Abstract
Objective:
Multimodality treatment for resectable non–small cell lung cancer has long remained at a therapeutic plateau. Immune checkpoint inhibitors are highly effective in advanced non–small cell lung cancer and promising preoperatively in small clinical trials for resectable non–small cell lung cancer. This large multicenter trial tested the safety and efficacy of neoadjuvant atezolizumab and surgery.
Methods:
Patients with stage IB to select IIIB resectable non–small cell lung cancer and Eastern Cooperative Oncology Group performance status 0/1 were eligible. Patients received atezolizumab 1200 mg intravenously every 3 weeks for 2 cycles or less followed by resection. The primary end point was major pathological response in patients without EGFR/ALK+ alterations. Pre- and post-treatment computed tomography, positron emission tomography, pulmonary function tests, and biospecimens were obtained. Adverse events were recorded by Common Terminology Criteria for Adverse Events v.4.0.
Results:
From April 2017 to February 2020, 181 patients were entered in the study. Baseline characteristics were mean age, 65.1 years; female, 93 of 181 (51%); nonsquamous histology, 112 of 181 (62%); and clinical stages IIB to IIIB, 147 of 181 (81%). In patients without EGFR/ALK alterations who underwent surgery, the major pathological response rate was 20% (29/143; 95% confidence interval, 14-28) and the pathological complete response rate was 6% (8/143; 95% confidence interval, 2-11). There were no grade 4/5 treatment-related adverse events preoperatively. Of 159 patients (87.8%) undergoing surgery, 145 (91%) had pathologic complete resection. There were 5 (3%) intraoperative complications, no intraoperative deaths, and 2 postoperative deaths within 90 days, 1 treatment related. Median disease-free and overall survival have not been reached.
Conclusions:
Neoadjuvant atezolizumab in resectable stage IB to IIIB non–small cell lung cancer was well tolerated, yielded a 20% major pathological response rate, and allowed safe, complete surgical resection. These results strongly support the further development of immune checkpoint inhibitors as preoperative therapy in locally advanced non–small cell lung cancer.
Keywords: immunotherapy, lung cancer, neoadjuvant therapy
Graphical Abstract
After neoadjuvant atezolizumab immunotherapy in patients with stage IB to IIIB NSCLC, the MPR rate was 20%.
Surgery is considered the primary curative therapy for patients with early-stage non–small cell lung cancer (NSCLC) who are medically fit for pulmonary resection. However, 5-year overall survival (OS) for patients with resectable, locally advanced NSCLC (stage IB to IIIB disease) is only 26% to 68%. Patients who progress after resection typically develop distant metastatic disease.1 During the past 35 years, multiple clinical trials have shown that the addition of either adjuvant or neoadjuvant platinum-based chemotherapy to surgical resection in patients with locally advanced NSCLC is feasible and increases the 5-year OS.2-13 Unfortunately, the absolute OS benefit with this approach is only approximately 5%, and patients experience significant toxicity. Although neoadjuvant therapy plus resection of locally advanced NSCLC has never been proven superior to postoperative adjuvant chemotherapy, it is widely used for locally advanced NSCLC. It has the potential advantages of early control of micrometastatic disease and is associated with better overall drug delivery because patients who start adjuvant therapy receive approximately 55% of the planned total dose. The neoadjuvant approach facilitates assessment of treatment safety and efficacy through serial scans before resection and pathologic evaluation of the degree of treatment response. It also enables pre- and post-treatment biomarker studies.14-16
Multimodality treatment using platinum-based chemotherapy and either surgery or radiation for locally advanced NSCLC has remained at a therapeutic plateau for over 2 decades. The discovery of immune regulatory pathways in cancer, including the important role of the programmed death-1/programmed death ligand 1 (PD-L1) pathways, and the development of drugs targeting those pathways, have revolutionized the treatment of many solid tumors, including NSCLC.17 These immune checkpoint inhibitors (IOs), particularly those targeting the programmed death-1/PD-L1 pathway, have been tested in large randomized clinical trials in metastatic NSCLC. In that setting, they were associated with significantly better OS when compared with cytotoxic chemotherapy as second- and then as first-line monotherapy, and when combined with platinum-based chemotherapy compared with chemotherapy alone.18-26 Consequently, combined chemotherapy and IO therapy (commonly termed “chemoIO”) has become standard of care for metastatic NSCLC without targetable oncogene driver gene mutations (eg, epidermal growth factor receptor [EGFR]).
Logically, the use of IO was next tested in locally advanced NSCLC. A large randomized clinical trial comparing the addition of IO (durvalumab) versus placebo as consolidation treatment after chemoradiotherapy for locally advanced, unresectable NSCLC yielded improved OS, thus altering the standard of care for this patient population.27-30 As a next step, studies examined the benefit of IO for resectable disease. Small neoadjuvant mono or dual immunotherapy trials demonstrated favorable rates of pathologic regression (major pathologic response [MPR] 14%-45% and pathological complete response 5%-16%) with acceptable treatment-related toxicity (grade ≥3 treatment-related adverse event [AE] 0%-14%) and low attrition of patients to surgery (0%-14%).29,31 Therefore, more detailed studies in larger clinical trials were needed to further confirm the safety and efficacy of IO followed by potentially curative surgery. We report the results of a large, multicenter trial of neoadjuvant atezolizumab with a focus on surgically related outcomes and view those results within the context of a recently published trial showing the superiority of neoadjuvant chemoIO to chemotherapy alone.32,33
MATERIALS AND METHODS
Study Design, Eligibility Criteria, and Required Interventions
This was a phase II multicenter single arm study investigating the efficacy and safety of atezolizumab (a PD-L1 checkpoint inhibitor) as neoadjuvant therapy in patients with previously untreated clinical stages IB to select IIIB (T3N2, but not T4 or N3 by 8th edition American Joint Commission on Cancer/Union for International Cancer Control staging) NSCLC. Pretreatment pathological documentation of NSCLC was required. Tumors were staged at screening by chest computed tomography (CT) scan, positron emission tomography imaging, and brain magnetic resonance imaging. Documentation of lymph node involvement by endobronchial ultrasound or mediastinoscopy for patients with clinical stages II and IIIA disease was encouraged. The primary tumor had to be measurable by Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Tumors of pure ground-glass opacity appearance on CT were excluded. All patients were evaluated jointly by a thoracic surgeon and a medical oncologist to verify study eligibility and to ensure that the primary tumor and involved lymph nodes were technically resectable. Patients needed to have adequate cardiopulmonary function for surgical resection. Postoperative forced expiratory volume in 1 second (FEV1) and diffusion capacity for carbon monoxide (DLCO) had to be at least 40% of predicted and/or maximal oxygen consumption greater than 10 mL/kg/min. Patients with a history of bone marrow or solid organ transplantation, autoimmune disease, idiopathic pulmonary fibrosis/pneumonitis, active hepatitis, active malignancy within 3 years of entry on study, or prior IO therapy were excluded. An Eastern Cooperative Oncology Group performance status score of 0 to 1 was required. PD-L1 testing on the primary tumor or lymph nodes was not required for study inclusion.
