Abstract
Background:
Alternatives to the Friedewald low-density lipoprotein cholesterol (LDL-C) equation have been proposed.
Objective:
To compare the accuracy of available LDL-C equations with ultracentrifugation measurement.
Methods:
We used the second harvest of the Very Large Database of Lipids (VLDbL), which is a population-representative convenience sample of adult and pediatric patients (N = 5,051,467) with clinical lipid measurements obtained via the vertical auto profile (VAP) ultracentrifugation method between October 1, 2015 and June 30, 2019. We performed a systematic literature review to identify available LDL-C equations and compared their accuracy according to guideline-based classification. We also compared the equations by their median error versus ultracentrifugation. We evaluated LDL-C equations overall and stratified by age, sex, fasting status, and triglyceride levels, as well as in patients with atherosclerotic cardiovascular disease, hypertension, diabetes, kidney disease, inflammation, and thyroid dysfunction.
Results:
Analyzing 23 identified LDL-C equations in 5,051,467 patients (mean±SD age, 56±16 years; 53.3% women), the Martin/Hopkins equation most accurately classified LDL-C to the correct category (89.6%), followed by the Sampson (86.3%), Chen (84.4%), Puavilai (84.1%), Delong (83.3%), and Friedewald (83.2%) equations. The other 17 equations were less accurate than Friedewald, with accuracy as low as 35.1%. The median error of equations ranged from –10.8 to 18.7 mg/dL, and was best optimized using the Martin/Hopkins equation (0.3, IQR–1.6 to 2.4 mg/dL). The Martin/Hopkins equation had the highest accuracy after stratifying by age, sex, fasting status, triglyceride levels, and clinical subgroups. In addition, one in five patients who had Friedewald LDL-C <70 mg/dL, and almost half of the patients with Friedewald LDL-C <70 mg/dL and triglyceride levels 150–399 mg/dL, had LDL-C correctly reclassified to >70 mg/dL by the Martin/Hopkins equation.
Conclusions:
Most proposed alternatives to the Friedewald equation worsen LDL-C accuracy, and their use could introduce unintended disparities in clinical care. The Martin/Hopkins equation demonstrated the highest LDL-C accuracy overall and across subgroups.
Keywords: LDL-C, atherosclerotic cardiovascular disease, Friedewald equation, Martin/Hopkins equation, ultracentrifugation
Introduction
The global burden of cardiovascular disease continued to increase over the past decade and is responsible for an estimated 17 million deaths annually [1]. A major prevention target is low-density lipoprotein cholesterol (LDL-C), a causative factor of atherosclerotic cardiovascular disease (ASCVD) [2]. Reducing LDL-C by 80 mg/dL, or 2 mmol/L, can lower ASCVD risk by 40–50%, and recent clinical guidelines have adopted combination therapy approaches towards lower LDL-C levels [3]. The 2018 American Heart Association/American College of Cardiology (AHA/ACC) guidelines recommend lowering LDL-C levels by ≥50% in patients with ASCVD and intensifying therapy if LDL-C is >70 mg/dL in very-high risk patients [4]. The European Society of Cardiology (ESC) and 2022 ACC non-statin consensus pathway recommend treating LDL-C to <55 mg/dL [5,6]. Clinical laboratories and accrediting institutions have an important role to play in aligning reference values in clinical laboratory reports with the current guidelines to best inform use of lipid lowering therapy to improve outcomes in patients with or at risk for ASCVD.
It is also imperative to have the most accurate means possible of assessing LDL-C to guide evidence-based therapy. Preparative ultracentrifugation, also known as beta quantification (BQ), is the gold standard for measuring LDL-C. However, this method is expensive, laborious, and unsuitable for clinical laboratories performing large volumes of lipid assays daily. An alternative ultracentrifugation-based method is the Vertical Auto Profile (VAP) method, which separates cholesterol into lipoprotein classes using ultracentrifugation and has been validated against BQ with accuracy meeting the requirements of the CDC-NHLBI (Centers for Disease Control-National Heart, Lung, and Blood Institute) Lipid Standardization Program [7,8]. While ultracentrifugation measured LDL-C by BQ or VAP can serve as a reliable reference measure, it is not widely available in clinical practice. Due to lower cost and ease of implementation, computational tools are the method of choice for LDL-C assessment, with the Friedewald equation being the most widely used [9]. The Friedewald equation is generally accurate for the average patient, but underestimates LDL-C at lower levels (especially LDL-C <100 mg/dL), particularly in patients with triglyceride (TG) levels >150 mg/dL, leading to missed prevention opportunities for more aggressive lipid control [10]. In the setting of TG levels >400 mg/dL, Friedewald estimation is increasingly compromised and clinical laboratories often rely on direct chemical-based LDL-C assays [11]. However, these assays add cost, and they lack standardization, accuracy, and traceability [12]. Among patients with dyslipidemia, where accuracy is of the greatest importance in guiding treatment, direct chemical-based LDL-C assays have especially unreliable accuracy [13]. Accuracy of the Friedewald equation is further impaired by non-fasting, while guidelines for clinical practice have increasingly endorsed added flexibility for testing in the non-fasting state [14].
With the current therapeutic armamentarium and the shortcomings of Friedewald estimation becoming more widely known, groups across the world have revisited their country’s LDL-C estimation approach [11,12,14,15]. A steady stream of new equations has entered the literature, attempting to replace the Friedewald equation, with these equations commonly using direct chemical-based assays as a reference LDL-C as opposed to BQ or VAP [11]. Many of these equations have not been externally validated, especially in a large, representative clinical population using ultracentrifugation as the reference. Several prior studies have evaluated a smaller subset of the new equations in various populations, most commonly comparing with direct chemical-based assays [16,17,18]. Due to the aforementioned concerns with these assays, conclusions from these prior studies are limited. Additionally, clinical adoption of new LDL-C equations without sufficient external validation could introduce unintended disparities in clinical care based on geography or access to clinical resources.
Therefore, we sought to identify LDL-C equations reported since 1972 and to compare the accuracy of these equations to ultracentrifugation-measured LDL-C by VAP in a large-scale analysis.
Methods
Search for Relevant Studies
The literature published through September 2021 was reviewed by searching PubMed and Web of Science. The following search strategy was developed with the input of a Johns Hopkins Welch Medical Library informationist: (measur*[ti] OR calculat*[ti] OR equation OR formula) AND (low density lipoprotein cholesterol OR LDL-C) in both databases. The initial search yielded 15,477 articles (Supplemental Figure 1), published from 1972 through 2021. Articles were evaluated for their report of new LDL-C equations. The reference lists of the final 23 articles were searched, and no additional LDL-C equations were identified.
