ABSTRACT
Temporomandibular (TM) disorders afflict many people globally and, despite the presence of existing peer-reviewed material that assists conservative orthopedic providers, recent advances in knowledge indicate that updated resources are required for students, clinicians, and educators. This two-part series builds off previously published material to present newer supplementary information that can be useful during the evaluation and management processes. Content in Part 1 of this series includes a discussion about the factors that have been shown to contribute to TM disorders, an updated perspective of relevant pain science, a discussion of self-report outcome measures, and various different topics related to the examination of patients with TM disorders. Part 2 addresses information related to the temporomandibular joint disc, joint hypermobility, oral splints, and clinical reasoning. In combination with other available publications, this two-part series provides clinicians an opportunity to improve their delivery of effective and efficient clinical services for people diagnosed with TM disorders.
KEYWORDS: Temporomandibular, disorders, physical therapy, orthopedics, physical examination, clinical reasoning
Introduction
Temporomandibular (TM) disorders involve a heterogeneous group of painful neuromusculoskeletal conditions associated with the masticatory system and related tissues. The prevalence of TM disorders has been estimated to be between 5 and 31% [1–3] with the age of onset ranging widely including birth to adult onset [4–12]. Of paramount clinical importance, TM disorders are known to negatively impact the quality of life [13,14].
Collectively, these points indicate a need for effective and efficient clinical services for TM disorders. While a rehabilitation-based clinical practice guideline is not currently available, previous peer-reviewed guidance is available for orthopedic physical therapists and other conservative clinicians seeking to learn about how to clinically evaluate and manage TM disorders [15–17]. However, while these sources are still worthwhile of attention, updates are warranted based on the perpetually advancing peer-reviewed science. As a result, the purpose of this two-part series is to provide orthopedic physical therapists and other providers with a supplementary framework that is instructive for the conservative examination and management of TM disorders.
Contributing factors
There are many potential contributing factors to TM disorders. Among the most important risk factors are the presence of greater numbers of comorbid conditions (e.g. fibromyalgia, lower back pain, depression, sleep apnea, etc.) and the presence of nonspecific orofacial symptoms (e.g. jaw stiffness and fatigue) [12,18]. Other contributing factors include but are not limited to oral behaviors, somatic symptom reporting (e.g. running nose, fatigue, and dizziness to a greater extent than psychological stress, anxiety, obsessive-compulsive feelings, and pain-coping strategies), and sleep dysfunction [12,18]. Additionally, an estimated 54% of females and 41% of males with TM disorders are likely to experience persistent pain 6 months after initial onset [12]. As a result, physical health, self-reported oral parafunction, frequent physical symptoms, and sleep disturbances are all important, modifiable risk factors in the management of TM disorders. Each of these factors should be considered during both the examination and management of patients seeking conservative services for acute and chronic TM disorders.
As appropriate to setting and scope of practice, practitioners can consider guiding patients through behavior change approaches to address these factors or develop interdisciplinary management teams to provide a multifaceted approach to care for patients with TM disorders. Furthermore, recognizing the complexity of the presentation allows the clinician to better facilitate the patient’s understanding of their condition truly as a biopsychosocial experience. By not placing blame on a single factor, the patient can better recognize that management of their condition may require modifications beyond musculoskeletal impairments.
TM disorders pain science
It is imperative to appreciate that patients afflicted with TM disorders frequently experience nociplastic pain. These aberrant changes typically involve both hyperalgesia and allodynia. While a full review of this topic is beyond the scope of this manuscript, several major points can be made. First, pain referral patterns associated with various temporomandibular joint (TMJ) structures are known to overlap to a great extent [19–21], which means that the use of pain location is of uncertain utility from a diagnostic standpoint. For example, this phenomenon includes overlapping pain referral patterns for TM disorders and myocardial infarction [22–24]. Therefore, care must be taken to ensure correct differential diagnosis by the clinician.
Second, patients with TM disorders demonstrate larger myofascial trigger point (MTrP) referred pain zones compared to controls [25]. Additionally, pain sensitivity is higher in those with TM disorders when compared to age- and sex-controlled matches, which even involves changes in forearm thermal pain threshold and tolerance values [26,27] as well as mechanical and thermal hypersensitivity in the face, neck, and anterior lower leg [28–30]. In summation, it is apparent that changes in the processing of nociception within the nervous system contribute to the pain experience in patients with TM disorders.
One potential reason for the level of nociplastic changes associated with TM disorders could be the proximity of the involved structures to the trigeminocervical complex. The trigeminocervical complex is an anatomically unique region of the brainstem and spinal cord in which there is a convergence of afferent input of the trigeminal nerve (cranial nerve V) and the spinal nerves of C1-C4 [31–33]. Thus, input from structures associated with the TMJ and cervical spine as well as the dura mater project to similar second-order ascending neurons. Sensitization at this synapse from nociceptive input from one or more of these areas may help explain the well-documented associations between neck pain and head/facial pain [34–36].
Understanding these centrally mediated pain processing variables can assist clinicians when preparing to evaluate and manage patients with TM disorders. For example, possessing sufficient pain science knowledge grants clinicians an opportunity to identify which patients may or may not have a lower tolerance to examination techniques prior to physically contacting the patient. The result can be the application of a more informed dosage of both examination and management techniques, thus improving the quality and accuracy of services. It also affords the clinician an opportunity to educate the patient on why they may be experiencing seemingly exaggerated symptoms such as hyperalgesia and allodynia. And, finally, an expert understanding of pain science variables can bring awareness to the behavior of TM disorders and their symptoms from a pain science perspective, which can ensure that the interdisciplinary team, often required for the management of TM disorders, is considering all factors of a complex presentation when developing and implementing the plan of care.
