Abstract
Aim
This study evaluates the cost–effectiveness of imipenem/cilastatin/relebactam (IMI/REL) for treating hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in an ‘early adjustment prescribing scenario’.
Methods
An economic model was constructed to compare two strategies: continuation of empiric piperacillin/tazobactam (PIP/TAZ) versus early adjustment to IMI/REL. A decision tree was used to depict the hospitalization period, and a Markov model used to capture long-term outcomes.
Results
IMI/REL generated more quality-adjusted life years than PIP/TAZ, at an increased cost per patient. The incremental cost–effectiveness ratio of $17,529 per QALY is below the typical US willingness-to-pay threshold.
Conclusion
IMI/REL may represent a cost-effective treatment for payers and a valuable option for clinicians, when considered alongside patient risk factors, local epidemiology, and susceptibility data.
Keywords: antimicrobial resistance, cost–effectiveness analysis, culture and susceptibility, HABP/VABP, health economics, imipenem/cilastatin/relebactam, infectious diseases
Plain language summary
What is this article about?
The article uses the phase 3 trial data to examine the economic value of a new antibiotic, imipenem/cilastatin/relebactam, over piperacillin/tazobactam for treating hospital-acquired pneumonia.
What were the results?
Imipenem/cilastatin/relebactam could yield more health benefits at a reasonably higher cost than piperacillin/tazobactam.
What do the results of the study mean?
Imipenem/cilastatin/relebactam could be a cost-effective antibiotic choice for treating hospital-acquired pneumonia.
Hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VAPB) are common nosocomial infections that are associated with high rates of morbidity and mortality. Furthermore, outcomes are often worse among patients with HABP/VABP caused by pathogens that are resistant to antibacterial agents. A particular cause for concern is resistance to carbapenems, a β-lactam antibacterial agent class effective against extended-spectrum β-lactamase producers, typically reserved for later line therapy; which have now become rooted in the management of HABP/VABP. As the prevalence of carbapenem resistant (CR) GN infections increases globally, limited treatment options remain, underpinning the need for access to novel agents.
Imipenem/cilastatin/relebactam (IMI/REL) combines an established carbapenem and a novel β-lactamase inhibitor (relebactam). Relebactam has no antibacterial activity itself but does exhibit inhibitory activity against various β-lactamases that commonly contribute to carbapenem non-susceptibility [1]. As such, IMI/REL has broad antibacterial activity, including carbapenem-resistant Enterobacterales and Pseudomonas aeruginosa. IMI/REL has been approved by the US FDA for treating HABP/VABP, complicated urinary tract infections (cUTI) and complicated intra-abdominal infections (cIAI) in adult patients [1]. Use of IMI/REL may help address antimicrobial resistance – an area of significant unmet medical need identified by regulators [2,3], public health entities [4,5], and professional societies [6].
To assess antimicrobial resistance, culture and susceptibility (C&S) testing is needed, with results typically available after 48 h. Prior to C&S test results, patients are treated empirically based on an awareness of local ecology, hospital antibiograms, and available patient history and risk factors. Certain broad-spectrum antibiotics such as piperacillin/tazobactam (PIP/TAZ), ceftazidime, or cefepime are often candidates for empiric treatment. Despite their broad spectrum, there remains a chance that the causative pathogen is resistant, rendering the empiric treatment inappropriate (i.e., initial inappropriate treatment). The literature reports that initial inappropriate antibacterial therapy is associated with an increased risk of in-hospital mortality, increased cost of care, and longer length of stay (LOS), compared with appropriate therapy [7–9].
To mitigate the potential consequences of initial inappropriate treatment, clinicians may make an ‘early adjustment’ by switching to an alternative antibacterial agent, even prior to the results of C&S testing, if a patient appears to deteriorate and/or the infection is suspected to be caused by a resistant pathogen. In such a scenario, IMI/REL could be considered as an option for early adjustment based on pathogen coverage. However, to support formulary inclusion and/or amendments to treatment protocols it is important to evaluate the economic value of IMI/REL and to explore the scenarios (e.g., how much additional resistance coverage IMI/REL can provide) in which early adjustment to IMI/REL proves cost effective. This study aims to achieve both objectives.
Methods
Model overview
A de novo economic model was developed to evaluate the cost–effectiveness of IMI/REL when used in the context of the early adjustment prescribing scenario. The modelled population were adult patients with HABP/VABP, whose condition was not improving or who experienced deterioration 12 to 48 h after receiving empiric treatment with PIP/TAZ (while pathogen susceptibility information was yet unknown). The two comparison strategies are continuation of empiric PIP/TAZ versus, early adjustment to IMI/REL.