Study Population
This study used data from the second harvest of the Very Large Database of Lipids (VLDbL), registered at http://www.clinicaltrials.gov (NCT01698489). The design of the VLDbL has been described in detail previously [19]. Patient samples were acquired by the VAP Diagnostics Laboratory from October 1, 2015 through June 30, 2019 in the United States. The study included both sexes, as well as children (aged <11 years), adolescents (aged 11 to <18 years), and adults (aged ≥18 years). Patient samples largely originated from primary care clinics (85%), with the remainder from inpatient settings and specialty centers. The lipid distributions in the VLDbL have been shown to reflect those in the population-representative National Health and Nutrition Examination Survey [19]. We excluded samples with missing total cholesterol (TC), high density low-density cholesterol (HDL-C), TG, and LDL-C. We included all samples regardless of their fasting status. The Johns Hopkins Institutional Review Board declared our study exempt.
Lipid Measurements
The VLDbL includes direct measurements of TC, LDL-C, very low-density lipoprotein cholesterol (VLDL-C), HDL-C, and other lipoprotein parameters. Measurements were performed by VAP to separate lipoprotein classes by density into all five classes (HDL, LDL-Real [LDL-R; the LDL without Lp(a) and IDL], VLDL, IDL, and Lp(a)) and then measure the cholesterol component via enzymatic analysis and spectrophotometric absorbance. The accuracy of VAP was validated by annual yearly random split-sample comparisons with preparative ultracentrifugation or BQ at the Washington University in St. Louis Core Laboratory for Clinical Studies (r = 0.986, r = 0.969, respectively; bias 0.6%, 1.7%, respectively). Correlation coefficients from an analysis using 40 split serum specimens comparing VAP to BQ were: 0.99 for total cholesterol, 0.99 for HDL-C, 0.98 for LDL-C, and 0.98 for VLDL-C [20]. TG levels were directly measured with the Architect C-8000 system (Abbott Laboratories, IL), which were compared with samples from the University of Alabama School of Medicine for quality assessment.
Equations for Estimating LDL-C
A total of 23 equations for estimating LDL-C are summarized in Supplemental Table 1. One class of LDL-C equations uses a fixed TG:VLDL-C ratio to estimate VLDL-C and extract estimated VLDL-C from non-HDL-C to estimate the LDL-C levels (Friedewald et al. [9], Puavilai et al. [21], Vujovic et al. [22], DeLong et al. [23], Ephraim et al. [24], Ghasemi et al. [25], Bauer et al. [26]). Another class of the equations uses TC, HDL-C, and TG levels as coefficients of linear models to estimate LDL-C (Hattori et al. [27], Anandaraja et al. [28], Chen et al. [29], Cordova et al. [30], Teerakanchana et al. [31], Ahmadi et al. [32], Rao et al. [33], Dansethakul et al. [34], Rasouli et al. [35], Lee & Hu [36], Choi et al. [37], Orejon et al. [38], and Molavi et al. [39]). Other equations use an adjustable factor for TG:VLDL-C ratio based on a lookup table (Martin et al. [40]) or interaction/quadratic terms (Sampson et al. [41], Saiedullah et al. [42]) to account for non-linearity. Furthermore, we used the extended Martin/Hopkins equation [43] for LDL-C estimation at high TG levels (400–799 mg/dL).
Statistical Analysis
We assessed the accuracy of the 23 equations for estimating LDL-C using VAP ultracentrifugation as the reference. Firstly, we compared the concordance in classification according to clinical guideline-based categories (<40, 40–54, 55–69, 70–99, 100–129, 130–159, 160–189, and ≥190 mg/dL) in patients with TG levels up to 399 mg/dL. Concordance was defined as the proportion of ultracentrifugation-measured LDL-C levels falling in the same category as estimated LDL-C. Secondly, we compared the magnitude of error of each equation compared to ultracentrifugation using median differences.
We separated the equations based on whether they performed better than Friedewald (Figure 1). We then conducted secondary analyses for the top equations as well as the Friedewald equation with ultracentrifugation as reference. We first compared the concordance of each equation in patients with TG levels of 150–399 mg/dL and 400–799 mg/dL. We further evaluated LDL-C equations stratified by age (<18, 18–59, ≥60), sex, and fasting status. We also performed analyses stratified by ASCVD (ICD-9 code 410.XX, 414.0X, 411.1, 433.XX, 434.XX, 435.9, 440.2X, 443.9) and hypertension (ICD-9 code 401.XX), and in patients with available laboratory data, we performed analyses stratified by kidney disease (eGFR <60 mL/min/1.73 m2), diabetes (ICD-9 250.XX, A1c >6.5%, or fasting glucose ≥126 mg/dL), inflammation (high-sensitivity C-reactive protein ≥ 2 mg/L), and thyroid dysfunction (TSH <0.5 or >4.5 uIU/mL). Finally, we evaluated the percentage of patients with Friedewald LDL-C <70 mg/dL correctly reclassified (confirmed by ultracentrifugation) to LDL-C >70 mg/dL using the other top performing equations.
Figure 1.
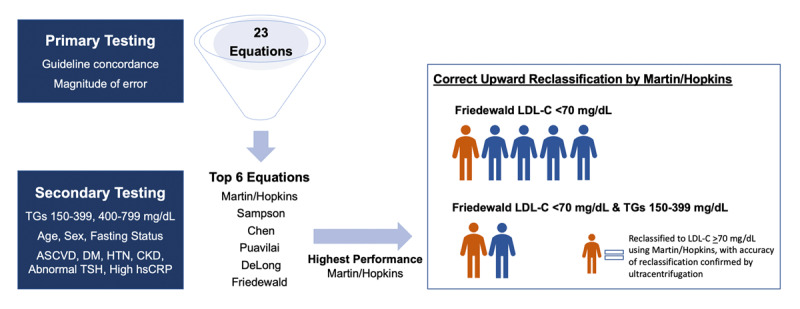
Diagram of Primary and Secondary Testing to Select the Highest Performing Equation. The figure shows the sequence of analysis, first comparing all 23 equations against ultracentrifugation for concordance in guideline-based LDL-C classification and overall magnitude of error (mg/dL units). In secondary testing, we compared Friedewald and the five equations that performed better than Friedewald in primary testing. These secondary tests evaluated performance at different levels of elevated triglycerides, by age (<18, 18–59, >60 years), sex, and fasting status strata, and across clinical subgroups (ASCVD, DM, HTN, CKD, abnormal TSH, high hsCRP). Finally, we assessed the top performing equation (Martin/Hopkins) for its impact in classification across an important clinical cutpoint in high risk patients (LDL-C 70 mg/dL) if a laboratory were to switch from the Friedewald equation. Patients highlighted in orange are ones with Friedewald LDL-C<70 mg/dL who have correct (confirmed by ultracentrifugation) upward reclassification to LDL-C >70 mg/dL by Martin-Hopkins. Patients highlighted in blue remain classified as LDL-C <70 mg/dL by both equations. Abbreviations: TGs = triglycerides; ASCVD = atherosclerotic cardiovascular disease; DM = diabetes mellitus; HTN = hypertension; CKD = chronic kidney disease; TSH = thyroid stimulating hormone; hsCRP = high-sensitivity C-reactive protein; LDL-C = low-density lipoprotein cholesterol.