Examination overview
Physical therapy services have been recommended for the conservative management of TM disorders [15,17,37,38]. Given the wide array of risk factors pertaining to TM disorders, thorough intake paperwork and subjective examination should be implemented prior to the physical examination. In isolation, no single clinical finding is sufficiently reliable, sensitive, or specific to classify TM disorders [39–44]. Bearing this in mind, a wide array of factors should be considered throughout the examination process, which must occur on an ongoing basis to ensure the delivery of effective and efficient services over time.
Self-report outcome measures
Self-Report Outcome Measures (SROMs) can be relied upon to help quantify related variables. With respect to TM disorders, the Oral Health Impact Profile-Temporomandibular Disorders (OHIP-TMD) [45], the 8- and 20-item versions of the Jaw Functional Limitation Scale (JFLS-8 and JFLS-20) [46], and the Mandibular Functional Impairment Questionnaire (MFIQ) [47] have each been reported to be internally consistent and reliable for this population [48]. The Pittsburgh Sleep Quality Index (PSQI) [49] has been shown to be reliable in the TM disorders population [50]. For region-specific quality of life (QoL), the OHIP-49 [51], OHIP-TMD/-22 [45], and OHIP-14 [52] have each been shown to be clinimetrically sound for TM disorders populations [14]. For investigating general QoL, the 36-item Short Form Health Survey (SF-36) [53] has been identified to be valid for TM disorders [14].
Subjective examination
Routine subjective examination must be completed. As with other anatomical regions, each patient should be screened for the presence of red flags [54,55]. Potential regional red flags are extensive [54–58] and beyond the scope of this paper. All of the traditional variables should be considered for each patient including but not limited to: 1. Symptom location, 2. Pain intensity, 3. Symptom type, 4. Symptom behavior, and 5. Related areas of involvement [16]. For assistance with this process, relying upon established frameworks for symptom Severity, Irritability, Nature, Stage, and Stability (SINSS) [59] can be helpful. Body diagrams can also assist when attempting to accurately identify the patient’s clinical presentation [60].
Specific to TM disorders, it can be important to ascertain the impact, if any, of corrective equipment used to alter the patient’s occlusal pattern, trauma to the face, head, and/or cervical spine, parafunctional habits, joint noises, and joint noises progression over time [16]. Some patients may also be impacted by dietary variables such as food consistency and chewing laterality [17]. While many of these patients appear to get better with conservative management without diet modification, some patients may require being switched to a soft diet during the initial states of rehabilitation and may have done so independently prior to seeking clinical services. When commencing this type of modification for management purposes, each patient should be closely monitored for potential impacts on TM disorders symptoms.
Observation
Observation can be useful when examining a patient with suspected TM disorders and should include but is not limited to mandible symmetry, chin deviations during mouth opening, temporalis and masseter muscle mass, anterior translation of the mandibular condyles, the size of the retrodiscal spaces, and oral anatomy. Regarding mandibular symmetry, two key variables to be observed include hemimandibular hyperplasia and hemimandibular elongation. These asymmetries involve enlargement of the mandibular ramus and body, respectively [61]. The pathophysiology of this process is partially understood and various classification systems exist [62,63]. While less severe cases of asymmetry pose little clinical significance beyond potentially minor interferences with movement quality, severe cases may require referral to an orthodontist and/or surgeon secondary to cosmetic concerns and/or alterations in occlusal patterns [64].
The precise diagnostic significance of chin deviations with mouth opening has rarely been studied. However, Table 1 provides a classification system that clinicians may find to be reasonably accurate and clinically useful. A recent movement analysis study noted deviations (i.e. a right or left movement of the chin during opening that returns to midline), deflections (i.e. a right or left movement of the chin during opening that does not return to midline), and limited range (i.e. insufficient movement of the mandible away from the maxilla during opening) to be three movement abnormalities associated with mouth opening [65]. Additionally, it has been noted that movement abnormalities of the mandibular condyles as detected by computer-assisted measurements correlate with clinical signs of TMJ dysfunction and are associated with a 28-times greater relative risk of dysfunction associated with TMJ structures [66]. Of note, condylar movement can also be palpated by clinicians without the assistance of computerized instruments [17]. Observation of hypertrophy and/or atrophy of the muscles of mastication is sometimes subtle [67] but can be very obvious in some cases [68]. Noting the relative size of the retrodiscal space upon full mouth opening can be useful, though variability in facial anthropometric details can render this form of observation ineffective with some patients. Regardless, if an asymmetry is observed, then the presence of a smaller retrodiscal space should indicate ipsilateral joint hypomobility with probable decreased anterior translation of the mandibular condyle.
Table 1.
