress-related factors including sleep deprivation, as well as reduced odds of ASM resistance associated with EEG-measured or self-reported photosensitivity, selectively in women. Although menstrual and stress-related triggers have long been recognized,75,76,125,126 their influence on prognosis in women has not previously been appreciated; this may warrant further research aiming to develop predictive models. Sex-stratified meta-analyses of existing data sets using individual patient data would be helpful to replicate these findings, and prospective studies in inception cohorts are necessary to validate them in real-world practice for JME and other epilepsies. Appropriately validated biomarkers could be incorporated into clinical guidelines and clinical trial designs.
In addition to identifying groups at differential risk of ASM resistance, stratifiers should ideally map onto specific evidence-based interventions. There is an urgent need for clinical trials of novel or existing/adjunctive ASMs stratified on prognostic factors such as the occurrence of absence seizures in JME. Catamenial seizures are also well recognized as a risk factor for ASM resistance; secondary analysis of previous hormonal trials suggests the need for further stratification according to seizure susceptibility at different times during the menstrual cycle.127 Although the female-specific prognostic significance of response to stressors including sleep deprivation needs replication, there is a notable lack of good quality evidence for the effectiveness of stress or sleep management strategies in epilepsy.128-130 Additionally, whilst lifestyle management improves stress, mental health and sleep, the evidence for sustained effects on primary disease measures is less solid.131-133 Pharmacological and non-pharmacological interventions such as cognitive behavioural therapy, mindfulness-based relaxation, yoga, play, biofeedback and exercise should be evaluated according to stress-response profiles.134 Connected to lifestyle modification is the seizure refractoriness due to ASM non-compliance (i.e. ‘pseudo-resistance’) that might be linked to the psychological challenges of regulating lifestyle or adhering to prescribed medications or to the impulsivity trait, as recently reported.135 This is especially salient in JME where we know that impulsivity is markedly elevated13,136-138 and increases general risk for multiple adverse psychosocial consequences related to lifestyle, relationships and behaviour.139
In contrast to ASM resistance, there are few validated predictors of ASM adverse events other than known HLA associations with drug-induced immune adverse response.114 Behavioural traits and psychiatric history might predict psychiatric adverse events to multiple ASMs10,15,27,57,86-88; however, prospective studies are needed to validate these hypotheses. Whilst valproate prescription has rightfully declined in recent years because of teratogenicity, it remains the most effective ASM in JME,59,98,140 ensuring its continued role in men. However, one of its main adverse events leading to withdrawal is weight gain, and predicting this risk would be a prioritized patient benefit.
Identifying molecular biomarkers is an alternative approach to prediction than using clinical and demographic variables. Molecular biomarkers are likely to contribute greatly to prognosis in future epilepsy practice. For example, there is evolving evidence that the circulating inflammatory marker HMGB1 may predict seizure severity and drug resistance at epilepsy diagnosis.141,142 Similarly, polygenic risk scores will assume an increasing role in enhancing risk prediction models and clinical pathways.143 EEG-based prognosis can be limited in resource-poor settings, or when relying on age-related EEG markers (such as GSW before ASM is started, or PPR in adolescence) but the opportunity for that EEG was missed, and the patient is now an adult. The prognostic EEG features reviewed here have current drawbacks in terms of human resources, but these could be solved by AI approaches that learn from high dimensional data sets. A machine learning model predicted drug resistance 2 years before epilepsy patients had failed two ASMs and could predict which patients would fail more than or equal to three ASMs at the time of first ASM prescription.144 Whilst we have focused on medical outcomes here, we should not neglect psychosocial outcomes, which typically arise from multiple genetic, early life and stressful life events interacting with adjustment styles and social support.145 Detailed lifespan studies or population linkage data sets would be indispensable to generate appropriate psychosocial prognostic models.
Conclusion
Our paper provides evidence-based diagnostic criteria for JME based on clinical data collected by the BIOJUME Consortium, supplemented by literature review, and it proposes a predictive model of ASM resistance that shows the prognostic value of variables such as absence seizures and female sex in predicting ASM resistance or the relevance of photosensitivity in females in reducing the odds of ASM refractoriness. These findings may assist in clinical practice by helping in the early diagnosis of drug-resistant patients or in the management of their treatment, particularly when drug withdrawal is considered after years of seizure freedom. Our findings can inspire future research either to replicate and validate them, to develop further prediction models in large-scale data sets and to assess whether the stratifying factors that we have outlined can serve to measure intervention-related outcomes and whether they can be meaningfully incorporated in clinical guidelines.