All statistical analyses were performed in Stata (StataCorp), version 15.1. Graphs were plotted using R (R Core Team) version 4.0.3.
Results
Characteristics of the Study Population
A total of 5,051,467 patients were analyzed with 4,939,528 patients included in the primary analyses of patients with TG levels up to 399 mg/dL. The mean (SD) age was 56±16 years and 53.3% of patients were women. The median directly measured LDL-C was 114 (IQR: 90–141) mg/dL and the median TG level was 116 (IQR: 82–169) mg/dL. Considering fasting status, 11.9% of patients were non-fasting, 19.4% were fasting, and we did not have data on fasting status for 68.7% of patients (Table 1). For patient subgroups, 32,223 had ASCVD, 274,286 had hypertension, 361,079 had kidney disease, 212,671 had diabetes, 417,561 had inflammation, 39,515 had TSH <0.5 uIU/mL and 27,350 had TSH ≥4.5 uIU/mL.
Table 1.
Demographic characteristics.
|
| |
|---|---|
| CHARACTERISTICS | STUDY POPULATION (N = 4,939,528) |
|
| |
| Age, mean (SD), y | 56 (16) |
|
| |
| Age category, no. (%), y | |
|
| |
| <18 | 59,838 (1.2) |
|
| |
| 18–59 | 2,879,747 (58.3) |
|
| |
| ≥60 | 1,966,468 (39.8) |
|
| |
| Not reported | 33,475 (0.7) |
|
| |
| Sex, no. (%) | |
|
| |
| Women | 2,635,486 (53.7) |
|
| |
| Men | 2,269,406 (46.3) |
|
| |
| Fasting status, no. (%) | |
|
| |
| Non-fasting | 586,256 (11.9) |
|
| |
| Fasting | 958,989 (19.4) |
|
| |
| Not reported | 3,394,283 (68.7) |
|
| |
| Lipid values, median (IQR) | |
|
| |
| TC, mg/dL | 193 (164–225) |
|
| |
| TG, mg/dL | 114 (81–164) |
|
| |
| HDL-C, mg/dL | 51 (42–63) |
|
| |
| LDL-C, mg/dL (ultracentrifugation) | 114 (90–141) |
|
| |
| Non-HDL-C, mg/dL | 138 (112–168) |
|
| |
| VLDL-C, mg/dL | 22 (17–29) |
|
| |
| Lp(a)-C, mg/dL | 6 (4–10) |
|
| |
| TC:VLDL-C ratio | 8.4 (6.5–10.9) |
|
| |
| TG:VLDL-C ratio | 5.0 (4.4–5.9) |
|
| |
| TG:TC ratio | 0.6 (0.4–0.9) |
|
| |
IQR = interquartile range; TC = total cholesterol; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; VLDL-C = very low-density lipoprotein cholesterol; Lp(a)-C = lipoprotein(a) cholesterol.
Concordance According to Clinical LDL-C Classification Guidelines
Among the LDL-C estimation methods, the Martin/Hopkins algorithm most accurately classified LDL-C to the correct clinical category (89.6%), followed by the Sampson (86.3%), Chen (84.4%), Puavilai (84.1%), Delong (83.3%), and Friedewald (83.2%) equations (Table 2). The other 17 equations were less accurate than Friedewald, with accuracy as low as 35.1%.
Table 2.
Percentage of patients correctly classified to LDL-C category.
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| EQUATION | LDL-C CATEGORY, MG/DL | ||||||||
|
| |||||||||
| <40 | 40–54 | 55–69 | 70–99 | 100–129 | 130–159 | 160–189 | ≥190 | OVERALL | |
|
| |||||||||
| Martin/Hopkins | 78.6 | 81.2 | 84.2 | 91.7 | 90.8 | 89.2 | 86.6 | 91.1 | 89.6 |
|
| |||||||||
| Sampson | 66.0 | 71.8 | 77.8 | 90.5 | 89.0 | 85.4 | 80.5 | 84.4 | 86.3 |
|
| |||||||||
| Chen | 85.9 | 81.1 | 80.2 | 86.0 | 84.3 | 83.2 | 81.7 | 95.7 | 84.4 |
|
| |||||||||
| Puavilai | 62.5 | 69.7 | 75.0 | 89.3 | 87.2 | 83.1 | 77.0 | 79.2 | 84.1 |
|
| |||||||||
| DeLong | 66.8 | 72.3 | 76.2 | 89.6 | 86.4 | 81.4 | 74.4 | 76.8 | 83.3 |
|
| |||||||||
| Friedewald | 42.1 | 52.5 | 62.6 | 83.8 | 87.0 | 87.7 | 86.1 | 90.0 | 83.2 |
|
| |||||||||
| Molavi | 44.6 | 54.2 | 63.0 | 82.8 | 85.6 | 87.2 | 87.6 | 95.2 | 82.9 |
|
| |||||||||
| Saiedullah | 72.2 | 65.8 | 66.0 | 81.1 | 83.6 | 85.8 | 86.6 | 95.4 | 82.1 |
|
| |||||||||
| Vujovic | 75.7 | 76.3 | 77.0 | 89.2 | 83.6 | 76.6 | 68.0 | 71.2 | 80.3 |
|
| |||||||||
| Teerakanchana | 83.5 | 70.8 | 67.0 | 83.8 | 78.4 | 76.6 | 75.7 | 83.8 | 78.5 |
|
| |||||||||
| Orejon | 71.5 | 67.8 | 68.0 | 85.3 | 80.4 | 76.1 | 71.1 | 76.4 | 78.5 |
|
| |||||||||
| Dansethakul | 52.1 | 58.1 | 62.1 | 82.7 | 77.9 | 72.9 | 67.1 | 73.3 | 75.2 |
|
| |||||||||
| Bauer | 87.2 | 77.6 | 73.6 | 86.5 | 77.5 | 68.3 | 57.8 | 63.2 | 73.8 |
|
| |||||||||
| Rao | 42.3 | 54.2 | 61.8 | 82.7 | 76.4 | 67.3 | 56.4 | 62.7 | 71.0 |
|
| |||||||||
| Ghasemi | 21.6 | 28.9 | 38.8 | 68.0 | 75.5 | 80.5 | 83.3 | 97.8 | 69.1 |
|
| |||||||||
| Ephraim | 93.0 | 74.3 | 67.0 | 83.0 | 71.7 | 61.1 | 49.8 | 57.3 | 67.5 |
|
| |||||||||
| Hattori | 33.9 | 38.1 | 43.5 | 67.7 | 70.9 | 73.1 | 73.9 | 99.1 | 67.0 |
|
| |||||||||
| Lee and Hu | 81.4 | 63.4 | 57.8 | 74.0 | 66.0 | 63.4 | 61.5 | 81.9 | 66.9 |
|
| |||||||||
| Rasouli | 74.9 | 65.4 | 62.3 | 68.1 | 59.8 | 48.6 | 29.0 | 99.5 | 60.3 |
|
| |||||||||
| Cordova | 86.8 | 64.5 | 54.6 | 62.6 | 56.6 | 50.1 | 41.1 | 96.7 | 57.5 |
|
| |||||||||
| Anandaraja | 29.9 | 36.0 | 43.3 | 67.6 | 59.4 | 52.1 | 45.3 | 56.5 | 55.9 |
|
| |||||||||
| Choi | 88.3 | 56.1 | 44.4 | 73.0 | 57.9 | 46.7 | 36.2 | 49.2 | 53.4 |
|
| |||||||||
| Ahmadi | 27.5 | 26.0 | 31.8 | 58.7 | 49.1 | 34.8 | 18.8 | 16.5 | 35.1 |
|
| |||||||||
Magnitude of Patient-Level Error
The median error of equations ranged from –10.8 to 18.7 mg/dL, and was best optimized using the Martin/Hopkins equation (0.3, IQR–1.6 to 2.4 mg/dL) (Table 3). In comparison, other equations that demonstrated higher overall accuracy compared to Friedewald had median differences of 1.7 mg/dL (Sampson), 3.3 mg/dL (Puavilai), 4.1 mg/dL (Delong), and –2.9 mg/dL (Chen). In patients with TG levels <400 mg/dL, 68.6% of patients had less than 5 mg/dL error using Friedewald compared to 70.8% and 82.8% using Sampson and Martin/Hopkins, respectively.
