ABSTRACT
Objective.
To map geographic clusters of rare disorders and congenital anomalies reported in South America.
Methods.
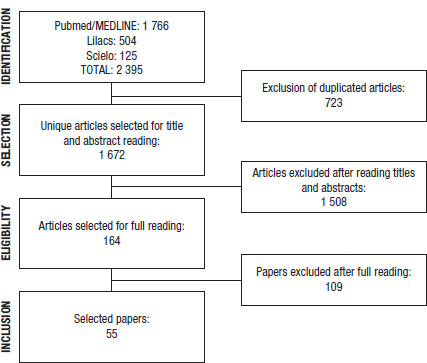
Qualitative systematic review conducted in Medline/PubMed, Lilacs, and Scielo electronic databases to identify studies meeting eligibility criteria. The strategy resulted in 1 672 unique articles, from which 164 were selected for full reading by a pair of reviewers.
Results.
Fifty-five articles reported at least one cluster of genetic disorders or congenital anomalies in South American territory. From these papers, 122 clusters were identified, of which half (61) were related to autosomal recessive disorders. Sixty-five (53.3%) of the clusters were located in Brazil.
Conclusions.
The results of the review reinforce that rare diseases and congenital anomalies can occur in a non-random way in space, which is discussed in the perspective of the complex history of formation, social organization, and genetic structure of the South American population. Mapping clusters in population medical genetics can be an important public health tool, given that such places concentrate cases of rare diseases that frequently require multiprofessional, specialized care. Therefore, these results can support important agendas in public health related to rare diseases and congenital anomalies, such as health promotion and surveillance.
Keywords: Disease hotspot, rare diseases, congenital abnormalities, systematic review, South America.
RESUMEN
Objetivo.
Trazar los conglomerados geográficos de los trastornos y las malformaciones congénitas poco frecuentes notificados en América del Sur.
Métodos.
Se realizó una revisión sistemática cualitativa en las bases de datos electrónicas Medline/PubMed, Lilacs y Scielo para encontrar los estudios que cumplieran con los criterios de selección. Se encontraron 1672 artículos originales, de los que se seleccionaron 164 para su lectura completa por un par de revisores.
Resultados.
En 55 artículos se informó de al menos un conglomerado de trastornos genéticos o malformaciones congénitas en América del Sur. A partir de estos artículos, se encontraron 122 conglomerados, de los cuales la mitad (61) se asociaron con trastornos autosómicos recesivos. Sesenta y cinco (53,3%) de los conglomerados se ubicaron en Brasil.
Conclusiones.
Los resultados de la revisión confirman que las enfermedades raras y las malformaciones congénitas pueden presentarse de una forma no aleatoria en el espacio, lo que se comenta desde la perspectiva de la complejidad histórica del proceso de formación, organización social y estructura genética de la población de América del Sur. Definir geográficamente los conglomerados en la genética médica poblacional puede ser una importante herramienta de salud pública, ya que en esos lugares se concentran casos de enfermedades raras que suelen requerir una atención especializada y multidisciplinaria. Por lo tanto, estos resultados pueden servir de apoyo a importantes programas de salud pública relacionados con las enfermedades raras y las malformaciones congénitas como, por ejemplo, la promoción de la salud y la vigilancia.
Palabras clave: Punto alto de contagio de enfermedades, enfermedades raras, anomalías congénitas, revisión sistemática, América del Sur
RESUMO
Objetivo.
Mapear agrupamentos geográficos de doenças raras e anomalias congênitas relatados na América do Sul.
Métodos.
Revisão sistemática qualitativa realizada nas bases de dados eletrônicos Medline/PubMed, Lilacs e Scielo para identificar estudos que atendessem aos critérios de elegibilidade. A estratégia resultou em 1.672 artigos únicos, dos quais 164 foram selecionados para leitura completa por uma dupla de revisores.
Resultados.
Cinquenta e cinco artigos relataram pelo menos um agrupamento de distúrbios genéticos ou anomalias congênitas no território sul-americano. A partir desses artigos, foram identificados 122 agrupamentos, dos quais metade (61) estava relacionada a doenças autossômicas recessivas. Sessenta e cinco (53,3%) dos agrupamentos estavam localizados no Brasil.
Conclusões.
Os resultados da revisão reforçam a observação de que doenças raras e anomalias congênitas podem ocorrer de forma não aleatória no espaço, o que é discutido na perspectiva da complexa história de formação, organização social e estrutura genética da população sul-americana. O mapeamento de agrupamentos em genética médica populacional pode ser uma importante ferramenta de saúde pública, visto que esses locais concentram casos de doenças raras que frequentemente requerem atendimento multiprofissional especializado. Portanto, esses resultados podem apoiar importantes agendas de saúde pública relacionadas a doenças raras e anomalias congênitas, como a vigilância e a promoção da saúde.
Palabras-chave: Hotspot de doença, doenças raras, anormalidades congênitas, revisão sistemática, América do Sul
Clusters of genetic disorders are defined as geographical areas that present a high frequency of genetic diseases (1, 2). This concept is close to “genetic isolates,” which are cultural and/or geographically isolated subpopulations, some of which may have high frequencies of genetic diseases as a consequence of processes related to their foundation (such as founder effect) and social organization (such as reproductive or cultural isolation and endogamy) (1, 3).
However, according to our experience in the National Census of Isolates (Censo Nacional de Isolados, CENISO), a nationwide, systematic register of human population clusters in Brazil, clusters of disorders related to medical genetics also may present environmental (such as thalidomide embryopathy and congenital Zika syndrome) and multifactorial (such as certain types of congenital anomalies) origins, and they do not occur only in isolates but also in large urban centers. Therefore, the definition of geographical clusters (from now on, only “clusters”) considered in this work is a place with an unexpectedly high frequency of rare diseases and congenital anomalies (4, 5).
Clusters are the main object of population medical genetics, an area of medical genetics that interacts with public health as it involves diagnosis, care, and surveillance of rare (and genetic, in most cases) disorders and congenital anomalies at the community level. Appropriate care of these communities can be a challenging task, especially when cases are concentrated in places far from reference centers and with poor socioeconomic indices (6). In addition, working with clusters has allowed advancing our knowledge about diseases and health care, including identification of genetic causes and risk factors for some disorders, improvement of diagnostic and therapeutic methods, studying of complex traits, among others (3, 7).
Clusters can be understood as biosocial phenomena, as their origin is related to a combination of biological, social, and historical factors of a given human populational group. In this sense, South America represents a unique opportunity to deepen our knowledge about the origin and biosocial dynamics of the clusters, considering its wide diversity of natural and geographical environments, with different ancestral origins of territorial occupation and socio-cultural organization (8). Its population presents a complex multiethnic admixture from the 15th century, with strong contributions of native South American populations (Amerindians), European settlers, and enslaved people from Africa brought with them (9).
Part of the South American population is organized in small rural semi-isolated centers, with little immigration (8). These features are commonly found in clusters, such as Maracaibo Lake, in Venezuela, where the world’s largest and best characterized population with Huntington’s disease (Mendelian inheritance in man [MIM] #143100) is found. Working with this community since the 1950s has contributed to mapping the HD gene (HTT, 4p16.3) and other molecular insights, searching for modifier factors, and characterizing the natural history of the disease (10). However, with a few other better-known examples, the literature on clusters in South America is diffuse and multilingual. In this work, our main goal was to describe clusters of rare disorders and congenital anomalies in South America.
MATERIALS AND METHODS
We carried out a qualitative systematic literature review through an extensive search by keywords in English and Spanish and countries (including “South America”) in three major scientific literature search engines, including two Latin American-specific search engines: Medline/PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Scielo (https://www.scielo.br/), and Lilacs (http://lilacs.bvsalud.org/), covering all the period previous to 31 December 2021. We used Biopython v1.79 Entrez and Medline modules in Python v3.9.7 for automated PubMed searches (11).
The keywords in English were: founder effect OR consanguineous marriages OR isolated population genetic diseases OR consanguinity marriage OR geographical cluster genetic disease OR geographic cluster genetic disease OR cluster genetic disease OR rumor AND [country], where country stands for “South America,” “Argentina,” “Brazil,” “Bolivia,” “Chile,” “Colombia,” “Ecuador,” “Guyana,” “Paraguay,” “Peru,” “Suriname,” “Uruguay,” and “Venezuela.” We also used the same terms in Spanish: efecto fundador OR matrimonios consanguineos OR poblaciones aisladas OR población aislada OR matrimonio consanguineo OR enfermedad genética cluster geográfico OR cluster enfermedad genética OR rumor AND [country].
For Scielo and Lilacs, we manually entered the keywords in their respective websites and downloaded the summary output. For Brazil, the search was performed from 2017 to 2021, in order to update the previous systematic review carried out by Cardoso et al. (5).
In the first phase, two authors (AS and GR) read all titles and abstracts independently, considering articles in English, Portuguese, and Spanish, without time restriction, and made a selection based on the following inclusion criteria: articles must (1) describe a human population living in one of the selected countries, and (2) describe a cluster of a rare disorder or congenital anomaly. We excluded articles without a title or abstract.
We then compared shortlisted articles and discussed discrepancies. Only articles deemed relevant by both researchers passed to the next phase, in which we read the articles in full to retrieve more details about suspected clusters. We read selected articles in detail and collected key information about the population’s location and characteristics.
Clusters were grouped by country and described according to the associated inheritance pattern. Detailed geographical location and molecular information were obtained from the articles. We created a map of the distribution of the populations identified in South America using rnaturalearth package v0.1.0 (South 2017) in R version v4.1.0 software.
RESULTS
The systematic review resulted in 1 672 unique articles, of which 164 were selected for full reading and 55 reported at least one cluster of genetic disorders or congenital anomalies in South American countries (Figure 1). From these papers, we identified 122 different clusters in 10 of the South American countries (no clusters identified for Guyana and Paraguay).
FIGURE 1. Flowchart of the selection of articles in the systematic review.
As shown in Table 1, more than half (n = 65) of these clusters were reported in Brazil, with another six clusters added to the previous review (5). Outside Brazil, Colombia and Venezuela showed the highest number of clusters (13; 10.7% each), followed by Argentina (12; 9.8%) and Ecuador (8; 6.6%). The majority were autosomal recessive genetic disorders (61; 50.0%), followed by autosomal dominant (27; 22.1%) and multifactorial (12; 9.8%). Two clusters of environmental disorders in Brazil (thalidomide embryopathy and microcephaly by Zika virus) and two of X-linked (fragile X syndrome, in Colombia, and progressive muscular dystrophy, in Brazil) were also found. In 18 (14.8%) cases, the inheritance pattern was not identified (individually, the highest proportion (5/8 or 62.5%) was found in Ecuador).
TABLE 1. Number of disease clusters according to the country and the inheritance pattern.
|
Country |
AD (%) |
AR (%) |
E (%) |
M (%) |
X (%) |
NI (%) |
Total (%) |
|---|---|---|---|---|---|---|---|
|
Argentina |
0 |
8 (66.7) |
0 |
2 (16.7) |
0 |
2 (16.7) |
12 (9.8) |
|
Bolivia |
0 |
0 |
0 |
0 |
0 |
1 (100) |
1 (0.8) |
|
Brazil |
13 (20.0) |
36 (55.4) |
2 (3.1) |
10 (15.4) |
1 (1.5) |
3 (4.6) |
65 (53.3) |
|
Chile |
2 (40.0) |
2 (40.0) |
0 |
0 |
0 |
1 (20.0) |
5 (4.1) |
|
Colombia |
6 (46.1) |
4 (30.8) |
0 |
0 |
1 (7.7) |
2 (15.4) |
13 (10.7) |
|
Ecuador |
0 |
3 (37.5) |
0 |
0 |
0 |
5 (62.5) |
8 (6.6) |
|
French Guianaa/Suriname |
0 |
0 |
0 |
0 |
0 |
1 (100) |
1 (0.8) |
|
Peru |
0 |
2 (66.7) |
0 |
0 |
0 |
1 (33.3) |
3 (2.5) |
|
Uruguay |
1 (100) |
0 |
0 |
0 |
0 |
0 |
1 (0.8) |
|
Venezuela |
5 (38.5) |
6 (46.1) |
0 |
0 |
0 |
2 (15.4) |
13 (10.7) |
|
Total |
27 (22.1) |
61 (50.0) |
2 (1.6) |
12 (9.8) |
2 (1.6) |
18 (14.8) |
122 (100) |
AD, autosomal dominant; AR, autosomal recessive; E, environmental; M, multifactorial; X, X-linked; NI, not identified.
