Abstract
Background
Altitude sojourns increasingly attract individuals of all ages and different health statuses due to the appeal of high-altitude destinations worldwide and easy access to air travel. The risk of acute mountain sickness (AMS) when flying to high-altitude destinations remains underemphasized. Thus, this review aims to evaluate the altitude-dependent AMS incidence depending on the mode of ascending, e.g. by air vs terrestrial travel.
Methods
A literature search was performed to identify the observational studies assessing AMS incidence after acute ascent of primarily healthy adults to real high altitude. In addition, placebo arms of interventional trials evaluating the prophylactic efficacy of various drugs have been separately analysed to confirm or refute the findings from the observational studies. Linear regression analyses were used to evaluate the altitude-dependent AMS incidence.
Results
Findings of 12 observational studies, in which the AMS incidence in 11 021 individuals ascending to 19 different altitudes (2200–4559 m) was evaluated, revealed an impressive 4.5-fold steeper increase in the AMS incidence for air travel as compared with slower ascent modes, i.e. hiking or combined car and/or air travel and hiking. The higher AMS incidence following transportation by flight vs slower means was also confirmed in placebo-treated participants in 10 studies of drug prophylaxis against AMS.
Conclusions
Due to the short time span in going from low to high altitude, reduced acclimatization likely is the main reason for a higher AMS risk when travelling to high-altitude destinations by flight. To avoid frustrating travel experiences and health risks, appropriate and timely medical advice on how to prepare for air travel to high altitude is of vital importance. Effective preparation options include the use of modern pre-acclimatization strategies and pharmacological prophylaxis by acetazolamide or dexamethasone, or even considering alternate itineraries with more gradual ascent.
Keywords: Air travel, high altitude, hypoxia, acute mountain sickness
Graphical Abstract
Graphical Abstract.

Introduction
High-altitude destinations all over the world (e.g. La Paz, 3640 m, Bolivia; Leh, 3500 m, Ladakh; Cusco, 3399 m, Peru; Lhasa, 3356 m, Tibet) are attracting millions of travellers and pilgrims every year. Reasons to travel include sightseeing and business activities, trekking or high-altitude climbing, study purposes or visiting relatives and friends to benefit from low allergen concentrations or to escape from the heat waves of low-altitude areas. People of all ages and both sexes, of a broad range of fitness and varying health conditions, expose themselves to high-altitude environments and are differentially affected based on individual characteristics and vulnerabilities.1–3 The decreasing availability of oxygen with increasing altitude can trigger the development of high-altitude illnesses (HAIs), i.e. AMS, high-altitude cerebral (HACE) and pulmonary oedema (HAPE).4–7 Besides individual susceptibility, the speed of ascent also determines the risk for such adverse altitude effects.3,4,7 AMS is by far the most prevalent HAI, affecting ~17% of all individuals who rapidly ascend to 2800 m8 and >50% if the target altitude is >4500 m.9 Shlim recently emphasized about the elevated risk for HAIs by rapid travel to high-altitude destinations and about the potential benefits of prophylactic drug use.10 Although >25 years ago, Murdoch et al. referred to an extraordinarily high AMS risk of 84% when rapidly ascending to high altitudes (3740 m) by airplane,11 whether fast passive (i.e. by airplane or car) or slower active (i.e. by hiking), ascents that are associated with a higher incidence of AMS requires further assessment. This factor, however, is of high clinical relevance for the huge numbers of air travellers visiting high-altitude destinations. In the present review, we therefore aimed to evaluate the altitude-dependent AMS incidence depending on the mode of ascending.
Methods
We searched the literature (PubMed and Web of Science) for original (observational) studies assessing the AMS incidence after acute ascent (1–3 days) to terrestrial high altitude. Search terms used were ‘acute mountain sickness’ and ‘incidence OR prevalence’. Studies with the main objective to evaluate altitude-dependent AMS in unacclimatized but primarily healthy adults, aged ≥18 years, were included. Additional inclusion criteria were that the AMS diagnosis was made within the first 24 hours after arrival at altitude and was based on at least three AMS symptoms12,13 or a Lake Louis Questionnaire Score (LLQS) of 3 or more.14,15 Studies were categorized according to the type of ascent, i.e. (i) passive (travel by plane or car), active (travel by foot) ascents or both and (ii) very rapid (1 day) and rapid (2–3 days) ascents. Studies on pilgrims and those without clear information on the type of ascent, or whether prophylaxis (by specific pre-acclimatization strategies or pharmacological interventions) was applied, were excluded. Linear regression analyses were used to evaluate the altitude-dependent AMS incidence and an extra sum of squares F-test was applied to compare the slopes of the regression models using Graphpad Prism, version 9.