Table 3.
Error between estimated LDL-C and VAP ultracentrifugation LDL-C.
|
| ||
|---|---|---|
| EQUATION | ERROR, MEDIAN (IQR), MG/DL | RELATIVE ERROR, MEDIAN (IQR), % |
|
| ||
| Friedewald | –0.2 (–4.4 to 2.6) | –0.2 (–4.0 to 2.2) |
|
| ||
| Martin/Hopkins | 0.3 (–1.6 to 2.4) | 0.2 (–1.4 to 2.1) |
|
| ||
| Sampson | 1.7 (–1.3 to 4.1) | 1.6 (–1.2 to 3.5) |
|
| ||
| Puavilai | 3.3 (0.5 to 5.5) | 2.9 (0.4 to 4.9) |
|
| ||
| Vujovic | 5.5 (3.2 to 7.7) | 4.8 (2.8 to 6.9) |
|
| ||
| Hattori | –7.6 (–11.8 to –4.6) | –6.4 (–10.0 to –4.1) |
|
| ||
| Anandaraja | 5.9 (–4.3 to 17.5) | 5.1 (–3.6 to 16.1) |
|
| ||
| Chen | –2.9 (–5.6 to –0.2) | –2.6 (–4.4 to –0.3) |
|
| ||
| Cordova | –10.8 (–17.2 to –4.5) | –9.8 (–13.4 to –4.7) |
|
| ||
| Teerakanchana | 5.2 (1.7 to 8.9) | 4.6 (1.3 to 8.7) |
|
| ||
| Ahmadi | 18.7 (–2.6 to 50.6) | 16.4 (–2.3 to 44.6) |
|
| ||
| DeLong | 4.1 (1.4 to 6.2) | 3.5 (1.2 to 5.5) |
|
| ||
| Rao | 7.7 (2.3 to 11.6) | 6.8 (2.1 to 9.9) |
|
| ||
| Ephraim | 9.4 (7.3 to 12.2) | 8.4 (6.4 to 11.1) |
|
| ||
| Saiedullah | –3.9 (–6.0 to –1.5) | –3.4 (–5.3 to –1.3) |
|
| ||
| Dansethakul | 6.9 (2.7 to 9.7) | 5.8 (2.2 to 8.8) |
|
| ||
| Rasouli | –10.1 (–16.6 to –4.0) | –8.8 (–12.3 to –4.3) |
|
| ||
| Ghasemi | –5.8 (–12.0 to –1.8) | –5.1 (–11.0 to –1.6) |
|
| ||
| Lee and Hu | 3.8 (–3.8 to 12.0) | 3.4 (–3.0 to 12.0) |
|
| ||
| Orejon | 6.2 (3.4 to 8.3) | 5.3 (2.7 to 8.0) |
|
| ||
| Bauer | 7.6 (5.6 to 10.1) | 6.8 (4.9 to 9.1) |
|
| ||
| Molavi | –1.1 (–5.2 to 1.7) | –0.9 (–4.5 to 1.5) |
|
| ||
| Choi | 14.5 (11.8 to 17.2) | 12.6 (9.8 to 16.0) |
|
| ||
Accuracy of Top Performing Equations at High Triglyceride Levels
In patients with TG levels 150–399 mg/dL, the Martin/Hopkins continued to be the best performing equation with accuracy of 83.5%, followed by Chen (81.3%), DeLong (80.0%), Puavilai (79.4%), Sampson (79.3%), and Friedewald (67.8%) (Supplemental Table 2). In patients with TG levels 400–799 mg/dL (n = 111,939), the extended Martin/Hopkins [43] had the highest accuracy (60.3%), followed by Martin/Hopkins (59.2%) and Chen (57.7%) (Supplemental Table 3). Some equations that demonstrated greater accuracy in patients with TG levels <400 mg/dL performed poorly in patients with hypertriglyceridemia. DeLong had a concordance of 42.2% and Sampson had a concordance of 37.3%.
Accuracy of Top Performing Equations in Patient Subgroups
The Martin/Hopkins equation was consistently the highest performing equation after stratifying by age, sex, and fasting status. The same pattern was observed when stratifying by ASCVD, hypertension, kidney disease, diabetes, inflammation, and thyroid dysfunction (Supplemental Table 4).
Percentage of Patients with Friedewald LDL-C <70 mg/dL Reclassified to LDL-C >70 mg/dL Using the Top Performing Equations
In patients with Friedewald LDL-C <70 mg/dL and TG levels <400 mg/dL, 19.8% were correctly reclassified to LDL-C >70 mg/dL using Martin/Hopkins, 17.6% using Chen, 17.4% using DeLong, 15.4% using Puavilai, and 13.9% using Sampson (Figure 2). In patients with Friedewald LDL-C <70 mg/dL and TG levels 150–399 mg/dL, a considerably greater percentage of patients were correctly reclassified to LDL-C >70 mg/dL, with the Martin/Hopkins having the highest reclassification percentage of 45.6%.