Note:
Properly, a French single territorial collectivity.
Source: Prepared by the authors based on the review data.
Individual details of each cluster, such as location aspects, phenotype, inheritance pattern, and molecular alteration, are shown in Table 2. Spatial distribution of clusters in South America are shown in Figure 2. In complement to Cardoso et al. (5), clusters from Brazil are shown separately in Table 3.
TABLE 2. Disease clusters in South America identified from the literature review (clusters in Brazil are shown in Table 3).
|
Country |
Location details |
Lat |
Long |
Phenotype |
MIM |
Etiology |
Reference |
|---|---|---|---|---|---|---|---|
|
ARGENTINA | |||||||
|
|
San Luis |
-33.41 |
-66.22 |
Citrullinemia type I |
#215700 |
AR |
(49) |
|
|
Aicuña |
-29.50 |
-67.76 |
Oculocutaneous albinism |
#203200 |
AR |
(50) |
|
|
Aicuña |
-29.50 |
-67.76 |
Ataxia–telangiectasia |
#208900 |
AR |
(50) |
|
|
La Caldera (Salta) |
-24.60 |
-65.38 |
Werner syndrome |
#277700 |
AR |
(50) |
|
|
Puna Jujeña (Jujuy) |
-22.75 |
-65.90 |
HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) |
NI |
M |
(51) |
|
|
San Luis del Palmar (Corrientes) |
-27.51 |
-58.56 |
Ellis van Creveld syndrome |
#225500 |
AR |
(50) |
|
|
San Luis del Palmar (Corrientes) |
-27.51 |
-58.56 |
Bloom syndrome |
#210900 |
AR |
(50) |
|
|
Patagoniaa |
-42.92 |
-71.33 |
Cleft lip with or without cleft palate |
NI |
NI |
(36) |
|
|
Catamarca, La Rioja, and Tucuman |
-28.47 |
-65.78 |
Cleft lip with or without cleft palate |
NI |
NI |
(36) |
|
|
West region of Córdoba |
NI |
NI |
Pediatric renal tumors, especially Wilms tumor |
NI |
M |
(52) |
|
|
Córdoba |
-32.04 |
-65.15 |
Sandhof disease |
#268800 |
AR |
(53) |
|
|
Córdoba |
-32.04 |
-65.15 |
Argininosuccinate synthetase deficiency |
#215700 |
AR |
(54) |
|
BOLIVIA | |||||||
|
|
La Paz, Cochabamba, Tarija |
-16.50 |
-68.15 |
Cleft lip with or without cleft palate |
NI |
NI |
(36) |
|
CHILE | |||||||
|
|
Robinson Crusoe Island |
-33.64 |
-78.83 |
Specific language impairment |
#602081 |
AD |
|
|
|
Chiloe Islands |
-42.63 |
-73.65 |
Chondrocalcinosis |
#600668 |
AR |
(28) |
|
|
Cachapoal |
-36.45 |
-71.73 |
Achromatopsia |
#262300 |
AR |
(55) |
|
|
Chillán |
-36.60 |
-72.10 |
Creutzfeldt–Jakob disease |
#123400 |
AD |
|
|
|
Maule |
-34.98 |
-71.23 |
Cleft lip with or without cleft palate |
NI |
NI |
(36) |
|
COLOMBIA | |||||||
|
|
Providencia Island |
13.35 |
-81.37 |
Non-syndromic deafness |
#220290 |
AR |
(19) |
|
|
Providencia Island |
13.35 |
-81.37 |
Waardenburg syndrome |
NI |
AD |
(19) |
|
|
Ricuarte (Bolívar Department) |
4.31 |
-76.21 |
Fragile X syndrome |
#300624 |
X |
(57) |
|
|
Cali |
3.43 |
-76.53 |
Sirenomelia |
NI |
NI |
(45) |
|
|
Antioquia |
6.27 |
-75.56 |
Lynch syndrome |
#609310 |
AD |
(25) |
|
|
Antioquia |
6.27 |
-75.56 |
Alzheimer’s disease |
#607822 |
AD |
(24) |
|
|
Antioquia |
6.27 |
-75.56 |
Renal tubular acidosis with deafness |
#267300 |
AR |
(23) |
|
|
Antioquia |
6.27 |
-75.56 |
Juvenile parkinsonism |
#600116 |
AR |
(21) |
|
|
Antioquia |
6.27 |
-75.56 |
Blepharophimosis–ptosis–epicanthus syndrome |
#110100 |
AD |
(22) |
|
|
Antioquia |
6.27 |
-75.56 |
Jarcho–Levin syndrome |
#277300 |
AR |
(26) |
|
|
Bogotá, Manizales, La Mesa |
5.05 |
-75.52 |
Preaxial polydactyly |
NI |
NI |
|
|
|
Cauca Department |
3.36 |
-76.63 |
Calpainopathy |
#253600 |
AR |
(35) |
|
|
Andean region |
3.36 |
-76.63 |
Mucopolysaccharidosis type IVA |
#253000 |
AR |
(58) |
|
ECUADOR | |||||||
|
|
Cañar |
-2.55 |
-78.93 |
Microtia |
NI |
NI |
|
|
|
Cañar and Azogues |
-2.55 |
-78.93 |
Oral clefts |
NI |
NI |
|
|
|
Manabi |
-0.99 |
-80.70 |
Lamellar ichthyosis |
#242300 |
AR |
(59) |
|
|
East Ecuador |
-1.27 |
-77.46 |
Hyperimmunoglobulinemia-E |
NI |
NI |
(32) |
|
|
Loja |
-4.00 |
-79.20 |
Laron syndrome |
#262500 |
AR |
(60) |
|
|
Quito |
-0.18 |
-78.47 |
Microtia |
NI |
NI |
(38) |
|
|
Multiple placesb |
0.62 |
80.43 |
Cleft lip with or without cleft palate |
NI |
NI |
(36) |
|
|
Pacific coast |
NI |
NI |
Mucopolysaccharidosis type IIIB |
#252920 |
AR |
(61) |
|
FRENCH GUIANA/SURINAME | |||||||
|
|
Maroni River (Bushinengue Maroons) |
4.43 |
-54.41 |
β-thalassemia |
NI |
NI |
(39) |
|
PERU | |||||||
|
|
Widespread Peru (native populations) |
NI |
NI |
Chitotriosidase deficiency |
#614122 |
AR |
(34) |
|
|
Trujillo |
-8.12 |
-79.03 |
Aplasia cutis congenita |
NI |
NI |
(62) |
|
|
Loma Negra (La Arena District of Piura Province) |
-5.40 |
-80.73 |
Berardinelli–Seip syndrome |
#269700 |
AR |
(63) |
|
URUGUAY | |||||||
|
|
Canelones County |
-34.53 |
-56.29 |
Oculopharyngeal muscular dystrophy |
#164300 |
AD |
(64) |
|
VENEZUELA | |||||||
|
|
Coro, Bolivar |
8.13 |
-63.55 |
Postaxial polydactyly |
NI |
NI |
|
|
|
Pregonero |
8.02 |
-71.76 |
Chediak–Higashi syndrome |
#214500 |
AR |
(65) |
|
|
Margarita Island (Macanao Peninsula) |
10.99 |
-63.84 |
Usher syndrome |
#276900 |
AR |
(18) |
|
|
Margarita Island (Macanao Peninsula) |
10.99 |
-63.84 |
Cleft lip/palate-ectodermal dysplasia syndrome (CLPED1) |
#225060 |
AR |
|
|
|
Margarita Island (Macanao Peninsula) |
10.99 |
-63.84 |
L-2-hydroxyglutaric aciduria |
#236792 |
AR |
(20) |
|
|
Western Venezuela (Barí indians) |
8.82 |
-72.69 |
Oral clefts |
NI |
AR |
(33) |
|
|
Colonia Tovar |
10.41 |
-67.29 |
Inherited deafness |
NI |
NI |
(66) |
|
|
Santa Lucia (Miranda State) |
10.33 |
-66.64 |
Acute intermittent porphyria |
#176000 |
AD |
(40) |
|
|
Nirgua (Yaracuy State) |
10.16 |
-68.56 |
Spinocerebellar ataxia 7 |
#164500 |
AD |
(67) |
|
|
El Tocuyo (Lara State) |
9.79 |
-69.79 |
Spinocerebellar ataxia 7 |
#164500 |
AD |
(67) |
|
|
Monagas, Anzoátegui, and Bolívar |
NI |
NI |
Spinocerebellar ataxia 1 |
#164400 |
AD |
(67) |
|
|
Pueblo Nuevo del Sur, Merida State |
-8.59 |
-71.15 |
5α-reductase type 2 deficiency |
#264600 |
AR |
(68) |
|
|
Lake Maracaibo (Zulia State) |
9.01 |
-71.93 |
Huntington's disease |
#143100 |
AD |
(10) |
MIM, Mendelian inheritance in man; AD, autosomal dominant; AR, autosomal recessive; M, multifactorial; X, X-linked; NI, not identified.
Notes:
This cluster involves other two places (Puerto Montt and Valdivia) in southern Chile.
This cluster involves other three places (Manizales, Cali, and Neiva) in Colombia.
Source: Prepared by the authors based on the review data.
FIGURE 2. Clusters of rare diseases and congenital anomalies in South America according to the inheritance pattern.
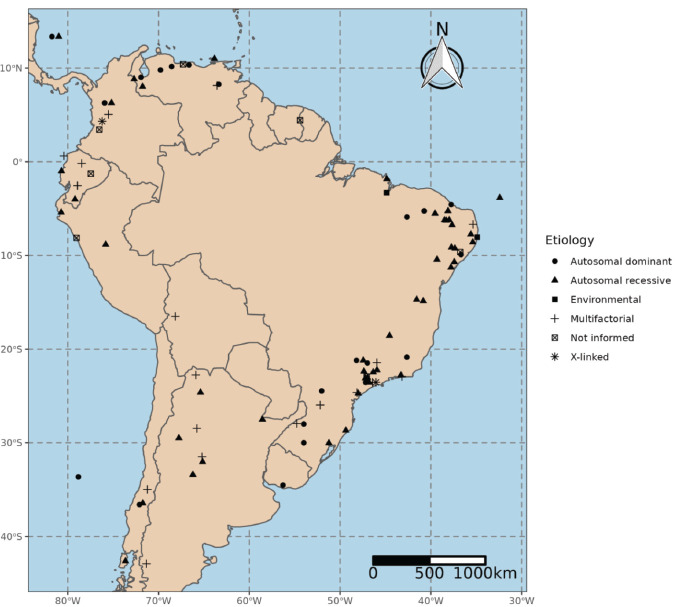
Disclaimer: Country borders or names do not necessarily reflect the PAJPH or PAHO’s official position. This map is for illustrative purposes only and does not imply the expression of any opinion concerning the legal status of any country or territory or concerning the delimitation of frontiers or boundaries.
Source: Prepared by the authors based on the review data.