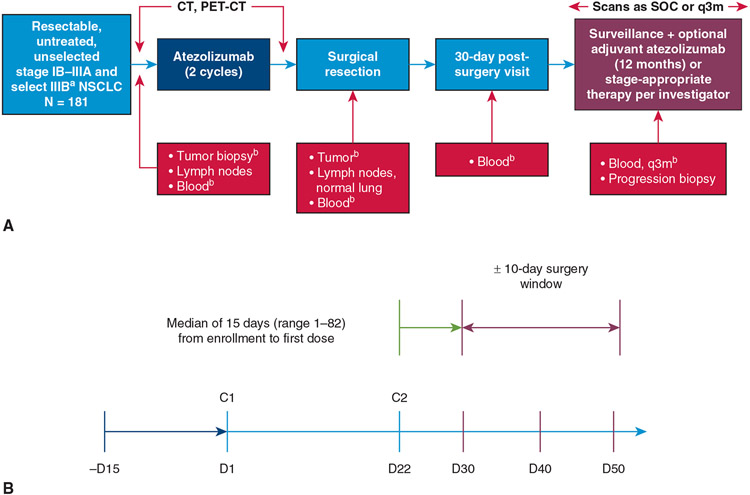
The study design is shown in Figure 1, A. A primary tumor core biopsy and blood samples for correlative biomarker studies were required before treatment initiation. Patients then received 2 cycles of atezolizumab at a dose of 1200 mg on days 1 and 22 followed by repeat imaging with CT and positron emission tomography, and repeat pulmonary function tests (PFTs). Surgical resection was allowed after an 8-day washout period from cycle 2 of atezolizumab within a 20-day window (ie, 30-50 days after cycle 1, Figure 1, B). Resection could be accomplished via thoracotomy, video-assisted thoracic surgery (VATS), or robotic VATS to allow pathologic complete (R0) resection. Anatomic resection via segmentectomy, lobectomy, bilobectomy, or pneumonectomy was strongly preferred, although wedge resection of peripheral tumors less than 2 cm in size was allowed provided at least a 1-cm circumferential margin could be obtained. Hilar and mediastinal lymph node dissection was required. For right-sided resections, this involved at least lymph nodes from levels 4R, 7, 10R, and 11R based on the International Association for the Study of Lung Cancer lymph node map.34 For left-sided resections, this involved at least lymph nodes from levels 5/6, 7, 10L, and 11L. Patients in whom a complete resection was not achieved were discontinued from the study. A sample of the primary tumor, draining lymph nodes, and blood samples were submitted at the time of surgery for additional post-treatment correlative biomarker studies.
FIGURE 1.
Study overview. A, Design. B, Protocol mandated timing of surgery in relationship to neoadjuvant therapy. aT4 due to mediastinal organ invasion and N3 disease were excluded. bMandatory: Driver mutations were removed. CT, Computed tomography; PET, positron emission tomography; SOC, standard of care; q3m, every 3 months; NSCLC, non–small cell lung cancer; C, cycle.
Part 1 of this study ended when the last data point required for primary efficacy analysis was obtained, which was expected to occur approximately 30 days after surgery. Patients were permitted to receive standard of care adjuvant chemotherapy and/or radiation therapy. Patients whose tumors showed evidence of pathologic response or lack of radiographic progression were eligible to participate in Part 2 of the study in which adjuvant atezolizumab was administered for up to a maximum of 12 additional months. Analysis of Part 2 will be reported in another publication. After completing all treatment, patients were followed for disease-free survival (DFS) and OS.
Study Objectives and End Points
The primary objective was to evaluate the efficacy of atezolizumab as neoadjuvant treatment based on MPR, defined as 10% or less viable tumor tissue scored by the designated institutional pathologist using standardized criteria, an end point previously reported to associate with OS.35 Secondary efficacy objectives included safety per National Cancer Institute Common Terminology Criteria for Adverse Events v.4.0,36 radiologic response (RECIST v1.1), pathologic complete response in the primary tumor, pathologic response according to tumor PD-L1 expression, and tumor mutation burden. Exploratory efficacy objectives included DFS and OS, as well as multiple exploratory biomarker objectives designed to identify predictors of response to atezolizumab. These will be the subject of future reports.
Statistical Considerations
The sample size was based on the primary objective of determining the MPR rate of 15%. The primary end point was assessed by a statistical test of a single proportion of responders against the simple alternative of 5% response. The design provided a 95% statistical power to detect a difference of 10% at a significance level of 0.05 (1-sided test). To achieve this, approximately 180 patients were required for the study. If there were at least 17 patients with primary tumor demonstrating MPR, then the statistical test would reject the null hypothesis of a 5% response rate in favor of a higher response rate. Two interim analyses were performed: a safety analysis after 30 patients and a futility analysis after 90 patients were accrued. For exploratory efficacy end points, DFS and OS were calculated using the Kaplan–Meier method.