Figure 2.
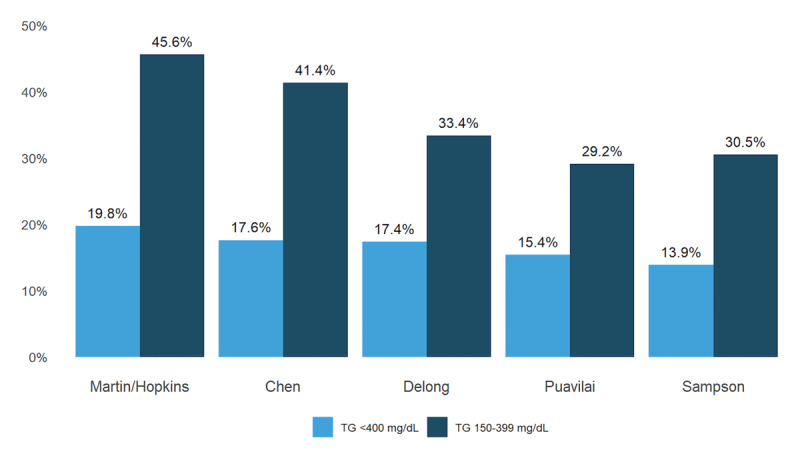
Upward Reclassification of Patients with Friedewald LDL-C < 70 mg/dL When Using an Alternative LDL-C Equation. The figure displays the percentage of patients with Friedewald LDL-C < 70 mg/dL who are reclassified to LDL-C > 70 mg/dL by top performing equations and confirmed to have a correct reclassification by ultracentrifugation. Reclassification for patients with TG levels of <400 mg/dL (n = 539,575) is highlighted in blue and for patients with TG levels of 150–399 mg/dL (n = 183,455) is highlighted in dark blue. Abbreviations: LDL-C = low-density lipoprotein cholesterol; TG = triglyceride.
Discussion
This study compared the performance of 23 LDL-C equations and generated several important findings: 1) most proposed alternatives to the Friedewald equation worsened accuracy of LDL-C; 2) the Martin/Hopkins most accurately classified LDL-C to the correct guideline-based LDL-C category followed by Sampson, Chen, Puavilai, Delong, and Friedewald, respectively; 3) across age, sex, TG levels, and patient subgroups, the Martin/Hopkins continued to have the highest accuracy; 4) the magnitude of error between calculated LDL-C and directly measured LDL-C was the smallest using the Martin/Hopkins compared with the other 22 equations; and 5) approximately one in five patients with Friedewald LDL-C <70 mg/dL and almost half of the patients with Friedewald LDL-C <70 mg/dL and TG levels 150–399 mg/dL were correctly reclassified to LDL-C >70 mg/dL using the Martin/Hopkins equation.
Approaches to LDL-C Determination
LDL-C is defined as TC – HDL-C – VLDL-C according to the landmark Friedewald equation [9] and the Centers for Disease and Prevention (CDC) definition of LDL-C. The two variables that are directly measured are TC and HDL-C, whereas VLDL-C is estimated. The Friedewald equation estimates VLDL-C using a fixed factor, thus assuming a consistent relationship between VLDL-C and TG levels. Some equations retain the basic backbone of Friedewald, which translates into a permutation of equations that substitute the factor 5 in mg/dL units with other predetermined ratios. For example, DeLong et al. proposed dividing TG levels by 6 [23] while Chen et al. in 2010 suggested that LDL-C = TC × 0.9 – HDL- C × 0.9 – TG × 0.1 [29]. These approaches do not account for heterogeneity in TG:VLDL-C ratios, which limits the capacity for substantial accuracy enhancement.
Other equations have deviated from the structure of the Friedewald equation.De Cordova et al. proposed that LDL-C = 3/4 (TC – HDL-C), which eliminated TG levels as an input variable and VLDL-C estimation [29,30]. Some equations, such as Anandaraja and Lee & Hu, do not include HDL-C as a component in their equations [28,36]. These equations seek to estimate LDL-C directly, which is unlike the focus of Friedewald on estimating VLDL-C to determine LDL-C. Friedewald LDL-C has guided key clinical trials that have shaped treatment recommendations for ASCVD patients [44]. Therefore, direct LDL-C estimation, which drifts away from the standard set by Friedewald, is inconsistent with our clinical standard for LDL-C.
In our analysis, all the equations that estimated LDL-C directly, except Sampson, performed poorly compared to Friedewald. Sampson used a bivariate equation, which included all the input variables used in Friedewald, unlike other equations that dropped key components of the original formula. Although Sampson demonstrated better accuracy than Friedewald, its deviation from the structure of Friedewald still poses the risk of a fundamental discrepancy—the definition of LDL-C used in treatment guidelines would not align with the one used in LDL-C calculation under Sampson. Furthermore, a subset of the equations assumed a non-zero baseline value by adding a y-intercept in their calculation of LDL-C from TC [28,31,32,34,38,42]. Such an approach implies that patients present with a standard pre-existing level of cholesterol, an assumption that may not have merit.
Of the 23 equations, the Martin/Hopkins demonstrated the highest accuracy across strata by age, sex, fasting status, and triglyceride levels, as well as in patients with atherosclerotic cardiovascular disease, hypertension, diabetes, kidney disease, inflammation, and thyroid dysfunction. The Martin/Hopkins equation has been validated extensively on a global scale [11,16,45,46,47]. Martin/Hopkins is the only formula to preserve the structure of Friedewald while leveraging an adjustable factor that improves VLDL-C estimation, catalyzing a more personalized risk assessment [40]. It thus maintains the same LDL-C definition that guided treatment trials, which offers internal consistency in treatment recommendations and calculation methods. The Martin-Hopkins equation was further validated across a wide spectrum of LDL-C levels, including LDL-C <70 mg/dL, using BQ in large-scale studies [10,48]. Given the development of the Martin-Hopkins equation based on VAP, these studies provide further validation of the VAP method.
The adjustable factor in the Martin/Hopkins, which can range from 3.1 to 9.5 in patients with TG levels <400 mg/dL, was derived from an analysis of TG-to-VLDL-C ratios in more than 1.3 million people, as opposed to the Friedewald equation which was derived in a sample of 448 patients [9,40]. Other independent, multi-national groups have reported that the Martin/Hopkins equation was more accurate than Friedewald in racially diverse populations [11,45,49] as well as for LDL-C values <70 mg/dL [11,47], and in patients taking proprotein convertase subtilisin/kexin (PCSK9) inhibitors [10], patients with diabetes [50], and patients with familial combined hyperlipidemia [51].