In order to further confirm or refute the findings derived from these observational non-intervention epidemiologic studies, we also separately analysed the placebo arms of a number of studies evaluating the prophylactic efficacy of various drugs (i.e. acetazolamide, corticosteroids and non-steroidal anti-inflammatory drugs) during rapid (1–2 days) ascents to terrestrial high altitude. Owing to the small sample sizes and the possibility of placebo and nocebo effects in these types of interventional studies,16 we did not include them in our primary analysis.
Results
We analysed a total of 12 studies in which the AMS incidence in 11 021 individuals ascending to 19 different altitudes (2200–4559 m) was evaluated. The findings of five studies of participants,11,17–20 who predominantly used air travel to high-altitude destinations, were compared with those of seven studies of travellers who primarily ascended by foot and/or car and plane.13,21–26 The characteristics of the selected studies are shown in Table 1. Overall, more study participants were male than female. While AMS scoring in one study was based on the presence of three AMS symptoms (before the availability of the LLQS), the other studies considered a LLQS of ≥3 (eight studies) or of ≥4 (three studies) as indicative for AMS. Young people and females seem to be more frequently affected by AMS than older individuals and men (Table 1). The AMS incidence further increased linearly with altitude, and this increase was significantly 4.5-fold steeper when ascending (rapidly) by air travel (slope = 0.094) compared with slower ascents by foot or a combination of air travel, car and foot (slope = 0.021) (Figure 1).
Table 1.
AMS incidence recorded at different altitudes depending on the type of ascent
| Reference | Altitude (m) | N total | Male (m), female (f) | AMS + (%) LLQS ≥3a | AMS + (%) LLQS ≥4 | Ascent in 1 (1) or 2–3 (2) days | Ascent by foot (1), car (2), airplane (3) | AMS + age differenceb Yes = 1, no = 2 |
AMS + sex differenceb Yes =1, no =2 |
|---|---|---|---|---|---|---|---|---|---|
| Maggiorini et al., 199013 | 2850–4559 | 466 | 83% m, 17% f | 34a | 1 + 2 | 1 | 1 (<20 and >40) | 2 | |
| 2850 | 47 | 9a | 1 | 1 | |||||
| 3050 | 128 | 13a | 1 | 1 | |||||
| 3650 | 82 | 34a | 1 | 1 | |||||
| 4559 | 209 | 52a | 2 | 1 | |||||
| Murdoch et al., 199511 | 3740 | 116 | 55% m, 45% f | 84 | 1 | 3 | NA | 1, female | |
| Mairer et al., 200921 | 2200–3500 | 431 | 76% m, 24% f | 16.2 | 1 | 1 | 2 | 2 | |
| 2200 | 159 | 6.9 | 1 | 1 | |||||
| 2500 | 55 | 9.1 | 1 | 1 | |||||
| 2800 | 138 | 17.4 | 1 | 1 | |||||
| 3500 | 79 | 38 | 1 | 1 | |||||
| Ren et al., 201017 | 3600 | 3628 | >99% m | 57.2 | 1 | 3 | NA, only young people included | NA | |
| Wang et al., 201022 | 3952 | 1066 | 67% m, 33% f | 36 | 28 | 2 | 1 | 1, young | 2 |
| Salazar et al., 201218 | 3400 | 991 | 44.5% m, 55.5% f | 48.5 | 1 | 3 | 1, young | 2 | |
| Gonggalanzi et al., 201623 | 3658 | 2385 | 55% m, 45% f | 36.7 | 1 + 2 | 2 + 3 | 1, young | 2 | |
| Horiuchi et al. 201624 | 3776 | 345 | 59% m, 41% f | 29.5 | 2 | 1 | 2 | 2 | |
| Shen et al., 202025 | 4100 | 99 | 71% m, 29% f | 47 | 2 | 1 | 2 | 1, female | |
| Yang et al., 202026 | 3272 | 345 | 62.4% m, 37.6% f | 23.9 | 1 | 1 | 1, young | 2 | |
| Caravedo et al., 202119 | 3350 | 123 | 43% m, 57% f | 39 | 1 | 3 | 2 | 1, female | |
| Chen et al., 202120 | 3700 | 1026 | 100% m | 68.3 | 1 | 3 | NA | NA |
NA, not assessed.
Three or more AMS symptoms indicative of AMS.12
The most affected populations are indicated.
Figure 1.
