Abstract
Human and nonhuman animal studies have established the importance of the amygdala and related structures in the processing of negative emotions. Indeed, there is sufficient published evidence to hypothesize that increased volume of the amygdala is related to greater neuroticism/negative emotionality in humans. Because negative emotionality is robustly and transdiagnostically associated with psychopathology, we tested the strong hypothesis that ratings of dispositional negative emotionality at 10–17 years on the Child and Adolescent Dispositions Scale (CADS) would predict larger volumes of the amygdala in adulthood. Additionally, we explored associations with other regions implicated in emotion processing. Participants were 433 twins strategically selected for neuroimaging during wave 2 from the Tennessee Twins Study (TTS) by oversampling on wave 1 psychopathology. Controlling for age, sex, race-ethnicity, handedness, scanner, and total brain volume, youth-rated negative emotionality positively predicted bilateral amygdala volumes after correction for multiple testing. Each unit difference of one standard deviation (SD) in negative emotionality was associated with a .12 SD unit difference in volumes of both amygdalae. Parent-rated negative emotionality predicted greater thickness of the caudal anterior cingulate cortex bilaterally, but did not predict volumes of other regions implicated in emotion regulation. These results are striking because dispositions assessed at 10–17 years of age were predictive of regional grey matter volumes measured 12–13 years later in adulthood. Future longitudinal research should examine the timing of the association of the amygdala with dispositional negative emotionality and psychopathology to determine when these associations emerge during development to elucidate causal influences underlying these correlations.
The trait-like tendency to react frequently and intensely with negative emotions to frustrations, threats, loss, and other challenges—variously termed neuroticism, dispositional negativity, or negative emotionality—is of substantial public health significance owing to its robust associations with every form of psychopathology and a broad range of physical health problems and longevity (Lahey, 2009; Shackman et al., 2016; Widiger & Oltmanns, 2017). This disposition can be measured early in life and is predictive of emotional behavior in adulthood (Rothbart, Ahadi & Evans 2000). Importantly, many theorists have posited that individual differences in childhood dispositions, including negative emotionality, influence the likelihood of developing psychopathology across the life span (Caspi, Henry, McGee, Moffitt, & Silva, 1995; Muris & Ollendick, 2005; Nigg, 2006; Thomas & Chess, 1957).
Current neural models of emotion distinguish processes of emotional appraisal, the generation of behavioral, physiological, subjective and communicative responses, implicit automatic emotion regulation, and explicit or effortful emotion regulation (Etkin, Buchel, & Gross, 2016; Gross, 2015; Gross & Barrett, 2011; Panksepp, 2004; Phelps & LeDoux, 2005; Phillips, Ladouceur, & Drevets, 2008; Rive et al., 2013; Shackman et al., 2016). These models are based on evidence from human and nonhuman animal studies of the roles of limbic and paralimbic brain regions in these processes, including the amygdala, orbitofrontal cortex, parts of the anterior cingulate cortex (ACC), and insula in emotional salience evaluation of stimuli and the direction of different aspects emotional expression, and the medial orbitofrontal and rostral/subgenual ACC in implicit emotion regulation, and more lateral prefrontal region in explicit emotion regulation (Etkin et al., 2016; Etkin, Egner, & Kalisch, 2011; Phillips et al., 2008; Ray & Zald, 2012; Seeley et al., 2007; Zald, 2003).
A fundamental question with theoretical and practical implications is whether individual differences in the structure of these regions are correlated with individual differences in negative emotionality in humans. Variations in the size of brain structures are readily and reliably measured using magnetic resonance imaging (Holmes et al., 2015; Holmes et al., 2012) and firmly established correlations between brain structure and behavior would facilitate research on risk factors and mechanisms in many ways. At present, the most widely tested and frequently observed structural neural correlate of dispositional negative emotionality/neuroticism is the volume of the amygdala (Allen & DeYoung, 2017; DeYoung & Krueger, 2018; Holmes et al., 2012; Mincic, 2015). This positive correlation between self-reported negative emotionality and amygdala volume has been observed in an activation likelihood estimation (ALE) analysis of voxelwise studies, particularly in the left hemisphere (Mincic, 2015). However, it has only emerged in about half of studies reporting region of interest (ROI) analyses (Mincic, 2015), possibly due to methodological issues, such as differences in scales utilized and sampling characteristics. For example, analyses of data from the Human Connectome Project, which was designed to be a study of healthy adults, did not find a significant correlation between amygdala volume and self-reported neuroticism (Gray, Owens, Hyatt, & Miller, 2018). Although having the advantages of a large sample, the exclusion of persons with significant psychopathology from Human Connectome Project may have underestimated the correlation by restricting the ranges of variation in both neuroticism and brain features given the known correlation between neuroticism and essentially every form of psychopathology (Krueger & Markon, 2006; Lahey, 2009). The age of the sample may also be important as most studies have focused on adults. Indeed, a recent study of adolescent girls (Delaparte et al., 2019) observed no association between amygdala volume and neuroticism. Similarly, a voxel based morphometry study of 16–17 year-olds observed no association between neuroticism and amygdala volume (Blankstein, Chen, Mincic, McGrath, & Davis, 2009). These inconsistencies argue for the importance of new tests of association between negative emotionality and amygdala volume.
In the present study, we conduct a prospective test of the association between dispositional negative emotionality measured at 10–17 years of age with amygdala volumes and related measures of cortical thickness measured approximately 12 years later at 22–31 years of age in a population enriched for psychopathology risk in a way that can be weighted to estimate population parameters. Three dispositions were measured by the Child and Adolescent Dispositions Scale (CADS) (Lahey, Applegate, et al., 2008; Lahey, Rathouz, Applegate, Tackett, & Waldman, 2010). Because the CADS was developed to study associations between dispositions and psychopathology, the CADS does not use items that are synonyms or antonyms of any externalizing or internalizing psychopathology symptoms to avoid item contamination when testing associations. The negative emotionality scale is composed of items tapping frequent, easily elicit, and intense response to frustrations or threats with any kind of negative emotion. Children rated high on the daring scale find intense and risky situations to be attractive and rewarding and are low in harm avoidance. The prosociality scale quantifies caring about the welfare of others, attempting to help and please them, and experiencing guilt over misbehaviors.
In cross-sectional studies, CADS negative emotionality scale has been found to correlate phenotypically and genetically with a broad range of both externalizing and internalizing psychopathology during childhood and adolescence (Lahey, Applegate, et al., 2008; Lahey et al., 2010; Tackett, Waldman, Van Hulle, & Lahey, 2011; Taylor, Allan, Mikolajewski, & Hart, 2013; Waldman et al., 2011). In longitudinal analyses based on the TTS, parent-rated negative emotionality at 10–17 years of age predicted the general factor of psychopathology based on self-reports at 23–31 years, whereas parent-rated prosociality and daring predicted specific externalizing psychopathology. Youth-rated negative emotionality and daring predicted externalizing psychopathology, but not the general factor (Class et al., 2019). Additionally, negative emotionality and other CADS dispositions prospectively predict antisocial and risky behavior during adolescence (Shaw, Hyde, & Brennan, 2012; Sitnick, Brennan, Forbes, & Shaw, 2014; Trentacosta, Hyde, Shaw, & Cheong, 2009), and adult antisocial personality disorder (Lahey et al., 2018). Thus, CADS dispositions have well-documented cross-sectional and prospective associations with psychopathology.
Based on reviews of previous findings of (a) a functional role of the amygdala in negative emotion (Shackman et al., 2016), and (b) an association of neuroticism with greater amygdalae volumes (DeYoung & Krueger, 2018; Mincic, 2015), we tested the strong prediction that child and adolescent dispositional negative emotionality would be associated with enlarged amygdalae in early adulthood. Additionally, based on less well-established hypotheses, we examined predictive associations with caudal ACC and anterior insula because of their role in emotion salience evaluation and close linkage to the amygdala. Furthermore, we tested associations with the medial orbitofrontal cortex (mOFC) and rostral ACC, which have been previously found to be structurally related to negative emotionality and implicated in implicit emotion regulation (Delaparte et al., 2019; Holmes et al. 2012; Mincic, 2015). Although early studies of the structural neural correlates of variations in trait negative emotionality measured volumes of cortical ROIs, volumes are a product of both the thickness and surface area of cortical structures and there is evidence thickness and surface area may be genetically independent (Panizzon et al., 2009; Winkler et al., 2010). Therefore, following others (Cha et al., 2014; Holmes et al., 2012), cortical thickness was used to measure the structure of cortical regions implicated in negative emotion. The subcortical amygdala could only be measured by volume, however.