TABLE 3. Disease clusters from Brazil.
|
ID |
UF |
Phase |
Location details |
Lat |
Long |
Phenotype |
MIM |
Etiology |
|---|---|---|---|---|---|---|---|---|
|
1 |
AL |
4 |
Agua Branca |
-5.89 |
-42.64 |
Aniridia |
106210 |
AD |
|
2 |
AL |
4 |
Mata Grande |
-9.12 |
-37.74 |
Chondrodysplasia, Blomstrand type |
215045 |
AR |
|
3 |
AL |
3 |
Craibas/ Marruas village |
-9.62 |
-36.77 |
Consanguinity and skeletal disorder |
NI |
NI |
|
4 |
AL |
3 |
Feira Grande |
-9.90 |
-36.68 |
Huntington disease |
143100 |
AD |
|
5 |
AL |
3 |
Maravilha |
-9.24 |
-37.35 |
Kindler syndrome |
173650 |
AR |
|
6 |
BA |
3 |
South of Bahia State |
|
|
Chondrodysplasia, Grebe type |
200700 |
AR |
|
7 |
BA |
4 |
Monte Santo |
-10.44 |
-39.33 |
Deafness autosomal recessive 1A (DFNB1A) |
220290 |
AR |
|
8 |
BA |
3 |
Vitória da Conquista/ Barra da Estiva/ Livramento de Nossa senhora |
-14.86 |
-40.84 |
Epidermolysis bullosa |
NI |
AR |
|
9 |
BA |
4 |
Monte Santo |
-10.44 |
-39.33 |
Mucopolysaccharidosis type VI (MPS6) |
253200 |
AR |
|
10 |
CE |
4 |
Tabuleiro do Norte |
-5.25 |
-38.12 |
Gaucher disease, type I |
230800 |
AR |
|
11 |
CE |
4 |
Jericoacara and North region |
NI |
NI |
Pycnodysostosis |
265800 |
AR |
|
12 |
CE |
3 |
Crateús |
-5.25 |
-40.74 |
Spinocerebellar ataxia 7 (SCA7) |
164500 |
AD |
|
13 |
CE |
4 |
Aracati |
-4.56 |
-37.77 |
Cutaneous CYLD syndrome |
NI |
AD |
|
14 |
GO |
4 |
Araras/ Faina village |
-22.36 |
-47.38 |
Xeroderma pigmentosum, complementation group D (XPD) |
278730 |
AR |
|
15 |
MA |
4 |
Cururupu/ Ilha dos Lençóis |
-1.83 |
-44.86 |
Albinism, oculocutaneous |
203200 |
AR |
|
16 |
MA |
4 |
Cajari/ Regada district |
-3.30 |
-44.88 |
Thalidomide embryopathy |
NI |
E |
|
17 |
MG |
4 |
Minas Gerais |
NI |
NI |
Acheiropodia |
200500 |
AR |
|
18 |
MG |
4 |
Pouso Alegre/ São José do Pântano |
-22.23 |
-45.94 |
Neu–Laxova syndrome (NLS) |
256520 |
AR |
|
19 |
MG |
4 |
Alfenas |
-21.42 |
-45.95 |
Oral clefts |
119530 |
M |
|
20 |
MG |
3 |
Bueno Brandão |
-22.44 |
-46.35 |
Osteogenesis imperfecta, type VI |
613982 |
AR |
|
21 |
MG |
3 |
Diamantina |
NI |
NI |
Enamel renal syndrome |
204690 |
AR |
|
22 |
MG |
4 |
Ervália |
-20.84 |
-42.65 |
Huntington’s disease |
143100 |
AD |
|
23 |
PB |
4 |
Lagoa |
-6.67 |
-35.36 |
Consanguinity with increased prevalence of disabilities (mental or physical) |
NI |
M |
|
24 |
PB |
3 |
Gado Bravo |
-6.73 |
-37.67 |
Usher syndrome |
NI |
AR |
|
25 |
PB |
4 |
Alagoa Nova, Cabeceiras, and Taperoa |
-7.07 |
-35.76 |
Mucopolysaccharidosis type IIIC |
252930 |
AR |
|
26 |
PB |
4 |
Campina Grande |
NI |
NI |
Mucopolysaccharidosis type IVA |
253000
|
AR |
|
27 |
PE |
4 |
Fernando de Noronha |
-3.84 |
-32.41 |
Alzheimer’s disease |
NI |
AR |
|
28 |
PE |
4 |
Orobó |
-7.74 |
-35.60 |
Laron syndrome |
262500 |
AR |
|
29 |
PE |
4 |
Brazil/ Recife |
-8.05 |
-34.90 |
Microcephaly by Zika virus |
NI |
E |
|
30 |
PE |
3 |
Gameleira |
-8.58 |
-35.39 |
Verma–Namouff Syndrome |
613091 |
AR |
|
31 |
PR |
4 |
Paraná |
NI |
NI |
Adrenocorticalcarcinoma, hereditary (ADCC) |
202300 |
AD |
|
32 |
PR |
4 |
Mangueirinha/ Reserva Kaingang |
-25.95 |
-52.19 |
Rheumatoid arthritis (RA) |
180300 |
M |
|
33 |
PR |
4 |
Curitiba and South Brazil |
NI |
NI |
p.R337H mutation in TP53 locus |
NI |
M |
|
34 |
RS |
4 |
Colônia Witmarsum, Palmeira (PR) |
NI |
NI |
Skin cancer in Mennonite communities |
NI |
M |
|
35 |
RJ |
4 |
Rio de Janeiro |
-22.91 |
-43.20 |
Breast cancer |
NI |
M |
|
36 |
RJ |
4 |
Duque de Caxias |
-22.78 |
-43.31 |
Periodontitis, aggressive 1 |
170650 |
AR |
|
37 |
RN |
4 |
São Miguel |
-6.22 |
-38.50 |
Lipodystrophy, congenital generalized, type 2 (CGL2) |
269700 |
AR |
|
38 |
RN |
4 |
Riacho de Santana |
-6.26 |
-38.32 |
Santos syndrome |
613005 |
AR |
|
39 |
RN |
4 |
Serrinha dos Pintos |
-6.20 |
-37.99 |
Spastic paraplegia, optic atrophy, and neuropathy (SPOAN) |
609541 |
AR |
|
40 |
RN |
4 |
Seridó territory (Carnaúba dos Dantas and Timbaúba dos Batistas) |
-6.55 |
-36.59 |
Berardinelli–Seip congenital lipodystrophy |
NI |
AR |
|
41 |
RS |
4 |
Geographically dispersed |
NI |
NI |
Breast and ovarian cancer, familial |
604370 |
AD |
|
42 |
RS |
4 |
Grande Porto Alegre |
-30.03 |
-51.23 |
GM1-gangliosidosis, type I |
230500 |
AR |
|
43 |
RS |
3 |
Geographically dispersed |
NI |
NI |
Machado Joseph disease (MJD) |
109150 |
AD |
|
44 |
RS |
4 |
Cândido Godói |
-27.95 |
-54.77 |
Twinning |
NI |
M |
|
45 |
RS |
4 |
Colônia Nova, Aceguá |
NI |
NI |
Skin cancer in Mennonite communities |
NI |
M |
|
46 |
SC |
4 |
Criciúma |
-28.68 |
-49.37 |
Growth hormone insensitivity with immunodeficiency |
245590 |
AR |
|
47 |
SC |
4 |
Coastal region (Itajaí) |
-26.90 |
-48.66 |
Spinocerebellar ataxia 10 (SCA10) |
603516 |
AD |
|
48 |
SE |
4 |
Itabaianinha |
-11.27 |
-37.79 |
Isolated growth hormone deficiency, type IA (IGHD1A) |
262400 |
AR |
|
49 |
SE |
4 |
Itabaiana |
-10.69 |
-37.42 |
Spectrum of pubertal delay |
NI |
AR |
|
50 |
SP |
4 |
São Paulo |
-23.53 |
-46.62 |
Breast and ovarian cancer |
NI |
M |
|
51 |
SP |
4 |
Indaiatuba |
-23.09 |
-47.21 |
Dandy–Walker syndrome (DWS) |
220200 |
AR |
|
52 |
SP |
4 |
Vinhedo |
-23.03 |
-46.98 |
Fraser syndrome 1 |
219000 |
AR |
|
53 |
SP |
4 |
Ribeirão Preto |
-21.18 |
-47.82 |
Gomez–Lopez–Hernandez syndrome (GLHS) |
601853 |
AR |
|
54 |
SP |
4 |
Campinas |
-22.91 |
-47.06 |
GAPO syndrome |
NI |
NI |
|
55 |
SP |
4 |
São Paulo |
-23.53 |
-46.62 |
Amyotrophic lateral sclerosis 8 (ALS8) |
NI |
AD |
|
56 |
SP |
3 |
Jacupiranga/ Vale do Ribeira |
-24.70 |
-48.01 |
Hypertension and consanguinity |
145500 |
AR |
|
57 |
SP |
4 |
São Paulo |
-23.53 |
-46.62 |
Isolated growth hormone deficiency |
NI |
AR |
|
58 |
SP |
3 |
Vale do Ribeira |
NI |
NI |
Obesity and consanguinity |
601665 |
M |
|
59 |
SP |
4 |
São Paulo |
-23.53 |
-46.62 |
Progressive muscular dystrophy |
NI |
X |
|
60 |
SP |
4 |
São Paulo |
-23.53 |
-46.62 |
R337H Mutation in TP53 gene in adrenocortical tumors |
NI |
M |
|
61 |
SP |
4 |
São Paulo |
-23.53 |
-46.62 |
Richieri–Costa–Pereira syndrome |
268305 |
AR |
|
62 |
SP |
4 |
Ribeirão Preto |
-21.18 |
-47.82 |
Spinocerebellar ataxia 1 (SCA1) |
164400 |
AD |
|
63 |
SP/MG |
4 |
Mococa e Guaxupe |
-21.47 |
-47.00 |
Multiple endocrine neoplasia type 1 (MEN1 |
131100 |
AD |
|
64 |
- |
4 |
South and southeast of Brazil |
NI |
NI |
Li–Fraumeni syndrome type 1 (LFS1) |
151623 |
AD |
|
65 |
- |
4 |
Geographically dispersed (Northeast of Brazil) |
NI |
NI |
Familial chylomicronemia syndrome |
612757 |
NI |
AD, autosomal dominant; AR, autosomal recessive; M, multifactorial; E, environmental; X, X-linked; NI, not identified; MIM, Mendelian inheritance in man; UF, Federative Units; AL, Alagoas; BA, Bahia; CE, Ceara; GO, Goiás; MA, Maranhão; MG, Minas Gerais; PB, Paraíba; PE, Pernambuco; PR, Paraná; RS, Rio Grande do Sul; RJ, Rio de Janeiro; RN, Rio Grande do Norte; SC, Santa Catarina; SE, Sergipe; SP; São Paulo.
Source: Table prepared by the authors. Data from Cardoso et al. (5) and from a(69); b(70); c(71); d(72); e(73); f(74).
DISCUSSION
The 122 clusters of rare diseases or congenital anomalies were reported in almost all South American countries. The multiplicity of peoples with diverse ancestry and cultural patterns, in combination with the wide range of natural environments, has allowed the creation of a complex scenario of clusters in South America, similar to what we have described for Brazil (4, 5). The population history and diversity have important medical genetic implications (12).
Most of the South American clusters were located in Brazil, consistent with it being the largest and most populous country, and its remarkable tradition in the study of communities with a high concentration of genetic diseases or their risk factors. In fact, some of the oldest works found by this review, published from the 1950s onwards, date back to the pioneering work of the Brazilian researcher Newton Freire-Maia, who greatly contributed to the studies of inbreeding, genetic diseases, and genetic isolates (13, 14).
In addition, Brazil has a national census (the CENISO) to map clusters by the National Institute of Population Medical Genetics (or INAGEMP). INAGEMP was created in 2008 supported by the Federal Government, with its headquarters located at the Hospital de Clínicas de Porto Alegre (HCPA) in Southern Brazil, with several associated institutions across the country (1). The cluster scenario in Brazil has been specifically discussed in previous works (4, 5) and from now on we will focus on the other South American countries.
Half of the South American clusters corresponded to autosomal recessive diseases, strongly associated with endogamy and consanguinity. Other works describing cluster sets or similar worldwide have obtained the same results (3, 5, 7, 15). For example, Charoute et al. (15) set a database of 219 Mendelian diseases caused by founder mutations across the Mediterranean basin (in which many clusters of different genetic diseases have been reported), of which 61.7% were autosomal recessive (15).
In this work, we have described some communities with more than one genetic disease; in other words, regions equivalent to “multi-clusters” (16–20). For instance, Antioquia, in northwestern Colombia, represented the most extreme example of a multi-cluster. With six identified clusters of Mendelian disorders (three autosomal recessive and three autosomal dominant), the population from Antioquia was established in the 16th–17th century through the admixture of Native Americans, Europeans (mainly Spanish), and Africans and grew in relative isolation until the late 19th century (21–26).
The Antioquian population has an Amerindian–Caucasian admixture with heterogeneous and specific patterns of sex-biased gene flow, experiencing cultural and geographical isolation from the total Colombian population (3, 27). With large and multigenerational genealogies, the Antioquian population can be compared to Finland, another classic example of a genetic isolate with multi-founder effects, in terms of potential contribution to studies regarding mapping genetic diseases and complex traits (3).
