Abstract
Aim:
To assess changes in outcomes and costs upon implementation of continuous vital sign monitoring in postsurgical patients.
Materials & methods:
Retrospective analysis of clinical outcomes and in-hospital costs compared with a control period.
Results:
During the intervention period patients were less frequently admitted to the intensive care unit (ICU) (p = 0.004), had shorter length of stay (p < 0.001) and lower costs (p < 0.001). The intervention was associated with a lower odds of ICU admission (odds ratio: 0.422; p = 0.007) and ICU related costs (coefficient: -622.6; p = 0.083).
Conclusion:
Continuous vital sign monitoring may have contributed to fewer ICU admissions and lower ICU costs in postsurgical patients.
Keywords: early warning score, outcomes research, real-world evidence, vital sign monitoring, wearable sensors
Plain language summary
What is this article about?
Continuous vital sign monitoring assists in identifying deteriorating patients outside intensive care. This study analyses changes in clinical outcomes and costs before and after implementation of a clinical vital sign monitoring device in postsurgical patients on a general ward in a Dutch hospital.
What were the results?
Results show that after implementation, patients were less likely to be admitted to the intensive care unit, had shorter length of stay and had lower hospital stay costs.
Tweetable abstract
A retrospective study of clinical outcomes and costs compared with a control period indicated that implementation of continuous vital sign monitoring in postsurgical patients in a general hospital in The Netherlands may have contributed to fewer intensive care unit admissions and lower intensive care unit costs.
Approximately 7 to 9% of all hospitalized patients suffer from an adverse event (AE) in Dutch hospitals each year [1,2]. These events, such as sepsis or respiratory failure, are unintended patient outcomes that arise from inadequate clinical management rather than the underlying disease of the patient [3] and frequently lead to intensive care unit (ICU) admissions or even death [4]. In addition, the direct medical costs associated with AEs in Dutch hospitals are substantial [1,5]. Importantly, the incidence of AEs is expected to increase as a result of shortages of nursing staff, increased complexity of in-hospital care, and an aging population [6].
A substantial share of AEs is preventable [1,2]. Physiological disturbances can be detected up to 24 h prior to a serious AE [7,8]. To prevent AEs, since the late 1990s early warning scores (EWS) are measured three times per day on general wards, a physiological track-and-trigger system that warns clinicians in case of physiological abnormalities [9,10]. The EWS is based on the vital signs respiratory rate (RR), oxygen saturation, temperature, blood pressure, heart rate (HR) and the alert, verbal, pain, unresponsive scale. Based on the EWS, corrective interventions like increasing nursing attention or activating a rapid response team (RRT) can be taken [10,11].
However, the current intermittent measurement intervals lead to monitoring gaps during which deterioration of the patient can go unnoticed [4]. Therefore, continuous vital sign monitoring has long been accepted on ICUs already [12]. In recent years, there has been a growing awareness that monitoring on general wards should be approached similarly to ICUs [13,14]. New developments in wearable devices have made ICU style monitoring possible on general wards, allowing for earlier identification of deteriorating patients [4,15], while these wearable devices are not invasive and do not inhibit patient mobility and recovery [16,17]. Until now, only few studies have assessed the impact of continuous vital sign monitoring on clinical outcomes. A Systematic Review performed by Sun and colleagues showed that non invasive monitoring devices improve clinical outcome measures in general wards outside the ICU [18]. In contrast, a Systematic Review by Areia et al., did not find significant evidence of wearable vital sign monitoring being superior to standard care [19]. According to the authors, this lack of significant evidence is caused by differing study designs, few available studies, diversity in the outcome measures analyzed and small patient samples [19]. Aside from effects on clinical outcomes, impacts on costs have rarely been included in studies evaluating the implementation of continuous vital sign monitoring devices. The few studies that did investigate financial outcomes found lower cost of patient stay in the hospital (attributed mostly to shortened length of stay and lower ICU costs) [20–22]. Together, these findings indicate a need for further investigation of the impact of wearable vital sign monitoring on clinical outcomes and costs.
One of the commercially available vital sign monitoring devices is the wearable biosensor of Philips (Philips Medical Systems, MA, USA). This biosensor is a lightweight patch, which is attached to the patient’s chest and monitors RR, HR, skin temperature, together with contextual information on posture, activity level and ambulation of the patient. The physiological data obtained by the biosensor are sent to the patient monitoring system (IntelliVue Guardian Solution [IGS], Philips Healthcare, Böblingen, Germany) backend, where an EWS is calculated. When the EWS exceeds a predefined threshold, the nursing staff will receive a notification via a mobile phone. The wearable biosensor is able to monitor RRs continuously and the acquired data are comparable to traditional capnography monitoring devices in patients on the emergency department [23]. However, no studies have investigated the impact of this particular wearable device on clinical outcomes and costs when deployed on a general ward. Therefore, this retrospective observational study assessed changes in clinical outcomes and in-hospital costs before and after the implementation of a non invasive continuous vital sign monitoring device, the wearable biosensor, in postsurgical patients on a general ward.
