Objective:
The objective of this study was to evaluate the clinical evolution of coronavirus disease 2019 (COVID-19) in children and adolescents with cancer.
Methods:
Cohort involving patients undergoing cancer treatment, 19 years old and under, with the diagnosis of COVID-19 by real-time polymerase chain reaction, in a reference hospital, between March 2020 and November 2021. Data were collected from medical records and interviews with patients and/or guardians. The primary outcomes studied were severe/critical COVID-19 presentation, deaths from any cause and overall survival. The Cox proportional hazards multivariate regression analysis was performed to determine the risk of death.
Results:
Sixty-two participants were included, most (67.7%) were male, with a median age of 6.8 years. Severe/critical forms of COVID-19, observed in 24.2%, seemed to indicate that the pediatric population undergoing cancer treatment has a higher morbidity rate than the general pediatric population (8–9.2%). During follow-up (4.5–18 months), 20 patients (32.3%) completed their cancer treatment and 18 died (29%)—6 during hospitalization and 12 after discharge. In total 61.1% of deaths occurred within 63 days of a detectable real-time polymerase chain reaction. Patients with a higher risk of death presented with severe/critical COVID-19 [adjusted hazard risk (aHR): 8.51; 95% confidence interval (CI): 2.91–24.80; P < 0.00] solid tumors (aHR: 3.99; 95% CI: 1.43–11.12; P = 0.008) and diarrhea as a symptom of COVID-19 (aHR: 3.9; 95% CI: 1.23–12.73; P = 0.021).
Conclusions:
These findings support the impact that severe acute respiratory syndrome-associated coronavirus 2 infection has on the population of children and adolescents with cancer, not only regarding immediate severity but also in their survival rate. Further studies evaluating long-term outcomes of COVID-19 in children and adolescents with cancer should be encouraged.
Keywords: coronavirus disease 2019, pediatric cancer, severe acute respiratory syndrome-associated coronavirus 2
In March 2020, the World Health Organization (WHO) classified coronavirus disease 2019 (COVID-19) as a pandemic.1 After almost 3 years, in February 2023, reported cases worldwide had exceeded 670 million, with 7.2 million deaths. Approximately 10% of all reported cases occurred in Brazil, the country with the second-highest death rate, with more than 690,000.2
During the COVID-19 pandemic, management of cancer patients has been a matter of concern, due to a greater risk of infection and to more severe forms of the disease. Additional issues regarding timely cancer diagnosis and meeting suitable treatment deadlines are also relevant.3,4 Most cancers in children present aggressive behavior, a short latency period, involve immediate treatment and may require long periods of intensive chemotherapy with multiple antineoplastic agents.5 In contrast, pediatric patients with cancer respond better to treatment and have a good prognosis when compared to adults.6
Adults with cancer and COVID-19, especially hematologic cancer or within a month after chemotherapy or surgery, presented with greater severity and mortality when compared with the general population.7,8 In pediatric cancer patients, however, data are conflicting. Although some studies have shown no increase in severity and lethality (4.9%),9–11 in developing countries with a high incidence of severe acute respiratory syndrome-associated coronavirus 2 (SARS-CoV-2), pediatric populations—particularly with comorbidities—may have different characteristics regarding the course of the disease.12,13 Likewise, there is still scarce data regarding survival and other long-term outcomes in children and adolescents with cancer.3–5,10–13
METHODS
Study Design, Period and Place
This was a cohort study, involving patients undergoing cancer treatment (or up to 3 months after completion), 19 years of age and under, diagnosed with COVID-19, recruited and monitored during the period from March 1, 2020, to November 30, 2021. The research was conducted at the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), a hospital located in Recife, Pernambuco, Brazil, which is a regional referral center for children and adolescents with cancer, with an intensive care unit (ICU) exclusively for cancer patients. Due to the pandemic, all patients with suspected COVID-19 and/or before being admitted to the hospital were tested. Sampling was consecutive, the sample size was not calculated and all eligible patients were included during the study period. The primary outcomes studied were severe/critical presentation of COVID-19, death from any cause and survival during the study period.
