ABSTRACT
We aimed to evaluate the effectiveness or efficacy of heterologous or homologous COVID-19 vaccine regimens against COVID-19-related outcomes after primary immunization with two doses of CoronaVac or Sinopharm COVID-19 vaccines. PubMed, EMBASE, Web of Science, and Cochrane Library databases were searched up to 31 October 2022. The primary measure was vaccine effectiveness against COVID-19 infection with homologous or heterologous booster. The results showed heterologous and homologous booster significantly improved effectiveness against COVID-19 infection compared to primary immunization. The effectiveness against COVID-19 infection was 89.19% (95%CI 78.49, 99.89) for heterologous mRNA vaccine booster, 87.00% (95%CI 82.14, 91.85) for non-replicating vector vaccine booster, 69.99% (95%CI 52.16, 87.82) for homologous booster, and 51.48% (95%CI 41.75, 61.21) for two doses of inactivated vaccine. Homologous and heterologous regimens were also effective against SARS-CoV-2 variants, and more evidence is still needed to confirm our findings.
KEYWORDS: SARS-CoV-2, COVID-19, inactivated vaccine, effectiveness, efficacy, meta-analysis
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the coronavirus disease 2019 (COVID-19) has posed an unprecedented hazard and a tremendous burden of disease to global public health.1 As of 16 March 2023, there have been more than 760,36 million confirmed cases of COVID-19 worldwide, with more than 6,87 million deaths and counting.2 The COVID-19 vaccination campaign remains an important preventive measure against COVID-19. As of 13 March 2023, more than 13.23 billion vaccine doses have been administered and more than 5.52 billion persons vaccinated with at least one dose all over the world.2
Sinopharm COVID-19 vaccines and Sinovac CoronaVac have been widely implemented in combating the COVID-19 pandemic. To date, Sinopharm COVID-19 vaccines and CoronaVac were included in the emergency use list by the World Health Organization on May 7, 2021 and June 1, 2021, respectively,3,4 and have been approved for use in 93 and 56 countries, respectively.5,6 In numerous nations, including Brazil,7 Chile,8 Turkey,9 Malaysia,10 Indonesia,11 and Argentina,12 real-world research and Phase III clinical trials have demonstrated the efficacy of both inactivated vaccines. But vaccine efficacy and effectiveness rates differed widely between nations.
Previous studies have found that the immunity of two doses of inactivated COVID-19 vaccine decreased rapidly over time,8,9,13–15 especially the continuous emergence of SARS-CoV-2 variants (such as Omicron), which significantly escaped the neutralizing antibodies induced by the original strain vaccine.10,16,17 The immune protection effect induced by primary immunization is greatly challenged. In view of this, WHO advised using a heterologous or homologous booster to restore and extend the protective effect in people who have completed the primary immunization with two doses of inactivated vaccine for 4–6 months.18,19 Studies in Chile and Brazil have found that heterologous booster vaccination produced a stronger immune response than homologous booster vaccination.20,21
Although there are many evidences for mRNA vaccines or non-replicating vector vaccines,15,22,23 there is still a lack of evidence for homologous and heterologous booster immunization of inactivated vaccines, which is not conducive to subsequent global vaccination and vaccination programs. Therefore, there is an urgent need for comprehensive data on the effectiveness of homologous or heterologous boosters after two doses of inactivated vaccine priming to provide evidence for strategic decision-making in China and the world.
Here, we conducted a meta-analysis to evaluate the vaccine effectiveness or efficacy (VE) of primary immunization with the Chinese COVID-19 inactivated vaccine, as well as homologous or heterologous booster immunization, in preventing COVID-19 infection, COVID-19 related hospitalization, ICU admission, death, and severe COVID-19 outcomes. Additionally, we analyzed the effectiveness of COVID-19 vaccine immunization regimens against various SARS-CoV-2 variants and populations.
Methods
Registration
This study was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA). This study was registered on PROSPERO (CRD42023400130).
Search strategy
We searched PubMed, EMBASE, Web of Science, and Cochrane Library databases for studies published up to October 31, 2022, without language restrictions, using the following search terms: (COVID-19 or SARS-CoV-2) and (vaccine or vaccination) AND (Sinovac OR Sinopharm OR inactivated vaccine) AND (effectiveness OR efficacy). The detailed search strategy can be found in Supplementary Table S1. To ensure the validity of the results of studies, studies published on preprint servers without peer review were not retrieved and included. Additionally, we reviewed the included studies’ references to identify any potentially missed relevant records.
The literature search, and the following subsections, identified eligible studies, data extraction, and risk of bias assessment were performed independently by two investigators and then checked by two other investigators, with any discrepancies resolved by group discussion until a consensus was reached.
Selection criteria
We first estimated the vaccine effectiveness of two doses of inactivated vaccine regimen (Sinovac and Sinopharm COVID-19 vaccine), or homologous or heterologous booster (three-dose regimen), using unvaccinated individuals as a comparison group. In our study, individuals were considered primarily immunized if they had received two doses of the vaccine at least 14 days prior. This is because evidence from across the globe indicated that an immune response of at least 14 days after the last dose of inactivated vaccine was required to provide sufficient defense against SARS-CoV-2.24 Individuals who completed the booster (third dose) vaccination at least 14 days prior were considered to be booster immunization. Those who have not received any doses of the COVID-19 vaccine are classified the unvaccinated controls. Then, randomized controlled trials (RCTs) and observational studies (cohort studies and case-control studies) were included in our study, including general or populations having a high risk of COVID-19 infection (e.g., healthcare workers, or COVID-19-positive cases and their close contacts) of all ages and genders. Next, when a study was conducted during an epidemic of multiple SARS-CoV-2 variants and no subgroup analysis for VOCs was performed, it was considered an effectiveness study against this variant if a specific VOC accounted for 50% or more of positive tests.25 Furthermore, all confirmed COVID-19 positive cases should be tested by polymerase chain reaction (PCR), antigen detection or genomic sequencing. The evaluated outcomes were the effectiveness of primary vaccination or booster vaccination for COVID-19, including infection (asymptomatic and symptomatic infection, or symptomatic infection), hospitalization, ICU admission, death, or severe outcomes. Severe cases were defined as at least one of the following: need for supplemental oxygen, admission to ICU, mechanical ventilation, or death (the interpretation of primary clinical outcomes was shown in Supplementary Table S3). Any studies that included one of the above five outcomes would be included.
The following studies would be excluded: (1) irrelevant studies, such as studies that did not use Sinovac or Sinopharm COVID-19 vaccine; (2) studies that did not report vaccine efficacy or effectiveness with corresponding 95% confidence intervals (CIs); (3) only reported the VE of partial vaccination (i.e., only one dose or less than 14 days after two doses); (4) study protocols, comments, reviews, meta-analyses, editorials, conference abstracts, case reports, animal experiments; (5) studies with specifically targeted participants (e.g., pregnant women, patients with chronic kidney disease, stem cell transplant recipients, cancer patients).