Materials & methods
Study design
This was a retrospective pre-post study conducted at Bravis Hospital (Roosendaal, The Netherlands), a general hospital located in the cities of Roosendaal and Bergen op Zoom in the South-Western part of The Netherlands. This study evaluated the association of implementation of the wearable biosensor with changes in clinical and financial outcomes. The biosensor was implemented on a general surgical ward with 48 beds. Patients admitted to the ward include postoperative gynecology and intestinal patients who qualify for the Enhanced Recovery After Surgery (ERAS) protocol [24]. This ward was selected because of its large proportion of patients with complex physiology and high rates of ICU transfers. The study included all patients admitted to the ward who had at least one overnight stay. Patients who were admitted for elective procedures or procedures other than the ERAS protocol were excluded. This study was reviewed and approved by the hospital’s institutional review board (PAC-2021-002) and granted exemption for patient consent.
Hospital policy stipulates the recording of vital signs in patients admitted to the surgical ward at least three times per day. The frequency of recording vital signs can be increased in case of or based on: deviations in one or more vital sign parameters; infections; a request by the head practitioner; nurses’ intuition; first week following ICU or cardiac care unit admission; the first 24 h post operation. Trained registered nurses obtain and record vital signs in patients’ electronic health record, where a corresponding EWS is calculated. In case of a score of 3 or higher, escalation to the RRT is performed. In order to avoid subjective judgement, all notifications are classified as independent events. Figure 1 shows the full escalation scheme.
Figure 1. . Early warning scores escalation scheme.
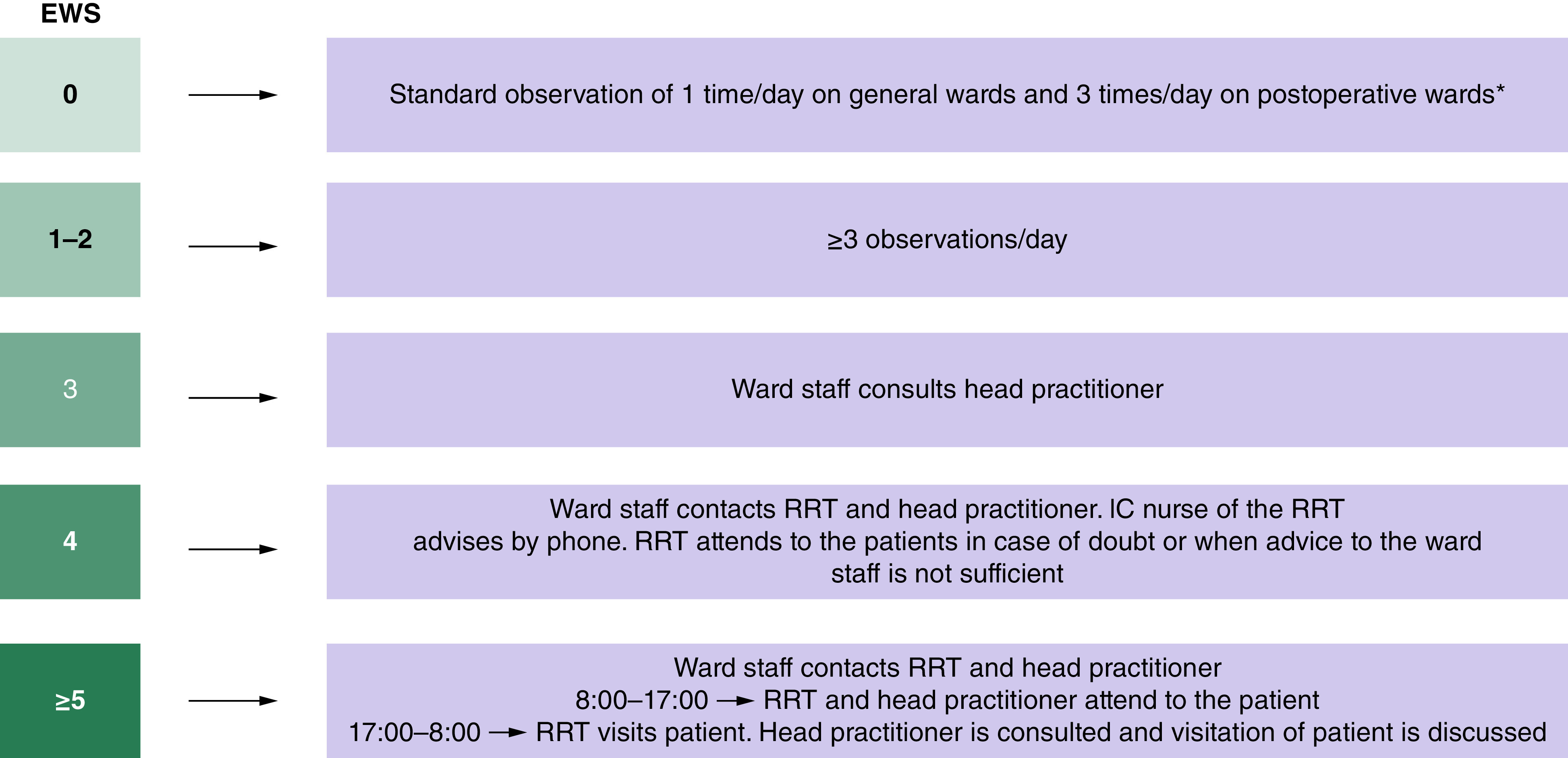
*The recommended observation interval for patients is 8 hours. Monitoring frequency can be increased in one of the following incidences: deviation of one or more of the vital parameters, patients with infections, upon request of the head practitioner, nurses' intuition, first week following intensive care or cardiac care unit admission and 24 h post-operative.