In another example, in the native people from Providencia Island (about 3 400 individuals), Colombia, at least 17 individuals were diagnosed with congenital deafness with two distinct genetic etiologies. A non-syndromic genetic deafness (MIM #220290; 35delG genetic variant in the GJB2 gene), found among individuals with Caucasian origin; and Waardenburg syndrome, found in families with African ancestry. Therefore, the authors argued that this finding was a “direct consequence of the multi-ethnic history of the island” (19).
Islands constitute a model of geographic isolation and, sometimes, with a small population showing high levels of inbreeding. Other studies in South America reported clusters in island communities, such as in the Macanao Peninsula of Margarita Island, Venezuela (Usher syndrome, #276900; cleft lip/palate-ectodermal dysplasia syndrome, #225060; and L-2-hydroxyglutaric aciduria, #236792) (16–18, 20); and Robinson Crusoe (language impairment, MIM #602081) and Chiloe Islands (chondrocalcinosis, #600668), in Chile (28–30). As they are generally derived from a few founding families and exposed to similar environmental factors, these populations also constitute a valuable source of information for the study of complex characteristics (3, 30).
Another important model of spatial and cultural isolation in South America is constituted by the native communities, as the Amazon Rainforest is home to some of the most isolated human groups in the world, many of which have remained relatively unknown until very recent times (31). The forest basin encompasses 7 000 000 km2 (2 700 000 square miles), with a territory belonging to nine nations and 3 344 formally acknowledged Indigenous territories. Some of these territories concentrate many small ancestral communities that are reciprocally isolated by both cultural (linguistic) and geographical barriers (32–34).
For instance, Manno et al. (34) have described a high prevalence of chitotriosidase deficiency (MIM #614122) among small, isolated Amerindian populations from Peru, in association with a very high frequency of 24-base pair duplication in CHIT1 gene (34). In other work, Landires et al. (35) reported the first Amerindian family with calpain 3-related, limb-girdle muscular dystrophy type r1 (MIM #253600) from an isolated, consanguineous, Indigenous community in Colombia (35). Affected people presented a novel deletion of four base pairs in CAPN3. Amerindian ethnic background was associated with high birth prevalence rates of cleft lip with or without cleft palate in clusters from Argentina, Bolivia, and Ecuador (36).
However, important bioethical issues restrict the development of studies (and, sometimes, any other type of contact) with native communities (33, 37). This helps to explain the scarcity of studies reporting clusters in communities from the North region in Brazil, which is sparsely populated by people with a strong Native American component, many of them in isolated or semi-isolated communities (5). Besides the Amazon Rainforest, other South American landscapes associated with clusters were related to high altitudes areas, which were hypothesized to be associated with the concentration of microtia in Quito, Ecuador, (38) and oral clefts in different places on the continent (36).
In addition, some clusters in South America occurred in communities whose origin is related to the escape from the slavery regime, which was established throughout Latin America based on the trafficking of Africans from the 16th century, such as concentration of spinocerebellar ataxias type 7 (MIM #164500) in Yaracuy state and β-thalassemia among Bushinengue Maroon people on the French Guiana–Suriname border (19, 39, 40). In Brazil, some studies have shown a high frequency of hemoglobinopathies and/or genetic variants related to them among these communities, commonly known as quilombos (41, 42). In recent work, we have found high rates of isonymy, congenital anomalies at birth, and clusters of genetic diseases in some places within the historic limits of Quilombo dos Palmares in the Brazilian Northeast, the largest conglomerate of escaped slaves in Latin America (43).
It is known that many of these clusters reported here (and mainly those not reported in the scientific literature) occur in regions with multiple social and health vulnerabilities. The concentration of many cases of uncommon, complex diseases, which are sometimes related to prejudice and social exclusion, can affect patients, their families, and community in multiple ways, sometimes requiring the reorganization of health care adjusted to the reality of each location. Therefore, mapping of clusters can contribute to the design of health policies, focusing on health promotion and equity (6, 44).
In terms of cluster detection and public health in South America, work using data from hospitals registered by the ECLAMC (Latin-American Collaborative Study of Congenital Malformations) network deserves to be highlighted, as many clusters of congenital anomalies (mainly, oral clefts) have been described there (36, 45–47). For instance, Gili et al. (47) applied spatial scan analysis in order to identify clusters from clinical epidemiological data by ECLAMC. With this approach, they have described five high birth prevalence rate regions associated with five congenital anomalies in South America. An additional study has investigated risk factors related to these (47).
The timely detection of these clusters of congenital anomalies can allow the identification of risk or etiological factors, mitigation of damages, and prevention of new potential cases. For this, different surveillance programs for congenital anomalies, such as the ECLAMC network, have alarms to systematically observe the fluctuations in the frequencies of different birth defects from birth registries (36, 48).
In fact, there is a growing interest in the subject of congenital anomalies and rare diseases across the South American continent. In South America, many countries promote the surveillance of congenital anomalies at the local and national levels, in addition to collaborating with international networking initiatives, such as the Latin American Network on Congenital Malformations (RELAMC) and the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR) (48).
Therefore, this work reinforces the importance of population medical genetics in the public health debate, as mapping clusters may support agendas such as health promotion and surveillance. It is important to consider that clusters published in the scientific literature may not have been captured by our search strategy, and this is a potential limitation of our work. However, in addition to constantly reviewing the scientific literature, we have mapped clusters in Brazil based on rumors; that is, by the report (based or not on evidence) of anyone about the possible presence of clusters, by filling out an online form: https://www.inagemp.bio.br/ceniso/. In the same link, it is possible to report populations in South America in four different languages. This can be an initial step toward creating a continental census of clusters of rare disorders and congenital anomalies in Latin America.
Footnotes
Author contributions.
LSF conceived the original idea. ACCS and GR planned the study, collected the data, analyzed the data, and interpreted the results. ACCS wrote the paper, and all authors reviewed it. All authors reviewed and approved the final version.
Conflict of interest.
None declared.
Disclaimer.
Authors hold sole responsibility for the views expressed in the manuscript, which may not necessarily reflect the opinion or policy of the RPSP/ PAHPH and/or the Pan American Health Organization (PAHO).
REFERENCIAS
- 1.Giugliani R, Bender F, Couto R, Bochernitsan A, Brusius-Facchin AC, Burin M, et al. Population Medical Genetics: Translating Science to the Community. Genet Mol Biol. 2019;;(suppl 1):42. doi: 10.1590/1678-4685-GMB-2018-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]; Giugliani R, Bender F, Couto R, Bochernitsan A, Brusius-Facchin AC, Burin M, et al. Population Medical Genetics: Translating Science to the Community. Genet Mol Biol. 2019;42(suppl 1). [DOI] [PMC free article] [PubMed]
- 2.Poletta FA, Orioli IM, Castilla EE. Genealogical data in population medical genetics: field guidelines. Genet Mol Biol. 2014;37(suppl 1):171–85. doi: 10.1590/s1415-47572014000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Poletta FA, Orioli IM, Castilla EE. Genealogical data in population medical genetics: field guidelines. Genet Mol Biol. 2014;37:171–85 (suppl 1). [DOI] [PMC free article] [PubMed]
- 3.Arcos-Burgos M, Muenke M. Genetics of population isolates. [cited 2022 Mar 1];Clin Genet. 2002 61(4):233–47. doi: 10.1034/j.1399-0004.2002.610401.x. Available from: https://pubmed.ncbi.nlm.nih.gov/12030885/ [DOI] [PubMed] [Google Scholar]; Arcos-Burgos M, Muenke M. Genetics of population isolates. Clin Genet. 2002;61(4):233–47 [cited 2022 Mar 1]. Available from: https://pubmed.ncbi.nlm.nih.gov/12030885/ [DOI] [PubMed]
- 4.Castilla EE, Schuler-Faccini L. From rumors to genetic isolates. [cited 2019 Jan 4];Genet Mol Biol. 2014; 37(1) suppl 1:186–93. doi: 10.1590/s1415-47572014000200005. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-47572014000200005&lng=pt&tlng=pt. [DOI] [PMC free article] [PubMed] [Google Scholar]; Castilla EE, Schuler-Faccini L. From rumors to genetic isolates. Genet Mol Biol. 2014;37(1 suppl 1):186–93 [cited 2019 Jan 4]. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-47572014000200005&lng=pt&tlng=pt [DOI] [PMC free article] [PubMed]
- 5.Cardoso GC, de Oliveira MZ, Paixão-Côrtes VR, Castilla EE, Schuler-Faccini L. Clusters of genetic diseases in Brazil. [cited 2020 Feb 18];J Community Genet. 2019 10(1):121–8. doi: 10.1007/s12687-018-0369-1. Available from: http://link.springer.com/10.1007/s12687-018-0369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cardoso GC, de Oliveira MZ, Paixão-Côrtes VR, Castilla EE, Schuler-Faccini L. Clusters of genetic diseases in Brazil. J Community Genet. 2019;10(1):121–8 [cited 2020 Feb 18]. Available from: http://link.springer.com/10.1007/s12687-018-0369-1 [DOI] [PMC free article] [PubMed]
- 6.Passos-Bueno MR, Bertola D, Dain D, Horovitz G, Evangelista De Faria Ferraz V, Brito LA. Genetics and genomics in Brazil: a promising future. [cited 2019 Aug 18];Mol Genet Genomic Med. 2014 2(4):280–291. doi: 10.1002/mgg3.95. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4113268/pdf/mgg30002-0280.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]; Passos-Bueno MR, Bertola D, Dain D, Horovitz G, Evangelista De Faria Ferraz V, Brito LA. Genetics and genomics in Brazil: a promising future. Mol Genet Genomic Med. 2014;2(4):280–291 [cited 2019 Aug 18]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4113268/pdf/mgg30002-0280.pdf [DOI] [PMC free article] [PubMed]