Data extraction
The following data were extracted from the selected studies: The first author, publish date, country of study, study design, study period, sample size, mean or median ages, sex (male), population characteristics, comorbidities, SARS-CoV-2 variants of concern investigated, vaccine name, vaccine brand, immunization regimens, dose interval, follow-up period, primary outcomes, diagnostic method, and outcome measures. The VE in various vaccination statuses (primary and booster) against a series of clinical outcomes caused by SARS-CoV-2 with corresponding 95%CIs was extracted. For studies that recorded the number of events at two or more time points, data were extracted for the period when the vaccine was the most effective.26
Risk of bias assessment
The quality of trials was evaluated according to the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials,27 using the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. For each criterion, the risk of bias was assessed at three levels “low,” “unclear” or “high” risk. We determined the overall risk-of-bias judgment as low risk of bias, some concerns, or high risk of bias considering the risk-of-bias judgment in the seven domains above. The risk of bias in cohort and case-control studies was assessed using the Newcastle-Ottawa scale (NOS).28 The NOS contains eight categories relating to methodological quality, with scores ranging from 0 to 9 points. A total score of 7–9 points is considered of good quality, while a score of 4–6 points is of moderate quality, and a score of 0–3 points is of low quality.
Statistical analysis
We estimated the overall effectiveness of each immunization regimen. The overall VE and 95%CIs were calculated using DerSimonian and Laird random-effects meta-analysis. We used the I2 statistical parameter to estimate the heterogeneity between studies included.29 Either I2 >50% or the p-value of χ2 test < .10 was deemed as statistically significant heterogeneity.29 We grouped the studies into two categories according to the follow-up period: studies with a follow-up period within 6 months, and over 6 months. Studies that did not mention a specific follow-up period were estimated based on the study duration. We conducted subgroup analysis for COVID-19 variants (Alpha, Gamma, Delta, and Omicron), study populations (children aged <18 years, general population, elderly people, healthcare workers, and close contacts of COVID-19-positive cases), and follow-up period (within six months, and over six months), depending on the data availability. Due to limited data, sex, age, and comorbidities were not analyzed in subgroups. To ensure the robustness of the results, we did not perform summary estimation for clinical outcomes or subgroups with fewer than three studies. Egger’s test and a visual assessment for funnel plot asymmetry were used to evaluate publication bias. If the p value of Egger’s test was > .05, these studies were considered to have no publication bias. Additionally, sensitivity analyses were performed by excluding the studies sequentially and repeating the meta-analysis. All analyses were conducted using Stata 17.0 (Stata Corp, College Station, TX, USA). A two-tailed p value < .05 was considered to be statistically significant.
Results
Search results and characteristics
For this study, we identified 4916 studies from four databases up to 31 October 2022 (1576 in PubMed, 1707 in Embase, 1467 in Web of Science, and 166 in Cochrane Library). A total of 2270 duplicates were excluded. After reading the titles and abstracts, 2559 articles were excluded based on the inclusion and exclusion criteria. Among the eighty-seven studies under full-text review, fifty-two studies were excluded. Eventually, thirty-five studies were included in this meta-analysis based on the inclusion criteria.7–10,12–14,16,17,20,21,30–53 The literature retrieval flow chart was shown in Figure 1.
Figure 1.
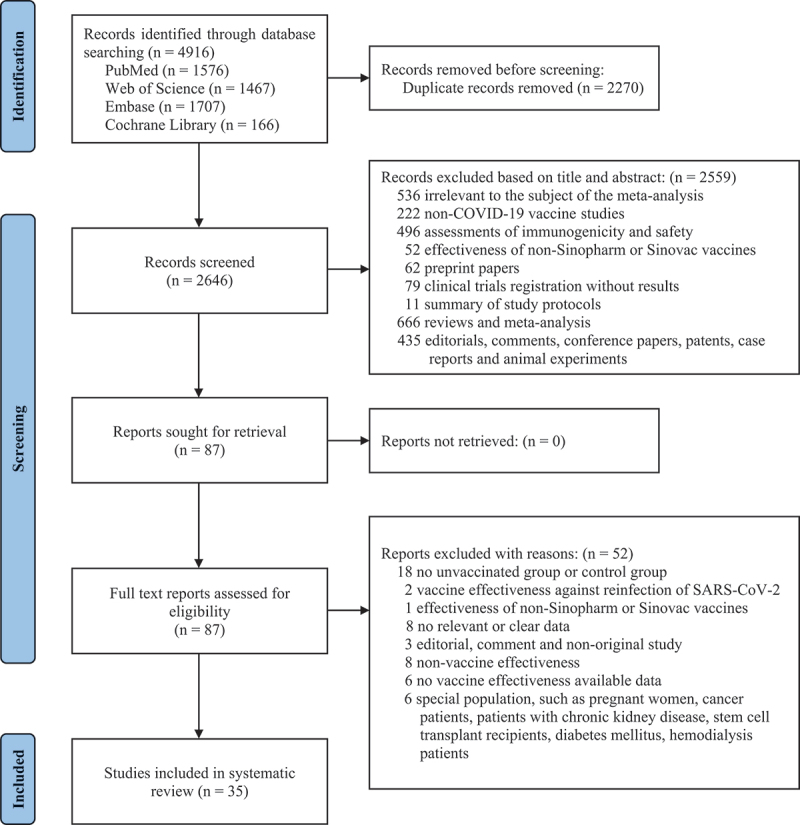
PRISMA flowchart for the study.
Of the thirty-five studies, a total of 45,589,056 people from fifteen countries were included. There were two RCTs (48,235 participants),9,13 twenty-two cohort studies (255,249,042 participants),8,10,12,14,16,20,30–45 and eleven case-control studies(11,712,615 participants).7,17,21,46–53 We identified four immunization regimens: two doses of inactivated vaccine, three doses of inactivated vaccine homologous booster, mRNA vaccine heterologous booster, and non-replicating vector vaccine heterologous booster. CoronaVac, HB02, WIV04, and BBIBP-CorV are inactivated vaccines used for the two-dose primary immunization. Following two doses of primary vaccination, four vaccines, namely mRNA-1273, BNT162b2, ChAdOx1 (AZD1222), and Ad26.COV2.S, were utilized for heterologous booster immunization. BNT162b2 and mRNA-1273 are mRNA vaccines; Ad26.COV2.S and ChAdOx1 are non-replicating vector vaccines. Moreover, we also identified four SARS-CoV-2 VOCs (Alpha, Gamma, Delta, and Omicron). Characteristics of included studies were shown in Supplementary Table S2A and Supplementary Table S2B.