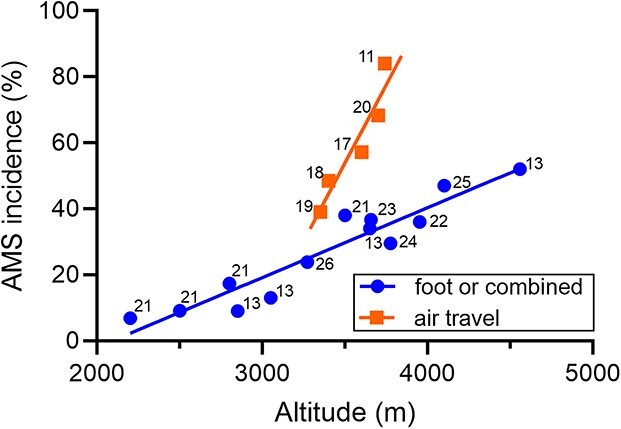
Altitude-dependent AMS incidence when travelling to high altitude by airplane or by foot and/or by car and plane; R2 for both regressions is 0.9 and the slopes are significantly different (P < 0.01), as determined by an extra sum of squares F-test (F(1, 14) = 21.90); numbers in the graph indicate references to the respective publications.
The AMS incidence reported in participants of the placebo arms of interventional studies evaluating various types of pharmacological AMS prophylaxis are shown in Table 2. For reasons of comparability (with Figure 1), the AMS incidence depending on the final altitude and mode of travel are depicted in Figure 2.
Table 2.
AMS incidence, mode and speed of ascent and the final altitude reached in participants of the placebo arm of interventional studies evaluating various types of pharmacological AMS prophylaxis
| Reference | Altitude (m) | Mode (and speed) of ascent | N, placebo arm; age; sex (% male) | AMS + (%) | Remarks |
|---|---|---|---|---|---|
| Hackett et al., 198827 | 4400 | Air travel (1 hour) | 8; 28 ± 1; 100 | 100 | Travel by helicopter |
| Maggiorini et al., 200628 | 4559 | Climb (1 day) | 9; 41 ± 8; 78 | 89 | HAPE susceptibles |
| van Patot et al., 200829 | 4300 | Car (2 hours) | 22; 24 ± 6; 43 | 77 | Staging at 2000 m |
| Wang et al., 201330 | 3561 | Air travel (3 hours) | 10; 25 ± 2; 100 | 70 | |
| Burki et al., 199231 | 4450 | Car (8 hours) | 6; 20 ± 1; 100 | 67 | |
| Parati et al., 201332 | 4559 | Climb (1 day) | 22; 37 ± 10; 50 | 64 | |
| Lipman et al., 201833 | 3810 | Car, climb (1 day) | 35; 32 ± 7; 40 | 63 | |
| Chow et al., 200534 | 3800 | Car (2 hours) | 20; 34 ± 10; 50 | 60 | Overnight stay at 1230 m |
| Moraga et al., 200735 | 3969 | Car (8.5 hours) | 12; 22 ± 1; 100 | 54 | |
| Ellsworth et al., 199136 | 4392 | Car, climb (2 days) | 18; 33 ± 4; 75 | 50 |
Figure 2.
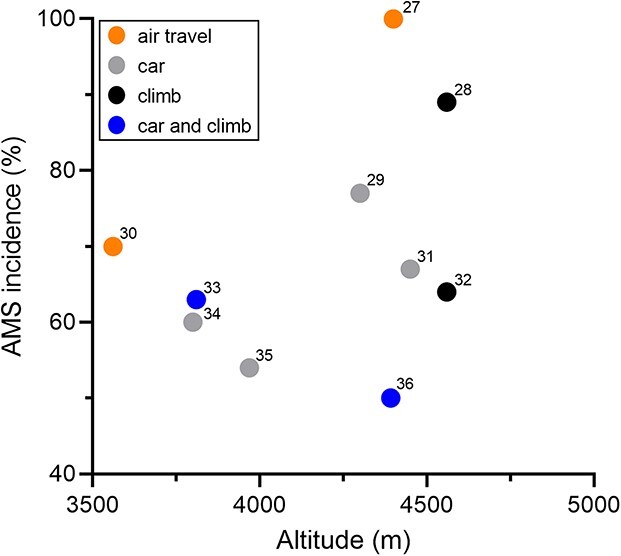
AMS incidence, speed of ascent and the final altitude reached in participants of the placebo arms of interventional studies evaluating various types of pharmacological AMS prophylaxis; numbers in the graph indicate references to the respective publications.