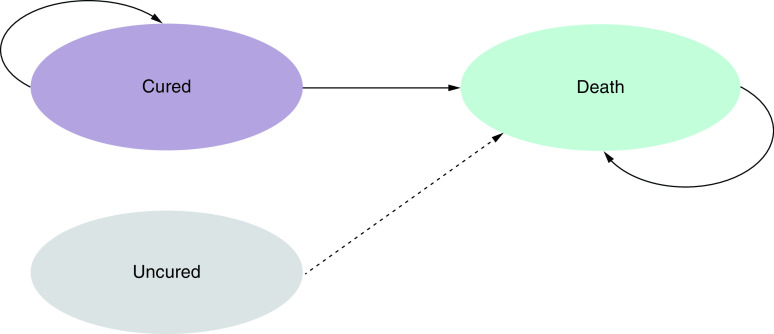
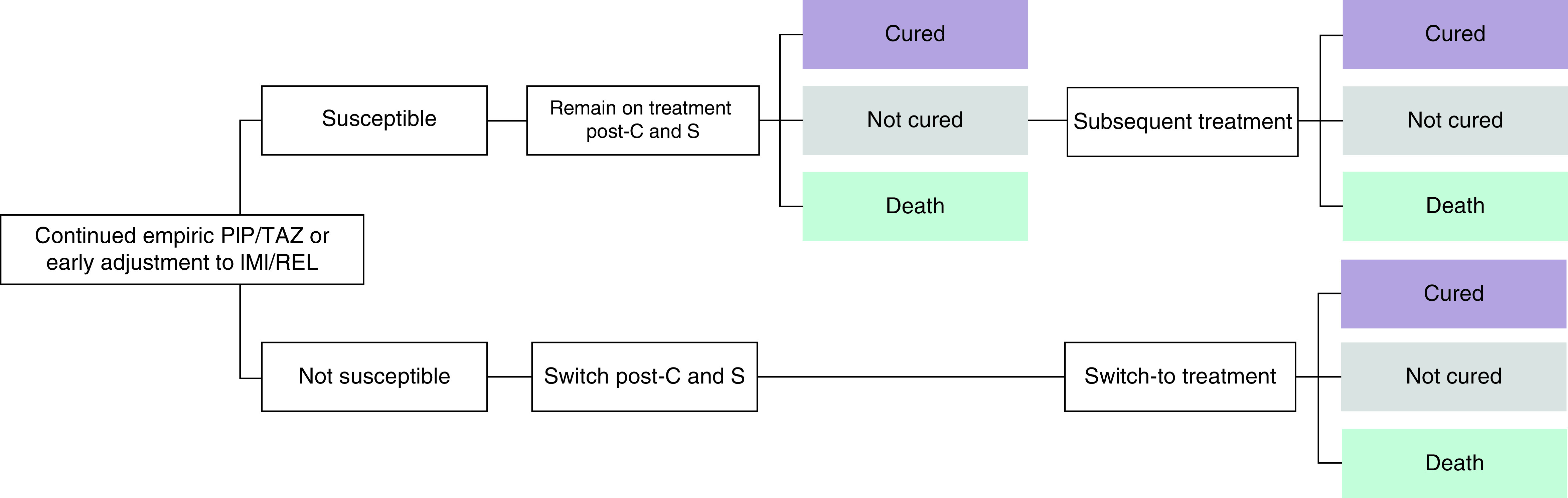
The model began with a short-term decision tree depicting treatment routes and outcomes during the hospitalization period (Figure 1). Patients discharged from the hospital enter a long-term Markov model (Figure 2), which extended beyond the RESTORE IMI-2 trial period to capture follow-up costs and health-related quality of life (HRQoL) of cured patients.
Figure 1. . Short-term decision tree.
Upon existing the decision tree, patients who are cured in the decision tree enter the long-term model; Patients who are alive but uncured at the end of the decision tree are assumed to die within a year.
IMI/REL: Imipenem/cilastatin/relebactam; PIP/TAZ: Piperacillin/tazobactam.
Figure 2. . Long-term Markov model.
In the decision tree, following C&S test results, patients follow one of two potential treatment pathways:
Organism susceptible to pre-C&S treatment: patient remains on pre-C&S treatment (either empiric PIP/TAZ or IMI/REL as the early adjustment). Patients alive but uncured receive subsequent salvage therapy.
Organism not susceptible to pre-C&S treatment: patient will switch to an alternative treatment based on susceptibility profile.
It is assumed that patients who remain alive but uncured following treatment with IMI/REL or PIP/TAZ receive colistin plus imipenem/cilastatin (CMS+IMI) as a subsequent salvage therapy. It is also assumed that patients who switch due to confirmed nonsusceptibility to either IMI/REL or PIP/TAZ following test results receive salvage CMS+IMI.
Patients who are cured in the short-term decision tree enter the long-term Markov model where they can remain in the cured state or experience death. Whereas those who are alive but uncured at the end of the treatment pathway are assumed to die within a year (half a year on average as a proxy), in line with solicited clinical expert opinion.
The model developed in this analysis represents an important and clinically relevant aspect of patient care early in the treatment pathway. However, it is acknowledged that, due to data limitations, this model is a simplification of a complex and multifaceted pathway. In practice, patients may receive multiple lines of antibacterial treatment (i.e., empiric treatment, combination therapy, early adjustment, switch and/or escalation either before or after the availability of susceptibility test results).
Model settings
This model considered a US perspective, and as such, model settings were aligned with the Institute for Clinical and Economic Review reference case [10]. A lifetime horizon of 40 years was adopted, beginning at the mean age (60 years) of patients in the RESTORE-IMI 2 trial. The analysis captured direct costs borne by third-party payers or integrated health systems and direct health effects for patients are captured. Costs and outcomes are time-preference discounted at a rate of 3%.
Clinical inputs
Key clinical inputs and values are provided in Table 1, categorized as efficacy data (determining the proportion of patients experiencing cure or death) [11], susceptibility data (determining the proportion of patients remaining on, or switching treatment) [12], resource use (in-hospital LOS) [11,13], and safety data (the proportion of patients experiencing Grade 3+ adverse events [AEs]) [11,14].
Table 1. . Model parameters.
