Abstract
Adolescent alcohol use can permanently alter brain function and lead to poor health outcomes in adulthood. Emerging evidence suggests that alcohol use can predispose individuals to pain disorders or exacerbate existing pain conditions, but the underlying neural mechanisms are currently unknown. Here we report that mice exposed to adolescent intermittent access to ethanol (AIE) exhibit increased pain sensitivity and depressive-like behaviors that persist for several weeks after alcohol cessation and are accompanied by elevated CD68 expression in microglia and reduced numbers of serotonin (5-HT)-expressing neurons in the dorsal raphe nucleus (DRN). 5-HT expression was also reduced in the thalamus, anterior cingulate cortex (ACC) and amygdala as well as the lumbar dorsal horn of the spinal cord. We further demonstrate that chronic minocycline administration after AIE alleviated hyperalgesia and social deficits, while chemogenetic activation of microglia in the DRN of ethanolnaïve mice reproduced the effects of AIE on pain and social behavior. Chemogenetic activation of microglia also reduced tryptophan hydroxylase 2 (Tph2) expression and was negatively correlated with the number of 5-HT-immunoreactive cells in the DRN. Taken together, these results indicate that microglial activation in the DRN may be a primary driver of pain, negative affect, and 5-HT depletion after AIE.
Keywords: Adolescence, Ethanol, Pain, Depression, Serotonin, Microglia, Dorsal raphe
1. Introduction
The adolescent brain is highly sensitive to the effects of ethanol, which may predispose individuals to a wide range of neuropsychiatric comorbidities that persist into adulthood (Crews et al., 2019, 2016a). Increased impulsivity (Gilpin et al., 2012), deficits in working memory and cognitive flexibility (Beaudet et al., 2016; Coleman et al., 2014; Macht et al., 2020; Towner and Spear, 2020; Wolstenholme et al., 2017), increased ethanol consumption (Gilpin et al., 2012; Younis et al., 2019) and enhanced ethanol sensitivity (Wolstenholme et al., 2017; Younis et al., 2019) have all been reported after adolescent alcohol. These behavioral adaptations are accompanied by changes in cerebral volume, myelin-related genes, chromatin structure, and neuronal excitability in the prefrontal cortex (PFC) and hippocampus, two brain regions implicated in cognition and emotional affect. Previous work by Linda Spear’s group also suggests that alcohol exposure during early adolescence can inhibit social investigation and preference in male rats (Broadwater et al., 2011; Varlinskaya et al., 2014) and reduce contextual fear recall (Broadwater and Spear, 2013), while late adolescent alcohol exposure increases contextual fear retention and promotes a shift from goal-oriented to habitual behavior (Towner and Spear, 2020). Other recent work suggests that 4 weeks of ethanol drinking in adolescence can potentiate anxiety-like behaviors in multiple assays (Sampedro-Piquero et al., 2022), although this was not consistently observed across all species and ethanol administration paradigms (Lee et al., 2016; Torcaso et al., 2017). These extensive behavioral changes suggest that multiple brain circuits are impacted during this critical developmental period, putting adolescent alcohol drinkers at higher risk for a wide range of neurological disorders.
Emerging evidence suggests that chronic alcohol exposure may also be a risk factor in chronic pain disorder, which is a condition that afflicts approximately 20 % of adults in the U.S. (Dahlhamer et al., 2018; Nasrallah et al., 2011; Risher et al., 2015; Schindler et al., 2014). Comorbidity between ethanol dependence and pain is often attributed to self-medication of symptoms (Brennan et al., 2005; Thompson et al., 2017), but some studies suggest that heavy alcohol drinking and other chronic stressors may exacerbate or even precipitate pain symptoms (Caniglia et al., 2020; de Oliveira et al., 2017; Egli et al., 2012; Fu et al., 2015; Marcinkiewcz et al., 2009). Several recent studies have also reported microglial activation and elevated levels of pro-inflammatory cytokines in the brain following adolescent alcohol exposure, all of which may adversely impact synaptic function in central pain circuits (Bajo et al., 2015; Crews et al., 2017, 2016b; Crews and Vetreno, 2014; Cruz et al., 2017; Marshall et al., 2020; Walter and Crews, 2017; Zhao et al., 2013). Together, these converging lines of evidence indicate that microglia may be a common cellular substrate linking alcohol use to chronic pain disorders, particularly in sensitive developmental periods like adolescence (Grace et al., 2018; Sawicki et al., 2019; Yi et al., 2021).
The dorsal raphe nucleus (DRN) is a brainstem nucleus that is enriched in serotonin (5-hydroxytryptamine; 5-HT) neurons and has been reported to play a central role in pain regulation and affect (Akil and Mayer, 1972; Dugé et al., 2014; Griffith and Gatipon, 1981; Horie et al., 1991; Mayer and Liebeskind, 1974; Oliveras et al., 1979). 5-HT neurons are acutely sensitive to the effects of acute and chronic alcohol (Lowery-Gionta et al., 2015; Thielen et al., 2001; Underwood et al., 2007) as well as pro-inflammatory cytokines such as those released by activated microglia in the brain (Brambilla et al., 2007; Hochstrasser et al., 2011; Hollis et al., 2006; Manfridi et al., 2003). Chronic alcohol exposure in adult mice results in hyperexcitability of 5-HT neurons (Lowery-Gionta et al., 2015), while alcohol exposure during adolescence reduces 5-HT expression and enhances neuroinflammation (Vetreno et al., 2017). A previous study found that lipopolysaccharide (LPS) or pro-inflammatory cytokines can reduce the number of tryptophan hydroxylase (Tph)-expressing neurons in the DRN, suggesting a link between inflammation and 5-HT loss in the DRN (Hochstrasser et al., 2011). Another study suggests that LPS induces c-fos expression in 5-HT neurons in subregions of the DRN (Hollis et al., 2006), which may indicate increased activity. These converging lines of evidence strongly suggest that chronic alcohol promotes hyperalgesia and negative affect by activating neuro-immune mechanisms in the DRN that ultimately perturb 5-HT neurotransmission.
The goal of the present study was to assess the role of DRN neuroinflammation in pain and depressive-like behaviors after adolescent alcohol exposure. These behaviors were the focus of this study since both have been associated with 5-HTergic dysregulation in the DRN. We first examined the effects of adolescent intermittent access to ethanol (AIE) on pain and affective behaviors, 5-HT expression, and neuroinflammation in the raphe nuclei as well as downstream targets of the DRN that have been implicated in nociception. The dorsal horn of the spinal cord was also included as it receives robust serotonergic input from the brain. Persistent hyperalgesia was accompanied by microglial activation in the DRN and median raphe nucleus (MRN) and reduced the number of 5-HT-immunoreactive neurons in the DRN. We also observed a significant reduction in 5-HT expression in nociceptive regions that receive 5-HT input from the DRN including the thalamus, anterior cingulate cortex (ACC), amygdala and dorsal horn (DH). In the thalamus, we focused on the posterior complex (Po), parafascicular nucleus (Pf), and paraventricular nucleus (Pvt) for their putative role in pain (Andersen and Dafny, 1983; Apkarian and Shi, 1994; Chang et al., 2019). This AIE-induced hyperalgesia and microglial activation was alleviated by minocycline treatment after alcohol cessation, while chemogenetic activation of microglia in the DRN was sufficient to induce hyperalgesia and social deficits in ethanol-naïve mice. We also found that chemogenetic activation of microglia reduced tryptophan hydroxylase 2 (Tph2) expression in the DRN, which may perturb 5-HTergic transmission. Together, these results suggest that AIE may predispose individuals to chronic pain disorders later in life by activating microglia in the DRN.
2. Methods
2.1. Animals
All procedures were carried out in accordance with the ethical guidelines for the use of animals in research and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Iowa. Adolescent male C57BL/6J mice (Jackson Laboratories, Stock # 000664) were used in voluntary ethanol access experiments, had ad libitum access to an irradiated diet (Prolab IsoPro RMH 3000, catalog # 5P75) and were maintained on a reverse light–dark cycle (lights off at 07:30). Adult male Cx3cr1-creER-GFP mice (Jackson Laboratories, Stock #021160) and CAG-LSL-Gq-DREADD mice (floxed hM3dq; Jackson Laboratories, Stock #026220) were used in DREADD experiments and maintained on a standard light–dark cycle (lights on at 06:00) with ad libitum access to standard chow. Mice were randomly assigned to a treatment group and data was analyzed by trained personnel blinded to the treatment.
2.2. Drugs
Ethanol solutions were prepared in tap water from 95 % ethyl alcohol (Decon Labs, catalog # 2801TP) and sucrose (Sigma Aldrich, catalog # BP220–212). Minocycline HCl (Sigma Aldrich, catalog # M9511) was dissolved in distilled water (0.0416 mg/mL) and administered via drinking bottles at 3 h into the dark cycle for an average daily dose of 7.1 ± 0.09 mg/kg/day. Tamoxifen (TM; Sigma Aldrich, catalog # T5648) was dissolved in Sunflowerseed Oil (Sigma Aldrich, catalog # S5007) and administered at a dose of 75 mg/kg (i.p.). Clozapine N-oxide (CNO) dihydrochloride (Hello Bio, catalog # HB6149) was dissolved in sterile saline and administered by intraperitoneal injection (3 mg/kg, i. p.) 45 min before behavioral testing. For pain management of mice undergoing surgery, animals were treated with meloxicam (0.4 mg/mL at 10 mL/kg, s.c.; Norbrook Laboratories Limited, NDC: 55529–040–10) immediately following, and 24 h after surgery.
2.3. Adolescent intermittent access to ethanol (AIE)
Mice were exposed to an intermittent access to ethanol paradigm as previously described (Hwa et al., 2011). Briefly, mice were individually housed and presented with two 50 mL plastic tubes that were fitted with No. 5 rubber stoppers. Three hours into the dark cycle on Mondays, Wednesdays, and Fridays, mice were presented with tubes containing ethanol or water for 24 h. The AIE protocol occurred for 4 weeks starting at postnatal day (PND) 25. Placement of the ethanol and water bottles was alternated each drinking session to control for side preferences. On the first four days of the AIE protocol, ethanol content was 3 %, 6 %, 10 %, and 20 % w/v with 5 % sucrose. On day 5, animals received 20 % EtOH w/v & 2 % sucrose. Thereafter, ethanol content was 20 % w/v. Water control animals did not receive sucrose in drinking water through this EtOH ramp-up period. Ethanol intake (g/kg/24 h) and preference were calculated after each drinking session for a daily average of 13.46 ± 1.78 g/kg/day.