METHOD
Imaging data were acquired during wave 2 from a selected subsample of the TTS (Lahey, Rathouz, et al., 2008), which was conducted 10–15 years (median = 12 years) after wave 1.
Wave 1 Sample and Methods
The wave 1 TTS sample was representative of 6–17 year-old twin pairs living in one of Tennessee’s five metropolitan statistical areas in the years 2000–2001. The Tennessee Department of Health identified 7794 birth records representing all twin pairs born in Tennessee in the eligible age range and used external databases to locate families; 2431 twin pairs were eliminated because they lived outside the 5 metropolitan statistical areas. A random sample was selected from the remaining families, stratified by age and 35 geographic subareas, proportional to the number of listed families in each subarea. Of 4012 selected households, 3592 (89.5%) were located and screened, with 2646 of the screened families being eligible (both twins co-resided with the adult caretaker at least 50% of the time during the past 6 months and the twins and caretaker spoke English). Biological mothers, biological fathers, stepmothers, and grandmothers were eligible to be interviewed as the adult caretaker. Interviews were completed with 2,063 adult caretakers (90.8% biological mothers), with a 70% response rate. When the caretaker was interviewed, both twins were interviewed 98% of the time. After exclusion of twin pairs in which either twin was reported to have been given a diagnosis of autism, psychosis, or seizure disorder, the sample consisted of 3,990 twins in 1,995 complete pairs. Consistent with the Tennessee population in 2001, caretakers classified 71% of the twins as non-Hispanic white, 24% African American, 2% as Hispanic, and 3% as other groups. There were no missing data on demographic variables, except that maternal education for two mothers was imputed from the mean of nonmissing values on that variable.
The reliable and well-validated CADS (Lahey, Applegate, et al., 2008; Lahey et al., 2010) was administered separately to each child and primary caretaker. CADS items were rated on a 1 (not at all) to 4 (very much) response scale in computer-assisted interview format.
Wave 2 Sample and Methods
Twin pairs for the wave 2 assessments were recruited from the wave 1 TTS sample in four replicates based on age in wave 1: 16–17, 14–15, 12–13, and 10–11 years. Twin pairs were eligible for recruitment if the last known address after initial searching of both twins was within 300 miles of Vanderbilt University (95.2% of twins). The replicates were selected by oversampling on wave 1 CAPS second-order psychopathology dimension scores based on the greater rating of each symptom from the parent or youth. All high-risk pairs were selected if either twin had symptom ratings on the total number of internalizing, attention-deficit/hyperactivity disorder, or the combination of ODD and CD symptoms in the top 10% of that age range. In addition, 19–23% of the remainder of each replicate was randomly selected with two constraints: (1) monozygotic pairs were oversampled by randomly excluding 40% of the randomly selected dizygotic pairs in the remainder of the sample, and (2) the exact number selected from the remainder of the sample varied slightly to equate replicate sizes. The resulting replicates were each 100 pairs of individuals, except for the fourth replicate, which had 105 pairs of individuals.
Three pairs of twins could not be located and 37 pairs declined to be screened for eligibility. Eighteen selected pairs of twins across replicates were declared out of scope due to previous participation in the pilot study for this study, mental or physical incapacity, residence outside the U.S., imprisonment, or death resulting in 387 screened twin pairs. A total of 114 screened individual twins were ineligible for neuroimaging because they or their co-twin was ineligible based on safety (e.g., metallic implants), body size, inadequate vision, back problems, neurological disorder, events causing unconsciousness, multiple concussions, or claustrophobia.
The four replicates (strata) were recruited in reverse order of wave 1 age over 3.5 years to minimize the age distribution of twins in wave 2. Interviews regarding symptoms of psychopathology were completed for 72% of the screened sample during 2013–2016. These interviews were completed for 248 complete twin pairs (49.6% monozygotic; 66.9% high risk) and 3 individuals without their twin. The 499 interviewed participants were 23–31 years of age (median 26 years); 52.1% female; 71.5% Non-Hispanic white, 25.2% African American, and 3.2% other groups.
MRI Acquisition and Processing
Imaging data were acquired on two identical 3T Intera-Achiava Phillips MRI scanners using a 32-channel head coil. T1-weighted images were acquired with a 3-D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence (TE/TR/TI=4.6/9.0/644(shortest) ms; SENSE=2.0; echo train=131; scan time=4 min 32 s; FOV: 256×256×170 mm, 1 mm isotropic resolution). Imaging data were processed on a flow basis during 2013–2016.
Segmentation of T1 images utilized several automated pipelines to optimally define regional cortical thickness and subcortical volume of key regions of interest. Cortical segmentations were derived using the recon-all script from FreeSurfer version 5.1.0 which is freely available online (https://surfer.nmr.mgh.harvard.edu/). These procedures have been described in detail elsewhere (Fischl, 2012; Fischl et al., 2002). All segmentations were visually inspected and manual edits were made for all subjects according to the standardized protocols on the software’s website. We excluded data from 10 subjects whose segmentations failed quality assurance checks after manual edits (excessive movement, processing errors, etc.), for a total of 438 subjects with useable data. Volume and thickness measures were extracted from regions in the Desikan-Killiany atlas, which divides each hemisphere into 34 regions (Desikan et al., 2006). We also extracted total-intracranial volume from this pipeline to use as a covariate in sensitivity analyses. After quality assurance 438 participants had nonmissing FreeSurfer segmentations.
Studies have identified biases in FreeSurfer subcortical segmentations as compared with manual segmentation, especially in clinical populations (Dewey et al., 2010; Morey et al., 2009; Perlaki et al., 2017; Schoemaker et al., 2016). Although manual segmentation by experts is the gold-standard, it is prohibitively time consuming in large datasets (Tsang et al., 2008). To limit error in automated segmentation, we applied multi-atlas segmentation, which consults multiple atlases to label brain regions (Asman & Landman, 2011). For the amygdala, we used a multi-atlas algorithm that incorporates 195 atlases and has been shown to outperform FreeSurfer (Plassard, McHugo, Heckers, & Landman, 2017) for medial temporal lobe structures, and is consistent with past evidence for the validity and reliability of the multi-atlas approach for segmenting the amygdala (Hanson et al., 2012). After quality assurance, 443 participants had nonmissing data on amygdala volumes derived from multi-atlas.
Statistical Analyses
Planned Tests of Predictions
In a time of legitimate concern about the replicability of many findings in psychology and neuroscience, it is important to conduct strong tests of even well-justified predictions. In this case, we tested predictions regarding the association of negative emotionality with individual differences in brain structure that were based on both reviews of previously published empirical findings and theories of neural substrates of emotion processing and regulation. All tests of associations were conducted using regions of interest (ROIs) selected prior to analyses. In addition to testing the strong hypothesis regarding volume of the amygdala, we tested associations with thickness of the caudal ACC, the rostral ACC, anterior insula, and medial orbitofrontal cortex (mOFC), and rostral ACC. In the Desikan atlas (Desikan et al., 2006), the mOFC encompasses both the medial orbital gyrus as well as the gyrus rectus on the ventral surface of the frontal lobe, as well as part of the ventromedial wall extending into the ventral frontal pole. The rostral ACC includes the portion of the cingulate surrounding the genu of the corpus of callosum, and the caudal ACC encompasses the dorsal ACC posterior to a perpendicular line through the genu of the corpus callosum.
Studies of the structure of ROIs related to emotion have typically controlled for total intracranial/total brain volume (TBV) along with sex and age (DeYoung et al., 2010; Holmes et al., 2012). This seems intuitively justified, as it is difficult to interpret volumetric results for a given region with specificity if the overall brain size is not accounted for given the high correlation between most regions and total brain size. Nonetheless, recent evidence that brain regions scale differentially based on total brain size (Reardon et al., 2018) leaves open the possibility that correcting for TBV might obscure meaningful differences. Therefore, we conducted two separate families of planned tests of four selected ROIs in the left and right hemispheres, controlling or not controlling for TBV in these analyses. Each set of tests was conducted separately for parent and youth ratings on the CADS because there is not yet an established method of combining the ratings of multiple informants of dispositions (Tackett, 2011), resulting in 48 tests in each family. To make the tests comparable across regions, analyses were limited to the 433 participants with nonmissing data on TBV, amygdala volumes, and cortical thicknesses.
