ABSTRACT
Temporomandibular (TM) disorders afflict many people globally and, despite the presence of existing peer-reviewed material that assists conservative orthopedic providers, recent advances in knowledge indicate that updated resources are required for students, clinicians, and educators. This two-part series builds off previously published material to present newer supplementary information that can be useful during the evaluation and management processes. Content in Part 1 of this series includes a discussion about the factors that have been shown to contribute to TM disorders, an updated perspective of relevant pain science, a discussion of self-report outcome measures, and various different topics related to the examination of patients with TM disorders. Part 2 addresses information related to the temporomandibular joint disc, joint hypermobility, oral splints, and clinical reasoning. In combination with other available publications, this two-part series provides clinicians an opportunity to improve their delivery of effective and efficient clinical services for people diagnosed with TM disorders.
KEYWORDS: Physical therapy, Physical examination, Review, Temporomandibular joint disorders, Clinical reasoning
Introduction
As it was stated in Part 1 of this two-part series, temporomandibular (TM) disorders involve a heterogeneous group of painful neuromusculoskeletal conditions associated with the masticatory system and related tissues. The prevalence of TM disorders is sufficiently high to warrant the availability of conservative management by physical therapists and other providers [1–3] across a wide age range [4–12]. The need for management strategies is not only associated with the presence of pain but also the deleterious effects TM disorders have on quality of life [13,14]. Of note, the content of this series should be complimented with other preexisting sources including but not limited to resources pertaining to evaluation and management procedures [15–17] and clinical reasoning [18]. The purpose of this two-part series is to provide orthopedic physical therapists and other providers with an updated framework that is instructive for the conservative examination and management of TM disorders.
The TMJ disc & retrodiscal tissue
The temporomandibular joint (TMJ) disc has been the target of extensive attention [19–21]. However, it has also been concluded that the TMJ disc is primarily a “noisy annoyance” that does not necessarily warrant close clinical scrutiny [22]. While this also seems generally consistent with anecdotal evidence, during treatment it can be the case that the clicking sound of a disc is resolved, goes unchanged, or at times even worsens. This indicates that clinicians should advise patients of the various possible outcomes before attempting to manage the clicking sound. This permits the acquisition of informed consent prior to proceeding with therapeutic services. In the event that a TMJ disc clicking does not resolve, it is important to note that invasive interventions, such as surgery for the purpose of recapturing the disc, have been shown to have limited success [23]. Additionally, through understanding these points the clinician and patient are able to direct their primary clinical focus on more important variables such as pain and functional performance.
Investigations have demonstrated that there is no correlation between the presence of degenerative changes and disc position [24,25]. However, self-reported joint noises and locking have been shown to be a risk factor for the development of TM disorders [26]. Additionally, knowing that a disc is displacing anteriorly does permit the clinician to know that the retrodiscal tissue is potentially being moved into a load bearing role, which can also be informative for the clinical reasoning process. This may be important because the retrodiscal tissue is susceptible to inflammation and the formation of adhesions [27], though there is a general void of scientific studies on this tissue.
TMJ hypermobility
As it was stated previously, active range of motion (AROM) of the TMJ is highly variable [28–30]. For this reason, it is imperative to distinguish between those individuals that possess above average mouth opening range but whom maintain joint stability and those who, regardless of their overall range, experience instability of one or both TMJs. Stated differently, high levels of mobility do not necessarily correlate to instability [31]. Generally speaking, people with systemic hypermobility syndromes such as Ehlers-Danlos Syndrome or Marfan Syndrome [32,33] who score high on the Beighton Scale [34–36] may be at elevated risk for joint dislocations, including TMJ instability [37]. As a result, when instability of the TMJ is suspected, a thorough medical history must be reviewed.
Dislocation of the TMJ can be spontaneous, iatrogenic, or traumatic and has been associated with an array of factors including but not limited to trauma, yawning, and laughing as well as poor development of the articular fossa(s), excessive activity of the lateral pterygoid and infrahyoid muscles secondary to medication usage (e.g. phenothiazines and metoclopramide), hypertonicity associated with epilepsy, joint laxity, and conditions associated with hypermobility (e.g. Ehlers-Danlos syndrome, Marfan syndrome, and Duchenne muscular dystrophy) [37–40].
While this area of TM disorders rehabilitation has not been studied well, a variety of clinical recommendations can be made when a TMJ is susceptible to dislocation. These recommendations include: 1. Avoid precipitating factors (e.g. sports/trauma), 2. Avoid end range mouth opening, 3. Use a period of relative immobilization, and 4. Seek medical consultation. When instructing a patient to avoid end-range opening, helpful hints can include performing cervical flexion while yawning, blocking mouth opening with a clenched fist, and cutting food smaller during eating. Wearing a soft cervical collar in reverse can also assist in avoiding end range opening.
When a TMJ is dislocated, manual reduction of the joint with or without anesthesia should be performed by a sufficiently qualified professional [41]. Various forms of TMJ immobilization have been described including bandaging, head-chin caps, and maxillomandibular fixation using arch bars [42]. Prolotherapy has been utilized with this population, though confident conclusions cannot be drawn based on the currently available evidence [43]. Medically speaking, maxillomandibular fixation mechanisms are reserved for the complex cases that do not respond to more conservative management strategies [41]. Fixation has been shown to be effective in the long term for the management of these cases [44].
Oral splints
Oral splints can be considered as an adjunctive intervention to conservative care. In general, the literature does not refute, or overwhelmingly support, the use of oral splints [45–48]. Oral splints can be used to protect the dentition of people with sleep bruxism and may improve symptoms in this population [47,48]. Anecdotally, oral splints are indicated for patients reporting increased pain during sleep or upon waking. A stabilization appliance with even, simultaneous contact on either all of the mandibular or maxillary teeth can be worn during
sleep for patients with bruxism and myogenic and/or arthrogenic TM disorders (Figure 1). Patients with anterior disc displacement and pain generated from the retrodiscal tissues may benefit further from an anterior repositioning appliance (Figure 2), which guides the mandibular condyles back atop the articular discs, off-loading the retrodiscal tissues during sleep bruxing. When using an anterior positioning appliance, or an appliance that does not provide coverage to all teeth simultaneously, care must be taken to avoid changes in dental occlusion, such as an anterior or posterior open bite. Similarly, a patient using a mandibular advancement appliance for sleep apnea should be monitored for the development of a posterior open bite. Overall, understanding the role and indications for oral splints in the conservative management of TM disorders can assist the physical therapist and other conservative orthopedic providers when engaging in collaborative care with appropriately trained dental professionals to best meet the needs of the patient.
Figure 1.