Risk of bias assessment
The risk of bias assessments for the included RCTs was shown in Supplement Table S4, and the results suggested that both the two studies9,13 were at low risk. The results of the quality assessment of the included cohort studies8,10,12,14,16,20,30–45 and case-control studies7,17,21,46–53 were presented in Supplement Table S5 and Supplement Table S6. All of these studies were of good or moderate quality with a low risk (≥7 points).
Vaccine effectiveness against COVID-19 infection
A total of twenty-eight studies investigated VE against COVID-19 infection, of which sixteen were cohort studies and ten were case-control studies and two were RCTs. The results of the effectiveness of all immunization regimens against COVID-19 related outcomes were presented in Supplementary Table S7. The results showed that summary effectiveness of the various immunization regimens against COVID-19 infection was 62.79% (95%CI 55.87, 69.71) (Table 1 and Supplementary Figure S1). Of these, the VE of two doses of inactivated vaccine was 51.48 (95%CI 41.75, 61.21) against COVID-19 infection and 69.99% (95%CI 52.16, 87.82) for homologous boosters. On top of completing two doses of inactivated vaccine, heterologous boosters with mRNA vaccine and non-replicating vector vaccine were more effective against COVID-19 infection with VE of 89.19 (95%CI 78.49, 99.89) and 87.00 (95%CI 82.14, 91.85), respectively.
Table 1.
Vaccine effectiveness of immunization regimens included in the study.
| Outcomes | Study (n) | VE% (95%CI) | I2 (%) | P value |
|---|---|---|---|---|
| COVID-19 infection | ||||
| Overall | 28 | 62.79 (55.87, 69.71) | 99.9 | <.001 |
| Two doses of inactivated vaccines | 26 | 51.48 (41.75, 61.21) | 99.9 | <.001 |
| Homologous booster of inactivated vaccines | 2 | 69.99 (52.16, 87.82) | 96.2 | <.001 |
| Heterologous booster of mRNA vaccines | 6 | 89.19 (78.49, 99.89) | 99.9 | <.001 |
| Heterologous booster of non-replicating vector vaccines | 4 | 87.00 (82.14, 91.85) | 97.7 | <.001 |
| COVID-19 related hospitalization | ||||
| Overall | 17 | 78.29 (74.15, 82.43) | 99.5 | <.001 |
| Two doses of inactivated vaccines | 16 | 70.00 (65.80, 74.19) | 98.3 | <.001 |
| Homologous booster of inactivated vaccines | 2 | 85.08 (79.11, 91.04) | 23.9 | .252 |
| Heterologous booster of mRNA vaccines | 3 | 95.94 (94.70, 97.18) | 73.7 | .010 |
| Heterologous booster of non-replicating vector vaccines | 2 | 97.67 (97.11, 98.22) | 1.7 | .313 |
| COVID-19 related ICU admission | ||||
| Overall | 8 | 82.27 (75.76, 88.78) | 99.3 | <.001 |
| Two doses of inactivated vaccines | 7 | 73.78 (63.94, 83.62) | 98.7 | <.001 |
| Homologous booster of inactivated vaccines | 1 | 92.20 (89.25, 95.15) | NA | NA |
| Heterologous booster of mRNA vaccines | 1 | 96.20 (94.85, 97.55) | NA | NA |
| Heterologous booster of non-replicating vector vaccines | 1 | 98.90 (98.55, 99.25) | NA | NA |
| COVID-19 related death | ||||
| Overall | 17 | 85.90 (82.60, 89.10) | 98.9 | <.001 |
| Two doses of inactivated vaccines | 17 | 81.35 (78.39, 84.31) | 96.9 | <.001 |
| Homologous booster of inactivated vaccines | 3 | 91.50 (83.67, 99.33) | 80.0 | .007 |
| Heterologous booster of mRNA vaccines | 5 | 95.71 (91.84, 99.59) | 96.7 | <.001 |
| Heterologous booster of non-replicating vector vaccines | 2 | 98.10 (97.45, 98.74) | 0.0 | .759 |
| Severe COVID-19 | ||||
| Overall | 9 | 86.37 (81.06, 91.67) | 99.4 | <.001 |
| Two doses of inactivated vaccines | 8 | 79.55 (70.80, 88.30) | 99.6 | <.001 |
| Homologous booster of inactivated vaccines | 1 | 88.00 (82.15, 93.85) | NA | NA |
| Heterologous booster of mRNA vaccines | 4 | 95.13 (89.49, 100.78) | 98.0 | <.001 |
| Heterologous booster of non-replicating vector vaccines | 1 | 99.10 (95.95, 102.25) | NA | NA |
Abbreviations: VE, vaccine effectiveness; CI, confidence interval; NA, not applicable.
Vaccine effectiveness against COVID-19 related hospitalization
The effectiveness of COVID-19-related hospitalization was assessed in seventeen studies with a summary VE of 78.29% (95%CI 74.15, 82.43), including twelve cohort studies, four case-control studies and one RCT (Table 1 and Figure S2).
The VE of the two doses of inactivated vaccine against COVID-19 related hospitalization was 70.00% (95%CI 65.80, 74.19) and 85.08% (95%CI 79.11, 91.04) for the homologous booster. Results of heterologous booster immunization showed that the VE for receiving non-replicating vector vaccines was 97.67% (95%CI 97.11, 98.22) and 95.94% (95%CI 94.70, 97.18) for mRNA vaccines. The results indicated that heterologous boosters were more effective than homologous boosters in preventing COVID-19-related hospitalization.
Vaccine effectiveness against COVID-19 related ICU admission
The effectiveness of the COVID-19 vaccine in preventing COVID-19-related ICU admissions was investigated in eight studies, all of which were cohort studies (Table 1 and Supplementary Figure S3). Seven studies evaluated the effectiveness of two doses of inactivated vaccine in preventing ICU admission with a VE of 73.78% (95%CI 63.94, 83.62). Only one study assessed the effectiveness of homologous and heterologous booster immunization with 92.20% (95%CI 89.25, 95.15) for homologous boosters, 96.20% (95%CI 94.85, 97.55) for heterologous mRNA vaccine boosters and 98.90% (95%CI 98.55, 99.25) for heterologous non-replicating vector vaccine boosters.20
Vaccine effectiveness against COVID-19 related death
We included seventeen studies to assess VE for COVID-19 related death (Table 1 and Supplementary Figure S4). We summarized the VE results for all immunization regimens showing an overall effectiveness of 85.90% (95%CI 82.60, 89.10) for prevention of COVID-19 related death. Among these, two doses of inactivated vaccine had a VE of 81.35% (95%CI 78.39, 84.31), homologous boosters 91.50% (95%CI 83.67, 99.33), mRNA vaccine boosters 95.71% (95%CI 91.84, 99.59) and non-replicating vector vaccine boosters 98.10% (95%CI 97.45, 98.74).