Analyses were performed using Mplus 8.1 (Muthén & Muthén, 2018). In separate SEMs for each CADS informant and side of the brain, regional brain volumes were simultaneously regressed on the three CADS scales of negative emotionality, daring, and prosociality, along with the covariates of no interest (sex, age in wave 1 at the time dispositions were measured, age in wave 2 when neuroimaging was conducted, race-ethnicity, handedness, and scanner). Although our empirically based hypotheses for these regions are focused on negative emotionality, inclusion of the other disposition scales provides an opportunity to obtain data that may support future hypotheses. Maximum likelihood estimation with robust standard errors (MLR) was used to account for any non-normality in the distributions and to adjust standard errors to reflect the clustering of twins within twin pairs. Except when presenting descriptive statistics in Table 1, each dispositional score, regional brain volume, and cortical thickness was standardized with a mean of 0 and a standard deviation of 1 to allow interpretation of regression coefficients in terms of standard deviation unit effect sizes. We corrected for multiple testing in each family of tests using a false discovery rate (FDR) of 5% applied to conservative two-tailed tests (Benjamini & Hochberg, 1995).
Table 1.
Means (standard deviations) and correlations among dispositional predictor variables and covariates of no interest.
| NE Youth 1.24 (0.49) | Prosociality Youth 2.36 (0.41) | Daring Youth 1.70 (0.62) | NE Parent 1.27 (0.58) | Prosociality Parent 2.25 (0.51) | Daring Parent 1.56 (0.67) | Wave 1 age 13.56 (2.46) | Wave 2 age 26.04 (1.78) | Female 0.53 (0.50) | NH White 0.72 (0.45) | |
|---|---|---|---|---|---|---|---|---|---|---|
| NE Youth | −0.03 | 0.02 | 0.26*** | −0.14** | 0.003 | −0.10* | −0.09 | −0.01 | 0.08 | |
| Posociality Youth | −0.12* | −0.11** | 0.37*** | −0.19**** | −0.04 | −0.05 | 0.35**** | 0.03 | ||
| Daring Youth | 0.07 | −0.20*** | 0.43*** | 0.04 | −0.05 | −0.25*** | 0.11* | |||
| NE Parent | −0.32*** | 0.17*** | −0.11 | −0.13** | −0.05 | 0.09 | ||||
| Prosociality Parent | −0.22*** | −0.06 | −0.04 | −0.24*** | 0.10* | |||||
| Daring Parent | −0.06 | −0.04 | −0.21*** | 0.02 | ||||||
| Wave 1 age | −0.07 | 0.02 | ||||||||
| Wage 2 age | −0.05 | 0.05 | ||||||||
| Female | 0.06 |
p < 0.05;
p < 0.01,
p < 0.001.
NE = negative emotionality; NH white = comparison of non-Hispanic white versus other race-ethnic groups.
Exploratory Tests of Sex-by-Disposition Interactions
Because of existing evidence of sexual dimorphism of the amygdala during childhood and adolescence (Giedd, Castellanos, Rajapakse, Vaituzis, & Rapoport, 1997), and evidence that sex differences may exist in the associations of amygdala or ACC volume or cortical thickness with behavior and personality (Blankstein et al., 2009; Sweeney, Tsapanou, & Stern, 2019; van der Plas, Boes, Wemmie, Tranel, & Nopoulos, 2010), we conducted similar families of exploratory tests of the interaction of each disposition with sex. In addition to controlling for TBV, we repeated the analyses without controlling for TBV to compare the results. This was based on the concern that the sex differences might be obscured when controlling for TBV, given that sex effects could be obscured given that males have larger amygdala than females, while those differences are largely removed when one controls for TBV (Marwha, Halari, & Eliot, 2017).
RESULTS
Table 1 shows that youth-rated CADS (CADS-Y) disposition scales were largely uncorrelated, with small to moderate correlations among scales of the parent-rated CADS (CADS-P) scales. Cross-informant crrelations of the same CADS disposition rated by parent and youth informants were modest (r = 0.26–0.43, all p < 0.0001).
The results of the simultaneous regression analyses presented in Table 2 show that CADS-Y negative emotionality was positively associated after FDR correction with the bilateral amygdala volumes when TBV was controlled. These effects were substantially smaller when not correcting for TBV. The analyses also revealed significant associations after FDR correction of CADS-P negative emotionality with the left and right rostral ACC thickness when TBV was controlled, and the association remained significant for the left rostral ACC even when TBV was not controlled. Scatterplots and slopes are presented for the significant associations between negative emotionality and amygdala volumes (Figure 1) and mean thickness of the rostral ACC (Figure 2). By contrast, neither daring, nor prosociality showed associations with any structural ROIs at corrected levels of statistical significance in the regression analyses reported in Table 2.
Table 2.
Standardized multiple regression coefficients and standard errors from regressions of each Desikan-Killiany atlas regions of interest on the three dispositions, controlling covariates of no interest that do not include total brain volume (top panels) or include total brain volume (all N =433).