EWS: Early warning scores; RRT: Rapid response team.
On 1 October 2019, an electronic automated advisory vital signs monitoring system (IntelliVue Guardian Solution [IGS] with wearable biosensors and MP5SC spot-check monitors, Philips Healthcare, Böblingen, Germany) was deployed on the ward. All components of this system are commercially available products (CE-marked, US FDA cleared) and have undergone all the required testing to fulfil the current standards for data privacy and cybersecurity. RR and HR are measured continuously by the biosensor. Other parameters of the EWS, in other words, blood pressure, oxygen saturation, body temperature and patient consciousness, are measured at the bedside by nurses using the spot-check monitors. The full set of vital sign measurements and the resulting EWS are electronically transferred to the IGS backend as well as to the patient's EMR. In between spot-check measurements, data of the biosensor are continuously transmitted to the IGS backend servers located on the hospital premises and consequently may trigger notifications on mobile devices worn by the nurse staff. The mobile devices are IGS clients and are only able to receive data from the IGS backend; they are not connected to telecommunication networks. The complete system operates within the hospitals IT infrastructure and is; therefore, protected by the hospital IT security measures against external threats.
Based on automated biosensor measurements, notifications of a patient’s deterioration are sent to the nurse’s mobile device when changes in the parameters resulted in a change of the EWS. Ward nurses received automated notifications when the EWS exceeded the threshold of 3 based on the HR and RR. According to clinical routine, upon notification, the nurse visits the patient, evaluates the situation and calls the RRT if necessary. In case automated notifications are missed by the nurse staff, fall back is ensured with standard care consisting of the routine EWS monitoring every 8 h. Implementation of electronic automated advisory vital signs monitoring system on the general ward did not result in a change of the nurse-to-patient ratio on the department.
Data collection
For all ERAS patients admitted to the ward during the study period, data were collected on patient characteristics, clinical outcomes and costs. The study period was divided into a control and an intervention period. Training in the new routine for all staff was performed for 3 months, from 1 October 2019 until 31 December 2019. This period was defined as a washout period and was not included in the control or intervention period. The control period ranges from 1 January 2017 to 30 September 2019 and the intervention period from 1 January 2020 to 30 April 2021.
Data were obtained on length of stay, number of RRT activations during stay, ICU transfers, resuscitations, readmission within 30 days, in-hospital mortality and mortality within 30 days after discharge. Length of stay data showed large outliers in both the control and intervention periods, with a maximum of 98 days that disproportionately affected the mean length of stay. Before analysis, length of stay was therefore set to 30 days for 7 patients. Data on patient characteristics included age, gender, BMI and disease categories. The latter reflect the underlying disease, for example, intestinal malignancies, non-malignant intestinal diseases and gynecological malignancies. BMI values were missing for some patients in both periods. For these patients, BMI was assumed to be equal to the mean BMI of other patients with the same gender and age in the corresponding group. Underlying parameters of the EWS (HR, RR, oxygen saturation, blood pressure, body temperature and patient consciousness) were obtained of those patients who had RRT activations during their hospitalization.
Costs were estimated using the costing tool developed by the institute for Medical Technology Assessment (iMTA) [25] and adjusted for inflation using the average consumer price index in the period of 2017–2021 as indicated by Statistics Netherlands. In addition to total in-hospital costs, costs of stay at the general ward and costs of stay at the ICU were analyzed separately. Costs of the implementation of the wearable biosensor include license costs of the system, hardware and software costs, installation, testing and training of employees. Annual costs include a yearly license of the software, labour required for system maintenance and wearable biosensor costs.
