Abstract
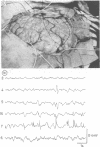
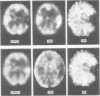
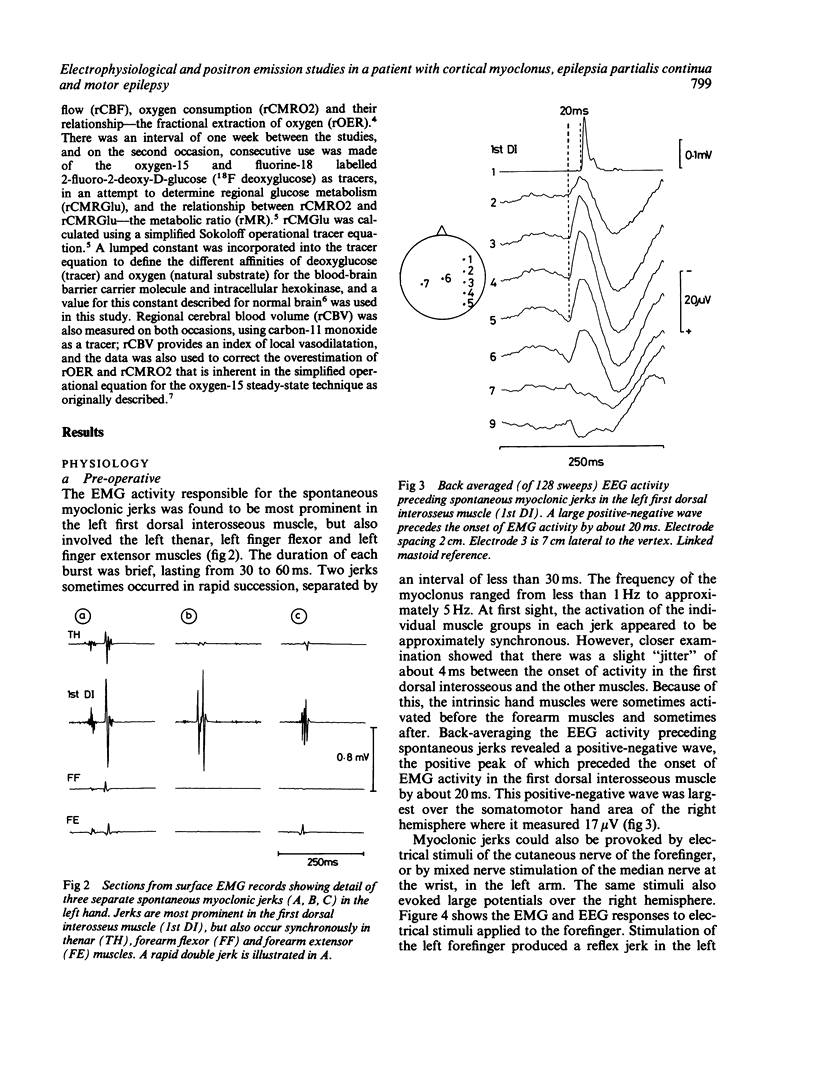
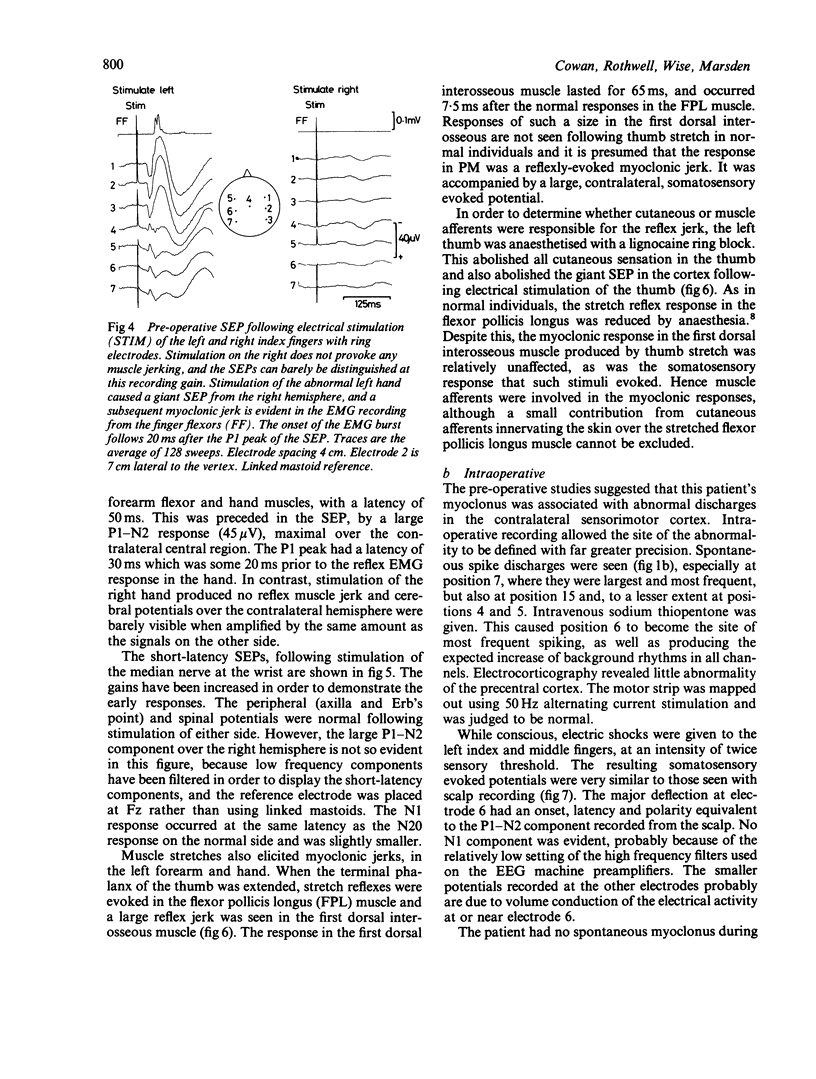
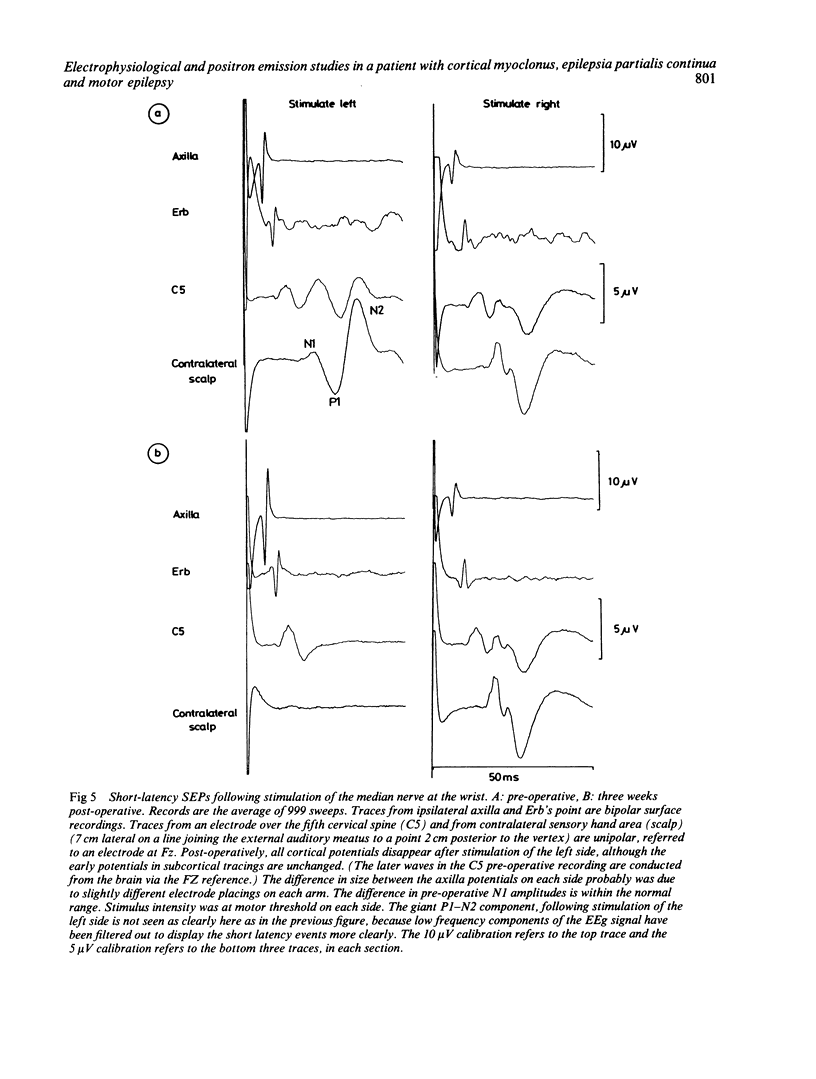
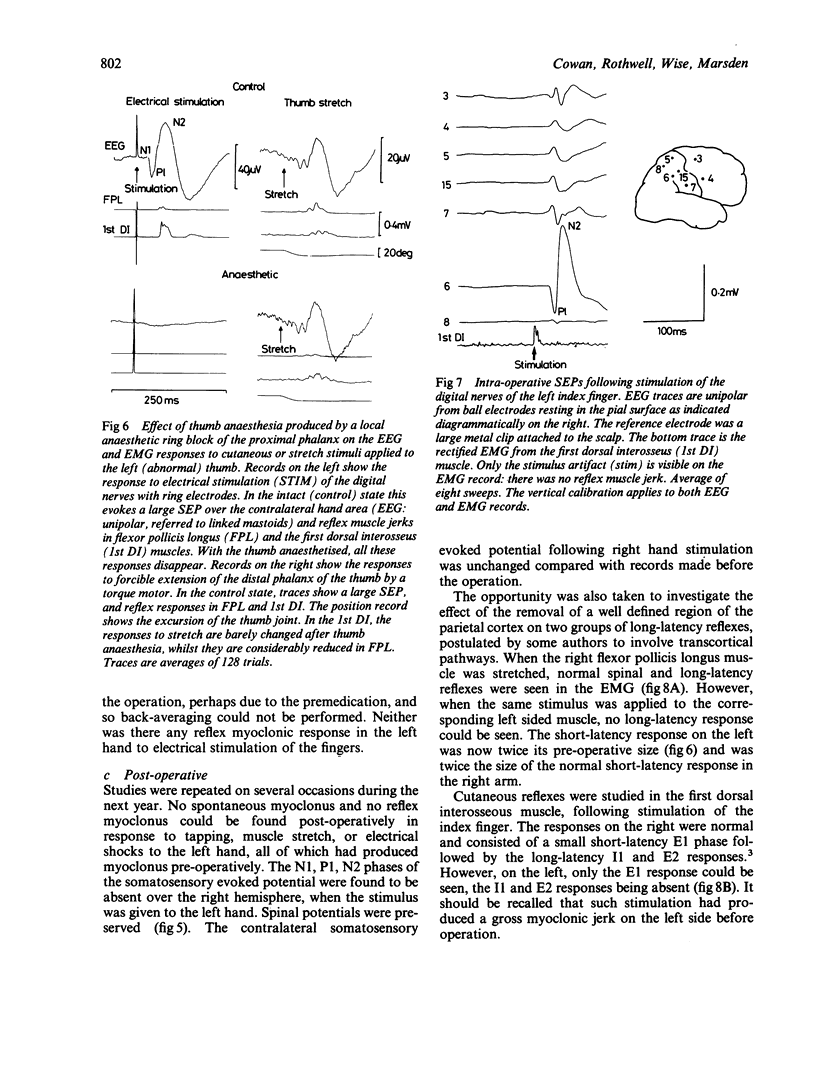
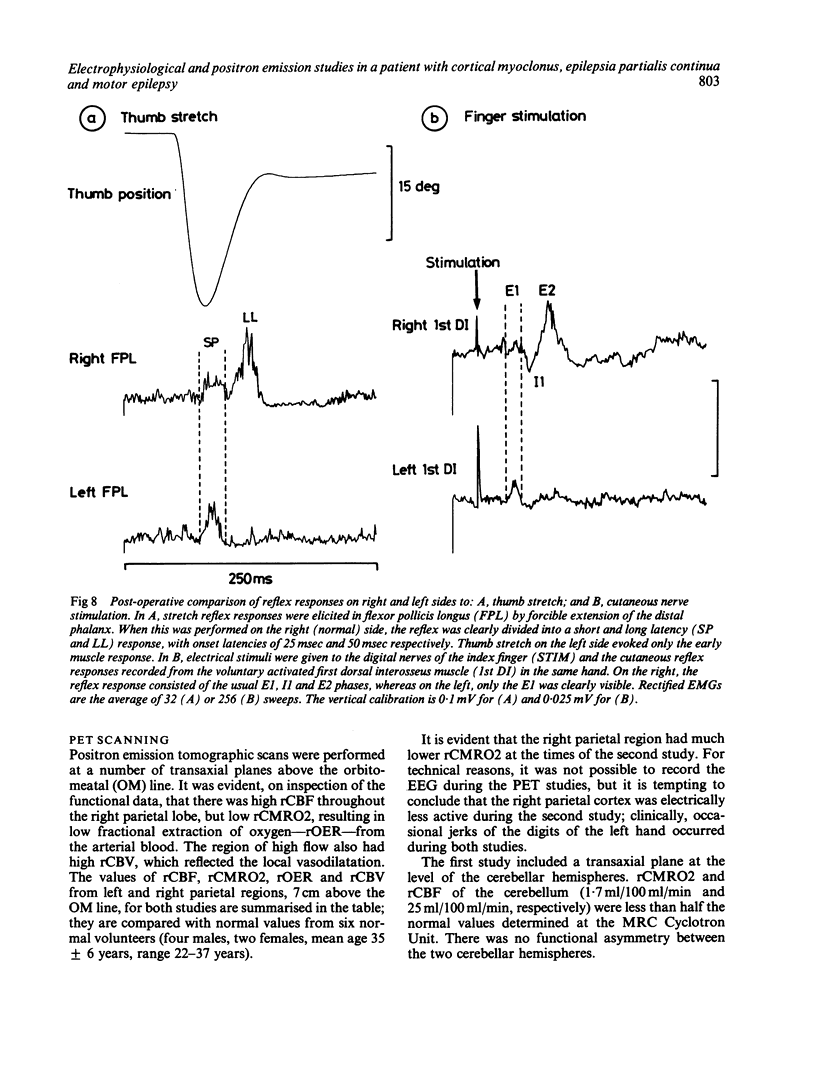
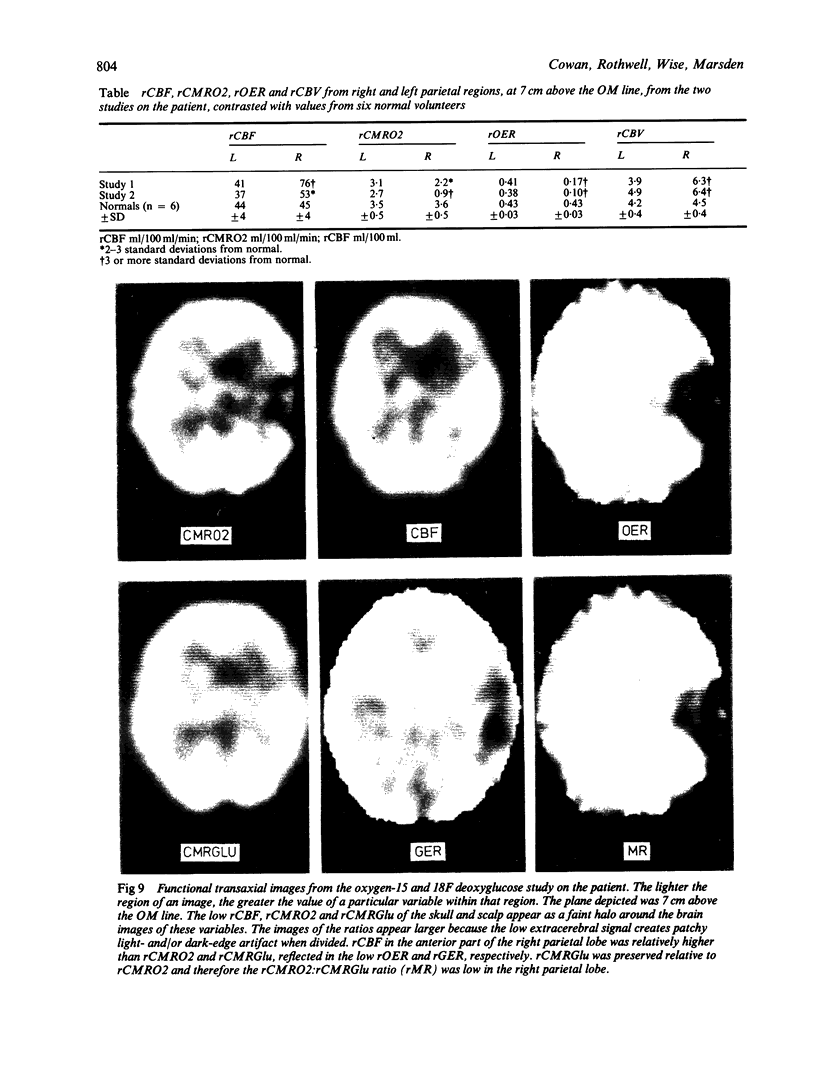
A patient is described who had a combination of stimulus-sensitive cortical myoclonus, epilepsia partialis continua, and Jacksonian motor epilepsy. He eventually required surgery because of the severity of his seizures. Electrophysiological recordings made before and during surgery, and PET scans performed before surgery identified an abnormal area of cerebral cortex in the postcentral parietal region. It is suggested that the stimulus-sensitive myoclonus arose because input into this region from peripheral sensory afferents produced an abnormal discharge