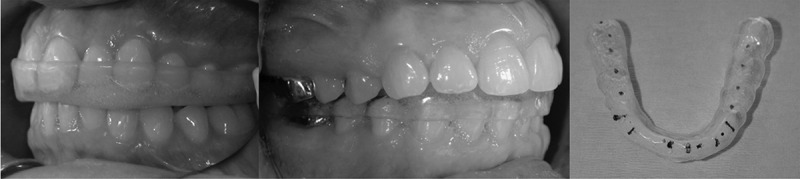
Mandibular and maxillary stabilization splints.
Description: A stabilization appliance is a full-coveragesplint that provides even, simultaneous tooth contacts on a flat-plane surface, directing force through the long axis of the teeth. The stabilization appliance also provides eccentric guidance, meaning that when the jaw moves intoexcursive motions (i.e. bruxing), the molars dis-occlude.
Figure 2.

Anterior positioning appliance.
Description: An anterior positioning appliance is fabricated similarly to the stabilization appliance, with the exception of the addition of an anterior ramp, which guides the mandible into a more protrusive position.
Clinical reasoning & management strategies
While clinical reasoning is not taught uniformly throughout physical therapy programs [49], attempts are being made to create a more universal understanding of the basic frameworks associated with this imperative process [50,51]. Furthermore, clinical reasoning has been discussed in the peer-reviewed scientific evidence as it relates to TM disorders [18]. The expert clinician should rely upon both general frameworks and content specific to TM disorders when providing services. In addition, presented here are a handful of a key topics that adjunct previously presented material.
Perhaps the most foundational clinical reasoning principle that can be made about TM disorders is that in many ways the TMJ is analogous to other anatomical regions. Based on this point, many of the same principles that apply to body regions elsewhere apply for the TMJ. Examples of this include but are not limited to: 1. Similarities of basic anatomical structures, 2. Regional interdependence, 3. Relevance of pain science variables, 4. Availability of multidisciplinary services, 5. Lack of consensus on evaluation and management strategies (within and between professions), and 6. Limitations of diagnostic imaging accuracy. More unique TMJ variables include but are not limited to: 1. Partially unique regional joint mechanics, 2. The intraarticular disc, 3. Bruxism, 4. Dentition considerations, 5. Salivary gland considerations, and 6. Contributions to headaches. Each of these topics can influence the clinical reasoning processes implemented by a clinician and, as a result, should be considered during evaluation and management processes.
Regarding anatomy from a clinical perspective, many of the same basic principles that are applicable elsewhere apply to the TMJ as well. These principles, for example, involve which basic anatomical structures and pathological processes occur in the painful joint complex. Relevant concepts include passive accessory joint restrictions, tendinopathy, myofascial trigger points, aberrant movement patterns, and joint instability. Prior published works can be sought for a review of basic TMJ anatomy [16,52].
In addition to what has been presented previously, it should be noted that the available studies pertaining to both the temporalis [53–58] and masseter tendons [59,60] are lacking. From a clinical reasoning standpoint, the critically thinking clinician must rely upon evidence and experience related to the management of other tendons. Furthermore, in the case of TM disorders it appears highly relevant to consider bruxism. Any patient, for example, that is clenching their teeth on a regular and/or consistent basis may already be applying undesirably high loads to the tendons. This could indicate that the application of additional load bearing activities via the prescription of therapeutic exercise will be counterproductive, though individual patients should be assessed for specific intervention appropriateness. Empirical evidence indicates that these tendons can benefit from examination via palpation and treatment via friction massage, though this is not a fully evidence informed approach.
Regional interdependence has been studied extensively in relation to TM disorders and several key factors can be described. TM disorders have been shown to be related to cervical spine dysfunction [61–68]. From a biomechanical perspective, head and cervical spine movements coordinate with mandibular movements during mouth opening and closing [69] and patients with TM disorders present with mechanical restrictions of the upper cervical spine [67]. Bearing this evidence in mind, it is not surprising that studies have shown cervical spine treatment techniques can assist in the alleviation of TM disorders symptoms [21,70–72].
Additionally, there is an interplay between TM disorders, the cervical spine, and various headache types [73–77]. For example, TM disorder symptoms are often concomitant with episodic tension-type headache, migraine headache, and chronic daily headache [74]. Cervical spine pathology has been linked to headache pain as well [78–83]. As a result of these findings, it appears obligatory to consider a wide array of TMJ, cervical spine, and headache-related variables when implementing a comprehensive evaluation and management strategy for people with TMJ, cervical spine, and headache complaints. Failure to do so may contribute to the implementation of a partial process that could lead to misdiagnosis and/or missed diagnoses.
Another regional interdependence consideration includes otological or ear-based symptoms. This category of potentially related clinical features includes symptoms such as otalgia, tinnitus, vertigo, and hearing loss [84]. In one investigation of 200 patients diagnosed with TM disorders, at least one of these four symptoms was reported by 78% of subjects with each of the symptoms experienced by 64%, 59%, 50%, and 36% of subjects, respectively [84].
Based on the established relationship between TM disorders and otological symptoms, each patient should be screened for concomitant otological concerns. Many such patients may have been examined by other providers previously for these symptoms. If not and when deemed appropriate, the patient should be referred to qualified clinicians for further examination. Additionally, otological symptoms can be followed over time to determine if they are changing throughout the administering of clinical services [85,86].
With respect to otological symptoms, only patients with vestibular complaints are likely to present to a rehabilitation clinic as a potential first line treatment option. For this reason, clinicians managing vestibular and TM disorders must be familiar with the association between vertigo and TMJ symptoms [84,87]. At times, it may be clinically important to consider collaboration with appropriately qualified vestibular professionals (e.g. rehabilitation and/or medical professionals) when a patient with TM disorder presents with vertigo and the clinician managing the TM disorder is not sufficiently qualified to examine the vestibular system and/or a rehabilitation examination of the vestibular system does not generate a rehabilitation diagnosis.
Of additional importance, there is a long history of the correlation between tinnitus and TM disorders being noted. Costen (1934) [88] was one of the early modern describers of TM disorders and tinnitus was part of the clinical patterns presented. Evidence suggests that there is a strong correlation between tinnitus and TM disorders [89,90]. Reports have varied with respect to how often patients with TM disorders experience tinnitus. Published data indicates that tinnitus could occur in 0% [91], 24% [92], 42% [93], or even up to 59% [84] of patients. However, at least one study indicates that approximately 80% of people experiencing tinnitus have signs of TM disorders [94]. This could insinuate that TM disorders are more prevalent in patients with tinnitus than tinnitus is in patients with TM disorders. While theories vary on the precise pathophysiological process unfolding during tinnitus in TM disorders patients [95–97], the precise origin(s) of this phenomenon remains unclear.