Statistical analysis
Descriptive statistics for binary variables are presented as numbers and percentages. For continuous variables, descriptive statistics are presented as means with standard deviation (SD). As continuous variables were not normally distributed according to the Kolmogorov–Smirnov test, pre-post analysis for continuous variables (age, BMI, length of stay, ICU days, ward costs, ICU costs and total costs) was performed using the Mann–Whitney test. For binary variables (gender, disease category, RRT activation, EWS scores and sub scores, ICU admission, readmission within 30 days, resuscitation and mortality), pre-post analysis was performed using the Chi-square test. In addition, the association of implementation of the biosensor with (changes in) continuous and binary outcomes was assessed using linear and logistic regression analysis, respectively, adjusting for patient characteristics, age, gender, BMI and disease categories. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Apple, Ver 27.0 (IBM Corp, NY, USA).
Results
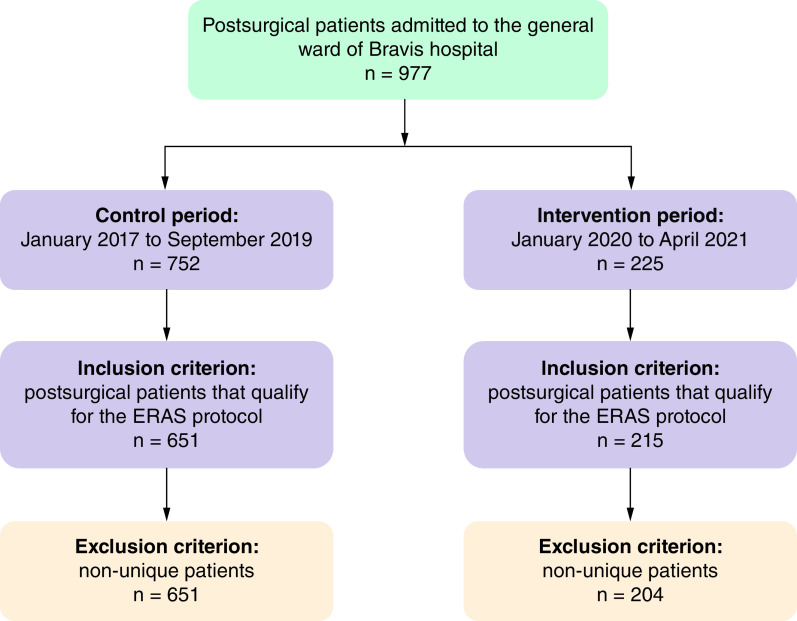
During the study period, 977 postsurgical patients were admitted to the ward. Of these, 752 and 225 patients were observed in the control and intervention period, respectively. Imposing the inclusion and exclusion criteria led to a final sample of 855 patients, 204 of whom were in the intervention period and 651 in the control period (see Figure 2).
Figure 2. . Sample population flow chart.
The control period was separated from the intervention period by a 3-month wash-out period as described in the methods section.
Patient characteristics are summarized for the intervention and control group in Table 1. The average age was 66.7 years (SD: 12.6 years) in the control group and 65.3 years (SD: 13.8 years) in the intervention group (p = 0.233). The percentage of males was 49.9% in the control group and 56.9% (p = 0.084) in the intervention group. Mean BMI in the control group was 26.6 kg/m2 (SD: 4.8) and 26.3 kg/m2 (SD: 4.5) in the intervention group (p = 0.456). In the control group, 67.4% of the patients underwent surgery for intestinal malignancies, 20.4% for other non-malignant intestinal diseases and 12.1% for gynecological malignancies. In the intervention group, 60.8% of the patients underwent surgery for intestinal malignancies, 26.5% for non-malignant intestinal diseases and 12.7% for gynecological malignancies (p = 0.159).
Table 1. . Patient characteristics.
| Control group (n = 651) | Intervention group (n = 204) | p-value | |
|---|---|---|---|
| Mean age in years (SD) | 66.7 (12.6) | 65.3 (13.8) | 0.233 |
| Male (%) | 49.9 | 56.9 | 0.084 |
| Mean BMI (kg/m2, SD) | 26.6 (4.8) | 26.3 (4.5) | 0.456 |
| Disease Categories (%) | |||
| Intestinal malignancies | 67.4 | 60.8 | |
| Non-malignant intestinal diseases | 20.4 | 26.5 | |
| Gynecological malignancies | 12.1 | 12.7 | |
| Overall | 0.159 | ||
p-values pertain to the differences between control and intervention period. Mann–Whitney tests were performed for the variables ‘age’ and ‘BMI’ and Chi-square tests were used ‘male’ and ‘disease categories’.