Supplementary Material
Abbreviations
- ASM =
antiseizure medications
- BIOJUME =
Biology of Juvenile Myoclonic Epilepsy
- BIS =
Barratt Impulsiveness Scale
- BMI =
body mass index
- CAE =
childhood absence epilepsy
- EGTCSA =
epilepsy with generalized tonic–clonic seizures alone
- GSW =
generalized spike wave
- GTCS =
generalized tonic–clonic seizure
- HLA =
human leucocyte antigen
- ICD-10 =
International Classification of Diseases 10th Revision
- IGE =
idiopathic generalized epilepsy
- ILAE =
International League Against Epilepsy
- IQ =
intelligence quotient
- JME =
juvenile myoclonic epilepsy
- NASR =
North American SUDEP Registry
- PPR =
photoparoxysmal response
- PPV =
positive predictive value
- SNOMED-CT =
Systematized Nomenclature of Medicine–Clinical Terms
- SUDEP =
sudden unexpected death in epilepsy
- TMS-EEG =
transcranial magnetic stimulation–electroencephalography
Appendix
BIOJUME Consortium:
Lisa Strug, Naim Panjwani, Fan Lin, Danielle Andrade, Jana Zarubova, Zuzana Šobíšková, Cechovaz, Pracoviste, Michaela Kajsova, Guido Rubboli, Rikke S. Møller, Elena Gardella, Christoph P. Beier, Joanna Gesche, Maria Miranda, Inga Talvik, Pasquale Striano, Alessandro Orsini, Choong Yi Fong, Ching Ching Ng, Kheng Seang Lim, Kaja K. Selmer, Marte Syvertsen, Pronab Bala, Amy Kitching, Kate Irwin, Lorna Walding, Lynsey Adams, Uma Jegathasan, Rachel Swingler, Rachel Wane, Julia Aram, Nikil Sudarsan, Dee Mullan, Rebecca Ramsay, Vivien Richmond, Mark Sargent, Paul Frattaroli, Matthew Taylor, Marie Home, Sal Uka, Susan Kilroy, Tonicha Nortcliffe, Halima Salim, Kelly Holroyd, Khalid Hamandi, Alison McQueen, Dympna Mcaleer, Dina Jayachandran, Dawn Egginton, Bridget MacDonald, Michael Chang, David Deekollu, Alok Gaurav, Caroline Hamilton, Jaya Natarajan Inyan Takon, Janet Cotta, Nick Moran, Jeremy Bland, Rosemary Belderbos, Heather Collier, Joanne Henry, Matthew Milner, Sam White, Michalis Koutroumanidis, William Stern, Mark P. Richardson, Jennifer Quirk, Javier Peña Ceballos, Anastasia, Papathanasiou, Ioannis Stavropoulos, Dora Lozsadi, Andrew Swain, Charlotte Quamina, Jennifer Crooks, Tahir Majeed, Sonia Raj, Shakeelah Patel, Michael Young, Melissa Maguire, Munni Ray, Caroline Peacey, Linetty Makawa, Asyah Chhibda, Eve Sacre, Shanaz Begum, Martin O’Malley, Lap Yeung, Claire Holliday, Louise Woodhead, Karen Rhodes, Rhys Thomas, Shan Ellawela, Joanne Glenton, Verity Calder, John Davis, Paul McAlinden, Sarah Francis, Lisa Robson, Karen Lanyon, Graham Mackay, Elma Stephen, Coleen Thow, Margaret Connon, Martin Kirkpatrick, Susan MacFarlane, Anne Macleod, Debbie Rice, Siva Kumar, Carolyn Campbell, Vicky Collins, William Whitehouse, Christina Giavasi, Boyanka Petrova, Thomas Brown, Catie Picton, Michael O’Donoghue, Charlotte West, Helen Navarra, Seán J. Slaght, Catherine Edwards, Andrew Gribbin, Liz Nelson, Stephen Warriner, Heather Angus-Leppan, Loveth Ehiorobo, Bintou Camara, Tinashe Samakomva, Rajiv Mohanraj, Vicky Parker, Rajesh Pandey, Lisa Charles, Catherine Cotter, Archana Desurkar, Alison Hyde, Rachel Harrison, Markus Reuber, Rosie Clegg, Jo Sidebottom, Mayeth Recto, Patrick Easton, Charlotte Waite, Alice Howell, Jacqueline Smith, Rosie Clegg, Shyam Mariguddi, Zena Haslam, Elizabeth Galizia, Hannah Cock, Mark Mencias, Samantha Truscott, Deirdre Daly, Hilda Mhandu, Nooria Said, Mark Rees, Seo-Kyung Chung, Owen Pickrell, Beata Fonferko-Shadrach, Mark Baker, Amy Whiting, Louise Swain, Kirsty O’Brien, Fraser Scott, Naveed Ghaus, Gail Castle, Jacqui Bartholomew, Ann Needle, Julie Ball, Andrea Clough, Shashikiran Sastry, Charlotte Busby Amit Agrawal, Debbie Dickerson, Almu Duran, Muhammad Khan, Laura Thrasyvoulou, Eve Irvine, Sarah Tittensor, Jacqueline Daglish, Sumant Kumar, Claire Backhouse, Claire Mewies, Julia Aram, Nikil Sudarsan, Dee Mullan, Rebecca Ramsay, Vivien Richmond, Denise Skinner, Mark Sargent, Rahul Bharat, Sarah-Jane Sharman, Arun Saraswatula, Helen Cockerill, David A. Greenberg.
For details of BIOJUME sites, see Supplementary Appendix 1.
Contributor Information
Guido Rubboli, Danish Epilepsy Centre, Filadelfia, Dianalund 4293, Denmark; Institute of Clinical Medicine, University of Copenhagen, Copenhagen 2200, Denmark.
Christoph P Beier, Department of Neurology, Odense University Hospital, Odense 5000, Denmark.
Kaja K Selmer, Department of Research and Innovation, Division of Clinical Neuroscience, Oslo University Hospital, Oslo 0372, Norway; National Centre for Epilepsy, Oslo University Hospital, Oslo 1337, Norway.
Marte Syvertsen, Department of Neurology, Drammen Hospital, Vestre Viken Health Trust, Oslo 3004, Norway.
Amy Shakeshaft, Department of Basic and Clinical Neurosciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK; MRC Centre for Neurodevelopmental Disorders, King’s College London, London SW1H 9NA, UK.
Amber Collingwood, Department of Basic and Clinical Neurosciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Anna Hall, Department of Basic and Clinical Neurosciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Danielle M Andrade, Adult Epilepsy Genetics Program, Krembil Research Institute, University of Toronto, Toronto M5T 0S8, Canada.
Choong Yi Fong, Division of Paediatric Neurology, Department of Pediatrics, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia.
Joanna Gesche, Department of Neurology, Odense University Hospital, Odense 5000, Denmark.
David A Greenberg, Abigail Wexner Research Institute, Nationwide Children’s Hospital, Columbus 43215, USA.
Khalid Hamandi, Department of Neurology, Cardiff & Vale University Health Board, Cardiff CF14 4XW, UK.
Kheng Seang Lim, Division of Neurology, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia.
Ching Ching Ng, Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur 50603, Malaysia.
Alessandro Orsini, Department of Clinical and Experimental Medicine, Pisa University Hospital, Pisa 56126, Italy.
Pasquale Striano, Pediatric Neurology and Muscular Disease Unit, IRCCS Istituto ‘G. Gaslini’, Genova 16147, Italy; Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genova, Genova 16132, Italy.
Rhys H Thomas, Newcastle upon Tyne NHS Foundation Trust, Newcastle upon Tyne NE7 7DN, UK; Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne NE1 7RU, UK.
Jana Zarubova, Department of Neurology, Second Faculty of Medicine, Charles University, Prague 150 06, Czech Republic; Motol University Hospital, Prague 150 06, Czech Republic.
Mark P Richardson, Department of Basic and Clinical Neurosciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK; MRC Centre for Neurodevelopmental Disorders, King’s College London, London SW1H 9NA, UK; School of Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King’s College, London SE5 8AF, UK.
Lisa J Strug, Program in Genetics and Genome Biology, The Hospital for Sick Children, Toronto M5G 1X8, Canada; Departments of Statistical Sciences and Computer Science and Division of Biostatistics, The University of Toronto, Toronto M5G 1Z5, Canada.