COVID-19: Diagnosis and Classification
Infection was laboratory confirmed by reverse transcription real-time polymerase chain reaction (RT-PCR), searching for SARS-CoV-2 in nasopharyngeal/oropharyngeal swabs, a method considered the gold standard to confirm the presence of the virus and current infection.14
To characterize the clinical presentation of COVID-19, the classification proposed by Dong et al.15 was used, that is, asymptomatic, mild, moderate, severe and critical disease. Severe cases were considered as those with early respiratory symptoms progressing to hypoxemia within a week, characterized by oxygen saturation levels lower than or equal to 92%. Critical cases were those that progressed rapidly to SARS, in addition to dysfunction in other systems, such as shock, encephalopathy, myocardial injury with heart failure, kidney and coagulation cascade changes.15,16 For the primary outcome analysis, the severe and critical forms were grouped together. The diagnostic criteria for the multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19, which is considered a late complication of the disease, were those adopted by the WHO.17
Data Collection and Analysis
Research data was obtained via electronic medical records and interviews with patients and/or guardians. Biologic and sociodemographic characteristics, diagnosis and cancer treatment (at the time of SARS-CoV-2 infection) were assessed. For the purpose of analysis, cancers were grouped into hematologic tumors (leukemias and lymphomas) and solid tumors. Cancer treatment was divided into curative and palliative treatment. Chemotherapy was subdivided into induction/consolidation and maintenance/reinduction and grouped according to the similarity in the intensity of chemotherapy used and the frequency with which patients attend the hospital during the treatment phase. Data regarding the follow-up of SARS-CoV-2 infection were also collected: household contact, clinical presentation, follow-up location, complementary examinations, medications used, respiratory support and use of vasoactive drugs (VADs).
In November 2021, patients were assessed for oncologic disease status and grouped into 4 categories: duration of cancer treatment, complete remission, recurrence/palliative care and death.
Frequency distribution measures were established for categorical variables and a central tendency for continuous variables, presented in tables. The Poisson regression was used to determine the prevalence ratio between the factors that influenced the severe/critical forms of COVID-19. The Cox proportional hazards analysis estimated the crude hazard risk (cHR) and the adjusted hazard risk (aHR) to determine the factors that influenced death. For the initial multivariate model, variables (clinical and laboratory) associated with each outcome were included, with a significance level lower than or equal to 20% in the univariate and with biologic plausibility. The COVID-19 symptoms were evaluated in a single block, controlled between each, and later incorporated into the multivariate analysis model, and in the final model the variables with a statistical significance (P values < 0.05) were considered, controlled by the age when admitted to the study.
Survival time was considered as the time between the date of the COVID-19 diagnosis and the date of the last consultation, the end of cancer treatment or the date of death until November 2021. The probability of overall survival was estimated by the Kaplan–Meier method and the survival curve was created with the respective 95% confidence intervals (CI). The difference in the probability of survival of the types of cancers was performed using the log-rank test, with a 95% CI.
Ethical Aspects
This research is part of a project submitted and approved by the IMIP Research Ethics Committee, CAAE: 31578520.8.0000.5201. The authors declare no conflicts of interest.
RESULTS
A total of 538 tests were performed to identify SARS-CoV-2 in 287 patients registered in pediatric oncology, during the period from March 1, 2020, to November 30, 2021. The pathogen was detected in 63 patients eligible for the study, the last one on May 28, 2021. There was data loss due to the incomplete medical records of 1 patient, thus totaling 62 participants in the cohort.
Characteristics of the sample are presented in Table, Supplemental Digital Content 1 (http://links.lww.com/INF/F11). The age of the participants ranged from 6 months to 18 years, with a median of 6.8 years, and most were male [42 (67.7%)]. Leukemias were the most frequent type of cancer [39 (62.9%)] and 32 patients (51.6%) were in the induction/consolidation phases of cancer treatment.
Clinical characteristics related to COVID-19 are presented in Table 1. Most patients [41 (66.1%)] presented with up to 3 symptoms, and the most frequent were: fever [36 (58%)], coryza [19 (30.6%)] and cough [16 (25.8%)]. Hypoxemia was present in 11 (17.7%) participants, 2 of whom (3.2%) presented with normal pulmonary imaging tests.
TABLE 1.