In general, there are no perfect clinical tests for determining if otological symptoms are generated by a TM disorder or not. As a result, the application of thorough subjective and objective examinations as well as the implementation of clinical interventions with subsequent follow up are required when deciding what the best clinical plan is. For example, when otological symptoms onset at the same time as other TM disorders symptoms, it implies that the two are related. If an otologic symptom is provoked or alleviated by TM disorders clinical testing then the implied relationship is yet further suggested. When an otologic symptom is alleviated by managing TM disorders variables then in hindsight it can be considered to be directly or partially associated with the TM disorder. Furthermore, clinicians should also bear in mind that otological concerns could be related not only to the ear and TMJ structures but also the cervical spine [98].
Of primary importance for TM disorders clinical reasoning is the variability in published approaches pertaining to evaluation and management strategies. Examples include textbooks [99,100], textbook chapters [101,102], and peer-reviewed scientific papers [15–17,103]. A recent Delphi study involving approximately two dozen leading global experts arrived at several conclusions pertaining to patient questionnaires, pain screening tools, and physical examination tests [104]. The preferred patient questionnaires included the Jaw Functional Limitation Scale (JFLS-8) [105], the Mandibular Function Impairment Questionnaire (MFIQ) [106], the Tampa Scale for Kinesiophobia for Temporomandibular disorders (TSK/TMD) [107], and the Neck Disability Index (NDI) [108]. The preferred pain screening tools included the Visual Analogue Scale (VAS) [109], the Numeric Pain Rating Scale (NPRS), and patient reported pain during mandibular movements. The preferred physical examination tests included physiological TMJ movements, myofascial trigger point (MTrP) palpation of the masticatory muscles, MTrP palpation away from the masticatory system, passive accessory movement testing, articular palpation, TMJ noise detection during movement, manual screening of the cervical spine, and the Neck Flexor Muscle Endurance Test [110,111]. Bearing in mind both the variability in approaches as well as the attempts to begin arriving at a consensus, the evaluating and managing clinician can pull from available expert opinions when attempting to devise a clinically effective and efficient plan.
Similar to other anatomical regions, diagnostic imaging has limitations when considering the TMJ. For example, degenerative findings seen on imaging often do not correlate with clinical findings [112–117]. This includes a lack of association between degenerative changes and TMJ disc position [24]. However, the presence of joint effusion seen on magnetic resonance imaging (MRI) can be indicative of pain [118]. The resulting conclusion should be that diagnostic imaging can play an important role in the evaluation and management of TM disorders but only when contextualized through the conclusions of a thorough examination and management process, which should include the implementation of clinical tools such as the Canadian C-spine Rule [119] when applicable.
Other diagnostic testing has also been found to have limited utility in the assessment of TM disorders. Electromyography (EMG) does not differentiate between patients with TM disorders and healthy controls [120–122]. Similarly, tests such as kinesiography and posturography have not been shown to be reliable or valid to guide clinical decision making in patients with TM disorders [123]. Dental occlusion has long been considered in the pathogenesis of TM disorders. A systematic review by Manfredini et al (2017) assessed the association between TM disorders and nearly 40 dental occlusal features in the 17 articles included. No consistent associations could be made and there was no evidence to support a causal relationship between occlusal features and the development of TM disorders; occlusal features could be a result of pain and structural changes related to TM disorders rather than the cause [124]. Therefore, while assessment of dental occlusion by a dental specialist can be warranted in patients with TM disorders, permanent modification to dental occlusion (e.g. orthodontics, occlusal carving/adjustments) is often not necessary to successfully manage these conditions.
The joint mechanics of the TMJ are similar to other joint complexes. During mouth opening a combination of mandibular condyle rotation and anterior translation occur [125–128]. When examining this kinematic motion physical therapist should keep two important details in mind. First, TMJ kinematics are often variable within and between subjects performing the same movement [129]. As a result, repeated palpation of the joint may be required during movement testing and it should not be surprising if two different palpation attempts result in different interpretations of joint mechanics. Second, mouth opening, which is used as a proxy measure for TMJ mechanics, is determined to a greater extent by mandibular condyle rotation than anterior translation [130,131]. It is therefore clinically useful to use the previously described variables to determine not only the quantity of interincisal gapping during mouth opening but also whether or not the quality of the movement involves aberrant components. For example, a joint restriction that prevents anterior translation of the mandibular condyle can be present and potentially result in a pathological presentation despite the patient maintaining overall mouth opening range. This can occur as a result of the rotational component generating a deceivingly normal mouth opening range even in the presence of a joint restriction that prohibits the mandibular condyle from translating anteriorly.
This type of restriction in movement constitutes a capsular pattern and can be noted by considering how each Range of Motion Testing variable described in Part 1 of this series may be affected. Possessing a detailed knowledge of passive accessory movement testing is crucial as well, which is partially covered in Part 1. Examples of findings that could indicate a capsular restriction include but are not limited to: 1. Reduced anterior translation of the mandibular condyle(s), 2. Restricted passive accessory joint motion of the TMJ(s), 3. Limited mouth opening range, 4. The perception of tightness or pain at/near the TMJ during active movements, 5. Improved range or ease of opening after joint mobilization techniques have been applied, 6. Improved mandibular condyle movement (e.g. anterior translation and/or accessory glides) after the application of joint mobilization, and 7. Improved oral function such as increased ease of eating large bites of food or less symptomatic yawning.
Salivary glands are relevant because of their potential impact on the differential diagnosis process. Two key diagnoses to consider include sialolithiasis and sialadenitis. Sialolithiasis involves the presence of stones (e.g. calcific stones similar to kidney stones) that can block the ducts of the salivary glands, thus leading to the retention of saliva that is accompanied by swelling and pain after a salivary stimulus is applied [132]. This diagnosis occurs most commonly in the submandibular glands [133] but is most relevant to a clinician working with TM disorders when the parotid gland is involved. The superior aspect of the parotid gland lies just superficial to the TMJ, which indicates that sialolithiasis can create pain and swelling over the TMJ during mastication. This could result in the false conclusion that the mechanical contributions of mastication are irritating musculoskeletal structures when in fact salivary stimulation is resulting in a glandular dysfunction. Palpation of the glands, questions pertaining to symptom provocation/alleviation, and the stimulation of salivation without mastication could each assist from a diagnostic perspective.