Various large laboratories have adopted the Martin/Hopkins equation, and the AHA/ACC/Multi-society Cholesterol Guideline provided a Class IIa recommendation for using the equation in patients with LDL-C <70 mg/dL [12]. The Martin/Hopkins equation has been supported by the National Lipid Association (NLA) [52], a consensus recommendation in Brazil [53], a joint consensus panel of the European Atherosclerosis Society (EAS), and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) [54]. It was also supported by the World Heart Federation Cholesterol Roadmap in 2022 [55]. It can be installed on lab information systems in a straightforward manner using line-by-line code for automatic calculation of LDL-C. There are no intellectual property restrictions. Laboratories can contact JHTT-Communications@jh.edu for assistance in implementation.
LDL-C Risk Reclassification at Low LDL-C Levels
We found that if a clinical laboratory were to switch from Friedewald to Martin-Hopkins, almost half of the patients with Friedewald LDL-C <70 mg/dL and TG levels 150–399 mg/dL would be correctly reclassified to LDL-C >70 mg/dL using Martin/Hopkins. Doing so would facilitate opportunities to further optimize LDL-C and ASCVD risk. Our results are consistent with prior studies reporting LDL-C underestimation using Friedewald estimation [10,47,56].
LDL-C Estimation in Hypertriglyceridemia
In patients with TG levels up to 799 mg/dL, the extended Martin/Hopkins performance was the most accurate. However, at such elevated TG levels, the clinical priority is to immediately reduce TG levels to prevent pancreatitis. Our results align with a previous study demonstrating that while the extended Martin/Hopkins is the most accurate of our LDL-C estimation tools at high TG levels, caution with LDL-C estimation in this elevated TG range should still be taken [43].
Limitations
This study has some limitations. Data on race and ethnicity or other clinical factors such as body mass index were not available for analysis due to using a clinical laboratory dataset. Our data also comes entirely from a US population. However, multiple national and international studies, including the ELSA Brazil study and a clinical trial conducted in 49 countries, reported results consistent with ours, which serves as an external validation of our findings [10,47,57]. Furthermore, we did not have patient treatment data. Accuracy of LDL-C estimation is important, nonetheless, both pre-treatment and on-treatment. A PCSK9 inhibitor trial and a CETP inhibitor trial showed better accuracy with Martin/Hopkins compared to Friedewald estimation [10,47].
Some studies suggested that LDL-C levels may vary by patients’ clinical characteristics [58,59,60]. We were able to use common biomarkers, such as HbA1c, hsCRP, TSH, and eGFR, as well as ICD codes to assess equation accuracy in subgroup analyses. We found consistent results across these groups. Finally, we used the VAP method as opposed to BQ to directly measure LDL-C. As noted above, studies have demonstrated excellent agreement between the two methods, which are both based on ultracentrifugation. Limited, inconclusive data has raised concern regarding the VAP method and its tendency to underestimate VLDL-C in samples with high TG due to adherence of TG-rich lipoproteins to the walls of test tubes [41,61,62]. However, this concern applies to all forms of ultracentrifugation and is only relevant at TG levels beyond the range in our analysis.
Conclusion
The influx of LDL-C equations in the literature reflects the importance of LDL-C in clinical medicine and the need for greater accuracy in LDL-C estimation given new therapies and new guideline recommendations. In our study, most proposed alternatives to the Friedewald equation worsened accuracy of LDL-C. Clinical use of LDL-C equations without appropriate validation using ultracentrifugation could result in geographic disparities in cardiovascular care. Of 23 LDL-C equations evaluated in 5,051,467 patients, the Martin/Hopkins equation was the most accurate.
Additional File
The additional file for this article can be found as follows:
Supplemental Table 1 to 4 and Figure 1.
Funding Statement
The Very Large Database of Lipids has received philanthropic funding from the David and June Trone Family Foundation.
Funding Information
The Very Large Database of Lipids has received philanthropic funding from the David and June Trone Family Foundation.
Competing Interests
Dr. Martin reports research support from American Heart Association (20SFRN35380046, 20SFRN35490003, 878924, 882415, 946222), PCORI (ME-2019C1-15 328, IHS-2021C3-24147), National Institutes of Health (R01AG071032, P01 HL108800), the David and June Trone Family Foundation, and Pollin Digital Health Innovation Fund, the PJ Schafer Cardiovascular Research Fund, Sandra and Larry Small, CASCADE FH, Apple, Google, and Amgen. Outside of this work, Dr. Martin reports consulting fees from Amgen, AstraZeneca, Dalcor, Esperion, iHealth, Kaneka, NewAmsterdam, Novartis, Novo Nordisk, Sanofi, and 89bio. Drs. Martin and Jones were listed as coinventors on a patent application filed by Johns Hopkins University for the Martin/Hopkins method of low-density lipoprotein cholesterol and that patent application has since been abandoned to enable use without intellectual property restrictions. Outside of this work, Dr. Michos reports consulting fees from AstraZeneca, Amarin, Bayer, Boehringer Ingelheim, Esperion, Novo Nordisk, Novartis, and Pfizer. The other authors do not report any additional disclosures.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: A report from the American Heart Association. Circulation. 2023; 147(8): e93–e621. DOI: 10.1161/CIR.0000000000001137 [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2019; 139(25): E1082–143. DOI: 10.1161/CIR.0000000000000624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010; 376(9753): 1670–1681. DOI: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019; 140(11): e596–e646. DOI: 10.1161/CIR.0000000000000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020; 41(1): 111–188. DOI: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022; 80(14): 1366–1418. DOI: 10.1016/j.jacc.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994; 35: 159–168. DOI: 10.1016/S0022-2275(20)40123-3 [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni KR, Garber DW, Jones MK, Segrest JP. Identification and cholesterol quantification of tow density lipoprotein subclasses in young adults by VAP-I I methodology. J Lipid Res. 1995; 36: 2291–2302. DOI: 10.1016/S0022-2275(20)39710-8 [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6): 499–502. DOI: 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 10.Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, friedewald estimation, and preparative ultracentrifugation insights from the FOURIER trial. JAMA Cardiol. 2018; 3(8): 749–753. DOI: 10.1001/jamacardio.2018.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownstein AJ, Martin SS. More accurate LDL-C calculation: Externally validated, guideline endorsed. Clinica Chimica Acta. 2020; 506: 149–153. DOI: 10.1016/j.cca.2020.03.030 [DOI] [PubMed] [Google Scholar]