The Distribution of Symptoms, Clinical Follow-up, and Cohort Evolution of 62 Pediatric Patients Undergoing Cancer Treatment with Detectable SARS-CoV-2 by RT-PCR Between March 01, 2020, and November 30, 2021, Followed up at the Pediatric Oncology Division at IMIP
| Variables | All Patients | March to December 2020 | January to October 2021 | ||
|---|---|---|---|---|---|
| n (%) | n = 50 | (%) | n = 12 | (%) | |
| Asymptomatic patients—systematic search | 12 (19.3) | 10 | (20.0) | 2 | (16.7) |
| Signs and symptoms during treatment for COVID-19 | 50 (80.6) | 40 | (80.0) | 10 | (83.3) |
| ▪ Fever | 36 (58.0) | 29 | (58.0) | 7 | (58.3) |
| ▪ Coryza | 19 (30.6) | 13 | (26.0) | 6 | (50.0) |
| ▪ Cough | 16 (25.8) | 13 | (26.0) | 3 | (25.0) |
| ▪ Dyspnea | 13 (21.0) | 10 | (20.0) | 3 | (25.0) |
| ▪ Nausea/vomits | 3 (4.8) | 2 | (4.0) | 1 | (8.3) |
| ▪ Diarrhea | 9 (14.5) | 8 | (16.0) | 1 | (8.3) |
| ▪ Headache | 5 (8.0) | 4 | (8.0) | 1 | (8.3) |
| ▪ Anosmia | 4 (6.4) | 4 | (8.0) | 0 | (0) |
| Follow-up | |||||
| ▪ Outpatients | 21 (33.9) | 16 | (32.0) | 5 | (41.7) |
| ▪ Hospitalization | 41 (66.1) | 34 | (68.0) | 7 | (58.3) |
| ICU | 12 (19.3) | 9 | (18.0) | 3 | (25.0) |
| History of COVID at home | 15 (24.2) | 9 | (18.0) | 6 | (50.0) |
| Complementary exams | |||||
| Changes in chest imagining exams (radiograph or CT) | 18 (29.0) | 13 | (26.0) | 5 | (41.7) |
| Lymphopenia (<300 cells/mm³) | 21 (33.9) | 17 | (34.0) | 4 | (33.3) |
| Neutropenia (<500 cells/mm³) | 31 (50.0) | 25 | (50.0) | 6 | (50.0) |
| Kidney changes | 6 (9.7) | 5 | (10.0) | 1 | (8.3) |
| Classification of severity (Dong et al.,2020) | |||||
| ▪ Mild/moderate | 47 (75.8) | 38 | (76.0) | 9 | (75.0) |
| Severe/critical COVID-19 | 15 (24.2) | 12 | (24.0) | 3 | (25.0) |
| MIS-C (Panigrahy et al., 2020) | 8 (12.9) | 6 | (12.0) | 2 | (16.6) |
| Treatment | |||||
| Respiratory support | 11 (17.7) | 9 | (18.0) | 2 | (16.6) |
| ▪ Mechanical ventilation | 6 (9.7) | 4 | (8.0) | 2 | (16.6) |
| Vasoactive drugs | 6 (9.7) | 4 | (8.0) | 2 | (16.6) |
| Death during hospitalization | 6 (9.7) | 4 | (8.0) | 2 | (16.6) |
| ▪ Cause of death ▪ severe acute respiratory syndrome ▪ Fungic infection | 6 (9.7) 5 (8.0) (1.6) | 3 1 | (6) (2) | 2 0 | (16.6) (0) |
COVID-19 indicates coronavirus disease 2019; ICU, intensive care unit.
Severe/critical acute forms of COVID-19 were observed in 15 patients (24.2%). Although some biologic and clinical variables entered the initial model (Poisson regression data are not shown in the tables) of the multivariate analysis, only dyspnea remained in the final model (prevalence ratio: 24.5; 95% CI: 6.23–96.26; P < 0.001), which may be explained by the sample size and the fact that dyspnea is part of the classification of severe forms of SARS-CoV-2 infection. PIMS-associated COVID-19 was diagnosed in 8 (12.9%) of the 62 patients.