| Parameter |
IMI/REL |
PIP/TAZ |
Source |
Ref. |
|---|---|---|---|---|
| Clinical parameters | ||||
|
Susceptibility
| ||||
| Susceptibility coverage |
93.8% |
86.0% |
SMART (US, 2017–19) |
[12] |
|
Clinical efficacy
| ||||
| Clinical response at Day 28 (MITT) |
51.9% |
50.6% |
RESTORE-IMI 2 |
[11] |
| All-cause mortality through Day 28 (MITT) |
15.9% |
21.3% |
||
| Subsequent CMS+IMI response rate |
40.0% |
|
RESTORE-IMI 1 |
[14] |
| Subsequent CMS+IMI mortality rate |
30.0% |
|
||
| Response odds ratio (switch due to nonsusceptibility) |
4.55 |
|
Raman et al. (2015) |
[8] |
| Mortality odds ratio (switch due to nonsusceptibility) |
2.92 |
|
Siempos et al. (2010) |
[15] |
|
Hospital LOS
| ||||
| Proportion of time intubated |
84.0% |
78.2% |
RESTORE-IMI 2 |
[11] |
| Average ICU LOS days - cured |
14.60 |
13.70 |
||
| Average ICU LOS days - uncured |
19.20 |
22.50 |
||
| Average ICU LOS days - death |
11.70 |
11.90 |
||
| Average general ward LOS days - cured |
5.30 |
6.10 |
||
| Average general ward LOS days - uncured |
3.80 |
2.10 |
||
| Average general ward LOS days - death |
0.20 |
0.20 |
||
| Additional LOS days due to nonsusceptibility |
6.50 |
|
Prabhu et al. (2015) |
[13] |
|
Adverse events
| ||||
| Thrombocytopenia |
0.38% |
0.00% |
RESTORE-IMI 2 |
[11] |
| Diarrhoea |
0.00% |
0.37% |
||
| Alanine aminotransferase increased |
0.75% |
0.00% |
||
| Aspartate aminotransferase increased |
0.75% |
0.00% |
||
| Generalised tonic-clonic seizure |
0.00% |
0.37% |
||
| Nephrotoxicity |
10.4% |
- |
RESTORE-IMI 1 | [14] |
| Subsequent CMS+IMI nephrotoxicity | 56.25% | |||
| Economic parameters | ||||
|---|---|---|---|---|
|
Drug costs
| ||||
| Treatment duration (days) – IMI/REL |
8.7 |
|
RESTORE-IMI 2 |
[11] |
| Treatment duration (days) – PIP/TAZ |
8.3 |
|
||
| Subsequent CMS+IMI treatment duration (days) |
6.1 |
|
RESTORE-IMI 1 |
[14] |
| IMI/REL – 500 mg/250 mg – 25 vials |
$6,687.50 |
|
NDC Code: 00006-3856-02 |
[17] |
| PIP/TAZ – 2 g/250 mg - 10 vials |
$30.00 |
|
NDC Code: 63323-0981-53 |
[17] |
| PIP/TAZ – 3 g/375 mg - 10 vials |
$30.00 |
|
NDC Code: 63323-0983-53 |
[17] |
| PIP/TAZ – 4 g/500 mg - 10 vials |
$56.10 |
|
NDC Code: 72572-0574-10 |
[17] |
| PIP/TAZ – 12 g/1500 mg - 1 vial |
$39.00 |
|
NDC Code: 61990-0140-01 |
[17] |
| PIP/TAZ – 36 g/4500 mg - 1 vial |
$65.00 |
|
NDC Code: 65219-0256-24 |
[17] |
|
Resource use costs
| ||||
| ICU cost on mechanical ventilator day 1 |
$19,800.90 |
|
Dasta et al. (2005), inflation adjusted to 2021. |
[18,21] |
| ICU cost on mechanical ventilator day 2 |
$8,797.95 |
|
||
| ICU cost on mechanical ventilator day 3+ |
$7,279.04 |
|
||
| ICU cost off mechanical ventilator day 1 |
$12,230.18 |
|
||
| ICU cost off mechanical ventilator day 2 |
$6,413.19 |
|
||
| ICU cost off mechanical ventilator day 3+ |
$5,837.17 |
|
||
| General ward unit cost per day |
$2,720.60 |
|
Kaiser Family Foundation (2018), inflation adjusted to 2021 |
[19,21] |
| Outpatient visit |
$183.19 |
|
2020 CMS Physician Fee Schedule |
[22] |
|
Adverse event costs
| ||||
| Thrombocytopenia |
$17,208.03 |
|
Healthcare Cost and Utilization Project (2017), inflation adjusted to 2021. |
[20,21] |
| Diarrhoea |
$6,936.34 |
|
||
| Alanine aminotransferase increased |
$8,684.18 |
|
||
| Aspartate aminotransferase increased |
$8,684.18 |
|
||
| Generalised tonic-clonic seizure |
$7,770.73 |
|
||
| Nephrotoxicity |
$14,051.62 |
|
||
|
Health-related quality of life
| ||||
| Utility value – ICU |
0.68 |
|
Whittington et al. (2017) |
[23] |
| Utility value – general ward | 0.73 | Lee et al. (2010) | [24] | |
CMS: Centers for Medicare and Medicaid Services; CMS+IMI: Colistin plus imipenem/cilastatin; ICU: Intensive care unit; IMI/REL: Imipenem/cilastatin/relebactam; LOS: Length of stay; MITT: Modified intent-to-treat; NDC: Nation Drug Code; PIP/TAZ: Piperacillin/tazobactam; SMART: Study for Monitoring Antimicrobial Resistance Trends.
Susceptibility
The proportion of patients susceptible to IMI/REL or PIP/TAZ, and conversely the proportion that switch treatment (i.e., not susceptible) was determined by a US subsample of the Study for Monitoring Antimicrobial Resistance Trends (SMART) database 2017–2019 with lower respiratory tract infection caused by Gram-negative pathogens (n = 2335). The SMART database monitors the in vitro susceptibility of clinical isolates to 12 commonly used antibacterial agents in different regions of the world [12]. Susceptibility is determined using the standard Clinical and Laboratory Standards Institute (CLSI) method and interpreted using 2018 CLSI breakpoint criteria. The US subsample, considered within this model, showed a higher proportion of causative pathogens to be susceptible to IMI/REL (93.8%) compared with PIP/TAZ (86.0%). While these data may be representative of the US overall, it is acknowledged that these data may not reflect local hospital antibiogram; alternative susceptibility scenarios are explored in sensitivity analyses.
Efficacy
To estimate the proportion of patients experiencing cure or death in the IMI/REL and PIP/TAZ arms of the model, data from the primary analysis population (modified intent-to-treat [MITT]) of the RESTORE-IMI 2 trial were used (see Appendix 1 for trial eligibility criteria) [11].
Although clinical response at the early follow-up visit was a key secondary end point in RESTORE-IMI 2, and is more favourable for IMI/REL versus PIP/TAZ (61.0% vs 55.8%), the model conservatively applied clinical response at Day 28 in the base case. Clinical response at day 28 is aligned with the key primary end point of all-cause mortality through day 28 (used to determine the proportion entering the death state). RESTORE-IMI 2 demonstrated favourable (not statistically significant) day 28 response rates (51.9% vs 50.6%) and mortality rates (15.9% vs 21.3%) for IMI/REL compared with PIP/TAZ [11].