- 7.Peltonen L, Palotie A, Lange K. Use of population isolates for mapping complex traits. [cited 2022 Mar 1];Nat Rev Genet. 2000 1:182–190. doi: 10.1038/35042049. Available from: https://www.nature.com/articles/35042049. [DOI] [PubMed] [Google Scholar]; Peltonen L, Palotie A, Lange K. Use of population isolates for mapping complex traits. Nat Rev Genet. 2000;1:182–190 [cited 2022 Mar 1]. Available from: https://www.nature.com/articles/35042049 [DOI] [PubMed]
- 8.Castilla EE, Adams J. Genealogical Information and the Structure of Rural Latin-American Populations: Reality and Fantasy. [cited 2022 Mar 1];Hum Hered. 1996 46(5):241–55. doi: 10.1159/000154361. Available from: https://www.karger.com/Article/FullText/154361. [DOI] [PubMed] [Google Scholar]; Castilla EE, Adams J. Genealogical Information and the Structure of Rural Latin-American Populations: Reality and Fantasy. Hum Hered. 1996;46(5):241–55 [cited 2022 Mar 1]. Available from: https://www.karger.com/Article/FullText/154361 [DOI] [PubMed]
- 9.Salzano FM, Sans M. Interethnic admixture and the evolution of Latin American populations. [cited 2022 Mar 2];Genet Mol Biol. 2014 37(Suppl. 1):151–70. doi: 10.1590/s1415-47572014000200003. Available from: http://www.scielo.br/j/gmb/a/4zvRkCTfqdpTSHSY7K3GgLm/abstract/?lang=en. [DOI] [PMC free article] [PubMed] [Google Scholar]; Salzano FM, Sans M. Interethnic admixture and the evolution of Latin American populations. Genet Mol Biol. 2014;37(Suppl. 1):151–70 [cited 2022 Mar 2]. Available from: http://www.scielo.br/j/gmb/a/4zvRkCTfqdpTSHSY7K3GgLm/abstract/?lang=en [DOI] [PMC free article] [PubMed]
- 10.Wexler NS. Huntington’s Disease: Advocacy Driving Science. [cited 2022 Mar 1];Ann Rev Med. 2012 63:1–22. doi: 10.1146/annurev-med-050710-134457. Available from: https://www.annualreviews.org/doi/abs/10.1146/annurev-med-050710-134457. [DOI] [PubMed] [Google Scholar]; Wexler NS. Huntington’s Disease: Advocacy Driving Science. Ann Rev Med. 2012;63:1–22 [cited 2022 Mar 1]. Available from: https://www.annualreviews.org/doi/abs/10.1146/annurev-med-050710-134457 [DOI] [PubMed]
- 11.Cock PJA, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. [cited 2022 Mar 6];Bioinformatics. 2009 25(11):1422–3. doi: 10.1093/bioinformatics/btp163. Available from: https://academic.oup.com/bioinformatics/article/25/11/1422/330687. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cock PJA, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25(11):1422–3 [cited 2022 Mar 6]. Available from: https://academic.oup.com/bioinformatics/article/25/11/1422/330687 [DOI] [PMC free article] [PubMed]
- 12.Laberge AM, Michaud J, Richter A, Lemyre E, Lambert M, Brais B, et al. Population history and its impact on medical genetics in Quebec. [cited 2022 Mar 2];Clin Genet. 2005 68(4):287–301. doi: 10.1111/j.1399-0004.2005.00497.x. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1399-0004.2005.00497.x. [DOI] [PubMed] [Google Scholar]; Laberge AM, Michaud J, Richter A, Lemyre E, Lambert M, Brais B, et al. Population history and its impact on medical genetics in Quebec. Clin Genet. 2005;68(4):287–301 [cited 2022 Mar 2]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1399-0004.2005.00497.x [DOI] [PubMed]
- 13.Freire-Maia N. Inbreeding in Brazil. Am J Hum Genet. 1957;9(4):284–98. [PMC free article] [PubMed] [Google Scholar]; Freire-Maia N. Inbreeding in Brazil. Am J Hum Genet. 1957;9(4):284–98. [PMC free article] [PubMed]
- 14.Freire-Maia A, Opitz JM. Historical note: The extraordinary handless and footless families of Brazil – 50 years of acheiropodia. [cited 2022 Mar 1];Am J Med Genet. 1981 9(1):31–41. doi: 10.1002/ajmg.1320090108. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.1320090108. [DOI] [PubMed] [Google Scholar]; Freire-Maia A, Opitz JM. Historical note: The extraordinary handless and footless families of Brazil – 50 years of acheiropodia. Am J Med Genet. 1981;9(1):31–41 [cited 2022 Mar 1]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.1320090108 [DOI] [PubMed]
- 15.Charoute H, Bakhchane A, Benrahma H, Romdhane L, Gabi K, Rouba H, et al. Mediterranean Founder Mutation Database (MFMD): Taking Advantage from Founder Mutations in Genetics Diagnosis, Genetic Diversity and Migration History of the Mediterranean Population. [cited 2022 Mar 1];Hum Mutat. 2015 36(11):E2441–53. doi: 10.1002/humu.22835. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/humu.22835. [DOI] [PubMed] [Google Scholar]; Charoute H, Bakhchane A, Benrahma H, Romdhane L, Gabi K, Rouba H, et al. Mediterranean Founder Mutation Database (MFMD): Taking Advantage from Founder Mutations in Genetics Diagnosis, Genetic Diversity and Migration History of the Mediterranean Population. Hum Mutat. 2015;36(11):E2441–53 [cited 2022 Mar 1]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/humu.22835 [DOI] [PubMed]
- 16.Bustos T, Simosa V, Pinto-Cisternas J, Abramovits W, Jolay L, Rodriguez L, et al. Autosomal recessive ectodermal dysplasia: I. An undescribed dysplasia/malformation syndrome. [cited 2022 Mar 6];Am J Med Genet. 1991 41(4):398–404. doi: 10.1002/ajmg.1320410403. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.1320410403. [DOI] [PubMed] [Google Scholar]; Bustos T, Simosa V, Pinto-Cisternas J, Abramovits W, Jolay L, Rodriguez L, et al. Autosomal recessive ectodermal dysplasia: I. An undescribed dysplasia/malformation syndrome. Am J Med Genet. 1991;41(4):398–404 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.1320410403 [DOI] [PubMed]
- 17.Suzuki K, Bustos T, Spritz RA. Linkage Disequilibrium Mapping of the Gene for Margarita Island Ectodermal Dysplasia (ED4) to 11q23. Am J Human Genet. 1998;63(4):1102–7. doi: 10.1086/302072. [DOI] [PMC free article] [PubMed] [Google Scholar]; Suzuki K, Bustos T, Spritz RA. Linkage Disequilibrium Mapping of the Gene for Margarita Island Ectodermal Dysplasia (ED4) to 11q23. Am J Human Genet. 1998;63(4):1102–7. [DOI] [PMC free article] [PubMed]
- 18.Keogh IJ, Godinho RN, Wu TP, de Palacios AMD, Palacios N, de Alford MB, et al. Clinical and genetic linkage analysis of a large Venezuelan kindred with Usher syndrome. Int J Pediatr Otorhinolaryngol. 2004;68(8):1063–8. doi: 10.1016/j.ijporl.2004.04.005. [DOI] [PubMed] [Google Scholar]; Keogh IJ, Godinho RN, Wu TP, de Palacios AMD, Palacios N, de Alford MB, et al. Clinical and genetic linkage analysis of a large Venezuelan kindred with Usher syndrome. Int J Pediatr Otorhinolaryngol. 2004;68(8):1063–8. [DOI] [PubMed]
- 19.Lattig M, Gelvez N, Plaza S, Tamayo G, Uribe J, Salvatierra I, et al. Deafness on the island of Providencia - Colombia: different etiology, different genetic counseling. Genet Couns. 2008;19(4):403–12. [PubMed] [Google Scholar]; Lattig M, Gelvez N, Plaza S, Tamayo G, Uribe J, Salvatierra I, et al. Deafness on the island of Providencia - Colombia: different etiology, different genetic counseling. Genet Couns. 2008;19(4):403–12. [PubMed]
- 20.Sass JO, Jobard F, Topçu M, Mahfoud A, Werlé E, Cure S, et al. L-2-hydroxyglutaric aciduria: identification of ten novel mutations in the L2HGDH gene. J Inherit Metab Dis. 2008. [cited 2022 Mar 6]. p. 31. Available from: https://pubmed.ncbi.nlm.nih.gov/18415700/ [DOI] [PubMed]; Sass JO, Jobard F, Topçu M, Mahfoud A, Werlé E, Cure S, et al. L-2-hydroxyglutaric aciduria: identification of ten novel mutations in the L2HGDH gene. J Inherit Metab Dis. 2008;31(Suppl 2) [cited 2022 Mar 6]. Available from: https://pubmed.ncbi.nlm.nih.gov/18415700/ [DOI] [PubMed]
- 21.Pineda-Trujillo N, Carvajal-Carmona LG, Buriticá O, Moreno S, Uribe C, Pineda D, et al. A novel Cys212Tyr founder mutation in parkin and allelic heterogeneity of juvenile Parkinsonism in a population from North West Colombia. Neurosci Lett. 2001;298(2):87–90. doi: 10.1016/s0304-3940(00)01733-x. [DOI] [PubMed] [Google Scholar]; Pineda-Trujillo N, Carvajal-Carmona LG, Buriticá O, Moreno S, Uribe C, Pineda D, et al. A novel Cys212Tyr founder mutation in parkin and allelic heterogeneity of juvenile Parkinsonism in a population from North West Colombia. Neurosci Lett. 2001;298(2):87–90. [DOI] [PubMed]
- 22.Ramírez-Castro JL, Pineda-Trujillo N, Valencia A v., Muñetón CM, Botero O, Trujillo O, et al. Mutations in FOXL2 underlying BPES (types 1 and 2) in Colombian families. [cited 2022 Mar 2];Am J Med Genet. 2002 113(1):47–51. doi: 10.1002/ajmg.10741. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.10741. [DOI] [PubMed] [Google Scholar]; Ramírez-Castro JL, Pineda-Trujillo N, Valencia A v., Muñetón CM, Botero O, Trujillo O, et al. Mutations in FOXL2 underlying BPES (types 1 and 2) in Colombian families. Am J Med Genet. 2002;113(1):47–51 [cited 2022 Mar 2]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.10741 [DOI] [PubMed]
- 23.Nikali K, Vanegas JJ, Burley MW, Martinez J, Lopez LM, Bedoya G, et al. Extensive founder effect for distal renal tubular acidosis (dRTA) with sensorineural deafness in an isolated South American population. [cited 2022 Mar 2];Am J Med Genet. 2008 146A(20):2709–12. doi: 10.1002/ajmg.a.32495. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.32495. [DOI] [PubMed] [Google Scholar]; Nikali K, Vanegas JJ, Burley MW, Martinez J, Lopez LM, Bedoya G, et al. Extensive founder effect for distal renal tubular acidosis (dRTA) with sensorineural deafness in an isolated South American population. Am J Med Genet. 2008;146A(20):2709–12 [cited 2022 Mar 2]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.32495 [DOI] [PubMed]
- 24.Lopera F, Ardilla A, Martínez A, Madrigal L, Arango-Viana JC, Lemere CA, et al. Clinical Features of Early-Onset Alzheimer Disease in a Large Kindred With an E280A Presenilin-1 Mutation. [cited 2022 Mar 2];JAMA. 1997 277(10):793–9. Available from: https://jamanetwork.com/journals/jama/fullarticle/414555. [PubMed] [Google Scholar]; Lopera F, Ardilla A, Martínez A, Madrigal L, Arango-Viana JC, Lemere CA, et al. Clinical Features of Early-Onset Alzheimer Disease in a Large Kindred With an E280A Presenilin-1 Mutation. JAMA. 1997;277(10):793–9 [cited 2022 Mar 2]. Available from: https://jamanetwork.com/journals/jama/fullarticle/414555 [PubMed]
- 25.Alonso-Espinaco V, Giráldez MD, Trujillo C, van der Klift H, Muñoz J, Balaguer F, et al. Novel MLH1 duplication identified in Colombian families with Lynch syndrome. [cited 2022 Mar 2];Genet Med. 2011 13(2):155–60. doi: 10.1097/GIM.0b013e318202e10b. Available from: http://www.gimjournal.org/article/S1098360021040703/fulltext. [DOI] [PubMed] [Google Scholar]; Alonso-Espinaco V, Giráldez MD, Trujillo C, van der Klift H, Muñoz J, Balaguer F, et al. Novel MLH1 duplication identified in Colombian families with Lynch syndrome. Genet Med. 2011;13(2):155–60 [cited 2022 Mar 2]. Available from: http://www.gimjournal.org/article/S1098360021040703/fulltext [DOI] [PubMed]