Most patients [41 (66.1%)] were hospitalized, with a length of stay between 1 and 40 days (a median of 9 days). Those who required ICU remained there between 3 and 28 days, with a median of 8 days. The following medications were used in the treatment of COVID-19: azithromycin [17 (27.4%)], corticosteroids [11 (17.7%)], antivirals [oseltamivir (11.3%) and acyclovir (8%)] and anticoagulants [3 (4.8%)], depending on the severity of the patients and the current recommendations. Six patients (9.7%) required VADs due to hemodynamic dysfunction. Changes in kidney function were observed in 6 (9.7%) patients, 1 of whom required dialysis. During hospitalization, 37 [59.7%] patients were treated for secondary bacterial infection and 24 required more than 1 course of antimicrobial drugs.
The follow-up time of the patients ranged from 4.5 to 18 months. During this period, 20 children (32.3%) completed their cancer treatment, 18 (29%) died during the cohort, 2 (3.2%) had recurrence of cancer and 2 (3.2%) are undergoing palliative care (Table 2).
TABLE 2.
The Follow-up of the Cohort and Oncological Status in October 2021 of the 62 Pediatric Patients Undergoing Cancer Treatment with Detectable SARS-CoV-2 by RT-PCR, Between March 1, 2020 and November 30, 2021, and the Distribution of the 18 Deaths During the Study Period by Type of Cancer, Stage of Cancer Treatment and Survival Time Between the Diagnosis of Cancer and COVID-19 and Death
| Variables | Absolute Frequency n | Relative Frequency % |
|---|---|---|
| All patients—status at the end of the study (follow-up 18 months) | n = 62 | % |
| Undergoing treatment | 20 | (32.3) |
| Complete remission | 20 | (32.3) |
| Recurrence/palliative care | 4 | (6.4) |
| Death | 18 | (29.0) |
| Patients who died before the end of the study | n = 18 | % |
| Type of cancer | ||
| Leukemias | 7 | (38.9) |
| Lymphomas | – | – |
| Solid tumors | 11 | (61.1) |
| Neuroblastoma | 3 | (16.6) |
| CNS tumor | 3 | (16.6) |
| Sarcomas and others | 5 | (27.8) |
| Phase of oncological treatment | ||
| Induction/consolidation | 13 | (72.2) |
| Maintenance/reinduction | 3 | (16.7) |
| Palliative care | 2 | (11.1) |
| Time between diagnosis of COVID-19 and death | ||
| Extreme (days and months) | 1 day to 13 months | |
| Mean (±SD) days | 215 (±155) | |
| Median (interquartile interval) days | 171 (99–320) | |
| Time between diagnosis of cancer and death | ||
| Extreme (days and months) | 20 days to 76 months | |
| Mean (±SD) days | 241 (±525) | |
| Median (interquartile interval) days | 275 (124–499) |
COVID-19 indicates coronavirus disease 2019.
Of the 18 deaths, 6 (9.7% of the cohort) occurred during acute COVID-19 hospitalization, in a median of 17 days between the date of detectable RT-PCR and death. Five of these (83.3%) had severe acute respiratory syndrome as the cause of death (4 with leukemia and 1 with rhabdomyosarcoma) and 1 leukemia patient (17.7%) died from widespread fungal infection (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/F11, and Fig. 1A). Regarding the 12 deaths after COVID-19 hospitalization, 8 were due to progression of oncologic disease (7 with solid tumors), 1 after tumor resection, 1 by MIS-C associated with COVID-19 and another after massive digestive tract hemorrhage. Cause of death data was missing in the medical records of 1 patient.