Chin deviations during mouth opening.
| Chin Movement | Potential Mandibular Condyle Arthrokinematics | Potential Diagnostic Implications |
|---|---|---|
| Deflects in one direction and stays there | The condyle ipsilateral to the chin deflection moves forward less | Joint hypomobility and/or disc displacement without reduction and/or adaptive shortening of the musculature on the side the chin deviates toward |
| Stays in midline with limited opening | Both mandibular condyles are not translating anteriorly | Bilateral joint hypomobility, and/or muscle guarding, and/or bilateral disc displacement without reduction |
| Deviates to one side and returns to midline | One condyle moves further forward first and then the other catches up | Lack of coordination unilaterally or bilaterally, possible joint hypomobility, possible disc displacement with reduction |
| Deviates in one direction and then to the other | One condyle moves further forward first and then the other does the same | Lack of coordination unilaterally or bilaterally, possible joint hypomobility, possible disc displacement with reduction |
| Small oscillations back and forth | Small/alternating forward movements of the condyles | Lack of coordination unilaterally or bilaterally, possible joint hypomobility |
Safety testing
Safety testing in patients with TM disorders can include both mandibular and cervical spine testing. While beyond the scope of this paper, any patient that experiences cervical spine or head trauma may require examination via the alar ligament stress test, transverse ligament stress test, and/or the Canadian C-spine Rule [69]. More specific to the TMJ, the tongue blade test can assist with determining if the mandible has sustained a fracture [70]. While described in various ways, the test generally involves the patient first unilaterally stabilizing a tongue depressor between their teeth and the examiner/patient then attempting to twist/break [70] (Figure 1) or pull to remove [71] the tongue depressor. The test should first be performed on the uninvolved side and, if negative, it can then be duplicated on the involved side. A bilateral negative test occurs when the tongue depressor breaks or is not removed on each side, which indicates that the bite force is not limited by acute pain. Negative tests indicate that diagnostic imaging is not required. If the patient cannot generate sufficient bite force to permit the tongue depressor to be broken, or, it is removed from between the teeth, then the patient should be examined radiographically. This test has been shown to have a sensitivity of 89–95% and a specificity of 65–95% [70–74].
Figure 1.

Tongue blade test with twisting of the tongue depressor.
Description:The patient self-twists the tongue depressor in order to permit the test to stop instantaneously if pain levels are too high.
Range of motion testing
Mouth opening is the most useful and informative active range of motion (AROM) measurement. While opening is commonly defined by the distance between the upper and lower incisors, which is an indirect measurement of joint mobility, an array of different variables can be used when considering the combined quantity and quality of mouth opening. These variables include but are not limited to: 1. Observations of the patient’s willingness to generate movement, 2. Chin movement during opening, 3. Observation and palpation of mandibular condyle anterior translation, 4. Observation (Figure 2) and palpation of the size of the retrodiscal spaces at end range opening, 5. Palpation for relative exposure of the coronoid processes at end range opening, and 6. The patient’s symptom experience during mouth opening (e.g. discomfort, tightness, pain, joint sounds, and/or apprehension). Each of these variables is directly related to mouth opening range of motion and should be taken into consideration.
Figure 2.

Lateral view of retrodiscal space at mouth closed (left) and end range mouth opening (Right).
Description:The arrow in the left image points toward a relative fullness because the mandibular condyle is positioned posteriorly with the mouth closed. The arrow in the right image points to a visible retrodiscal space because the mandibular condyle has translated anteriorly during mouth opening.
Two appropriate instruments for quantifying mouth opening range are a boley gauge caliper and a single use range of motion scale [16]. When selecting a boley gauge caliper for clinical use it is helpful to ensure that it is made of stainless steel, that the sliding mechanism moves smoothly, and that each of the two protrusions that will contact the incisors has a notch to help the examiner stabilize the instrument against the teeth. Similarly, a disposable measurement tool should have one notch that can be stabilized on the lower incisors during AROM measurements. Metal instruments should be sanitized according to jurisdictional sanitization requirements. However, despite being recommended elsewhere [16], plastic instruments may be inappropriate for clinical practice due to the risk of contact with a mucous membrane in combination with an inability to properly disinfect the material. Each clinician should take care to understand and follow required sanitization procedures in their jurisdiction.
Less than or equal to 40 millimeters (mm) of mouth opening is often relied upon as a clinical cutoff point for determining the presence of TMJ hypomobility as measured by interincisal gapping during mouth opening [75,76]. However, within physical therapy practice this cutoff point is insufficiently accurate. Normal opening range has been reported to vary widely, including as high as 73–77 mm [77–79] (Table 2). Furthermore, the averages listed in Table 2, when compared to the identified ranges, indicate that more individuals trend toward the lower portion of the normal range while a subset of people have a higher-than-average baseline range. Taking this normal variability into account is vital to providing individualized care for each patient. For example, a patient measuring 40 mm of mouth opening may at their baseline have 60 mm of mouth opening and thus a measurement of 40 mm is demonstrating hypomobility rather than normal range.
Table 2.
Average normal mouth opening movement.
| Age in Years | 6 | 12-14 | 18–25 (female) | 18-25 (male) |
|---|---|---|---|---|
| Average Opening (SD) and Range in mm | 44.8 (± 4.3) Range 33-60 |
53.9 (± 5.9) Range 41-73 |
51.0 (± 5.7) Range 39-75 |
55.5 (± 7.1) Range 42-77 |
Key: mm = millimeters, SD = standard deviation.
Data from: Agerberg 1974a, Agerberg 1974b, Agerberg 1974c.