Vaccine effectiveness against severe COVID-19 outcomes
We analyzed nine studies of VE against severe COVID-19 outcomes (Table 1 and Supplementary Figure S5). All were observational studies, four cohort studies, and five case-control studies. Aggregated VE results showed that the VE for two doses of inactivated vaccine, homologous booster, mRNA vaccine booster, and non-replicating vector vaccine booster was 79.55% (95%CI 70.80, 88.30), 88.00% (95%CI 82.15, 93.85), 95.13% (95%CI 89.49, 100.78), and 99.10% (95%CI 95.95, 102.25), respectively. The results suggested that all immunization regimens were effective in preventing severe COVID-19 outcomes, with a summary VE of 86.37% (95%CI 81.06, 91.67).
Vaccine effectiveness against Alpha and Gamma variants
There were four and three studies assessed the VE of primary immunization against infection caused by the Alpha and Gamma variants, with a VE of 69.28% (95%CI 57.99, 80.58) and 47.64% (95%CI 40.97, 54.30), respectively (Table 2 and Supplementary table S9). Owing to limited evidence, only one study evaluated the VE against hospitalization, death, and severe illness caused by the Alpha variant, and the VE was 88.50% (95%CI 86.05, 90.95), 86.00% (95%CI 83.90, 88.10) and 90.50% (95%CI 89.00, 92.00), respectively (Supplementary Table S10). In the analysis for the Gamma variant, the VE against hospitalization and death was 55.50% (95%CI 47.30, 63.70), and 61.20% (95%CI 50.40, 72.00), respectively.
Table 2.
Vaccine effectiveness against SARS-CoV-2 variants infection.
| Outcomes | Study (n) | VE% (95% CI) | I2 (%) | p value |
|---|---|---|---|---|
| Alpha | ||||
| Two doses of inactivated vaccines | 4 | 69.28 (57.99, 80.58) | 98.9 | <.001 |
| Gamma | ||||
| Two doses of inactivated vaccines | 3 | 47.64 (40.97, 54.30) | 0.0 | .821 |
| Delta | ||||
| Overall | 8 | 75.76 (70.73, 80.79) | 99.7 | <.001 |
| Two doses of inactivated vaccines | 6 | 48.68 (26.86, 70.49) | 98.5 | <.001 |
| Homologous booster of inactivated vaccines | 2 | 69.99 (52.16, 87.82) | 96.2 | <.001 |
| Heterologous booster of mRNA vaccines | 4 | 93.44 (89.03, 97.85) | 99.1 | <.001 |
| Heterologous booster of non-replicating vector vaccines | 4 | 87.00 (82.14, 91.85) | 97.7 | <.001 |
| Omicron | ||||
| Overall | 4 | 34.67 (6.75, 62.60) | 100.0 | <.001 |
| Two doses of inactivated vaccines | 4 | 27.37 (8.23, 46.52) | 99.8 | <.001 |
| Heterologous booster of mRNA vaccines | 1 | 63.60 (62.85, 64.35) | NA | NA |
Abbreviations: VE, vaccine effectiveness; CI, confidence interval; NA, not applicable.
Vaccine effectiveness against Delta variant
Nine studies assessed effectiveness against the Delta variant (Table S9). Against Delta-induced infection, the heterologous mRNA vaccine booster had the highest VE at 93.44% (95%CI 89.03, 97.85), followed by heterologous non-replicating vector vaccine booster with a VE of 87.00 (95%CI 82.14, 91.85) and homologous inactivated vaccine booster with a VE of 69.99% (95%CI 52.16, 87.82) (Table 2). However, the two-dose inactivated vaccine regimen was shown to be ineffective against Delta infection with a VE of 48.68% (95%CI 26.86, 70.49). Booster immunization provided significant protection against Delta-induced death and heterologous booster was higher than homologous booster (Supplementary Table S11). The effectiveness against death was 86.82 (95%CI 81.64, 92.00) for homologous boosters, 96.32% (95%CI 94.82, 97.82) for heterologous mRNA vaccine boosters, and heterologous non-replicating vector vaccine boosters were the highest at 98.10% (95%CI 97.45, 98.74).
Vaccine effectiveness against Omicron variant
The effectiveness against the Omicron variant was assessed in six studies (Supplementary Table S9). We found that the two doses of inactivated vaccine were ineffective in preventing Omicron infection with a VE of 27.37% (95%CI 8.23, 46.52) (Table 2). Heterologous mRNA vaccine boosters had a VE of 63.60% (95%CI 62.85, 64.35) for the prevention of Omicron infection, however only one study contributed. Two studies assessed the effectiveness against Omicron-induced death with a VE of 93.79% (95%CI 87.32, 100.25) for heterologous mRNA vaccine boosters (Supplementary Table S12). Three studies evaluated the effectiveness against severe outcomes caused by Omicron variants. Homologous booster and heterologous mRNA vaccine booster were highly effective in preventing severe outcomes related to Omicron, with VE of 88.00% (95%CI 82.15, 93.85) and 90.47% (95%CI 86.49, 94.44), respectively.
Vaccine effectiveness against COVID-19 in different populations
Two, fourteen, four, five, and three studies presented VE against COVID-19 infection in children aged <18 years, the general population, elderly people aged ≥60 years, healthcare workers, and close contacts of COVID-19-positive cases, respectively (Table 3). Because of data limitations, the VE of the booster immunization regimen was not assessed in children, healthcare workers, and close contacts against COVID-19. The VE of the two doses of inactivated vaccine in preventing COVID-19 infection was 38.36% (95%CI 36.73, 40.00) for children, 44.45% (95%CI 24.63, 64.27) for healthcare workers, 57.51% (95%CI 42.74, 72.29) for close contacts. In the general population, for COVID-19 infection and death, the VE of homologous boosters was 69.99% (95%CI 52.16, 87.82) and 86.82% (95%CI 81.64, 92.00), mRNA vaccine boosters were 89.19% (95%CI 78.49, 99.89) and 95.36% (95%CI 90.92, 99.80), and non-replicating vector vaccine boosters were 87.00% (95%CI 82.14, 91.85) and 98.10% (95%CI 97.45, 98.74), respectively (Table 3 and Supplementary Table S13). In the elderly population, two doses of the inactivated vaccine had an effectiveness of 68.11% (95%CI 43.14, 93.09) against COVID-19 infection, 79.17% (95%CI 70.44, 87.89) against death, and 74.95% (95%CI 43.98, 105.91) against the severe outcome. Only one study assessed the VE of heterologous mRNA booster immunization for death and severe outcomes, 97.20% (95%CI 94.70, 99.70), 95.20% (95%CI 86.05, 104.35) respectively.
Table 3.