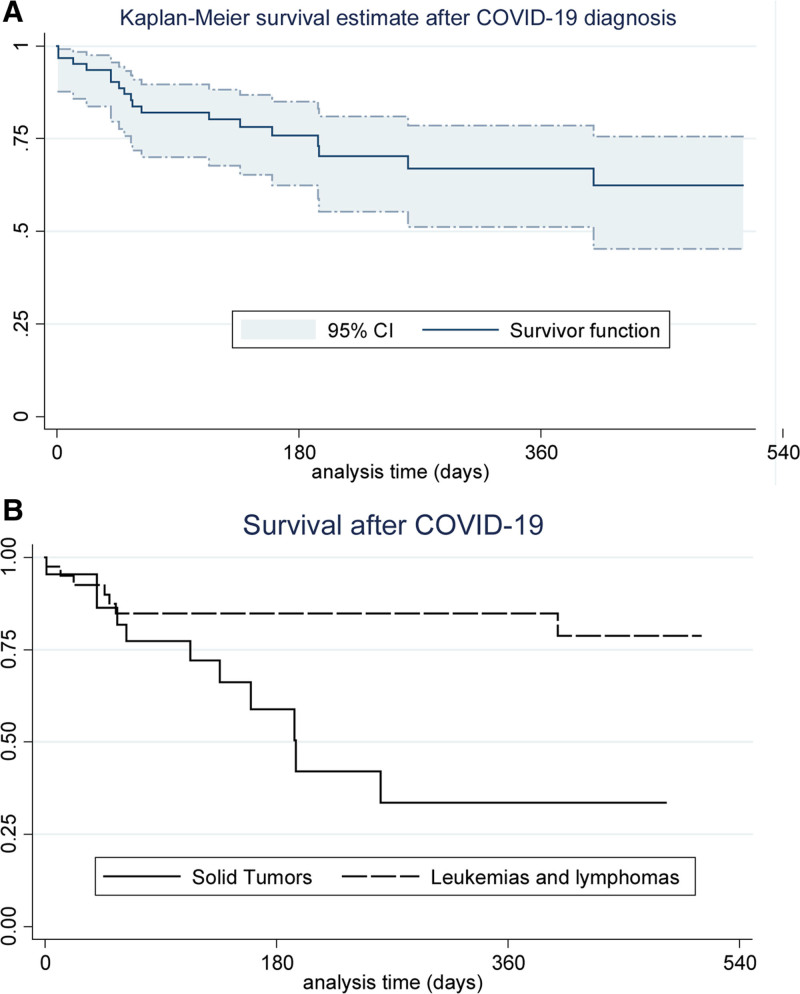
FIGURE 1.
Overall survival curves of the 62 patients undergoing cancer treatment who had detectable SARS-CoV-2 by RT-PCR between March 1, 2020, and November 30, 2021, followed up at the pediatric oncology division at IMIP. A: All patients; (B) according to the type of tumor.a
Delay in cancer treatment related to SARS-CoV-2 infection happened in 12 of the 18 patients that died. Four delays occurred in the group of children that died during hospitalization (3 leukemia and 1 solid tumor patient) and 8 in the group that died after hospital discharge (2 leukemia and 6 solid tumors). However, there was no statistically significant association between delayed treatment and death by any cause.
In the Cox proportional hazards multivariate analysis (Table 3), when considering all deaths in the sample, these were associated with a higher risk of death: severe/critical COVID-19 (aHR: 8.51; 95% CI: 2.91–24.80; P < 0.001), diagnosis of solid tumors (aHR: 3.99; P = 0.008; 95% CI: 1.43–11.12) and diarrhea as a symptom of SARS-CoV-2 infection (aHR: 3.97; 95% CI: 1.23–12.73; P = 0.021).
TABLE 3.
The Bivariate and Multivariate Analyses of the Cox Proportional Hazards for the Deaths of 62 Patients Undergoing Cancer Treatment who had Detectable SARS-CoV-2 by RT-PCR Between March 1, 2020, and November 30, 2021, Followed up at the Pediatric Oncology Division at IMIP
| Hazard Risk (CI 95%) | P value | aHR (CI 95%) | P value | |
|---|---|---|---|---|
| Age (years) | 0.99 (0.90–1.08) | 0.775 | 0.95 (0.86–1.06) | 0.371 |
| Nutritional status | – | – | ||
| Underweight | 3.18 (1.10–9.20) | 0.032 | – | – |
| Overweight/obese | 2.66 (0.8–8.93) | 0.113 | – | – |
| Solid tumor | 3.62 (1.37–9.59) | 0.009 | 3.99 (1.43–11.12) | 0.008 |
| Induction/consolidation phase of the oncological treatment | 2.89 (1.03–8.10) | 0.044 | – | – |
| Palliative care | 3.01 (0.67–13.50) | 0.149 | – | – |
| Dyspnea | 5.00 (1.94–12.88) | 0.001 | – | – |
| Diarrhea | 3.43 (1.21–9.74) | 0.021 | 3.97 (1.23–12.73) | 0.021 |
| Lymphopenia (<300 mm/cel³) | 1.92 (0.76–4.87) | 0.170 | – | – |
| Severe/critical COVID-19 | 6.57 (2.53–17.02) | <0.001 | 8.51 (2.91–24.80) | <0.001 |
aHR indicates adjusted hazard risk; Ci, confidence interval; COVID-19, coronavirus disease 2019.
