Abstract
Background
Helicobacter pylori (H. pylori) has increasingly been shown to be related to extragastric diseases. Glycated hemoglobin A1c (HbA1c), an indicator of glycemic control, is closely linked to the event of diabetes. The purpose of this research was to analyze the association between H. pylori and HbA1c through a cohort study.
Methods
The population who underwent multiple physical checkups in the physical examination center of Taizhou Hospital was included. All of them underwent urea breath test, serological examination and physical parameter measurement. Multiple regression was used for analyzing the influencing factors of HbA1c. In addition, the result of HbA1c on H. pylori infection was studied by restricted cubic spline (RCS) analysis. The triglyceride glucose (TyG) index represents the level of insulin resistance (IR) in the population. The population was classified on the basis of primary and last H. pylori infection, therefore, the variations of HbA1c and TyG index among totally different teams were investigated.
Results
Multiple regression demonstrated that H. pylori was an influential factor in HbA1c. RCS analysis showed a nonlinear relationship between HbA1c and H. pylori infection. When HbA1c>5.7%, the chance of H. pylori infection was considerably enlarged. Additionally, long-term H. pylori infection increased HbA1c levels, while HbA1c levels decreased after H. pylori eradication. Similarly, long-term H. pylori infection also increased the TyG index.
Conclusion
Prediabetes increases the danger of H. pylori infection, long-term H. pylori infection increases HbA1c and IR levels, and wipeout of H. pylori could have a positive impact for glycemic control in the population.
Keywords: Helicobacter pylori, triglyceride glucose index, insulin resistance, cohort study, glycated hemoglobin
Introduction
Helicobacter pylori (H. pylori), a chronic infection of the gastrointestinal tract, has a higher prevalence in developing countries and is a global public health problem (Zamani et al., 2018). H. pylori can make functional dyspepsia, chronic gastritis, gastric ulcers, and even gastric cancer (McColl, 2010). In recent years, there are more and more studies on H. pylori and extra-gastric diseases, such as dermatological, cardiovascular, neurological, immune, respiratory, blood system, and metabolic diseases (Wong et al., 2014; Yu et al., 2019; Santos et al., 2020; Wang et al., 2020; Doheim et al., 2021; Reuter et al., 2023). H. pylori can turn out a variety of inflammatory factors, like C-reactive protein, interleukin, tumor necrosis factor α (TNF- α), and reduce the level of leptin (Noach et al., 1994; Perri et al., 1999; Chen et al., 2021). High levels of inflammatory factors and leptin deficiency are key mechanisms of insulin resistance (IR) and metabolic syndrome (Longo-Mbenza et al., 2007; Li et al., 2022).
Diabetes is a chronic metabolic disease with a rising global prevalence and a huge economic burden (Yazdanpanah et al., 2017). Glycated hemoglobin A1c (HbA1c) ≥6.5% has been included in the diagnostic criteria for diabetes mellitus, representing last 2 or 3 months of glycemic control (American Diabetes Association, 2009; Hinzmann et al., 2012). IR plays a crucial part in a variety of diseases, including diabetes and cardiovascular disease (Stumvoll et al., 2005; Wang et al., 2018; Adeva-Andany et al., 2019). Hyperinsulinemic-Euglycemic Clamp (HIEC) is that the gold criterion for designation of IR, however, owing to its complexity and high cost, it is difficult to be introduced in the physical examination population (Cersosimo et al., 2014). The triglyceride glucose (TyG) index is a novel clinical alternative index for IR (Guerrero-Romero et al., 2010; Lee et al., 2016). Because of its simplicity, accessibility and reproducibility, it has received much attention in risk and diagnostic studies for the development of type 2 diabetes (Lee et al., 2014; Wang et al., 2021).
However, the connection between H. pylori and HbA1c as well as IR is controversial. In a cross-sectional study involving 37,263 people, H. pylori infection was associated only with dyslipidemia, not fasting blood glucose (FBG) or HbA1c (Kim et al., 2016). In addition, H. pylori infection failed to take issue between diabetic and non-diabetic populations, and H. pylori eradication had no result on blood glucose and IR (Park et al., 2005; Demir et al., 2008). However, in some researches, H. pylori infection has been related to glycemic management in diabetic populations, and wipeout of H. pylori may benefit glycemic management (Cheng et al., 2019; Song et al., 2021).