While movements such as lateral deviation and protrusion can be measured [16], the clinical utility of this process may be in doubt. One difficulty is that recommended measurement strategies are variable, which includes techniques relying upon the frenula or teeth [16,80], the former of which is difficulty to rely upon based on tissue location and the latter of which can both move over time and/or be substantially different person to person (e.g. it can be difficult to account for variables such as measurement error within an already small range, an overbite, an underbite, asymmetry, missing/damaged teeth, or changes over time due to orthodontic work). Likewise, passive range of motion testing and the application of overpressure may have limited utility. Complicating variables such as simultaneously moving two joint complexes as well as the potential difficulty of the patient relaxing while their mandible if handled can hinder testing. For those clinicians who find these procedures to be difficult to implement, using extraoral passive accessory joint testing at the joint line may be a sufficient alternative (e.g. see undefined Figures 3 and 4). For these reasons, the measurement of active mouth opening can be relied upon as the primary range of motion value. However, visual inspection of movements such as lateral deviation and protrusion can be informative when categorizing each as either equal bilaterally vs. hyper-/hypomobile relative to the contralateral side and/or asymptomatic vs. symptomatic. Measurement of protrusion can be indicated for some professionals, for example, when a dentist is determining if a sleep apnea appliance is appropriate [81].
Figure 3.

Temporomandibular joint medial glide with fingertip contact just distal to the joint line.
Description:The right hand serves as a mobilization contact point and the left as a stabilization contact. Note that the contact points are lateral to the mandibular condyles.
Figure 4.
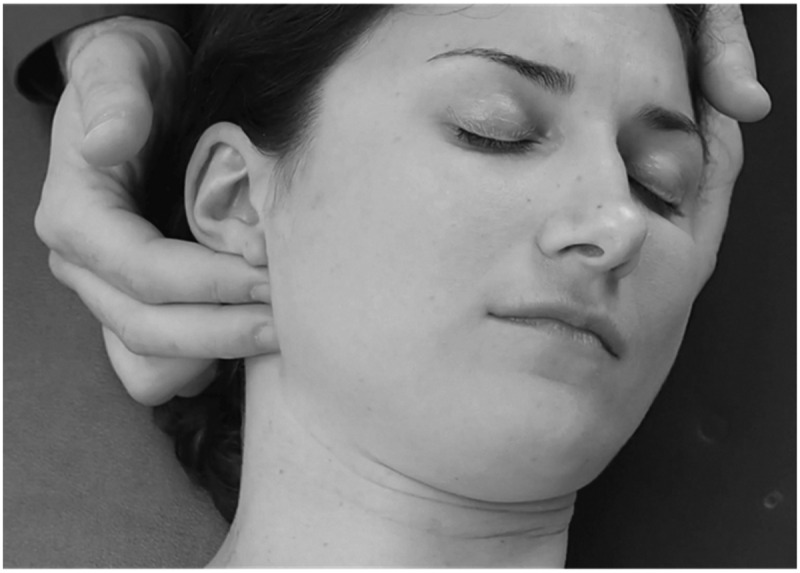
Temporomandibular joint anterior glide with fingertip contact just distal to the joint line.
Description:The right hand serves as a mobilization contact point and the left as a stabilization contact. Note that the contact points are posterior to the mandibular condyles.
Physical testing – “Top-Down Approach”
The examination approach described here will present a “Top-Down Approach” to physical testing. It has been named as such because the majority of physical testing can be conducted with the patient lying supine with the testing beginning cranially and progressing toward more distal structures. The list of structures/movements to be tested include: 1. The temporalis muscles, 2. The proximal-posterior temporalis tendons, 3. Anterior translation of the mandibular condyles, 4. The retrodiscal spaces, 5. The retrodiscal tissues, 6. Passive accessory joint mobility (medial glides, anterior glides, caudal-anterior-medial or CAM glides, and combined glides in any direction), 7. The masseter tendons, 8. The masseter muscles, and 9. The coronoid processes, which serve as the insertion point of the temporalis tendons. Testing these structures in the order they are presented in can assist in ensuring that a thorough physical examination is conducted on each patient. Of note, additional structures may require examination and management strategies if addressing this list of baseline structures does not suffice.
Accessible muscles of mastication should be palpated for irregularities such as MTrPs and taut bands. The temporalis tendons can be palpated in two locations. First, the proximal-posterior temporalis tendons, which are located just cranial and anterior to the cranial aspect of the ear as it connects to the head, demonstrate a tendency to develop palpable irregularities. These irregularities may present as painful enlargements, the palpation of which may reproduce the patient’s symptoms. The second accessible location for the temporalis tendon is at its insertion upon the coronoid process of the mandible, which will be described in greater depth later in this manuscript.
Palpation of both mandibular condyle anterior translation and the subsequent opening (or lack thereof) of the retrodiscal spaces provides information that supplements the measurement of mouth opening. When considering these variables, multiple factors should be tested including: 1. The quantity of mandibular condyle anterior translation bilaterally, 2. The size of the resulting retrodiscal spaces, 3. Any lateral or medial bias of condyle motion relative to the contralateral side, 4. The presence of popping, clicking, or crepitus, and 5. Palpable tenderness or small irregularities of the retrodiscal spaces.
Passive accessory joint glide testing has been described in detail elsewhere [17]. However, several additional points should be made. First, while it appears that joint distraction is a commonly relied upon technique for the TMJ, it likely should not be considered as a first-line joint technique. The primary problem with joint distraction is that it requires the clinician to use an intraoral technique so that the distraction force can be placed through the ipsilateral lower molars and premolars [17]. This technique requires the patient to preposition in mouth opening, which places the ligamentous capsule of the TMJs on stretch. As a result, this technique becomes a more advanced approach when compared to those maneuvers that permit the TMJs to be closer to a resting position (i.e. mouth closed, teeth not in contact) when implemented.