- 12.Nordestgaard BG, Langlois MR, Langsted A, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: Consensus-based recommendations from EAS and EFLM. Clin Chem Lab Med. 2020; 58(4): 496–517. DOI: 10.1515/cclm-2019-1253 [DOI] [PubMed] [Google Scholar]
- 13.Miller WG, Myers GL, Sakurabayashi I, et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010; 56(6): 977–986. DOI: 10.1373/clinchem.2009.142810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathiyakumar V, Park J, Golozar A, et al. Fasting versus nonfasting and low-density lipoprotein cholesterol accuracy. Circulation. 2018; 137(1): 10–19. DOI: 10.1161/CIRCULATIONAHA.117.030677 [DOI] [PubMed] [Google Scholar]
- 15.Martin SS, Blaha MJ, Elshazly MB, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013; 62(8): 732–739. DOI: 10.1016/j.jacc.2013.01.079 [DOI] [PubMed] [Google Scholar]
- 16.Rim JH, Lee YH, Lee MH, et al. Comparison and validation of 10 equations including a novel method for estimation of LDL-cholesterol in a 168,212 Asian population. Medicine. 2016; 95(14): e3230. DOI: 10.1097/MD.0000000000003230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piani F, Cicero AFG, Ventura F, et al. Evaluation of twelve formulas for LDL-C estimation in a large, blinded, random Italian population. Int J Cardiol. 2021; 330: 221–227. DOI: 10.1016/j.ijcard.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Lee HS, Baik SJ, et al. Comparison of the effectiveness of Martin’s equation, Friedewald’s equation, and a novel equation in low-density lipoprotein cholesterol estimation. Sci Rep. 2021; 11(1): 13545. DOI: 10.1038/s41598-021-92625-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SS, Blaha MJ, Toth PP, et al. Very Large Database of Lipids: Rationale and design. Clin Cardiol. 2013; 36(11): 641–648. DOI: 10.1002/clc.22214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006; 26(4): 787–802. DOI: 10.1016/j.cll.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 21.Puavilai W, Laorugpongse D, Deerochanawong C, Muthapongthavorn Rn N, Rn S. The accuracy in using modified Friedewald equation to calculate LDL from non-fast triglyceride: A pilot study. J Med Assoc Thai. 2009; 92(2): 182–187. [PubMed] [Google Scholar]
- 22.Vujovic A, Kotur-Stevuljevic J, Spasic S, et al. Evaluation of different formulas for LDL-C calculation. Lipids Health Dis. 2010; 9: 27. DOI: 10.1186/1476-511X-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delong DM, Delong ER, Wood PD, Lippel K, Rifkind BM. A comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol: The Lipid Research Clinics Prevalence Study. JAMA. 1986; 256(17): 2372–2377. DOI: 10.1001/jama.1986.03380170088024 [DOI] [PubMed] [Google Scholar]
- 24.Ephraim RKD, Acheampong E, Swaray SM, et al. Developing a modified low-density lipoprotein (M-LDL-C) Friedewald’s equation as a substitute for direct LDL-C measure in a Ghanaian population: A comparative study. J Lipids. 2018; 2018: 7078409. DOI: 10.1155/2018/7078409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghasemi A, Asgari S, Hadaegh F, et al. New modified Friedewald formulae for estimating low-density lipoprotein cholesterol according to triglyceride levels: extraction and validation. Endocrine. 2018; 62(2): 404–411. DOI: 10.1007/s12020-018-1685-2 [DOI] [PubMed] [Google Scholar]
- 26.Bauer F, Seibert FS, Rohn B, Babel N, Westhoff TH. Estimation of LDL cholesterol in chronic kidney disease. Eur J Prev Cardiol. 2021; 28(12): 1402–1408. DOI: 10.1093/eurjpc/zwaa003 [DOI] [PubMed] [Google Scholar]
- 27.Hattori Y, Suzuki M, Tsushima M, et al. Development of approximate formula for LDL-chol, LDL-apo B and LDL-chol/LDL-apo B as indices of hyperapobetalipoproteinemia and small dense LDL. Atherosclerosis. 1998; 138(2): 289–299. DOI: 10.1016/S0021-9150(98)00034-3 [DOI] [PubMed] [Google Scholar]
- 28.Anandaraja S, Narang R, Godeswar R, Laksmy R, Talwar KK. Low-density lipoprotein cholesterol estimation by a new formula in Indian population. Int J Cardiol. 2005; 102(1): 117–120. DOI: 10.1016/j.ijcard.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Zhang X, Pan B, et al. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis. 2010; 9: 52. DOI: 10.1186/1476-511X-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauricio Mendes de Cordova C, Mendes de Cordova M. A new accurate, simple formula for LDL-cholesterol estimation based on directly measured blood lipids from a large cohort. Ann Clin Biochem. 2013; 50(Pt 1): 13–19. DOI: 10.1258/acb.2012.011259 [DOI] [PubMed] [Google Scholar]
- 31.Teerakanchana T, Puavilai W, Suriyaprom K, Tungtrongchitr R. Comparative study of LDL-cholesterol levels in Thai patients by the direct method and using the Friedewald formula. Southeast Asian J Trop Med Public Health. 2007; 38(3): 519–527. [PubMed] [Google Scholar]
- 32.Ahmadi SA, Boroumand MA, Gohari-Moghaddam K, Tajik P, Dibaj SM. The impact of low serum triglyceride on LDL-cholesterol estimation. Arch Iran Med. 2008; 11(3): 318–321. [PubMed] [Google Scholar]
- 33.Rao A, Parker AH, El-Sheroni NA, Babelly MM. Calculation of low-density lipoprotein cholesterol with use of triglyceride/cholesterol ratios in lipoproteins compared with other calculation methods. Clin Chem. 1988; 34(12): 2532–2534. DOI: 10.1093/clinchem/34.12.2532 [DOI] [PubMed] [Google Scholar]
- 34.Dansethakul P, Thapanathamchai L, Saichanma S, Worachartcheewan A, Pidetcha P. Determining a new formula for calculating low-density lipoprotein cholesterol: data mining approach. EXCLI J. 2015; 14: 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasouli M, Mokhtari H. Calculation of LDL-cholesterol vs. direct homogenous assay. J Clin Lab Anal. 2017; 31(3): e22057. DOI: 10.1002/jcla.22057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu CY, Lee CL, Sheu WHH, et al. A new formula for estimation of low-density lipoprotein cholesterol in an ethnic Chinese population. Clin Chem Lab Med. 2015; 53(11): 1871–1879. DOI: 10.1515/cclm-2014-1029 [DOI] [PubMed] [Google Scholar]
