Abstract
Objective
To evaluate the effectiveness of vaccination on reducing household transmission of SARS-CoV-2 among common household types in Japan during the Omicron variant wave.
Methods
This retrospective study was conducted using vaccination records, COVID-19 infection data, and resident registry data from two Japanese municipalities. Households that experienced their first COVID-19 case between January and April 2022 were categorized into two groups according to the presence/absence of children aged ≤11 years. We constructed multivariable logistic regression models with generalized estimating equations to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for household transmission according to the vaccination statuses of primary cases and household contacts.
Results
We analyzed 7,326 households with 17,586 contacts. In all households, the OR for household transmission was <0.6 (P<0.001) when the primary case and/or contact were vaccinated. In households with children aged ≤11 years, the OR was 0.71 (P<0.001) when only the contact was vaccinated. In households with all members aged ≥12 years, the OR was <0.5 (P<0.001) when the primary case and/or contact were vaccinated.
Conclusions
COVID-19 vaccination effectively reduced household transmission in Japan during the Omicron variant wave.
Keywords: Household transmission, COVID-19 vaccination status, infectiousness, susceptibility, children, SARS-CoV-2 Omicron variant
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 (Omicron) variant was designated by the World Health Organization as a variant of concern in November 2021, and quickly became the dominant strain within the coronavirus disease 2019 (COVID-19) pandemic [1]. The Omicron variant and its subvariants have demonstrated a higher household secondary attack risk (SAR) than the previously dominant B.1.617.2 (Delta) variant [2, 3], which indicates the need for more effective measures to reduce the formation of household clusters.
The following three factors are needed to enable secondary transmission of SARS-CoV-2: an infected primary case to transmit the virus, a potential secondary case that is susceptible to infection, and a contact event between these two individuals [4]. Accordingly, secondary transmission must be assessed from the dual perspectives of infectiousness in primary cases and susceptibility in potential secondary cases. Previous studies on the factors associated with household transmission of the Delta variant have reported that children have higher susceptibility than adults [5, 6, 7, 8], infants have higher infectiousness and susceptibility than older children [5, 9, 10], and residing in a household with young children increases the risk of infection among adults [11, 12].
Other studies have reported that COVID-19 vaccination is effective in reducing secondary transmission within households. This effect may be mediated through two mechanisms: (i) the vaccine-induced reduction of an infected person's infectiousness to uninfected household contacts and (ii) the vaccine-induced reduction of an uninfected person's susceptibility to infection from infected household contacts [13, 14]. The reduction in infectiousness may be influenced by decreasing transmission from breakthrough infection cases [15]. Much of the current literature on the effects of vaccination on SARS-CoV-2 infection risks have focused either on evaluating the infectiousness of primary cases or the susceptibility of secondary cases according to vaccination status. Studies on the infectiousness of primary cases have been conducted in England [16], the Netherlands [17], Germany [15], and Israel [18] for the Delta and earlier variants, and in Norway after the Omicron variant was designated [19]. The Israeli study noted that the vaccination of parents decreased the risk of infecting their unvaccinated children, thereby indirectly conferring protection against COVID-19 [18]. More recent reports have analyzed both SARS-CoV-2 infectiousness and susceptibility in Denmark [4], Singapore [6], Israel [20], and the UK [21] for the Delta and earlier variants; as well as in Denmark [3, 22] and the US [12] in the Omicron era. All these studies have indicated that vaccinations are, to varying degrees, effective in reducing the infectiousness of primary cases and the susceptibility of household contacts.
However, few studies have analyzed both the infectiousness of primary cases and the susceptibility of household contacts according to vaccination status after the emergence of the Omicron variant. The simultaneous estimation of both infectiousness and susceptibility may also help to avoid bias that can occur when either one is estimated by itself. In addition, little is known about these effects in children and with consideration to common household structures. To address these gaps in knowledge, we conducted this study to simultaneously analyze the effectiveness of COVID-19 vaccination on infectiousness in primary cases and susceptibility in their household contacts during the Omicron variant wave. Specifically, we analyzed households according to the presence or absence of young children aged ≤11 years as the COVID-19 vaccine was approved much later in Japan for children aged 5–11 years than for older children and adults.