Deb K Pal, Department of Basic and Clinical Neurosciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK; MRC Centre for Neurodevelopmental Disorders, King’s College London, London SW1H 9NA, UK; School of Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King’s College, London SE5 8AF, UK.
BIOJUME Consortium:
Lisa Strug, Naim Panjwani, Fan Lin, Danielle Andrade, Jana Zarubova, Zuzana Šobíšková, Cechovaz, Pracoviste, Michaela Kajsova, Guido Rubboli, Rikke S Møller, Elena Gardella, Christoph P Beier, Joanna Gesche, Maria Miranda, Inga Talvik, Pasquale Striano, Alessandro Orsini, Choong Yi Fong, Ching Ching Ng, Kheng Seang Lim, Kaja K Selmer, Marte Syvertsen, Pronab Bala, Amy Kitching, Kate Irwin, Lorna Walding, Lynsey Adams, Uma Jegathasan, Rachel Swingler, Rachel Wane, Julia Aram, Nikil Sudarsan, Dee Mullan, Rebecca Ramsay, Vivien Richmond, Mark Sargent, Paul Frattaroli, Matthew Taylor, Marie Home, Sal Uka, Susan Kilroy, Tonicha Nortcliffe, Halima Salim, Kelly Holroyd, Khalid Hamandi, Alison McQueen, Dympna Mcaleer, Dina Jayachandran, Dawn Egginton, Bridget MacDonald, Michael Chang, David Deekollu, Alok Gaurav, Caroline Hamilton, Jaya Natarajan Inyan Takon, Janet Cotta, Nick Moran, Jeremy Bland, Rosemary Belderbos, Heather Collier, Joanne Henry, Matthew Milner, Sam White, Michalis Koutroumanidis, William Stern, Mark P Richardson, Jennifer Quirk, Javier Peña Ceballos, Anastasia, Papathanasiou, Ioannis Stavropoulos, Dora Lozsadi, Andrew Swain, Charlotte Quamina, Jennifer Crooks, Tahir Majeed, Sonia Raj, Shakeelah Patel, Michael Young, Melissa Maguire, Munni Ray, Caroline Peacey, Linetty Makawa, Asyah Chhibda, Eve Sacre, Shanaz Begum, Martin O’Malley, Lap Yeung, Claire Holliday, Louise Woodhead, Karen Rhodes, Rhys Thomas, Shan Ellawela, Joanne Glenton, Verity Calder, John Davis, Paul McAlinden, Sarah Francis, Lisa Robson, Karen Lanyon, Graham Mackay, Elma Stephen, Coleen Thow, Margaret Connon, Martin Kirkpatrick, Susan MacFarlane, Anne Macleod, Debbie Rice, Siva Kumar, Carolyn Campbell, Vicky Collins, William Whitehouse, Christina Giavasi, Boyanka Petrova, Thomas Brown, Catie Picton, Michael O’Donoghue, Charlotte West, Helen Navarra, Seán J Slaght, Catherine Edwards, Andrew Gribbin, Liz Nelson, Stephen Warriner, Heather Angus-Leppan, Loveth Ehiorobo, Bintou Camara, Tinashe Samakomva, Rajiv Mohanraj, Vicky Parker, Rajesh Pandey, Lisa Charles, Catherine Cotter, Archana Desurkar, Alison Hyde, Rachel Harrison, Markus Reuber, Rosie Clegg, Jo Sidebottom, Mayeth Recto, Patrick Easton, Charlotte Waite, Alice Howell, Jacqueline Smith, Rosie Clegg, Shyam Mariguddi, Zena Haslam, Elizabeth Galizia, Hannah Cock, Mark Mencias, Samantha Truscott, Deirdre Daly, Hilda Mhandu, Nooria Said, Mark Rees, Seo-Kyung Chung, Owen Pickrell, Beata Fonferko-Shadrach, Mark Baker, Amy Whiting, Louise Swain, Kirsty O’Brien, Fraser Scott, Naveed Ghaus, Gail Castle, Jacqui Bartholomew, Ann Needle, Julie Ball, Andrea Clough, Shashikiran Sastry, Charlotte Busby Amit Agrawal, Debbie Dickerson, Almu Duran, Muhammad Khan, Laura Thrasyvoulou, Eve Irvine, Sarah Tittensor, Jacqueline Daglish, Sumant Kumar, Claire Backhouse, Claire Mewies, Julia Aram, Nikil Sudarsan, Dee Mullan, Rebecca Ramsay, Vivien Richmond, Denise Skinner, Mark Sargent, Rahul Bharat, Sarah-Jane Sharman, Arun Saraswatula, Helen Cockerill, and David A Greenberg
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This work was supported by the Canadian Institutes of Health Research: Biology of Juvenile Myoclonic Epilepsy 201503MOP-342469 (D.K.P. and L.J.S.) and 201809FDN-407295 (L.J.S.); UK Medical Research Council, Centre for Neurodevelopmental Disorders MR/N026063/1 (D.K.P. and M.P.R.); UK Medical Research Council, Programme grant MR/K013998/1 (M.P.R.); PhD stipend from UK Medical Research Council and the Sackler Institute for Translational Neurodevelopment (A.S.); National Institute for Health and Care Research Specialist Biomedical Research Centre for Mental Health of South London and Maudsley National Health Service Foundation Trust (D.K.P. and M.P.R.); UK Engineering and Physical Sciences Research Council, Centre for Predictive Modelling in Healthcare (EP/N014391/1) (M.P.R.); Grant 21/17483 from Region Southern Denmark (J.G.); Ministero della Salute - Ricerca corrente 2022; and DINOGMI. Dipartimento di Eccellenza MIUR 2018–2022 (P.S.).
Competing interests
G.R. received speaker honoraria from UCB, EISAI, Arvelle and consultancy honoraria from Ology Medical Education. C.P.B. received honoraria from UCB, EISAI and Arvelle and travel support from Arvelle. K.K.S. received speaker honoraria and travel support from UCB. M.S. received speakers honoraria from EISAI and Angelini Pharma. R.T. received honoraria from Arvelle/Angelini, Bial, Eisai, GW/Jazz, Sanofi, UCB Pharma, UNEEG, Zogenix.
Data availability
The data supporting the findings and conclusions of this study are available from the corresponding authors upon reasonable request.