Given the unspecified association between H. pylori and HbA1c, the research explored the influence of H. pylori infection on HbA1c and IR through a large cohort study.
Materials and methods
Data collection
In this study, people who underwent physical examination at the Health Examination Center of Taizhou Hospital between 2017 and 2022 were included. The age, sex, smoking and alcohol history, laboratory indicators and carbon breath test results of the patients were collected. Laboratory parameters included triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), FBG and HbA1c. We excluded patients who lacked complete clinical information or who had a history of malignancy, severe cardiovascular disease, or gastrointestinal surgery. All people had undergone multiple medical examinations, with an interval of >6 months between the first and last examination. A total of 9,266 individuals participated in the study.
Clinical indicators collection
Trained nurses inquired the patient’s age, gender, smoking and drinking history, and measured diastolic blood pressure (DBP) and systolic blood pressure (SBP) in the quiet state. After fasting for 8 hours during the night, venous blood was collected from people under fasting condition for testing laboratory parameters such as TC, TG, LDL, HDL, FBG, and HbA1c. Glycated hemoglobin analyzer was used to measure HbA1c levels in the population. The calculation formula of TyG index was [Tg (mg/dL) ×FBG (mg/dL)/2] (Guerrero-Romero et al., 2010).
Detection of Helicobacter pylori
H. pylori was detected by 13C or 14C urease breath tests (Fischbach and Malfertheiner, 2018). The process of the 13C breath test was as follow: the first breath sample was collected in the fasting state, then the capsule labeled with 13C was taken, the respiratory sample was collected after waiting 30 minutes, and finally the two samples were analyzed on the apparatus. The procedure of the 14C breath test was as follow: taken a 14C urea capsule and added water, waited for 15 minutes, evenly blown air through the conduit for 1-3 minutes, and obtained the results by inserting the gas collecting card into the detector.
Statistical analysis
For continuous variables, Mann-Whitney test or independent T test were used to analyze the differences between groups. Categorical variables were tested by chi-square test and expressed as counts and percentages. Multiple regression was used to analyze the influencing factors of HbA1c. Additionally, we investigated the link between HbA1c and H. pylori using restricted cubic spline (RCS) analysis with nodes set at the 5th, 35th, 65th, and 95th percentiles. Statistical analysis was performed in R software (version 4.1.3), and p<0.05 was deemed significant.
Result
Baseline characteristics
The baseline characteristics of the population were shown in Table 1 . All medical examiners were divided into H. pylori negative and positive groups depending on breath test. The positive group was more likely to be male, older, and included more smokers than negative group. Additionally, compared to negative group, positive group had higher HbA1c and lower HDL levels (p<0.05).
Table 1.
Baseline characteristics of all physical examination populations.
| Variables | H. pylori negative (n=5328) | H. pylori positive (n=3938) | p-value |
|---|---|---|---|
| Gender (n, %) | 0.020 | ||
| Female | 1925 (36.1) | 1331 (33.8) | |
| Male | 3403 (63.9) | 2607 (66.2) | |
| Smoke (n, %) | 0.003 | ||
| No | 3997 (75.0) | 2847 (72.3) | |
| Yes | 1331 (25.0) | 1091 (27.7) | |
| Drink (n, %) | 0.136 | ||
| No | 4300 (80.7) | 3129 (79.5) | |
| Yes | 1028 (19.3) | 809 (20.5) | |
| Age (year) | 48.33 ± 11.78 | 49.33 ± 11.82 | <0.001 |
| Triglycerides (mmol/L) | 1.87 ± 1.59 | 1.92 ± 1.67 | 0.133 |
| Total cholesterol (mmol/L) | 5.04 ± 0.96 | 5.03 ± 0.95 | 0.950 |
| High density lipoprotein (mmol/L) | 1.42 ± 0.30 | 1.40 ± 0.29 | 0.009 |
| Low density lipoprotein (mmol/L) | 2.69 ± 0.73 | 2.68 ± 0.71 | 0.264 |
| Diastolic blood pressure (mmHg) | 76.07 ± 11.74 | 77.38 ± 11.71 | 0.215 |
| Systolic blood pressure (mmHg) | 126.53 ± 17.46 | 127.18 ± 17.98 | 0.080 |
| Fasting blood glucose (mmol/L) | 5.38 ± 1.43 | 5.42 ± 1.48 | 0.128 |
| Glycated hemoglobin A1c (%) | 5.83 ± 0.87 | 5.89 ± 0.90 | 0.004 |
Multiple regression analysis
In order to study the factors affecting the level of HbA1c, multiple regression was conducted for gender, age, H. pylori infection, smoking, drinking, lipids, blood pressure, and glucose. In the results, H. pylori remained the hazard factor for HbA1c (p=0.025), Table 2 .