Ethical Considerations
The study was approved by the Institutional Review Board at each of the participating institutions, and all patients provided informed consent before entry on study. (Number and dates of Institutional Review Board approval: 295131 4/19/2022; 294898 1/24/2018; 294886 4/15/2022; 294889 4/1/2021; 295677 6/2/2021; 301840 5/19/2022; 294903 3/21/2022; 294899 4/15/2022; 294887 3/9/2022; 295326 4/27/2022; 295678 5/11/2021; 301242 6/1/2021; 294888 8/5/2021; 294904 8/21/2022; 294895 4/14/2022.)
RESULTS
Patient Demographics and Study Flow
From April 20, 2017, to February 3, 2020, a total of 181 eligible patients with NSCLC from 13 sites were enrolled. The final data cut for this study was October 15, 2021. Baseline patient and tumor characteristics are shown in Table 1. Approximately half of patients were female, and 81% were White. Only 18 tumors (10%) were clinical stage IB, and 147 tumors (81%) were stages IIB to select IIIB. Forty-nine of 181 patients had pretreatment staging via mediastinoscopy or endobronchial ultrasound. Most patients (n = 163, 90%) were former or current smokers. A minority of tumors showed squamous histology (n = 69, 38%). Fifty-two patients (29%) had greater than 50% of the tumor cells stain positive for PD-L1, and an additional 30 tumors (17%) showed immunostaining in the 1% to 49% range.
TABLE 1.
Baseline patient and tumor characteristics
| Patients (N = 181) | |
|---|---|
| Median age, y (range) | 65.0 (37-83) |
| Female, n (%) | 93 (51) |
| Race, n (%) | |
| White | 145 (81) |
| Black/African American | 13 (7) |
| Asian | 9 (5) |
| Unknown | 12 (7) |
| ECOG performance status score, n (%) | |
| 0 | 104 (57) |
| 1 | 77 (43) |
| Clinical stage, n (%) | |
| IB | 18 (10) |
| IIA | 16 (9) |
| IIB | 55 (30) |
| IIIA | 70 (39) |
| IIIB* | 22 (12) |
| Histology, n (%) | |
| Nonsquamous | 112 (62) |
| Squamous | 69 (38) |
| History of tobacco use, n (%) | |
| Never | 18 (10) |
| Current | 35 (19) |
| Former | 128 (71) |
| Median pack-y, n (range) | 22.75 (0-162.0) |
| PD-L1 TPS at screening, n (%)† | |
| <1% | 69 (38) |
| 1%-49% | 28 (15) |
| ≥50% | 49 (27) |
| Unknown‡ | 35 (19) |
| EGFR mutation, n (%)§ | |
| Positive | 11 (6) |
| Negative | 154 (85) |
| Unknown¶ | 16 (9) |
| ALK rearrangement, n (%) | |
| Positive | 6 (3) |
| Negative | 162 (90) |
| Unknown ¶ | 13 (7) |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1; TPS, tumor proportion score; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
Select IIIB includes T3N2 or T4 (by size criteria, not by mediastinal invasion) per the American Joint Committee on Cancer Staging System (8th edition).
PD-L1 status was centrally determined by immunohistochemistry using the DAKO PD-L1 (22C3) assay.
The large number of patients with “unknown” PD-L1 status was due to missing samples and failed testing.
Determined either locally or centrally from screening tissue (when adequate) or resected tumor tissue.
EGFR status was unknown in 16 patients (nonsquamous, n = 5; squamous, n = 11).
ALK rearrangement status was unknown in 13 patients (nonsquamous, n = 5; squamous, n = 8).
The patient populations at primary analysis are shown in Figure E1. All 181 patients with NSCLC received at least 1 cycle of atezolizumab with 171 completing the planned 2 cycles (safety population) and 159 (88%) proceeding to surgery. Reasons for no surgery (Figure E1) included radiographic disease progression (n = 10, 6%), physician decision (n = 2, 3%), and other, not specified (n = 3, 2%). The sites of radiographic disease progression in 10 patients were local (lung) in 3, regional (mediastinal or supraclavicular lymph nodes) in 3, and distant (brain, pleura, bone, duodenum) in 4. Of the 159 patients who underwent surgery, 16 proved to have a tumor EGFR mutation or anaplastic lymphoma kinase (ALK) translocation and were excluded per protocol. At the time of the original study design, data on the effect of EGFR mutation/ALK alterations on IOs were only beginning to emerge, and only in the metastatic setting. The effect of PD-L1 therapy on early-stage disease in patients with EGFR mutations had not yet been studied. Subsequently, these molecular alterations were shown to associate with failure to respond to IO,35 and the protocol was amended accordingly. Thus, the primary efficacy population (without EGFR or ALK alterations) for pathological response is considered to be 143 patients (79%).37
Primary Outcomes
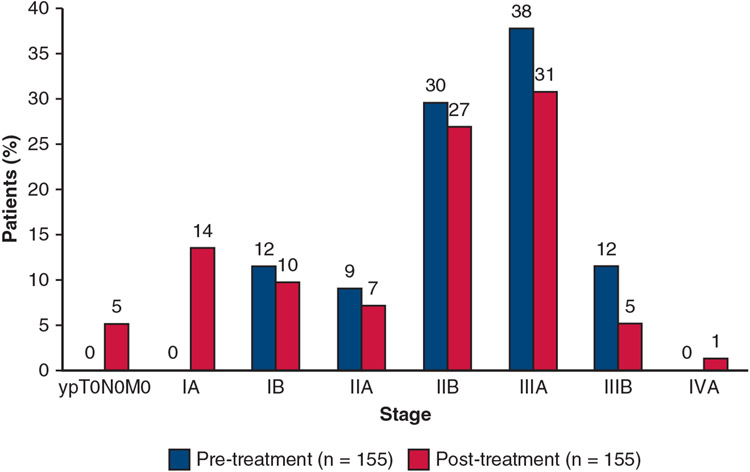
During the study, both the prespecified interim safety (at 30 patients) and futility analyses (at 90 patients) were successfully met. At completion, the study met the primary end point (Figure E2) with an MPR seen in 29 of 143 patients (20%) and pathologic complete response in 8 of 143 patients (6%) in the primary efficacy population (Figure E3). The pretreatment clinical stage (cStage) in relationship to the post-treatment pathological stage (yp-Stage) is shown in Figure 2. The pretreatment (cN) and post-treatment (ypN) nodal status are shown in Figure E2. Recognizing the discrepancies that may occur between clinical and pathological staging, these results indicate that 66 of the 155 tumors (43%) for which both c and ypStage were available were downstaged after atezolizumab and 19% were upstaged. The lack of association between radiologic response and final pathological TNM and stage is shown in Table E1. Of the 29 patients who had an MPR, 25 had stable disease and 4 had a partial response by RECIST criteria. Additional results, including information about adjuvant therapies, subgroup analyses of factors associated with MPR, and key biomarker correlative data are presented in a previous manuscript.38
FIGURE 2.