In addition to RESTORE IMI-2 MITT population in the base case, a subset of MITT cohort (n = 118; 28.9%) who received and failed antibiotic treatment prior to study drug (i.e., study drug was provided as an adjustment) was analysed in a scenario analysis. In this sub-cohort, IMI/REL had more favourable outcomes (day 28 response: 50.9% IMI/REL vs 34.9% PIP/TAZ; day 28 mortality rates: 29.1% IMI/REL vs 34.1% PIP/TAZ) compared with overall MITT population.
For salvage treatment with CMS+IMI, response (40%) and mortality (30%) rates were sourced from the phase III RESTORE-IMI 1 study [14]. For patients who switch to CMS+IMI due to nonsusceptibility to IMI/REL or PIP/TAZ, odds ratios of 4.55 and 2.92 were used to adjust naive response and mortality rates, respectively; thus, reflecting the impact of inappropriate therapy [8,15]. In the long-term Markov model, age- and sex-matched general population mortality rates were applied to cured patients (Appendix 2) [16].
In-hospital length of stay
Total in-hospital LOS comprised of days in the intensive care unit (ICU) plus days spent in a general ward setting; this was primarily informed using data from RESTORE-IMI 2 [11]. ICU stay was further split according to patients with and without mechanical ventilation. Hospital LOS was stratified by health status at the end of hospitalization (cured, uncured or death). Analysis of RESTORE IMI-2 LOS data indicated that patients who remain uncured experienced on average a longer LOS than those who were cured. Patients that died experienced on average a shorter LOS than those who were cured. Table 1 summarizes in-hospital LOS data applied in the model. The model includes an additional LOS penalty of 6.5 days for those who switch due to inappropriate therapy following susceptibility results [13].
Adverse events
Safety data for IMI/REL and PIP/TAZ were primarily taken from RESTORE-IMI 2; the analysis considered serious drug-related AEs during IV therapy and 14-day follow-up (Table 1). Additionally, for IMI/REL and CMS+IMI, treatment-emergent nephrotoxicity observed in the RESTORE-IMI 1 trial were included as an additional AE (Table 1) [14]. This additional AE was included in the model due to potential renal toxicity concern associated with colistin, included as a salvage treatment.
Economic inputs
Costs
The cost–effectiveness model considered the following direct costs (see full list and values in Table 1) borne by the US healthcare system: treatment-acquisition costs, hospital resource use costs, AE management costs, and long-term monitoring costs.
Drug unit costs were based on wholesale acquisition costs from the Micromedex REDBOOK [17]. Total treatment costs were calculated by combining pack costs with recommended daily dose and the trial-observed average treatment duration. Drug administration costs were not included in the base case analysis, as it is assumed these costs are implicitly captured by hospital LOS costs.
As previously described, in-hospital LOS (ICU and general ward) was captured in RESTORE-IMI 2 (stratified by ventilation status and health status at the end of hospitalization). Dasta et al. report ICU costs for non-ventilated and ventilated patients at days 1, 2 and 3 or more [18]. The inpatient general ward cost was sourced from the Kaiser Family Foundation database 2018 [19]. The costs associated with the management of AEs were taken from HCUPNet [20]. Lastly, all cost items were inflated to 2021 dollars using the Consumer Price Index sourced from the US Bureau of Labor Statistics [21].
It is assumed that 80% of cured HABP/VABP patients use monthly outpatient services for long-term monitoring for a duration of 1 year. The unit cost of an outpatient visit is $183.19 based on the CMS Physician Fee Schedule 2020 [22].
Health related quality-of-life
The RESTORE IMI-2 did not include any patient quality of life measure. Therefore, Health-related quality of life data were taken from the literature, and were conditional on hospital setting. The base case utility value for patients in the ICU was 0.68, while a utility value of 0.73 was applied for patients in the general ward [23,24]. The analysis did not consider utility decrements associated with AEs during the initial hospitalization period; as patient utility during the hospitalization period is based on hospital setting (ICU and general ward), including further per-AE-incidence utility loss risks double-counting. Furthermore, due to the short-term nature of the decision tree, the impact of incorporating AE utility decrements would be considered negligible. In the base case model, for cured patients who are cleared of infection, it is assumed that health-related quality of life returns to that of the general population [25].
Analysis
The following clinical and economic outcomes are considered in the analysis: cure and mortality rates by treatment pathway, total direct healthcare costs (by health status at the end of hospitalization and cost category), life years (LYs) and quality-adjusted life years (QALYs; a composite measure of duration and quality of life), and incremental cost–effectiveness ratio (ICER) in terms of additional cost per QALY gained and incremental net monetary benefit (INMB). The INMB is an informative metric when negative ICERs occur in sensitivity analysis results, as ICERs spanning different quadrants of a cost–effectiveness plane may be difficult to interpret. An INMB greater than zero is indicative of cost–effectiveness at a given willingness-to-pay threshold (here $100,000 per QALY gained).
Sensitivity analyses were conducted to capture parametric and methodological uncertainty, and test robustness of results. First, in one-way sensitivity analysis (OWSA), inputs with uncertainty were tested at their lower and upper bound in turn, and model results recorded to identify the most influential parameters. A summary of model inputs and uncertainty information (mean, distribution, 95% confidence interval) is provided in Appendix 3.
Second, given the importance of pathogen susceptibility on patient and model outcomes, a two-way sensitivity analysis (TWSA) was also conducted, varying the susceptibility coverage of IMI/REL and PIP/TAZ simultaneously in increments of 10%. Third, in probabilistic sensitivity analysis (PSA), all inputs were simultaneously varied from an assigned probability distribution and results recorded over 5,000 iterations. PSA results were presented as cost–effectiveness acceptability curve, which plotted a range of cost–effectiveness thresholds on the horizontal axis against the probability that the intervention will be cost-effective at that threshold on the vertical axis. Lastly, a set of alternative scenarios were analysed to evaluate different model assumptions on the cost–effectiveness outcomes.
Results
Deterministic results
As shown in Table 2, a higher proportion of patients enter the long-term Markov model as cured (62% vs 55%) in the early adjustment to IMI/REL arm. Similarly, the in-hospital mortality rate is lower in the early adjustment to IMI/REL arm (27% vs 33%).