Ten studies, including 162 participants (placebo arms), report an AMS incidence between 50 and 100% when rapidly (1 hour–2 days) ascending to altitudes between 3651 and 4559 m. Not surprisingly, the number of participants of the placebo arms in these clinical trials is much lower compared with that of the observational studies shown in Table 1. Only two trials evaluated the AMS incidence after air travel to high altitude, but incidence data fit well with those presented in Figure 1, i.e. air travel (helicopter) within 1 hour to 4400 m was associated with an AMS incidence of 100%27 and that to 3561 m within 3 hours was associated with an AMS incidence of 70%.30 However, similarly, high AMS incidence was observed after rapid ascent (within a few hours) by car and/or strenuous climbing to altitudes >4000 m,29 particularly in susceptible individuals, e.g. those prone to suffer from HAPE.28 The remaining six studies report an average AMS incidence of 60% when arriving at an average altitude of 4163 m.31–36 This is higher than the AMS incidence observed in the non-flight studies but clearly lower than that of the observational air travel studies (Table 1, Figure 1).
Discussion
The present findings, derived from mostly large observational studies, indicate an impressive 4.5-fold steeper increase in AMS incidence when quickly ascending to high-altitude destinations by air travel, i.e. from 39% at 3350 m to 84% at 3740 m (~11.5% per 100 m gain in altitude). By contrast, slower ascent by foot or combined walking with car and air travel was associated with an only 2.1% increase in AMS incidence per 100 m (Figure 1). Younger people and females suffer more frequently from AMS. Despite earlier reports of the high risk of developing AMS11 or headache37 when flying to high altitude, the remarkable differences between the AMS incidence when travelling to high altitude by airplane compared with slower ascent modes have previously been underemphasized. Travellers flying to high-altitude destinations are thus mostly not aware of the higher AMS risk they may experience.
The increasing accessibility of appealing high-altitude destinations worldwide by air travel1 contributes to growing numbers of high-altitude sojourners and reduces age- and health-related barriers. The insufficiently understood interactions of chronic illnesses and age with HAIs add to the clinical relevance of these developments.
Although the risk of altitude illness is higher when flying, health consequences may be less severe compared with trekking or climbing, likely due to better access to medical care in the cities. Still, flying to high altitude remains a considerable health risk, as confirmed, e.g. by the study of Ren and colleagues, who reported that 12% of air travellers needed hospitalization and 2% suffered from HAPE when flying to an altitude of 3600 m.17 Moreover, the risk of HAIs increases considerably in subjects who continue with trekking/climbing too fast after arrival by air travel.12
The lack or minimal acclimatization to high altitude (hypoxia) due to the very short time span from low to high altitude likely is the main reason for the high AMS risk when flying to high altitude, which is supported by the demonstrated short-term benefits of high-altitude acclimatization.38,39 Cabin air pressure of commercial flights corresponds to an altitude of 1981–2438 m (6500–8000 ft) and is associated with a respective decrease in arterial oxygen saturation (from 97% at sea level to ~92.5% at 2438 m).40 These hypoxemic levels are usually well tolerated by healthy passengers and lead to AMS development in <10% of passengers.40 In-flight hypoxia may even initiate acclimatization processes, but those are not sufficient when landing at high altitudes with a considerably lower atmospheric pressure. While this remains to be evaluated, it is conceivable that the symptoms of general discomfort developing during the flight,40 and/or jet lag following long haul travel, might even adversely affect the acclimatization process and aggravate the AMS risk.41
The slightly higher AMS incidence observed in the placebo-treated participants of non-flight studies (Table 2, Figure 2) might also be the result of a more rapid ascent to higher altitudes which is associated with the study participants being unable to customize their ascent speed due to the protocols that require equal ascent rates as of those on AMS prophylaxis. Slower and individually tailored ascent rates are certainly the main reason explaining the much lower AMS incidence, (i.e. 20–34% in those without pharmacological prophylaxis) during many-day high-altitude treks.42–45 Kayser and colleagues nicely demonstrated the steep increase of AMS incidence from 34 to 86% in mountaineers hiking slowly (average = 14 m/hour) to 5896 m when compared with acute exposure to the same simulated altitude.46 The impact of rapid ascent from 2000 to 4500 m has also been convincingly quantified by Beidleman and colleagues based on observations from 20 studies (10 at real and 10 at simulated altitude).47 These authors found an almost linear increase of AMS incidence (from <10% to >80%) in this large cohort (N = 308) of young and middle-aged (18–45 years) unacclimatized but fit males and females, who rapidly ascended (<2 hours) from ~2000–4500 m.47 Importantly, AMS severity peaked between 18 and 22 hours of exposure and was (in these studies) more pronounced in males and aggravated by physical activity but returned to baseline (regardless of sex and physical activity) after around 48 hours.47 In the studies of rapid active ascents, these all entailed physical exertion, which is known to increase the AMS severity, likely by the greater degree of hypoxemia over that at rest that occurs with exercise at high altitude.47,48 This is in addition to the lack of time to physiologically acclimatize by steadily increasing ventilation, a process that starts immediately and becomes complete after several days at high altitude.49 Although AMS is usually benign and self-limited (disappears during the first days at altitude), in rare cases, it may progress to or be accompanied by life-threatening HACE or HAPE.3–7 In addition, AMS symptoms (i.e. headache, nausea, vomiting, dizziness and fatigue) may by themselves curtail otherwise pleasant experiences during high-altitude sojourns, such as enjoying food, sleep, landscape, sightseeing and other physical activities. For rather short stays at high altitude, AMS can be responsible for not only adverse health effects but also a frustrating travel experience.