Association between pretreatment clinical stage and final pathologic stage at resection. Figure reflects patients with both clinical stage and pathologic stage per American Joint Commission on Cancer 8th edition staging system. Four patients did not have a pathologic stage evaluation.
Surgical Outcomes
Of the 159 patients who underwent surgery, 140 (88%) had the operation performed within the prespecified window at a median time of 22 days after cycle 2 of atezolizumab. In only 4 patients were the delays to surgery considered treatment related (Table E2).
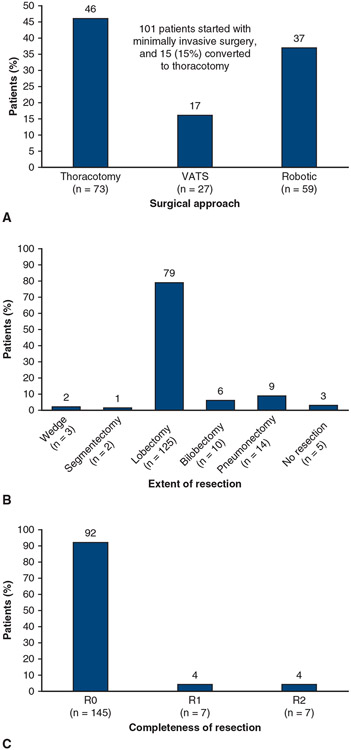
The surgical approach and the extent and completeness of resection are shown in Figure 3. Of 159 patients, 73 (46%) underwent thoracotomy, and the remaining 86 (54%) had the operation performed via minimally invasive surgery (MIS), either VATS or robotic VATS. Fifteen of 101 patients (15%) underwent MIS that was then converted to thoracotomy. Most patients received a lobectomy (125 patients, 79%). Of these, 5 patients underwent sleeve lobectomies (4 bronchial, 1 bronchial and vascular). Only 3 patients had a wedge resection, and 5 patients had no resection due to the extent of disease. A complete resection (R0) was achieved in 145 patients (91%). Among the patients who had resection and lymph node dissection (n = 140), the median (range) number of sampled lymph node stations per patients was 6 (2-9), including a median of 3 (1-5) N1 stations and a median of 3 (1-6) N2 stations.
FIGURE 3.
Surgical overview in patients who underwent surgery (n = 159). A, Surgical approach used. B, Extent of resection. C, Completeness of resection. Five of the patients who underwent lobectomy had sleeve lobectomies (4 bronchial, 1 bronchial and vascular). VATS, Video-assisted thoracoscopic surgery.
Pre- and postneoadjuvant treatment PFTs are shown in Table 2. The mean change in percent predicted FEV1, forced vital capacity, and DLCO were 3.0% or less. Five intraoperative complications (4 vascular injuries, 1 bronchial injury) occurred and were successfully repaired. Four of 159 patients (2.5%) had chest tube air leak reported as an AE (1 grade 1, 3 grade 3). Ten patients (6.2%) experienced postoperative atrial fibrillation (7 had grade 2, 2 had grade 3, 1 had grade 4, 0 had grade 5). Among the 14 patients receiving pneumonectomy, 14 had AEs: 3 grade 4 AEs (21.4%) and 1 grade 5 AE (7.1%). Among the overall surgery population, excluding the patients who underwent pneumonectomy (n = 145), 10 patients had grade 4 AEs (6.9%) and 4 patients had grade 5 AEs (2.8%). Because of the small number of pneumonectomies, we did not perform statistical comparisons with the rest of the patients who underwent surgery. The median length of hospitalization was 7.5 days. Within 30 days of surgery, 1 patient died of an unexplained cardiac arrest after discharge from the hospital. One patient died 2.5 months postoperatively with pneumonitis, considered treatment related.
TABLE 2.
Pre- and post-atezolizumab pulmonary function tests*
| PFT factor | Pre-atezolizumab (mean values) | Post-atezolizumab (mean values) | Mean change (95% CI) |
|---|---|---|---|
| FEV1 (n = 150) | 85.6% | 84.3% | −1.3% (−3.1% to 0.5%) |
| FVC (n = 152) | 93.0% | 92.9% | −0.1% (−1.8% to 1.7%) |
| DLCO (n = 126) | 79.2% | 76.2% | −3.0% (−4.9% to −1.2%) |
Results are shown as percent predicted. PFT, Pulmonary function test; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusion capacity for carbon monoxide.
Patients with paired values at screening and surgery visits.