| CADS Informant: | Parent | Youth | Parent | Youth | ||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p < | β (SE) | p < | β (SE) | p < | β (SE) | p < | |
| Dispositions | ||||||||
| Covariates: Sex, age in wave 1, age in wave 2, race-ethnicity, handedness, scanner | ||||||||
| ROIs: | Left Amygdala Volume | Right Amygdala Volume | ||||||
| Negative Emotionality | −0.05 (0.06) | 0.4082 | 0.10 (0.05) | 0.0405 | −0.08 (0.09) | 0.3568 | 0.11 (0.06) | 0.0680 |
| Prosociality | 0.04 (0.08) | 0.6306 | −0.08 (0.09) | 0.3711 | 0.04 (0.10) | 0.6698 | −0.11 (0.09) | 0.2054 |
| Daring | −0.01 (0.08) | 0.8624 | −0.03 (0.07) | 0.6350 | 0.00 (0.09) | 0.9671 | −0.02 (0.08) | 0.8425 |
| ROIs: | Left Caudal ACC Thickness | Right Caudal ACC Thickness | ||||||
| Negative Emotionality | 0.24 (0.06) | 0.0002 | −0.07 (0.07) | 0.3456 | 0.17 (0.06) | 0.0076 | −0.04 (0.07) | 0.5778 |
| Prosociality | 0.07 (0.09) | 0.4569 | −0.09 (0.07) | 0.2013 | 0.15 (0.06) | 0.0147 | 0.02 (0.06) | 0.6597 |
| Daring | 0.07 (0.06) | 0.2525 | 0.08 (0.07) | 0.2975 | 0.03 (0.07) | 0.6767 | 0.02 (0.05) | 0.7651 |
| ROIs: | Left Rostral ACC Thickness | Right Rostral ACC Thickness | ||||||
| Negative Emotionality | 0.08 (0.07) | 0.2519 | 0.12 (0.10) | 0.2117 | 0.07 (0.08) | 0.3990 | −0.04 (0.09) | 0.6560 |
| Prosociality | 0.08 (0.06) | 0.1867 | 0.04 (0.06) | 0.4482 | 0.04 (0.08) | 0.6348 | 0.09 (0.06) | 0.1576 |
| Daring | 0.10 (0.09) | 0.2494 | −0.04 (0.09) | 0.6085 | −0.02 (0.05) | 0.7597 | 0.13 (0.06) | 0.0272 |
| ROIs: | Left Medial Orbitofrontal Cortex Thickness | Right Medial Orbitofrontal Cortex Thickness | ||||||
| Negative Emotionality | 0.10 (0.07) | 0.1673 | 0.04 (0.07) | 0.5227 | 0.09 (0.06) | 0.1531 | 0.04 (0.06) | 0.5444 |
| Prosociality | 0.13 (0.08) | 0.0992 | 0.11 (0.06) | 0.0876 | 0.04 (0.07) | 0.5690 | 0.06 (0.05) | 0.3072 |
| Daring | 0.02 (0.07) | 0.7578 | 0.06 (0.09) | 0.5138 | −0.05 (0.06) | 0.4244 | 0.10 (0.06) | 0.1087 |
| Covariates: Sex, age in wave 1, age in wave 2, race-ethnicity, handedness, scanner, total brain volume | ||||||||
| ROIs: | Left Amygdala Volume | Right Amygdala Volume | ||||||
| Negative Emotionality | 0.06 (0.04) | 0.0894 | 0.12 (0.03) | 0.0007 | 0.04 (0.05) | 0.3986 | 0.12 (0.03) | 0.0001 |
| Prosociality | −0.00 (0.05) | 0.9685 | −0.07 (0.06) | 0.2224 | −0.00 (0.06) | 0.9329 | −0.11 (0.06) | 0.0554 |
| Daring | 0.01 (0.05) | 0.7617 | −0.00 (0.05) | 0.9455 | 0.04 (0.06) | 0.5599 | 0.02 (0.04) | 0.6470 |
| ROIs: | Left Caudal ACC Thickness | Right Caudal ACC Thickness | ||||||
| Negative Emotionality | 0.28 (0.06) | 0.0001 | −0.07 (0.07) | 0.3408 | 0.19 (0.07) | 0.0038 | −0.04 (0.07) | 0.6045 |
| Prosociality | 0.05 (0.08) | 0.5057 | −0.09 (0.07) | 0.2071 | 0.15 (0.06) | 0.0261 | 0.03 (0.06) | 0.6432 |
| Daring | 0.08 (0.06) | 0.1544 | 0.09 (0.07) | 0.2146 | 0.03 (0.06) | 0.6116 | 0.02 (0.05) | 0.7040 |
| ROIs: | Left Rostral ACC Thickness | Right Rostral ACC Thickness | ||||||
| Negative Emotionality | 0.08 (0.06) | 0.2546 | 0.12 (0.10) | 0.2188 | 0.10 (0.08) | 0.2060 | −0.03 (0.08) | 0.6736 |
| Prosociality | 0.08 (0.06) | 0.1857 | 0.04 (0.06) | 0.4511 | 0.03 (0.07) | 0.7112 | 0.09 (0.06) | 0.1445 |
| Daring | 0.10 (0.09) | 0.2587 | −0.05 (0.09) | 0.5911 | −0.01 (0.05) | 0.8922 | 0.14 (0.06) | 0.0213 |
| ROIs: | Left Medial Orbitofrontal Cortex Thickness | Right Medial Orbitofrontal Cortex Thickness | ||||||
| Negative Emotionality | 0.09 (0.07) | 0.1751 | 0.04 (0.07) | 0.5344 | 0.09 (0.07) | 0.1576 | 0.04 (0.06) | 0.5505 |
| Prosociality | 0.14 (0.08) | 0.0964 | 0.11 (0.06) | 0.0908 | 0.04 (0.07) | 0.5787 | 0.06 (0.05) | 0.3076 |
| Daring | 0.02 (0.07) | 0.7882 | 0.05 (0.09) | 0.5355 | −0.05 (0.06) | 0.4251 | 0.10 (0.06) | 0.1155 |
AMYGDALA CADS LOCKWW180309 RDOC DX TASK NETWORKS W1W2 4 SEPT 18.SAS
CADS dimensions and brain volumes normalized before analysis to facilitate interpretation of betas in terms of effect sizes. Coefficients in bold are significant (two-tailed) after FDR correction for 48 tests (adopting a 5% false discovery rate) in the family of analyses without controlling TBV (top rows) and controlling TBV (bottom rows).
Figure 1.
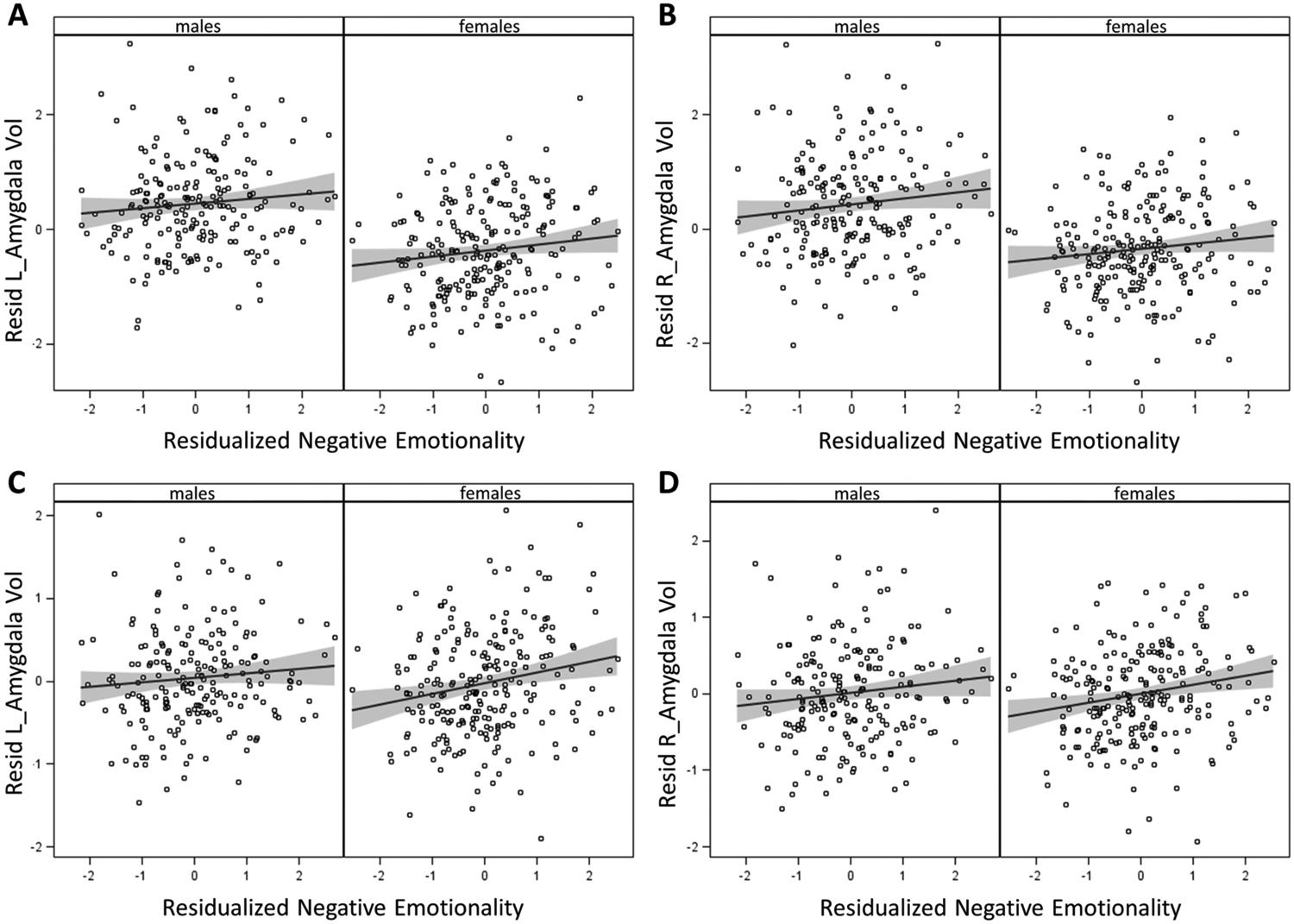
Residual-residual plots of youth-rated negative emotionality at 10–17 years by left and right amygdala volumes at 22–31 years. Variables on each axis were residualized in wave 1, age in wave 2, race-ethnicity, handedness, scanner, and CADS prosociality and daring scores. Panels a and b present findings when negative emotionality and amygdala volumes were not also residualized on total brain volume. Panels c and d present findings when negative emotionality and amygdala volumes were also residualized on brain volume. Gray shading indicates 95% confidence intervals for regression lines. Note the vertical scales vary.
Figure 2.