Table 2.
Multiple regression analysis.
| Variables | β | p-value |
|---|---|---|
| Gender (%) | -0.028 | <0.001 |
| Smoke (%) | 0.043 | <0.001 |
| Drink (%) | 0.010 | 0.095 |
| Age (>50) | 0.099 | <0.001 |
| H. pylori infection (+) | 0.012 | 0.025 |
| Triglycerides (mmol/L) | -0.020 | 0.060 |
| Total cholesterol (mmol/L) | 0.060 | 0.006 |
| High density lipoprotein (mmol/L) | -0.061 | <0.001 |
| Low density lipoprotein (mmol/L) | -0.011 | 0.568 |
| Diastolic blood pressure (mmHg) | -0.042 | <0.001 |
| Systolic blood pressure (mmHg) | 0.008 | 0.337 |
| Fasting blood glucose (mmol/L) | 0.834 | <0.001 |
Nonlinear relationship between HbA1c and H. pylori infection
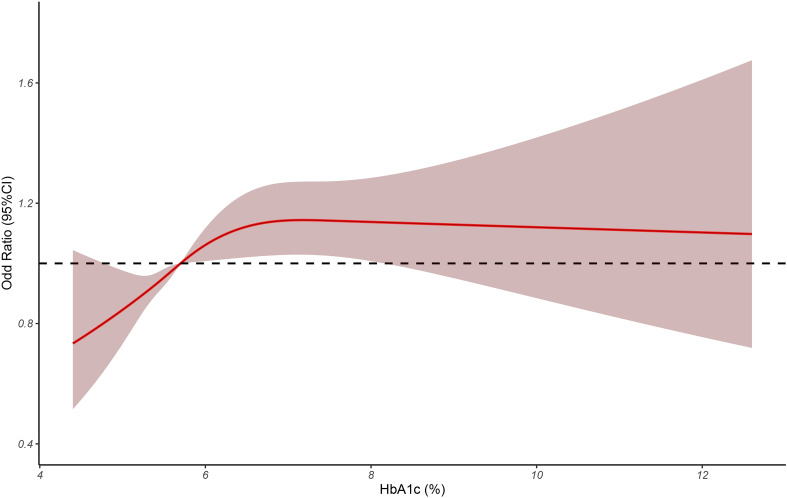
We used RCS model to evaluate the relevance between HbA1c and H. pylori infection. As shown in Figure 1 , when HbA1c>5.7%, the hazard of H. pylori infection elevated markedly with the increase of HbA1c (p<0.05).
Figure 1.
The restricted cubic spline was used to analyze the relationship between HbA1c and H. pylori infection.
Longitudinal association between HbA1c and H. pylori infection
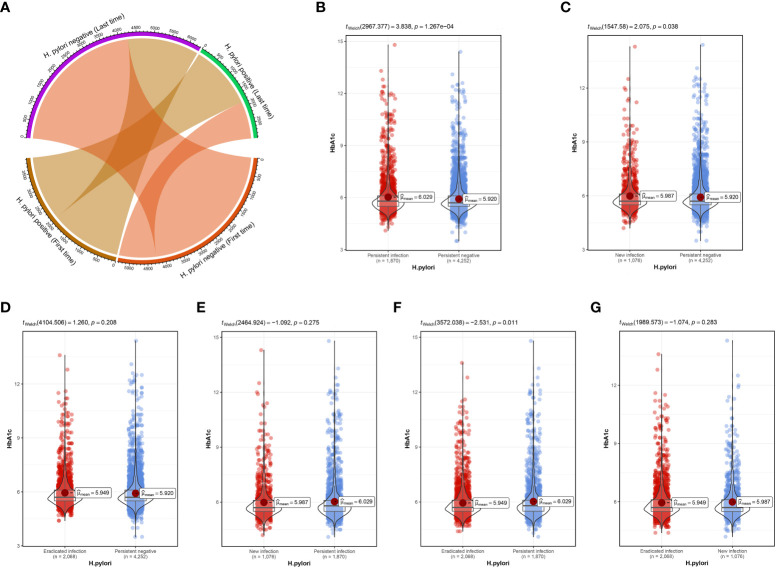
The median follow-up time for the study population was 1.77 years. Based on status of H. pylori infection at primary and last physical examination, all people were divided into H. pylori persistent infection, persistent negative, new infection and eradicated infection groups. During the follow-up period, the first and last H. pylori infection status changes were shown in Figure 2A . Compared with persistent negative group, HbA1c in persistent infection and new infection groups was remarkably higher, p<0.05 ( Figure 2B, C ). The difference between the eradicated infection and the persistent negative groups was not statistically significant, p=0.208 ( Figure 2D ). Further, there was no vital distinction in HbA1c between the persistent infection and the new infection groups ( Figure 2E ), but HbA1c in the eradicated infection group decreased significantly ( Figure 2F ). In addition, no marked difference in HbA1c was seen between new infection and eradicated infection groups, Figure 2G .