Building off that point, external fingertip or hand placements on the mandible should be considered the starting point for TMJ passive accessory joint testing (Figures 3–4). There are several reasons for this including closer or direct manual contact to the joint on the mandibular condyles, increased feasibility of testing/treatment in patients with dental impairments, the ability to apply the techniques throughout a larger TMJ range of motion, and patient/clinician preference (i.e no gloves required and no/rare intraoral techniques used). Of additional importance given the global pandemic of COVID-19, these techniques can be implemented while the patient wears an appropriately fitting face mask, though caution should be used in these scenarios such that smaller masks may not suffice given a general tendency to move off the nose during mouth opening.
Continuing with the Top-Down Approach, the masseter tendons can be palpated at the inferior margin of the zygomatic arches. These tissues should be tested for both palpable irregularities and the generation of symptoms. Care should be taken during palpation by attempting to find even small irregularities that correlate to painful reports by the patient. This can be accomplished by palpating millimeter by millimeter. Upon testing the masseter tendons, the muscle bellies should be tested as well. Next, the insertion of the temporalis tendon on the coronoid process should be palpated when feasible. This location is only easily accessible when sufficient anterior translation of the mandibular condyle is present and therefore testing and/or treatment may have to be delayed in situations where joint restrictions are present until more normal articular mechanics are restored.
When learning how to palpate this location, the following steps can be helpful: 1. Begin with the patient’s mouth closed, 2. Gently palpate just inferior to the zygomatic bones, 3. Ask the patient to slowly open their mouth (Figure 5), 4. If the coronoid processes do not move forward to contact the examiner’s fingers, then ask the patient to open further and/or adjust the fingers’ locations, 5. When the coronoid process is moving forward far enough, check all easily accessible coronoid aspects including the anterior and lateral surfaces, 6. Remember that verbal cues are generally required for the patient to maintain an opened mouth, 7. Provide frequent rest breaks to permit the patient to swallow, and 8. Intraoral palpation can assist with making contact on the medial aspect of the tendon’s insertion, though this portion of the technique is generally not required.
Figure 5.

Palpation location for the insertion point of the temporalis tendon on the coronoid process.
In each instance, it is important to investigate whether or not pain or other symptoms generated during these physical tests reproduce a familiar symptom or some other complaint that is not believed to be directly associated with the reason the patient sought healthcare services. Additionally, the palpable irregularities associated with some of these structures can be difficult to identify by the novice or inexperienced orthopedic clinician. As is always the case, repeated practice and mentoring are invaluable when learning a new skillset. It is also important to remember that patients with TM disorders frequently have highly sensitized tissues, which indicates that all testing should be initially implemented gently and only increased in aggressiveness when tolerated and/or indicated to be necessary as a result of thorough clinical reasoning.
Of note, each of the examination techniques, with minor modifications, can also serve as management techniques. For example, identifying palpable tenderness or an enlargement of a tendon can easily be transitioned into friction massage. Or, the identification of a passive accessory glide restriction will easily be implemented at a higher dosage to generate a joint mobilization technique. And, in many instances, not only can an examination technique be utilized as in-clinic treatment but the patient can also be taught how to use these procedures as a self-care component when deemed appropriate.
Conclusion
The evaluation of TM disorders is a multifaceted endeavor. In addition to routine orthopedic examination components such as intake paperwork and both the subjective and objection examination procedures, many of which are presented here, other pertinent factors that should be considered include but are not limited to physical health, self-reported oral parafunction, frequent physical symptoms, and sleep disturbances. Of equally importance, clinicians must sufficiently understand the extent to which nociplastic pain states often affect this patient population. By paying attention to these and other variables physical therapists and other clinical professionals hold the potential to positively impact TM disorders. Finally, while this process should be grounded in a conservative approach, an interdisciplinary treatment plan is often warranted.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Al-Jundi MA, John MT, Setz JM, et al. Meta-analysis of treatment need for temporomandibular disorders in adult nonpatients. J Orofac Pain. 2008;22(2):97–107. [PubMed] [Google Scholar]
- [2].NIDCR. National Institute of Health (NIH) . National Institute of Dental and Craniofacial Research (NIDCR). Research. Data and Statistics. Facial Pain. 2018. July [cited 2021 May 19]. Available from: https://www.nidcr.nih.gov/research/data-statistics/facial-pain