Table 2. . Deterministic results.
| Outcome |
IMI/REL |
PIP/TAZ |
Incremental |
|---|---|---|---|
| Clinical outcomes | |||
| Clinical cure rate |
61.5% |
54.9% |
6.6% |
| In-hospital mortality rate | 27.4% | 33.4% | -6.0% |
| Long-term outcomes | |||
|---|---|---|---|
| Life years |
9.64 |
8.62 |
1.02 |
| QALYs | 7.92 | 7.08 | 0.84 |
| Economic outcomes | |||
|---|---|---|---|
| Treatment costs |
$9,130 |
$410 |
$8,720 |
| Resource use costs |
$170,600 |
$166,137 |
$4,464 |
| AE costs |
$4,527 |
$3,072 |
$1,455 |
| Monitoring costs |
$997 |
$895 |
$102 |
| Total costs |
$185,254 |
$170,513 |
$14,741 |
| ICER (cost per QALY) | - | - | $17,529 |
ICER: Incremental cost–effectiveness ratio; IMI/REL: Imipenem/cilastatin/relebactam; PIP/TAZ: Piperacillin/tazobactam; QALY: Quality-adjusted life year.
Consequently, when translating to long-term outcomes, IMI/REL generates more LYs (9.64 vs 8.62) and QALYs (7.92 vs 7.08) compared with PIP/TAZ, at an increased cost per patient ($185,254 vs $170,513). The corresponding ICER (cost per QALY gained) of $17,529 falls below the typical US willingness-to-pay threshold of $100,000 per QALY gained, reported in the Institute for Clinical and Economic Review reference case [10].
Hospital LOS costs were relatively consistent across treatment arms and were the primary contributor to total costs in both arms. The difference in total costs between the IMI/REL and PIP/TAZ treatment arms was primarily driven by a higher drug acquisition cost associated with IMI/REL.
Sensitivity analysis
Figure 3 presents the results of the OWSA based on INMB. The results of the OWSA indicate that the parameters with the largest impact on cost–effectiveness are the SMART-derived proportion of patients whose infections are susceptible to treatment, followed by the RESTORE-IMI 2 clinical response rates. This is unsurprising given that susceptibility parameters determine the proportion of patients who remain or switch treatment due to inappropriate therapy (which is in turn associated with worsened clinical and economic outcomes). Furthermore, the response rates determine the proportion of patients who enter the “cured” health state, influencing treatment and resource use costs during the hospital stay and long-term QALY gains.
Figure 3. . Deterministic sensitivity analysis.
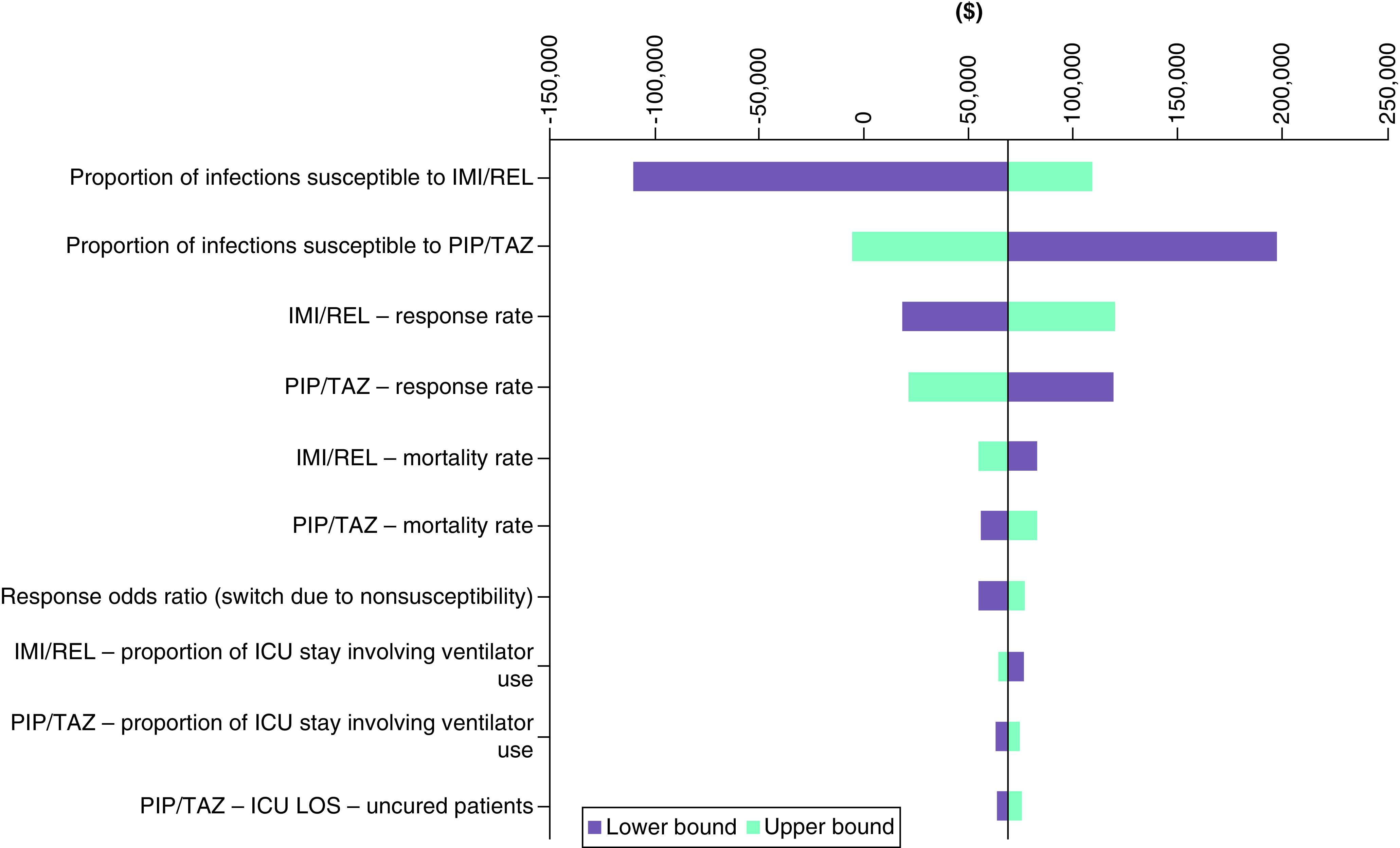
CMS+IMI: Colistimethate sodium plus imipenem/cilastatin; cUTI: Complicated urinary tract infection; HABP/VABP: Hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia; IMI/REL: Imipenem/Cilastatin/Relebactam; mMITT: Microbiological modified intent-to-treat.