References
- 1. Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: Strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov. 2007;6:287–293. [DOI] [PubMed] [Google Scholar]
- 2. SNOMED CT Browser . Juvenile myoclonic epilepsy (disorder). Accessed 21 August 2022. https://snomedbrowser.com/Codes/Details/6204001
- 3. ICD10Data.com . Juvenile myoclonic epilepsy, intractable, without status epilepticus. Accessed 21 August 2022. https://www.icd10data.com/ICD10CM/Codes/G00-G99/G40-G47/G40-/G40.B09
- 4. Kasteleijn-Nolst Trenite DG, Schmitz B, Janz D, et al. Consensus on diagnosis and management of JME: From founder's observations to current trends. Epilepsy Behav. 2013;28(Suppl 1):S87–S90. [DOI] [PubMed] [Google Scholar]
- 5. Hirsch E, French J, Scheffer IE, et al. ILAE definition of the idiopathic generalized epilepsy syndromes: Position statement by the ILAE task force on nosology and definitions. Epilepsia. 2022;63(6):1475–1499. [DOI] [PubMed] [Google Scholar]
- 6. Bureau M, Genton P, Delgado-Escueta AV, et al. Epileptic syndromes in infancy, childhood and adolescence. 6th edn. John Libbey; 2019. [Google Scholar]
- 7. EpilepsyDiagnosis.org . Accessed 21 August 2022. https://www.ilae.org/education/diagnostic-manual
- 8. Wirrell EC, Nabbout R, Scheffer IE, et al. Methodology for classification and definition of epilepsy syndromes with list of syndromes: Report of the ILAE task force on nosology and definitions. Epilepsia. 2022;63:1333–1348. [DOI] [PubMed] [Google Scholar]
- 9. Martinez-Juarez IE, Alonso ME, Medina MT, et al. Juvenile myoclonic epilepsy subsyndromes: Family studies and long-term follow-up. Brain. 2006;129:1269–1280. [DOI] [PubMed] [Google Scholar]
- 10. Guaranha MS, Filho GM, Lin K, Guilhoto LM, Caboclo LO, Yacubian EM. Prognosis of juvenile myoclonic epilepsy is related to endophenotypes. Seizure. 2011;20:42–48. [DOI] [PubMed] [Google Scholar]
- 11. Baykan B, Wolf P. Juvenile myoclonic epilepsy as a spectrum disorder: A focused review. Seizure. 2017;49:36–41. [DOI] [PubMed] [Google Scholar]
- 12. Genton P GP, Thomas P, Serafini A, et al. Juvenile myoclonic epilepsies. In: Bureau MGenton P and Delgado-Escueta AV, eds. Epileptic syndromes in infancy, childhood and adolescence. John Libbey; 2019:329–356. [Google Scholar]
- 13. Shakeshaft A, Panjwani N, McDowall R, et al. Trait impulsivity in juvenile myoclonic epilepsy. Ann Clin Transl Neurol. 2021;8:138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shakeshaft A, Panjwani N, Collingwood A, et al. Sex specific disease modifiers in juvenile myoclonic epilepsy. Sci Rep. 2022;12:2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevelink R, Koeleman BPC, Sander JW, Jansen FE, Braun KPJ. Refractory juvenile myoclonic epilepsy: A meta-analysis of prevalence and risk factors. Eur J Neurol. 2019;26:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janz D, Christian W. Impulsiv-peti mal. Dtsch Z Nervenheilk. 1957;176:348–386. [Google Scholar]
- 17. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on classification and terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 18. Asconapé J, Penry JK. Some clinical and EEG aspects of benign juvenile myoclonic epilepsy. Epilepsia. 1984;25:108–114. [DOI] [PubMed] [Google Scholar]
- 19. Tsuboi T, Christian W. On the genetics of the primary generalized epilepsy with sporadic myoclonias of impulsive petit mal type. Humangenetik. 1973;19:155–182. [DOI] [PubMed] [Google Scholar]
- 20. Janz D. Epilepsy with impulsive petit mal (juvenile myoclonic epilepsy). Acta Neurol Scand. 1985;72:449–459. [DOI] [PubMed] [Google Scholar]
- 21. Syvertsen M, Hellum MK, Hansen G, et al. Prevalence of juvenile myoclonic epilepsy in people <30 years of age—A population-based study in Norway. Epilepsia. 2017;58:105–112. [DOI] [PubMed] [Google Scholar]
- 22. Wirrell EC, Camfield CS, Camfield PR, Gordon KE, Dooley JM. Long-term prognosis of typical childhood absence epilepsy: Remission or progression to juvenile myoclonic epilepsy. Neurology. 1996;47:912–918. [DOI] [PubMed] [Google Scholar]
- 23. Loiseau J, Crespel A, Picot MC, et al. Idiopathic generalized epilepsy of late onset. Seizure. 1998;7:485–487. [DOI] [PubMed] [Google Scholar]
- 24. Delgado-Escueta AV, Enrile-Bacsal F. Juvenile myoclonic epilepsy of Janz. Neurology. 1984;34:285–294. [DOI] [PubMed] [Google Scholar]
- 25. Gilsoul M, Grisar T, Delgado-Escueta AV, de Nijs L, Lakaye B. Subtle brain developmental abnormalities in the pathogenesis of juvenile myoclonic epilepsy. Front Cell Neurosci. 2019;13:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leu C, Stevelink R, Smith AW, et al. Polygenic burden in focal and generalized epilepsies. Brain. 2019;142:3473–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Höfler J, Unterberger I, Dobesberger J, Kuchukhidze G, Walser G, Trinka E. Seizure outcome in 175 patients with juvenile myoclonic epilepsy—a long-term observational study. Epilepsy Res. 2014;108:1817–1824. [DOI] [PubMed] [Google Scholar]
- 28. Gesche J, Christensen J, Hjalgrim H, Rubboli G, Beier CP. Epidemiology and outcome of idiopathic generalized epilepsy in adults. Eur J Neurol. 2020;27:676–684. [DOI] [PubMed] [Google Scholar]
- 29. Asadi-Pooya AA, Emami M, Sperling MR. Age of onset in idiopathic (genetic) generalized epilepsies: Clinical and EEG findings in various age groups. Seizure. 2012;21:417–421. [DOI] [PubMed] [Google Scholar]
- 30. Brigo F, Tavernelli V, Nardone R, Trinka E. De novo late-onset absence status epilepticus or late-onset idiopathic generalized epilepsy? A case report and systematic review of the literature. Epileptic Disord. 2018;20:123–131. [DOI] [PubMed] [Google Scholar]
- 31. Reichsoellner J, Larch J, Unterberger I, et al. Idiopathic generalised epilepsy of late onset: A separate nosological entity? J Neurol Neurosurg Psychiatry. 2010;81(11):1218–1222. [DOI] [PubMed] [Google Scholar]