The survival curve and table, using the Kaplan–Meier method (presented in Fig. 1A), demonstrated that 11 (61.1%) of the 18 deaths occurred within 63 days of the laboratory diagnosis of COVID-19. The survival probability of solid tumors (Fig. 1B) was 33.6% (95% CI: 11.4–57.8) and of hematologic neoplasms was 78.7% (95% CI: 58.1–90.0), with a statistically significant difference (log-rank, P = 0.0056).
DISCUSSION
From a cohort of 62 pediatric patients with cancer, 24.2% had severe/critical forms of COVID-19 and 29% died, 1/3 of the deaths during hospitalization for COVID-19 and the others during the follow-up period for up to 18 months.
Although systematic reviews with adolescents and children reveal a lower incidence of severe/critical forms of COVID-19 (8%–12% in general and 9.6% in oncologic population), in our study, the incidence of severe/critical forms was close to the 19% found in a multicenter cohort of 45 countries, involving 1301 children and adolescents with cancer.10,11,18,19 These findings seem to indicate that the pediatric population undergoing cancer treatment may be predisposed to severe presentations of COVID-19 in a similar proportion, or just slightly less, to adults (13%–28%).7,8,20 Although some biologic and clinical variables entered the initial model of the Poisson multivariate analysis, only dyspnea remained in the final model. It is interesting to note, however, that dyspnea did not lead to a higher risk of death for patients and did not remain in the final Cox proportional hazards model (Table 3).
In our study, there was a lower number of diagnosed cases of COVID-19 in 2021 (Table 1), a fact that was probably related to the beginning of the vaccination program in the adult population (as of January 2021), with an indirect impact on the population studied through health professionals and carers.21 It is also relevant that the first cases of omicron subvariant in Brazil were reported after the study’s period (December 2021), which may have influenced the relatively low incidence during 2021.22
MIS-C was diagnosed in 12.9% of patients, a slightly higher number than reported in a systematic review (8%) of 87 children with cancer.10 Although a significant risk factor for death was identified in the bivariate analysis of our data, due to the excessively wide CI (possibly due to the sample size), MIS-C was excluded from the final Cox proportional-hazards model. It should be noted that the frequency of deaths reported with MIS-C is higher than among children with the acute form of COVID-19, and further studies in children and adolescents with cancer are needed since this complication has been poorly reported in studies of this population.11,18,23–26
Lethality during hospital admission (9.7%) was higher than expected in the general pediatric population (0.1%–0.7%) and in children and adolescents with cancer (3.8%–4.9%), although it is similar to other studies conducted in South America, in pediatric cancer patients.10–13,25,28
In the Cox proportional hazards multivariate analysis (Table 3), age was maintained in the model for biologic plausibility and was identified as a protective factor. For each year of age, the risk of death in patients was reduced by 5%. The literature reports that children under the age of 6 years, especially those under the age of 1 year, present more severe forms of COVID-19 and greater lethality from SARS-CoV-2.10,15,28
Severe acute forms of COVID-19 were associated with a higher risk of death, which is expected, given that this classification includes hypoxemic patients or those with multiple system dysfunctions, thereby presenting a greater need for intensive care and VADs.15 It is interesting to note that diarrhea was associated with a higher risk of death, which was also observed in another study, where pediatric patients with gastrointestinal manifestations presented more severe forms of COVID-19.29
Hematologic neoplasms are the most frequent forms in the pediatric age group and present more severe conditions and higher mortality rates from complications (including infections) when compared to solid tumors. However, with proper treatment, they have a high chance of being cured.30–32 Perhaps for this reason, more patients with hematologic neoplasms died during hospitalization for COVID-19, while in the total cohort follow-up, solid tumors presented a higher risk of death, as evidenced in Fig. 1B.