Figure 2.
(A) Changes in the status of first and last H pylori infections. (B) Difference in HbA1c between persistent infection and persistent negative. (C) Difference in HbA1c between new infection and persistent negative. (D) Difference in HbA1c between eradicated infection and persistent negative. (E) Difference in HbA1c between new infection and persistent infection. (F) Difference in HbA1c between eradicated infection and persistent infection. (G) Difference in HbA1c between eradicated infection and new infection.
Relationship between HbA1c and TyG index
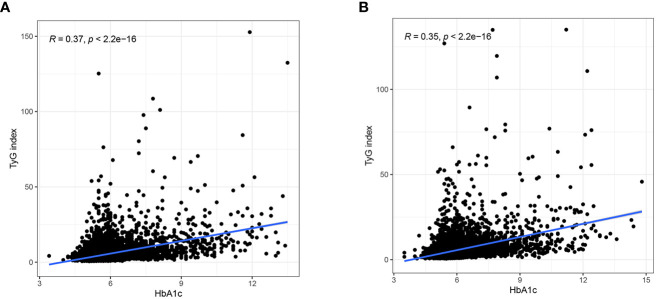
TyG index can represent insulin resistance, and we further studied the link between HbA1c and IR. At the first physical examination, there was a correlation between HbA1c and TyG index (r=0.37), Figure 3A . Similarly, at the last physical examination, HbA1c was still significantly correlated with TyG index (r=0.35), Figure 3B .
Figure 3.
(A) Relationship between HbA1c and TyG index at the first physical examination. (B) Relationship between HbA1c and TyG index at the last physical examination.
Effect of H. pylori infection on TyG index
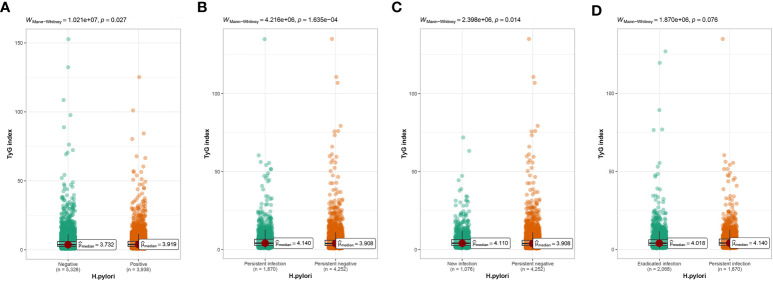
According to cross-sectional research, we observed that TyG index was considerably higher within H. pylori positive group than that in negative group ( Figure 4A ). In longitudinal study, the TyG index in the persistent infection group was apparently increased compared to the persistent negative group ( Figure 4B ). In the same way, TyG index in the new infection group was obviously increased than the persistent negative group ( Figure 4C ). The TyG index in eradicated group showed a downward trend compared with that in persistent infection group, but no difference on statistics ( Figure 4D ).
Figure 4.
(A) Difference in TyG index between H pylori negative and positive groups. (B) Difference in TyG index between persistent infection and persistent negative. (C) Difference in TyG index between new infection and persistent negative. (D) Difference in TyG index between eradicated infection and persistent infection.