- 32. Ratcliffe C, Wandschneider B, Baxendale S, Thompson P, Koepp MJ, Caciagli L. Cognitive function in genetic generalized epilepsies: Insights from neuropsychology and neuroimaging. Front Neurol. 2020;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orsini A, Valetto A, Bertini V, et al. The best evidence for progressive myoclonic epilepsy: A pathway to precision therapy. Seizure. 2019;71:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thakran S, Guin D, Singh P, et al. Genetic landscape of common epilepsies: Advancing towards precision in treatment. Int J Mol Sci. 2020;21:7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mullen SA, Carvill GL, Bellows S, et al. Copy number variants are frequent in genetic generalized epilepsy with intellectual disability. Neurology. 2013;81:1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hatton SN, Huynh KH, Bonilha L, et al. White matter abnormalities across different epilepsy syndromes in adults: An ENIGMA-Epilepsy study. Brain. 2020;143:2454–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Why do seizures in generalized epilepsy often occur in the morning? Neurology. 2009;73:218–222. [DOI] [PubMed] [Google Scholar]
- 38. Manganotti P, Bongiovanni LG, Fuggetta G, Zanette G, Fiaschi A. Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: A combined transcranial magnetic stimulation and EEG study. J Neurol Neurosurg Psychiatry. 2006;77:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panzica F, Rubboli G, Franceschetti S, et al. Cortical myoclonus in Janz syndrome. Clin Neurophysiol. 2001;112:1803–1809. [DOI] [PubMed] [Google Scholar]
- 40. Niedermeyer E. Primary (idiopathic) generalized epilepsy and underlying mechanisms. Clin Electroencephalogr. 1996;27:1–21. [DOI] [PubMed] [Google Scholar]
- 41. Avanzini G, Vergnes M, Spreafico R, Marescaux C. Calcium-dependent regulation of genetically determined spike and waves by the reticular thalamic nucleus of rats. Epilepsia. 1993;34:1–7. [DOI] [PubMed] [Google Scholar]
- 42. Re CJ, Batterman AI, Gerstner JR, Buono RJ, Ferraro TN. The molecular genetic interaction between circadian rhythms and susceptibility to seizures and epilepsy. Front Neurol. 2020;23(11):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uchida CG, de Carvalho KC, Guaranha MS, et al. Phenotyping juvenile myoclonic epilepsy. Praxis induction as a biomarker of unfavorable prognosis. Seizure. 2015;32:62–68. [DOI] [PubMed] [Google Scholar]
- 44. Greenberg DA, Durner M, Resor S, Rosenbaum D, Shinnar S. The genetics of idiopathic generalized epilepsies of adolescent onset: Differences between juvenile myoclonic epilepsy and epilepsy with random grand mal and with awakening grand mal. Neurology. 1995;45:942–946. [DOI] [PubMed] [Google Scholar]
- 45. Clement MJ, Wallace SJ. Juvenile myoclonic epilepsy. Arch Dis Child. 1988;63:1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panayiotopoulos CP, Obeid T, Tahan AR. Juvenile myoclonic epilepsy: A 5-year prospective study. Epilepsia. 1994;35:285–296. [DOI] [PubMed] [Google Scholar]
- 47. Murthy JM, Rao CM, Meena AK. Clinical observations of juvenile myoclonic epilepsy in 131 patients: A study in South India. Seizure. 1998;7:43–47. [DOI] [PubMed] [Google Scholar]
- 48. Dhanuka AK, Jain BK, Daljit S, Maheshwari D. Juvenile myoclonic epilepsy: A clinical and sleep EEG study. Seizure. 2001;10:374–378. [DOI] [PubMed] [Google Scholar]
- 49. Watila MM, Balarabe SA, Ojo O, Keezer MR, Sander JW. Overall and cause-specific premature mortality in epilepsy: A systematic review. Epilepsy Behav. 2018;87:213–225. [DOI] [PubMed] [Google Scholar]
- 50. Quintana M, Sanchez-Lopez J, Mazuela G, et al. Incidence and mortality in adults with epilepsy in northern Spain. Acta Neurol Scand. 2021;143:27–33. [DOI] [PubMed] [Google Scholar]
- 51. Callaghan B, Choi H, Schlesinger M, et al. Increased mortality persists in an adult drug-resistant epilepsy prevalence cohort. J Neurol Neurosurg Psychiatry. 2014;85:1084–1090. [DOI] [PubMed] [Google Scholar]
- 52. Blank LJ, Acton EK, Willis AW. Predictors of mortality in older adults with epilepsy: Implications for learning health systems. Neurology. 2021;96:e93–e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu Y, Wu H, Wang MD. Feature exploration and causal inference on mortality of epilepsy patients using insurance claims data. 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI). 2019; 1–4,
- 54. Deaths associated with neurological conditions in England 2001 to 2014 . Data analysis report. Accessed 1 June 2022. https://www.gov.uk/government/publications/deaths-associated-with-neurological-conditions
- 55. Verducci C, Friedman D, Donner E, Devinsky O. Genetic generalized and focal epilepsy prevalence in the North American SUDEP Registry. Neurology. 2020;94:e1757–e1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Genton P, Gelisse P. Premature death in juvenile myoclonic epilepsy. Acta Neurol Scand. 2001;104:125–129. [DOI] [PubMed] [Google Scholar]
- 57. Baykan B, Altindag EA, Bebek N, et al. Myoclonic seizures subside in the fourth decade in juvenile myoclonic epilepsy. Neurology. 2008;70:2123–2129. [DOI] [PubMed] [Google Scholar]
- 58. Brodie MJ. Modern management of juvenile myoclonic epilepsy. Expert Rev Neurother. 2016;16:681–688. [DOI] [PubMed] [Google Scholar]
- 59. Gesche J, Hjalgrim H, Rubboli G, Beier CP. Patterns and prognostic markers for treatment response in generalized epilepsies. Neurology. 2020;95:e2519–e2528. [DOI] [PubMed] [Google Scholar]