Methods
Study Design and Setting
This retrospective observational study was conducted using a database acquired from the Vaccine Effectiveness, Networking, and Universal Safety (VENUS) Study [23], which is a Japanese database platform that enables comparative analyses of infection occurrence and adverse events in both vaccinated and unvaccinated groups at the municipal level. The VENUS Study is a sub-project of the Longevity Improvement & Fair Evidence (LIFE) Study [24], and utilizes a multi-source database that links health-related data from municipal governments, vaccination records, and COVID-19–related data from healthcare providers.
In the VENUS Study, individual residents are assigned unique research identification codes within each municipal government's building, and these codes allow the linkage of residents across the different data types. The data sources used in the VENUS Study include a national cloud-based COVID-19 Vaccination Record System (VRS), a national information-sharing and management support system for COVID-19 patients (Health Center Real-time Information-sharing System on COVID-19, or HER-SYS), and municipal-level Basic Resident Registers. Each municipality in Japan maintains a Basic Resident Register that includes each resident's age, sex, address, and household information (including household members). We obtained data from two municipalities (designated City A and City B) with populations of 250,000–400,000 across 130,000–180,000 households. All data were anonymized before analysis. The study was approved by the Kyushu University Institutional Review Board for Clinical Research (Approval Number: 2021-399).
Study Population
The study focused on households that experienced their first COVID-19 case between January and April 2022 (i.e., following the emergence of the Omicron variant). Study subjects were selected using a two-stage process. First, we identified individual COVID-19 cases using the HER-SYS data. We excluded cases with missing research identification codes and duplicate positive test dates, as well as cases who could not be linked with their household information in the Basic Resident Registers. Second, we identified households with a single positive COVID-19 case using the HER-SYS data. We excluded households in which the primary case or contacts had missing positive test dates, households in which the primary case had an unclear age, and households in which the primary case had tested positive in December 2021 or earlier (i.e., before the spread of the Omicron variant). We also excluded households with ≥2 simultaneously occurring COVID-19 cases, single-member households, and households that included members with missing vaccination dates.
Next, we categorized the households into two groups: Household Group 1 comprised households with children aged ≤11 years, and Household Group 2 comprised households with all members aged ≥12 years. In households with children aged ≤11 years, we included households with 3–5 members (i.e., a man and woman aged 20–59 years and ≥1 children aged ≤11 years); some households also included older offspring aged 12–19 years. As there were relatively few households in which children aged ≤11 years were vaccinated against COVID-19, these households were excluded from the analysis. In households with all members aged ≥12 years, we included households with 2–5 members where at least one member was aged ≥20 years.
Within each study household, we defined a primary case as the first individual to test positive (either from a nucleic acid amplification test or antigen test) for SARS-CoV-2 as recorded in the HER-SYS. All other household members were regarded as susceptible household contacts, who formed the study population included in the analysis.
Exposures
Using VRS data from the municipal governments, each individual's COVID-19 vaccination status was identified through the numbers and dates of vaccination doses. During the study period, the following three vaccines were approved for use in Japan: BNT162b2 vaccine (Pfizer–BioNTech), mRNA-1273 vaccine (Moderna/Takeda), and ChAdOx1-S recombinant vaccine (Oxford–AstraZeneca). For this study, we defined fully vaccinated individuals as those for whom seven days had passed after receiving a third COVID-19 vaccine dose. All other individuals were considered unvaccinated; these included those who had received one dose (0.9%), two doses (43.9%), and three doses with fewer than seven days after the third dose (22.2%). Based on combinations of the vaccination statuses of each primary case and each household contact, we set the following four categories: (i) neither the primary case nor household contact was fully vaccinated, (ii) only the household contact was fully vaccinated, (iii) only the primary case was fully vaccinated, and (iv) both the primary case and household contact were fully vaccinated.
Outcome
The outcome measure was the secondary transmission of SARS-CoV-2 to a household contact (secondary case), which was defined as a positive test in a household contact within seven days of the primary case's positive test date [12].
Statistical Analysis
The statistical analysis was performed for all households and according to household group. The two household groups were chosen to simulate typical real-world Japanese households based on the presence or absence of children aged ≤11 years. The age cutoff of 11 years was selected because the COVID-19 vaccine was only approved for children aged 5–11 years in January 2022. Therefore, the vaccination coverage of children aged ≤11 years would have been much lower than persons aged ≥12 years during our study period. We considered that these households would have different infection control behaviors, and that this group-specific analysis would help to account for the fundamental differences between the households.