Vaccine effectiveness against COVID-19 infection in different populations.
| Outcomes | Study (n) | VE% (95% CI) | I2 (%) | p value |
|---|---|---|---|---|
| Children aged <18 years | ||||
| Two doses of inactivated vaccines | 2 | 38.36 (36.73, 40.00) | 0.0 | .499 |
| General population | ||||
| Overall | 14 | 66.85 (58.66, 75.03) | 100.0 | <.001 |
| Two doses of inactivated vaccines | 12 | 49.31 (35.58, 63.04) | 99.9 | <.001 |
| Homologous booster of inactivated vaccines | 2 | 69.99 (52.16, 87.82) | 96.2 | <.001 |
| Heterologous booster of mRNA vaccines | 6 | 89.19 (78.49, 99.89) | 99.9 | <.001 |
| Heterologous booster of non-replicating vector vaccines | 4 | 87.00 (82.14, 91.85) | 97.7 | <.001 |
| Elderly people aged ≥60 years | ||||
| Two doses of inactivated vaccines | 4 | 68.11 (43.14, 93.09) | 99.9 | <.001 |
| Healthcare workers | ||||
| Two doses of inactivated vaccines | 5 | 44.45 (24.63, 64.27) | 91.5 | <.001 |
| Close contacts | ||||
| Two doses of inactivated vaccines | 3 | 57.51 (42.74, 72.29) | 7.6 | .339 |
Abbreviations: VE, vaccine effectiveness; CI, confidence interval; NA, not applicable.
Publication bias and sensitivity analysis
Supplementary Figure S6 showed funnel plots for all COVID-19 related outcomes. Egger’s test showed that there exists publication bias for COVID-19 infection (Egger’s testt = −2.80, p = .008), for COVID-19 related hospitalization (Egger’s testt = −2.17, p = .041) and for COVID-19 related ICU admission (Egger’s testt = −3.10, p = .015) among individuals with primary and booster immunization, whereas for COVID-19-related death (Egger’s testt = −1.23, p = .229) and severe COVID-19 infection (Egger’s testt = −0.07, p = .947), no significant publication bias was found, either qualitative based on funnel plot or visual bias based on funnel plot. We performed a sensitivity analysis using the one-by-one elimination method. The overall results for the five primary outcomes did not change significantly, suggesting our results were relatively stable (Supplementary Figure S7).
Discussion
In this systematic review and meta-analysis, we found that homologous and heterologous booster regimens reduced the risk of SARS-CoV-2 infection and were effective in preventing severe outcomes and death after primary immunization with two doses of inactivated vaccine. We confirmed that homologous and heterologous booster regimens were effective in reducing the risk of hospitalization, death, and severe outcomes with the delta and omicron variants. The effectiveness of mRNA vaccines and non-replicating vector vaccines for any COVID-19-related outcome was similar in boosting immune protection. Both types of vaccine can be used as a booster dose, based on the availability of vaccines.
Although the effectiveness against COVID-19 infection of the two-dose regimen seemed to be mediocre, the protection of death and severe outcomes was still obvious which justifies the merit of massive and speedy vaccine rollout in the population. Homologous and heterologous COVID-19 vaccine booster regimens provided high levels of protection against COVID-19 infection, hospitalization, death and severe outcomes for individuals completing primary immunization. Furthermore, compared to homologous boosters, the heterologous boosters showed higher effectiveness, which may be related to levels of antibody titer and T-cell responses. Zuo et al. demonstrated that heterologous booster with mRNA vaccine after two doses of inactivated vaccine induced higher RBD-IgG antibody levels (GMT: 462.3 BAU/ml VS. 57.6 BAU/ml) and more S1-specific T cells (median: 43.1 VS. 11.5) compared to homologous boosters about 3 months after the boost dose.54 Sablerolles et al. showed that at 28 days post-immunization, heterologous booster immunization (1 dose of Ad26.COV2.S and BNT162b2 booster) induced higher levels of S-specific neutralizing antibodies compared to homologous booster immunization (2 doses of Ad26.COV2.S).55 Neutralizing antibody level was 8.5-fold increase over that for the homologous booster (GMT: 2007 IU/ml VS. 235 IU/ml). The levels of interferon-γ were also higher for the heterologous booster, compared to the homologous booster (response: 91.7% VS.72.7%). Additionally, the safety of heterologous booster immunization regimens has been widely demonstrated to be safe and tolerable,56–58 providing additional support for a mix-and-match approach.
Two doses of inactivated vaccine provided high protection against severe Alpha infection with a VE of 90.50%, and failed to prevent infection with the Gamma variant. This may be due to the fact that studies occurred in elderly populations with compromised immune systems,7 and among healthcare workers with more exposure to SARS-CoV-2.43,47
Although less effective against infections caused by Omicron variants, vaccination was essential to prevent serious disease. Homologous and heterologous booster immunizations provided high protection against Delta variant and Omicron-associated death and severe outcomes. However, prevention of Delta infection appears to be more effective than Omicron, which may be related to the partial neutralizing antibody escape of Omicron variant.59,60 Although omicron is less virulent than the alpha and delta variant strains, it still causes significant morbidity and mortality.61 The Omicron spectrum of SARS-CoV-2 continues to evolve, and the emerging Omicron subspectrum is not only more transmissible but more likely to evade neutralization,59 posing a challenge to vaccine effectiveness. Given the significantly increased transmissibility of the Omicron variant, some countries started a fourth dose of vaccine booster as soon as it was available.62–64 A recent study of a fourth dose of mRNA vaccine reported improved efficacy against confirmed infection and severe COVID-19 in adults aged 19 years or older, from receiving the third to the fourth dose.62 In the long term, in the absence of an Omicron variant-specific vaccine, ongoing booster vaccination with an existing vaccine remains the optimal choice for reducing infection and disease severity in Omicron variant.
We performed subgroup analyses of different populations to explore the population impact on effectiveness. Most studies enrolled the general population, followed by the elderly, and healthcare workers, and only three studies analyzed vaccine effectiveness in people aged <18 years. In the general population, both the heterologous mRNA vaccine and the non-replicating viral vector vaccine booster regimen provided high levels of protection, with vaccine effectiveness higher than the inactivated vaccine homologous booster regimen, especially, against COVID-19-related hospitalization, ICU admission.20,50 Due to data limitations, we only found a subgroup aged under 18 years with two-dose inactivated vaccine primary immunization regimen performed 70.47% of VE against hospitalization, which were in line with previous studies of VE in adult and adolescent populations.30,65 Both homologous and heterologous mRNA booster immunization regimens had high vaccine effectiveness for COVID-19 related death and severe outcomes in the elderly population, suggesting that booster immunization is necessary to reduce severe illness and death in the elderly population. However, the results were inconclusive as only one study contributed to the results,53 so more studies on children and adolescents are needed.