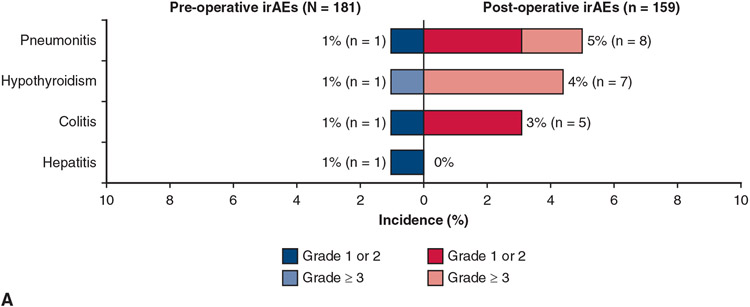
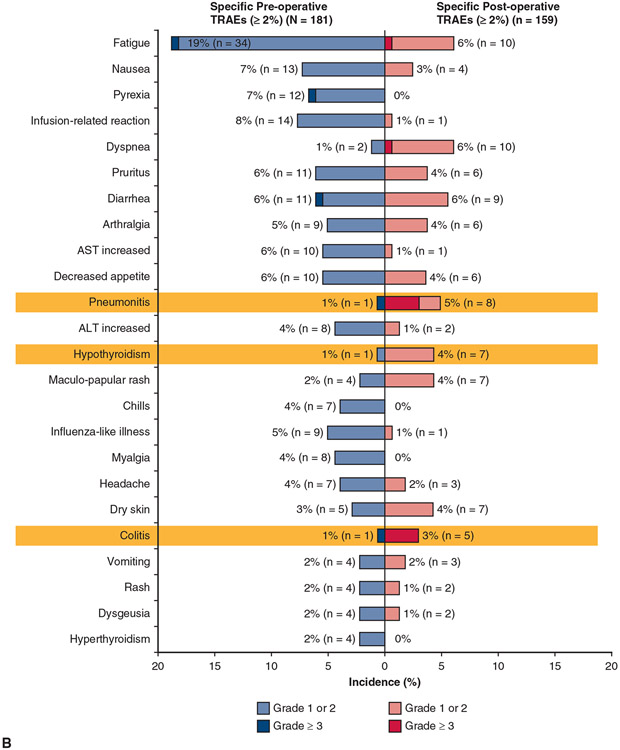
Pre- and postoperative treatment-related and immune-related AEs are shown in Figure 4. There were no grade 4/5 treatment-related AEs preoperatively and only 4 grade 4 AEs and, as noted, 2 grade 5 AEs postoperatively. By contrast with the toxicities usually seen with neoadjuvant chemotherapy, the types of AEs reported, although generally mild, are consistent with those known to occur with IO, specifically pneumonitis, hypothyroidism, colitis, and hepatitis.
FIGURE 4.
Pre- and postoperative treatment-related AEs. A, AEs considered to be immune-related are summarized. B, One patient had hypothyroidism preoperatively and postoperatively. irAE, Immune-related adverse event; TRAE, treatment-related adverse event; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Exploratory End Points: Efficacy Outcomes
For the 145 patients who had R0 resections, DFS and OS in relationship to initial clinical disease stage and nodal status are shown in Figure E4. Median DFS and OS were not reached. Three-year DFS and OS for patients with stage I/II disease were 73% and 79%, and 68% and 78% for those with stage III disease, respectively.
DISCUSSION
To date, Lung Cancer Mutation Consortium (LCMC) 3 is the largest multicenter Phase II trial showing the safety and efficacy of neoadjuvant monotherapy IO and surgery in patients with locally advanced NSCLC. Notably, in this group of predominantly clinical stage II to IIIB NSCLC, short-course, single-agent, low-toxicity neoadjuvant therapy was associated with an MPR rate of 20% and a complete pathologic response rate of 6% in the primary efficacy patient population. Surgery was performed within a short interval after neoadjuvant therapy and was associated with low morbidity and mortality. By contrast, the established approach of longer-duration (3-4 cycles) neoadjuvant platinum-based chemotherapy is associated with at least moderate toxicity, the need for longer recovery time to surgery, and a pathologic complete response rate in the range of 5%.2-13
As reported in this article, patients who receive neoadjuvant IO may have toxicities that are different from what surgeons are familiar with from preoperative chemotherapy. The risk of pneumonitis appears to be low with the approach used in this trial. A unique feature of this study was the requirement for pre- and postneoadjuvant therapy PFTs, specifically to assess the potential impact of IO on lung function. We did not identify clinically significant differences in the key parameters percent predicted FEV1, forced vital capacity, and DLCO. Endocrinopathies including hyper- or hypothyroidism and pituitary or adrenal insufficiency, although uncommon, can occur, as can immune-related hepatitis, pancreatitis, myocarditis, or colitis. Although the frequency of these AEs is generally in the single digits, it is important for surgeons to be alert to them because they can affect perioperative care.
Based on the unequivocal improvement in OS observed in advanced NSCLC with combined chemoIO, 6 Phase III global clinical trials are now testing this approach in resectable NSCLC.39 With several of these trials, using various IO agents in combination with chemotherapy, expected to be reported during the next few years, the results of the CheckMate 816 trial comparing induction nivolumab plus platinum-doublet chemotherapy with chemotherapy alone for resectable NSCLC were presented at the 2021 American Association for Cancer Research and American Society of Clinical Oncology meetings and were recently published, establishing neoadjuvant chemoIO as a standard of care.32,33 However, the toxicities of the single-agent IO in large phase II trials are needed to assess the toxicities contributed by the IO in the chemotherapy plus IO regimens. In a patient population relatively similar to what we report, CheckMate 816 found a pathologic complete response of 2.2% and an MPR rate of 8.9% with chemotherapy alone. For patients receiving chemoIO, these were 24% and 37%, respectively. There did not appear to be significantly greater toxicity or delays to surgery with chemoIO. Indeed, the greater depth of pathological response seen with chemoIO appeared to be associated with a higher frequency of MIS to resection, fewer pneumonectomies, and similar postoperative complications and length of hospital stay. However, relative to this study, patients received longer (3 cycles over 9 weeks) duration neoadjuvant therapy with a longer delay (median of 5.3 weeks) to surgery. Surgery was performed outside of the defined protocol window in 12% of the LCMC3 study versus 21 % in the CheckMate 816 trial. Although preoperative attrition was similar, serious treatment-related toxicity was expectantly higher (grade ≥3; 33.5%) in the CheckMate 816 chemoIO arm. In the context of multimodality therapy, more intense treatment is not always superior if toxicity or intolerance outweighs the benefits of higher response rates. This is particularly true in the usually older patient population with NSCLC being considered for surgical resection. Ultimately, as trial data mature and all of the correlative analyses from the LCMC 3 trial become available, the most salient result may be improved patient selection. Just as we now recognize that NSCLC with driver mutations (eg, EGFR mutations) is most effectively treated with tyrosine kinase inhibitors rather than chemotherapy, it will be pivotal to identify the approximately 20% of patients who, based on this study, need only single-agent IO rather than more intensive preoperative regimens. Another important finding from this study is the discrepancy between radiological response and final pathological stage after neoadjuvant therapy. This has been noted in previous trials using neoadjuvant chemoradiotherapy40 and in the early experience with resection after IO.41 Thus, radiological response cannot be used to predict final pathologic stage.
