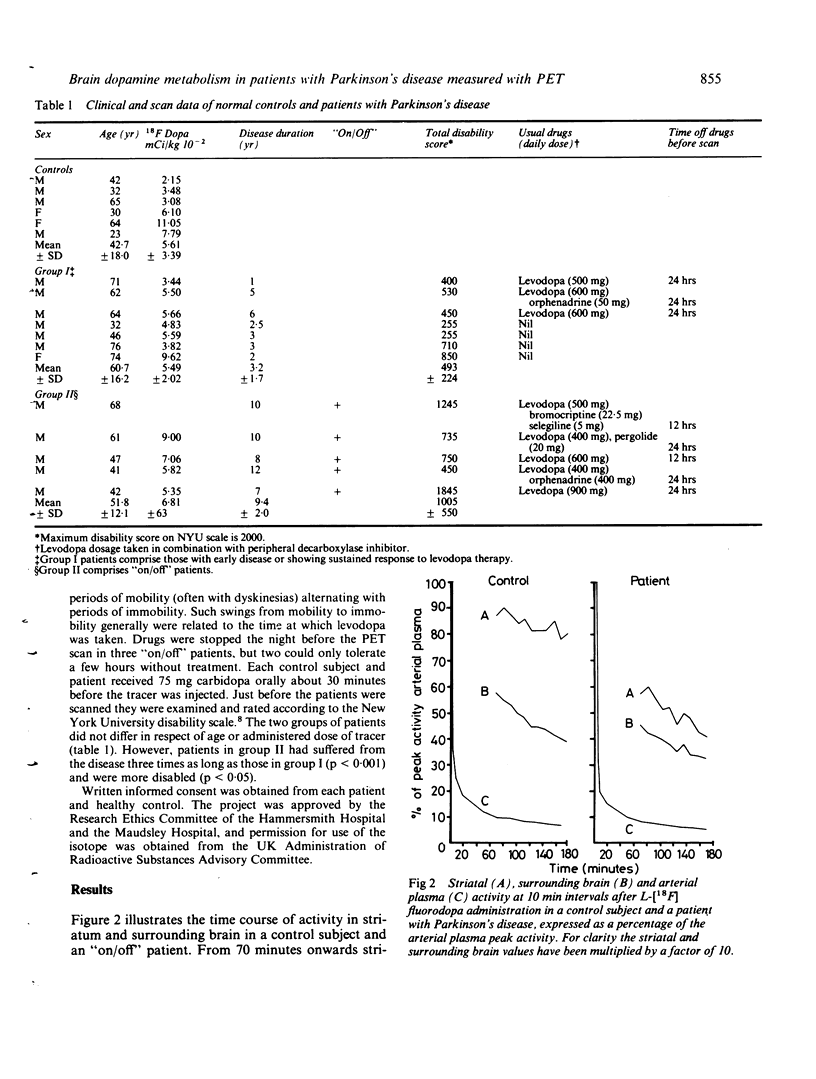
Abstract
L-[18F] fluorodopa was administered in trace amounts intravenously to healthy control subjects and to patients with Parkinson's disease. Striatal uptake of radioactivity was measured using positron emission tomography. The capacity of the striatum to retain tracer was severely impaired in patients compared to controls. This may reflect a reduction of striatal dopamine storage in Parkinson's disease. Patients showing the "on/off" phenomenon had an even greater decrease of striatal storage capacity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Firnau G., Chirakal R., Garnett E. S. Aromatic radiofluorination with [18F]fluorine gas: 6-[18F]fluoro-L-dopa. J Nucl Med. 1984 Nov;25(11):1228–1233. [PubMed] [Google Scholar]
- Firnau G., Garnett E. S., Chan P. K., Belbeck L. W. Intracerebral dopamine metabolism studied by a novel radioisotope technique. J Pharm Pharmacol. 1976 Jul;28(7):584–585. doi: 10.1111/j.2042-7158.1976.tb02801.x. [DOI] [PubMed] [Google Scholar]
- Garnett E. S., Firnau G., Chan P. K., Sood S., Belbeck L. W. [18F]fluoro-dopa, an analogue of dopa, and its use in direct external measurements of storage, degradation, and turnover of intracerebral dopamine. Proc Natl Acad Sci U S A. 1978 Jan;75(1):464–467. doi: 10.1073/pnas.75.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett E. S., Firnau G., Nahmias C. Dopamine visualized in the basal ganglia of living man. Nature. 1983 Sep 8;305(5930):137–138. doi: 10.1038/305137a0. [DOI] [PubMed] [Google Scholar]
- Garnett E. S., Firnau G., Nahmias C., Sood S., Belbeck L. Blood-brain barrier transport and cerebral utilization of dopa in living monkeys. Am J Physiol. 1980 May;238(5):R318–R327. doi: 10.1152/ajpregu.1980.238.5.R318. [DOI] [PubMed] [Google Scholar]
- Garnett E. S., Nahmias C., Firnau G. Central dopaminergic pathways in hemiparkinsonism examined by positron emission tomography. Can J Neurol Sci. 1984 Feb;11(1 Suppl):174–179. doi: 10.1017/s0317167100046369. [DOI] [PubMed] [Google Scholar]
- Garnett S., Firnau G., Nahmias C., Chirakal R. Striatal dopamine metabolism in living monkeys examined by positron emission tomography. Brain Res. 1983 Nov 28;280(1):169–171. doi: 10.1016/0006-8993(83)91187-3. [DOI] [PubMed] [Google Scholar]
- Gervas J. J., Muradás V., Bazán E., Aguado E. G., de Yébenes J. G. Effects of 3-OM-dopa on monoamine metabolism in rat brain. Neurology. 1983 Mar;33(3):278–282. doi: 10.1212/wnl.33.3.278. [DOI] [PubMed] [Google Scholar]