Discussion
Diabetes is a chronic metabolic disease whose progression can result in a series of complications such as retinopathy, cardiovascular-related problem, and neuropathy (Cole and Florez, 2020). Blood glucose control is essential in the management of diabetes to delay the emergence and progression of its complications (Vilsbøll et al., 2018; Wang and Hng, 2021). HbA1c, as a serological indicator of glycemic control, is important for preclinical diagnosis of diabetes and prevention of vascular complications (Heianza et al., 2012; Krhač and Lovrenčić, 2019; Halalau et al., 2023). HbA1c between 5.7-6.4% has been used by American Diabetes Association (ADA) as one of the diagnostic criteria for prediabetes (American Diabetes Association, 2021). Poor blood glucose control can lead to an increased prevalence of prediabetes, further increasing the risk of diabetes and its complications (Tabák et al., 2012).
H. pylori and diabetes are linked in many aspects, but studies on H. pylori and HbA1c are still relatively few, and there are different conclusions. In some meta-analyses, H. pylori was not detected to be related to glycemic control in diabetic population (Horikawa et al., 2014). However, in certain studies, H. pylori infection was positively correlated to HbA1c levels (Chen and Blaser, 2012). In our study, differences in HbA1c were identified in H. pylori negative and positive populations. Nonlinear relationship showed a striking increase in risk of H. pylori infection when the HbA1c >5.7%. This means that the population is more susceptible to H. pylori infection when there is a tendency towards early diabetes. Our further cohort study confirmed that long-term H. pylori infection resulted in apparently elevated HbA1c levels, but after eradication of H. pylori, HbA1c levels in the population decreased. It was consistent with some previous studies (Devrajani et al., 2010; Maluf et al., 2020). In addition, in our paper, the IR of the population was significantly higher with the increase of HbA1c. After prolonged H. pylori infection, IR levels were significantly higher than that of uninfected population, which may be closely related to the influence of H. pylori on glycemic control. However, the mechanisms by which H. pylori infection affects glycemic control and IR are complex. Type 1 diabetes mellitus (T1DM) is due to certain factors mediating autoimmune destruction of the pancreas, resulting in insufficient insulin secretion, while type 2 diabetes mellitus (T2DM) is mainly caused by impaired islet function and IR (Ilonen et al., 2019; Bonora et al., 2022). H. pylori may modulate the autoimmune response and IR syndrome through chronic inflammation outside the stomach (Polyzos et al., 2011; Marietti et al., 2013; Blosse et al., 2018). It can lead to the destruction of pancreatic islet cells and aggravate insulin secretion deficiency (Wang et al., 2022). In addition, its induced gastritis may affect the abnormal secretion of various hormones and further aggravate IR (Chen et al., 2021). Similarly, abnormal secretion of hormones such as ghrelin and leptin may cause changes in dietary intake, further affecting glycemic control (Aimasso et al., 2019).
Our cohort study, confirmed the nonlinear relationship between H. pylori and HbA1c, indicating that H. pylori can increase HbA1c levels and have an effect on IR. However, this research still has some limitations. First, it was a single-center study, which may require a multicenter longitudinal study to provide additional evidence. Second, mechanism by which H. pylori affects glycemic control is uncertain and perhaps needs to be further explored by biological experiments. Third, there was a lack of analysis of certain confounding factors, such as exercise and obesity, which may affect HbA1c levels (de Moura et al., 2023). In addition, although we did not use the homoeostasis model assessment of IR (HOMA-IR) to measure IR in the physically examined population because fasting insulin levels were not measured, the TyG index has been extensively studied as an alternative indicator of IR (Minh et al., 2021; Lopez-Jaramillo et al., 2023).