Therefore, appropriate and timely medical advice on how to prepare for air travel to high altitude is of vital importance. Such preparation includes pre-acclimatization and pharmacological prophylaxis1,50,51 and possibly suggesting alternate itineraries with more gradual ascent.
Pre-acclimatization strategies include both exposures to real altitude near home and/or normobaric hypoxia (hypoxia rooms and breathing hypoxic gas mixtures).50–52 Hiking and sleeping at altitudes >2500 m for at least 3 days close to the planned start of air travel and/or repeated exposures in hypoxia rooms (intermittently for at least 60 hours at a simulated altitude >2500 m) reduces the AMS risk associated with subsequent air travel to high altitude.50
Beside avoiding intense exercise during the first days at high altitude,48,53 the preventive use of acetazolamide supports acclimatization by increasing ventilatory drive to achieve better arterial oxygenation due to renal excretion of bicarbonate that counteracts the respiratory alkalosis.3,10,54 To achieve this effect, acetazolamide (125 mg bd) should be initiated 8–24 hours before ascent and should be continued for 48 hours at the final high-altitude destination.55 Travellers should further be advised not to ascend higher until mild symptoms have resolved and should descend or seek medical assistance when severe symptoms develop.56
Dexamethasone can be considered as an alternative to acetazolamide for adult travellers rapidly ascending by air. Although unlike acetazolamide, it does not facilitate acclimatization, studies show it to be very effective in preventing AMS with 2 mg every 6 hours or 4 mg every 12 hours, starting 4–6 hours before the ascent.4 Some experts recommend a higher dose of 4 mg every 6 hours for very rapid deployment over ~4000 m, such as for rescue work or military deployment that requires physical exertion.57 Owing to potential side effects (hyperglycaemia and mood changes), its use should be limited to no more than 5 days. It is most often used for 2–3 days, while natural acclimatization occurs (without facilitating it).
Limitations
Although we tried to limit study inclusion on rapid ascent (1–3 days) to high altitude without essential pre-acclimatization and/or pharmacological prophylaxis, and the availability of clear information on the AMS incidence, confounding cannot entirely be excluded due to individual differences in pre-ascent, within-ascent and post-ascent conditions (e.g. chronic and/or acute diseases, physical activity patterns, dietary and sleeping habits) and differences in methods used for and interpretation of AMS diagnosis. However, the large study population of the observational studies and affirmation of the high AMS incidence after air travel in placebo arms of studies evaluating pharmacological AMS prophylaxis are the strengths of the present review.
Conclusion
Visiting high-altitude destinations by air travel is associated with a higher risk of AMS development when compared with slower ascent by foot or combined travelling (walking, car and/or plane). To avoid frustrating travel experiences and health risks, appropriate and timely medical advice on how to prepare for air travel to high altitude is of vital importance. Besides the use of modern pre-acclimatization strategies, options of pharmacological prophylaxis or alternate itineraries with more gradual ascent should be considered.
Funding
None.
Acknowledgements
None.
Contributor Information
Johannes Burtscher, Institute of Sport Sciences, University of Lausanne, Lausanne 1015, Switzerland; Department of Biomedical Sciences, University of Lausanne, Lausanne 1005, Switzerland.
Erik R Swenson, VA Puget Health Care System, University of Washington, Seattle, WA, USA.
Peter H Hackett, Altitude Research Center, Division of Pulmonary Sciences and Critical Care Medicine, Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Grégoire P Millet, Institute of Sport Sciences, University of Lausanne, Lausanne 1015, Switzerland; Department of Biomedical Sciences, University of Lausanne, Lausanne 1005, Switzerland.