During the past 2 decades, the thoracic surgical community has gradually transitioned from thoracotomy to minimally invasive approaches, VATS, and robotic VATS for the resection of early-stage NSCLC. This paradigm shift has taken time as surgeons gradually acquired the technical skill sets that are radically different from performing a thoracotomy and gained the confidence that they could offer patients comparable oncologic outcomes. The recently published Video-Assisted Thoracoscopic Versus Open Lobectomy in Patients With Early-Stage Lung Cancer (VIOLET) study was a large randomized trial in the United Kingdom comparing VATS with thoracotomy and lobectomy for resection of early-stage NSCLC. It established the superiority of VATS in terms of postoperative pain, functional recovery, complications, length of hospital stay, and economic outcomes. There were no differences in short- or long-term oncologic outcomes.42 A significant proportion of patients in both the LCMC3 and CheckMate 816 trials were able to have resections performed by VATS or robotic VATS. Whether the increasing use of robotic VATS will influence the use of MIS after neoadjuvant therapy remains to be seen. As surgeons gain more experience with MIS in this context, it will be important to assess whether these techniques are associated with improved outcomes in patients with locally advanced NSCLC.
One of the sometimes technically challenging aspects of surgical resection in patients who have received neoadjuvant therapy for locally advanced NSCLC is perihilar fibrosis, particularly when this involves hilar vessels and can lead to intraoperative hemorrhage. Although a phenomenon well known to thoracic surgeons, perihilar fibrosis is not predictable and has never been quantified with respect to frequency or severity. The LCMC3 trial was not designed to assess this issue. The low frequency of intraoperative AEs suggests a lack of severity of this problem. However, future neoadjuvant trials should systematically analyze the frequency and severity of perihilar fibrosis, the presence of which can affect surgical approach, extent of lung resection, and operative morbidity and mortality. We propose that for future studies, the severity of perihilar fibrosis be systematically recorded according to a 0 to 3 grading scale, in which 0 would indicate normal hilar anatomy with no fibrosis; 3 would describe dense fibrosis with obliteration of anatomical planes; and 1 and 2 would describe intermediate findings (Table E3).
CONCLUSIONS
The outcomes in this large multicenter trial provide a strong rationale for neoadjuvant IO and surgery in the management of some patients with resectable NSCLC. Correlative biomarker studies from the LCMC3 study, still under analysis, will hopefully identify which patients may be treated with monotherapy alone and which may require more intensive preoperative regimens.
Supplementary Material
CENTRAL MESSAGE.
In this large multicenter trial, neoadjuvant atezolizumab immunotherapy plus surgical resection for stage IB to IIIB NSCLC was well tolerated, associated with a 20% MPR rate and encouraging OS.
PERSPECTIVE.
In metastatic NSCLC, immunotherapy has improved OS. This phase II multicenter trial, the largest reported to date, showed that neoadjuvant atezolizumab immunotherapy plus surgical resection in stage IB to IIIB NSCLC was well tolerated, associated with a 20% MPR rate and encouraging OS, thus providing a strong rationale for this treatment approach in locally advanced, resectable NSCLC.
Acknowledgments
This study was sponsored by Genentech, Inc. Illustrative support was provided by Tiffany DeSimone, PhD, of Ashfield MedComms, an Ashfield Health Company, and was funded by F. Hoffmann-La Roche/Genentech. The work of Drs Rusch, Kris, and Chaft is supported in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Abbreviations and Acronyms
- AE
adverse event
- ALK
anaplastic lymphoma kinase
- CT
computed tomography
- DFS
disease-free survival
- DLCO
diffusion capacity for carbon monoxide
- EGFR
epidermal growth factor receptor
- FEV1
forced expiratory volume in 1 second
- IO
immune checkpoint inhibitor
- LCMC
Lung Cancer Mutation Consortium
- MIS
minimally invasive surgery
- MPR
major pathologic response
- NSCLC
non–small cell lung cancer
- OS
overall survival
- PD-L1
programmed death ligand 1
- PFT
pulmonary function test
- RECIST
Response Evaluation Criteria In Solid Tumors
- R0
pathologic complete resection
- VATS
video-assisted thoracic surgery
Footnotes
Clinicaltrials.gov Registry Number: 02927301.
This study was approved by the Institutional Review Board at each of the participating institutions, and all patients provided informed consent before entry on study. Number and Dates of Institutional Review Board approval: 295131 4/19/2022; 294898 1/24/2018; 294886 4/15/2022; 294889 4/1/2021; 295677 6/2/2021; 301840 5/19/2022; 294903 3/21/2022; 294899 4/15/2022; 294887 3/9/2022; 295326 4/27/2022; 295678 5/11/2021; 301242 6/1/2021; 294888 8/5/2021; 294904 8/21/2022; 294895 4/14/2022.