- 26.Montoya JH, Morales OL. Four cases of Jarcho-Levin’s syndrome in the province of Antioquia, Colombia. [cited 2022 Mar 2];Biomedica. 2009 29(1):25–32. Available from: https://revistabiomedica.org/index.php/biomedica/article/view/38. [PubMed] [Google Scholar]; Montoya JH, Morales OL. Four cases of Jarcho-Levin’s syndrome in the province of Antioquia, Colombia. Biomedica. 2009;29(1):25–32 [cited 2022 Mar 2]. Available from: https://revistabiomedica.org/index.php/biomedica/article/view/38 [PubMed]
- 27.Mooney JA, Huber CD, Service S, Sul JH, Marsden CD, Zhang Z, et al. Understanding the Hidden Complexity of Latin American Population Isolates. [cited 2022 Mar 1];Am J Hum Genet. 2018 103(5):707–26. doi: 10.1016/j.ajhg.2018.09.013. Available from: http://www.cell.com/article/S0002929718303513/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mooney JA, Huber CD, Service S, Sul JH, Marsden CD, Zhang Z, et al. Understanding the Hidden Complexity of Latin American Population Isolates. Am J Hum Genet. 2018;103(5):707–26 [cited 2022 Mar 1]. Available from: http://www.cell.com/article/S0002929718303513/fulltext [DOI] [PMC free article] [PubMed]
- 28.Reginato AJ, Hollander JL, Martinez V, Valenzuela F, Schiapachasse V, Covarrubias E, et al. Familial chondrocalcinosis in the Chiloe Islands, Chile. [cited 2022 Mar 6];Ann Rheum Dis. 1975 34(3):260–8. doi: 10.1136/ard.34.3.260. Available from: https://ard.bmj.com/content/34/3/260. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reginato AJ, Hollander JL, Martinez V, Valenzuela F, Schiapachasse V, Covarrubias E, et al. Familial chondrocalcinosis in the Chiloe Islands, Chile. Ann Rheum Dis. 1975;34(3):260–8 [cited 2022 Mar 6]. Available from: https://ard.bmj.com/content/34/3/260 [DOI] [PMC free article] [PubMed]
- 29.Villanueva P, de Barbieri Z, Palomino HM, Palomino H. Alta prevalencia de trastorno específico de lenguaje en isla Robinson Crusoe y probable efecto fundador. [cited 2022 Mar 6];Rev Med Chil. 2008 136(2):186–92. Available from: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0034-98872008000200007&lng=es&nrm=iso&tlng=es. [PubMed] [Google Scholar]; Villanueva P, de Barbieri Z, Palomino HM, Palomino H. Alta prevalencia de trastorno específico de lenguaje en isla Robinson Crusoe y probable efecto fundador. Rev Med Chil. 2008;136(2):186–92 [cited 2022 Mar 6]. Available from: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0034-98872008000200007&lng=es&nrm=iso&tlng=es [PubMed]
- 30.Villanueva P, Newbury DF, Jara L, de Barbieri Z, Mirza G, Palamino HM, et al. Genome-wide analysis of genetic susceptibility to language impairment in an isolated Chilean population. [cited 2022 Mar 6];Eur J Hum Genet. 2011 19(6):687–95. doi: 10.1038/ejhg.2010.251. Available from: https://www.nature.com/articles/ejhg2010251. [DOI] [PMC free article] [PubMed] [Google Scholar]; Villanueva P, Newbury DF, Jara L, de Barbieri Z, Mirza G, Palamino HM, et al. Genome-wide analysis of genetic susceptibility to language impairment in an isolated Chilean population. Eur J Hum Genet. 2011;19(6):687–95 [cited 2022 Mar 6]. Available from: https://www.nature.com/articles/ejhg2010251 [DOI] [PMC free article] [PubMed]
- 31.Cardoso S, Alfonso-Sánchez MA, Valverde L, Sánchez D, Zarrabeitia MT, Odriozola A, et al. Genetic uniqueness of the Waorani tribe from the Ecuadorian Amazon. [cited 2022 Mar 6];Heredity. 2012 108(6):609–15. doi: 10.1038/hdy.2011.131. Available from: https://www.nature.com/articles/hdy2011131. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cardoso S, Alfonso-Sánchez MA, Valverde L, Sánchez D, Zarrabeitia MT, Odriozola A, et al. Genetic uniqueness of the Waorani tribe from the Ecuadorian Amazon. Heredity. 2012;108(6):609–15 [cited 2022 Mar 6]. Available from: https://www.nature.com/articles/hdy2011131 [DOI] [PMC free article] [PubMed]
- 32.Kaplan JE, Larrick JW, Yost JA. Hyperimmunoglobulinemia E in the Waorani, an Isolated Amerindian Population. [cited 2022 Mar 6];Am J Trop Med Hyg. 1980 29(5):1012–7. doi: 10.4269/ajtmh.1980.29.1012. Available from: https://www.ajtmh.org/view/journals/tpmd/29/5/article-p1012.xml. [DOI] [PubMed] [Google Scholar]; Kaplan JE, Larrick JW, Yost JA. Hyperimmunoglobulinemia E in the Waorani, an Isolated Amerindian Population. Am J Trop Med Hyg. 1980;29(5):1012–7 [cited 2022 Mar 6]. Available from: https://www.ajtmh.org/view/journals/tpmd/29/5/article-p1012.xml [DOI] [PubMed]
- 33.Ballew C, Beckerman SJ, Lizarralde R. High Prevalence of Cleft Lip among the Barí Indians of Western Venezuela. [cited 2022 Mar 6];Cleft Palate Craniofac J. 1993 30(4):411–3. doi: 10.1597/1545-1569_1993_030_0411_hpocla_2.3.co_2. Available from: https://journals.sagepub.com/doi/10.1597/1545-1569_1993_030_0411_hpocla_2.3.co_2. [DOI] [PubMed] [Google Scholar]; Ballew C, Beckerman SJ, Lizarralde R. High Prevalence of Cleft Lip among the Barí Indians of Western Venezuela. Cleft Palate Craniofac J. 1993;30(4):411-3 [cited 2022 Mar 6]. Available from: https://journals.sagepub.com/doi/10.1597/1545-1569_1993_030_0411_hpocla_2.3.co_2 [DOI] [PubMed]
- 34.Manno N, Sherratt S, Boaretto F, Coico FM, Camus CE, Campos CJ, et al. High prevalence of chitotriosidase deficiency in Peruvian Amerindians exposed to chitin-bearing food and enteroparasites. Carbohydr Polym. 2014 Nov 26;113:607–14. doi: 10.1016/j.carbpol.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Manno N, Sherratt S, Boaretto F, Coico FM, Camus CE, Campos CJ, et al. High prevalence of chitotriosidase deficiency in Peruvian Amerindians exposed to chitin-bearing food and enteroparasites. Carbohydr Polym. 2014 Nov 26;113:607–14. [DOI] [PMC free article] [PubMed]
- 35.Landires I, Núñez-Samudio V, Fernandez J, Sarria C, Villareal V, Córdoba F, et al. Calpainopathy: Description of a Novel Mutation and Clinical Presentation with Early Severe Contractures. [cited 2022 Mar 6];Genes. 2020 11(2):129. doi: 10.3390/genes11020129. Available from: https://www.mdpi.com/2073-4425/11/2/129/htm. [DOI] [PMC free article] [PubMed] [Google Scholar]; Landires I, Núñez-Samudio V, Fernandez J, Sarria C, Villareal V, Córdoba F, et al. Calpainopathy: Description of a Novel Mutation and Clinical Presentation with Early Severe Contractures. Genes. 2020;11(2):129 [cited 2022 Mar 6]. Available from: https://www.mdpi.com/2073-4425/11/2/129/htm [DOI] [PMC free article] [PubMed]
- 36.Poletta FA, Castilla EE, Orioli IM, Lopez-Camelo JS. Regional analysis on the occurrence of oral clefts in South America. [cited 2022 Mar 2];Am J Med Genet A. 2007 143A(24):3216–27. doi: 10.1002/ajmg.a.32076. Available from: https://pubmed.ncbi.nlm.nih.gov/18000905/ [DOI] [PubMed] [Google Scholar]; Poletta FA, Castilla EE, Orioli IM, Lopez-Camelo JS. Regional analysis on the occurrence of oral clefts in South America. Am J Med Genet A. 2007;143A(24):3216–27 [cited 2022 Mar 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/18000905/ [DOI] [PubMed]
- 37.D’Angelo CS, Hermes A, McMaster CR, Prichep E, Richer E, van der Westhuizen FH, et al. Barriers and Considerations for Diagnosing Rare Diseases in Indigenous Populations. Front Pediatr. 2020;8:797. doi: 10.3389/fped.2020.579924. [DOI] [PMC free article] [PubMed] [Google Scholar]; D’Angelo CS, Hermes A, McMaster CR, Prichep E, Richer E, van der Westhuizen FH, et al. Barriers and Considerations for Diagnosing Rare Diseases in Indigenous Populations. Front Pediatr. 2020;8:797. [DOI] [PMC free article] [PubMed]
- 38.Castilla EE, Orioli IM. Prevalence Rates of Microtia in South America. [cited 2022 Mar 6];Int J Epidemiol. 1986 15(3):364–8. doi: 10.1093/ije/15.3.364. Available from: https://academic.oup.com/ije/article/15/3/364/658691. [DOI] [PubMed] [Google Scholar]; Castilla EE, Orioli IM. Prevalence Rates of Microtia in South America. Int J Epidemiol. 1986;15(3):364–8 [cited 2022 Mar 6]. Available from: https://academic.oup.com/ije/article/15/3/364/658691 [DOI] [PubMed]
- 39.Broquere C, Brudey K, Harteveld CL, Saint-Martin C, Elion J, Giordano PC, et al. Phenotypic Expression and Origin of the Rare β-Thalassemia Splice Site Mutation HBB:c.315 + 1G>T. [cited 2022 Mar 6];Hemoglobin. 2010 34(3):322–6. doi: 10.3109/03630269.2010.484956. Available from: https://www.tandfonline.com/doi/abs/10.3109/03630269.2010.484956. [DOI] [PubMed] [Google Scholar]; Broquere C, Brudey K, Harteveld CL, Saint-Martin C, Elion J, Giordano PC, et al. Phenotypic Expression and Origin of the Rare β-Thalassemia Splice Site Mutation HBB:c.315 + 1G>T. Hemoglobin. 2010;34(3):322–6 [cited 2022 Mar 6]. Available from: https://www.tandfonline.com/doi/abs/10.3109/03630269.2010.484956 [DOI] [PubMed]
- 40.Paradisi I, Arias S. Marked geographic aggregation of acute intermittent porphyria families carrying mutation Q180X in Venezuelan populations, with description of further mutations. [cited 2022 Mar 6];J Inherit Metab Dis. 2010 33(Suppl. 3):455–63. doi: 10.1007/s10545-010-9228-x. Available from: https://onlinelibrary.wiley.com/doi/full/10.1007/s10545-010-9228-x. [DOI] [PubMed] [Google Scholar]; Paradisi I, Arias S. Marked geographic aggregation of acute intermittent porphyria families carrying mutation Q180X in Venezuelan populations, with description of further mutations. J Inherit Metab Dis. 2010;33(Suppl. 3):455–63 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1007/s10545-010-9228-x [DOI] [PubMed]
- 41.Soares LF, Lima EM, da Silva JA, Fernandes SS, Silva KM da C, Lins SP, et al. Prevalence of hemoglobin variants in quilombola communities in the state of Piauí, Brazil. [cited 2022 Mar 5];Cien Saude Colet. 2017 22(11):3773–80. doi: 10.1590/1413-812320172211.04392016. Available from: https://pubmed.ncbi.nlm.nih.gov/29211182/ [DOI] [PubMed] [Google Scholar]; Soares LF, Lima EM, da Silva JA, Fernandes SS, Silva KM da C, Lins SP, et al. [Prevalence of hemoglobin variants in quilombola communities in the state of Piauí, Brazil]. Cien Saude Colet. 2017;22(11):3773–80 [cited 2022 Mar 5]. Available from: https://pubmed.ncbi.nlm.nih.gov/29211182/ [DOI] [PubMed]
- 42.Santiago RP, Oliveira RM, Soares LF, Figueiredo CVB, Silva DO, Hurtado-Guerrero AF, et al. Hemoglobin Variant Profiles among Brazilian Quilombola Communities. [cited 2022 Mar 6];Hemoglobin. 2017 41(2):83–8. doi: 10.1080/03630269.2017.1321014. Available from: https://www.tandfonline.com/doi/abs/10.1080/03630269.2017.1321014. [DOI] [PubMed] [Google Scholar]; Santiago RP, Oliveira RM, Soares LF, Figueiredo CVB, Silva DO, Hurtado-Guerrero AF, et al. Hemoglobin Variant Profiles among Brazilian Quilombola Communities. Hemoglobin. 2017;41(2):83–8 [cited 2022 Mar 6]. Available from: https://www.tandfonline.com/doi/abs/10.1080/03630269.2017.1321014 [DOI] [PubMed]