Martin Burtscher, Department of Sport Science, University of Innsbruck, Innsbruck A-6020, Austria; Austrian Society for Alpine and High-Altitude Medicine, Innsbruck A-6020, Austria.
Authors’ contributions
Johannes Burtscher and Martin Burtscher (conceived the presented idea, literature search, manuscript preparation, final proof reading), Johannes Burtscher (visualization), Erik R. Swenson, Peter H Hackett and Grégoire P. Millet (provided critical feedback, contributed to the final version of the manuscript, final proof reading).
Conflict of interest
None declared.
References
- 1. Luks AM, Hackett PH. Medical conditions and high-altitude travel. N Engl J Med 2022; 386:364–73. [DOI] [PubMed] [Google Scholar]
- 2. Burtscher M, Philadelphy M, Gatterer H, Burtscher J, Likar R. Submaximal exercise testing at low altitude for prediction of exercise tolerance at high altitude. J Travel Med 2018; 25:tay011. [DOI] [PubMed] [Google Scholar]
- 3. Richalet JP, Larmignat P, Poitrine E, Letournel M, Canouï-Poitrine F. Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med 2012; 185:192–8. [DOI] [PubMed] [Google Scholar]
- 4. Hackett PH, Roach RC. High-altitude illness. N Engl J Med 2001; 345:107–14. [DOI] [PubMed] [Google Scholar]
- 5. Luks AM, Swenson ER, Bartsch P. Acute high-altitude sickness. Eur Respir Rev 2017; 26:160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartsch P, Swenson ER. Clinical practice: acute high-altitude illnesses. N Engl J Med 2013; 368:2294–302. [DOI] [PubMed] [Google Scholar]
- 7. Netzer N, Strohl K, Faulhaber M, Gatterer H, Burtscher M. Hypoxia-related altitude illnesses. J Travel Med 2013; 20:247–55. [DOI] [PubMed] [Google Scholar]
- 8. Mairer K, Wille M, Burtscher M. The prevalence of and risk factors for acute mountain sickness in the Eastern and Western Alps. High Alt Med Biol 2010; 11:343–8. [DOI] [PubMed] [Google Scholar]
- 9. Wu TY, Ding SQ, Liu JL, Jia JH, Chai ZC, Dai RC. Who are more at risk for acute mountain sickness: a prospective study in Qinghai-Tibet railroad construction workers on mt. Tanggula. Chin Med J (Engl) 2012; 125:1393–400. [PubMed] [Google Scholar]
- 10. Shlim DR. The use of acetazolamide for the prevention of high-altitude illness. J Travel Med 2020; 27:taz106. [DOI] [PubMed] [Google Scholar]
- 11. Murdoch DR. Altitude illness among tourists flying to 3740 meters elevation in the Nepal Himalayas. J Travel Med 1995; 2:255–6. [DOI] [PubMed] [Google Scholar]
- 12. Hackett PH, Rennie D, Levine HD. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet 1976; 2:1149–55. [DOI] [PubMed] [Google Scholar]
- 13. Maggiorini M, Bühler B, Walter M, Oelz O. Prevalence of acute mountain sickness in the Swiss Alps. BMJ 1990; 301:853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roach RC, Bärtsch P, Hackett PH, Oelz O. The lake louise acute mountain sickness scoring system. In: Sutton JRHC, Coates G (eds). Hypoxia and Molecular Medicine. Burlington: Queen City Press, 1993, pp. 272–4. [Google Scholar]
- 15. Roach RC, Hackett PH, Oelz O et al. The 2018 Lake Louise acute mountain sickness score. High Alt Med Biol 2018; 19:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bärtsch P. The impact of nocebo and placebo effects on reported incidence of acute mountain sickness. High Alt Med Biol 2022; 23:8–17. [DOI] [PubMed] [Google Scholar]
- 17. Ren Y, Fu Z, Shen W et al. Incidence of high altitude illnesses among unacclimatized persons who acutely ascended to Tibet. High Alt Med Biol 2010; 11:39–42. [DOI] [PubMed] [Google Scholar]
- 18. Salazar H, Swanson J, Mozo K et al. Acute mountain sickness impact among travelers to Cusco. Peru J Travel Med 2012; 19:220–5. [DOI] [PubMed] [Google Scholar]
- 19. Caravedo MA, Mozo K, Morales ML et al. Risk factors for acute mountain sickness in travelers to Cusco, Peru: coca leaves, obesity, and sex. J Travel Med 2022; 29:taab102. [DOI] [PubMed] [Google Scholar]