2.4. Minocycline treatment
Chronic minocycline treatment began after the AIE protocol. Minocycline (0.0416 mg/mL) was presented via drinking bottles, 3 h into the dark cycle. Fresh solutions were presented daily for an average daily dose of 7.1 ± 0.09 mg/kg/day. Treatment continued through chronic withdrawal.
2.5. Stereotaxic surgeries
All surgeries were performed using aseptic technique. Before the surgery, mice were deeply anesthetized with 3 % isoflurane and placed on a heating pad in a stereotactic frame. During surgery, isoflurane was maintained at 1.5–2 %. To selectively target microglia, AAV encoding Cre-inducible Gq-coupled DREADDs (AAVDJ-ef1α-DIO-hM3DqmCherry, Stanford viral vector core) were microinjected into the DRN of Cx3cr1-creER mice using a 1 μL Neuros Hamilton syringe at a rate of 100 nl/min. After infusion, the needle was left in place for an additional 10 min to allow diffusion in the target area. Coordinates from bregma were AP: −4.65, ML: 0, DV: −3.3, at an angle 23.58⁰ to ML. The incision site was closed, and mice were returned to their regular housing room.
2.6. Behavior
Following intermittent access to ethanol, animals entered into a chronic withdrawal period. Animals were tested between weeks 3 and 5 of chronic withdrawal. Animals were first tested on anxiety measures (Elevated Plus Maze; EPM, and Open Field; OF). Next, we evaluated sensitivity to mechanical and thermal stimuli (von Frey and Hargreaves assays), and finally we tested social behavior and depressive-like behavior (forced swim test). Behavior tests were spaced at least 48 hr apart.
2.6.1. Open field
Mice were placed in the corner of a 50 × 50 × 25 cm plexiglass container as described previously and allowed to freely explore the chamber for 10 min (Marcinkiewcz et al., 2019, 2016, 2014). The time spent in the center of the container as well as total distance traveled was recorded by an overhead camera and quantified with Ethovision XT15 software. The center of the open field was defined as the central 15 % of the arena.
2.6.2. Elevated plus maze
Mice were placed in the center of an elevated plus maze and allowed to freely explore the arena for 5 min. The maze was 60 cm above the floor and consisted of two open and two closed arms (5 × 35 cm) and a neutral zone (center; 5 × 5 cm). The light intensity in the open arms were roughly ~ 20 lux. Time spent in the open arms and the number of transitions between closed and open arms was recorded by an overhead camera and quantified with Ethovision software.
2.6.3. Von Frey
Mice were placed inside a clear acrylic box (100 × 100 × 150 mm) and acclimatized to the test apparatus which consisted of a raised wire mesh platform (holes in mesh were 5×5 mm) for at least 3-h on two consecutive days (Warwick et al., 2019). Mechanical sensitivity was evaluated by applying von Frey filaments (Stoelting, Wood Dale, IL) of varying strength to the plantar surface of each hindpaw. The number of responses to each filament out of 5 applications was recorded and used to calculate the 50 % threshold.
2.6.4. Hargreaves
Mice were placed in a clear acrylic box (100 × 100 × 150 mm) on a IITC Life Science heated base (Model 400; temperature maintained at 30°C) and were acclimated to the test apparatus for 2 consecutive days before testing. Thermal sensitivity was evaluated by focusing a light beam to generate heat on the plantar surface of each hindpaw. The time required for the stimulus to evoke a withdrawal was measured 3 times per paw and averaged to calculate the paw withdrawal latency.
2.6.5. Social interaction
The social interaction test was performed as described previously (Lowery-Gionta et al., 2014; Marcinkiewcz et al., 2014; Moy et al., 2008). Briefly, mice were placed in a clear three-chambered plexiglass arena (~20 lx) with small openings to allow movement between chambers. Following a 10-min habituation phase, two small metal enclosures were placed in each side chamber and a novel mouse was placed inside of one of the enclosures. Placement of the novel mouse was altered between trials to control for side preferences. Behavior was recorded by an overhead camera and scored by a researcher blind to experimental conditions. The time spent interacting with the novel mouse and the empty cage was analyzed over a 10-minute period for each trial.
2.6.6. Forced swim
Mice were gently placed in a tall cylinder (32 cm height × 20 cm diameter) filled with tap water (24°C) to a height of 25 cm, for 6 min. After the 6 min, animals were removed from the test cylinder and placed in a clean cage under a heating lamp for 5-minutes to warm them up and allow them to dry off. Behaviors were recorded from a side-view camera and analyzed with Ethovision software. Latency to immobility, as well as number of immobile bouts + total immobile time during minutes 0–2 (‘pretest’) and min 3–6 (‘test’) were evaluated.
2.6.7. DREADD experiments
Adult ethanol-naïve Cx3cr1-creER mice (3mo+) underwent stereotaxic infusion of Gq-coupled DREADDs and were used to assess the role of DRN microglial activation on pain and social behaviors. Animals were treated with tamoxifen two weeks following viral infusion into the DRN and used in behavior tests ~ 1 month following surgery.
To evaluate how the activation of Cx3cr1+ cells affects 5-HT neurons, Cx3cr1-creER mice were crossed with floxed hM3Dq mice to induce expression of hM3Dq in microglia. Adult, ethanol naïve, Cx3cr1-creER and Cx3cr1-creER × hM3Dq mice were injected with tamoxifen (75 mg/kg, i.p.) once daily for 5 days to induce Cre-meditated recombination and DREADD expression in microglia. Two weeks following the final tamoxifen injection, mice were treated with CNO (3 mg/kg, i.p.) in a light and sound controlled environment. Mice were sacrificed 2.25 h following CNO treatment. Brains were processed for histological analysis of 5-HT, tph2, and microglia expression.
2.7. Immunohistochemistry and microscopy
Mice were anesthetized with Avertin and transcardially perfused with 0.01 M PBS followed by 4 % paraformaldehyde (PFA). Brains were extracted, fixed in PFA for 24 h at 4 °C, and stored in PBS at 4 °C. Brains were sliced on a Leica VT1000S at 45 μm and stored in 50/50 PBS/Glycerol at −20 °C. For each region of interest (ROI), four slices were used across the caudal-rostral axis. Slices were washed in PBS and incubated in 0.5 % Triton X-100/PBS for 30 min, blocked in 10 % Normal Donkey Serum in 0.1 % Triton X-100/PBS and then incubated with the respective primary and secondary antibodies (Supplementary Table 1). Slices were subsequently washed in PBS, mounted on glass slides and coverslipped with Vectashield.
Confocal z-stacks (1 μm) were captured on a confocal microscope (40 sections/z-stack), at 20X (for raphe nuclei) or 40X (for downstream regions and microglia analysis). After imaging, confocal stacks were converted to maximum projection images using Image J software. Images were analyzed by trained researchers blind to experimental conditions for cell counts, % immunoreactive (IR) area, cell body size, and optical density using ImageJ.
2.8. Image Analysis
The intensity of serotonergic cells and glia were estimated via optical density measurement in ImageJ. Briefly, images were converted to 8-bit gray scale images and background was corrected. An optical density calibration was performed in ImageJ with a 21-step table using the Rodbard function. Mean gray values were obtained from regions of Interest (ROIs) that were overlaid on the grayscale images, with an effort to avoid obvious artifacts. Percent immunoreactive area was measured on thresholded 8-bit images, with an effort to avoid obvious artifacts. Within the raphe nuclei, 20X images were used to quantify the number of serotonergic neurons and astrocytes. For microglia analyses (cell counts and morphological analysis), 40X confocal stacks were used. Morphological analysis was performed on GFAP and P2Y12 positive cells by observers blind to the experimental groups who traced outlines of the glial cells, making sure to avoid dendritic processes.
2.9. Statistical analyses
Experiments comparing two groups were analyzed with a Student ttest, with α < 0.05; social interaction and minocycline experiments were analyzed using a two-way ANOVA and Bonferroni corrections for post hoc analyses using Graphpad Prism 8 software. Data are reported as means ± standard error of the mean (SEM).
3. Results
3.1. AIE promotes hyperalgesia and depressive-like behaviors in adult mice.
Adolescent male C57BL/6J mice were exposed to 4 weeks of AIE starting at postnatal day 25 and averaged an ethanol intake of 13.46 ± 1.78 g/kg/day and an ethanol preference of 50.8 ± 0.07 % by their final week (Fig. 1A–C). While we did not observe significant effects of AIE on anxiety-like behavior or locomotor activity in the EPM (Fig. 1D–E), there was a reduction in time spent in the center of the open field (t17 = 2.57, p < 0.05) without any change in locomotor activity (Fig. 1F–G).
Fig. 1.