First, we examined the following characteristics: presence/absence of children aged ≤11 years in the household, primary case age (≤11 or ≥12 years), household contact age and sex (≤11 years, male or female; 12–19 years, male or female; 20–59 years, male; 20–59 years, female; or ≥60 years, male or female), household size (2, 3, 4, or 5 members), infection month of the primary case (January, February, March, or April 2022), and municipality (City A or City B). The 20–59-year age group was separated by sex because we hypothesized that their household roles may be different, especially for women aged 20–59 years as they typically play a major caregiving role in households with young children. The infection month of the primary case was set as the month in which they tested positive for SARS-CoV-2. Next, we calculated the SAR as the proportion of secondary cases to the total number of household contacts within each household group. The 95% confidence interval (CI) for each SAR was also calculated.
To analyze the effect of COVID-19 vaccination on household transmission, we constructed multivariable logistic regression models with generalized estimating equations (GEEs) to calculate the odds ratios (ORs) and 95% CIs. We used an exchangeable correlation structure for each household to account for household-based clustering [5, 12, 17]. In the regression models, the outcome was the secondary transmission of SARS-CoV-2 to a household contact. The predictor of interest was the combinations of vaccination statuses for the primary case and household contacts (reference category: neither primary case nor household contact was vaccinated). Other predictors included the presence/absence of children aged ≤11 years in the household, primary case age, household contact age and sex, household size, infection month of the primary case, and municipality. We considered these to be the minimum necessary predictors for assessing household transmission that were available in the study data.
All statistical analyses were performed using STATA version 17 (StataCorp LP, College Station, TX, US). Statistical significance was set at P < 0.05 (two-tailed).
Results
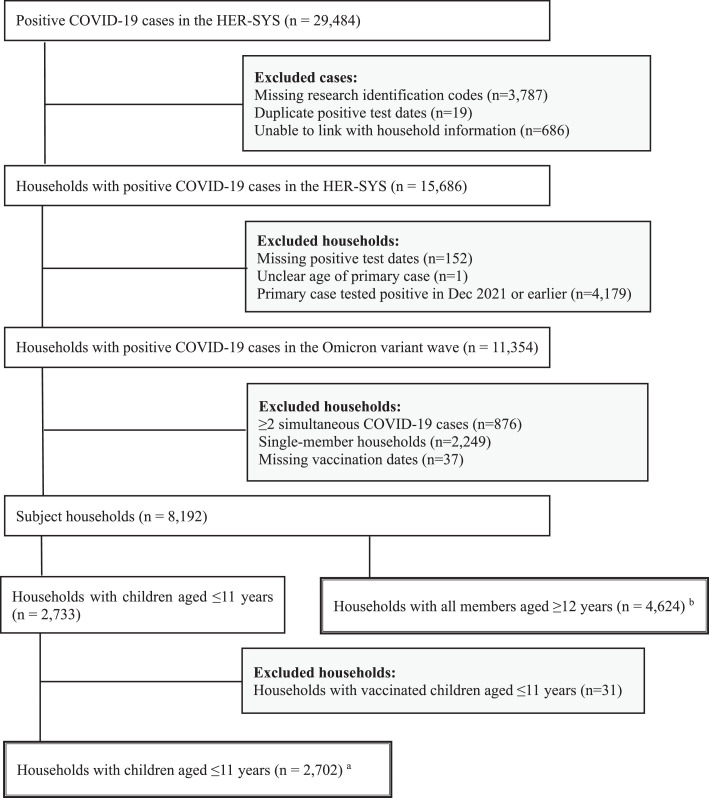
The selection of the study population is presented in Figure 1 . From 29,484 positive COVID-19 cases in the two municipalities between January and April 2022, we identified 7,326 households (17,586 contacts) for analysis, with 2,702 households (8,014 contacts) in Household Group 1 and 4,624 households (9,572 contacts) in Household Group 2.
Figure 1.
Flow Diagram of Study Population Selection
a Household Group 1: Households with ≥1 children aged ≤11 years. b Household Group 2: Households with all members aged ≥12 years. Abbreviation: HER-SYS, Health Center Real-time Information-sharing System on COVID-19.
Table 1 summarizes the characteristics of the primary cases, household contacts, and households. The percentage of household contacts that became secondary cases was 24.3% in all households, 35.0% in Household Group 1, and 15.4% in Household Group 2. In all households, persons aged ≥12 years accounted for 74.4% of primary cases. In Household Group 1, children aged ≤11 years accounted for 56.3% of primary cases.