It has been reported in the medical literature that the incidence of parotid sialolithiasis is 4–28% and that approximately 90% of cases involve both swelling and pain [133]. When suspected, a conservative musculoskeletal providers should fully understand their jurisdiction’s scope of practice and likely refer to a medical provider for the management of this condition. However, in less severe cases some of the medical management strategies might be safely and legally implemented by physical therapists including gland massage, extracorporeal shock-wave therapy, and the usage of a sialogogue [133]. A sialogogue is an agent that stimulates salivation, which can be helpful when attempting to flush out the blockage. While prescription medications are available, sour candies can serve this function [133]. For more severe cases, additional medical management options involve prescription medications, irrigation, and endoscopy [133].
Sialadenitis involves a bacterial infection of a salivary gland [134]. Stasis is believed to be a primary contributing factor with risk factors including dehydration, decreased oral intake (i.e. less promotion of salivation), diabetes mellitus, hypothyroidism, renal failure, and Sjögren syndrome [134]. Similar to sialolithiasis, local pain and swelling near the joint (Figure 3) can contribute to clinical reasoning errors during the differential diagnosis process. Management strategies include massaging the gland to remove the infectious material, hydration, improved oral hygiene, promoting salivation, hot packs, antibiotics, and, in rare occasions, surgery [134]. An important, discerning clinical factor in sialadenitis when compared to both sialolithiasis and TM disorders is the presence of a foul taste in the oral cavity after manual palpation of the enlarged portion of the salivary gland. This experience should decrease if the infectious material is fully evacuated. As with cases of sialolithiasis, physical therapist and other similar providers should be aware of their practice limitations and refer to medical providers when appropriate.
Figure 3.

Lateral view of Sialadenitis.
Description: The arrow indicates a visible enlargement in the parotid gland just distal and anterior to the temporomandibular joint secondary to a mild case of sialadenitis.
Each of the variables presented here should be considered during the clinical reasoning process. By understanding, considering, and informing decisions on pertinent variables such as those described here, clinicians can attempt to ensure that they are delivering the most effective, efficient services possible, which holds the potential to benefit all stakeholders involved. Likewise, a failure to properly consider the most relevant variables holds the potential to ensure the delivery of inadequate services, which should be avoided whenever possible.
Conclusions
The evaluation of TM disorders is a multifaceted endeavor and various well-written sources exist to help inform educators, clinicians, and researchers on details related to this process [15–18]. As an update, this two-part series was written to introduce or reintroduce readers to the changing landscape of TM disorders content as it pertains to not only routine orthopedic examination components but also modifiable risk factors, pain science, the TMJ disc, TMJ hypermobility, oral splints, and clinical reasoning. By paying attention to these and other variables, physical therapists and other clinical professionals hold the potential to positively impact TM disorders. Lastly, while this process should be grounded in a conservative approach, an interdisciplinary treatment plan is often warranted.
Acknowledgement
The authors would like to thank Jeffrey P. Okeson, DMD from the University of Kentucky for supplying images related to oral appliances.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Al-Jundi MA, John MT, Setz JM, et al. Meta-Analysis of treatment need for temporomandibular disorders in adult nonpatients. J Orofac Pain. 2008;22(2):97–107. [PubMed] [Google Scholar]
- [2].NIDCR. National Institute of Health. National Institute of Dental and Craniofacial Research. Research . Data and statistics. Facial Pain. July 2018. cited May 19, 2021. Available from: https://www.nidcr.nih.gov/research/data-statistics/facial-pain.
- [3].Valesan LF, Da-Cas CD, Réus JC, et al. Prevalence of temporomandibular joint disorders: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(2):441–453. DOI: 10.1007/s00784-020-03710-w [DOI] [PubMed] [Google Scholar]
- [4].Keeling SD, McGorray S, Wheeler TT, et al. Risk factors associated with temporomandibular joint sounds in children 6 to 12 years of age. Am J Orthod Dentofacial Orthop. 1994;105(3):279–287. [DOI] [PubMed] [Google Scholar]
- [5].Allori AC, Chang CC, Fariña R, et al. Current concepts in pediatric temporomandibular joint disorders: part 1. Etiology, epidemiology, and classification. Plast Reconstr Surg. 2010;126(4):1263–1275. DOI: 10.1097/PRS.0b013e3181ebe207 [DOI] [PubMed] [Google Scholar]
- [6].Vierola A, Suominen AL, Ikavalko T, et al. Clinical signs of temporomandibular disorders and various pain conditions among children 6 to 8 years of age: the PANIC study. J Orofac Pain. 2012;26(1):17–25. [PubMed] [Google Scholar]
- [7].Lauriti L, Motta LJ, Silva PF, et al. Are occlusal characteristics, headache, parafunctional habits and clicking sounds associated with the signs and symptoms of temporomandibular disorder in adolescents? J Phys Ther Sci. 2013;25(10):1331–1334. DOI: 10.1589/jpts.25.1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sena MF, Mesquita KS, Santos FR, et al. Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paul Pediatr. 2013;31(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fernandes G, van Selms MK, Gonçalves DA, et al. Factors associated with temporomandibular disorders pain in adolescents. J Oral Rehabil. 2015;42(2):113–119. [DOI] [PubMed] [Google Scholar]
- [10].Slade G, Ohrbach R, Greenspan J, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016;95(10):1084–1092. DOI: 10.1177/0022034516653743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Østensjø V, Moen K, Storesund T, et al. Prevalence of painful temporomandibular disorders and correlation to lifestyle factors among adolescents in Norway. Pain Res Manag. 2017;2017:2164825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vierola A, Suominen AL, Eloranta AM, et al. Determinants for craniofacial pains in children 6-8 years of age: the PANIC study. Acta Odontol Scand. 2017;75(6):453–460. DOI: 10.1080/00016357.2017.1339908 [DOI] [PubMed] [Google Scholar]