SD: Standard deviation.
Clinical outcomes
Mean total length of stay in the control group was 8.7 days (SD: 6.9 days) and 8.0 days (SD: 6.15 days) in the intervention group (p < 0.001) (Table 2). In the control group, 13.2% (86/651) patients were admitted to the ICU, which was significantly higher than in the intervention group (5.9%; p = 0.004). Length of stay in the ICU was lower in the intervention group (3.8 days, SD: 2.7) than in the control group (4.2 days, SD: 5.42), but this difference was not statistically significant (p = 0.331). The shorter length of stay that was observed after implementation of the biosensor was only observed in patients who underwent surgery for intestinal (p < 0.001) or gynecological malignancies (p = 0.002), but not for non-malignant intestinal diseases (p = 0.667), whereas the decrease in ICU transfers was only observed in patients with intestinal malignancies (Supplementary Table 1). No significant differences between intervention and control group were observed in the rates of RRT activation, readmission, resuscitation and mortality.
Table 2. . Clinical outcomes.
| Outcomes | Control group (n = 651) | Intervention group (n = 204) | p-value |
|---|---|---|---|
| Mean length of stay in days (SD) | 8.7 (6.15) | 8.0 (6.89) | <0.001 |
| ICU admission (%) | 13.2 | 5.9 | 0.004 |
| Mean number of ICU days (SD) | 4.2 (5.42) | 3.8 (2.70) | 0.331 |
| RRT activation (%) | 7.4 | 9.8 | 0.263 |
| Readmission (30 days) (%) | 20.7 | 23.5 | 0.396 |
| Resuscitation (%) | 0.9 | 0 | 0.169 |
| In-hospital mortality (%) | 3.1 | 2 | 0.402 |
| Mortality (30 days) (%) | 1.4 | 1 | 0.657 |
p-value pertain to the differences between control and intervention period. Statistical significance was determined using a Mann–Whitney test for ‘Length of stay’ and ‘Mean number of ICU days’. A Chi-square test was used for the other outcome variables.
RRT: Rapid response team; SD: Standard deviation.
To determine whether the differences in clinical outcomes between the two groups can be explained by differences in patient characteristics, a multivariable logistic regression analysis was performed. After adjustment for age, gender, BMI and disease categories, implementation of the biosensor was only significantly associated with the odds of being admitted to the ICU (OR: 0.422; p = 0.007) (see Table 3 & Supplementary Tables 2 & 3 for a summary of the regression results for the outcome variables length of stay and ICU admission, respectively).
Table 3. . Regression results for clinical and financial outcomes.
| Estimated coefficient/odds ratio of Biosensor | p-value | |
|---|---|---|
| Length of stay | -0.530 | 0.296 |
| ICU admission | 0.422 | 0.007 |
| Ward costs | -47.238 | 0.833 |
| ICU costs | -622.648 | 0.083 |
| Total costs | -669.886 | 0.127 |
Results based on a multivariable logistic regression for ICU admission and a multivariable linear regression for length of stay, ward costs, ICU costs and total costs. Estimated coefficients (length of stay, costs) and odds ratio (ICU admission) for Biosensor adjusted for patient age, gender, BMI and disease category.
ICU: Intensive care unit.
Individual vital sign parameter input to EWS
We also analyzed the individual vital sign parameters (specifically HR and RR as these are continuously monitored by the biosensor) of the EWS in case of a RRT activation in the control and intervention period. In the intervention period, the frequency of RRT activations where both HR and RR contributed to an EWS alarm was 82.1% compared with 60.5% in the control period (p = 0.019) (Supplementary Table 2). EWS sub scores of HR and RR were on average also higher in the intervention period (1.67 and 1.82, respectively) than in control period (1.37 and 1.50, respectively), but these differences were not statistically significant (Supplementary Table 3).
Costs
The implementation costs of the IntelliVue Guardian Solution (IGS) with wearable biosensors and MP5SC spot-check monitors at the general ward in Bravis Hospital were €88,286. Annual costs for the system were €16,453 for the intervention period with additional costs of €63 per wearable biosensor. Mean total costs of the system per patient during the intervention period were €144 (€81 annual costs per patient and €63 per biosensor) (Table 4). Both ward costs and ICU costs per patients were lower in the intervention period, resulting in mean total costs of €4064 per patient (SD: €4231) in the intervention group compared with €4855 per patient (SD: €5827) during the control period (p < 0.001). This indicates patient ward and ICU costs were €647 lower per patient after correction for the mean costs of the system per patient of €144 after implementation (Table 4). After adjustment for age, gender, BMI and disease categories in a multivariable linear regression analysis, implementation of the biosensor was not significantly associated with a change in ward costs (coefficient €47.2; p = 0.833) or total costs per patient (coefficient €669.9; p = 0.127). However, it did appear to be associated with lower ICU costs, although the estimated coefficient (€622.6) was not significant when using a significance level of 5% (p = 0.083) (see Table 3 & Supplementary Tables 6–8 for a summary of the regression results for the outcome variable ward costs, ICU costs and total costs, respectively).