TWSA results, varying IMI/REL and PIP/TAZ susceptibility coverage while holding all else constant, are presented as a heat map in Figure 4. The results demonstrate that, at a willingness-to-pay threshold of $100,000 per QALY gained, IMI/REL remains a cost-effective treatment option compared with PIP/TAZ with equal or greater susceptibility coverage (regardless of the starting susceptibility value between 10 and 100%). For example, the ICER for IMI/REL compared with PIP/TAZ when susceptibility coverage is assumed to be as low as 10% for both treatments is $24,508, with the ICER improving as the assumed difference in the proportion of infections susceptible to treatment increases.
Figure 4. . Two-way sensitivity analysis: relationship between cost–effectiveness and clinical efficacy of IMI/REL relative to CMS+IMI.
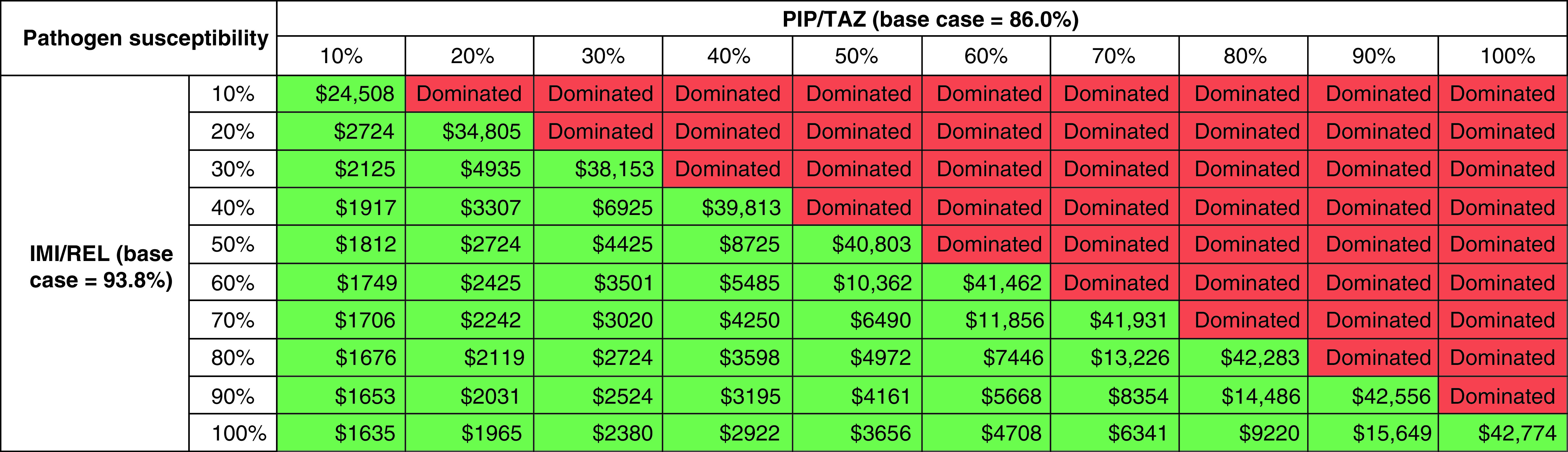
Green cells indicate that IMI/REL is considered cost-effective at a willingness-to-pay threshold of $100,000 per QALY gained; red cells indicate that IMI/REL is not considered cost-effective at a willingness-to-pay threshold of $100,000 per QALY gained.
IMI/REL: Imipenem/cilastatin/relebactam; PIP/TAZ: Piperacillin/tazobactam.
PSA results showed an average QALY gain of 0.81 for IMI/REL and an increased average cost of $14,300. The resulting ICER of $17,623 is close to the deterministic equivalent ($17,529), demonstrating the robustness of model results to parametric uncertainty. The cost–effectiveness acceptability curve in Appendix 4 demonstrates that, compared with PIP/TAZ, IMI/REL is more likely to be the cost-effective treatment option at willingness-to-pay thresholds of approximately $20,000 per QALY gained or higher in a US setting.
Model results were robust to methodological uncertainty in scenario sensitivity analysis, with IMI/REL remaining cost–effectiveness at willingness-to-pay thresholds commonly used by the Institute of Clinical and Economic Review when model settings were varied. A list of scenarios and corresponding results is provided in the supplementary materials (Appendix 5). It is note-worthy that, when the MITT sub-cohort who experienced prior treatment failure (i.e., study drugs were provided as an adjustment), IMI/REL was expected to cost less while yielding a positive QALY gain; this scenario provides reassurance of the value of IMI.REL – in this case dominant versus PIP/TAZ.
Discussion
Critically ill patients with HABP/VABP often require treatment and early adjustment of their antibacterial therapy prior to the availability of pathogen identification and susceptibility results. PIP/TAZ is a routine empiric treatment option due to its broad coverage; however, susceptibility analysis using the SMART 2017-19 US cohort indicated a collective susceptibility of 86%. Therefore, 14% of empiric treatment with PIP/TAZ could be considered ‘inappropriate if used as an initial treatment’ and may result in detrimental clinical outcomes. Jones et al. reported on an international sample of lower respiratory tract specimens from patients in the ICU and indicated that Pseudomonas aeruginosa and Enterobacterales were most common (∼80%), of which only 73% were considered susceptible to PIP/TAZ [26]. In contrast, IMI/REL has demonstrated potent in vitro activity against both Pseudomonas aeruginosa and Enterobacterales isolates, providing approximately up to 45% higher susceptibility rate than tested comparators for Enterobacterales [27], and 15–25% higher than comparators for Pseudomonas aeruginosa [28,29].