- [13].Bitiniene D, Zamaliauskiene R, Kubilius R, et al. Quality of life in patients with temporomandibular disorders. A systematic review. Stomatologija. 2018;20(1):3–9. [PubMed] [Google Scholar]
- [14].Shaffer SM, Emerson AJ, Burr M, et al. Quality of life in painful temporomandibular disorders onset: a systematic review of outcome measure clinimetrics and predictive properties. Phys Ther Rev. 2021;26(4):284–298. [Google Scholar]
- [15].Harrison AL, Thorp JN, Ritzline PD.. A proposed diagnostic classification of patients with temporomandibular disorders: implications for physical therapists. J Orthop Sports Phys Ther. 2014;44(3):182–197. [DOI] [PubMed] [Google Scholar]
- [16].Shaffer SM, Brismée JM, Sizer PS, et al. Temporomandibular disorders. Part 1: anatomy and examination/diagnosis. J Man Manip Ther. 2014;22(1):2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shaffer SM, Brismée JM, Sizer PS, et al. Temporomandibular disorders. Part 2: conservative management. J Man Manip Ther. 2014;22(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fernández-de-Las-Peñas C, Von Piekartz H. Clinical reasoning for the examination and physical therapy treatment of temporomandibular disorders (TMD): a narrative literature review. J Clin Med. 2020;9(11):3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al-Baghdadi M, Durham J, Araujo-Soares V, et al. TMJ disc displacement without reduction management: a systematic review. J Dent Res. 2014;93(7 Suppl):37S–51S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tocaciu S, McCullough MJ, Dimitroulis G. Surgical management of recurrent TMJ dislocation-a systematic review. Oral Maxillofac Surg. 2019;23(1):35–45. [DOI] [PubMed] [Google Scholar]
- [21].La Touche R, Boo-Mallo T, Zarzosa-Rodríguez J, et al. Manual therapy and exercise in temporomandibular joint disc displacement without reduction. A systematic review. Cranio. 2020;40(5):440–450. [DOI] [PubMed] [Google Scholar]
- [22].Naeije M, Te Veldhuis AH, Te Veldhuis EC, et al. Disc displacement within the human temporomandibular joint: a systematic review of a ‘noisy annoyance’. J Oral Rehabil. 2013;40(2):139–158. [DOI] [PubMed] [Google Scholar]
- [23].Okeson JP. Joint intracapsular disorders: diagnostic and nonsurgical management considerations. Dent Clin North Am. 2007;51(1):85–103. [DOI] [PubMed] [Google Scholar]
- [24].Kondoh T, Westesson PL, Takahashi T, et al. Prevalence of morphological changes in the surfaces of the temporomandibular joint disc associated with internal derangement. J Oral Maxillofac Surg. 1998;56(3):339–343. [DOI] [PubMed] [Google Scholar]
- [25].Schiffman EL, Ahmad M, Hollender L, et al. Longitudinal stability of common TMJ structural disorders. J Dent Res. 2017;96(3):270–276. DOI: 10.1177/0022034516679396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ohrbach R, Bair E, Fillingim RB, et al. Clinical orofacial characteristics associated with risk of first-onset TMD: the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T33–50. DOI: 10.1016/j.jpain.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee SH, Yoon HJ. The relationship between MRI findings and the relative signal intensity of retrodiscal tissue in patients with temporomandibular joint disorders. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):113–115. [DOI] [PubMed] [Google Scholar]
- [28].Agerberg G. Maximal mandibular movements in children. Acta Odontol Scand. 1974;32(2):147–159. [DOI] [PubMed] [Google Scholar]
- [29].Agerberg G. Maximal mandibular movements in teen-agers. Acta Morphol Neerl Scand. 1974;12(2):79–102. [PubMed] [Google Scholar]
- [30].Agerberg G. Maximal mandibular movements in young men and women. Sven Tandlak Tidskr. 1974;67(2):81–100. [PubMed] [Google Scholar]
- [31].Rybalov OV, Yatsenko PI, Yatsenko OI, et al. Hypermobility of the articular heads of the temporomandibular joint: pathology or variant of the norm? Wiad Lek. 2019;72(10):1883–1889. DOI: 10.36740/WLek201910105 [DOI] [PubMed] [Google Scholar]
- [32].Malfait F, Wenstrup RJ, De Paepe A. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. 2010;12(10):597–605. [DOI] [PubMed] [Google Scholar]
- [33].Cattalini M, Khubchandani R, Cimaz R. When flexibility is not necessarily a virtue: a review of hypermobility syndromes and chronic or recurrent musculoskeletal pain in children. Pediatr Rheumatol Online J. 2015;13(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444–453. [PubMed] [Google Scholar]
- [35].Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32(5):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boyle KL, Witt P, Riegger-Krugh C. Intrarater and Interrater Reliability of the Beighton and Horan joint mobility index. J Athl Train. 2003;38(4):281–285. [PMC free article] [PubMed] [Google Scholar]
- [37].Papoutsis G, Papoutsi S, Klukowska-Rötzler J, et al. Temporomandibular joint dislocation: a retrospective study from a Swiss urban emergency department. Open Access Emerg Med. 2018;10:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].El Bouazzaoui A, Labib S, Derkaoui A, et al. Dislocation of temporo-mandibular joint - an uncommon circumstance of occurrence: vaginal delivery. Pan Afr Med J. 2010;5:23. [PMC free article] [PubMed] [Google Scholar]
- [39].Sailors ME. Evaluation of sports-related temporomandibular dysfunctions. J Athl Train. 1996;31(4):346–350. [PMC free article] [PubMed] [Google Scholar]
- [40].Liddell A, Perez DE. Temporomandibular joint dislocation. Oral Maxillofac Surg Clin North Am. 2015;27(1):125–136. [DOI] [PubMed] [Google Scholar]
- [41].Ruiz S, Lim R. Spontaneous temporomandibular joint dislocation. J Craniofac Surg. 2019;30(3):e265–e267. [DOI] [PubMed] [Google Scholar]
- [42].Jaisani MR, Pradhan L, Sagtani A. Use of cervical collar in temporomandibular dislocation. J Maxillofac Oral Surg. 2015;14(2):470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nagori SA, Jose A, Gopalakrishnan V, et al. The efficacy of dextrose prolotherapy over placebo for temporomandibular joint hypermobility: a systematic review and meta-analysis. J Oral Rehabil. 2018;45(12):998–1006. DOI: 10.1111/joor.12698 [DOI] [PubMed] [Google Scholar]