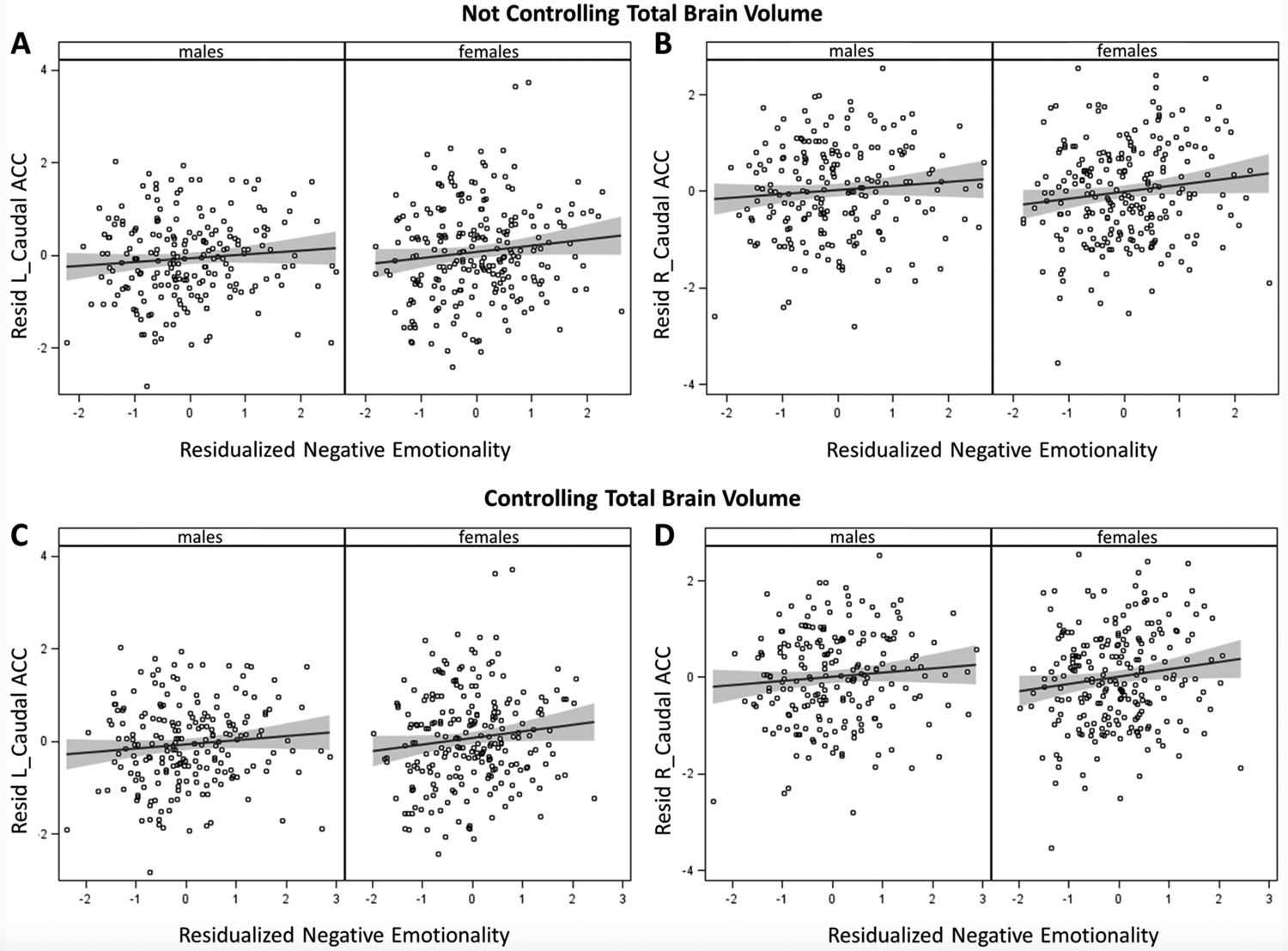
Residual-residual plots of youth-rated negative emotionality at 10–17 years by left and right caudal (dorsal) anterior cingulate cortex (ACC) volumes at 22–31 years. Variables on each axis were residualized in wave 1, age in wave 2, race-ethnicity, handedness, scanner, and CADS prosociality and daring scores. Panels a and b present findings when negative emotionality and caudal ACC volumes were not also residualized on total brain volume. Panels c and d present findings when negative emotionality and caudal ACC volumes were also residualized on brain volume. Gray shading indicates 95% confidence intervals for regression lines. Note the vertical scales vary.
As shown in Table 3, an exploratory family of tests suggested that associations of parent and youth CADS negative emotionality ratings with nearly all ROIs were not significantly moderated by sex. Nonetheless, there was a significant unpredicted interaction with sex in the association of parent ratings on dispositional daring and the volume of the left amygdala when TBV was controlled. There was also a nominally significant interaction between sex and parent ratings of daring in the association with the volume of the right amygdala (p < .0077). Follow-up tests for each sex assessed separately revealed a nominally significant positive association between left amygdala volume and parent daring ratings among females, β = 0.16, p < .0181, and a nominally significant inverse association between left amygdala volume and parent daring ratings among males, β = −0.13, p < .0128.
Table 3.
Standardized regression coefficients and standard errors for tests of sex-by-disposition interactions for Desikan-Killiany atlas regions of interest on the three dispositions, controlling covariates of no interest that do not include total brain volume (top panels) or include total brain volume (bottom panels) (all N =433).
| CADS Informant: | Parent | Youth | Parent | Youth | ||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p < | β (SE) | p < | β (SE) | p < | β (SE) | p < | |
| Dispositions | ||||||||
| Covariates: Sex, age in wave 1, age in wave 2, race-ethnicity, handedness, scanner | ||||||||
| ROIs: | Left Amygdala Volume | Right Amygdala Volume | ||||||
| Negative Emotionality | −0.09 (0.11) | 0.4307 | 0.18 (0.09) | 0.0578 | −0.12 (0.16) | 0.4313 | 0.15 (0.11) | 0.1975 |
| Prosociality | −0.01 (0.16) | 0.9597 | 0.10 (0.17) | 0.5744 | −0.12 (0.20) | 0.5516 | 0.05 (0.18) | 0.7691 |
| Daring | 0.34 (0.15) | 0.0263 | −0.07 (0.13) | 0.6040 | 0.32 (0.17) | 0.0618 | −0.02 (0.13) | 0.9051 |
| ROIs: | Left Caudal ACC Thickness | Right Caudal ACC Thickness | ||||||
| Negative Emotionality | 0.21 (0.14) | 0.1262 | −0.18 (0.10) | 0.0713 | 0.19 (0.12) | 0.1182 | −0.03 (0.13) | 0.8077 |
| Prosociality | 0.29 (0.15) | .0539 | −0.03 (0.17) | 0.8394 | −0.05 (0.14) | .7135 | 0.07 (0.12) | 0.5698 |
| Daring | 0.04 (0.12) | .7787 | −0.05 (0.14) | 0.7424 | −0.06 (0.12) | .6374 | 0.15 (0.13) | 0.2335 |
| ROIs: | Left Rostral ACC Thickness | Right Rostral ACC Thickness | ||||||
| Negative Emotionality | 0.26 (0.12) | 0.0407 | 0.12 (0.14) | 0.4220 | 0.07 (0.18) | 0.6907 | −0.12 (0.12) | 0.3046 |
| Prosociality | 0.25 (0.11) | 0.0321 | 0.03 (0.12) | 0.7919 | −0.16 (0.17) | 0.3419 | −0.20 (0.14) | 0.1604 |
| Daring | 0.09 (0.18) | 0.5923 | −0.18 (0.16) | 0.2640 | 0.15 (0.10) | 0.1329 | 0.16 (0.12) | 0.1850 |
| ROIs: | Left Medial Orbitofrontal Cortex Thickness | Right Medial Orbitofrontal Cortex Thickness | ||||||
| Negative Emotionality | 0.24 (0.13) | 0.0532 | 0.12 (0.12) | 0.3472 | 0.15 (0.12) | 0.2087 | −0.02 (0.10) | 0.8343 |
| Prosociality | 0.37 (0.14) | 0.0077 | 0.17 (0.15) | 0.2439 | 0.12 (0.12) | 0.3583 | 0.12 (0.13) | 0.3592 |
| Daring | 0.16 (0.14) | 0.2604 | −0.22 (0.14) | 0.1176 | −0.02 (0.14) | 0.8845 | −0.14 (0.13) | 0.2949 |
| Covariates: Sex, age in wave 1, age in wave 2, race-ethnicity, handedness, scanner, total brain volume | ||||||||
| ROIs: | Left Amygdala Volume | Right Amygdala Volume | ||||||
| Negative Emotionality | −0.03 (0.08) | 0.6659 | 0.10 (0.07) | 0.1674 | −0.06 (0.09) | 0.5343 | 0.06 (0.08) | 0.4501 |
| Prosociality | 0.06 (0.11) | 0.5808 | −0.00 (0.11) | 0.9580 | −0.04 (0.12) | 0.7100 | −0.07 (0.09) | 0.4738 |
| Daring | 0.29 (0.08) | 0.0006 | −0.12 (0.08) | 0.1277 | 0.26 (0.10) | 0.0077 | −0.07 (0.07) | 0.2902 |
| ROIs: | Left Caudal ACC Thickness | Right Caudal ACC Thickness | ||||||
| Negative Emotionality | 0.23 (0.13) | 0.0790 | −0.20 (0.10) | 0.0442 | 0.20 (0.13) | 0.1140 | −0.04 (0.13) | 0.7589 |
| Prosociality | 0.31 (0.13) | 0.0198 | −0.06 (0.17) | 0.7176 | −0.04 (0.14) | 0.7763 | 0.06 (0.13) | 0.6457 |
| Daring | 0.01 (0.11) | 0.8975 | −0.06 (0.14) | 0.6601 | −0.07 (0.12) | 0.5600 | 0.15 (0.13) | 0.2480 |
| ROIs: | Left Rostral ACC Thickness | Right Rostral ACC Thickness | ||||||
| Negative Emotionality | 0.26 (0.12) | 0.0386 | 0.12 (0.14) | 0.3917 | 0.09 (0.16) | 0.5955 | −0.14 (0.11) | 0.2158 |
| Prosociality | 0.25 (0.11) | 0.0299 | 0.04 (0.12) | 0.7686 | −0.14 (0.16) | 0.3720 | −0.23 (0.14) | 0.1025 |
| Daring | 0.09 (0.18) | 0.5894 | −0.18 (0.17) | 0.2784 | 0.14 (0.11) | 0.2004 | 0.15 (0.12) | 0.2111 |
| ROIs: | Left Medial Orbitofrontal Cortex Thickness | Right Medial Orbitofrontal Cortex Thickness | ||||||
| Negative Emotionality | 0.01 (0.14) | 0.9243 | 0.28 (0.13) | 0.0381 | 0.07 (0.12) | 0.5444 | 0.04 (0.10) | 0.6540 |
| Prosociality | 0.02 (0.14) | 0.9130 | 0.04 (0.10) | 0.6762 | −0.09 (0.12) | .4726 | −0.10 (0.09) | 0.2927 |
| Daring | −0.02 (0.15) | 0.9095 | 0.01 (0.10) | 0.9395 | −0.01 (0.08) | 0.8916 | 0.01 (0.11) | 0.9445 |
AMYGDALA CADS LOCKWW180309 RDOC DX TASK NETWORKS W1W2 4 SEPT 18.SAS
CADS dimensions and brain volumes normalized before analysis to facilitate interpretation of betas in terms of effect sizes. Coefficients in bold are significant (two-tailed) after FDR correction for 48 tests (adopting a 5% false discovery rate) in the family of analyses without controlling TBV (top rows) and controlling TBV (bottom rows).
Discussion