Conclusion
H. pylori is closely related to glycemic control in the population, and prediabetic populations may be more likely to be infected with H. pylori. Long-term H. pylori infection can increase HbA1c levels, worsen glycemic control, and exacerbate IR. Eradication of H. pylori has a positive impact on glycemic control in the population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Taizhou Hospital (K20220790). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JZ made great contributions to the concept, design and data acquisition of the article. YC and CY was mainly involved in data analysis. NY was involved in article writing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adeva-Andany M. M., Martínez-Rodríguez J., González-Lucán M., Fernández-Fernández C., Castro-Quintela E. (2019). Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 13, 1449–1455. doi: 10.1016/j.dsx.2019.02.023 [DOI] [PubMed] [Google Scholar]
- Aimasso U., D’Onofrio V., D’Eusebio C., Devecchi A., Pira C., Merlo F. D., et al. (2019). Helicobacter pylori and nutrition: a bidirectional communication. Minerva Gastroenterol. Dietol 65, 116–129. doi: 10.23736/s1121-421x.19.02568-6 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . (2009). Diagnosis and classification of diabetes mellitus. Diabetes Care 32 Suppl 1, S62–7. doi: 10.2337/dc09-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association . (2021). 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care 44, S15–s33. doi: 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- Blosse A., Lehours P., Wilson K. T., Gobert A. P. (2018). Helicobacter: inflammation, immunology, and vaccines. Helicobacter 23 Suppl 1, e12517. doi: 10.1111/hel.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E., Trombetta M., Dauriz M., Brangani C., Cacciatori V., Negri C., et al. (2022). Insulin resistance and beta-cell dysfunction in newly diagnosed type 2 diabetes: expression, aggregation and predominance. Verona newly diagnosed type 2 diabetes study 10. Diabetes Metab. Res. Rev. 38, e3558. doi: 10.1002/dmrr.3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo E., Solis-Herrera C., Trautmann M. E., Malloy J., Triplitt C. L. (2014). Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr. Diabetes Rev. 10, 2–42. doi: 10.2174/1573399810666140214093600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Blaser M. J. (2012). Association between gastric helicobacter pylori colonization and glycated hemoglobin levels. J. Infect. Dis. 205, 1195–1202. doi: 10.1093/infdis/jis106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. W., Chien C. H., Lin C. L., Chien R. N. (2021). Increased glycated hemoglobin but decreased cholesterol after a loss of helicobacter pylori infection: a community-based longitudinal metabolic parameters follow-up study. J. Pers. Med. 11. doi: 10.3390/jpm11100997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. P., Yang Y. J., Hung H. C., Lin C. H., Wu C. T., Hung M. H., et al. (2019). Helicobacter pylori eradication improves glycemic control in type 2 diabetes patients with asymptomatic active helicobacter pylori infection. J. Diabetes Investig. 10, 1092–1101. doi: 10.1111/jdi.12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. B., Florez J. C. (2020). Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 16, 377–390. doi: 10.1038/s41581-020-0278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir M., Gokturk H. S., Ozturk N. A., Kulaksizoglu M., Serin E., Yilmaz U. (2008). Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis. Sci. 53, 2646–2649. doi: 10.1007/s10620-007-0185-7 [DOI] [PubMed] [Google Scholar]
- de Moura S. S., de Menezes-Júnior L. A. A., Rocha A. M. S., Batista A. P., de Menezes M. C., Carraro J. C. C., et al. (2023). High levels of glycated hemoglobin (HbA1c) are associated with physical inactivity, and part of this association is mediated by being overweight. Nutrients 15. doi: 10.3390/nu15051191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devrajani B. R., Shah S. Z., Soomro A. A., Devrajani T. (2010). Type 2 diabetes mellitus: a risk factor for helicobacter pylori infection: a hospital based case-control study. Int. J. Diabetes Dev. Ctries 30, 22–26. doi: 10.4103/0973-3930.60008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doheim M. F., Altaweel A. A., Elgendy M. G., Elshanbary A. A., Dibas M., Ali A., et al. (2021). Association between helicobacter pylori infection and stroke: a meta-analysis of 273,135 patients. J. Neurol. 268, 3238–3248. doi: 10.1007/s00415-020-09933-x [DOI] [PubMed] [Google Scholar]