Conflict of Interest Statement
V.W.R. is a member of the Data Safety and Monitoring Committee for the MARS2 trial (UK), serves as Co-Chair of the National Cancer Institute Thoracic Staging Malignancy Committee, and reports institutional funding from Genentech. A.N. is an employee of Genentech and reports stock ownership with Roche. S.N.W. reports research support grants from AbbVie Inc, Ariad Pharmaceuticals, Genentech, ImmunoMedics, Inc, Millennium Pharmaceuticals Inc, Roche, Astellas Pharma Inc, Daiichi Sankyo, Cullinan Pearl, Verastem Inc, GlaxoSmithKline/GSK, Janssen Research & Development, LLC, Elevation Oncology, Daiichi Sankyo, Genentech, Loxo Oncology, Takeda Pharmaceuticals Company Limited, grant from the SWOG Clinical Trials Partnership, honorarium from ASCO, and Chair of Data Safety Monitoring Board for the Hoosier Cancer Research Network. E.M.T. reports honoraria from Intuitive Surgical. E.B.H. serves in a consulting or advisory role to Amgen, Ellipses Pharma, Janssen Oncology, Janssen Research & Development, and Revolution Medicines; reports research funding (paid to his institution) from AstraZeneca, Genentech, Incyte, Janssen, Novartis, Revolution Medicines, and Spectrum Pharmaceuticals; and reports patents, royalties, or other intellectual property from Protein-Protein Interactions as Biomarkers Patent. K.L.R. reports personal fees from Amgen, Calithera, AstraZeneca, Blueprint, Boehringer Ingelheim, Daiichi Sankyo, EMD Soreno, Genentech, GlaxoSmithKline, Janssen, Lilly, Merck KGA, Mirati, Takeda, Tesaro, and nonfinancial support from Seattle Genetics; research support to institution from CALITHERA, Blueprint, Daiichi Sankyo, Genentech, Elevation Oncology, and Janssen outside the submitted work. R.E.M. reports speaker fees from Intuitive Surgical. D.H.O. reports funding to his institution from Genentech, Merck, Pfizer, Palobiofarma, and Bristol Myers Squibb. E.B.G. reports grant and research support from ABL Bio, AstraZeneca, Bristol Myers Squibb, Dynavax Technologies, EMD Serono, Genentech, Iovance Biotherapeutics, Eli Lilly & Co, Merck, Mirati Therapeutics, Neon Therapeutics, and Novartis; and consulting or advisory roles with ABL Bio, Boehringer Ingelheim, Bristol Myers Squibb, Dracen Pharmaceuticals, Eisai, Eli Lilly, EMD Serono, Gilead, GSK, Merck, Natera, Novartis, Personalis, Regeneron, Sanofi, Shionogi, and Xilio Therapeutics. R.C.D. is an employee and shareholder of Rain Therapeutics and has received consulting fees, travel reimbursement, and licensing fees from Genentech/Roche; and has received funding from the National Institutes of Health/National Cancer Institute University of Colorado Lung SPORE (5P50CA058187). M.N. reports honoraria from Astra Zeneca (ad-board), Caris Life Sciences (consultant), Lilly (consultant), Daiichi Sankyo (ad-board), Takeda (speaker), Novartis (ad-board), EMD Serono (ad-board), Blueprint Medicines (speaker), Janssen (ad-board), Pfizer (ad-board), Genentech (ad-board), and has received travel support from AnHeart Therapeutics. H.I.P. is a member of the Genentech Steering Committee for IMPOWER030 and the SKYSCRAPER Trial. K.S. is an employee of Genentech and reports stock ownership with Roche. A.J. is an employee of Genentech and reports stock ownership with Roche. P.A.B. is on Advisory or Data Safety Monitoring Boards for BMS and Merck, and reports membership on the Board of Directors for Verastem, Inc., with funding to his institution. B.E.J. reports consultant fees from Checkpoint Therapeutics, Genentech, Hummingbird Diagnostics and Hengrui Therapeutics. M.G.K. reports speaking fees from AstraZeneca and Pfizer, consultant fees for Janssen, and in-kind support for medical writing from Hoffman-La Roche. D.J.K. serves as an advisor to AstraZeneca and Genentech/Roche and reports research funding from AADi. I.I.W. reports grants and personal fees from Genentech/Roche, Bayer, Bristol-Myers Squibb, AstraZeneca, Pfizer, HTG Molecular, GlaxoSmithKline, Guardant Health, Merck, Novartis, Sanofi, and Amgen; personal fees from Asuragen, Flame, Daiichi Sankyo, Oncocyte, MSD, and Platform Health; grants from Adaptive, Adaptimmune, EMD Serono, Takeda, Karus, Johnson & Johnson, 4D, Iovance, and Akoya, outside the submitted work. J.E.C. serves as an advisor to Genentech/Roche, AstraZeneca/MedImmune, Merck, Bristol Myers Squibb, Flame Biosciences, Janssen Oncology, Guardant Health, Regeneron/Sanofi, and Novartis; and reports research funding from Genentech/Roche, Bristol Myers Squibb, AstraZeneca/MedImmune, and Merck. D.P.C. serves in a consultant or advisory role to AbbVie, Agenus, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, EMD Serono, Genentech/Roche, Helsinn Healthcare, Incyte, Inivata, Inovio Pharmaceuticals, Janssen, Kyowa Hakko Kirin, Merck, Novartis, Pfizer, prIME Oncology, and Takeda, and research funding from Bristol Myers Squibb and Genentech. J.M.L. reports grants, consulting fees, and honoraria from AstraZeneca, BMS, Genentech, and Novartis, and leadership roles at AstraZeneca, Genentech, and Novartis. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Data Availability Statement
Qualified researchers may request access to individual patient-level clinical data through Vivli (data request platform being used at the time of this writing): https://vivli.org/ourmember/roche/.
For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing.
Anonymized records for individual patients across more than 1 data source external to Roche cannot and should not be linked because of potential increase in risk of patient reidentification.
Data will be available within 2 years after last patient survival follow-up visit and indefinitely.