Most deaths (72.2%) occurred in the induction/consolidation phases of cancer treatment. Although the P value was not significant in the multivariate analysis (probably due to the number of patients in the sample), it is important to mention that during these phases cancer treatment is more intense and there is a greater need of attending to the hospital, which may contribute to a greater impairment of the immune system and infections in this group (Tables 2 and 3).
The findings of our study highlight the impact that SARS-CoV-2 infection has on the population of children and adolescents with cancer, possibly not only on the immediate severity but also on the survival of these patients after dealing with the acute condition. The estimated 5-year survival of children and adolescents with cancer is 80% in developed countries but does not exceed 30% when low- and middle-income countries are assessed, where 89% of pediatric cancers occur.32 In Latin America, the estimated survival rate is 55%, and in Brazil, 65%.33,34 However, in our study, the overall survival after COVID-19 was 62.4% (95% CI: 45.20–75.62) over a maximum period of 1.5 years, which may express a decrease in the expected survival of this population in the period of 5 years. More studies, with a longer follow-up time, are necessary to confirm this possibility.
It is important to emphasize that the present study has limitations due to the small sample size and was performed in a single health center. Furthermore, another limitation is the lack of differentiation concerning morbidity and/or risk of death as caused by COVID-19 or by the underlying malignant disease itself. In the multivariate analysis, deaths were included as a single outcome since the aim of our study was to determine patient survival overall. Our study design did not provide a precise distinction of which deaths were directly caused by COVID-19 (such as SARS and MIS-C associated with SARS-CoV-2 infection) and which were an indirect effect of COVID-19 (eg, other infections during COVID-19 hospitalization or delay in treatment and progression of oncologic disease). For this purpose, more studies are required.
Children and adolescents with cancer constitute a group that requires specific health care and attention. When diagnosed and treated in a timely manner, they present a good outcome with a high chance of being cured. However, the COVID-19 pandemic—especially in developing countries—has aggravated problems in the care of this population, such as a delay in their diagnosis (due to overcrowding in health services) and in starting cancer treatment as well. The presented study showed that SARS-CoV-2 infection resulted in greater severity and mortality in this population, which probably impacted their long-term survival. Nonetheless, further studies are doubtless necessary to investigate this matter, and future research on delay in diagnosing cancer and the long-term outcomes of COVID-19 in children and adolescents with cancer should be encouraged.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
A.L.M.A.L., M.J.G.M. and M.M.L. contributed with conception, planning, tabulation, data analysis and interpretation, writing and article review. K.M.M.O. contributed to the conception, planning, analysis and interpretation of the data, writing and reviewing the article. M.C.D.B. and A.P.R.M. contributed to data collection, writing and review of the article. All authors approved the final version of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Mecneide Mendes Lins, Email: mecneide.mendes@gmail.com.
Maria Júlia Gonçalves Mello, Email: mjuliagmello@gmail.com.
REFERENCES
- 1.Dhama K, Khan S, Tiwari R, et al. Coronavirus disease 2019–COVID-19. Clin Microbiol Rev. 2020;33:e00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center. COVID-19 Map. 2023. Available at: https://coronavirus.jhu.edu/map.html. Accessed February 3, 2023.