- Fischbach W., Malfertheiner P. (2018). Helicobacter pylori infection. Dtsch Arztebl Int. 115, 429–436. doi: 10.3238/arztebl.2018.0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Romero F., Simental-Mendía L. E., González-Ortiz M., Martínez-Abundis E., Ramos-Zavala M. G., Hernández-González S. O., et al. (2010). The product of triglycerides and glucose, a simple measure of insulin sensitivity. comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- Halalau A., Roy S., Hegde A., Khanal S., Langnas E., Raja M., et al. (2023). Risk factors associated with glycated hemoglobin A1c trajectories progressing to type 2 diabetes. Ann. Med. 55, 371–378. doi: 10.1080/07853890.2022.2164347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heianza Y., Arase Y., Fujihara K., Hsieh S. D., Saito K., Tsuji H., et al. (2012). Longitudinal trajectories of HbA1c and fasting plasma glucose levels during the development of type 2 diabetes: the toranomon hospital health management center study 7 (TOPICS 7). Diabetes Care 35, 1050–1052. doi: 10.2337/dc11-1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinzmann R., Schlaeger C., Tran C. T. (2012). What do we need beyond hemoglobin A1c to get the complete picture of glycemia in people with diabetes? Int. J. Med. Sci. 9, 665–681. doi: 10.7150/ijms.4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa C., Kodama S., Fujihara K., Yachi Y., Tanaka S., Suzuki A., et al. (2014). Association of helicobacter pylori infection with glycemic control in patients with diabetes: a meta-analysis. J. Diabetes Res. 2014, 250620. doi: 10.1155/2014/250620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilonen J., Lempainen J., Veijola R. (2019). The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 15, 635–650. doi: 10.1038/s41574-019-0254-y [DOI] [PubMed] [Google Scholar]
- Kim T. J., Lee H., Kang M., Kim J. E., Choi Y. H., Min Y. W., et al. (2016). Helicobacter pylori is associated with dyslipidemia but not with other risk factors of cardiovascular disease. Sci. Rep. 6, 38015. doi: 10.1038/srep38015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krhač M., Lovrenčić M. V. (2019). Update on biomarkers of glycemic control. World J. Diabetes 10, 1–15. doi: 10.4239/wjd.v10.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Kwon H. S., Park Y. M., Ha H. S., Jeong S. H., Yang H. K., et al. (2014). Predicting the development of diabetes using the product of triglycerides and glucose: the chungju metabolic disease cohort (CMC) study. PloS One 9, e90430. doi: 10.1371/journal.pone.0090430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Yang H. K., Lee J., Kang B., Yang Y., Lee S. H., et al. (2016). Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 15, 155. doi: 10.1186/s12944-016-0324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chi X., Wang Y., Setrerrahmane S., Xie W., Xu H. (2022). Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 7, 216. doi: 10.1038/s41392-022-01073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo-Mbenza B., Nkondi Nsenga J., Vangu Ngoma D. (2007). Prevention of the metabolic syndrome insulin resistance and the atherosclerotic diseases in africans infected by helicobacter pylori infection and treated by antibiotics. Int. J. Cardiol. 121, 229–238. doi: 10.1016/j.ijcard.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Lopez-Jaramillo P., Gomez-Arbelaez D., Martinez-Bello D., Abat M. E. M., Alhabib K. F., Avezum Á, et al. (2023). Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev 4, e23–e33. doi: 10.1016/s2666-7568(22)00247-1 [DOI] [PubMed] [Google Scholar]
- Maluf S., Salgado J. V., Cysne D. N., Camelo D. M. F., Nascimento J. R., Maluf B. V. T., et al. (2020). Increased glycated hemoglobin levels in patients with helicobacter pylori infection are associated with the grading of chronic gastritis. Front. Immunol. 11, 2121. doi: 10.3389/fimmu.2020.02121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marietti M., Gasbarrini A., Saracco G., Pellicano R. (2013). Helicobacter pylori infection and diabetes mellitus: the 2013 state of art. Panminerva Med. 55, 277–281. [PubMed] [Google Scholar]
- McColl K. E. (2010). Clinical practice. helicobacter pylori infection. N Engl. J. Med. 362, 1597–1604. doi: 10.1056/NEJMcp1001110 [DOI] [PubMed] [Google Scholar]
- Minh H. V., Tien H. A., Sinh C. T., Thang D. C., Chen C. H., Tay J. C., et al. (2021). Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J. Clin. Hypertens. (Greenwich) 23, 529–537. doi: 10.1111/jch.14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noach L. A., Bosma N. B., Jansen J., Hoek F. J., van Deventer S. J., Tytgat G. N. (1994). Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with helicobacter pylori infection. Scand. J. Gastroenterol. 29, 425–429. doi: 10.3109/00365529409096833 [DOI] [PubMed] [Google Scholar]