References
- 1.Goldstraw P, Crowley J, Rami-Porta R, Rusch VW, Postmus PE, The IASLC Prognostic Factors Committee, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the eighth edition of the TNM classification of malignant tumors. J Thorac Oncol. 2015;11:39–51. [Google Scholar]
- 2.Pless M, Stupp R, Ris H-B, Stahel RA, Weder W, Thierstein S, et al. Induction chemoradiation in stage IIIA/N2 non-small cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386:1049–56. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT III, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small cell lung cancer: mature results of Southwest Oncology Group Phase II study 8805. J Clin Oncol. 1995;13:1880–92. [DOI] [PubMed] [Google Scholar]
- 4.Van Meerbeeck JP, Kramer GWPM, Van Schil PEY, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Nat Cancer Inst. 2007;99:442–50. [DOI] [PubMed] [Google Scholar]
- 5.Pisters KMW, Vallières E, Crowley JJ, Franklin WA, Bunn PA Jr, Ginsberg RJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early stage non-small cell lung cancer: Southwest Oncology Group trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosell R, Gómez-Codina J, Camps C, Maestre J, Padille J, Cantó A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–8. [DOI] [PubMed] [Google Scholar]
- 7.Roth JA, Atkinson EN, Fossella F, Komaki R, Ryan MB, Putnam JB Jr, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small cell lung cancer. Lung Cancer. 1998;21:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Martini N, Kris MG, Flehinger BJ, Gralla RJ, Bains MS, Burt ME, et al. Preoperative chemotherapy for stage IIIa (N2) lung cancer: the Sloan-Kettering experience with 136 patients. Ann Thorac Surg. 1993;55:1365–74. [DOI] [PubMed] [Google Scholar]
- 9.Pignon J-P, Tribodet H, Scagliotti GV, Douillard J-Y, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9. [DOI] [PubMed] [Google Scholar]
- 10.Pisters KMW, Ginsberg RJ, Giroux DJ, Putnam JB Jr, Kris MG, Johnson DH, et al. Induction chemotherapy before surgery for early-stage lung cancer: a novel approach. J Thorac Cardiovasc Surg. 2000;119:429–39. [DOI] [PubMed] [Google Scholar]
- 11.Kris MG, Pisters KMW, Ginsberg RJ, Rigas JR, Miller VA, Grant SC, et al. Effectiveness and toxicity of preoperative therapy in stage IIIA non-small cell lung cancer including the Memorial Sloan-Kettering experience with induction MVP in patients with bulky mediastinal lymph node metastases (clinical N2). Lung Cancer. 1995;12(Suppl 1):S47–57. [DOI] [PubMed] [Google Scholar]
- 12.Burdett S, The NSCLC Meta-Analysis Collaborative Group. Preoperative chemotherapy for non-small cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilligan D, Nicolson M, Smith I, Groen H, Dalesio O, Goldstraw P, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet. 2007;369:1929–37. [DOI] [PubMed] [Google Scholar]
- 14.McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis. 2014;6(Suppl 2):S224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felip E, Rosell R, Maestre JA, Rodriguez-Paniagua JM, Morán T, Astudillo J, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small cell lung cancer. J Clin Oncol. 2010;28:3138–45. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Blake SJ, Yong CR, Harjunpää H, Nglow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382–99. [DOI] [PubMed] [Google Scholar]
- 17.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways. Similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cha BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled phase 3 trial. Lancet. 2019;393:1819–30. [DOI] [PubMed] [Google Scholar]
- 20.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. [DOI] [PubMed] [Google Scholar]
- 21.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csöszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felib E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 23.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüs M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–51. [DOI] [PubMed] [Google Scholar]
- 24.Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer. Medicine. 2018;97:e11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazières J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. [DOI] [PubMed] [Google Scholar]
- 26.Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Aix SP, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1156–70. [DOI] [PubMed] [Google Scholar]
- 27.Antonia SJ, Villegas A, Vicente DD, Murakami S, Hui R, Kurata T, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 28.Cacsone T, William WN Jr, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOASTAR trial. Nat Med. 2021;27:504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellman MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (sinitilimab) in NSCLC. J Thorac Oncol. 2020;15:816–26. [DOI] [PubMed] [Google Scholar]
- 31.Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2019;158:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spicer J, Wang C, Tanaka F, Saylors GB, Chen K-N, Liberman M, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: nivolumab + platinum-doublet chemotherapy vs chemotherapy alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer. J Clin Oncol. 2021;39(Suppl 15):abstract 8503. [Google Scholar]
- 33.Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77. [DOI] [PubMed] [Google Scholar]
- 35.Hellman MD, Chaft JE, William WN Jr, Rusch V, Pisters KMW, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small cell lung cancers: proposals for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CTCAE, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v.4.03; 2010. Accessed April 1, 2022. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf [Google Scholar]
- 37.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer–a meta-analysis. J Thorac Oncol. 2017;12:403–7. [DOI] [PubMed] [Google Scholar]
- 38.Chaft JE, Oezkan F, Kris MG, Bunn PA, Wistuba II, Kwiatkowski DJ, et al. Neoadjuvant treatment of non-small cell lung cancer with atezolizumab. Nat Med. 2022;28:2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JM, Tsuboi M, Brunelli A. Surgical perspective on neoadjuvant immunotherapy in non-small cell lung cancer. Ann Thorac Surg. 2022;114:1505–15. [DOI] [PubMed] [Google Scholar]
- 40.Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, et al. Induction chemoradiation and surgical resection for superior sulcus non-small cell lung carcinomas: long-term results of Southwest Oncology Group trial 9416 (Intergroup trial 0160). J Clin Oncol. 2007;25:313–8. [DOI] [PubMed] [Google Scholar]
- 41.Chaft JE, Hellmann MD, Velez MJ, Travis WD, Rusch VW. Initial experience with lung cancer resection after treatment with T-cell checkpoint inhibitors. Ann Thorac Surg. 2017;104:e217–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim E, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NEJM Evid. 2022;1(3). 10.1056/EVIDoa2100016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient-level clinical data through Vivli (data request platform being used at the time of this writing): https://vivli.org/ourmember/roche/.
For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing.
Anonymized records for individual patients across more than 1 data source external to Roche cannot and should not be linked because of potential increase in risk of patient reidentification.
Data will be available within 2 years after last patient survival follow-up visit and indefinitely.