- [44].Sefidroodi M, Lobekk OK, Løes S, et al. Temporomandibular joint function 10-15 years after mandibular setback surgery and six weeks of intermaxillary fixation. J Appl Oral Sci. 2019;27:e20180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Forssell H, Kalso E. Application of principles of evidence-based medicine to occlusal treatment for temporomandibular disorders: are there lessons to be learned? J Orofac Pain. 2004;18(1): 9–22. discussion 23-32. [PubMed] [Google Scholar]
- [46].Al-Ani MZ, Davies SJ, Gray RJ, et al. Stabilisation splint therapy for temporomandibular pain dysfunction syndrome. Cochrane Database Syst Rev. 2004;1:CD002778. DOI: 10.1002/14651858.CD002778.pub2 [DOI] [PubMed] [Google Scholar]
- [47].Klasser GD, Greene CS. Oral appliances in the management of temporomandibular disorders. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod. 2009;107(2):212–223. [DOI] [PubMed] [Google Scholar]
- [48].Manfredini D, Bucci MB, Montagna F, et al. Temporomandibular disorders assessment: medicolegal considerations in the evidence-based era. J Oral Rehabil. 2011;38(2):101–119. [DOI] [PubMed] [Google Scholar]
- [49].Christensen N, Black L, Furze J, et al. Clinical reasoning: survey of teaching methods, integration, and assessment in entry-level physical therapist academic education. Phys Ther. 2017;97(2):175–186. DOI: 10.2522/ptj.20150320 [DOI] [PubMed] [Google Scholar]
- [50].Huhn K, Gilliland SJ, Black LL, et al. Clinical reasoning in physical therapy: a concept analysis. Phys Ther. 2019;99(4):440–456. [DOI] [PubMed] [Google Scholar]
- [51].Petersen EJ, Thurmond SM, Jensen GM. Severity, irritability, nature, stage, and stability (SINSS): a clinical perspective. J Man Manip Ther. 2021;29:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Alomar X, Medrano J, Cabratosa J, et al. Anatomy of the temporomandibular joint. Semin Ultrasound CT MR. 2007;28(3):170–183. DOI: 10.1053/j.sult.2007.02.002 [DOI] [PubMed] [Google Scholar]
- [53].Chierici G, Miller AJ. Experimental study of muscle reattachment following surgical detachment. J Oral Maxillofac Surg. 1984;42(8):485–490. [DOI] [PubMed] [Google Scholar]
- [54].Shankland WE 2nd. Temporal tendinitis: a modified Levandoski panoramic analysis of 21 cases. Cranio. 2011;29(3):204–210. [DOI] [PubMed] [Google Scholar]
- [55].Dupont JS Jr, Brown CE. The concurrency of temporal tendinitis with TMD. Cranio. 2012;30(2):131–135. [DOI] [PubMed] [Google Scholar]
- [56].Bressler HB, Friedman T, Friedman L. Ultrasound-Guided injection of the temporalis tendon: a novel technique. J Ultrasound Med. 2017;36(10):2125–2131. [DOI] [PubMed] [Google Scholar]
- [57].Duffin PS, Smith A, Hawkins JM. Nonodontogenic odontalgia referred from the temporal tendon: a case report. J Endod. 2020;46(10):1530–1534. [DOI] [PubMed] [Google Scholar]
- [58].Elsayed N, Shimo T, Harada F, et al. Masticatory muscle tendon-aponeurosis hyperplasia diagnosed as temporomandibular joint disorder: a case report and review of literature. Int J Surg Case Rep. 2021;78:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Friedman MH. Tenomyositis of the masseter muscle: report of cases. J Am Dent Assoc. 1985;110(2):201–202. [DOI] [PubMed] [Google Scholar]
- [60].DuPont JS Jr, Brown CE. Masseter tenomyositis. Cranio. 2009;27(3):180–184. [DOI] [PubMed] [Google Scholar]
- [61].de Wijer A, Steenks MH, de Leeuw JR, et al. Symptoms of the cervical spine in temporomandibular and cervical spine disorders. J Oral Rehabil. 1996;23(11):742–750. [DOI] [PubMed] [Google Scholar]
- [62].De Laat A, Meuleman H, Stevens A, et al. Correlation between cervical spine and temporomandibular disorders. Clin Oral Investig. 1998;2(2):54–57. [DOI] [PubMed] [Google Scholar]
- [63].Visscher CM, Lobbezoo F, de Boer W, et al. Prevalence of cervical spinal pain in craniomandibular pain patients. Eur J Oral Scii. 2001;109(2):76–80. [DOI] [PubMed] [Google Scholar]
- [64].Stiesch-Scholz M, Fink M, Tschernitschek H. Comorbidity of internal derangement of the temporomandibular joint and silent dysfunction of the cervical spine. J Oral Rehabil. 2003;30(4):386–391. [DOI] [PubMed] [Google Scholar]
- [65].Armijo Olivo S, Magee DJ, Parfitt M, et al. The association between the cervical spine, the stomatognathic system, and craniofacial pain: a critical review. J Orofac Pain. 2006;20(4):271–287. [PubMed] [Google Scholar]
- [66].Kraus S. Temporomandibular disorders, head and orofacial pain: cervical spine considerations. Dent Clin North Am. 2007;51(1):161–193. [DOI] [PubMed] [Google Scholar]
- [67].Grondin F, Hall T, Laurentjoye M, et al. Upper cervical range of motion is impaired in patients with temporomandibular disorders. Cranio. 2015;33(2):91–99. [DOI] [PubMed] [Google Scholar]
- [68].Flores HF, Ottone NE, Fuentes R. Analysis of the morphometric characteristics of the cervical spine and its association with the development of temporomandibular disorders. Cranio. 2016;4:1–7. [DOI] [PubMed] [Google Scholar]
- [69].Eriksson PO, Häggman-Henrikson B, Nordh E, et al. Co-Ordinated mandibular and head-neck movements during rhythmic jaw activities in man. J Dent Res. 2000;79(6):1378–1384. [DOI] [PubMed] [Google Scholar]
- [70].La Touche R, Fernández-de-Las-Peñas C, Fernández-Carnero J, et al. The effects of manual therapy and exercise directed at the cervical spine on pain and pressure pain sensitivity in patients with myofascial temporomandibular disorders. J Oral Rehabil. 2009;36(9):644–652. DOI: 10.1111/j.1365-2842.2009.01980.x [DOI] [PubMed] [Google Scholar]
- [71].Calixtre LB, Moreira RF, Franchini GH, et al. Manual therapy for the management of pain and limited range of motion in subjects with signs and symptoms of temporomandibular disorder: a systematic review of randomised controlled trials. J Oral Rehabil. 2015;42(11):847–861. [DOI] [PubMed] [Google Scholar]
- [72].Giacalone A, Febbi M, Magnifica F, et al. The effect of high velocity low amplitude cervical manipulations on the musculoskeletal system: literature review. Cureus. 2020;12(4):e7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bertoli FM, Antoniuk SA, Bruck I, et al. Evaluation of the signs and symptoms of temporomandibular disorders in children with headaches. Arq Neuropsiquiatr. 2007;65(2A):251–255. DOI: 10.1590/S0004-282X2007000200012 [DOI] [PubMed] [Google Scholar]