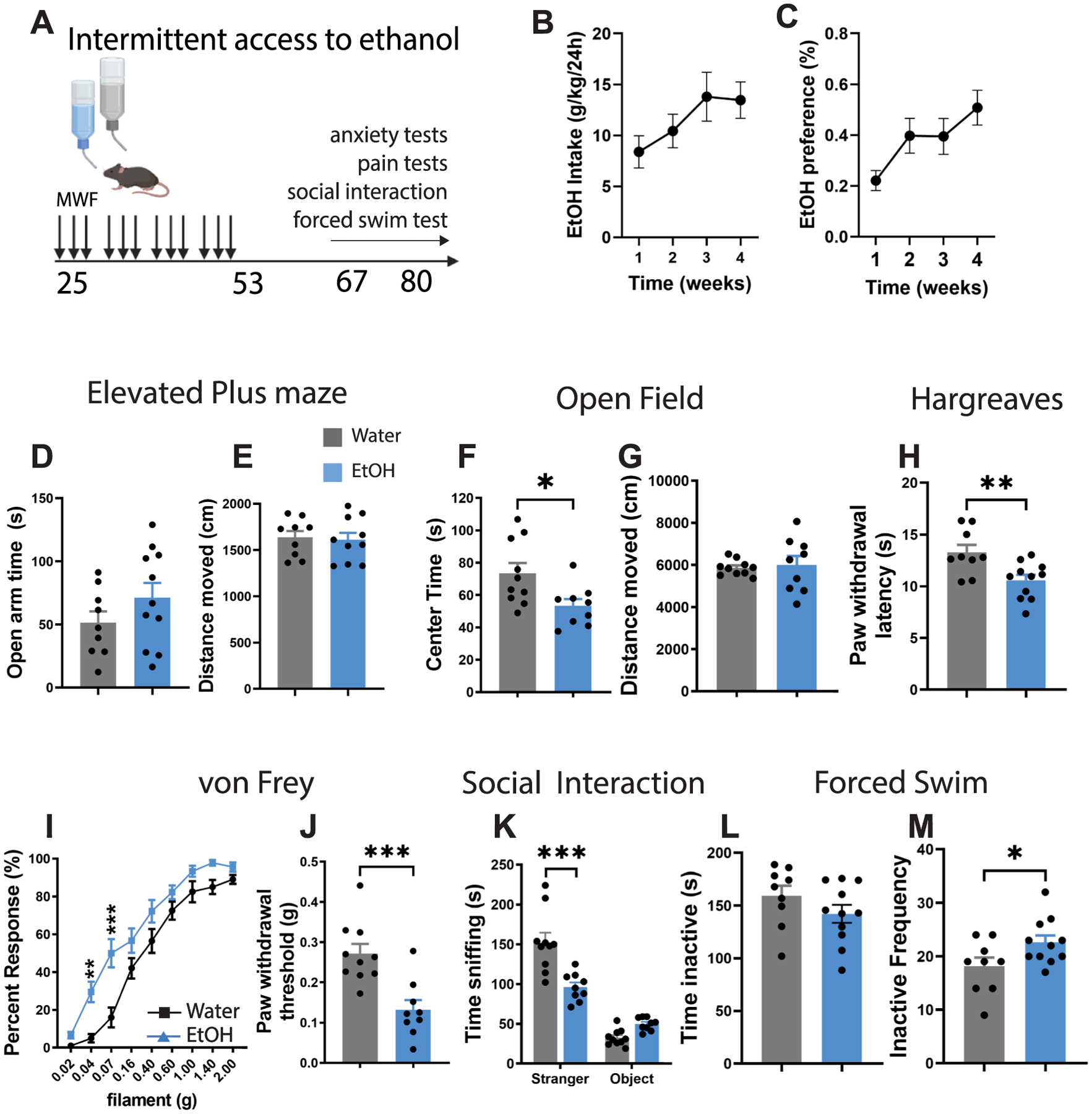
Hyperalgesia and social deficits in adult mice after adolescent alcohol exposure. (A) Experimental timeline for adolescent alcohol exposure and behavioral testing. (B) Average ethanol intake (g/kg/day) and (C) preference (%) over the 4-week alcohol drinking paradigm. (D-E) Time spent in the open arms and distance traveled in the elevated plus maze (EPM). (F-G) Time spent in the center and distance traveled in an open field. (H) Thermal pain threshold in the Hargreaves test and (I-J) mechanical pain thresholds in the Von Frey test. (K) Time spent in social interaction in the 3-chambered social interaction test. (L-M) Time spent immobile and frequency of inactive bouts in the forced swim test. *p < 0.05, **p < 0.01, ***p < 0.001. Figure 1A was created with BioRender.
AIE also reduced pain thresholds in the Hargreaves and Von Frey tests at 12 weeks of age, which is indicative of hyperalgesia (Hargreaves t18 = 3.05, p < 0.01; Von Frey: t17 = 4.11, p < 0.001) (Fig. 1H–J). These mice also exhibited significant deficits in social interaction which may be indicative of depressive-like behavior (F1,17 = 12.56, p < 0.001, Stranger × Ethanol interaction) (Fig. 1K). This is congruent with previous reports in the literature in rats exposed to alcohol in early adolescence (Varlinskaya et al., 2014). Surprisingly, there was no effect of AIE on immobility time in the forced swim test, although we did see an increase in the frequency of inactive bouts (t18 = 2.19, p < 0.05) which aligns with depressive-like behavior (Fig. 1L–M). Together, these results suggest that long-term plasticity in neural circuits mediating pain and affective behavior may occur following AIE.
3.2. AIE reduces the number of 5-HT neurons in the DRN and induces microglial activation in the raphe nuclei.
We then asked whether AIE depletes 5-HT in the DRN as previously suggested after adolescent alcohol gavage (Vetreno et al., 2017). We first quantified the number of 5-HT-immunoreactive cells in the raphe nuclei and found this was significantly depleted in the DRN (t26 = 2.48, p < 0.05) but not the MRN (t26 = 1.24, ns). Tryptophan hydroxylase 2 is the rate limiting enzyme in 5-HT biosynthesis and is often used as a marker of 5-HT neurons. We found a non-significant trend toward a reduction in the number of Tph2-IR neurons in the DRN (t27 = 2.02, p = 0.053). However if we confine ourselves to the rostral and mid subregions of the DRN where the vast majority of 5-HT neurons reside, we observe a significant reduction in Tph2-IR neurons (t28 = 2.65, p < 0.05; data not shown). No changes in Tph2-IR neurons were observed in the MRN (t27 = 0.92, ns) (Fig. 2A–E). The density of CD68-positive microglia in the DRN and MRN were also increased after AIE (DRN: t23 = 2.32, p < 0.05 and MRN: t20 = 2.33 with Welch’s correction, p < 0.05; Fig. 2F), and there was a significant negative correlation between CD68 optical density and the number of 5-HT-IR cells in the DRN (r = −0.4425, p < 0.05) (Fig. 2G). These results suggest that microglial activation in the DRN may drive 5-HT depletion after AIE.
Fig. 2.
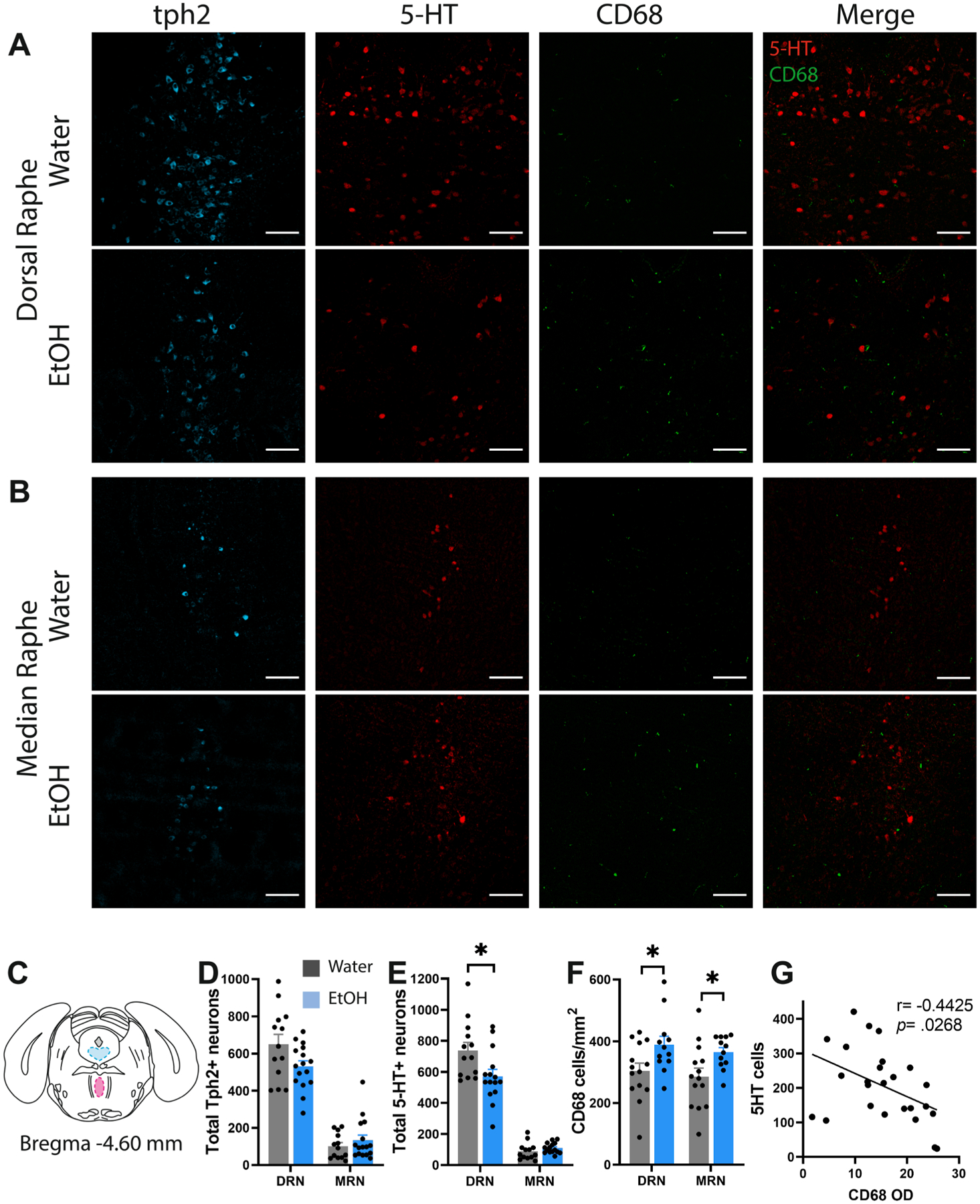
5-HT depletion and microglial activation in the DRN following AIE. (A) Representative confocal images of Tph2, 5-HT, CD68 and 5-HT-CD68 overlay in the DRN and (B) MRN of water control and alcohol drinking mice. (C) Brain atlas schematic illustrating the approximate positions of the DRN (blue) and MRN (magenta) included in this analysis. (D) Histograms of total cell counts in the DRN and MRN for (D) Tph2-IR neurons, (E) 5-HT-IR neurons, and (F) density of CD68-IR microglia. (G) Correlation between CD68 optical density and 5-HT-IR cells in the DRN. Scale bar = 100 μm. *p < 0.05.
3.3. AIE reduces 5-HT immunoreactivity and increases neuroinflammation in the spinal cord and central pain processing regions.