- 3.Amicucci M, Mastronuzzi A, Ciaralli I, et al. The management of children with cancer during the COVID-19 pandemic: a rapid review. J Clin Med. 2020;9:3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathania AS, Prathipati P, Abdul BA, et al. COVID-19 and cancer comorbidity: therapeutic opportunities and challenges. Theranostics. 2021;11:731–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotecha RS. Challenges posed by COVID-19 to children with cancer. Lancet Oncol. 2020;21:e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministério da Saúde. Protocolo do diagnóstico precoce do câncer pediátrico. [Google Scholar]
- 7.Jarahzadeh MH, Asadian F, Farbod M, et al. Cancer and coronavirus disease (COVID-19): comorbidity, mechanical ventilation, and death risk. J Gastrointest Cancer. 2021;52:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai A, Sachdeva S, Parekh T, et al. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisogno G, Provenzi M, Zama D, et al. Clinical characteristics and outcome of severe acute respiratory syndrome coronavirus 2 infection in Italian pediatric oncology patients: a study from the infectious diseases working group of the Associazione Italiana di Oncologia e Ematologia Pediatrica. J Pediatric Infect Dis Soc. 2020;9:530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meena JP, Kumar Gupta A, Tanwar P, et al. Clinical presentations and outcomes of children with cancer and COVID-19: a systematic review. Pediatr Blood Cancer. 2021;68:e29005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukkada S, Bhakta N, Chantada GL, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. 2021;22:1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corso MCM, Soares VJ, Amorim AMP, et al. SARS-CoV-2 in children with cancer in Brazil: results of a multicenter national registry. Pediatr Blood Cancer. 2021;68:e29223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montoya J, Ugaz C, Alarcon S, et al. COVID-19 in pediatric cancer patients in a resource-limited setting: national data from Peru. Pediatr Blood Cancer. 2021;68:e28610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumavath R, Barh D, Andrade BS, et al. The spike of SARS-CoV-2: uniqueness and applications. Front Immunol. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 16.Freire N de M, Garros D. Covid-19 em pediatria: diagnóstico, recomendações e condutas: uma revisão sistemática da literatura. Pantanal Editora; 2021. Available at: https://www.editorapantanal.com.br/ebooks-capitulo.php?ebook_id=covid-19-em-pediatria-diagnostico-recomendacoes-e-condutas-uma-revisao-sistematica-da-literatura&ebook_ano=2021&ebook_caps=0&ebook_org=0. Accessed March 2022. [Google Scholar]
- 17.Panigrahy N, Policarpio J, Ramanathan R. Multisystem inflammatory syndrome in children and SARS-CoV-2: a scoping review. McLaughlin M, Vercler C, editors. J Pediatr Rehabil Med. 2020;13:301–316. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Rinaldi E, Zusi C, et al. Coronavirus disease 2019 (COVID-19) in children and/or adolescents: a meta-analysis. Pediatr Res. 2021;89:733–737. [DOI] [PubMed] [Google Scholar]
- 19.Belsky JA, Tullius BP, Lamb MG, et al. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021;82:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021;93:6486–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloete J, Kruger A, Masha M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health. 2022;6:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabeerdoss J, Pilania RK, Karkhele R, et al. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Zhang S, Zhang R, et al. Epidemiological and clinical characteristics of COVID-19 in children: a systematic review and meta-analysis. Front Pediatr. 2020;8:591132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulad F, Kamboj M, Bouvier N, et al. COVID-19 in children with cancer in New York City. JAMA Oncol. 2020;6:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari A, Zecca M, Rizzari C, et al. Children with cancer in the time of COVID-19: an 8-week report from the six pediatric onco-hematology centers in Lombardia, Italy. Pediatr Blood Cancer. 2020;67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo Marin B, Aghagoli G, Lavine K, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Q, Wang Z, Liu J, et al. Risk factors for poor prognosis in children and adolescents with COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;41:101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraiva DDCA, Santos SDS, Monteiro GTR. Tendência de mortalidade por leucemias em crianças e adolescentes nas capitais dos estados brasileiros: 1980-2015. Epidemiol e Serviços Saúde. 2018;27:e2017310. [DOI] [PubMed] [Google Scholar]
- 31.Stephano K, Dubbs SB. Pediatric hematologic and oncologic emergencies. Emerg Med Clin N Am. 2021;39:555–571. [DOI] [PubMed] [Google Scholar]
- 32.Society AC. Key Statistics for Childhood Cancers. 2022. Available at: https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Accessed January 2023.
- 33.Instituto Nacional do Câncer. Sobrevida de pacientes infantojuvenis com câncer é de 64% no Brasil. 2022. Available at: https://www.inca.gov.br/en/node/297. Accessed January 2023.
- 34.Burki T. Inequality in childhood cancer care worldwide. Lancet Oncol. 2022;23:456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.