- [74].Gonçalves DA, Dal Fabbro AL, Campos JA, et al. Symptoms of temporomandibular disorders in the population: an epidemiological study. J Orofac Pain. 2010;24(3):270–278. [PubMed] [Google Scholar]
- [75].Anderson GC, John MT, Ohrbach R, et al. Influence of headache frequency on clinical signs and symptoms of TMD in subjects with temple headache and TMD pain. Pain. 2011;152(4):765–771. DOI: 10.1016/j.pain.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tomaz-Morais JF, Lucena LB, Mota IA, et al. Temporomandibular disorder is more prevalent among patients with primary headaches in a tertiary outpatient clinic. Arq Neuropsiquiatr. 2015;73(11):913–917. DOI: 10.1590/0004-282X20150145 [DOI] [PubMed] [Google Scholar]
- [77].Tchivileva IE, Ohrbach R, Fillingim RB, et al. Temporal change in headache and its contribution to the risk of developing first-onset temporomandibular disorder in the orofacial pain: prospective evaluation and risk assessment (OPPERA) study. Pain. 2017;158(1):120–129. DOI: 10.1097/j.pain.0000000000000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man Ther. 2006;11(2):118–129. [DOI] [PubMed] [Google Scholar]
- [79].Bevilaqua-Grossi D, Pegoretti KS, Goncalves MC, et al. Cervical mobility in women with migraine. Headache. 2009;49(5):726–731. DOI: 10.1111/j.1526-4610.2008.01233.x [DOI] [PubMed] [Google Scholar]
- [80].Florencio LL, de Oliveira AS, Carvalho GF, et al. Cervical muscle strength and muscle coactivation during isometric contractions in patients with migraine: a cross-sectional study. Headache. 2015;55(10):1312–1322. DOI: 10.1111/head.12644 [DOI] [PubMed] [Google Scholar]
- [81].Rubio-Ochoa J, Benítez-Martínez J, Lluch E, et al. Physical examination tests for screening and diagnosis of cervicogenic headache: a systematic review. Man Ther. 2016;21:35–40. [DOI] [PubMed] [Google Scholar]
- [82].Ferracini GN, Florencio LL, Dach F, et al. Musculoskeletal disorders of the upper cervical spine in women with episodic or chronic migraine. Eur J Phys Rehabil Med. 2017;53(3):342–350. DOI: 10.23736/S1973-9087.17.04393-3 [DOI] [PubMed] [Google Scholar]
- [83].Park SK, Yang DJ, Kim JH, et al. Analysis of mechanical properties of cervical muscles in patients with cervicogenic headache. J Phys Ther Sci. 2017;29(2):332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tuz HH, Onder EM, Kisnisci RS. Prevalence of otologic complaints in patients with temporomandibular disorder. Am J Orthod Dentofacial Orthop. 2003;123(6):620–623. [DOI] [PubMed] [Google Scholar]
- [85].Wright EF. Otologic symptom improvement through TMD therapy. Quintessence Int. 2007;38(9):e564–571. [PubMed] [Google Scholar]
- [86].de Felício CM, Melchior Mde O, Ferreira CL, et al. Otologic symptoms of temporomandibular disorder and effect of orofacial myofunctional therapy. Cranio. 2008;26(2):118–125. [DOI] [PubMed] [Google Scholar]
- [87].Guimarães TOC, Oliveira AJ, Araújo RR, et al. The influence of degrees of severity of temporomandibular dysfunction on report of vestibular symptoms - a cross-sectional study. J Man Manip Ther. 2022;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Costen JB. A syndrome of ear and sinus symptoms dependent upon disturbed function of the temporomandibular joint. 1934. Ann Otol Rhinol Laryngol. 1997;106:805–819. [DOI] [PubMed] [Google Scholar]
- [89].Buergers R, Kleinjung T, Behr M, et al. Is there a link between tinnitus and temporomandibular disorders? J Prosthet Dent. 2014;111(3):222–227. [DOI] [PubMed] [Google Scholar]
- [90].Omidvar S, Jafari Z. Association between tinnitus and temporomandibular disorders: a systematic review and meta-analysis. Ann Otol Rhinol Laryngol. 2019;128(7):662–675. [DOI] [PubMed] [Google Scholar]
- [91].Ciancaglini R, Loreti P, Radaelli G. Ear, nose and throat symptoms in patients with TMD: the association of symptoms according to severity of arthropathy. J Orofacial Pain. 1994;8:293–297. [PubMed] [Google Scholar]
- [92].Sheppard IM, Sheppard SM. Characteristics of temporomandibular joint problems. J Prosthet Dent. 1977;38(2):180–191. [DOI] [PubMed] [Google Scholar]
- [93].Atkinson TA, Vossler S, Hart DL. The evaluation of facial, head, neck, and temporomandibular joint pain patients. J Orthop Sports Phys Ther. 1982;3:193–199. [DOI] [PubMed] [Google Scholar]
- [94].Tullberg M, Ernberg M. Long-Term effect on tinnitus by treatment of temporomandibular disorders: a two-year follow-up by questionnaire. Acta Odontol Scand. 2006;64(2):89–96. [DOI] [PubMed] [Google Scholar]
- [95].Myrhaug H. The incidence of ear symptoms in cases of malocclusion and temporo-mandibular joint disturbances. Br J Oral Surg. 1964;2(1):28–32. [DOI] [PubMed] [Google Scholar]
- [96].Salvinelli F, Casale M, Paparo F, et al. Subjective tinnitus, temporomandibular joint dysfunction, and serotonin modulation of neural plasticity: causal or casual triad? Med Hypotheses. 2003. Oct;61(4):446–448. [DOI] [PubMed] [Google Scholar]
- [97].Ralli M, Greco A, Turchetta R, et al. Somatosensory tinnitus: current evidence and future perspectives. J Int Med Res. 2017;45(3):933–947. DOI: 10.1177/0300060517707673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jaber JJ, Leonetti JP, Lawrason AE, et al. Cervical spine causes for referred otalgia. Otolaryngol Head Neck Surg. 2008;138(4):479–485. [DOI] [PubMed] [Google Scholar]
- [99].Fernández-de-Las-Peñas C, Mesa-Jiménez J, editors. Temporomandibular Disorders: manual therapy, exercise and needling. Pencaitland, East Lothian, Scotland: Handspring Publishing; 2018. [Google Scholar]
- [100].Okeson JP. Management of temporomandibular disorders and occlusion. 8th ed. St. Louis, Missouri: Elsevier; 2019. [Google Scholar]
- [101].Wise CH. Orthopaedic manual physical therapy: from art to evidence. Philadelphia, Pennsylvania: FA Davis Company; 2015. [Google Scholar]
- [102].Magee DJ, Manske RC. Orthopedic physical assessment. 7th ed. St. Louis, Missouri: Elsevier; 2021. [Google Scholar]