Table 4. . Financial outcomes.
| Parameter | Control group (n = 651) | Intervention group (n = 204) | p-value |
|---|---|---|---|
| Mean annual system costs (SD) | €0 | €81 (0) | |
| Mean biosensor costs (SD) | €0 | €63 (0) | |
| Mean ward costs (SD) | €3713 (2654) | €3611 (3200) | 0.003 |
| Mean ICU costs (SD) | €1142 (4977) | €453 (2229) | 0.005 |
| Mean total costs (SD) | €4855 (5827) | €4064 (4231) | <0.001 |
p-values reflect the difference between control and intervention period based on a Mann–Whitney test. Annual system costs were determined based on annual license costs, labor costs and costs per biosensor and divided by the total number of patients in the intervention group. Ward costs and ICU costs were based on the costs stated in the costing tool developed by the institute for Medical Technology Assessment (iMTA) [25] and adjusted for inflation using the average consumer price index in the period of 2017–2021 as indicated by Statistics Netherlands and multiplied by the number of days on the ward or ICU per patient.
ICU: Intensive care unit; SD: Standard deviation.
Discussion
Wearable technologies that allow for continuous vital sign monitoring in ambulatory patients on general wards have the potential to positively impact patient outcomes by early detection of clinical deterioration. However, studies reporting on the effects of wearable vital sign monitoring on clinical and financial outcomes are sparse, and consequently, the impact of these novel devices remains largely unknown. Therefore, the purpose of this retrospective observational study was to assess changes in clinical outcomes and in-hospital costs before and after implementation of a non invasive continuous vital sign monitoring device, the wearable biosensor, in postsurgical patients on a general ward.
Our analysis revealed that after adjusting for differences in relevant patient characteristics, implementation of the wearable biosensor was associated with a lower ICU admission rate. Specifically, patients with the biosensor were found to be less than half as likely to be admitted to the ICU than patients without the device. In turn, this also seems to have impacted ICU costs, with patients with the biosensor on average incurring €622 lower costs on the ICU than patients without the biosensor (although this difference only reached statistical significance at a 10% significance level). Overall length of stay was also significantly lower in the time period when patients received the biosensor, but this appeared to be driven by a difference in patient characteristics as compared with the control group. Furthermore, decreases in the rate of resuscitations, readmissions and mortality were observed upon implementation of continuous vital sign monitoring on the ward, but these were not statistically significant.
Our finding of a reduced ICU admission rates following the implementation of the wearable biosensor, is in line with findings from previous studies [18,26]. In addition, Stellpflug et al., found that fewer patients were admitted to the ICU after having triggered a RRT [27]. Other studies had similar findings [13,16,28,29], although the effects reported in these studies did not reach statistical significance. This might have been caused by small patient sample sizes in these studies and/or analysis of low-risk patient groups (e.g., orthopedic and neurological patients) with low chances of being admitted to ICU.
Reductions in length of stay were also found by other studies [18], but like in our study not always reaching statistical significance [16]. Findings of reduced length of stay are often debated. Randomized controlled trials (RCTs) tend to not find significant effects which might also be due to limited numbers of patients included in these trials, while observational studies using a pre-post analyses often do find significant effects but which cannot be interpreted as causal as other factors (e.g., altered discharge policies) could also have affected length of stay during the study period.
We found no significant effects on RRT activation, 30-day readmission rate and in-hospital and 30-day mortality. Prior studies found varying results regarding the number of RRT activations. Our finding for this outcome is in line with the result of one study [29], while others found either an increase [13,21,26,30] or a decline [18,27,28] in RRT activations. This inconsistency in findings may be the result of differences in study setting and in escalation protocol following an early warning by a continuous monitoring device, as well as differences in the type of patients included in the study. Similar to our study, prior work also did not find effects on readmission or mortality rates [13,16,28,29], which appears to be related to small sample sizes and low numbers of events. Interestingly, the Meta-analysis by Sun et al. did find a reduction in mortality [18], which they attribute primarily to new studies with more high-risk patients included in their analysis compared with an earlier Review by Cardona-Morrell et al. [13].