which was fed forwards via cortico-cortical connections to the precentral motor cortex, to produce a reflex muscle jerk. The epilepsia partialis continua may have been caused by spontaneous discharges arising in the same region of parietal cortex. Both forms of jerking disappeared after resection of this part of the cortex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWSON G. D. Cerebral responses to nerve stimulation in man. Br Med Bull. 1950;6(4):326–329. doi: 10.1093/oxfordjournals.bmb.a073624. [DOI] [PubMed] [Google Scholar]
- Frackowiak R. S., Lenzi G. L., Jones T., Heather J. D. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980 Dec;4(6):727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Ghez C., Shinoda Y. Spinal mechanisms of the functional stretch reflex. Exp Brain Res. 1978 May 12;32(1):55–68. doi: 10.1007/BF00237390. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hägglund J. V., Wallin E. U., Young R. R. Grouped spindle and electromyographic responses to abrupt wrist extension movements in man. J Physiol. 1981 Mar;312:81–96. doi: 10.1113/jphysiol.1981.sp013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. C., Phelps M. E., Hoffman E. J., Sideris K., Selin C. J., Kuhl D. E. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980 Jan;238(1):E69–E82. doi: 10.1152/ajpendo.1980.238.1.E69. [DOI] [PubMed] [Google Scholar]
- Jenner J. R., Stephens J. A. Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol. 1982 Dec;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma A. A., Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. Description of the method. J Cereb Blood Flow Metab. 1983 Dec;3(4):416–424. doi: 10.1038/jcbfm.1983.67. [DOI] [PubMed] [Google Scholar]