Table 1.
Characteristics of the Primary Cases, Household Contacts, and Households
| All Households | Household Group 1a | Household Group 2b | |
|---|---|---|---|
| Households, n | 7,326 | 2,702 | 4,624 |
| Household contacts, n | 17,586 | 8,014 | 9,572 |
| Secondary cases | 4,282 (24.3) | 2,808 (35.0) | 1,474 (15.4) |
| Vaccination status c | |||
| Primary = No / Contact = No | 14,878 (84.6) | 7,299 (91.1) | 7,579 (79.2) |
| Primary = No / Contact =Yes | 1,556 (8.8) | 523 (6.5) | 1,033 (10.8) |
| Primary = Yes / Contact = No | 640 (3.6) | 177 (2.2) | 463 (4.8) |
| Primary = Yes / Contact = Yes | 512 (2.9) | 15 (0.2) | 497 (5.2) |
| Children aged ≤11 years in household | |||
| Yes | 8,014 (45.6) | 8,014 (100.0) | 0 (0.0) |
| Primary case age | |||
| ≥12 years | 13,077 (74.4) | 3,505 (43.7) | 9,572 (100.0) |
| ≤11 years | 4,509 (25.6) | 4,509 (56.3) | 0 (0.0) |
| Contact age and sex | |||
| ≤11 years, male or female | 3,048 (17.3) | 3,048 (38.0) | - |
| 12–19 years, male or female | 2,091 (11.9) | 598 (7.5) | 1,493 (15.6) |
| 20–59 years, male | 4,824 (27.4) | 2,117 (26.4) | 2,707 (28.3) |
| 20–59 years, female | 5,368 (30.5) | 2,251 (28.1) | 3,117 (32.6) |
| ≥60 years, male or female | 2,255 (12.8) | - | 2,255 (23.6) |
| Household size | |||
| 2 | 1,660 (9.4) | - | 1,660 (17.3) |
| 3 | 4,058 (23.1) | 1,360 (17.0) | 2,698 (28.2) |
| 4 | 8,040 (45.7) | 4,302 (53.7) | 3,738 (39.1) |
| 5 | 3,828 (21.8) | 2,352 (29.3) | 1,476 (15.4) |
| Infection month of the primary case | |||
| Jan 2022 | 3,532 (20.1) | 1,540 (19.2) | 1,992 (20.8) |
| Feb 2022 | 6,404 (36.4) | 2,887 (36.0) | 3,517 (36.7) |
| Mar 2022 | 4,806 (27.3) | 2,483 (31.0) | 2,323 (24.3) |
| Apr 2022 | 2,844 (16.2) | 1,104 (13.8) | 1,740 (18.2) |
| Municipality | |||
| City A | 5,457 (31.0) | 2,886 (36.0) | 2,571 (26.9) |
| City B | 12,129 (69.0) | 5,128 (64.0) | 7,001 (73.1) |
Data are presented as n (%) unless otherwise indicated.
Household Group 1: Households with ≥1 children aged ≤11 years.
Household Group 2: Households with all members aged ≥12 years. c “Primary” refers to the primary case, and “Contact” refers to the household contact. For vaccination status, “Yes” refers to individuals for whom seven days had passed after receiving a third COVID-19 vaccine dose; all other individuals were designated “No”.
eFigure 1 shows the number of days between the positive test dates of the primary and secondary cases. In all households and both household groups, the majority of secondary cases occurred within 2–3 days of the primary case, with few cases occurring after 7 days.