The present findings confirmed the prediction that higher youth-reported dispositional negative emotionality in childhood/adolescence would be associated with greater amygdala volumes when assessed in adulthood. This extends the previous findings on which the planned tests were based that variations in the size of amygdala (after correcting for TBV) is a biomarker for dispositional negative emotionality (DeYoung & Krueger, 2018; Holmes et al., 2012; Mincic, 2015) and converges with theories emphasizing the involvement of the amygdala in the processing of negatively valenced emotions (Gross & Barrett, 2011; Shackman et al., 2016; Zald, 2003). Although past ROI studies and an ALE analysis have more frequently observed left than right amygdala associations (Mincic, 2015), we observed effects bilaterally. Given the robust correlations of negative emotionality with diverse aspects of mental and physical health, the observed linkage to the size of the amygdala has broad theoretical and practical significance (Lahey, 2009; Widiger & Oltmanns, 2017).
Our results stand in contrast to two previous studies that found no association between amygdala volume and self-reported neuroticism in adolescents (Blankstein et al., 2009; Delaparte et al., 2019). Although the methods of these studies differed in a number of ways from the present study, including the measures of dispositions used and the nature of amygdala measurement, the two major differences were (1) the samples 15 males and 20 females (Blankstein et al. (2009) and 223 females, but no males (Delaparte et al., 2019), and (2) the timing of amygdala measurement (concurrent with assessment of negative emotionality assessment or in adulthood).
The association between negative emotionality and amygdala volume is particularly remarkable in the present study because the measurement of amygdala volume was made 12–13 years after the measurement of negative emotional disposition. Unfortunately, because we lacked an MRI assessment in adolescence, we cannot infer whether the association was stable over developmental time in our sample, and thus leaves open the question of the timing and causal nature of the association. Because amygdala volume and regional cortical thickness are highly heritable (Jansen, Mous, White, Posthuma, & Polderman, 2015) and that heritability is already expressed by at least late childhood (Swagerman, Brouwer, de Geus, Pol, & Boomsma, 2014), it is possible that genetically influenced differences in brain structures are present relatively early in life and are part of the substrate of individual differences in negative emotionality across the lifespan.
Alternatively, it is possible that the observed correlation is the result of higher levels of negative emotionality over this period of development causing increases in the size of the amygdala in a way that is not yet understood. In considering the mechanisms that might contribute to such relations, it is notable that stress during specific developmental periods can alter stereological features of the amygdala, in some cases in subnuclei-specific ways. For instance, in rodents, prenatal stress produced an enhancement of the lateral nucleus assessed in adulthood, in part due to greater numbers of neurons and glia (Salm et al., 2004), while postnatal chronic stress has been found to increase dendritic spine density in the basolateral amygdala as well as changes in cortico-amygdala excitability and anxiety like behaviors (Zhang et al., 2018). Because there is good reason to believe that persons with higher levels of negative emotionality are more likely to generate stressful events (Hammen, 1991, 2006), it is possible that negative emotionality becomes linked to variation in amygdala volumes and the thickness of the caudal ACC through the effects of negative emotionality on stress and the effects of stress on the developing brain. There are numerous alternative possibilities that remain to be explored, of course. For example, greater acute or chronic stress may cause both greater negative emotionality and larger amygdala volumes. Although this possibility remains speculative, it might help explain why studies such as those by Blankstein et al. (2009) and Delaparte et al. (2019) failed to observe a relationship between the amygdala volume and negative emotionality in adolescents, when the present study could detect a brain-disposition association with a dozen year separation in time.
In addition to confirming the hypothesis of an association between greater negative emotionality and larger amygdala volume for the youth ratings, we detected an unpredicted positive association between parent-rated dispositional negative emotionality and individual differences in the thickness of the bilateral caudal region of the ACC. Like the amygdala, this region has been implicated in evaluation of emotional salience (Etkin et al., 2011; Seeley et al., 2007), and its close connections with the amygdala (Ghashghaei, Hilgetag, & Barbas, 2007) places it at a critical junction in the interface between cingulate-mediated control functions and emotional processes. Consistent with the direction of the effect, some studies have reported associations of greater neuroticism in healthy adults with increased volume of the caudal and other regions of the ACC (Pujol et al., 2002)) or whole ACC volume (DeYoung et al., 2010; Pujol et al., 2002), although this association has not consistently emerged in the literature. This pattern of enhanced cortical thickness is notable because, when examined over time, greater neuroticism has been associated with reduced cortical thinning in several frontotemporal regions across development, which could be predicted to result in areas with enhanced cortical thickness in adulthood (Ferschmann et al., 2018). A prior meta-analysis of studies of fear learning indicates an association between neuroticism and fMRI activations in a similar caudal ACC region (Servaas et al., 2013), suggesting a convergence of functional and structural associations in this region.
Considering the amygdala and caudal ACC findings together, significant positive associations of dispositional negative emotionality as measured by parent and youth ratings on the CADS were found with the morphometry of two brain regions implicated with emotion evaluation and aspects of driving emotional responses. Both findings are important, but the association of parent-rated negative emotionality with the thickness of the bilateral caudal ACC is particularly interesting since parent-rated CADS negative emotionality in childhood and adolescence has been found to predict all forms of psychopathology in adulthood through the general factor of psychopathology (Class et al., 2019). Thus, parent-rated negative emotionality and its associated individual differences in the ACC could constitute a nonspecific risk factor for a broad range of dimensions of psychopathology (Lahey, Krueger, Rathouz, Waldman, & Zald, 2017).