Our results further suggest a potential negative impact on in-hospital costs, specifically lower ICU costs (€622), after implementation of the wearable biosensor. The few previous studies that also looked at financial outcomes following the implementation of a wireless vital sign monitoring system also found lower cost of inpatient stay, attributed mostly to shortened length of stay and lower ICU costs [20–22]. Costs savings ranged between $224 and $710 saved per person, dependent on the type of prediction model used by Slight et al. [20] and $2,897 saved per person by Mohr et al. [22] Large differences in cost savings can potentially be explained by the incorporation of costs attributed to implementation of the system.
A key strength of the current study is the length of the study period (around 4.5 years). Due to the small number of papers reporting on clinical outcomes and their contradictory results, it remained unclear at the outset of this research which clinical parameters to focus on to obtain an insight into the clinical impact of continuous vital sign monitoring devices. Therefore, this study focused on a broad set of clinical outcomes, including conventional clinical outcomes reflecting severe deterioration like mortality and ICU admissions as well as clinical outcomes that reflect milder deterioration, such as RRT activations. All in all, this study contributes to the scarce, but growing literature on the clinical impact of continuous vital sign monitoring devices.
Several limitations should also be acknowledged. First, the hospital’s implementation of the wearable biosensor in all ERAS patients made it impossible to conduct a RCT. Therefore, the study was designed as an observational pre-post analysis and our results should be interpreted with caution as time trends and unobserved confounding factors might have affected changes in the outcomes. Specifically, patient risk factors known to be associated with ICU admission and length of stay, such as Charlson’s comorbidity index and the American Society of Anesthesiologists score, were incompletely registered in the electronic health record of the included patients during the control and intervention periods and could therefore not be adjusted for in the analysis. Second, in this study the biosensor was implemented in postsurgical intestinal and gynecological patients admitted to a ward in a Dutch general hospital; the effect of this monitoring device might be different in other patient groups and other settings (for example, the impact might differ according to how the device is implemented in routine protocols). Therefore, further research in other hospitals, patient groups and settings is warranted. At last, this study may have been underpowered to detect differences in clinical outcomes with low number of events (e.g., resuscitation and mortality rates). These results should therefore be interpreted with caution.
Conclusion
In conclusion, this study suggests significant clinical benefits of the introduction of an ambulatory continuous vital sign monitoring device, the wearable biosensor, in postoperative intestinal and gynecological patients in addition to the conventional manual intermittent monitoring on the general surgical ward in a general hospital in The Netherlands. The biosensor detects clinical deterioration in these patients at an earlier stage, which appears to have contributed to patients being less likely to be admitted to the ICU, in turn contributing to numerically lower ICU costs.
Future perspective
Further investigation of the clinical and financial impact of continuous vital sign monitoring is strongly recommended. Ideally, multicenter international RCTs are conducted to provide insight into the causal effects on outcomes and costs. Future research should also determine the effects of continuous vital sign monitoring on different outcomes in different patient populations, since the impact might differ across types of care (e.g., orthopedic or trauma) or specific patient populations. These future analyses should also investigate cost–effectiveness among a broader population, as these data are essential to evaluate the additional benefit relative to the additional costs of continuous monitoring.
Summary points.
A wearable technology that allows for continuous vital sign monitoring in ambulatory patients was implemented in postsurgical patients on a general ward with the intention to positively impact patient outcomes by early detection of clinical deterioration.
Implementation of the wearable biosensor at Bravis hospital (Roosendaal, The Netherlands) was significantly associated with a reduced intensive care unit admission rate, relative to a control period.
Implementation of the wearable biosensor may lower in-hospital cost per patient via a decrease of intensive care unit costs (in this study: €622).
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/cer-2022-0176
Author contributions
All authors contributed to the design, interpretation, writing and approval of the manuscript. H Vroman and D Mosch acquired and analyzed the data. All authors agree to be accountable for all aspects of the work.