Despite higher rates of in vitro susceptibility for IMI/REL, its use as an alternative empiric option versus PIP/TAZ is challenged for two reasons; the higher drug acquisition cost, and the belief that new antibacterial agents should be reserved for patients with limited or no alternative treatment choice. Nonetheless, our analyses suggest that empiric treatment using PIP/TAZ should be carefully assessed by clinicians and when appropriate subject to early adjustment prescribing to IMI/REL to mitigate the risk of initial inappropriate treatment. This study simulated such a strategy using an economic model and demonstrated its clinical and economic viability.
This study uses the best available evidence to simulate real-world prescribing practices, with diverse scenario sensitivity analyses to demonstrate the potential impact of IMI/REL as an early adjustment prescribing option. As acknowledged in the ‘methods’ section, data inputs specific to an “early adjustment prescribing” pathway were lacking. Therefore, to populate this model, it was necessary to consider expert inputs and the available data from the RESTORE IMI-2 trial, in which patients were not provided with IMI/REL as an early adjustment but treated empirically. As such, it should be recognized that the results of these analysis may not directly align with real-world early adjustment for IMI/REL. Nevertheless, in places where direct evidence was missing, inputs that were in favour of PIP/TAZ were chosen (see Appendix 6 for more detailed discussion). One example of this conservate approach was that the susceptibility data from SMART included all lower respiratory tract specimens, rather than those from a subgroup of patients that were PIP/TAZ resistant and therefore subject to early adjustment. Another example is the use of efficacy data from RESTORE IMI-2 MITT cohort instead of the sub-cohort that experienced prior antibiotic treatment failure and showed more favourable results for IMI/REL. Hence, it is expected that the conclusion of IMI/REL being a cost-effective option for early adjustment treatment would be conservative and applicable to real-world practice.
This study is subject to several limitations. The first relates to the application of the trial data used in the model. As shown in the sensitivity analysis, comparative efficacy data taken from RESTORE-IMI 2 are one of the key drivers of cost–effectiveness results. Although IMI/REL was associated with a higher response rate (mean difference = 1.3%) and lower mortality rate (mean difference = -5.4%), it should be noted that RESTORE-IMI 2 was a noninferiority trial, and thus not designed to demonstrate superiority in efficacy end points. Furthermore, it was assumed that clinical data from the MITT population of RESTORE-IMI 2 was generalizable to the population considered in this analysis; that is, those experiencing early adjustment to IMI/REL due to deterioration or suspected nonsusceptibility to empiric PIP/TAZ. Ideally, this analysis would have incorporated data from a population of patients treated with PIP/TAZ followed by early adjustment to IMI/REL; however, such data are not available, and it is not possible to isolate the true effect of the modelled pathway from the available data.
Secondly, the analysis does not consider any of the wider external costs or benefits associated with the introduction of a new antimicrobial agent, beyond that of the cohort of treated patients. For example, this analysis cannot quantify the potential impact of slowing the build-up of antibacterial resistance with another treatment option or reducing onward population transmission rates, and as such, it could be the case that these analyses provide a conversative estimate of the full value of IMI/REL as an early adjustment treatment option for patients with HABP/VABP.
Thirdly, as a simplifying assumption, the model assumes no difference in future risk of recurrence between two treatment arms once patients are initially cured. Neither recurrence or readmission are incorporated in the analysis, and it is assumed that those who remain alive but uncured at the end of the decision-tree enter the death state within a year given the severity of HABP/VABP.
Conclusion
This analysis is the first to our knowledge to consider the real-world challenge of managing inappropriate initial (empiric) therapy among patients with HABP/VABP. In the absence of data pertaining to early treatment adjustment, we utilized US specific susceptibility data to inform a treatment pathway populated with available efficacy and safety data to determine a likely outcome among patients experiencing early treatment adjustment. Our results, as tested, suggest that IMI/REL, when considered alongside the principles of antimicrobial stewardship and an awareness of local susceptibility data, may represent a valuable and cost-effective treatment option to clinicians and decision makers to improve patient outcomes.
Summary points.
HABP/VAPB are common nosocomial infections associated with high rates of morbidity and mortality; outcomes are often worse when causative pathogens are resistant to antibacterial agents.
Due to its broad antibacterial activity, IMI/REL (which is approved by the US FDA for treating HABP/VABP), may help to address some of the unmet need caused by antimicrobial resistance for safe and efficacious treatments.
The effective management of HABP/VABP often relies on the selection of appropriate initial therapy, which in clinical practice may need to be adjusted. This study evaluated the cost–effectiveness of IMI/REL for the treatment of patients with HABP/VABP when used in an ‘early adjustment prescribing scenario’; that is, before the results of pathogen susceptibility, but after the initiation of an empirical treatment that is deemed inadequate.
An economic model (comprising a short-term decision tree followed by long-term Markov model) was constructed to compare costs and outcomes from a US payer perspective for two treatment strategies: continuation of empiric PIP/TAZ versus early adjustment to IMI/REL.
Clinical inputs informing the economic model were sourced from the phase III RESTORE-IMI 2 trial comparing IMI/REL with PIP/TAZ, and a US subsample of the Study for Monitoring Antimicrobial Resistance Trends (SMART) database 2017–2019.
RESTORE-IMI 2 demonstrated favourable (not statistically significant) day 28 response and mortality rates for IMI/REL versus PIP/TAZ, while the SMART database showed a higher proportion of causative pathogens to be susceptible to IMI/REL compared with PIP/TAZ.
Our cost–effectiveness analysis estimated that a higher proportion of HABP/VABP patients were cured in the early adjustment to IMI/REL arm. Consequently, IMI/REL generated more LYs and QALYs than PIP/TAZ, at an increased cost per patient. The difference in total costs was primarily driven by a higher drug acquisition cost associated with IMI/REL.
The corresponding ICER of $17,529 is below the typical US willingness-to-pay threshold. Our findings suggest that IMI/REL, when considered alongside the principles of antimicrobial stewardship and an awareness of local susceptibility data, may represent a valuable and cost-effective treatment option to clinicians and decision makers.