- [3].Valesan LF, Da-Cas CD, Réus JC, et al. Prevalence of temporomandibular joint disorders: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(2):441–453. DOI: 10.1007/s00784-020-03710-w [DOI] [PubMed] [Google Scholar]
- [4].Allori AC, Chang CC, Fariña R, et al. Current concepts in pediatric temporomandibular joint disorders: part 1. Etiology, epidemiology, and classification. Plast Reconstr Surg. 2010;126(4):1263–1275. DOI: 10.1097/PRS.0b013e3181ebe207 [DOI] [PubMed] [Google Scholar]
- [5].Keeling SD, McGorray S, Wheeler TT, et al. Risk factors associated with temporomandibular joint sounds in children 6 to 12 years of age. Am J Orthod Dentofacial Orthop. 1994;105(3):279–287. [DOI] [PubMed] [Google Scholar]
- [6].Sena MF, Mesquita KS, Santos FR, et al. Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paul Pediatr. 2013;31(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vierola A, Suominen AL, Ikavalko T, et al. Clinical signs of temporomandibular disorders and various pain conditions among children 6 to 8 years of age: the PANIC study. J Orofac Pain. 2012;26(1):17–25. [PubMed] [Google Scholar]
- [8].Vierola A, Suominen AL, Eloranta AM, et al. Determinants for craniofacial pains in children 6-8 years of age: the PANIC study. Acta Odontol Scand. 2017;75(6):453–460. DOI: 10.1080/00016357.2017.1339908 [DOI] [PubMed] [Google Scholar]
- [9].Lauriti L, Motta LJ, Silva PF, et al. Are occlusal characteristics, headache, parafunctional habits and clicking sounds associated with the signs and symptoms of temporomandibular disorder in adolescents? J Phys Ther Sci. 2013;25(10):1331–1334. DOI: 10.1589/jpts.25.1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fernandes G, van Selms MK, Gonçalves DA, et al. Factors associated with temporomandibular disorders pain in adolescents. J Oral Rehabil. 2015;42(2):113–119. [DOI] [PubMed] [Google Scholar]
- [11].Østensjø V, Moen K, Storesund T, et al. Prevalence of painful temporomandibular disorders and correlation to lifestyle factors among adolescents in norway. Pain Res Manag. 2017;2017:2164825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Slade G, Ohrbach R, Greenspan J, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016;95(10):1084–1092. DOI: 10.1177/0022034516653743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bitiniene D, Zamaliauskiene R, Kubilius R, et al. Quality of life in patients with temporomandibular disorders. A systematic review. Stomatologija. 2018;20(1):3–9. [PubMed] [Google Scholar]
- [14].Shaffer SM, Emerson AJ, Burr M, et al. Quality of life in painful temporomandibular disorders onset: a systematic review of outcome measure clinimetrics and predictive properties. Phys Ther Rev. 2021;26(4):284–298. [Google Scholar]
- [15].Harrison AL, Thorp JN, Ritzline PD.. A proposed diagnostic classification of patients with temporomandibular disorders: implications for physical therapists. J Orthop Sports Phys Ther. 2014;44(3):182–197. [DOI] [PubMed] [Google Scholar]
- [16].Shaffer SM, Brismée JM, Sizer PS, et al. Temporomandibular disorders. Part 1: anatomy and examination/diagnosis. J Man Manip Ther. 2014;22(1):2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shaffer SM, Brismée JM, Sizer PS, et al. Temporomandibular disorders. Part 2: conservative management. J Man Manip Ther. 2014;22(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bair E, Ohrbach R, Fillingim RB, et al. Multivariable modeling of phenotypic risk factors for first-onset TMD: the OPPERA prospective cohort study. J Pain. 2013;14(12 0):T102–T115. DOI: 10.1016/j.jpain.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wright EF. Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc. 2000;131(9):1307–1315. [DOI] [PubMed] [Google Scholar]
- [20].Kuć J, Szarejko KD, Sierpińska T. Evaluation of orofacial and general pain location in patients with temporomandibular joint disorder-myofascial pain with referral. Front Neurol. 2019. 29;10:546. DOI: 10.3389/fneur.2019.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Serrano-Hernanz G, Futarmal Kothari S, Castrillón E, et al. Importance of standardized palpation of the human temporomandibular joint. J Oral Facial Pain Headache. 2019;33(2):220–226. DOI: 10.11607/ofph.2235 [DOI] [PubMed] [Google Scholar]
- [22].Rusiecki RS. Chest pain as result of temporomandibular disorder (TMD). Gen Dent. 1998;46(4):352–355. [PubMed] [Google Scholar]
- [23].Kreiner M, Falace D, Michelis V, et al. Quality difference in craniofacial pain of cardiac vs. dental origin. J Dent Res. 2010;89(9):965–969. [DOI] [PubMed] [Google Scholar]
- [24].Lichtman JH, Leifheit EC, Safdar B, et al. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the virgo study (Variation in recovery: role of gender on outcomes of young AMI patients). Circulation. 2018;137(8):781–790. DOI: 10.1161/CIRCULATIONAHA.117.031650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fernández-de-Las-Peñas C, Galán-Del-Río F, Alonso-Blanco C, et al. Referred pain from muscle trigger points in the masticatory and neck-shoulder musculature in women with temporomandibular disorders. J Pain. 2010;11(12):1295–1304. DOI: 10.1016/j.jpain.2010.03.005 [DOI] [PubMed] [Google Scholar]
- [26].Maixner W, Fillingim R, Booker D, et al. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63(3):341–351. [DOI] [PubMed] [Google Scholar]