- 60. Chen Y, Chen J, Chen X, et al. Predictors of outcome in juvenile myoclonic epilepsy. Risk Manag Health Policy. 2020;13:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Healy L, Moran M, Singhal S, O’Donoghue MF, Alzoubidi R, Whitehouse WP. Relapse after treatment withdrawal of antiepileptic drugs for juvenile absence epilepsy and juvenile myoclonic epilepsy. Seizure. 2018;59:116–122. [DOI] [PubMed] [Google Scholar]
- 62. Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: A population-based study. Neurology. 2009;73:1041–1045. [DOI] [PubMed] [Google Scholar]
- 63. Geithner J, Schneider F, Wang Z, et al. Predictors for long-term seizure outcome in juvenile myoclonic epilepsy: 25–63 years of follow-up. Epilepsia. 2012;53:1379–1386. [DOI] [PubMed] [Google Scholar]
- 64. Arntsen V, Sand T, Syvertsen MR, Brodtkorb E. Prolonged epileptiform EEG runs are associated with persistent seizures in juvenile myoclonic epilepsy. Epilepsy Res. 2017;134:26–32. [DOI] [PubMed] [Google Scholar]
- 65. von Podewils F, Lapp S, Wang ZI, et al. Natural course and predictors of spontaneous seizure remission in idiopathic generalized epilepsy: 7–27 years of follow-up. Epilepsy Res. 2014;108:1221–1227. [DOI] [PubMed] [Google Scholar]
- 66. Cerulli Irelli E, Cocchi E, Morano A, et al. The potential impact of enhanced hygienic measures during the COVID-19 outbreak on hospital-acquired infections: A pragmatic study in neurological units. J Neurol Sci. 2020;418:117111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vorderwulbecke BJ, Kirschbaum A, Merkle H, Senf P, Holtkamp M. Discontinuing antiepileptic drugs in long-standing idiopathic generalised epilepsy. J Neurol. 2019;266:2554–2559. [DOI] [PubMed] [Google Scholar]
- 68. Pietrafusa N, La Neve A, de Palma L, et al. Juvenile myoclonic epilepsy: Long-term prognosis and risk factors. Brain Dev. 2021;43:688–697. [DOI] [PubMed] [Google Scholar]
- 69. Irelli E C, Morano A, Orlando B, et al. Seizure outcome trajectories in a well-defined cohort of newly diagnosed juvenile myoclonic epilepsy patients. Acta Neurol Scand. 2022;145:314–321. [DOI] [PubMed] [Google Scholar]
- 70. Giuliano L, Mainieri G, Aguglia U, et al. Long-term prognosis of juvenile myoclonic epilepsy: A systematic review searching for sex differences. Seizure. 2021;86:41–48. [DOI] [PubMed] [Google Scholar]
- 71. Berg AT, Levy SR, Testa FM, Blumenfeld H. Long-term seizure remission in childhood absence epilepsy: Might initial treatment matter? Epilepsia. 2014;55:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crunelli V, Lorincz ML, McCafferty C, et al. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain. 2020;143:2341–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol Ther. 2019;201:77–93. [DOI] [PubMed] [Google Scholar]
- 74. Dezsi G, Ozturk E, Stanic D, et al. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia. 2013;54:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. da Silva Sousa P, Lin K, Garzon E, Sakamoto AC, Yacubian EM. Self-perception of factors that precipitate or inhibit seizures in juvenile myoclonic epilepsy. Seizure. 2005;14:340–346. [DOI] [PubMed] [Google Scholar]
- 76. Kasteleijn-Nolst Trenité DGA, de Weerd A, Beniczky S. Chronodependency and provocative factors in juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S25–S29. [DOI] [PubMed] [Google Scholar]
- 77. Ferlisi M, Shorvon S. Seizure precipitants (triggering factors) in patients with epilepsy. Epilepsy Behav. 2014;33:101–105. [DOI] [PubMed] [Google Scholar]
- 78. Nakken KO, Solaas MH, Kjeldsen MJ, Friis ML, Pellock JM, Corey LA. Which seizure-precipitating factors do patients with epilepsy most frequently report? Epilepsy Behav. 2005;6:85–89. [DOI] [PubMed] [Google Scholar]
- 79. Sperling MR, Schilling CA, Glosser D, Tracy JI, Asadi-Pooya AA. Self-perception of seizure precipitants and their relation to anxiety level, depression, and health locus of control in epilepsy. Seizure. 2008;17:302–307. [DOI] [PubMed] [Google Scholar]
- 80. Kasteleijn-Nolst Trenité D, Rubboli G, Hirsch E, et al. Methodology of photic stimulation revisited: Updated European algorithm for visual stimulation in the EEG laboratory. Epilepsia. 2012;53:16–24. [DOI] [PubMed] [Google Scholar]
- 81. Bartolini E, Pesaresi I, Fabbri S, et al. Abnormal response to photic stimulation in juvenile myoclonic epilepsy: An EEG-fMRI study. Epilepsia. 2014;55:1038–1047. [DOI] [PubMed] [Google Scholar]
- 82. Moeller F, Muthuraman M, Stephani U, Deuschl G, Raethjen J, Siniatchkin M. Representation and propagation of epileptic activity in absences and generalized photoparoxysmal responses. Hum Brain Mapp. 2013;34:1896–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vollmar C, O’Muircheartaigh J, Symms MR, et al. Altered microstructural connectivity in juvenile myoclonic epilepsy: The missing link. Neurology. 2012;78:1555–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Poleon S, Szaflarski JP. Photosensitivity in generalized epilepsies. Epilepsy Behav. 2017;68:225–233. [DOI] [PubMed] [Google Scholar]
- 85. Jain S, Tripathi M, Srivastava AK, Narula A. Phenotypic analysis of juvenile myoclonic epilepsy in Indian families. Acta Neurol Scand. 2003;107:356–362. [DOI] [PubMed] [Google Scholar]
- 86. Gelisse P, Genton P, Thomas P, Rey M, Samuelian JC, Dravet C. Clinical factors of drug resistance in juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry. 2001;70:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gomez-Ibañez A, McLachlan RS, Mirsattari SM, Diosy DC, Burneo JG. Prognostic factors in patients with refractory idiopathic generalized epilepsy. Epilepsy Res. 2017;130:69–73. [DOI] [PubMed] [Google Scholar]
- 88. Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. [DOI] [PubMed] [Google Scholar]
- 89. Mangaard S, Gesche J, Delcomyn L, Beier CP. The burden of disease of idiopathic/genetic generalized epilepsy—A nationwide online survey. Epilepsy Behav. 2021;123:108232. [DOI] [PubMed] [Google Scholar]