Financial & competing interests disclosure
B Mohr, E Naujokat and G Medic are the employees of Philips. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The institutional review board waived informed consent due to the large number of patients included, and the retrospective nature of the study.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Hoogervorst-Schilp J, Langelaan M, Spreeuwenberg P, De Bruijne MC, Wagner C. Excess length of stay and economic consequences of adverse events in Dutch hospital patients. BMC Health Serv. Res. 15(1), (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual. Saf. Heal. Care 17(3), 216–223 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwendimann R, Blatter C, Dhaini S, Simon M, Ausserhofer D. The occurrence, types, consequences and preventability of in-hospital adverse events - a scoping review. BMC Health Serv. Res. 18(1), (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weenk M, van Goor H, Frietman B et al. Continuous monitoring of vital signs using wearable devices on the general ward: pilot study. JMIR mHealth uHealth. 5(7), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoonhout LHF, De Bruijne MC, Wagner C et al. Direct medical costs of adverse events in Dutch hospitals. BMC Health Serv. Res. 9(1), (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leenen JPL, Leerentveld C, van Dijk JD, van Westreenen HL, Schoonhoven L, Patijn GA. Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: systematic review. J. Med. Internet Res. 22(6), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hillman K. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom - The ACADEMIA study. Resuscitation 62(3), 275–282 (2004). [DOI] [PubMed] [Google Scholar]
- 8.McGaughey J, Alderdice F, Fowler R, Kapila A, Mayhew A, Moutray M. Outreach and early warning systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards. Cochrane Database Syst. Rev. (3), (2007). [DOI] [PubMed] [Google Scholar]
- 9.Burch VC, Tarr G, Morroni C. Modified early warning score predicts the need for hospital admission and inhospital mortality. Emerg. Med. J. 25(10), 674–678 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Ludikhuize J, Smorenburg SM, de Rooij SE, de Jonge E. Identification of deteriorating patients on general wards; measurement of vital parameters and potential effectiveness of the Modified Early Warning Score. J. Crit. Care 27(4), 424.e7-13 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Smith MEB, Chiovaro JC, O'Neil M et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann. Am. Thorac. Soc. 11(9), 1454–1465 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Nates JL, Nunnally M, Kleinpell R et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit. Care Med. 44(8), 1553–1602 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Cardona-Morrell M, Prgomet M, Turner RM, Nicholson M, Hillman K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta-analysis. Int. J. Clin. Pract. 70(10), 806–824 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Jones D, Mitchell I, Hillman K, Story D. Defining clinical deterioration. Resuscitation 84(8), 1029–1034 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Eddahchouri Y, Koeneman M, Plokker M et al. Low compliance to a vital sign safety protocol on general hospital wards: a retrospective cohort study. Int. J. Nurs. Stud. 115 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Downey C, Randell R, Brown J, Jayne DG. Continuous versus intermittent vital signs monitoring using a wearable, wireless patch in patients admitted to surgical wards: pilot cluster randomized controlled trial. J. Med. Internet Res. 20(12), (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Liu M, Bai Y, Zhang J, Liu H, Zhu W. Recent progress in flexible wearable sensors for vital sign monitoring. Sensors (Switzerland) 20(14), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun L, Joshi M, Khan SN, Ashrafian H, Darzi A. Clinical impact of multi-parameter continuous non-invasive monitoring in hospital wards: a systematic review and meta-analysis. J. R. Soc. Med. 113(6), 217–224 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Areia C, Young L, Vollam S et al. Wearability testing of ambulatory vital sign monitoring devices: prospective observational cohort study. JMIR mHealth uHealth 8(12), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slight SP, Franz C, Olugbile M, Brown HV, Bates DW, Zimlichman E. The return on investment of implementing a continuous monitoring system in general medical-surgical units. Crit. Care Med. 42(8), 1862–1868 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Bellomo R, Ackerman M, Bailey M et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit. Care Med. 40(8), 2349–2361 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Mohr BA, Bartos D, Dickson S, Bucsi L, Vente M, Medic G. Economics of implementing an early deterioration detection solution for general care patients at a US hospital. J. Comp. Eff. Res. 11(4), 251–261 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Li T, Divatia S, McKittrick J, Moss J, Hijnen NM, Becker LB. A pilot study of respiratory rate derived from a wearable biosensor compared with capnography in emergency department patients. Open Access Emerg. Med. 11, 103–108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery a review. JAMA Surg. 152(3), 292–298 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Van Roijen LH. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health 18(6), 753–758 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Heller AR, Mees ST, Lauterwald B, Reeps C, Koch T, Weitz J. Detection of deteriorating patients on surgical wards outside the ICU by an automated MEWS-based early warning system with paging functionality. Ann. Surg. 271(1), 100–105 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Stellpflug C, Pierson L, Roloff D et al. Continuous physiological monitoring improves patient outcomes. Am. J. Nurs. 121(4), 40–46 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Weller RS, Foard KL, Harwood TN. Evaluation of a wireless, portable, wearable multi-parameter vital signs monitor in hospitalized neurological and neurosurgical patients. J. Clin. Monit. Comput. 32(5), 945–951 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Verrillo SC, Cvach M, Hudson KW, Winters BD. Using continuous vital sign monitoring to detect early deterioration in adult postoperative inpatients. J. Nurs. Care Qual. 34(2), 107–113 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Soar J, Subbe CP. Identifying the patient at risk of deterioration, intensive care unit admission, or cardiac arrest: stop predicting, start preventing. Crit. Care Med. 40(7), 2243–2244 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.