- [27].Maixner W, Fillingim R, Sigurdsson A, et al. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76(1–2):71–81. [DOI] [PubMed] [Google Scholar]
- [28].Svensson P, Graven-Nielsen T, Arendt-Nielsen L. Mechanical hyperesthesia of human facial skin induced by tonic painful stimulation of jaw-muscles. Pain. 1998;74(1):93–100. [DOI] [PubMed] [Google Scholar]
- [29].Fernández-de-Las-Peñas C, Galán-Del-Río F, Fernández-Carnero J, et al. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: evidence of impairment in central nociceptive processing. J Pain. 2009;10(11):1170–1178. DOI: 10.1016/j.jpain.2009.04.017 [DOI] [PubMed] [Google Scholar]
- [30].Park JW, Clark GT, Kim YK, et al. Analysis of thermal pain sensitivity and psychological profiles in different subgroups of TMD patients. Int J Oral Maxillofac Surg. 2010;39(10):968–974. [DOI] [PubMed] [Google Scholar]
- [31].Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: current concepts and synthesis. Curr Pain Headache Rep. 2003;7(5):371–376. [DOI] [PubMed] [Google Scholar]
- [32].Edvinsson JCA, Viganò A, Alekseeva A, et al. The fifth cranial nerve in headaches. J Headache Pain. 2020;21(1):65. DOI: 10.1186/s10194-020-01134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gillies C, Tyagi A. Understanding the trigeminal cervical connections; an observation. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.25346970 [Google Scholar]
- [34].Piovesan EJ, Kowacs PA, Tatsui CE, et al. Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia. 2001;21(2):107–109. DOI: 10.1046/j.1468-2982.2001.00166.x [DOI] [PubMed] [Google Scholar]
- [35].Busch V, Jakob W, Juergens T, et al. Functional connectivity between trigeminal and occipital nerves revealed by occipital nerve blockade and nociceptive blink reflexes. Cephalalgia. 2006;26(1):50–55. DOI: 10.1111/j.1468-2982.2005.00992.x [DOI] [PubMed] [Google Scholar]
- [36].Weber P, Corrêa EC, Ferreira Fdos S, et al. Cervical spine dysfunction signs and symptoms in individuals with temporomandibular disorder. J Soc Bras Fonoaudiol. 2012;24(2):134–139. DOI: 10.1590/S2179-64912012000200008 [DOI] [PubMed] [Google Scholar]
- [37].Martins WR, Blasczyk JC, Aparecida Furlan de Oliveira M, et al. Efficacy of musculoskeletal manual approach in the treatment of temporomandibular joint disorder: a systematic review with meta-analysis. Man Ther. 2016;21:10–17. [DOI] [PubMed] [Google Scholar]
- [38].Kraus S, Prodoehl J. Outcomes and patient satisfaction following individualized physical therapy treatment for patients diagnosed with temporomandibular disc displacement without reduction with limited opening: a cross-sectional study. Cranio. 2019;37(1):20–27. [DOI] [PubMed] [Google Scholar]
- [39].Lobbezoo-Scholte AM, Steenks MH, Faber JA, et al. Diagnostic value of orthopedic tests in patients with temporomandibular disorders. J Dent Res. 1993;72(10):1443–1453. [DOI] [PubMed] [Google Scholar]
- [40].Lobbezoo-Scholte AM, de Wijer A, Steenks MH, et al. Interexaminer reliability of six orthopaedic tests in diagnostic subgroups of craniomandibular disorders. J Oral Rehabil. 1994;21(3):273–285. [DOI] [PubMed] [Google Scholar]
- [41].de Wijer A, Lobbezoo-Scholte AM, Steenks MH, et al. Reliability of clinical findings in temporomandibular disorders. J Orofac Pain. 1995;9(2):181–191. [PubMed] [Google Scholar]
- [42].Hesse JR, van Loon LA, Naeije M. Subjective pain report and the outcome of several orthopaedic tests in craniomandibular disorder patients with recent pain complaints. J Oral Rehabil. 1997;24(7):483–489. [DOI] [PubMed] [Google Scholar]
- [43].John MT, Zwijnenburg AJ. Interobserver variability in assessment of signs of TMD. Int J Prosthodont. 2001;14(3):265–270. [PubMed] [Google Scholar]
- [44].Masumi S, Kim YJ, Clark GT. The value of maximum jaw motion measurements for distinguishing between common temporomandibular disorder subgroups. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod. 2002;93(5):552–559. [DOI] [PubMed] [Google Scholar]
- [45].Durham J, Steele JG, Wassell RW, et al. Creating a patient-based condition-specific outcome measure for temporomandibular disorders (TMDs): oral health impact profile for TMDs (OHIP-TMDs). J Oral Rehabil. 2011;38(12):871–883. DOI: 10.1111/j.1365-2842.2011.02233.x [DOI] [PubMed] [Google Scholar]
- [46].Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. 2008;22(3):219–230. [PubMed] [Google Scholar]
- [47].Stegenga B, de Bont LG, Boering G. Temporomandibular joint pain assessment. J Orofac Pain. 1993;7(1):23–37. [PubMed] [Google Scholar]
- [48].Emerson Kavchak AJ, Mischke JJ, Lulofs-MacPherson K, et al. The psychometric properties of self-report outcome measures in temporomandibular dysfunction. Phys Ther Rev. 2014;19(3):174–185. [Google Scholar]
- [49].Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- [50].Burr M, Naze GS, Shaffer SM, et al. The role of sleep dysfunction in temporomandibular onset and progression: a systematic review and meta-analyses. J Oral Rehabil. 2021;48(2):183–194. [DOI] [PubMed] [Google Scholar]
- [51].Yule PL, Durham J, Playford H, et al. OHIP-Tmds: a patient-reported outcome measure for temporomandibular disorders. Community Dent Oral Epidemiol. 2015;43(5):461–470. DOI: 10.1111/cdoe.12171 [DOI] [PubMed] [Google Scholar]
- [52].Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 1997;25(4):284–290. [DOI] [PubMed] [Google Scholar]
- [53].Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- [54].Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med. 1997;101(5). 46-50, 55-56, 626-4. DOI: 10.3810/pgm.1997.05.217. [DOI] [PubMed] [Google Scholar]