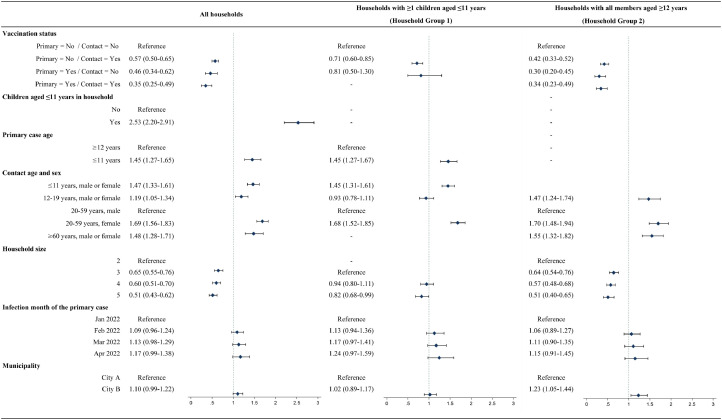
The effect of COVID-19 vaccination on household transmission was assessed using GEE multivariable logistic regression models. The variance inflation factors of all predictors in the models were below 2.0, and the Wald statistic was significant (P<0.001) in all models. The ORs and 95% CIs of the vaccination status combinations and other predictors for household transmission are shown in Figure 2 . In all households, the OR was <0.6 (P<0.001) when the primary case and/or contact were vaccinated. In Household Group 1, the OR was 0.71 (P<0.001) when only the contact was vaccinated. There were no cases of household transmission when both parties were vaccinated. In Household Group 2, the OR was <0.5 (P<0.001) when the primary case and/or contact were vaccinated. In all households, those with children aged ≤11 years had a significantly higher odds (OR: 2.53, P<0.001) for household transmission than those without children aged ≤11 years. Next, the odds for household transmission were significantly higher for primary cases aged ≤11 years than for primary cases aged ≥12 years (OR: 1.45, P<0.001) in all households and Household Group 1. Furthermore, the odds for household transmission were significantly higher when the household contact was a woman aged 20–59 years than for men aged 20–59 years (OR: ≥1.68, P<0.05) in all households and both household groups.
Figure 2.
Odds Ratios and 95% Confidence Intervals for Household Transmission According to COVID-19 Vaccination Status
a Household Group 1: Households with ≥1 children aged ≤11 years. b Household Group 2: Households with all members aged ≥12 years. “Primary” refers to the primary case, and “Contact” refers to the household contact. For vaccination status, “Yes” refers to individuals for whom seven days had passed after receiving a third COVID-19 vaccine dose; all other individuals were designated “No”. Diamonds represent the odds ratios, and bars indicate the 95% CIs. The variance inflation factors of all predictors in the models were below 2.0, and the Wald statistic was significant (P<0.001) in all models. Abbreviation: CI, confidence interval.
Discussion
Using a multi-source database that linked VRS data, HER-SYS data, and Basic Resident Registers, this retrospective observational study was conducted on 7,326 households from two Japanese municipalities to analyze the effect of COVID-19 vaccination on household transmission during the Omicron variant wave in early 2022. The analysis was conducted for all households and two household groups based on the presence/absence of young children. Our main findings were as follows: vaccination in the household contacts was associated with a reduced susceptibility to infection in Household Group 1. In all households and Household Group 2, vaccination was associated with both a reduced infectiousness in primary cases and a reduced susceptibility in household contacts. Moreover, vaccination in both parties was associated with a further reduction in infectiousness and susceptibility in all households.
While previous studies [15, 16, 17, 19] have reported that COVID-19 vaccination reduces the infectiousness of primary cases in household transmission, our analysis did not observe this relationship in Household Group 1. The effect of vaccination on reducing household transmission may be exerted through the shortening of the viral shedding period [21, 25]. An Israeli study reported that parental vaccination reduces the risk of SARS-CoV-2 infection in their unvaccinated children [18]. Although we had expected our study's Household Group 1 to confirm the results of that study [18], our analysis showed no significant reduction in the odds of secondary transmission when the primary case was vaccinated but the household contact was not. However, this discrepancy may have been due to a lack of power in our study and should be examined further. In contrast, our finding that vaccination reduced the susceptibility of household contacts in all household groups was consistent with previous studies [3, 4, 6, 10, 12, 20, 21, 22]. Our analysis showed that vaccination of household contacts reduced the odds of household transmission by approximately 60% in all households, 30% in Household Group 1, and 60% in Household Group 2. Notably, the OR was high when the primary case was a young child aged ≤11 years, suggesting that further studies are needed to investigate the infectiousness and susceptibility of children according to vaccination status.
We calculated the overall SAR to be 24.3% (95% CI: 23.5–25.2) in all households. Among the household groups, the SAR was 35.0% (95% CI: 33.6–36.5) in Household Group 1 and 15.4% (14.5–16.3) in Household Group 2. These SAR values were lower than those of previous studies conducted in Denmark (29%) [3] and Norway (51%) [19] during the Omicron variant wave. In addition, we found that the SAR in households with children aged ≤11 years was approximately twice that of households with all members aged ≥12 years, which was similar to the findings from studies in Denmark [11] and the US [12]. The general trends in the number of days between the positive test dates of the primary and secondary cases were similar to those of a US study [12].