By contrast, cortical thickness in other cortical areas implicated in the processing of emotion, including the insula and ventromedial frontal regions, did not show a relationship with CADS negative emotionality, at least not using the large ROIs defined in the Desikan atlas, which encompass several cytoarchitectural areas. Some theorists (Etkin et al., 2016; Etkin et al., 2011; Phillips et al., 2008) have emphasized the role of the ventromedial cortical regions in implicit or automatic emotion regulation. In such models, the present results may suggest that dispositional negative emotionality as defined by the CADS is related more to brain regions involved in aspects of emotional evaluation and the direction of emotional responses than those involved in implicit emotion regulation. In considering this interpretation, it is critical to note that the present study differs from past studies that have observed reductions in grey matter volume or thickness in ventromedial regions to be associated with negative emotion (Holmes, et al. 2012; Mincic, 2015), as past studies measured concurrent, not prospective relations, and the definitions of negative emotionality were heavily focused on fears and anxiety rather than the broader definition of negative emotionality used in the CADS. The CADS has not been previously utilized in studies of neurobiological correlates of psychopathology, but the current data suggest its potential utility in this domain.
The implications of the present findings based on the CADS are complicated by differences in the predictive associations between parent and youth informants. These observed differences between the raters may reflect differences in maturity, experiences, perspectives, the situations in which each informant observes the child, the covertness of some important experiences, and response style differences between the informants. New research is needed that can shed light on informant differences, but because no resolution has been reached at present on the most valid method of combining discrepant ratings from parents and youth on dispositional traits (Tackett, 2011), we analyzed the data separately for parent and youth CADS ratings in the primary analyses. CADS negative emotionality measured by youth ratings was found to be associated with greater left and right amygdala volumes, but parent-rated negative emotionality was associated with greater volume of the bilateral caudal ACC. Although both regions are implicated in emotion generation, the informant difference raises questions about the meaning of the findings. The correlation between parent and youth ratings on CADS negative emotionality was only r = 0.26. Even allowing for lack of perfect reliability of these measures, this correlation suggests that parents and youth report only partially overlapping aspects of emotional behavior.
In the present study, we performed analyses both controlling for TBV and providing parallel analyses without controlling for TBV. The results in Figures 1 and 2 are presented separately based on whether TBV was controlled (lower panels) or was not controlled (upper panels) to help visualize the effects of controlling TBV. For amygdala volumes, the slopes shown Figure 1 are similar when TBV is and is not controlled, although as previously reported (Marwha et al., 2017), the normative sex difference in mean amygdala volumes when TBV is not controlled is reduced when TBV is controlled. When considered with the results in Table 2, however, it is clear that the standard errors of the regression coefficients are 67–100% larger when TBV is not controlled, resulting in differences in the statistical significance of associations of negative emotionality with amygdala volumes. Consistent with previous findings of little or no sex differences in mean cortical thickness in this age range (Gennatas et al., 2017), controlling TBV had less effect on tests of associations of parent-rated negative emotionality with mean thickness of the caudal ACC (Figure 2 and Table 2). The only difference was a very small difference in the p level of the association for the caudal ACC, which resulted in the association with right caudal ACC being significant after FDR adjustment when TBV was controlled, but falling just short when TBV was not controlled.
Finally, the exploratory tests of interactions between sex and the CADS dispositions revealed little evidence of sex differences in the predictive associations between adolescent CADS dispositions and later brain structure for the selected ROIs. The one exception was that parent-rated daring may predict left amygdala volume in different directions in males and females. It is important to note, however, that these exploratory tests of interactions were not based on previous findings and were conducted in a sample that was not well powered for testing interactions. Thus, the absence of statistically significant interactions with other demographic variables should not be interpreted as evidence for the absence of such interactions.
Contributor Information
Benjamin B. Lahey, University of Chicago
Xiaochan Yang, University of Chicago.
Paul J. Rathouz, University of Texas at Austin
Brooks Applegate, Western Michigan University.
David H. Zald, Vanderbilt University
References
- Allen TA, & DeYoung CG (2017). Personality neuroscience and the Five Factor Model. In Widiger TA (Ed.), Oxford handbook of the Five Factor Model (pp. 319–349). New York: Oxford University Press. [Google Scholar]
- Asman AJ, & Landman BA (2011). Characterizing spatially varying performance to improve multi-atlas multi-label segmentation. Paper presented at the Biennial International Conference on Information Processing in Medical Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B, 57, 289–300. [Google Scholar]
- Blankstein U, Chen JYW, Mincic AM, McGrath PA, & Davis KD (2009). The complex minds of teenagers: Neuroanatomy of personality differs between sexes. Neuropsychologia, 47(2), 599–603. doi: 10.1016/j.neuropsychologia.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Caspi A, Henry B, McGee RO, Moffitt TE, & Silva PA (1995). Temperamental origins of child and adolescent behavior problems: From age 3 to age 15. Child Development, 66(1), 55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x [DOI] [PubMed] [Google Scholar]
- Cha J, Greenberg T, Carlson JM, DeDora DJ, Hajcak G, & Mujica-Parodi LR (2014). Circuit-wide structural and functional measures predict ventromedial prefrontal cortex rear generalization: Implications for generalized anxiety disorder. Journal of Neuroscience, 34(11), 4043–4053. doi: 10.1523/jneurosci.3372-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Rathouz PJ, Van Hulle CA, Applegate B, Waldman ID, Zald DH, & Lahey BB (2019). Socioemotional dispositions of children and adolescents predict general and specific second-order factors of psychopathology in early adulthood across informants: A 12-year prospective study. Journal of Abnormal Psychology, 128, 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaparte L, Bartlett E, Grazioplene R, Perlman G, Gardus J, DeLorenzo C, … Kotov R (2019). Structural correlates of the orbitofrontal cortex and amygdala and personality in female adolescents. Psychophysiology, e13376. doi: 10.1111/psyp.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Hyman BT (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, … Navia B (2010). Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage, 51(4), 1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, & Gray JR (2010). Testing Predictions From Personality Neuroscience: Brain Structure and the Big Five. Psychological Science, 21(6), 820–828. doi: 10.1177/0956797610370159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, & Krueger RF (2018). A cybernetic theory of psychopathology. Psychological Inquiry, 29(3), 117–138. [Google Scholar]
- Etkin A, Buchel C, & Gross JJ (2016). Emotion regulation involves both model-based and model-free processes. Nature Reviews Neuroscience, 17(8). doi: 10.1038/nrn.2016.79 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. doi: 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferschmann L, Fjell AM, Vollrath ME, Grydeland H, Walhovd KB, & Tamnes CK (2018). Personality Traits Are Associated With Cortical Development Across Adolescence: A Longitudinal Structural MRI Study. Child Development, 89(3), 811–822. doi: 10.1111/cdev.13016 [DOI] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. Neuroimage, 62(2), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Klaveness S (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Gennatas ED, Avants BB, Wolf DH, Satterthwaite TD, Ruparel K, Ciric R, … Gur RC (2017). Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adulthood. Journal of Neuroscience, 37(20), 5065–5073. doi: 10.1523/jneurosci.3550-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag CC, & Barbas H (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage, 34(3), 905–923. doi: 10.1016/j.neuroimage.2006.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, & Rapoport JL (1997). Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 21, 1185–1201. [DOI] [PubMed] [Google Scholar]
- Gray JC, Owens MM, Hyatt CS, & Miller JD (2018). No evidence for morphometric associations of the amygdala and hippocampus with the five-factor model personality traits in relatively healthy young adults. Plos One, 9. doi: 10.1371/journal.pone.0204011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (2015). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26(1), 1–26. doi: 10.1080/1047840x.2014.940781 [DOI] [Google Scholar]
- Gross JJ, & Barrett LF (2011). Emotion generation and emotion regulation: One or two depends on your point of view. Emotion Review, 3(1), 8–16. doi: 10.1177/1754073910380974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (1991). Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology, 100(4), 555–561. doi: 10.1037//0021-843x.100.4.555 [DOI] [PubMed] [Google Scholar]
- Hammen C (2006). Stress generation in depression: Reflections on origins, research, and future directions. Journal of Clinical Psychology, 62(9), 1065–1082. doi: 10.1002/jclp.20293 [DOI] [PubMed] [Google Scholar]