- [55].Sizer PS Jr, Brismée JM, Cook C. Medical screening for red flags in the diagnosis and management of musculoskeletal spine pain. Pain Pract. 2007;7(1):53–71. [DOI] [PubMed] [Google Scholar]
- [56].Gaini SM, Fiori L, Cesana C, et al. The headache in the Emergency Department. Neurol Sci. 2004;25(Suppl 3):S196–201. [DOI] [PubMed] [Google Scholar]
- [57].Teichtahl AJ, McColl G. An approach to neck pain for the family physician. Aust Fam Physician. 2013;42(11):774–777. [PubMed] [Google Scholar]
- [58].International Headache Society . International classification of headache disorders (ICHD) guidelines. 2018. [cited 2021 June 17]. Available from: http://www.ihs-headache.org/ichd-guidelines
- [59].Petersen EJ, Thurmond SM, Gm J. Severity, irritability, nature, stage, and stability (SINSS): a clinical perspective. J Man Manip Ther. 2021;29(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jamison RN, Fanciullo GJ, Baird JC. Usefulness of pain drawings in identifying real or imagined pain: accuracy of pain professionals, nonprofessionals, and a decision model. J Pain. 2004;5(9):476–482. [DOI] [PubMed] [Google Scholar]
- [61].Kaneyama K, Segami N, Hatta T. Congenital deformities and developmental abnormalities of the mandibular condyle in the temporomandibular joint. Congenit Anom (Kyoto). 2008;48(3):118–125. [DOI] [PubMed] [Google Scholar]
- [62].Nolte JW, Verhoeven TJ, Schreurs R, et al. 3-Dimensional CBCT analysis of mandibular asymmetry in unilateral condylar hyperplasia. J Craniomaxillofac Surg. 2016;44(12):1970–1976. DOI: 10.1016/j.jcms.2016.09.016 [DOI] [PubMed] [Google Scholar]
- [63].Vernucci RA, Mazzoli V, Galluccio G, et al. Unilateral hemimandibular hyperactivity: clinical features of a population of 128 patients. J Craniomaxillofac Surg. 2018;46(7):1105–1110. [DOI] [PubMed] [Google Scholar]
- [64].Bennett SC, Goonewardene MS. Long-Term surgical-orthodontic management of hemimandibular hyperplasia. Aust Orthod J. 2016;32(1):97–108. [PubMed] [Google Scholar]
- [65].Jeon KJ, Kim YH, Ha EG, et al. Quantitative analysis of the mouth opening movement of temporomandibular joint disorder patients according to disc position using computer vision: a pilot study. Quant Imaging Med Surg. 2022;12(3):1909–1918. DOI: 10.21037/qims-21-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kordass B, Hugger A, Bernhardt O. Correlation between computer-assisted measurements of mandibular opening and closing movements and clinical symptoms of temporomandibular dysfunction. Int J Comput Dent. 2012; 15(2):93–107. [PubMed] [Google Scholar]
- [67].Hwang SW, Abozed MM, Antoniou AJ, et al. Postoperative temporalis muscle atrophy and the use of electrocautery: a volumetric MRI comparison. Skull Base. 2010;20(5):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ranasinghe JC, Wickramasinghe C, Rodrigo G. Isolated unilateral temporalis muscle hypertrophy in a child: a case report with literature review. BMC Pediatr. 2018;18(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. Jama. 2001;286(15):1841–1848. DOI: 10.1001/jama.286.15.1841 [DOI] [PubMed] [Google Scholar]
- [70].Alonso LL, Purcell TB. Accuracy of the tongue blade test in patients with suspected mandibular fracture. J Emerg Med. 1995;13(3):297–304. [DOI] [PubMed] [Google Scholar]
- [71].Neiner J, Free R, Caldito G, et al. Tongue blade bite test predicts mandible fractures. Craniomaxillofac Trauma Reconstr. 2016;9(2):121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Schwab RA, Genners K, Robinson WA. Clinical predictors of mandibular fractures. Am J Emerg Med. 1998;16(3):304–305. [DOI] [PubMed] [Google Scholar]
- [73].Malhotra R, Dunning J. The utility of the tongue blade test for the diagnosis of mandibular fracture. Emerg Med J. 2003;20(6):552–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Caputo ND, Raja A, Shields C, et al. Re-Evaluating the diagnostic accuracy of the tongue blade test: still useful as a screening tool for mandibular fractures? J Emerg Med. 2013;45(1):8–12. [DOI] [PubMed] [Google Scholar]
- [75].Schiffman EL, Ohrbach R, Truelove EL, et al. The research diagnostic criteria for temporomandibular disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J Orofac Pain. 2010;24(1):63–78. [PMC free article] [PubMed] [Google Scholar]
- [76].International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research Applications: recommendations of the International RDC/TMD consortium network* and orofacial pain special interest group†. J Oral Facial Pain Headache. 2014;28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Agerberg G. Maximal mandibular movements in children. Acta Odontol Scand. 1974;32(2):147–159. [DOI] [PubMed] [Google Scholar]
- [78].Agerberg G. Maximal mandibular movements in teen-agers. Acta Morphol Neerl Scand. 1974;12(2):79–102. [PubMed] [Google Scholar]
- [79].Agerberg G. Maximal mandibular movements in young men and women. Sven Tandlak Tidskr. 1974;67(2):81–100. [PubMed] [Google Scholar]
- [80].Kraus SL. Temporomandibular Disorders. In: Saunders H, and Ryan R, editors. Evaluation, treatment and prevention of musculoskeletal disorders volume 1 spine. 4th ed. Chaska: The Saunders Group; 2004. p. 173-210. [Google Scholar]
- [81].García NM, Blaya F, Urquijo EL, et al. Oral appliance for obstructive sleep apnea: prototyping and optimization of the mandibular protrusion device. J Med Syst. 2019;43(5):107. [DOI] [PubMed] [Google Scholar]