- Hardie R. J., Lees A. J., Stern G. M. On-off fluctuations in Parkinson's disease. A clinical and neuropharmacological study. Brain. 1984 Jun;107(Pt 2):487–506. doi: 10.1093/brain/107.2.487. [DOI] [PubMed] [Google Scholar]
- Hefti F., Melamed E., Wurtman R. J. The site of dopamine formation in rat striatum after L-dopa administration. J Pharmacol Exp Ther. 1981 Apr;217(1):189–197. [PubMed] [Google Scholar]
- Horne M. K., Cheng C. H., Wooten G. F. The cerebral metabolism of L-dihydroxyphenylalanine. An autoradiographic and biochemical study. Pharmacology. 1984;28(1):12–26. doi: 10.1159/000137938. [DOI] [PubMed] [Google Scholar]
- Leenders K. L., Gibbs J. M., Frackowiak R. S., Lammertsma A. A., Jones T. Positron emission tomography of the brain: new possibilities for the investigation of human cerebral pathophysiology. Prog Neurobiol. 1984;23(1-2):1–38. doi: 10.1016/0301-0082(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Lieberman A., Dziatolowski M., Gopinathan G., Kupersmith M., Neophytides A., Korein J. Evaluation of Parkinson's disease. Adv Biochem Psychopharmacol. 1980;23:277–286. [PubMed] [Google Scholar]
- Melamed E., Hefti F., Bitton V., Globus M. Suppression of L-dopa-induced circling in rats with nigral lesions by blockade of central dopa-decarboxylase: implications for mechanism of action of L-dopa in parkinsonism. Neurology. 1984 Dec;34(12):1566–1570. doi: 10.1212/wnl.34.12.1566. [DOI] [PubMed] [Google Scholar]
- Nutt J. G., Woodward W. R., Hammerstad J. P., Carter J. H., Anderson J. L. The "on-off" phenomenon in Parkinson's disease. Relation to levodopa absorption and transport. N Engl J Med. 1984 Feb 23;310(8):483–488. doi: 10.1056/NEJM198402233100802. [DOI] [PubMed] [Google Scholar]
- Phelps M. E., Hoffman E. J., Huang S. C., Kuhl D. E. ECAT: a new computerized tomographic imaging system for positron-emitting radiopharmaceuticals. J Nucl Med. 1978 Jun;19(6):635–647. [PubMed] [Google Scholar]
- Quinn N., Parkes J. D., Marsden C. D. Control of on/off phenomenon by continuous intravenous infusion of levodopa. Neurology. 1984 Sep;34(9):1131–1136. doi: 10.1212/wnl.34.9.1131. [DOI] [PubMed] [Google Scholar]
- Reches A., Fahn S. 3-O-methyldopa blocks dopa metabolism in rat corpus striatum. Ann Neurol. 1982 Sep;12(3):267–271. doi: 10.1002/ana.410120310. [DOI] [PubMed] [Google Scholar]
- Wooten G. F., Horne M. K. A new autoradiographic approach for imaging forebrain dopamine distribution. Ann Neurol. 1982 Aug;12(2):163–168. doi: 10.1002/ana.410120206. [DOI] [PubMed] [Google Scholar]