When examining the household structure factors included as predictors in the GEE logistic regression models, we found that the odds for household transmission for women aged 20–59 years as household contacts was approximately 70% higher than men aged 20–59 years in all households and both household groups. We also noted that the odds for household transmission for children aged ≤11 years as the primary case were approximately 45% higher than persons aged ≥12 years in Household Group 1. This could indicate that young children as primary cases require an unavoidable need for care that is frequently fulfilled by mothers (represented by women aged 20–59 years in this study), thereby increasing their contact and risk of transmission [3]. In addition, when the household contact was a child aged ≤11 years, the odds for household transmission were approximately 45–47% higher than men aged 20–59 years. When a household included children aged ≤11 years, the odds were 153% higher than households without similarly aged children. This indicates that the risk of transmission is particularly high among children aged ≤11 years.
This study sought to address the general lack of evidence about secondary transmission of SARS-CoV-2 in households with children. While we were unable to conduct an in-depth analysis that included vaccinated children aged ≤11 years, we identified households that we considered to be fairly representative of Japan, and categorized them into typical scenarios based on the presence/absence of young children. We believe the trends observed here further our understanding of household transmission of COVID-19 in the Omicron era, and may guide the refinement of transmission prevention measures.
Limitations
Our study is characterized by the following limitations. First, household structure was determined based on information from the Basic Resident Registers, but some households may have contained non-family members that are not reflected in these registers. However, we attempted to minimize this possibility by analyzing common household structures. Second, we assumed that all infections in the household contacts were transmitted within the household, but a portion of these infections could have originated from external sources. Therefore, our use of multivariable logistic regression models may have overestimated the SAR as these are based on the assumption that all secondary infections were infected directly by the primary case. To focus on household transmission, we excluded households with ≥2 simultaneously occurring COVID-19 cases. In addition, persons identified as close contacts of a COVID-19 case were obligated to self-quarantine for a period of time during the study period, which may have reduced the exposure of household members to external sources of infection. Third, the retrospective design of this study limited the types of data that we could use to adjust for variations in households and household members. For example, we could not account for COVID-19 symptoms or disease severity, preexisting conditions, or specific transmission prevention measures undertaken in each household. The lack of these data could have introduced confounding bias. Fourth, our study was conducted using data from only two Japanese municipalities, which limits the generalizability of its findings. Nevertheless, the use of household information from the municipalities enabled an overall assessment of their residents. In addition, this study focused on households that experienced their first COVID-19 case relatively late in the pandemic, which may suggest that these household members are more cautious than persons who had been infected earlier. This could have introduced a degree of selection bias into the study.
Conclusions
Through an analysis of common household structures, this study demonstrated the effectiveness of COVID-19 vaccination as a strategy to prevent secondary transmission within households. Our study also sheds light on the differential effectiveness of vaccination on infectiousness and susceptibility for different household members, such as children and mothers. As our results highlight the elevated risk of household transmission between children aged ≤11 years, further studies are needed to investigate transmission prevention measures—including vaccination—for this age group.
Author Contributions
Concept and design: Maeda, Fukuda.
Acquisition, analysis, and interpretation of data: Maeda, Murata, Fukuda.
Drafting of the manuscript: Maeda.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Maeda.
Obtained funding: Fukuda.
Supervision: Fukuda.
Funding
This research was supported by an AMED grant (Grant Number JP21nf0101635).
References
-
1
Khandia R, Singhal S, Alqahtani T, Kamal MA, El-Shall NA, Nainu F, et al. Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ Res. 2022;209:112816.
-
2
Madewell ZJ, Yang Y, Longini IM, Jr., Halloran ME, Dean NE. Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status: An Updated Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5(4):e229317.
-
3
Lyngse FP, Mortensen LH, Denwood MJ, Christiansen LE, Møller CH, Skov RL, et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun. 2022;13(1):5573.
-
4
Lyngse FP, Molbak K, Denwood M, Christiansen LE, Moller CH, Rasmussen M, et al. Effect of vaccination on household transmission of SARS-CoV-2 Delta variant of concern. Nat Commun. 2022;13(1):3764.
-
5
Li F, Li YY, Liu MJ, Fang LQ, Dean NE, Wong GWK, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021;21(5):617-28.
-
6
Ng OT, Koh V, Chiew CJ, Marimuthu K, Thevasagayam NM, Mak TM, et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination and Pediatric Age on Delta Variant Household Transmission. Clin Infect Dis. 2022;75(1):e35-e43.