- 90. Jayalakshmi S, Vooturi S, Bana AK, Sailaja S, Somayajula S, Mohandas S. Factors associated with lack of response to valproic acid monotherapy in juvenile myoclonic epilepsy. Seizure. 2014;23:527–532. [DOI] [PubMed] [Google Scholar]
- 91. Jayalakshmi SS, Srinivasa Rao B, Sailaja S. Focal clinical and electroencephalographic features in patients with juvenile myoclonic epilepsy. Acta Neurol Scand. 2010;122(2):115–123. [DOI] [PubMed] [Google Scholar]
- 92. Japaridze G, Kasradze S, Lomidze G, et al. Focal EEG features and therapeutic response in patients with juvenile absence and myoclonic epilepsy. Clin Neurophysiol. 2016;127:1182–1187. [DOI] [PubMed] [Google Scholar]
- 93. Sun Y, Seneviratne U, Perucca P, et al. Generalized polyspike train: An EEG biomarker of drug-resistant idiopathic generalized epilepsy. Neurology. 2018;91:e1822–e1830. [DOI] [PubMed] [Google Scholar]
- 94. Kamitaki BK, Janmohamed M, Kandula P, et al. Clinical and EEG factors associated with antiseizure medication resistance in idiopathic generalized epilepsy. Epilepsia. 2022;63:150–161. [DOI] [PubMed] [Google Scholar]
- 95. Pegg EJ, Taylor JR, Laiou P, Richardson M, Mohanraj R. Interictal electroencephalographic functional network topology in drug-resistant and well-controlled idiopathic generalized epilepsy. Epilepsia. 2021;62:492–503. [DOI] [PubMed] [Google Scholar]
- 96. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: An open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397(10282):1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Holmes EAF, Plumpton C, Baker GA, et al. Patient-focused drug development methods for benefit-risk assessments: A case study using a discrete choice experiment for antiepileptic drugs. Clin Pharmacol Ther. 2019;105:672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Silvennoinen K, de Lange N, Zagaglia S, et al. Comparative effectiveness of antiepileptic drugs in juvenile myoclonic epilepsy. Epilepsia Open. 2019;4:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kossoff EH, Bergey GK, Freeman JM, Vining EP. Levetiracetam psychosis in children with epilepsy. Epilepsia. 2001;42:1611–1613. [DOI] [PubMed] [Google Scholar]
- 100. Isojarvi JI, Laatikainen TJ, Pakarinen AJ, Juntunen KT, Myllyla VV. Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med. 1993;329:1383–1388. [DOI] [PubMed] [Google Scholar]
- 101. Josephson CB, Engbers JDT, Jette N, et al. Prediction tools for psychiatric adverse effects after levetiracetam prescription. JAMA Neurol. 2019;76:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Helmstaedter C, Mihov Y, Toliat MR, et al. Genetic variation in dopaminergic activity is associated with the risk for psychiatric side effects of levetiracetam. Epilepsia. 2013;54:36–44. [DOI] [PubMed] [Google Scholar]
- 103. Campbell C, McCormack M, Patel S, et al. A pharmacogenomic assessment of psychiatric adverse drug reactions to levetiracetam. Epilepsia. 2022;63:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pinckaers FME, Boon ME, Majoie M. Risk factors predisposing to psychotic symptoms during levetiracetam therapy: A retrospective study. Epilepsy Behav. 2019;100(Pt A):106344. [DOI] [PubMed] [Google Scholar]
- 105. Mula M, Trimble MR, Yuen A, Liu RS, Sander JW. Psychiatric adverse events during levetiracetam therapy. Neurology. 2003;61:704–706. [DOI] [PubMed] [Google Scholar]
- 106. Kanner AM, Wuu J, Faught E, et al. A past psychiatric history may be a risk factor for topiramate-related psychiatric and cognitive adverse events. Epilepsy Behav. 2003;4:548–552. [DOI] [PubMed] [Google Scholar]
- 107. Kanner AM. Can antiepileptic drugs unmask a susceptibility to psychiatric disorders? Nat Clin Pract Neurol. 2009;5:132–133. [DOI] [PubMed] [Google Scholar]
- 108. Li H, Wang X, Zhou Y, et al. Association of LEPR and ANKK1 gene polymorphisms with weight gain in epilepsy patients receiving valproic acid. Int J Neuropsychopharmacol. 2015;18(7):pyv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noai M, Soraoka H, Kajiwara A, et al. Cytochrome P450 2C19 polymorphisms and valproic acid-induced weight gain. Acta Neurol Scand. 2016;133:216–223. [DOI] [PubMed] [Google Scholar]
- 110. Mani B, Nair PP, Sekhar A, Kamalanathan S, Narayan SK, Kesavan R. CYP2C19 & UGT1A6 genetic polymorphisms and the impact on valproic acid-induced weight gain in people with epilepsy: Prospective genetic association study. Epilepsy Res. 2021;177:106786. [DOI] [PubMed] [Google Scholar]
- 111. Bai X, Xu C, Wen D, et al. Polymorphisms of peroxisome proliferator-activated receptor gamma (PPARγ) and cluster of differentiation 36 (CD36) associated with valproate-induced obesity in epileptic patients. Psychopharmacology (Berl). 2018;235:2665–2673. [DOI] [PubMed] [Google Scholar]
- 112. Chen PS, Chang HH, Huang CC, et al. A longitudinal study of the association between the GNB3 C825T polymorphism and metabolic disturbance in bipolar II patients treated with valproate. Pharmacogenomics J. 2017;17:155–161. [DOI] [PubMed] [Google Scholar]
- 113. Chang HH, Gean PW, Chou CH, et al. C825t polymorphism of the GNB3 gene on valproate-related metabolic abnormalities in bipolar disorder patients. J Clin Psychopharmacol. 2010;30:512–517. [DOI] [PubMed] [Google Scholar]
- 114. Edinoff AN, Nguyen LH, Fitz-Gerald MJ, et al. Lamotrigine and Stevens-Johnson syndrome prevention. Psychopharmacol Bull. 2021;51:96–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shi YW, Min FL, Zhou D, et al. HLA-A*24:02 as a common risk factor for antiepileptic drug-induced cutaneous adverse reactions. Neurology. 2017;88:2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Moon J, Park HK, Chu K, et al. The HLA-A*2402/Cw*0102 haplotype is associated with lamotrigine-induced maculopapular eruption in the Korean population. Epilepsia. 2015;56:e161–e167. [DOI] [PubMed] [Google Scholar]