Supplementary Material
Acknowledgment
The authors thank Dr. Eilish McCann, Dr. Sanjay Merchant and Alex Watanabe for their discussion and support of this study.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/cer-2022-0113
Author contributions
All authors are responsible for the work described in this article. All authors were involved in the design/development of the analysis and drafting/critically reviewing the manuscript.
Financial & competing interests disclosure
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA (MSD). At the time this study was conducted, Jaesh Naik, Matthew Massello, and Lewis Ralph were employees of Bresmed, which received a collaborative contract from Merck & Co., Inc., Rahway, NJ, USA. Zhuo Yang is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) and may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. At the time this study was conducted, Ryan Dillon was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) and may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Food and Drug Administration. RECARBRIO. July 2021. Available from: www.merck.com/product/usa/pi_circulars/r/recarbrio/recarbrio_pi.pdf (Accessed: 2 March 2022).
- 2.Food and Drug Administration. Antibacterial Therapies for Patients With an Unmet Medical Need for the Treatment of Serious Bacterial Diseases. August 2017. Available from: www.fda.gov/regulatory-information/search-fda-guidance-documents/antibacterial-therapies-patients-unmet-medical-need-treatment-serious-bacterial-diseases
- 3.European Medicines Agency. Evaluation of medicinal products indicated for treatment of bacterial infections. Available from: www.ema.europa.eu/en/evaluation-medicinal-products-indicated-treatment-bacterial-infections-scientific-guideline
- 4.Centers for Disease Control and Prevention. 2019 AR Threats Report. Available from: www.cdc.gov/drugresistance/biggest-threats.html
- 5.World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics (2018). [Google Scholar]
- 6.Infectious Diseases Society of America (IDSA) , Spellberg B, Blaser M, Guidos RJ et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin. Infect. Dis. 52(Suppl. 5), S397–S428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant S, Proudfoot EM, Quadri HN et al. Risk factors for Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 14, 33–44 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Raman G, Avendano E, Berger S, Menon V. Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: systematic review and meta-analysis. BMC Infect. Dis. 15, 395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassetti M, Rello J, Blasi F et al. Systematic review of the impact of appropriate versus inappropriate initial antibiotic therapy on outcomes of patients with severe bacterial infections. Int. J. Antimicrob. Agents 56(6), 106184 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Institute for Clinical and Economic Review (ICER). ICER's Reference Case for Economic Evaluations: Principles and Rationale. 31 January 2020. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_Reference_Case_013120.pdf (Accessed: 26 May 2021).
- 11.Titov I, Wunderink RG, Roquilly A et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 Study). Clin. Infect. Dis. 73(11), e4539–e4548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Study for Monitoring Antimicrobial Resistance Trends (SMART). Merck & Co. Inc, NJ, USA: (2019). [Google Scholar]
- 13.Prabhu V, Sen S, Miller B, Basu A, Medic G. Using an economic model to choose initial appropriate antibiotic therapy based on differences in in-vitro susceptibility to ceftolozane/tazobactam and piperacillin/tazobactam. Presented at: ISPOR 18th Annual European Congress. Milan, Italy: (2015). [Google Scholar]
- 14.Motsch J, Murta de Oliveira C, Stus V et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin. Infect. Dis. 70(9), 1799–1808 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siempos II, Vardakas KZ, Kyriakopoulos CE, Ntaidou TK, Falagas ME. Predictors of mortality in adult patients with ventilator-associated pneumonia: a meta-analysis. Shock 33(6), 590–601 (2010). [DOI] [PubMed] [Google Scholar]
- 16.National Vital Statistics Reports. United States Life Tables. 2018. Available from: www.cdc.gov/nchs/data/nvsr/nvsr69/nvsr69-12-508.pdf [PubMed]
- 17.IBM Microdex. The Red Book Online. 2020. Available from: www.micromedexsolutions.com/home/dispatch/ssl/true (Accessed: 20 November 2020).
- 18.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit. Care Med. 33(6), 1266–1271 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day. 2018. Available from: www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D# (Accessed: 16 November 2020).
- 20.Agency for Health and Research Quality. Healthcare Cost and Utilization Project (HCUP). 2020. Available from: https://hcupnet.ahrq.gov/#setup [PubMed]
- 21.US Bureau of Labor Statistics. Consumer Price Index. 2020. Available from: www.bls.gov/cpi/
- 22.Centers for Medicare and Medicaid Services (CMS). Physician's fee schedule. 2020. Available from: www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html (Accessed: 20 November 2020).
- 23.Whittington MD, Atherly AJ, Curtis DJ et al. Recommendations for methicillin-resistant infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J. Glob. Antimicrob. Resist. 14, 33–44 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Lee BY, Wiringa AE, Bailey RR et al. The economic effect of screening orthopaedic surgery patients preoperatively for methicillin-resistant. Infect. Control Hosp. Epidemiol. 31(11), 1130–1138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szende A, Janssen B, Cabases J. (Eds). Self-reported population health: an international perspective based on EQ-5D. SpringerOpen, The Netherlands: (2014). [PubMed] [Google Scholar]
- 26.Moise PA, Gonzalez M, Alekseeva I et al. Collective assessment of antimicrobial susceptibility among the most common Gram-negative respiratory pathogens driving therapy in the ICU. JAC Antimicrob. Resist. 3(1), dlaa129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heo YA. Imipenem/cilastatin/relebactam: a review in Gram-negative bacterial infections. Drugs 81(3), 377–388 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlowsky JA, Lob SH, Raddatz J et al. In vitro Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 31(11), 1130–1138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlowsky JA, Lob SH, Young K, Motyl MR, Sahm DF. Activity of imipenem/relebactam against activity of imipenem/relebactam and ceftolozane/tazobactam against clinical isolates of Gram-negative bacilli with difficult-to-treat resistance and multidrug-resistant phenotypes-study for monitoring antimicrobial resistance trends, United States 2015–2017. Clin. Infect. Dis. 72(12), 2112–2120 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.