-
7
Dattner I, Goldberg Y, Katriel G, Yaari R, Gal N, Miron Y, et al. The role of children in the spread of COVID-19: Using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol. 2021;17(2):e1008559.
-
8
Madewell ZJ, Yang Y, Longini IM, Jr., Halloran ME, Dean NE. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(12):e2031756.
-
9
Paul LA, Daneman N, Schwartz KL, Science M, Brown KA, Whelan M, et al. Association of Age and Pediatric Household Transmission of SARS-CoV-2 Infection. JAMA Pediatr. 2021;175(11):1151-8.
-
10
Ng OT, Koh V, Chiew CJ, Marimuthu K, Thevasagayam NM, Mak TM, et al. Impact of Delta Variant and Vaccination on SARS-CoV-2 Secondary Attack Rate Among Household Close Contacts. Lancet Reg Health West Pac. 2021;17:100299.
-
11
Husby A, Corn G, Grove Krause T. SARS-CoV-2 infection in households with and without young children: Nationwide cohort study, Denmark, 27 February 2020 to 26 February 2021. Euro Surveill. 2022;27(32).
-
12
Baker JM, Nakayama JY, O'Hegarty M, McGowan A, Teran RA, Bart SM, et al. SARS-CoV-2 B.1.1.529 (Omicron) Variant Transmission Within Households - Four U.S. Jurisdictions, November 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):341-6.
-
13
Richterman A, Meyerowitz EA, Cevik M. Indirect Protection by Reducing Transmission: Ending the Pandemic With Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination. Open Forum Infect Dis. 2022;9(2):ofab259.
-
14
Vanderweele TJ, Tchetgen Tchetgen EJ, Halloran ME. Components of the indirect effect in vaccine trials: identification of contagion and infectiousness effects. Epidemiology. 2012;23(5):751-61.
-
15
Hsu L, Grune B, Buess M, Joisten C, Klobucnik J, Niessen J, et al. COVID-19 Breakthrough Infections and Transmission Risk: Real-World Data Analyses from Germany's Largest Public Health Department (Cologne). Vaccines (Basel). 2021;9(11).
-
16
Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385(8):759-60.
-
17
de Gier B, Andeweg S, Backer JA, surveillance RC-, epidemiology t, Hahne SJ, et al. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), the Netherlands, August to September 2021. Euro Surveill. 2021;26(44).
-
18
Hayek S, Shaham G, Ben-Shlomo Y, Kepten E, Dagan N, Nevo D, et al. Indirect protection of children from SARS-CoV-2 infection through parental vaccination. Science. 2022;375(6585):1155-9.
-
19
Jalali N, Brustad HK, Frigessi A, MacDonald EA, Meijerink H, Feruglio SL, et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun. 2022;13(1):5706.
-
20
Prunas O, Warren JL, Crawford FW, Gazit S, Patalon T, Weinberger DM, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science. 2022;375(6585):1151-4.
-
21
Singanayagam A, Hakki S, Dunning J, Madon KJ, Crone MA, Koycheva A, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. The Lancet Infectious Diseases. 2022;22(2):183-95.
-
22
Lyngse FP, Kirkeby CT, Denwood M, Christiansen LE, Molbak K, Moller CH, et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat Commun. 2022;13(1):5760.
-
23
Ishiguro C, Mimura W, Murata F, Fukuda H. Development and application of a Japanese vaccine database for comparative assessments in the post-authorization phase: The Vaccine Effectiveness, Networking, and Universal Safety (VENUS) study. Vaccine. 2022;40(42):6179-86.
-
24
Fukuda H, Ishiguro C, Ono R, Kiyohara K. The Longevity Improvement & Fair Evidence (LIFE) Study: Overview of the Study Design and Baseline Participant Profile. J Epidemiol. 2022.
-
25
Jung J, Kim JY, Park H, Park S, Lim JS, Lim SY, et al. Transmission and Infectious SARS-CoV-2 Shedding Kinetics in Vaccinated and Unvaccinated Individuals. JAMA Netw Open. 2022;5(5):e2213606.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the municipal government staff for their cooperation in providing the data for this study. We also thank Mr. S. Yamakawa (Denno Labo Corporation, Tokyo, Japan) for his programming support in data collection and processing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.06.017.
Appendix. Supplementary materials
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.