- 43.Cardoso-Dos-Santos AC, Ramallo V, Zagonel-Oliveira M, Veronez MR, Navarro P, Monlleó IL, et al. An invincible memory: What surname analysis tells us about history, health and population medical genetics in the Brazilian Northeast. [cited 2021 Apr 26];J Biosoc Sci. 2020 53(2):183–98. doi: 10.1017/S0021932020000127. Available from: https://www.cambridge.org/core/journals/journal-of-biosocial-science/article/abs/an-invincible-memory-what-surname-analysis-tells-us-about-history-health-and-population-medical-genetics-in-the-brazilian-northeast/BEAAE4230B47C1BDA2DFE31D1D04CBF1. [DOI] [PubMed] [Google Scholar]; Cardoso-Dos-Santos AC, Ramallo V, Zagonel-Oliveira M, Veronez MR, Navarro P, Monlleó IL, et al. An invincible memory: What surname analysis tells us about history, health and population medical genetics in the Brazilian Northeast. J Biosoc Sci. 2020;53(2):183–98 [cited 2021 Apr 26]. Available from: https://www.cambridge.org/core/journals/journal-of-biosocial-science/article/abs/an-invincible-memory-what-surname-analysis-tells-us-about-history-health-and-population-medical-genetics-in-the-brazilian-northeast/BEAAE4230B47C1BDA2DFE31D1D04CBF1 [DOI] [PubMed]
- 44.Bronberg R, Gili J, Gimenez L, Dipierri J, Lopez Camelo J. Biosocial correlates and spatial distribution of consanguinity in South America. [cited 2022 Mar 6];Am J Hum Biol. 2016 28(3):405–11. doi: 10.1002/ajhb.22802. Available from: https://pubmed.ncbi.nlm.nih.gov/26515926/ [DOI] [PubMed] [Google Scholar]; Bronberg R, Gili J, Gimenez L, Dipierri J, Lopez Camelo J. Biosocial correlates and spatial distribution of consanguinity in South America. Am J Hum Biol. 2016;28(3):405–11 [cited 2022 Mar 6]. Available from: https://pubmed.ncbi.nlm.nih.gov/26515926/ [DOI] [PubMed]
- 45.Orioli IM, Mastroiacovo P, López-Camelo JS, Saldarriaga W, Isaza C, Aiello H, et al. Clusters of sirenomelia in South America. [cited 2022 Mar 6];Birth Defects Res A Clin Mol Teratol. 2009 85(2):112–8. doi: 10.1002/bdra.20492. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/bdra.20492. [DOI] [PubMed] [Google Scholar]; Orioli IM, Mastroiacovo P, López-Camelo JS, Saldarriaga W, Isaza C, Aiello H, et al. Clusters of sirenomelia in South America. Birth Defects Res A Clin Mol Teratol. 2009;85(2):112–8 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/bdra.20492 [DOI] [PubMed]
- 46.Gili JA, Poletta FA, Pawluk M, Gimenez LG, Campaña H, Castilla E, et al. High Birth Prevalence Rates for Congenital Anomalies in South American Regions. [cited 2022 Mar 2];Epidemiology. 2015 26(5):e53–5. doi: 10.1097/EDE.0000000000000345. Available from: https://pubmed.ncbi.nlm.nih.gov/26134350/ [DOI] [PubMed] [Google Scholar]; Gili JA, Poletta FA, Pawluk M, Gimenez LG, Campaña H, Castilla E, et al. High Birth Prevalence Rates for Congenital Anomalies in South American Regions. Epidemiology. 2015;26(5):e53–5 [cited 2022 Mar 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/26134350/ [DOI] [PubMed]
- 47.Gili JA, Poletta FA, Giménez LG, Pawluk MS, Campaña H, Castilla EE, et al. Descriptive analysis of high birth prevalence rate geographical clusters of congenital anomalies in South America. [cited 2022 Mar 1];Birth Defects Res A Clin Mol Teratol. 2016 106(4):257–66. doi: 10.1002/bdra.23481. Available from: https://pubmed.ncbi.nlm.nih.gov/26887535/ [DOI] [PubMed] [Google Scholar]; Gili JA, Poletta FA, Giménez LG, Pawluk MS, Campaña H, Castilla EE, et al. Descriptive analysis of high birth prevalence rate geographical clusters of congenital anomalies in South America. Birth Defects Res A Clin Mol Teratol. 2016;106(4):257–66 [cited 2022 Mar 1]. Available from: https://pubmed.ncbi.nlm.nih.gov/26887535/ [DOI] [PubMed]
- 48.Cardoso-dos-Santos AC, Magalhães VS, Medeiros-de-Souza AC, Bremm J, Alves R, Araujo V, et al. International collaboration networks for the surveillance of congenital anomalies: a narrative review. Revista do Sistema Único de Saúde do Brasil. 2020;29(4):14. doi: 10.5123/s1679-49742020000400003. Available from: https://bit.ly/3vjlScB. [DOI] [PubMed] [Google Scholar]; Cardoso-dos-Santos AC, Magalhães VS, Medeiros-de-Souza AC, Bremm J, Alves R, Araujo V, et al. International collaboration networks for the surveillance of congenital anomalies: a narrative review. Revista do Sistema Único de Saúde do Brasil. 2020;29(4):14. Available from: https://bit.ly/3vjlScB [DOI] [PubMed]
- 49.Laróvere LE, Angaroni CJ, Antonozzi SL, Bezard MB, Shimohama M, Dodelson de Kremer R. Citrullinemia Type I, Classical Variant. Identification of ASS~p.G390R (c.1168G > A) mutation in families of a limited geographic area of Argentina: A possible population cluster. Clin Biochem. 2009;42(10–11):1166–8. doi: 10.1016/j.clinbiochem.2009.03.024. [DOI] [PubMed] [Google Scholar]; Laróvere LE, Angaroni CJ, Antonozzi SL, Bezard MB, Shimohama M, Dodelson de Kremer R. Citrullinemia Type I, Classical Variant. Identification of ASS∼p.G390R (c.1168G > A) mutation in families of a limited geographic area of Argentina: A possible population cluster. Clin Biochem. 2009;42(10–11):1166–8. [DOI] [PubMed]
- 50.Dipierri J, Rodríguez-Larralde A, Barrai I, Camelo JL, Redomero EG, Rodríguez CA, et al. Random inbreeding, isonymy, and population isolates in Argentina. J Community Genet. 2014;5(3):241–8. doi: 10.1007/s12687-013-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dipierri J, Rodríguez-Larralde A, Barrai I, Camelo JL, Redomero EG, Rodríguez CA, et al. Random inbreeding, isonymy, and population isolates in Argentina. J Community Genet. 2014;5(3):241–8. [DOI] [PMC free article] [PubMed]
- 51.Biglione MM, Pizarro M, Puca A, Salomón HE, Berría MI. A Cluster of Human T-Cell Lymphotropic Virus Type I-myelopathy/tropical spastic paraparesis in Jujuy, Argentina. J Acquir Immune Defic Syndr. 2003;32(4):441–5. doi: 10.1097/00126334-200304010-00015. [DOI] [PubMed] [Google Scholar]; Biglione MM, Pizarro M, Puca A, Salomón HE, Berría MI. A Cluster of Human T-Cell Lymphotropic Virus Type I-myelopathy/tropical spastic paraparesis in Jujuy, Argentina. J Acquir Immune Defic Syndr. 2003;32(4):441–5. [DOI] [PubMed]
- 52.Agost L. Tumores renales pediátricos y su posible asociación con poblaciones endogámicas en el centro de Argentina Pediatric renal tumors and their possible relation with endogamous populations in central Argentina. [cited 2022 Mar 2];Rev Cubana Pediatr. 2019 91(1) Available from: http://scielo.sld.cu. [Google Scholar]; Agost L. Tumores renales pediátricos y su posible asociación con poblaciones endogámicas en el centro de Argentina Pediatric renal tumors and their possible relation with endogamous populations in central Argentina. Rev Cubana Pediatr. 2019;91(1) [cited 2022 Mar 2]. Available from: http://scielo.sld.cu
- 53.Brown CA, McInnes B, Dodelson de Kremer R, Mahuran DJ. Characterization of two HEXB gene mutations in Argentinean patients with Sandhoff disease. Biochim Biophys Acta. 1992;1180(1):91–8. doi: 10.1016/0925-4439(92)90031-h. [DOI] [PubMed] [Google Scholar]; Brown CA, McInnes B, Dodelson de Kremer R, Mahuran DJ. Characterization of two HEXB gene mutations in Argentinean patients with Sandhoff disease. Biochim Biophys Acta. 1992;1180(1):91–8. [DOI] [PubMed]
- 54.Silvera-Ruiz SM, Arranz JA, Häberle J, Angaroni CJ, Bezard M, Guelbert N, et al. Urea cycle disorders in Argentine patients: Clinical presentation, biochemical and genetic findings. [cited 2022 Mar 6];Orphanet J Rare Dis. 2019 14(1):1–8. doi: 10.1186/s13023-019-1177-3. Available from: https://ojrd.biomedcentral.com/articles/10.1186/s13023-019-1177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Silvera-Ruiz SM, Arranz JA, Häberle J, Angaroni CJ, Bezard M, Guelbert N, et al. Urea cycle disorders in Argentine patients: Clinical presentation, biochemical and genetic findings. Orphanet J Rare Dis. 2019;14(1):1–8 [cited 2022 Mar 6]. Available from: https://ojrd.biomedcentral.com/articles/10.1186/s13023-019-1177-3 [DOI] [PMC free article] [PubMed]
- 55.Cruz-Coke R, Moreno RS, Aguirre J, Cortes G. Genetic epidemiology of single gene defects in Chile. [cited 2022 Mar 6];J Med Genet. 1994 31(9):702. doi: 10.1136/jmg.31.9.702. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1050080/ [DOI] [PMC free article] [PubMed] [Google Scholar]; Cruz-Coke R, Moreno RS, Aguirre J, Cortes G. Genetic epidemiology of single gene defects in Chile. J Med Genet. 1994;31(9):702 [cited 2022 Mar 6]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1050080/ [DOI] [PMC free article] [PubMed]
- 56.Goldfarb LG, Brown P, Mitrovà E, Cervenáková L, Goldin L, Korczyn AD, et al. Creutzfeldt-Jacob disease associated with the PRNP codon 200LYS mutation: An analysis of 45 families. [cited 2022 Mar 6];Eur J Epidemiol. 1991 7(5):477–86. doi: 10.1007/BF00143125. Available from: https://link.springer.com/article/10.1007/BF00143125. [DOI] [PubMed] [Google Scholar]; Goldfarb LG, Brown P, Mitrovà E, Cervenáková L, Goldin L, Korczyn AD, et al. Creutzfeldt-Jacob disease associated with the PRNP codon 200LYS mutation: An analysis of 45 families. Eur J Epidemiol. 1991;7(5):477–86 [cited 2022 Mar 6]. Available from: https://link.springer.com/article/10.1007/BF00143125 [DOI] [PubMed]
- 57.Saldarriaga W, Forero-Forero JV, González-Teshima LY, Fandiño-Losada A, Isaza C, Tovar-Cuevas JR, et al. Genetic cluster of fragile X syndrome in a Colombian district. [cited 2022 Mar 6];J Hum Genet. 2018 63(4):509–16. doi: 10.1038/s10038-017-0407-6. Available from: https://www.nature.com/articles/s10038-017-0407-6. [DOI] [PubMed] [Google Scholar]; Saldarriaga W, Forero-Forero JV, González-Teshima LY, Fandiño-Losada A, Isaza C, Tovar-Cuevas JR, et al. Genetic cluster of fragile X syndrome in a Colombian district. J Hum Genet. 2018;63(4):509–16 [cited 2022 Mar 6]. Available from: https://www.nature.com/articles/s10038-017-0407-6 [DOI] [PubMed]
- 58.Pachajoa H, Acosta MA, Alméciga-Díaz CJ, Ariza Y, Diaz-Ordoñez L, Caicedo-Herrera G, et al. Molecular characterization of mucopolysaccharidosis type IVA patients in the Andean region of Colombia. [cited 2022 Mar 6];Am J Med Genet C Semin Med Genet. 2021 187(3):388–95. doi: 10.1002/ajmg.c.31936. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.c.31936. [DOI] [PubMed] [Google Scholar]; Pachajoa H, Acosta MA, Alméciga-Díaz CJ, Ariza Y, Diaz-Ordoñez L, Caicedo-Herrera G, et al. Molecular characterization of mucopolysaccharidosis type IVA patients in the Andean region of Colombia. Am J Med Genet C Semin Med Genet. 2021;187(3):388–95 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.c.31936 [DOI] [PubMed]