Depletion of 5-HT-IR neurons could impact downstream 5-HT transmission in the brain and spinal cord, so next we examined nociceptive regions that receive substantial 5-HT input from the DRN. We initially focused on the posterior complex (Po) of the thalamus as a previous study indicated a high proportion of nociresponsive neurons in this subnucleus (Apkarian and Shi, 1994). We also examined the medullary raphe which contains 5-HT neurons that project to the spinal cord. Surprisingly, AIE did not modify the number of 5-HT-IR neurons in the medullary raphe (Fig. 3A,G). We did observe a significant reduction in 5-HT immunoreactive area in the Po (t7 = 2.92, p < 0.05) and dorsal horn (t18 = 2.54, p < 0.05), and there was also a reduction in 5-HT optical density in the Po (t7 = 2.98, p < 0.05), ACC (t7 = 2.47p < 0.05), and amygdala (t7 = 2.50, p < 0.05) (Fig. 3B–F, 3H-I). We then examined 5-HT immunoreactivity in the parafascicular (Pf) and paraventricular nucleus (Pvt) to determine whether reduced 5-HT input to the thalamus was more widespread. Here we found that AIE reduced 5-HT optical density in the Pf (t7 = 2.91, p < 0.05) and PVT (t7 = 2.86, p < 0.05) without altering 5-HT immunoreactive area in either subregion (Supplementary Fig. 1).
Fig. 3.
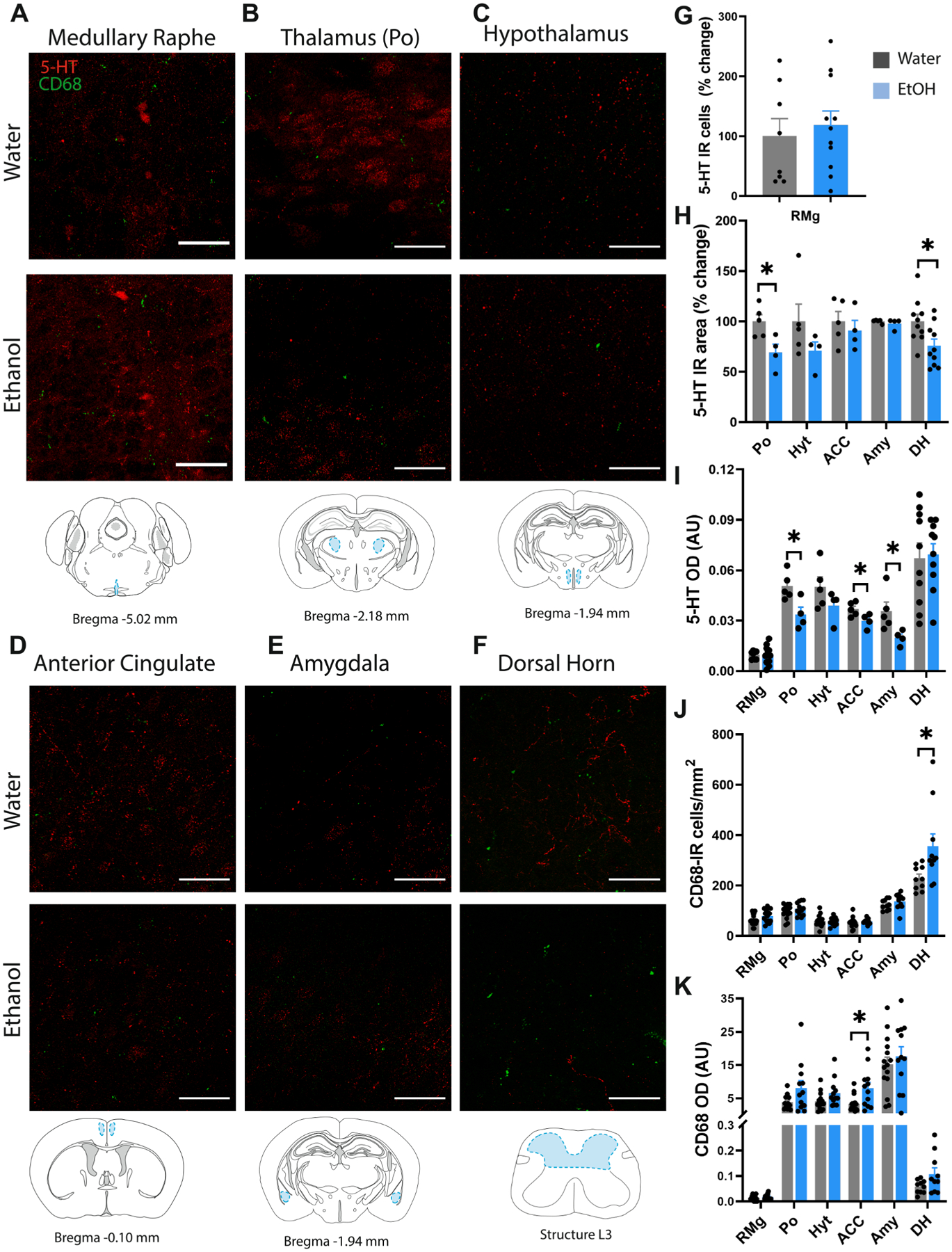
Loss of 5-HT input and microglial activation in pain processing regions of the brain and spinal cord. Representative confocal images of 5-HT and CD68 from water control and alcohol drinking mice and approximate brain atlas coordinates for the (A) medullary raphe, (B) posterior complex of the thalamus (Po), (C) hypothalamus (Hyt), (D) anterior cingulate cortex (ACC), (E) amygdala (Amy), and (F) dorsal horn (DH) of the spinal cord. (G) Histogram of total 5-HT-IR neurons in the medullary raphe. Histograms of (H) 5-HT-IR area and (I) 5-HT optical density, (J) CD68-IR cell density, and (K) CD68 optical density in the Po, hyt, ACC, Amy and DH. Scale bar = 50 μm *p < 0.05.
There was also a significant increase in CD68 + microglial density in the dorsal horn (t18 = 3.28, p < 0.01) and increased optical density of CD68 in the ACC (t14 = 2.61, p < 0.05 with Welch’s correction) of AIE mice (Fig. 3J–K). Surprisingly, there was no change in CD68 + microglial density or CD68 optical density in any other region examined.
P2Y12 is a purinergic receptor that is involved in platelet aggregation and is expressed at high levels in resting microglia. Once microglia are activated, P2Y12 expression decreases (Haynes et al., 2006). We found that adolescent ethanol reduced P2Y12 + microglial density in the posterior complex of the thalamus (t25 = 3.01, p < 0.01), amygdala (t25 = 2.20, p < 0.05) and ACC (t20 = 2.46, p < 0.05 with Welch’s correction), suggesting that microglia in these regions may also be in an activated state (Supplementary Fig. 2). P2Y12 is also transiently decreased in cortical microglia in response to an acute ethanol challenge in neonatal mice (Ahlers et al., 2015), so it is interesting that this P2Y12 reduction persists for several weeks after AIE cessation. There was also an increase in the P2Y12 + soma area (μm2) in the amygdala (t25 = 4.00, p < 0.001) and the medullary raphe (t22 = 3.25, p < 0.01 with Welch’s correction), which is consistent with an activated state. Given the essential role of the spinal cord in nociception, we also examined P2Y12 expression in the dorsal horn but found no change in the cell density of P2Y12 + microglia or soma size (Supplementary Fig. 2).
The clinical and preclinical literature also suggests that chronic alcohol may reduce astrocyte density and nuclei size in the prefrontal and orbitofrontal cortex, ACC and hippocampus (Adermark and Bowers, 2016; Bull et al., 2015, 2014; Korbo, 1999; Miguel-Hidalgo et al., 2002). We did not observe a change in GFAP + cell number or optical density in the DRN or MRN after AIE, but we did observe a reduction in cell body area in the DRN (t18 = 3.13, p < 0.05 with Welch’s correction). We also observed an increase in the number of GFAP + cells in the medullary raphe (t17 = 2.18, p < 0.05) without any concomitant change in soma area (Supplementary Fig. 3).
3.4. Minocycline treatment alleviates pain and depressive-like behavior after AIE.
Minocycline inhibits microglial activation in vivo and may have clinical utility in a variety of neurological conditions involving chronic inflammation. We next asked whether chronic minocycline treatment after AIE could reverse the effect on pain thresholds and social behavior (Fig. 4A). Mice consumed an average of 7.1 ± 0.09 mg/kg/day over the course of the minocycline treatment (Fig. 4B). Here we found that AIE reduced mechanical pain thresholds in the Von Frey in vehicle-treated mice (Main effect of Ethanol: F1,36 = 5.50, p < 0.05, Bonferroni posttest t36 = 2.73, p < 0.05) which was abolished by chronic minocycline treatment after AIE cessation (t36 = 0.585, ns) (Fig. 4C–D). Minocycline also normalized thermal sensitivity in the Hargreaves test (Main effect of Ethanol: F1,56 = 7.77, Ethanol × minocycline interaction: F1,56 = 5.86, p < 0.05) (Fig. 4E). Bonferroni post-tests revealed an effect of AIE in vehicle-treated mice in the Hargreaves (t56 = 3.68, p < 0.01) that was abolished in minocycline-treated mice (t56 = 0.26, ns). Social interaction deficits were also reversed by minocycline treatment (F1,36 = 22.47, p < 0.001 Ethanol × minocycline interaction). Pairwise comparisons revealed social deficits in ethanol vehicle-treated vs water vehicletreated mice (t36 = 6.088, p < 0.0001) that were abolished by minocycline treatment (t36 = 0.6163, ns) (Fig. 4F).
Fig. 4.