- 20. Chen R, Wang Y, Zhang C et al. Assessment of acute mountain sickness using 1993 and 2018 versions of the Lake Louise score in a large Chinese cohort. High Alt Med Biol 2021; 22:362–8. [DOI] [PubMed] [Google Scholar]
- 21. Mairer K, Wille M, Bucher T, Burtscher M. Prevalence of acute mountain sickness in the Eastern Alps. High Alt Med Biol 2009; 10:239–45. [DOI] [PubMed] [Google Scholar]
- 22. Wang SH, Chen YC, Kao WF et al. Epidemiology of acute mountain sickness on Jade Mountain, Taiwan: an annual prospective observational study. High Alt Med Biol 2010; 11:43–9. [DOI] [PubMed] [Google Scholar]
- 23. Gonggalanzi L, Nafstad P et al. Acute mountain sickness among tourists visiting the high-altitude city of Lhasa at 3658 m above sea level: a cross-sectional study. Arch. Public Health 2016; 74:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horiuchi M, Endo J, Akatsuka S, Uno T, Jones TE. Prevalence of acute mountain sickness on Mount Fuji: a pilot study. J Travel Med 2016; 23:taw024. [DOI] [PubMed] [Google Scholar]
- 25. Shen Y, Yang YQ, Liu C et al. Association between physiological responses after exercise at low altitude and acute mountain sickness upon ascent is sex-dependent. Mil Med Res 2020; 7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang SL, Ibrahim NA, Jenarun G, Liew HB. Incidence and determinants of acute mountain sickness in mount Kinabalu. Malaysia High Alt Med Biol 2020; 21:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hackett PH, Roach RC, Wood RA et al. Dexamethasone for prevention and treatment of acute mountain sickness. Aviat Space Environ Med 1988; 59:950–4. [PubMed] [Google Scholar]
- 28. Maggiorini M, Brunner-La Rocca HP, Peth S et al. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: a randomized trial. Ann Intern Med 2006; 145:497–506. [DOI] [PubMed] [Google Scholar]
- 29. van Patot MC, Leadbetter G 3rd, Keyes LE, Maakestad KM, Olson S, Hackett PH. Prophylactic low-dose acetazolamide reduces the incidence and severity of acute mountain sickness. High Alt Med Biol 2008; 9:289–93. [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Ke T, Zhang X et al. Effects of acetazolamide on cognitive performance during high-altitude exposure. Neurotoxicol Teratol 2013; 35:28. [DOI] [PubMed] [Google Scholar]
- 31. Burki NK, Khan SA, Hameed MA. The effects of acetazolamide on the ventilatory response to high altitude hypoxia. Chest 1992; 101:736–41. [DOI] [PubMed] [Google Scholar]
- 32. Parati G, Revera M, Giuliano A et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J 2013; 34:759–66. [DOI] [PubMed] [Google Scholar]
- 33. Lipman GS, Pomeranz D, Burns P et al. Budesonide versus acetazolamide for prevention of acute mountain sickness. Am J Med 2018; 131:200.e9–16. [DOI] [PubMed] [Google Scholar]
- 34. Chow T, Browne V, Heileson HL, Wallace D, Anholm J, Green SM. Ginkgo biloba and acetazolamide prophylaxis for acute mountain sickness: a randomized, placebo-controlled trial. Arch Intern Med 2005; 165:296–301. [DOI] [PubMed] [Google Scholar]
- 35. Moraga FA, Flores A, Serra J, Esnaola C, Barriento C. Ginkgo biloba decreases acute mountain sickness in people ascending to high altitude at Ollagüe (3696m) in northern Chile. Wilderness Environ Med 2007; 18:251–7. [DOI] [PubMed] [Google Scholar]
- 36. Ellsworth AJ, Meyer EF, Larson EB. Acetazolamide or dexamethasone use versus placebo to prevent acute mountain sickness on Mount Rainier. West J Med 1991; 154:289–93. [PMC free article] [PubMed] [Google Scholar]
- 37. Bian SZ, Zhang JH, Gao XB et al. Risk factors for high-altitude headache upon acute high-altitude exposure at 3700 m in young Chinese men: a cohort study. J Headache Pain 2013; 14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burtscher M, Faulhaber M, Flatz M, Likar R, Nachbauer W. Effects of short-term acclimatization to altitude (3200 m) on aerobic and anaerobic exercise performance. Int J Sports Med 2006; 27:629–35. [DOI] [PubMed] [Google Scholar]