The greatest strength of this study is to evaluate the effectiveness of CoronaVac or Sinopharm COVID-19 vaccines against five COVID-19 related outcomes by combining randomized controlled trials and real-world studies. Moreover, this study included a large number of people, providing sufficient evidence for this topic. In addition, in order to reduce the impact of heterogeneity, we conducted rigorous subgroup analysis, such as COVID-19 variant, study population and follow-up period, to obtain more precise and detailed results. In the meantime, we must acknowledge that our study has some limitations. First, vaccine effectiveness may be affected by factors such as study population (e.g., age and race), study region, pandemic intensity, and vaccine type, so there was considerable heterogeneity in our summary results. Although we performed subgroup analyses with different stratifications, such as variants and study population, the heterogeneity remains high and must be considered when interpreting the results. Besides, in the original study design, we attempted to conduct subgroup analyses of effectiveness across age groups, sex, and comorbidities, but we were unable to do so due to limited data. Furthermore, most studies against COVID-19-related outcomes predominantly accepted two doses of inactivated vaccine, while studies of homologous or heterologous booster immunization were scarce. Therefore, our findings should be interpreted in a very cautious way, and in the future, additional studies are needed to validate the results. In addition, in our study, homologous and heterologous booster immunization regimens focused mainly on summarizing COVID-19 vaccine effectiveness within 6 months, with a few studies with follow-up beyond 6 months and only in the two-dose inactivated vaccine arm (Supplementary Table S8). Knowing whether and to what extent the long-term effectiveness of vaccine wanes is crucial to inform vaccine policy decisions. Unfortunately, our data were not sufficient to assess the effectiveness of the vaccine over a longer duration, and more studies and longer follow-up periods would be needed to know how long the protective effect of the vaccine persists. Finally, but not least, our study did not assess the safety or immunogenicity of COVID-19 vaccines, nor the virulence or transmission of SARS-CoV-2 variants were not evaluated in our study, which could result in a partial understanding of COVID-19 vaccines against SARS-CoV-2 variants. Due to insufficient data, some recent vaccines and variants were not included in our study. As a result, it is unclear whether our findings are generalizable.
For now, immunization remains the most effective way to stop the outbreak. Booster immunization offers excellent defense against various SARS-CoV-2 variants and aids in preventing serious illness and death associated with COVID-19. In the process of improving immunization strategies, heterologous booster immunization provides a way to improve immunogenicity.66 Few original studies have been conducted on the effectiveness of heterologous or homologous boosters of inactivated COVID-19 vaccines, and future immunization schedules would benefit from more pertinent data.
In conclusion, the results of our study indicated that whether heterologous or homologous COVID-19 boosters provided good protection, with heterologous boosters being more desirable. This would contribute to scientific decision making by the government on public health issues, and effective use of COVID-19 vaccine resources, and it would also serve as a reference for future studies.
Supplementary Material
Acknowledgments
Our special gratitude goes to the authors of included studies who helped us to do this systematic review and meta-analysis.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Xiaoyin Zhang, Jingxin Li and Fengcai Zhu designed the study. Xiaoyin Zhang, Jiayue Xia, Lairun Jin and Xiang Cao conducted the literature search. Xiaoyin Zhang, Jiayue Xia, Lairun Jin, Xiuyu Zheng, Xingchen Meng and Yanfei Wu identified the study, data extraction, and quality assessment. Xiaoyin Zhang, Jiayue Xia and Lairun Jin participated in the statistical analysis. Xiaoyin Zhang wrote the original draft, and Jingxin Li and Fengcai Zhu contributed critical reviews and made revisions. All authors contributed to data interpretation and writing, reviewing and editing this manuscript.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2221146.
References
- 1.Akande OW, Akande TM.. COVID-19 pandemic: a global health burden. Niger Postgrad Med J. 2020;27(3):147–10. doi: 10.4103/npmj.npmj_157_20. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO coronavirus (COVID-19) dashboard. 2023. Mar 13 [accessed 2023 Mar 20]. https://covid19.who.int/table.
- 3.World Health Organization . Recommendation for an emergency use listing of COVID-19 vaccine BIBP submitted by Beijing Institute of Biological Products Co., Ltd. 2021. May 7 [accessed 2023 Mar 20]. https://extranet.who.int/pqweb/vaccines/who-recommendation-covid-19-vaccine-bibp.
- 4.World Health Organization . Recommendation for an emergency use listing of COVID-19 vaccine (vero cell), Inactivated submitted by sinovac. 2021. Jun 1 [accessed 2023 Mar 20]. https://extranet.who.int/pqweb/vaccines/who-recommendation-sinovac-covid-19-vaccine-vero-cell-inactivated-coronavac.
- 5.COVID-19 vaccine tracker. Sinopharm (Beijing): Covilo; 2022. Dec 02 [accessed 2023 Mar 20]. https://covid19.trackvaccines.org/vaccines/5/.
- 6.COVID-19 vaccine tracker. Sinovac: CoronaVac; 2022. Dec 02 [accessed 2023 Mar 20]. https://covid19.trackvaccines.org/vaccines/7/.
- 7.Ranzani O, Hitchings MDT, Dorion M, D’Agostini TL, Paula R, Paula O, Villela EFDM, Torres MSS, de Oliveira SB, Schulz W, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: test negative case-control study. Br Med J. 2021;374. doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jara A, Undurraga EA, Gonzalez C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–84. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanriover MD, Doganay HL, Akova M, Guner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suah JL, Husin M, Tok PSK, Tng BH, Thevananthan T, Low EV, Appannan MR, Muhamad Zin F, Mohd Zin S, Yahaya H, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int J Infect Dis. 2022;119:69–76. doi: 10.1016/j.ijid.2022.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, Khrisna CV, Sari RM, Setyaningsih L, Surachman F, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520–8. doi: 10.1016/j.vaccine.2021.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez S, Olszevicki S, Gaiano A, Baino ANV, Regairaz L, Salazar M, Pesci S, Marín L, Martínez VVG, Varela T, et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273 vaccines against hospitalisations among children and adolescents during the Omicron outbreak in Argentina: a retrospective cohort study. Lancet Reg Health Am. 2022;13:100316–. doi: 10.1016/j.lana.2022.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, et al. Effect of 2 Inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults a randomized clinical trial. J Am Med Assoc. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Belayachi J, Yang Y, Fu Q, Rodewald L, Li H, Yan B, Wang Y, Shen Y, Yang Q, et al. Real-world study of the effectiveness of BBIBP-CorV (Sinopharm) COVID-19 vaccine in the Kingdom of Morocco. BMC Public Health. 2022;22(1). doi: 10.1186/s12889-022-14016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Can G, Acar HC, Aydin SN, Balkan II, Karaali R, Budak B, Saltoglu N. Waning effectiveness of CoronaVac in real life: a retrospective cohort study in health care workers. Vaccine. 2022;40(18):2574–9. doi: 10.1016/j.vaccine.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranzani OT, Hitchings MDT, de Melo RL, de Franca GVA, Fernandes C, Lind ML, Torres MSS, Tsuha DH, David LCS, Said RFC, et al. Effectiveness of an inactivated covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil. Nat Commun. 2022;13(1). doi: 10.1038/s41467-022-33169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . The Sinovac-CoronaVac COVID-19 vaccine: what you need to know. 2022. Jun 10 [accessed 2023 Mar 20]. https://www.who.int/zh/news-room/feature-stories/detail/the-sinovac-covid-19-vaccine-what-you-need-to-know.