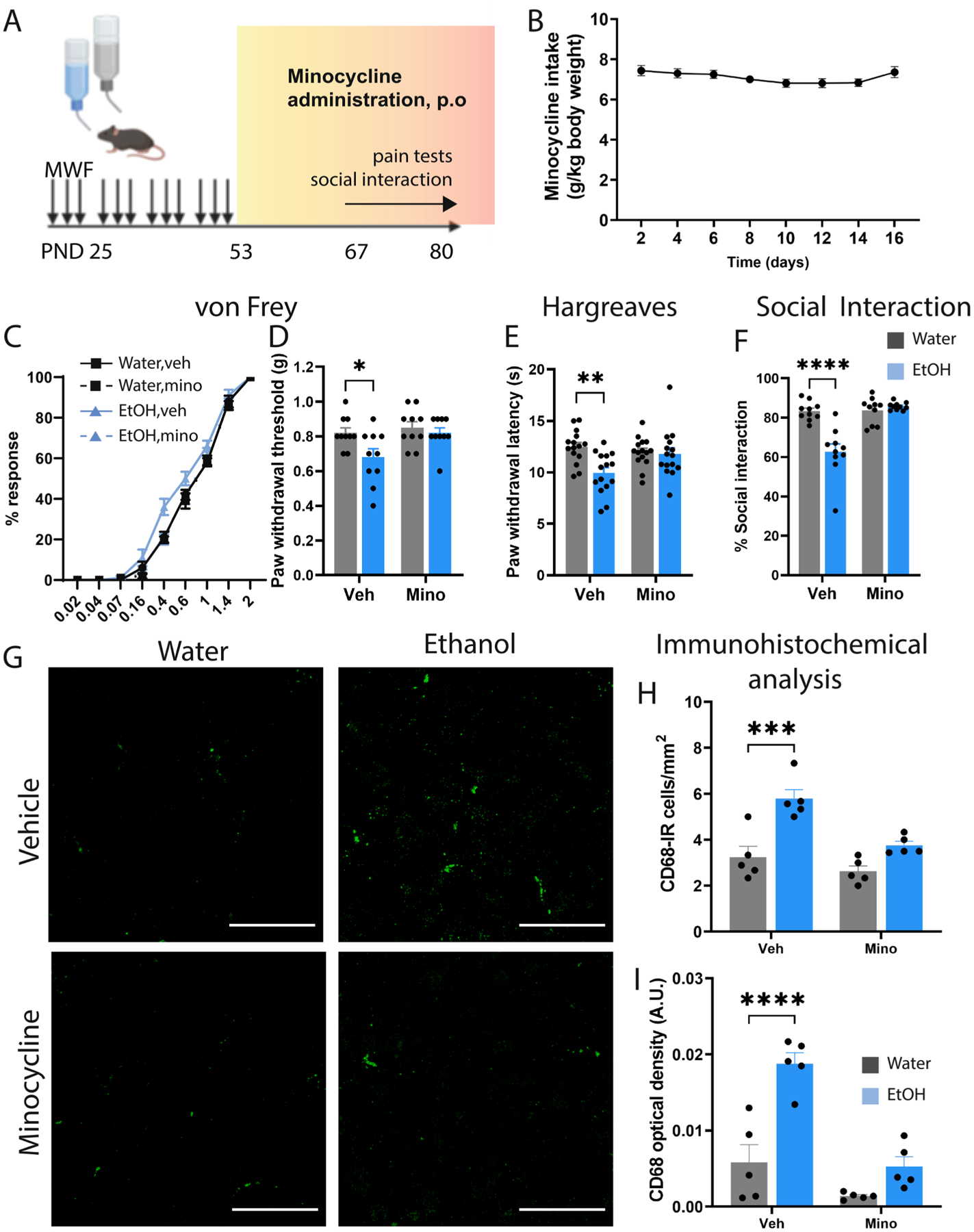
Minocycline alleviates pain and depressive-like behaviors after AIE. (A) Experimental time of alcohol exposure, minocycline administration and behavioral tests. (B) Average minocycline dose over the exposure period. (C-D) Mechanical pain thresholds in the Von Frey and (E) Hargreaves tests showing that minocycline alleviates hyperalgesia after AIE. (F) Time spent in social interaction in the 3-chambered social interaction test indicating that minocycline can mitigate depressivelike behaviors after AIE. (G) Representative confocal images of CD68-IR microglia in vehicle and minocycline treated water control and AIE mice. (H-I) Immunohistochemical analysis of CD68-IR microglia in the DRN following AIE and chronic minocycline administration. Scale bar = 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Figure 4A was created with BioRender.
We next examined CD68 expression in these mice and found that minocycline significantly attenuated the AIE-induced increase in CD68-positive microglial density in the DRN (Main effect of Ethanol F1,16 = 28.55, p < 0.0001, Bonferroni post-test t16 = 5.22, p < 0.001) that was attenuated by chronic minocycline treatment (t16 = 2.34, ns) (Fig. 4G–H). Likewise, minocycline also reversed the increase in CD68 optical density in the DRN (Main effect of Ethanol: F1,16 = 30.59, p < 0.0001, Ethanol × minocycline interaction: F1,16 = 8.88, p < 0.01). Post-tests indicate an effect of AIE in vehicle-treated mice on CD68 optical density (t16 = 6.02, p < 0.0001) that was abolished by chronic minocycline (t16 = 1.80, ns) (Fig. 4I).
3.5. Chemogenetic activation of microglia in the DRN induces hyperalgesia and reduces social preference
We next asked whether direct activation of microglia in the DRN could phenocopy the effects of AIE. Male Cx3cr1-creER mice were microinjected with AAV-ef1α-DIO-hM3Dq-mCherry or a control virus (AAV-ef1α-DIO-mCherry) in the DRN at 8 weeks of age and then received i.p. injections of tamoxifen to enable Cre-mediated recombination and expression of the DREADDS in microglia (Fig. 5A–B). This resulted in hM3Dq-mCherry expression in 52.17 ± 2.74 % of Cx3cr1 + cells in the DRN, while 71.67 ± 1.69 % of DREADD-expressing cells also expressed Cx3cr1 (Fig. 5C–G). Chemogenetic activation of microglia in ethanol-naïve Cx3cr1-creER::hM3DqDRN mice reduced mechanical and thermal pain thresholds in the Von Frey (t14 = 2.44, p < 0.05) and Hargreaves test (t14 = 2.84, p < 0.05) (Fig. 5H–J). Social preference was also attenuated in these mice after microglial activation (t14 = 8.51, p < 0.0001) (Fig. 5K).
Fig. 5.
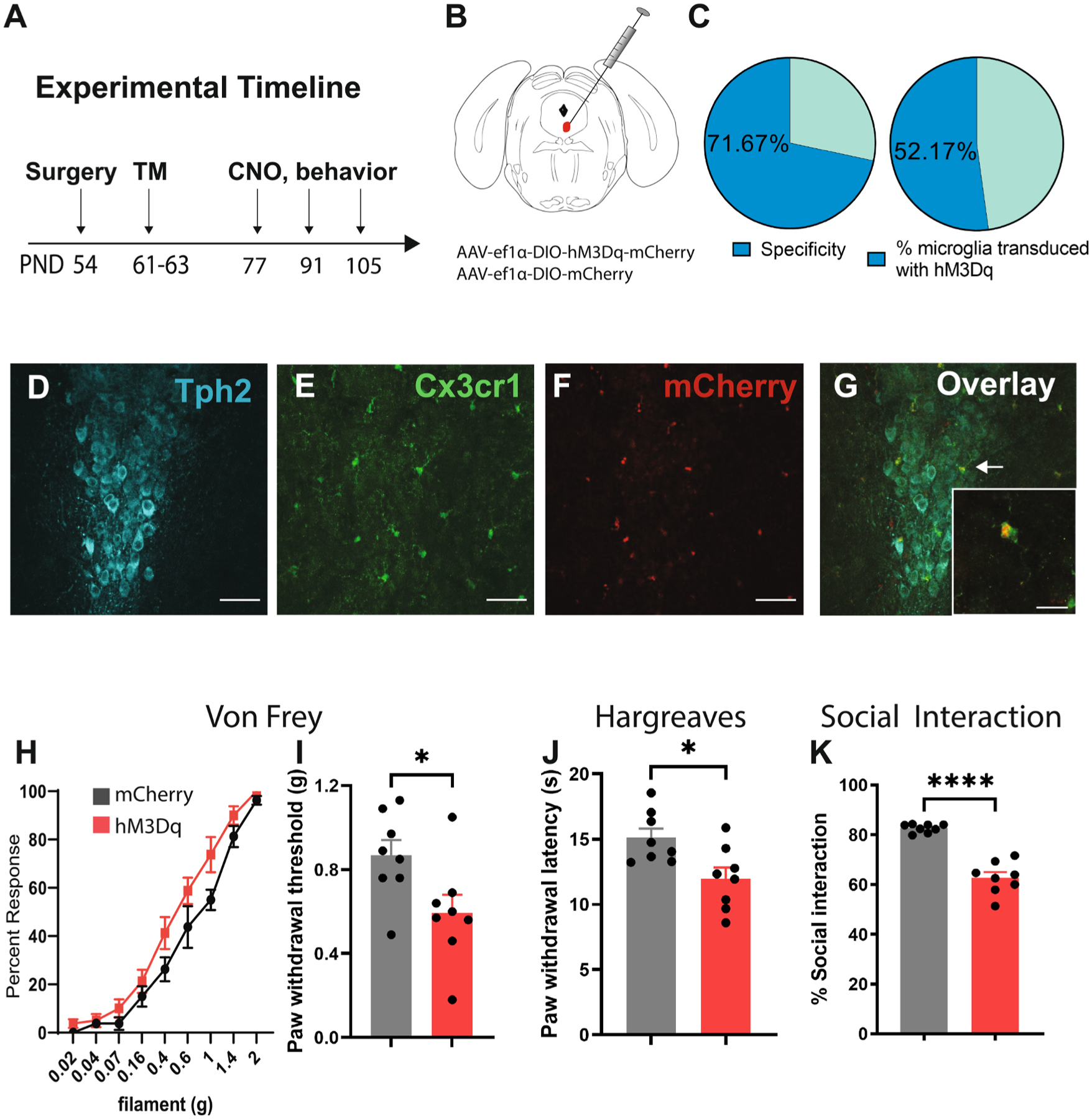
Chemogenetic activation of microglia in the DRN induces hyperalgesia and depressive-like behaviors in ethanol-naïve mice. (A) Experimental timeline for surgeries, injections and behavioral experiments in Cx3cr1-cre::hM3DqDRN mice. (B) Schematic of stereotaxic injections of AAV into the DRN. (C) Specificity of DREADD expression in microglia and transduction efficiency of AAV-mediated hM3Dq expression in DRN microglia. (D-G) Representative confocal images of Tph2, Cx3cr1-eGFP, hM3Dq-mCherry and overlay in the DRN. (H-I) Mechanical pain thresholds in the Von Frey test, (J) thermal pain thresholds in the Hargreaves test and (K) social investigation time in the 3-chambered social interaction test in Cx3cr1-cre::hM3DqDRN relative to Cx3cr1-cre::mCherryDRN mice following CNO injection. Scale bar = 50 μm (15 μm for inset). *p < 0.05, ****p < 0.0001.