- 117. Li LJ, Hu FY, Wu XT, An DM, Yan B, Zhou D. Predictive markers for carbamazepine and lamotrigine-induced maculopapular exanthema in Han Chinese. Epilepsy Res. 2013;106:296–300. [DOI] [PubMed] [Google Scholar]
- 118. Koomdee N, Pratoomwun J, Jantararoungtong T, et al. Association of HLA-A and HLA-B alleles with lamotrigine-induced cutaneous adverse drug reactions in the Thai population. Front Pharmacol. 2017;8:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jang HW, Kim SW, Cho YJ, et al. GWAS identifies two susceptibility loci for lamotrigine-induced skin rash in patients with epilepsy. Epilepsy Res. 2015;115:88–94. [DOI] [PubMed] [Google Scholar]
- 120. Hirsch LJ, Weintraub DB, Buchsbaum R, et al. Predictors of lamotrigine-associated rash. Epilepsia. 2006;47:318–322. [DOI] [PubMed] [Google Scholar]
- 121. Egunsola O, Choonara I, Sammons HM. Safety of lamotrigine in paediatrics: A systematic review. BMJ Open. 2015;5(6):e007711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. [DOI] [PubMed] [Google Scholar]
- 123. Shlobin NA, Singh G, Newton CR, Sander JW. Classifying epilepsy pragmatically: Past, present, and future. J Neurol Sci. 2021;427:117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Steriade C, Sperling MR, DiVentura B, et al. Proposal for an updated seizure classification framework in clinical trials. Epilepsia. 2022;63:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wolf P, Goosses R. Relation of photosensitivity to epileptic syndromes. J Neurol Neurosurg Psych. 1986;49:1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shiraishi H, Fujiwara T, Inoue Y, Yagi K. Photosensitivity in relation to epileptic syndromes: A survey from an epilepsy center in Japan. Epilepsia. 2001;42:393–397. [DOI] [PubMed] [Google Scholar]
- 127. Maguire MJ, Nevitt SJ. Treatments for seizures in catamenial (menstrual-related) epilepsy. Cochrane Database Syst Rev. 2019;10:CD013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Edward KL, Cook M, Stephenson J, Giandinoto JA. The impact of brief lifestyle self-management education for the control of seizures. Br J Nurs. 2019;28:348–354. [DOI] [PubMed] [Google Scholar]
- 129. Wolf P. The role of nonpharmaceutic conservative interventions in the treatment and secondary prevention of epilepsy. Epilepsia. 2002;43(Suppl 9):2–5. [DOI] [PubMed] [Google Scholar]
- 130. Ramaratnam S, Baker GA, Goldstein L. Psychological treatments for epilepsy. Cochrane Database Syst Rev. 2001(4):CD002029. [DOI] [PubMed] [Google Scholar]
- 131. Sun Y, You W, Almeida F, Estabrooks P, Davy B. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: A systematic review and meta-analysis. J Acad Nutr Diet. 2017;117:404–421.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Burgess E, Hassmen P, Welvaert M, Pumpa KL. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: A systematic review and meta-analysis. Clin Obes. 2017;7:105–114. [DOI] [PubMed] [Google Scholar]
- 133. Wu Y, Johnson BT, Acabchuk RL, et al. Yoga as antihypertensive lifestyle therapy: A systematic review and meta-analysis. Mayo Clin Proc. 2019;94:432–446. [DOI] [PubMed] [Google Scholar]
- 134. Leeman-Markowski BA, Schachter SC. Cognitive and behavioral interventions in epilepsy. Curr Neurol Neurosci Rep. 2017;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gesche J, Cornwall CD, Delcomyn L, Rubboli G, Beier CP. Pseudoresistance in idiopathic/genetic generalized epilepsies—Definitions, risk factors, and outcome. Epilepsy Behav. 2022;130:108633. [DOI] [PubMed] [Google Scholar]
- 136. Walsh J, Thomas RH, Church C, Rees MI, Marson AG, Baker GA. Executive functions and psychiatric symptoms in drug-refractory juvenile myoclonic epilepsy. Epilepsy Behav. 2014;35:72–77. [DOI] [PubMed] [Google Scholar]
- 137. Smith A, Syvertsen M, Pal DK. Meta-analysis of response inhibition in juvenile myoclonic epilepsy. Epilepsy Behav. 2020;106:107038. [DOI] [PubMed] [Google Scholar]
- 138. Wandschneider B, Centeno M, Vollmar C, et al. Risk-taking behavior in juvenile myoclonic epilepsy. Epilepsia. 2013;54:2158–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chamorro J, Bernardi S, Potenza MN, et al. Impulsivity in the general population: A national study. J Psychiatr Res. 2012;46:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ascoli M, Mastroianni G, Gasparini S, et al. Diagnostic and therapeutic approach to drug-resistant juvenile myoclonic epilepsy. Expert Rev Neurother. 2021;21:1265–1273. [DOI] [PubMed] [Google Scholar]
- 141. Zhu M, Chen J, Guo H, Ding L, Zhang Y, Xu Y. High mobility group protein B1 (HMGB1) and interleukin-1beta as prognostic biomarkers of epilepsy in children. J Child Neurol. 2018;33:909–917. [DOI] [PubMed] [Google Scholar]
- 142. Ravizza T, Terrone G, Salamone A, et al. High mobility group box 1 is a novel pathogenic factor and a mechanistic biomarker for epilepsy. Brain Behav Immun. 2018;72:14–21. [DOI] [PubMed] [Google Scholar]
- 143. Polygenic Risk Score Task Force of the International Common Disease Alliance . Responsible use of polygenic risk scores in the clinic: Potential benefits, risks and gaps. Nat Med. 2021;27:1876–1884. [DOI] [PubMed] [Google Scholar]
- 144. An S, Malhotra K, Dilley C, et al. Predicting drug-resistant epilepsy—A machine learning approach based on administrative claims data. Epilepsy Behav. 2018;89:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lacey CJ, Salzberg MR, D’Souza WJ. What factors contribute to the risk of depression in epilepsy? Tasmanian Epilepsy Register Mood Study (TERMS). Epilepsia. 2016;57:516–522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings and conclusions of this study are available from the corresponding authors upon reasonable request.