- 39. Nussbaumer-Ochsner Y, Ursprung J, Siebenmann C, Maggiorini M, Bloch KE. Effect of short-term acclimatization to high altitude on sleep and nocturnal breathing. Sleep 2012; 35:419–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muhm JM, Rock PB, McMullin DL et al. Effect of aircraft-cabin altitude on passenger discomfort. N Engl J Med 2007; 357:18–27. [DOI] [PubMed] [Google Scholar]
- 41. Burtscher J, Strasser B, Millet GP, Burtscher M. Can melatonin be used as a potential antioxidant and sleep aid supplement for high-altitude travelers? J Travel Med 2022; 29. [DOI] [PubMed] [Google Scholar]
- 42. Basnyat B, Gertsch JH, Holck PC. Low-dose acetylsalicylic acid analog and acetazolamide for prevention of acute mountain sickness. High Alt Med Biol 2008; 9:349.author reply 351-2–349; author reply 352. [DOI] [PubMed] [Google Scholar]
- 43. Basnyat B, Holck PS, Pun M et al. Spironolactone does not prevent acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial by space trial group (spironolactone and acetazolamide trial in the prevention of acute mountain sickness group). Wilderness Environ Med 2011; 22:15–22. [DOI] [PubMed] [Google Scholar]
- 44. Gertsch JH, Basnyat B, Johnson EW, Onopa J, Holck PS. Randomised, double blind, placebo controlled comparison of ginkgo biloba and acetazolamide for prevention of acute mountain sickness among himalayan trekkers: the prevention of high altitude illness trial (phait). BMJ 2004; 328:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gertsch JH, Lipman GS, Holck PS et al. Prospective, double-blind, randomized, placebo-controlled comparison of acetazolamide versus ibuprofen for prophylaxis against high altitude headache: the headache evaluation at altitude trial (heat). Wilderness Environ Med 2010; 21:236–43. [DOI] [PubMed] [Google Scholar]
- 46. Kayser B, Hulsebosch R, Bosch F. Low-dose acetylsalicylic acid analog and acetazolamide for prevention of acute mountain sickness. High Alt Med Biol 2008; 9:15–23. [DOI] [PubMed] [Google Scholar]
- 47. Beidleman BA, Tighiouart H, Schmid CH et al. Predictive models of acute mountain sickness after rapid ascent to various altitudes. Med Sci Sports Exerc 2013; 45:792–800. [DOI] [PubMed] [Google Scholar]
- 48. Roach RC, Maes D, Sandoval D et al. Exercise exacerbates acute mountain sickness at simulated high altitude. J Appl Physiol 19852000; 88:581–5. [DOI] [PubMed] [Google Scholar]
- 49. Lenfant C, Sullivan K. Adaptation to high altitude. N Engl J Med 1971; 284:1298–309. [DOI] [PubMed] [Google Scholar]
- 50. Burtscher M, Millet GP, Burtscher J. Hypoxia conditioning for high-altitude pre-acclimatization. J Sci Med Sport Exerc 2022; 4:331–45. [Google Scholar]
- 51. Tannheimer M, Lechner R. Rapid ascents of Mt Everest: Normobaric hypoxic preacclimatization. J Travel Med 2020; 27:taaa099. [DOI] [PubMed] [Google Scholar]
- 52. Millet GP, Jornet K. On top to the top-acclimatization strategy for the “fastest known time” to Mount Everest. Int J Sports Physiol Perform 2019; 14:1438–41. [DOI] [PubMed] [Google Scholar]
- 53. Beidleman BA, Muza SR, Rock PB et al. Exercise responses after altitude acclimatization are retained during reintroduction to altitude. Med Sci Sports Exerc 1997; 29:1588–95. [DOI] [PubMed] [Google Scholar]
- 54. Swenson ER. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell Biochem 2014; 75:361–86. [DOI] [PubMed] [Google Scholar]
- 55. Basnyat B, Gertsch JH, Johnson EW, Castro-Marin F, Inoue Y, Yeh C. Efficacy of low-dose acetazolamide (125 mg bid) for the prophylaxis of acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial. High Alt Med Biol 2003; 4:45–52. [DOI] [PubMed] [Google Scholar]
- 56. Croughs M, Nyakunga GB, Sakita FM, Kilonzo K, Mmbaga BT, Soentjens P. Incidence and predictors of severe altitude illness symptoms in mt. Kilimanjaro hikers: a prospective cohort study. J Travel Med 2022; 29:taac044. [DOI] [PubMed] [Google Scholar]
- 57. Luks AM, Auerbach PS, Freer L et al. Wilderness medical society clinical practice guidelines for the prevention and treatment of acute altitude illness: 2019 update. Wilderness Environ Med 2019; 30:S3–s18. [DOI] [PubMed] [Google Scholar]