We then asked whether chemogenetic activation of microglia could alter 5-HT expression or activity in the DRN. Cx3cr1-creER × hM3Dq mice were first injected with tamoxifen to induce Cre-mediated recombination. Mice were then injected with CNO and perfused 2.25 hr later (Fig. 6A). These mice exhibited robust DREADD expression in the DRN (Fig. 6B), with 57 ± 3 % of microglia expressing hM3Dq (Fig. 6C). Of DREADD-expressing cells, 99 ± 1 % were microglia (Fig. 6C). We then verified that chemogenetic activation elevated CD68 expression in the DRN (t26 = 2.39, p < 0.05; Fig. 6D, 6L). We also observed an increase in the density of fos-positive 5-HT neurons in the DRN after microglial activation (t26 = 2.90, p < 0.01; Fig. 6E), which is congruent with previous reports following LPS injection (Hollis et al., 2006). Tph2 optical density in the DRN was also significantly reduced in these mice (t26 = 2.09, p < 0.05; Fig. 6F) and was negatively correlated with CD68 optical density (r = −0.5094, p < 0.01); Fig. 6H). There was also non-significant trend toward a reduction in 5-HT optical density (Fig. 6F). Surprisingly, the density of Tph2-IR and 5-HT-IR neurons was not significantly reduced (Fig. 6G), but there was negative correlation between CD68 optical density and 5-HT neuronal density (r = −0.4421, p < 0.05; Fig. 6I). In agreement with these results, a previous study reported immunogenic stimuli reduced Tph2-IR neurons in the DRN (Hochstrasser et al., 2011). Overall, these data provide converging evidence that reduced 5-HTergic tone in the DRN following AIE may be mediated by microglial activation in the DRN. There is also accumulating evidence in the literature that depletion of 5-HT neurons in the DRN and loss of 5-HT input to downstream targets can promote pain sensitivity in rodents and may drive hyperalgesia following AIE.
Fig. 6.
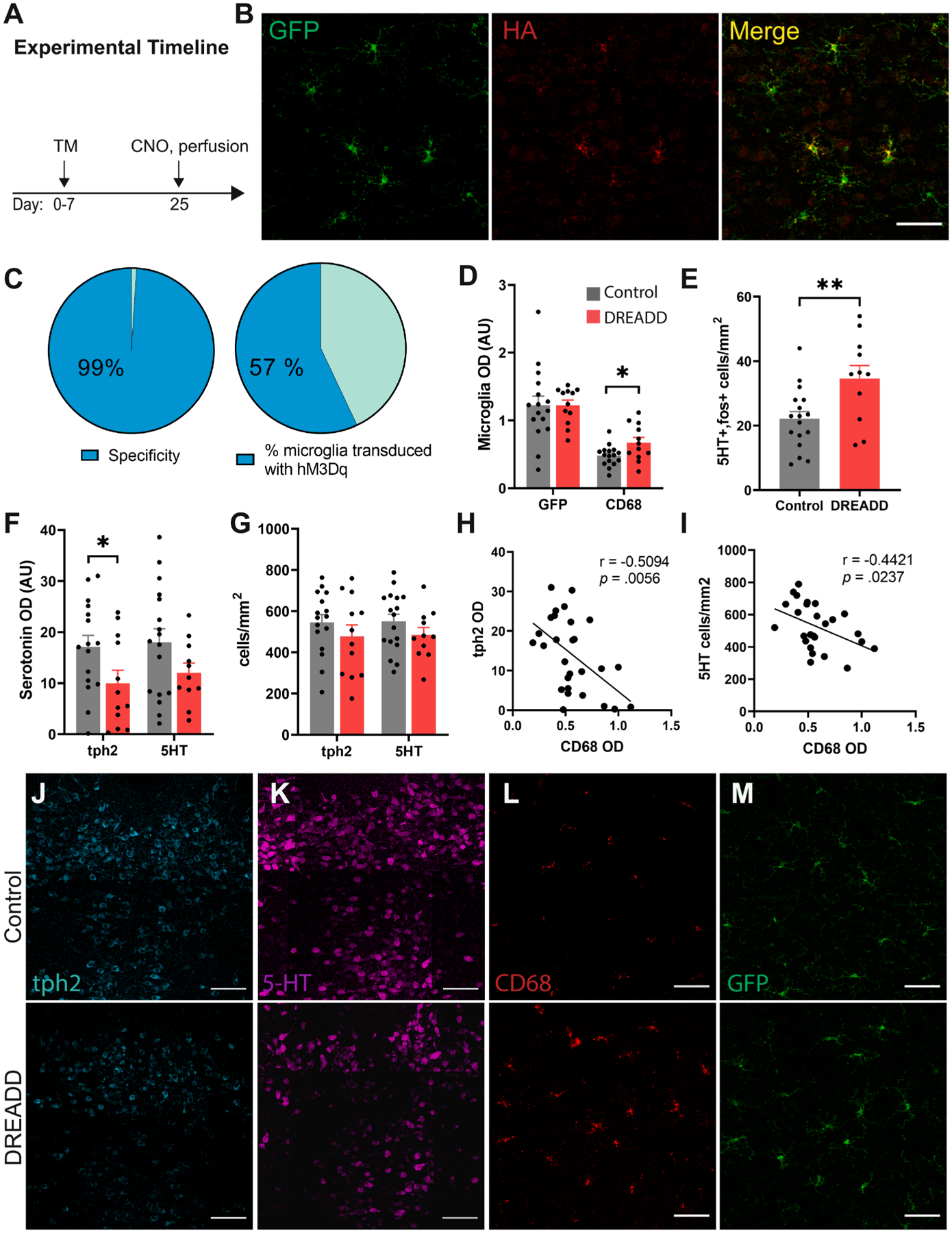
Chemogenetic activation of microglia induces CD68 and inhibits Tph2 expression in the DRN. (A) Experimental timeline of injections and perfusions for histology in Cx3cr1-cre × hM3Dq mice. (B) Representative confocal images of Cx3cr1-GFP, HA, and overlay in the DRN. (C) Specificity of DREADD expression in microglia and % of microglia transduced with hM3Dq in the DRN. (D) Histogram of GFP and CD68 expression, (E) Cell density of 5-HT-fos double positive neurons, (F) Tph2 and 5-HT optical density and (G) neuronal density in the DRN of Cx3cr1-cre × hM3Dq and control (Cx3cr1-cre) mice following CNO injection. Correlation analysis between CD68 OD and (H) Tph2 optical density and (I) 5-HT neuronal density. Representative confocal images of (J)Tph2, (K) 5-HT, (L) CD68 and (M) GFP in control and DREADD-expressing mice after CNO. Scale bar = 50 μm in panels B, L-M, and 100 μm in panels J-K. *p < 0.05, **p < 0.01.
4. Discussion
Our results indicate that AIE induces long-lasting adaptations in pain sensitivity and affective behavior. While there are numerous reports of affective and cognitive disturbances in mice and rats after adolescent alcohol exposure, to our knowledge this is the first report of hyperalgesia in a mouse model of intermittent adolescent alcohol exposure. The reported effects on pain were robust and consistent across multiple cohorts of mice and accompanied by changes in microglial density in the dorsal and median raphe nuclei. While CD68-IR cell density was not significantly altered in other brain regions, there was an increase in CD68 optical density in the ACC and a decrease in the density of P2Y12-expressing microglia in the Po, amygdala and ACC which are implicated in central pain processing. This was accompanied by an increase in the soma area of P2Y12 + microglia in the amygdala only. Surprisingly, there was no change in P2Y12 + microglial density or soma area in the raphe nuclei, indicating that microglial activation states may differ between these brain regions. There was also a significant increase in CD68 + microglial density in the dorsal horn of the spinal cord, suggesting that microglial activity in spinal and supraspinal pain circuits may contribute to persistent pain after adolescent alcohol exposure.
Given the robust changes in microglial density in the raphe nuclei and the sensitivity of 5-HT neurons to inflammatory stimuli (Hochstrasser et al., 2011; Zhang et al., 2001), we sought to examine 5-HT expression in the DRN, MRN, and medullary raphe. The number of 5-HT-IR neurons was reduced in the DRN and negatively correlated with CD68 OD, but there was no apparent reduction in 5-HT neurons in the MRN or the medullary raphe. However, we did observe a reduction in 5-HT expression in several projection targets of the DRN including the thalamus (Po, Pf, and PVT), ACC and amygdala. These regions are of particular interest because they mediate descending modulation of pain and receive 5-HT input from the DRN (Ab Aziz and Ahmad, 2006; Chao et al., 2021; Duan et al., 2021; Gandhi et al., 2021; Gilpin et al., 2021; Heijmans et al., n.d.; Juarez-Salinas et al., 2019; Li et al., 2019; Price, 2000; Zargarani et al., 2021). There was also a decrease in 5-HT expression in the dorsal horn, which may have nociceptive or antinociceptive effects depending on which 5-HT receptors are prevalent (Sommer, 2010; Zhang et al., 2011). 5-HT neurons in the DRN were previously found to mediate anti-nociception (Dugé et al., 2014; Ruan et al., 1990), suggesting that depletion of 5-HT may contribute to increased pain thresholds in AIE mice. A previous study also found that 5-HT projections from the DRN to the parafascicular nucleus of the thalamus inhibits nociceptive responses (Andersen and Dafny, 1983), so loss of 5-HT expression in this subregion may also account for heightened pain sensitivity after AIE. Other thalamic subregions including the PVT and Po may also mediate hyperalgesia in this context as both were found to have reduced 5-HT expression after AIE. Together, these studies suggest that the 5-HT DRN inputs to thalamic nuclei or other downstream targets may be promising targets for treating pain disorders with co-morbid alcohol dependence.
Depressive-like behaviors in the social interaction test following AIE were also consistent with previous reports in male rats (Varlinskaya et al., 2014). This study found that social deficits were associated with early rather than late adolescent alcohol exposure, whereas our study took place over the entire duration of adolescence. It is possible that exposure in the first 2 weeks is critical for the development of social deficits in our model, whereas pain could be the result of early or late adolescent exposure. The DRN has been implicated in social behavior and stimulation of 5-HT neurons in this region rescues social deficits in mice (Luo et al., 2017), so 5-HT depletion in the DRN and its downstream targets may account for social deficits in our model. We also observed a depressive phenotype in the forced swim test, although this was only apparent in the frequency of immobile bouts and not the total time spent immobile. This stands in contrast to a previous report in adult and adolescent mice exposed to 14 consecutive days of 4-bottle choice alcohol (5, 10, 15 and 40 %) for 2 hrs/day which resulted in a reduction in immobility time and the number of immobile episodes (Lee et al., 2016). This discrepancy may be attributed to the difference in timing of alcohol administration as well as the age of the animals. We did not see substantial effects of AIE in anxiety measures, although there was a decrease in time spent in the center of the open field. Other reports in mice suggest that anxiety-like behaviors may occur in mice exposed to a continuous 4-day ethanol drinking paradigm for 4 weeks in adolescence (Sampedro-Piquero et al., 2022), whereas our model was intermittent drinking every other day for 3 days per week. They also measured behavior in the elevated zero maze (EZM) and marble burying tests which were not included in our battery of tests. The absence of an anxiety phenotype in the EPM in our model was consistent with that reported in rats undergoing intragastric alcohol administration in adolescence (Torcaso et al., 2017).