- 19.World Health Organization . The Sinopharm COVID-19 vaccine: what you need to know. 2022. June 10 [accessed 2023 Mar 20]. https://www.who.int/zh/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know.
- 20.Jara A, Undurraga EA, Zubizarreta JR, Gonzalez C, Pizarro A, Acevedo J, Leo K, Paredes F, Bralic T, Vergara V, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10(6):E798–E806. doi: 10.1016/S2214-109X(22)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerqueira-Silva T, de Araujo Oliveira V, Paixao ES, Bertoldo Junior J, Penna GO, Werneck GL, Pearce N, Barreto ML, Boaventura VS, Barral-Netto M, et al. Duration of protection of CoronaVac plus heterologous BNT162b2 booster in the Omicron period in Brazil. Nat Commun. 2022;13(1). doi: 10.1038/s41467-022-31839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386(16):1532–46. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, Smatti MK, Tang P, Hasan MR, Coyle P, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386(19):1804–16. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):200. doi: 10.1186/s12916-022-02397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Au WY, Cheung PP. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: living systematic review with network meta-analysis. Bmj. 2022;377:e069989. doi: 10.1136/bmj-2022-069989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2008. [accessed 2023 Mar 20]. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 29.Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses . In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. London (UK): Cochrane; 2022. [Google Scholar]
- 30.Jara A, Undurraga EA, Zubizarreta JR, Gonzalez C, Acevedo J, Pizarro A, Vergara V, Soto-Marchant M, Gilabert R, Flores JC, et al. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. 2022;28(7):1377–80. doi: 10.1038/s41591-022-01874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voko Z, Kiss Z, Surjan G, Surjan O, Barcza Z, Palyi B, Formanek-Balku E, Molnár GA, Herczeg R, Gyenesei A, et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary—the HUN-VE study. Clin Microbiol Infect. 2022;28(3):398–404. doi: 10.1016/j.cmi.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edith Solis-Castro M, Jaramillo-Corrales A, Gonzalez Seminario RV, Janampa Grados N, Mamani Pilco IE, Vargas Quispe KE, La Torre Rosillo LY, Vásquez Dominguez MN, Enriquez Cusi DT, Minaya P, et al. Effectiveness of the Inactivated SARS-CoV-2 (vero cell) vaccine in Peruvian health workers. Life-Basel. 2022;12(9). doi: 10.3390/life12091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovic V, Vukovic V, Markovic M, Ristic M. Early effectiveness of four SARS-CoV-2 vaccines in preventing COVID-19 among adults aged ≥ 60 years in Vojvodina, Serbia. Vaccines. 2022;10(3). doi: 10.3390/vaccines10030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, Zhang Y, Tang L, Wang F, Ye Y, Ma C, Zheng H, Yu W, Cao L, Song Y, et al. Effectiveness of inactivated COVID-19 vaccines against symptomatic, pneumonia, and severe disease caused by the Delta variant: real world study and evidence — China, 2021. china Cdc Weekly. 2022;4(4):57–65. doi: 10.46234/ccdcw2022.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voko Z, Kiss Z, Surjan G, Surjan O, Barcza Z, Wittmann I, Molnár GA, Nagy D, Müller V, Bogos K, et al. Effectiveness and waning of protection with different SARS-CoV-2 primary and booster vaccines during the Delta pandemic wave in 2021 in Hungary (HUN-VE 3 Study). Front Immunol. 2022;13. doi: 10.3389/fimmu.2022.919408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paternina-Caicedo A, Jit M, Alvis-Guzman N, Fernandez JC, Hernandez J, Paz-Wilches JJ, Rojas-Suarez J, Dueñas-Castell C, Alvis-Zakzuk NJ, Smith AD, et al. Effectiveness of CoronaVac and BNT162b2 COVID-19 mass vaccination in Colombia: a population-based cohort study. Lancet Reg Health Am. 2022;12:100296. doi: 10.1016/j.lana.2022.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suah JL, Tok PSK, Ong SM, Husin M, Tng BH, Sivasampu S, Thevananthan T, Appannan MR, Muhamad Zin F, Mohd Zin S, et al. PICK-ing Malaysia’s epidemic apart: effectiveness of a diverse COVID-19 vaccine portfolio. Vaccines. 2021;9(12):1381. doi: 10.3390/vaccines9121381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arregoces-Castillo L, Fernandez-Nino J, Rojas-Botero M, Palacios-Clavijo A, Galvis-Pedraza M, Rincon-Medrano L, Pinto-Álvarez M, Ruiz-Gómez F, Trejo-Valdivia B. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longevity. 2022;3(4):E242–E52. doi: 10.1016/S2666-7568(22)00035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma C, Sun W, Tang T, Jia M, Liu Y, Wan Y, Han J, Rodewald L, Li J, Song Y, et al. Effectiveness of adenovirus type 5 vectored and inactivated COVID-19 vaccines against symptomatic COVID-19, COVID-19 pneumonia, and severe COVID-19 caused by the B.1.617.2 (Delta) variant: evidence from an outbreak in Yunnan, China, 2021. Vaccine. 2022;40(20):2869–74. doi: 10.1016/j.vaccine.2022.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Kaabi N, Oulhaj A, Ganesan S, Al Hosani FI, Najim O, Ibrahim H, Acuna J, Alsuwaidi AR, Kamour AM, Alzaabi A, et al. Effectiveness of BBIBP-CorV vaccine against severe outcomes of COVID-19 in Abu Dhabi, United Arab Emirates. Nat Commun. 2022;13(1). doi: 10.1038/s41467-022-30835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirahmadizadeh A, Heiran A, Bagheri Lankarani K, Serati M, Habibi M, Eilami O, Heiran F, Moghadami M. Effectiveness of coronavirus disease 2019 vaccines in preventing infection, hospital admission, and death: a historical cohort study using Iranian registration data during vaccination program. Open Forum Infect Dis. 2022;9(6). doi: 10.1093/ofid/ofac177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.AlHosani FI, Stanciole AE, Aden B, Timoshkin A, Najim O, Zaher WA, AlSayedsaleh AlDhaheri F, Al Mazrouie S, Rizvi TA, Mustafa F, et al. Impact of the Sinopharm’s BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE). Vaccine. 2022;40(13):2003–10. doi: 10.1016/j.vaccine.2022.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marra AR, Miraglia JL, Malheiros DT, Guozhang Y, Teich VD, da Silva Victor E, Sheeler LL, Abosi O, Holley S, Kukla MB, et al. Coronavirus disease 2019 (COVID-19) among nonphysician healthcare personnel by work location at a tertiary-care center, Iowa, 2020–2021. Infect Control Hosp Epidemiol. 2022:1–20. doi: 10.1017/ice.2022.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Copur B, Surme S, Sayili U, Tuncer G, Pehlivanoglu F, Sengoz G. Effectiveness of CoronaVac vaccination against COVID-19 development in healthcare workers: real-life data. Future Microbiol. 2022;17(17):1381–91. doi: 10.2217/fmb-2022-0134. [DOI] [PubMed] [Google Scholar]