- 59.Esperón-Moldes US, Pardo-Seco J, Montalván-Suárez M, Fachal L, Ginarte M, Rodríguez-Pazos L, et al. Biogeographical origin and timing of the founder ichthyosis TGM1 c.1187G > A mutation in an isolated Ecuadorian population. [cited 2022 Mar 6];Scientific Reports. 2019 9(1):1–10. doi: 10.1038/s41598-019-43133-6. Available from: https://www.nature.com/articles/s41598-019-43133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Esperón-Moldes US, Pardo-Seco J, Montalván-Suárez M, Fachal L, Ginarte M, Rodríguez-Pazos L, et al. Biogeographical origin and timing of the founder ichthyosis TGM1 c.1187G > A mutation in an isolated Ecuadorian population. Scientific Reports. 2019;9(1):1–10 [cited 2022 Mar 6]. Available from: https://www.nature.com/articles/s41598-019-43133-6 [DOI] [PMC free article] [PubMed]
- 60.Berg MA, Guevara-Aguirre J, Rosenbloom AL, Rosenfeld RG, Francke U. Mutation creating a new splice site in the growth hormone receptor genes of 37 Ecuadorean patients with Laron syndrome. [cited 2022 Mar 6];Hum Mutat. 1992 1(1):24–34. doi: 10.1002/humu.1380010105. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/humu.1380010105. [DOI] [PubMed] [Google Scholar]; Berg MA, Guevara-Aguirre J, Rosenbloom AL, Rosenfeld RG, Francke U. Mutation creating a new splice site in the growth hormone receptor genes of 37 Ecuadorean patients with Laron syndrome. Hum Mutat. 1992;1(1):24–34 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/humu.1380010105 [DOI] [PubMed]
- 61.Montenegro YHA, de Souza CFM, Kubaski F, Trapp FB, Burin MG, Michelin-Tirelli K, et al. Sanfilippo syndrome type B: Analysis of patients diagnosed by the MPS Brazil Network. [cited 2022 Mar 5];Am J Med Genet A. 2022 188(3):760–7. doi: 10.1002/ajmg.a.62572. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.62572. [DOI] [PubMed] [Google Scholar]; Montenegro YHA, de Souza CFM, Kubaski F, Trapp FB, Burin MG, Michelin-Tirelli K, et al. Sanfilippo syndrome type B: Analysis of patients diagnosed by the MPS Brazil Network. Am J Med Genet A. 2022;188(3):760–7 [cited 2022 Mar 5]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.62572 [DOI] [PubMed]
- 62.Wong OT, Guevara GM, López JV, Granja CZ, Plasencía PR. Aplasia Cutis Congénita Acervo clínico de Trujillo, 1982-2002. [cited 2022 Mar 6];Folia Dermatol. 2003 13(2):102–12. Available from: https://sisbib.unmsm.edu.pe/bvrevistas/dermatologia/v13_n2/rsm_aplasia.htm. [Google Scholar]; Wong OT, Guevara GM, López JV, Granja CZ, Plasencía PR. Aplasia Cutis Congénita Acervo clínico de Trujillo, 1982-2002. Folia Dermatol. 2003;13(2):102–12 [cited 2022 Mar 6]. Available from: https://sisbib.unmsm.edu.pe/bvrevistas/dermatologia/v13_n2/rsm_aplasia.htm
- 63.Purizaca-Rosillo N, Mori T, Benites-Cóndor Y, Hisama FM, Martin GM, Oshima J. High incidence of BSCL2 intragenic recombinational mutation in Peruvian type 2 Berardinelli–Seip syndrome. [cited 2022 Mar 6];Am J Med Genet A. 2017 173(2):471–8. doi: 10.1002/ajmg.a.38053. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.38053. [DOI] [PMC free article] [PubMed] [Google Scholar]; Purizaca-Rosillo N, Mori T, Benites-Cóndor Y, Hisama FM, Martin GM, Oshima J. High incidence of BSCL2 intragenic recombinational mutation in Peruvian type 2 Berardinelli–Seip syndrome. Am J Med Genet A. 2017;173(2):471–8 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.38053 [DOI] [PMC free article] [PubMed]
- 64.Rodríguez M, Camejo C, Bertoni B, Braida C, Rodríguez MM, Brais B, et al. (GCG)11 founder mutation in the PABPN1 gene of OPMD Uruguayan families. [cited 2022 Mar 6];Neuromuscular Disorders. 2005 15(2):185–90. doi: 10.1016/j.nmd.2004.10.012. Available from: http://www.nmd-journal.com/article/S0960896604003049/fulltext. [DOI] [PubMed] [Google Scholar]; Rodríguez M, Camejo C, Bertoni B, Braida C, Rodríguez MM, Brais B, et al. (GCG)11 founder mutation in the PABPN1 gene of OPMD Uruguayan families. Neuromuscular Disorders. 2005;15(2):185–90 [cited 2022 Mar 6]. Available from: http://www.nmd-journal.com/article/S0960896604003049/fulltext [DOI] [PubMed]
- 65.Ramirez-Duque P, Arends T, Merino F. Chediak-Higashi syndrome: description of a cluster in a Venezuelan-Andean isolated region. [cited 2022 Mar 6];J Med. 1982 13(5–6):431–51. Available from: https://pubmed.ncbi.nlm.nih.gov/6763069/ [PubMed] [Google Scholar]; Ramirez-Duque P, Arends T, Merino F. Chediak-Higashi syndrome: description of a cluster in a Venezuelan-Andean isolated region. J Med. 1982;13(5–6):431–51 [cited 2022 Mar 6]. Available from: https://pubmed.ncbi.nlm.nih.gov/6763069/ [PubMed]
- 66.Arias S, Paradisi I, Hernández A, Kanzler D. Undescribed GJB2 c.35dupG homozygous prelingual distinguished from c.35delG homozygous/compound heterozygous deafs, dwelling a German ancestry Venezuelan isolate. [cited 2022 Mar 6];Egypt J Med Hum Genet. 2021 22(1):1–14. Available from: https://jmhg.springeropen.com/articles/10.1186/s43042-021-00159-8. [Google Scholar]; Arias S, Paradisi I, Hernández A, Kanzler D. Undescribed GJB2 c.35dupG homozygous prelingual distinguished from c.35delG homozygous/compound heterozygous deafs, dwelling a German ancestry Venezuelan isolate. Egypt J Med Hum Genet. 2021;22(1):1–14 [cited 2022 Mar 6]. Available from: https://jmhg.springeropen.com/articles/10.1186/s43042-021-00159-8
- 67.Paradisi I, Ikonomu V, Arias S. Spinocerebellar ataxias in Venezuela: genetic epidemiology and their most likely ethnic descent. [cited 2022 Mar 2];J Hum Genet. 2016 61(3):215–22. doi: 10.1038/jhg.2015.131. Available from: https://www.nature.com/articles/jhg2015131. [DOI] [PubMed] [Google Scholar]; Paradisi I, Ikonomu V, Arias S. Spinocerebellar ataxias in Venezuela: genetic epidemiology and their most likely ethnic descent. J Hum Genet. 2016;61(3):215–22 [cited 2022 Mar 2]. Available from: https://www.nature.com/articles/jhg2015131 [DOI] [PubMed]
- 68.Avendaño A, González-Coira M, Paradisi I, Rojas A, da Silva G, Gómez-Pérez R, et al. 5α-Reductase type 2 deficiency in families from an isolated Andean population in Venezuela. [cited 2022 Mar 6];Ann Hum Genet. 2020 84(2):151–60. doi: 10.1111/ahg.12358. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/ahg.12358. [DOI] [PubMed] [Google Scholar]; Avendaño A, González-Coira M, Paradisi I, Rojas A, da Silva G, Gómez-Pérez R, et al. 5α-Reductase type 2 deficiency in families from an isolated Andean population in Venezuela. Ann Hum Genet. 2020;84(2):151–60 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/ahg.12358 [DOI] [PubMed]
- 69.Dourado MR, dos Santos CRR, Dumitriu S, Iancu D, Albanyan S, Kleta R, et al. Enamel renal syndrome: A novel homozygous FAM20A founder mutation in 5 new Brazilian families. Eur J Med Genet. 2019;62(11):103561. doi: 10.1016/j.ejmg.2018.10.013. [DOI] [PubMed] [Google Scholar]; Dourado MR, dos Santos CRR, Dumitriu S, Iancu D, Albanyan S, Kleta R, et al. Enamel renal syndrome: A novel homozygous FAM20A founder mutation in 5 new Brazilian families. Eur J Med Genet. 2019;62(11):103561. [DOI] [PubMed]
- 70.Martins C, de Medeiros PF v., Leistner-Segal S, Dridi L, Elcioglu N, Wood J, et al. Molecular characterization of a large group of Mucopolysaccharidosis type IIIC patients reveals the evolutionary history of the disease. [cited 2022 Mar 6];Hum Mutat. 2019 40(8):1084–100. doi: 10.1002/humu.23752. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/humu.23752. [DOI] [PubMed] [Google Scholar]; Martins C, de Medeiros PF v., Leistner-Segal S, Dridi L, Elcioglu N, Wood J, et al. Molecular characterization of a large group of Mucopolysaccharidosis type IIIC patients reveals the evolutionary history of the disease. Hum Mutat. 2019;40(8):1084–100 [cited 2022 Mar 6]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/humu.23752 [DOI] [PubMed]
- 71.dos Santos-Lopes SS, de Oliveira JMF, de Queiroga Nascimento D, Montenegro YHA, Leistner-Segal S, Brusius-Facchin AC, et al. Demographic, clinical, and ancestry characterization of a large cluster of mucopolysaccharidosis IV A in the Brazilian Northeast region. [cited 2022 Mar 5];Am J Med Genet A. 2021 185(10):2929–40. doi: 10.1002/ajmg.a.62375. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.62375. [DOI] [PubMed] [Google Scholar]; dos Santos-Lopes SS, de Oliveira JMF, de Queiroga Nascimento D, Montenegro YHA, Leistner-Segal S, Brusius-Facchin AC, et al. Demographic, clinical, and ancestry characterization of a large cluster of mucopolysaccharidosis IV A in the Brazilian Northeast region. Am J Med Genet A. 2021;185(10):2929–40 [cited 2022 Mar 5]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.a.62375 [DOI] [PubMed]
- 72.de Azevedo Medeiros LB, Cândido Dantas VK, Craveiro Sarmento AS, Agnez-Lima LF, Meireles AL, Xavier Nobre TT, et al. High prevalence of Berardinelli-Seip Congenital Lipodystrophy in Rio Grande do Norte State, Northeast Brazil. [cited 2022 Mar 6];Diabetol Metab Syndr. 2017 9(1):1–6. doi: 10.1186/s13098-017-0280-7. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-017-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; de Azevedo Medeiros LB, Cândido Dantas VK, Craveiro Sarmento AS, Agnez-Lima LF, Meireles AL, Xavier Nobre TT, et al. High prevalence of Berardinelli-Seip Congenital Lipodystrophy in Rio Grande do Norte State, Northeast Brazil. Diabetol Metab Syndr. 2017;9(1):1–6 [cited 2022 Mar 6]. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-017-0280-7 [DOI] [PMC free article] [PubMed]
- 73.Nascimento FA, Rodrigues VOR, Pelloso FC, Camargo CHF, Moro A, Raskin S, et al. Spinocerebellar ataxias in Southern Brazil: Genotypic and phenotypic evaluation of 213 families. Clin Neurol Neurosurg. 2019;184:105427. doi: 10.1016/j.clineuro.2019.105427. [DOI] [PubMed] [Google Scholar]; Nascimento FA, Rodrigues VOR, Pelloso FC, Camargo CHF, Moro A, Raskin S, et al. Spinocerebellar ataxias in Southern Brazil: Genotypic and phenotypic evaluation of 213 families. Clin Neurol Neurosurg. 2019;184:105427. [DOI] [PubMed]
- 74.Lima JG, Helena C, Nobrega L, Moura Bandeira FT, Pires Sousa AG, Medeiros de Araujo Macedo TB, Cavalcante Nogueira AC, et al. A novel GPIHBP1 mutation related to familial chylomicronemia syndrome: A series of cases. [cited 2022 Mar 6];Atherosclerosis. 2021 322:31–8. doi: 10.1016/j.atherosclerosis.2021.02.020. Available from: http://www.atherosclerosis-journal.com/article/S0021915021000927/fulltext. [DOI] [PubMed] [Google Scholar]; Lima JG, Helena C Nobrega L, Moura Bandeira FT, Pires Sousa AG, Medeiros de Araujo Macedo TB, Cavalcante Nogueira AC, et al. A novel GPIHBP1 mutation related to familial chylomicronemia syndrome: A series of cases. Atherosclerosis. 2021;322:31–8 [cited 2022 Mar 6]. Available from: http://www.atherosclerosis-journal.com/article/S0021915021000927/fulltext [DOI] [PubMed]