We also found that chronic systemic minocycline treatment could alleviate hyperalgesia and social deficits and reverse microglial activation in the DRN, suggesting that targeting microglia may be an effective treatment strategy for chronic pain and depression after AIE. This is further supported by the finding that chemogenetic activation of microglia in the DRN increased mechanical and thermal sensitivity while reducing social preference. While previous studies have reported that activating microglia in the entire brain and spinal cord can modulate nociception (Binning et al., 2020), our results demonstrate that regional activation of microglia in the DRN is sufficient to recapitulate hyperalgesia following AIE. Chemogenetic activation of microglia also stimulates the release of pro-inflammatory cytokines which can modulate 5-HT neuronal activity (Brambilla et al., 2007; Manfridi et al., 2003). In another study, overexpression of IL-1β in the DRN resulted in manic-like behavior characterized by reduced fearfulness and increased stress-induced locomotor activity (Howerton et al., 2014). While we did not test mania-like behaviors directly or measure IL-1β levels in the DRN, previous studies have shown that IL-1β is elevated in other brain regions after chronic ethanol exposure (Bajo et al., 2015). We also demonstrate that chemogenetic activation of microglia reduces Tph2 expression in the DRN and that CD68 was negatively correlated with Tph2 optical density and 5-HT cell density in the DRN, which strongly suggests that activated microglia can modulate pain and affective behavior by perturbing 5-HT transmission.
One potential limitation of the current study is that we did not directly compare the effects of alcohol in adolescent mice with those in adult mice, so it is possible that the behavioral and 5-HTergic dysregulation observed here may also occur in adult-onset drinking. However, based on previous literature suggesting that the DRN serotonin system may be more vulnerable to environmental insults during early development (Deneris and Gaspar, 2018), future studies are warranted to determine if hyperalgesia and 5-HT depletion are limited to alcohol exposure during adolescence. It could also be the case that the effects observed here are transient and would resolve at later time points, so further studies will help to resolve these outstanding questions. A previous study in rats exposed to adolescent alcohol gavage suggests that depletion of 5-HT neurons persists through PND 220 (Vetreno et al., 2017), so we might expect 5-HT loss to persist well after cessation of alcohol exposure. Finally, this study was limited to male mice as our initial experiments revealed that these behavioral phenotypes were inconsistent in females and likely to be dependent on estrus state, which requires further investigation that is beyond the scope of the current study.
Overall, our studies support the conclusion that adolescent alcohol drinking can increase the risk of chronic pain and social deficits by activating microglia in the DRN, which may drive reduced 5-HTergic tone in the DRN. Based on previous studies indicating that 5-HT neurons promote analgesia and social behavior, loss of 5-HTergic neurotransmission in the DRN and its downstream targets may be critically involved in these adverse outcomes of AIE. Further studies are warranted to delineate the specific 5-HT circuits that mediate these behaviors and how 5-HT neurotransmission modulates activity in nociresponsive neurons in downstream targets, particularly in the thalamus, ACC, amygdala and dorsal horn. Pharmacological interventions aimed at reducing neuroinflammation in the DRN or increasing 5-HTergic tone may have therapeutic potential in the treatment of pain disorders in patients with a history of adolescent alcohol use.
Supplementary Material
Acknowledgements
This work was funded through NIH grants R00 AA024215, R01 AA028931 and BBRF grant #27530 to C.M. K.K. was supported by T32 NS045549.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2022.07.160.
Data availability
Data will be made available on request.
References
- Ab Aziz CB, Ahmad AH, 2006. The role of the thalamus in modulating pain. Malays. J. Med. Sci 13, 11–18. [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Bowers MS, 2016. Disentangling the Role of Astrocytes in Alcohol Use Disorder. Alcohol. Clin. Exp. Res 40, 1802–1816. 10.1111/acer.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers KE, Karaçay B, Fuller L, Bonthius DJ, Dailey ME, 2015. Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia 63, 1694–1713. 10.1002/glia.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Mayer DJ, 1972. Antagonism of stimulation-produced analgesia by p-CPA, a serotonin synthesis inhibitor. Brain Res. 44, 692–697. 10.1016/0006-8993(72)90338-1. [DOI] [PubMed] [Google Scholar]
- Andersen E, Dafny N, 1983. An ascending serotonergic pain modulation pathway from the dorsal raphe nucleus to the parafascicularis nucleus of the thalamus. Brain Res. 269, 57–67. 10.1016/0006-8993(83)90962-9. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T, 1994. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J. Neurosci 14, 6779–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Varodayan FP, Madamba SG, Robert AJ, Casal LM, Oleata CS, Siggins GR, Roberto M, 2015. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front. Pharmacol 6 10.3389/fphar.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet G, Valable S, Bourgine J, Lelong-Boulouard V, Lanfumey L, Freret T, Boulouard M, Paizanis E, 2016. Long-Lasting Effects of Chronic Intermittent Alcohol Exposure in Adolescent Mice on Object Recognition and Hippocampal Neuronal Activity. Alcohol. Clin. Exp. Res 40, 2591–2603. 10.1111/acer.13256. [DOI] [PubMed] [Google Scholar]
- Binning W, Hogan-Cann AE, Yae Sakae D, Maksoud M, Ostapchenko V, AlOnaizi M, Matovic S, Lu W-Y, Prado MAM, Inoue W, Prado VF, 2020. Chronic hM3Dq signaling in microglia ameliorates neuroinflammation in male mice. Brain Behav. Immun 88, 791–801. 10.1016/j.bbi.2020.05.041. [DOI] [PubMed] [Google Scholar]
- Brambilla D, Franciosi S, Opp MR, Imeri L, 2007. Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur. J. Neurosci 26, 1862–1869. 10.1111/j.1460-9568.2007.05796.x. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH, 2005. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction (Abingdon, England) 100, 777–786. 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Spear LP, 2013. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav. Brain Res 256, 10–19. 10.1016/j.bbr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP, 2011. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol. Clin. Exp. Res 35, 1392–1403. 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Freitas KCC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS, 2014. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology 39, 2835–2845. 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Syed WA, Minter SC, Bowers MS, 2015. Differential response of glial fibrillary acidic protein-positive astrocytes in the rat prefrontal cortex following ethanol self-administration. Alcohol. Clin. Exp. Res 39, 650–658. 10.1111/acer.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniglia EC, Stevens ER, Khan M, Young KE, Ban K, Marshall BDL, Chichetto NE, Gaither JR, Crystal S, Edelman EJ, Fiellin DA, Gordon AJ, Bryant KJ, Tate J, Justice AC, Braithwaite RS, 2020. Does Reducing Drinking in Patients with Unhealthy Alcohol Use Improve Pain Interference, Use of Other Substances, and Psychiatric Symptoms? Alcohol. Clin. Exp. Res 44, 2257–2265. 10.1111/acer.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-T, Chen W-H, Shih H-C, Min M-Y, Shyu B-C, Chen C-C, 2019. Anterior nucleus of paraventricular thalamus mediates chronic mechanical hyperalgesia. Pain 160, 1208–1223. 10.1097/j.pain.0000000000001497. [DOI] [PubMed] [Google Scholar]
- Chao C-C, Tseng M-T, Hsieh P-C, Lin C-H-J, Huang S-L, Hsieh S-T, Chiang M-C, 2021. Brain mechanisms of pain and dysautonomia in diabetic neuropathy: connectivity changes in thalamus and hypothalamus. J. Clin. Endocrinol. Metab 10.1210/clinem/dgab754. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Liu W, Oguz I, Styner M, Crews FT, 2014. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav 116, 142–151. 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, 2014. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol 118, 315–357. 10.1016/B978-0-12-801284-0.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL, 2016. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol. Rev 68, 1074–1109. 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG, 2017. The role of neuroimmune signaling in alcoholism. Neuropharmacology 122, 56–73. 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS, Vetreno RP, 2019. Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcohol. Clin. Exp. Res 43, 1806–1822. 10.1111/acer.14154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C, Meireles M, Silva SM, 2017. Chronic ethanol intake induces partial microglial activation that is not reversed by long-term ethanol withdrawal in the rat hippocampal formation. Neurotoxicology 60, 107–115. 10.1016/j.neuro.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, von Korff M, Porter L, Helmick C, 2018. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR. Morbidity and Mortality Weekly Report 67, 1001–1006. 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira BMT, Telles TMBB, Lomba LA, Correia D, Zampronio AR, 2017. Effects of binge-like ethanol exposure during adolescence on the hyperalgesia observed during sickness syndrome in rats. Pharmacol. Biochem. Behav 160, 63–69. 10.1016/j.pbb.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Deneris E, Gaspar P, 2018. Serotonin neuron development: shaping molecular and structural identities. Wiley Interdiscip Rev Dev Biol 7. 10.1002/wdev.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Shen F, Li L, Tu Z, Chen P, Chen P, Wang Z, Liang W, Wang Y, 2021. Activation of the Notch signaling pathway in the anterior cingulate cortex is involved in the pathological process of neuropathic pain. Pain 162, 263–274. 10.1097/j.pain.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugé GP, Lörincz ML, Lottem E, Audero E, Matias S, Correia PA, Ĺena C, Mainen ZF, 2014. Optogenetic recruitment of dorsal raphe serotonergic neurons acutely decreases mechanosensory responsivity in behaving mice. PLoS One 9, e105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S, 2012. Alcohol dependence as a chronic pain disorder. Neurosci. Biobehav. Rev 36, 2179–2192. 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J, 2015. Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int. J. Physiol. Pathophysiol. Pharmacol. 7, 136–144. [PMC free article] [PubMed] [Google Scholar]