- 37.Choi R, Park MJ, Oh Y, Kim SH, Lee SG, Lee EH. Validation of multiple equations for estimating low-density lipoprotein cholesterol levels in Korean adults. Lipids Health Dis. 2021; 20(1): 111. DOI: 10.1186/s12944-021-01525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saldaña Orejón IM, Benites Ricra MA, Chipana Huallpa JA. Derivación y validación de una ecuación para estimar el colesterol ligado a lipoproteínas de baja densidad en una población de Lima, Perú. Anales de la Facultad de Medicina. 2017; 78(1): 41–48. DOI: 10.15381/anales.v78i1.13020 [DOI] [Google Scholar]
- 39.Molavi F, Namazi N, Asadi M, et al. Comparison common equations for LDL-C calculation with direct assay and developing a novel formula in Iranian children and adolescents: the CASPIAN V study. Lipids Health Dis. 2020; 19(1): 129. DOI: 10.1186/s12944-020-01306-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013; 310(19): 2061–2068. DOI: 10.1001/jama.2013.280532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020; 5(5): 540–548. DOI: 10.1001/jamacardio.2020.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdhury N, Saiedullah M, Khan MAH, Rahman MR. Comparison of modified Friedewald’s formula with direct measurement of low-density lipoprotein cholesterol in Bangladeshi population. Bangladesh Med Res Counc Bull. 2013; 39(3): 120–123. DOI: 10.3329/bmrcb.v39i3.20312 [DOI] [PubMed] [Google Scholar]
- 43.Sajja A, Park J, Sathiyakumar V, et al. Comparison of methods to estimate low-density lipoprotein cholesterol in patients with high triglyceride levels. JAMA Netw Open. 2021; 4(10): e2128817–e2128817. DOI: 10.1001/jamanetworkopen.2021.28817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giugliano RP, Keech A, Murphy SA, et al. Clinical efficacy and safety of evolocumab in high-risk patients receiving a statin: Secondary analysis of patients with low LDL cholesterol levels and in those already receiving a maximal-potency statin in a randomized clinical trial. JAMA Cardiol. 2017; 2(12): 1385–1391. DOI: 10.1001/jamacardio.2017.3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang M, Kim J, Lee SY, Kim K, Yoon J, Ki H. Martin’s equation as the most suitable method for estimation of low-density lipoprotein cholesterol levels in Korean adults. Korean J Fam Med. 2017; 38(5): 263–269. DOI: 10.4082/kjfm.2017.38.5.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrinho C, Alves AC, Bourbon M, Duarte S. Applicability of Martin-Hopkins formula and comparison with Friedewald formula for estimated low-density lipoprotein cholesterol in e_COR study population. Revista Portuguesa de Cardiologia. 2021; 40(10): 715–724. DOI: 10.1016/j.repc.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 47.Martin SS, Ditmarsch M, Simmons M, et al. Comparison of low-density lipoprotein cholesterol equations in patients with dyslipidaemia receiving cholesterol ester transfer protein inhibition. Eur Heart J Cardiovasc Pharmacother. 2022; pvac056. DOI: 10.1093/eurheartj/ehac544.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meeusen JW, Lueke AJ, Jaffe AS, Saenger AK. Validation of a proposed novel equation for estimating LDL cholesterol. Clin Chem. 2014; 60(12): 1519–1523. DOI: 10.1373/clinchem.2014.227710 [DOI] [PubMed] [Google Scholar]
- 49.Pallazola VA, Sathiyakumar V, Ogunmoroti O, et al. Impact of improved low-density lipoprotein cholesterol assessment on guideline classification in the modern treatment era-Results from a racially diverse Brazilian cross-sectional study. J Clin Lipidol. 2019; 13(5): 804–811.e2. DOI: 10.1016/j.jacl.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 50.Chaen H, Kinchiku S, Miyata M, et al. Validity of a novel method for estimation of low-density lipoprotein cholesterol levels in diabetic patients. J Atheroscler Thromb. 2016; 23(12): 1355–1364. DOI: 10.5551/jat.35972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta R, Reyes-Rodríguez E, Yaxmehen Bello-Chavolla O, et al. Performance of LDL-C calculated with Martin’s formula compared to the Friedewald equation in familial combined hyperlipidemia. Atherosclerosis. 2018; 277: 204–210. DOI: 10.1016/j.atherosclerosis.2018.06.868 [DOI] [PubMed] [Google Scholar]
- 52.Wilson PW, Jacobson TA, Martin SS, et al. Lipid measurements in the management of cardiovascular diseases: Practical recommendations a scientific statement from the national lipid association writing group. J Clin Lipidol. 2021; 15(5): 629–648. DOI: 10.1016/j.jacl.2021.09.046 [DOI] [PubMed] [Google Scholar]
- 53.Scartezini M, Ferreira CE dos S, Izar MCO, et al. Positioning about the flexibility of fasting for lipid profiling. Arq Bras Cardiol. 2017; 108(3): 195–197. DOI: 10.5935/abc.20170039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langlois MR, Nordestgaard BG, Langsted A, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: Consensus-based recommendations from EAS and EFLM. Clin Chem Lab Med. 2020; 58(4): 496–517. DOI: 10.1515/cclm-2019-1253 [DOI] [PubMed] [Google Scholar]
- 55.Ray KK, Ference BA, Séverin T, et al. World Heart Federation cholesterol roadmap 2022. Glob Heart. 2022; 17(1): 75. DOI: 10.5334/gh.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whelton SP, Meeusen JW, Donato LJ, et al. Evaluating the atherogenic burden of individuals with a Friedewald-estimated low-density lipoprotein cholesterol <70 mg/dL compared with a novel low-density lipoprotein estimation method. J Clin Lipidol. 2017; 11(4): 1065–1072. DOI: 10.1016/j.jacl.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 57.Al Rifai M, Martin SS, McEvoy JW, et al. The prevalence and correlates of subclinical atherosclerosis among adults with low-density lipoprotein cholesterol. Atherosclerosis. 2018; 274: 61–66. DOI: 10.1016/j.atherosclerosis.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 58.Bauer F, Seibert FS, Rohn B, Babel N, Westhoff TH. Estimation of LDL cholesterol in chronic kidney disease. Eur J Prev Cardiol. 2021; 28(12): 1402–1408. DOI: 10.1093/eurjpc/zwaa003 [DOI] [PubMed] [Google Scholar]
- 59.Choi SY, Park HE, Kim MK, et al. Difference between calculated and direct-measured low-density lipoprotein cholesterol in subjects with diabetes mellitus or taking lipid-lowering medications. J Clin Lipidol. 2012; 6(2): 114–120. DOI: 10.1016/j.jacl.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 60.Fawwad A, Sabir R, Riaz M, Moin H. Measured versus calculated LDL-cholesterol in subjects with type 2 diabetes. Pak J Med Sci. 2016; 32(4): 955. DOI: 10.12669/pjms.324.9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hopkins PN, Brinton EA, Nanjee MN. Hyperlipoproteinemia type 3: The forgotten phenotype. Curr Atheroscler Rep. 2014; 16(9). DOI: 10.1007/s11883-014-0440-2 [DOI] [PubMed] [Google Scholar]
- 62.Chung BH, Wilkinson T, Geer JC, Segrest JP. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980; 21(3): 284–291. DOI: 10.1016/S0022-2275(20)39807-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 to 4 and Figure 1.