- Hanson JL, Suh JW, Nacewicz BM, Sutterer MJ, Cayo AA, Stodola DE, … Davidson RJ (2012). Robust automated amygdala segmentation via multi-atlas diffeornorphic registration. Frontiers in Neuroscience, 6. doi: 10.3389/fnins.2012.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead MO, O’Keefe TM, Petrov VI, Fariello GR, Wald LL, … Buckner RL (2015). Brain genomics superstruct project initial data release with structural, functional, and behavioral measures. Scientific data, 2. doi: 10.1038/sdata.2015.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, & Buckner RL (2012). Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. Journal of Neuroscience, 32(50), 18087–18100. doi: 10.1523/jneurosci.2531-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AG, Mous SE, White T, Posthuma D, & Polderman TJC (2015). What Twin Studies Tell Us About the Heritability of Brain Development, Morphology, and Function: A Review. Neuropsychology Review, 25(1), 27–46. doi: 10.1007/s11065-015-9278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, & Markon KE (2006). Understanding psychopathology: Melding behavior genetics, personality, and quantitative psychology to develop an empirically based model. Current Directions in Psychological Science, 15, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB (2009). Public health significance of neuroticism. American Psychologist, 64, 241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Chronis AM, Jones HA, Williams SH, Loney J, & Waldman ID (2008). Psychometric characteristics of a measure of emotional dispositions developed to test a developmental propensity model of conduct disorder. Journal of Clinical Child and Adolescent Psychology, 37, 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Class QA, Zald DH, Rathouz PJ, Applegate B, & Waldman ID (2018). Prospective test of the developmental propensity model of antisocial behavior: from childhood and adolescence into early adulthood. Journal of Child Psychology and Psychiatry, 59, 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, & Zald DH (2017). A hierarchical causal taxonomy of psychopathology across the life span. Psychological Bulletin, 143, 142–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Applegate B, Tackett JL, & Waldman ID (2010). Psychometrics of a self-report version of the child and adolescent dispositions scale. Journal of Clinical Child and Adolescent Psychology, 39, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Applegate B, Van Hulle C, Garriock HA, Urbano RC, … Waldman ID (2008). Testing structural models of DSM-IV symptoms of common forms of child and adolescent psychopathology. Journal of Abnormal Child Psychology, 36, 187–206. [DOI] [PubMed] [Google Scholar]
- Marwha D, Halari M, & Eliot L (2017). Meta-analysis reveals a lack of sexual dimorphism in human amygdala volume. Neuroimage, 147, 282–294. doi: 10.1016/j.neuroimage.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Mincic AM (2015). Neuroanatomical correlates of negative emotionality-related traits: A systematic review and meta-analysis. Neuropsychologia, 77, 97–118. doi: 10.1016/j.neuropsychologia.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner II HR, Lewis DV, … McCarthy G (2009). A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage, 45(3), 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, & Ollendick TH (2005). The role of temperament in the etiology of child psychopathology. Clinical Child and Family Psychology Review, 8(4), 271–289. doi: 10.1007/s10567-005-8809-y [DOI] [PubMed] [Google Scholar]
- Muthén B, & Muthén L (2018). Mplus 8.1. Los Angeles: Muthén & Muthén. [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47, 395–422. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, … Kremen WS (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex, 19(11), 2728–2735. doi: 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J (2004). Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press. [Google Scholar]
- Perlaki G, Horvath R, Nagy SA, Bogner P, Doczi T, Janszky J, & Orsi G (2017). Comparison of accuracy between FSL’s FIRST and Freesurfer for caudate nucleus and putamen segmentation. Scientific reports, 7(1), 2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, & LeDoux JE (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48(2), 175–187. doi: 10.1016/j.neuron.2005.09.025 [DOI] [PubMed] [Google Scholar]
- Phillips M, Ladouceur C, & Drevets W (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833–857. doi: 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassard AJ, McHugo M, Heckers S, & Landman BA (2017). Multi-scale hippocampal parcellation improves atlas-based segmentation accuracy. Paper presented at the Medical Imaging 2017: Image Processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, & Paus T (2002). Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage, 15(4), 847–855. doi: 10.1006/nimg.2001.1004 [DOI] [PubMed] [Google Scholar]
- Ray RD, & Zald DH (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience and Biobehavioral Reviews, 26, 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon PK, Seidlitz J, Vandekar S, Liu SY, Patel R, Park MTM, … Raznahan A (2018). Normative brain size variation and brain shape diversity in humans. Science, 360(6394), 1222–1226. doi: 10.1126/science.aar2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, & Ruhe HG (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37(10), 2529–2553. doi: 10.1016/j.neubiorev.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, & Birkle DL (2004). Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Developmental Brain Research, 148(2), 159–167. doi: 10.1016/j.devbrainres.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, … Pruessner JC (2016). Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage, 129, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi: 10.1523/jneurosci.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, van der Velde J, Costafreda SG, Horton P, Ormel J, Riese H, & Aleman A (2013). Neuroticism and the brain: A quantitative meta-analysis of neuroimaging studies investigating emotion processing. Neuroscience and Biobehavioral Reviews, 37(8), 1518–1529. doi: 10.1016/j.neubiorev.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DPM, Stockbridge MD, Kaplan CM, Tillman RM, & Fox AS (2016). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142(12), 1275–1314. doi: 10.1037/bul0000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, & Brennan LM (2012). Early predictors of boys’ antisocial trajectories. Development and Psychopathology, 24(3), 871–888. doi: 10.1017/s0954579412000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick SL, Brennan LM, Forbes E, & Shaw DS (2014). Developmental pathways to sexual risk behavior in high-risk adolescent boys. Pediatrics, 133(6), 1038–1045. doi: 10.1542/peds.2013-3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swagerman SC, Brouwer RM, de Geus EJC, Pol HEH, & Boomsma DI (2014). Development and heritability of subcortical brain volumes at ages 9 and 12. Genes Brain and Behavior, 13(8), 733–742. doi: 10.1111/gbb.12182 [DOI] [PubMed] [Google Scholar]
- Sweeney M, Tsapanou A, & Stern Y (2019). Regional cortical thickness and neuroticism across the lifespan. Psychiatry Research-Neuroimaging, 286, 39–44. doi: 10.1016/j.pscychresns.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL (2011). Parent Informants for Child Personality: Agreement, Discrepancies, and Clinical Utility. Journal of Personality Assessment, 93(6), 539–544. doi: 10.1080/00223891.2011.608763 [DOI] [PubMed] [Google Scholar]
- Tackett JL, Waldman ID, Van Hulle CA, & Lahey BB (2011). Shared genetic influences on negative emotionality and major depression/conduct disorder comorbidity. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Allan N, Mikolajewski AJ, & Hart SA (2013). Common genetic and nonshared environmental factors contribute to the association between socioemotional dispositions and the externalizing factor in children. Journal of Child Psychology and Psychiatry, 54, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, & Chess S (1957). An approach to the study of sources of individual differences in child behavior. Journal of clinical and experimental psychopathology, 18, 347–357. [PubMed] [Google Scholar]
- Trentacosta CJ, Hyde LW, Shaw DS, & Cheong JW (2009). Adolescent dispositions for antisocial behavior in context: The roles of neighborhood dangerousness and parental knowledge. Journal of Abnormal Psychology, 118, 564–575. doi: 10.1037/a0016394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang O, Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, & Panahi I (2008). Comparison of tissue segmentation algorithms in neuroimage analysis software tools. Paper presented at the Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE. [DOI] [PubMed] [Google Scholar]
- van der Plas EAA, Boes AD, Wemmie JA, Tranel D, & Nopoulos P (2010). Amygdala volume correlates positively with fearfulness in normal healthy girls. Social Cognitive and Affective Neuroscience, 5(4), 424–431. doi: 10.1093/scan/nsq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID, Tackett JL, Van Hulle CA, Applegate B, Pardini D, Frick PJ, & Lahey BB (2011). Child and adolescent conduct disorder substantially shares genetic influences with three socioemotional dispositions. Journal of Abnormal Psychology, 120, 57–70. doi: 10.1037/a0021351 [DOI] [PubMed] [Google Scholar]
- Widiger TA, & Oltmanns JR (2017). Neuroticism is a fundamental domain of personality with enormous public health implications. World Psychiatry, 16(2), 144–145. doi: 10.1002/wps.20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, … Glahn DC (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage, 53, 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews, 41(1), 88–123. doi: 10.1016/s0165-0173(02)00248-5 [DOI] [PubMed] [Google Scholar]
- Zhang JY, Liu TH, He Y, Pan HQ, Zhang WH, Yin XP, … Pan BX (2018). Chronic stress remodels synapses in an amygdala circuit-specific manner. Biological Psychiatry. doi:doi: 10.1016/j.biopsych.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]