- Gandhi PJ, Gawande DY, Shelkar GP, Gakare SG, Kiritoshi T, Ji G, Misra B, Pavuluri R, Liu J, Neugebauer V, Dravid SM, 2021. Dysfunction of Glutamate Delta-1 Receptor-Cerebellin 1 Trans-Synaptic Signaling in the Central Amygdala in Chronic Pain. Cells 10. 10.3390/cells10102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Yu W, Kash TL, 2021. Forebrain-Midbrain Circuits and Peptides Involved in Hyperalgesia After Chronic Alcohol Exposure. Alcohol. Res 41, 13. 10.35946/arcr.v41.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN, 2012. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS ONE 7, e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Wang X, Strand KA, Baratta MV, Zhang Y, Galer EL, Yin H, Maier SF, Watkins LR, 2018. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp. Neurol 304, 125–131. 10.1016/j.expneurol.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JL, Gatipon GB, 1981. A comparative study of selective stimulation of raphe nuclei in the cat in inhibiting dorsal horn neuron responses to noxious stimulation. Brain Res. 229, 520–524. 10.1016/0006-8993(81)91015-5. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan W-B, Julius D, 2006. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci 9, 1512–1519. 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Heijmans L, Mons MR, Joosten EA, n.d. A systematic review on descending serotonergic projections and modulation of spinal nociception in chronic neuropathic pain and after spinal cord stimulation. Mol Pain 17, 17448069211043964. 10.1177/17448069211043965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C, 2011. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience 184, 128–138. 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- Hollis JH, Evans AK, Bruce KPE, Lightman SL, Lowry CA, 2006. Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons. Brain Behav. Immun 20, 569–577. 10.1016/j.bbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Horie H, Pamplin PJ, Yokota T, 1991. Inhibition of nociceptive neurons in the shell region of nucleus ventralis posterolateralis following conditioning stimulation of the periaqueductal grey of the cat. Evidence for an ascending inhibitory pathway. Brain Res. 561, 35–42. 10.1016/0006-8993(91)90746-I. [DOI] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Bale TL, 2014. Dorsal raphe neuroinflammation promotes dramatic behavioral stress dysregulation. J. Neurosci 34, 7113–7123. 10.1523/JNEUROSCI.0118-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA, 2011. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcoholism: Clin. Exp. Res 35, 1938–1947. 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez-Salinas DL, Braz JM, Etlin A, Gee S, Sohal V, Basbaum AI, 2019. GABAergic cell transplants in the anterior cingulate cortex reduce neuropathic pain aversiveness. Brain 142, 2655–2669. 10.1093/brain/awz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbo L, 1999. Glial cell loss in the hippocampus of alcoholics. Alcohol. Clin. Exp. Res 23, 164–168. [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK, 2016. Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Front. Cell. Neurosci 10, 265. 10.3389/fncel.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-N, Sun Y, Ji S-L, Chen Y-B, Ren J-H, He C-B, Wu Z-Y, Li H, Dong Y-L, Li Y-Q, 2019. Collateral Projections from the Medullary Dorsal Horn to the Ventral Posteromedial Thalamic Nucleus and the Parafascicular Thalamic Nucleus in the Rat. Neuroscience 410, 293–304. 10.1016/j.neuroscience.2019.04.050. [DOI] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, Kash TL, 2014. Functional Alterations in the Dorsal Raphe Nucleus Following Acute and Chronic Ethanol Exposure. Neuropsychopharmacology 1–11. 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, Kash TL, 2015. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology 40, 590–600. 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Feng Q, Wei L, Luo M, 2017. Optogenetic activation of dorsal raphe neurons rescues the autistic-like social deficits in Shank3 knockout mice. Cell Res. 27, 950–953. 10.1038/cr.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht V, Elchert N, Crews F, 2020. Adolescent Alcohol Exposure Produces Protracted Cognitive-Behavioral Impairments in Adult Male and Female Rats. Brain Sci 10. 10.3390/brainsci10110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L, 2003. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur. J. Neurosci 18, 1041–1049. 10.1046/j.1460-9568.2003.02836.x. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Green MK, Devine DP, Duarte P, Vierck CJ, Yezierski RP, 2009. Social defeat stress potentiates thermal sensitivity in operant models of pain processing. Brain Res. 1251, 112–120. 10.1016/j.brainres.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL, 2014. Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: Implications for anxiety during ethanol withdrawal. Neuropharmacology 89C, 157–167. 10.1016/j.neuropharm.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Wooden JI, Nixon K, 2020. Microglia Dystrophy Following Binge-Like Alcohol Exposure in Adolescent and Adult Male Rats. Front. Neuroanat. 14 10.3389/fnana.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DJ, Liebeskind JC, 1974. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res. 68, 73–93. 10.1016/0006-8993(74)90534-4. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G, 2002. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol. Psychiatry 52, 1121–1133. 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR, 2008. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res 191, 118–129. 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL, 2011. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc. Natl. Acad. Sci 108, 5466–5471. 10.1073/pnas.1017732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras JL, Guilbaud G, Besson JM, 1979. A map of serotoninergic structures involved in stimulation producing analgesia in unrestrained freely moving cats. Brain Res. 164, 317–322. 10.1016/0006-8993(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Price DD, 2000. Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772. 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Risher M-L, Fleming RL, Risher WC, Miller KM, Klein RC, Wills T, Acheson SK, Moore SD, Wilson WA, Eroglu C, Swartzwelder HS, 2015. Adolescent Intermittent Alcohol Exposure: Persistence of Structural and Functional Hippocampal Abnormalities into Adulthood. Alcohol. Clin. Exp. Res 39, 989–997. 10.1111/acer.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Li X, Li H, Yuan H, 1990. Effect of monoamine neurotransmitters, enkephalin and morphine on substance P contents of several brain regions and pain threshold in rats. Zhen Ci Yan Jiu 15. [PubMed] [Google Scholar]
- Sampedro-Piquero P, Moreno-Fernández RD, Begega A, López M, Santín LJ, 2022. Long-term consequences of alcohol use in early adolescent mice: Focus on neuroadaptations in GR, CRF and BDNF. Addict. Biol 27, e13158. [DOI] [PubMed] [Google Scholar]
- Sawicki CM, Kim JK, Weber MD, Faw TD, McKim DB, Madalena KM, Lerch JK, Basso DM, Humeidan ML, Godbout JP, Sheridan JF, 2019. Microglia Promote Increased Pain Behavior through Enhanced Inflammation in the Spinal Cord during Repeated Social Defeat Stress. J. Neurosci 39, 1139–1149. 10.1523/JNEUROSCI.2785-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, Clark JJ, 2014. Chronic Alcohol Intake During Adolescence, but not Adulthood, Promotes Persistent Deficits in Risk-Based Decision Making. Alcohol. Clin. Exp. Res 38, 1622–1629. 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, 2010. Serotonin in Pain and Pain Control. 10.1016/S1569-7339(10)70096-5. [DOI] [Google Scholar]
- Thielen RJ, Morzorati SL, McBride WJ, 2001. Effects of ethanol on the dorsal raphe nucleus and its projections to the caudate putamen. Alcohol 23, 131–139. 10.1016/S0741-8329(01)00126-4. [DOI] [PubMed] [Google Scholar]
- Thompson T, Oram C, Correll CU, Tsermentseli S, Stubbs B, 2017. Analgesic Effects of Alcohol: A Systematic Review and Meta-Analysis of Controlled Experimental Studies in Healthy Participants. The Journal of Pain 18. 10.1016/j.jpain.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Torcaso A, Asimes A, Meagher M, Pak TR, 2017. Adolescent binge alcohol exposure increases risk assessment behaviors in male Wistar rats after exposure to an acute psychological stressor in adulthood. Psychoneuroendocrinology 76, 154–161. 10.1016/j.psyneuen.2016.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner TT, Spear LP, 2020. Rats exposed to intermittent ethanol during late adolescence exhibit enhanced habitual behavior following reward devaluation. Alcohol 91, 11–20. 10.1016/j.alcohol.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MD, Mann JJ, Arango V, 2007. Morphometry of dorsal raphe nucleus serotonergic neurons in alcoholism. Alcohol. Clin. Exp. Res 31, 837–845. 10.1111/j.1530-0277.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP, 2014. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol 48, 433–444. 10.1016/j.alcohol.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Patel Y, Patel U, Walter TJ, Crews FT, 2017. Adolescent intermittent ethanol reduces serotonin expression in the adult raphe nucleus and upregulates innate immune expression that is prevented by exercise. Brain Behav. Immun 60, 333–345. 10.1016/j.bbi.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Crews FT, 2017. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J. Neuroinflamm 14, 86. 10.1186/s12974-017-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick CA, Shutov LP, Shepherd AJ, Mohapatra DP, Usachev YM, 2019. Mechanisms underlying mechanical sensitization induced by complement C5a. Pain 160, 702–711. 10.1097/j.pain.0000000000001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Mahmood T, Harris GM, Abbas S, Miles MF, 2017. Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC. Front. Mol. Neurosci 10 10.3389/fnmol.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M-H, Liu YU, Liu K, Chen T, Bosco DB, Zheng J, Xie M, Zhou L, Qu W, Wu L-J, 2021. Chemogenetic manipulation of microglia inhibits neuroinflammation and neuropathic pain in mice. Brain Behav. Immun 92, 78–89. 10.1016/j.bbi.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis RM, Wolstenholme JT, Bagdas D, Bettinger JC, Miles MF, Damaj MI, 2019. Adolescent but not adult ethanol binge drinking modulates ethanol behavioral effects in mice later in life. Pharmacol. Biochem. Behav 184, 172740 10.1016/j.pbb.2019.172740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargarani A, Karimi-Haghighi S, Haghparast A, 2021. Role of hippocampal orexin receptors in antinociception elicited by chemical stimulation of the lateral hypothalamus in the tail-flick test. Behav. Brain Res 414, 113492 10.1016/j.bbr.2021.113492. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li A, Xin J, Lao L, Ren K, Berman BM, Tan M, Zhang R-X, 2011. Involvement of spinal serotonin receptors in electroacupuncture anti-hyperalgesia in an inflammatory pain rat model. Neurochem. Res 36 10.1007/s11064-011-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Terreni L, De Simoni MG, Dunn AJ, 2001. Peripheral interleukin-6 administration increases extracellular concentrations of serotonin and the evoked release of serotonin in the rat striatum. Neurochem. Int 38, 303–308. 10.1016/s0197-0186(00)00099-1. [DOI] [PubMed] [Google Scholar]
- Zhao Y-N, Wang F, Fan Y-X, Ping G-F, Yang J-Y, Wu C-F, 2013. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav. Brain Res 236, 270–282. 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