- Park S. H., Jeon W. K., Kim S. H., Kim H. J., Park D. I., Cho Y. K., et al. (2005). Helicobacter pylori eradication has no effect on metabolic and inflammatory parameters. J. Natl. Med. Assoc. 97, 508–513. [PMC free article] [PubMed] [Google Scholar]
- Perri F., Clemente R., Festa V., De Ambrosio C. C., Quitadamo M., Fusillo M., et al. (1999). Serum tumour necrosis factor-alpha is increased in patients with helicobacter pylori infection and CagA antibodies. Ital J. Gastroenterol. Hepatol. 31, 290–294. [PubMed] [Google Scholar]
- Polyzos S. A., Kountouras J., Zavos C., Deretzi G. (2011). The association between helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter 16, 79–88. doi: 10.1111/j.1523-5378.2011.00822.x [DOI] [PubMed] [Google Scholar]
- Reuter S., Raspe J., Uebner H., Contoyannis A., Pastille E., Westendorf A. M., et al. (2023). Treatment with helicobacter pylori-derived VacA attenuates allergic airway disease. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1092801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. L. C., de Brito B. B., da Silva F. A. F., Sampaio M. M., Marques H. S., Oliveira E. S. N., et al. (2020). Helicobacter pylori infection: beyond gastric manifestations. World J. Gastroenterol. 26, 4076–4093. doi: 10.3748/wjg.v26.i28.4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Cai C., Jin Q., Chen X., Yu C. (2021). The efficacy of helicobacter pylori eradication in diabetics and its effect on glycemic control: a systematic review and meta-analysis. Helicobacter 26, e12781. doi: 10.1111/hel.12781 [DOI] [PubMed] [Google Scholar]
- Stumvoll M., Goldstein B. J., van Haeften T. W. (2005). Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365, 1333–1346. doi: 10.1016/s0140-6736(05)61032-x [DOI] [PubMed] [Google Scholar]
- Tabák A. G., Herder C., Rathmann W., Brunner E. J., Kivimäki M. (2012). Prediabetes: a high-risk state for diabetes development. Lancet 379, 2279–2290. doi: 10.1016/s0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsbøll T., Bain S. C., Leiter L. A., Lingvay I., Matthews D., Simó R., et al. (2018). Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes. Metab. 20, 889–897. doi: 10.1111/dom.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Cao Z. M., Zhang L. L., Dai X. C., Liu Z. J., Zeng Y. X., et al. (2022). Helicobacter pylori and autoimmune diseases: involving multiple systems. Front. Immunol. 13. doi: 10.3389/fimmu.2022.833424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Hng T. M. (2021). HbA1c: more than just a number. Aust. J. Gen. Pract. 50, 628–632. doi: 10.31128/ajgp-03-21-5866 [DOI] [PubMed] [Google Scholar]
- Wang C., Li F., Guo J., Li C., Xu D., Wang B. (2018). Insulin resistance, blood glucose and inflammatory cytokine levels are risk factors for cardiovascular events in diabetic patients complicated with coronary heart disease. Exp. Ther. Med. 15, 1515–1519. doi: 10.3892/etm.2017.5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu J., Cheng Z., Zhong Y., Chen X., Song W. (2021). Triglyceride glucose-body mass index and the risk of diabetes: a general population-based cohort study. Lipids Health Dis. 20, 99. doi: 10.1186/s12944-021-01532-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Yu M., Zhang R., Chen S., Xi Y., Duan G. (2020). A meta-analysis of the association between helicobacter pylori infection and risk of atherosclerotic cardiovascular disease. Helicobacter 25, e12761. doi: 10.1111/hel.12761 [DOI] [PubMed] [Google Scholar]
- Wong F., Rayner-Hartley E., Byrne M. F. (2014). Extraintestinal manifestations of helicobacter pylori: a concise review. World J. Gastroenterol. 20, 11950–11961. doi: 10.3748/wjg.v20.i34.11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah S., Rabiee M., Tahriri M., Abdolrahim M., Rajab A., Jazayeri H. E., et al. (2017). Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: a comprehensive review. Crit. Rev. Clin. Lab. Sci. 54, 219–232. doi: 10.1080/10408363.2017.1299684 [DOI] [PubMed] [Google Scholar]
- Yu L. Y., Hu K. C., Liu C. J., Hung C. L., Bair M. J., Chen M. J., et al. (2019). Helicobacter pylori infection combined with non-alcoholic fatty liver disease increase the risk of atherosclerosis: focus in carotid artery plaque. Med. (Baltimore) 98, e14672. doi: 10.1097/md.0000000000014672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani M., Ebrahimtabar F., Zamani V., Miller W. H., Alizadeh-Navaei R., Shokri-Shirvani J., et al. (2018). Systematic review with meta-analysis: the worldwide prevalence of helicobacter pylori infection. Aliment Pharmacol. Ther. 47, 868–876. doi: 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.