- Lee R. G., Tatton W. G. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975 Aug;2(3):285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Is the human stretch reflex cortical rather than spinal? Lancet. 1973 Apr 7;1(7806):759–761. doi: 10.1016/s0140-6736(73)92141-7. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. The sensory mechanism of servo action in human muscle. J Physiol. 1977 Feb;265(2):521–535. doi: 10.1113/jphysiol.1977.sp011728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Brooks V. B. Late muscular responses to arm perturbations persist during supraspinal dysfunctions in monkeys. Exp Brain Res. 1981;41(2):146–158. doi: 10.1007/BF00236604. [DOI] [PubMed] [Google Scholar]
- Obeso J. A., Rothwell J. C., Marsden C. D. The spectrum of cortical myoclonus. From focal reflex jerks to spontaneous motor epilepsy. Brain. 1985 Mar;108(Pt 1):193–124. doi: 10.1093/brain/108.1.193. [DOI] [PubMed] [Google Scholar]
- Rasmussen T. Further observations on the syndrome of chronic encephalitis and epilepsy. Appl Neurophysiol. 1978;41(1-4):1–12. doi: 10.1159/000102395. [DOI] [PubMed] [Google Scholar]
- Rhodes C. G., Wise R. J., Gibbs J. M., Frackowiak R. S., Hatazawa J., Palmer A. J., Thomas D. G., Jones T. In vivo disturbance of the oxidative metabolism of glucose in human cerebral gliomas. Ann Neurol. 1983 Dec;14(6):614–626. doi: 10.1002/ana.410140604. [DOI] [PubMed] [Google Scholar]
- Rothwell J. C., Obeso J. A., Marsden C. D. On the significance of giant somatosensory evoked potentials in cortical myoclonus. J Neurol Neurosurg Psychiatry. 1984 Jan;47(1):33–42. doi: 10.1136/jnnp.47.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. E., Reagan T. J., Klass D. W. Epilepsia partialis continua. A review of 32 cases. Arch Neurol. 1977 May;34(5):266–275. doi: 10.1001/archneur.1977.00500170020003. [DOI] [PubMed] [Google Scholar]