- [103].Wright EF, North SL. Management and treatment of temporomandibular disorders: a clinical perspective. J Man Manip Ther. 2009;17(4):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].von Piekartz H, Schwiddessen J, Reineke L, et al. International consensus on the most useful assessments used by physical therapists to evaluate patients with temporomandibular disorders: a Delphi study. J Oral Rehabil. 2020;47(6):685–702. DOI: 10.1111/joor.12959 [DOI] [PubMed] [Google Scholar]
- [105].Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. 2008;22(3):219–230. [PubMed] [Google Scholar]
- [106].Stegenga B, de Bont LG, Boering G. Temporomandibular joint pain assessment. J Orofac Pain. 1993;7(1):23–37. [PubMed] [Google Scholar]
- [107].Visscher CM, Ohrbach R, van Wijk AJ, et al. The Tampa Scale for Kinesiophobia for Temporomandibular disorders (TSK-TMD). Pain. 2010;150(3):492–500. [DOI] [PubMed] [Google Scholar]
- [108].Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7): 409–415. Erratum in: J Manipulative Physiol Ther. 1992;15(1):followi. [PubMed] [Google Scholar]
- [109].Hayes MHS, Patterson DG. Experimental development of the graphic rating method. Psychol Bull. 1921;18:98–99. [Google Scholar]
- [110].Grimmer K. Measuring the endurance capacity of the cervical short flexor muscle group. Aust J Physiother. 1994;40(4):251–254. [DOI] [PubMed] [Google Scholar]
- [111].Harris KD, Heer DM, Roy TC, et al. Reliability of a measurement of neck flexor muscle endurance. Phys Ther. 2005;85(12):1349–1355. DOI: 10.1093/ptj/85.12.1349 [DOI] [PubMed] [Google Scholar]
- [112].Nordahl S, Alstergren P, Appelgren A, et al. Pain, tenderness, mandibular mobility, and anterior open bite in relation to radiographic erosions in temporomandibular joint disease. Acta Odontol Scand. 1997;55(1):18–22. DOI: 10.3109/00016359709091935 [DOI] [PubMed] [Google Scholar]
- [113].Al-Ekrish AA, Al-Juhani HO, Alhaidari RI, et al. Comparative study of the prevalence of temporomandibular joint osteoarthritic changes in cone beam computed tomograms of patients with or without temporomandibular disorder. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(1):78–85. [DOI] [PubMed] [Google Scholar]
- [114].Cömert Kiliç S, Kiliç N, Sümbüllü MA. Temporomandibular joint osteoarthritis: cone beam computed tomography findings, clinical features, and correlations. Int J Oral Maxillofac Surg. 2015;44(10):1268. [DOI] [PubMed] [Google Scholar]
- [115].Türp JC, Schlenker A, Schröder J, et al. Disk displacement, eccentric condylar position, osteoarthrosis - misnomers for variations of normality? Results and interpretations from an MRI study in two age cohorts. BMC Oral Health. 2016;16(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bae S, Park MS, Han JW, et al. Correlation between pain and degenerative bony changes on cone-beam computed tomography images of temporomandibular joints. Maxillofac Plast Reconstr Surg. 2017;39(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Arayasantiparb R, Mitrirattanakul S, Kunasarapun P, et al. Association of radiographic and clinical findings in patients with temporomandibular joints osseous alteration. Clin Oral Investig. 2020;24(1):221–227. DOI: 10.1007/s00784-019-02945-6 [DOI] [PubMed] [Google Scholar]
- [118].Park HN, Kim KA, Koh KJ. Relationship between pain and effusion on magnetic resonance imaging in temporomandibular disorder patients. Imaging Sci Dent. 2014;44(4):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. Jama. 2001;286(15):1841–1848. DOI: 10.1001/jama.286.15.1841 [DOI] [PubMed] [Google Scholar]
- [120].Lund JP, Widmer CG. An evaluation of the use of surface electromyography in the diagnosis, documentation, and treatment of dental patients. J Craniomandib Disord. 1989;3(3):125–137. [PubMed] [Google Scholar]
- [121].Carlson CR, Okeson JP, Falace DA, et al. Comparison of psychologic and physiologic functioning between patients with masticatory muscle pain and matched controls. J Orofac Pain. 1993;7(1):15–22. [PubMed] [Google Scholar]
- [122].Klasser GD, Okeson JP. The clinical usefulness of surface electromyography in the diagnosis and treatment of temporomandibular disorders. J Am Dent Assoc. 2006;137(6):763–771. [DOI] [PubMed] [Google Scholar]
- [123].Manfredini D, Castroflorio T, Perinetti G, et al. Dental occlusion, body posture and temporomandibular disorders: where we are now and where we are heading for. J Oral Rehabil. 2012;39(6):463–471. [DOI] [PubMed] [Google Scholar]
- [124].Manfredini D, Lombardo L, Siciliani G. Temporomandibular disorders and dental occlusion. A systematic review of association studies: end of an era? J Oral Rehabil. 2017;44(11):908–923. [DOI] [PubMed] [Google Scholar]
- [125].Smith RJ. Functions of condylar translation in human mandibular movement. Am J Orthod. 1985;88(3):191–202. [DOI] [PubMed] [Google Scholar]
- [126].Rayne J. Functional anatomy of the temporomandibular joint. Br J Oral Maxillofac Surg. 1987;25(2):92–99. [DOI] [PubMed] [Google Scholar]
- [127].Merlini L, Palla S. The relationship between condylar rotation and anterior translation in healthy and clicking temporomandibular joints. Schweiz Monatsschr Zahnmed. 1988;98(11):1191–1199. [PubMed] [Google Scholar]
- [128].Gallo LM, Airoldi GB, Airoldi RL, et al. Description of mandibular finite helical axis pathways in asymptomatic subjects. J Dent Res. 1997;76(2):704–713. [DOI] [PubMed] [Google Scholar]
- [129].Lindauer SJ, Sabol G, Isaacson RJ, et al. Condylar movement and mandibular rotation during jaw opening. Am J Orthod Dentofacial Orthop. 1995;107(6):573–577. [DOI] [PubMed] [Google Scholar]
- [130].Travers KH, Buschang PH, Hayasaki H, et al. Associations between incisor and mandibular condylar movements during maximum mouth opening in humans. Arch Oral Biol. 2000;45(4):267–275. [DOI] [PubMed] [Google Scholar]
- [131].Ferrario VF, Sforza C, Lovecchio N, et al. Quantification of translational and gliding components in human temporomandibular joint during mouth opening. Arch Oral Biol. 2005;50(5):507–515. [DOI] [PubMed] [Google Scholar]
- [132].Delli K, Spijkervet FK, Vissink A. Salivary gland diseases: infections, sialolithiasis and mucoceles. Monogr Oral Sci. 2014;24:135–148. [DOI] [PubMed] [Google Scholar]
- [133].Kraaij S, Karagozoglu KH, Forouzanfar T, et al. Salivary stones: symptoms, aetiology, biochemical composition and treatment. Br Dent J. 2014;217(11):E23. [DOI] [PubMed] [Google Scholar]
- [134].Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882–888. [PubMed] [Google Scholar]