- 45.Kang M, Yi Y, Li Y, Sun L, Deng A, Hu T, Zhang J, Liu J, Cheng M, Xie S, et al. Effectiveness of Inactivated COVID-19 Vaccines against illness caused by the B.1.617.2 (Delta) variant during an outbreak in Guangdong, China. Ann Intern Med. 2022;175(4):533–40. doi: 10.7326/M21-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rearte A, Manuel Castelli J, Rearte R, Fuentes N, Pennini V, Pesce M, Barbeira PB, Iummato LE, Laurora M, Bartolomeu ML, et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. Lancet. 2022;399(10331):1254–64. doi: 10.1016/S0140-6736(22)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, Borg R, Schulz WL, de Oliveira RD, da Silva PV, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am. 2021;1:100025–. doi: 10.1016/j.lana.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nadeem I, Ul Munamm SA, Rasool MU, Fatimah M, Abu Bakar M, Rana ZK, Khatana UF, Jordon L, Saqlain M, Mahdi N, et al. Safety and efficacy of Sinopharm vaccine (BBIBP-CorV) in elderly population of Faisalabad district of Pakistan. Postgrad Med J. 2022. doi: 10.1136/postgradmedj-2022-141649. [DOI] [PubMed] [Google Scholar]
- 49.Florentino PTV, Alves FJO, Cerqueira-Silva T, Oliveira V, Junior JBS, Jantsch AG, Penna GO, Boaventura V, Werneck GL, Rodrigues LC, et al. Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the Omicron period. Nat Commun. 2022;13(1). doi: 10.1038/s41467-022-32524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerqueira-Silva T, Katikireddi SV, Oliveira V, Flores-Ortiz R, Bertoldo Junior J, Paixao ES, Robertson C, Penna GO, Werneck GL, Barreto ML, et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022;28(4):838–43. doi: 10.1038/s41591-022-01701-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suphanchaimat R, Nittayasoot N, Jiraphongsa C, Thammawijaya P, Bumrungwong P, Tulyathan A, Cheewaruangroj N, Pittayawonganon C, Tharmaphornpilas P. Real-world effectiveness of mix-and-match vaccine regimens against SARS-CoV-2 Delta variant in Thailand: a nationwide test-negative matched case-control study. Vaccines. 2022;10(7):1080. doi: 10.3390/vaccines10071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, Chaiyakulsil C, Sinlapamongkolkul P, Tangsathapornpong A, Bunjoungmanee P, et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: a test-negative case-control study. Emerging Microbes Infect. 2022;11(1):585–92. doi: 10.1080/22221751.2022.2037398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan VKC, Wan EYF, Ye X, Mok AHY, Lai FTT, Chui CSL, Li X, Wong CKH, Li PH, Ma T, et al. Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case-control study. Emerg Microbes Infect. 2022;11(1):2304–14. doi: 10.1080/22221751.2022.2114854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo F, Abolhassani H, Du L, Piralla A, Bertoglio F, de Campos-Mata L, Wan H, Schubert M, Cassaniti I, Wang Y, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun. 2022;13(1):2670. doi: 10.1038/s41467-022-30340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, Visser LG, Geers D, Schmitz KS, Garcia Garrido HM, Koopmans MPG, Dalm VASH, et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N Engl J Med. 2022;386(10):951–63. doi: 10.1056/NEJMoa2116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grenfell RFQ, Almeida NBF, Filgueiras PS, Corsini CA, Gomes SVC, de Miranda DAP, Lourenço AJ, Martins-Filho OA, de Oliveira JG, Teixeira-Carvalho A, et al. Immunogenicity, effectiveness, and safety of inactivated virus (CoronaVac) vaccine in a two-dose primary protocol and BNT162b2 heterologous booster in Brazil (Immunita-001): a one year period follow up phase 4 study. Front Immunol. 2022;13:918896. doi: 10.3389/fimmu.2022.918896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, Pan H, Wang X, Song Z, Wan J, et al. Heterologous AD5-Ncov plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022;28(2):401–9. doi: 10.1038/s41591-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li JX, Wu SP, Guo XL, Tang R, Huang BY, Chen XQ, Chen Y, Hou L-H, Liu J-X, Zhong J, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir Med. 2022;10(8):739–48. doi: 10.1016/S2213-2600(22)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Guo Y, Iketani S, Nair MS, Li Z, Mohri H, Wang M, Yu J, Bowen AD, Chang JY, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–8. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCallum M, Czudnochowski N, Rosen LE, Zepeda SK, Bowen JE, Walls AC, Hauser K, Joshi A, Stewart C, Dillen JR, et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022;375(6583):864–8. doi: 10.1126/science.abn8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, Alroy-Preis S, Ash N, Huppert A, Milo R, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386(18):1712–20. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Regev-Yochay G, Gonen T, Gilboa M, Mandelboim M, Indenbaum V, Amit S, Meltzer L, Asraf K, Cohen C, Fluss R, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against Omicron. N Engl J Med. 2022;386(14):1377–80. doi: 10.1056/NEJMc2202542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magen O, Waxman JG, Makov-Assif M, Vered R, Dicker D, Hernán MA, Lipsitch M, Reis BY, Balicer RD, Dagan N, et al. Fourth Dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603–14. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Florentino PTV, Millington T, Cerqueira-Silva T, Robertson C, de Araújo Oliveira V, Júnior JBS, Alves FJO, Penna GO, Vital Katikireddi S, Boaventura VS, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis. 2022;22(11):1577–86. doi: 10.1016/S1473-3099(22)00451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng J, Ma Y, Liu Q, Du M, Liu M, Liu J. Comparison of the effectiveness and safety of heterologous booster doses with homologous booster doses for SARS-CoV-2 vaccines: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(17):10752. doi: 10.3390/ijerph191710752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


