Abstract
Many patients suffer from complaints of dyspnea, cough, and sputum production, clinical symptoms that hallmark the structural abnormalities that are present in patients with COPD. Although pharmacologic and non-pharmacologic medical therapies help reduce these symptoms, many of these symptoms, especially dyspnea, remain unchecked and contribute to the burden of disease in patients with COPD. Over the last 3 decades, several surgical and interventional treatments delivered via a bronchoscopic approach have been developed to complement medical therapies and show promise to improve patient outcomes. Surgical and interventional treatments target structural abnormalities of the airway and lung parenchyma that can be identified with a combination of imaging and physiological testing, factors that are key to select patients most likely to benefit from these treatments. This paper reviews surgical and bronchoscopic interventional treatment options for patients with emphysema and airways disorders.
Keywords: COPD, emphysema, chronic bronchitis, LVRS, BLVR
Introduction
Much of the morbidity that is found in patients with COPD is related to complaints of dyspnea, cough, and sputum production, symptoms that are prevalent in more than half the patients with COPD and constantly present upon arising in the morning, throughout the daytime hours, and then again at bedtime.1 When patients are more severely obstructed, almost 90% complain of dyspnea, cough, or sputum production; and in most patients, several of these symptoms concurrently coexist. In fact, most patients with COPD describe dyspnea as their most troublesome symptom and the one symptom that never is effectively treated with current medical treatment. In fact, most patients state they are willing to accept a higher mortality for an effective treatment that alleviates dyspnea.2
The increased morbidity and mortality of COPD are largely attributable to structural abnormalities that occur within the airways and lung parenchyma. Air flow obstruction due to increased airway wall inflammation and thickness, bronchoconstriction, mucous hypersecretion, and emphysematous destruction of the lung parenchyma all contribute to the development of hyperinflation and the symptoms of dyspnea, cough, and mucus production (Fig. 1). Hyperinflation is associated with an increase in the frequency and severity of COPD exacerbations, impaired cardiopulmonary function, and skeletal muscle dysfunction.3–5 Severe emphysema alters the pulmonary capillary bed, and impairments in ventilation and perfusion matching cause hypoxemia and hypercapnia and can lead to pulmonary hypertension. Increases in intrathoracic pressure decrease cardiac chamber filling and can reduce cardiac output, further contributing to limitations in exercise tolerance and reduced quality of life.6
Fig. 1.
Airway and lung parenchymal structural derangements contributing to symptoms of dyspnea in patients with COPD.
Structural abnormalities that occur within the airways and lung parenchyma (ie, emphysema, mucus hypersecretion, and airway constriction) provide potential targets for treatment to alleviate dyspnea, reduce cough and mucus production, and improve quality of life (Fig. 2). Newer methods in imaging and physiological characterization of lung structure and function that characterize lung perfusion and ventilation to the lobar level provide sensitive and specific information to subphenotypes for specific interventions. Clinical information coupled with physiological and imaging testing is key to select appropriate candidates and to assess the risks and benefits of the type of intervention to be performed. Multidisciplinary review of the patient’s clinical information by specialists in pulmonary medicine, thoracic surgery, imaging, and the respiratory and physical therapy disciplines ensures quality outcomes.
Fig. 2.
Overview of structural targets for treatment: emphysema and airways diseases. LVRS = lung volume reduction surgery; BLVR = bronchoscopic lung volume reduction; EBV = endobronchial valve; TLD = targeted lung denervation.
Over the past 70 years, treatments to reduce lung volumes medically or surgically, or more recently via bronchoscopic techniques, have been implemented to reduce hyperinflation in patients with emphysema. More recently, bronchoscopic treatments that are directed to the airway to alleviate bronchoconstriction and mucus production have been the subject of recent investigations.
This article will succinctly review the treatment options for patients with emphysema and airways disorders. Treatments that target emphysema include bullectomy, lung volume reduction surgery (LVRS), bronchoscopic lung volume reduction (BLVR), and in select cases lung transplantation. Potential airway-predominant treatments include metered-dose nitrogen cryospray, rheoplasty, and targeted lung denervation (TLD); these later therapies are the focus of ongoing phase 3 clinical trials.
Surgical Treatments for Emphysema
Patients with COPD who suffer predominately from emphysema have a loss of tethering of the distal airways due to a loss of lung elastic recoil. The loss of lung recoil coupled with the small airways disorders that commonly coexist in patients with emphysema (ie, airway inflammation, mucus plugging, epithelial hyperplasia, and smooth muscle hypertrophy) contributes to airways obstruction that results in significant air trapping and hyperinflation at rest (eg, static hyperinflation). During exercise, an increase in breathing frequency reduces the time for lung emptying during expiration, which leads to further increases in hyperinflation (eg, dynamic hyperinflation) and as a result a reduction in exercise performance. As emphysema progresses, further increases in static hyperinflation and dynamic hyperinflation cause progression reductions in exercise performance, increase dyspnea, and further contribute to a decrease in the patient’s quality of life.
Bullectomy
Bullectomy is a rare but effective procedure for removing giant bulla that occupies more than one third of a hemithorax. Post bullectomy, improvements in dyspnea, lung, and cardiac performance as well as exercise activity have been reported.3,4 Physiologic improvements in lung, respiratory muscle, and cardiac mechanics have all been reported following giant bullectomy to be responsible for improved clinical outcomes.3,4
Lung Volume Reduction Surgery
LVRS was first proposed as an intervention for patients with severe emphysema and associated hyperinflation in 1959 by surgeon Otto Branigan.5 Although outcomes were potentially promising, unacceptably high morbidity and mortality halted the broad adoption of the therapy as a viable treatment option until Cooper revised the procedure and in 1995 reported significantly improved outcomes in 20 subjects with severe emphysema.6 Subsequently, the same group reported outcomes in 150 consecutive subjects who underwent LVRS and reported significant improvements in FEV1 and reductions in residual volume (RV) with a 4% 90-d mortality.7 Following Cooper’s publication, LVRS became adopted by many clinical centers; however, in contrast to the earlier reports, significantly poorer results were observed with increased morbidity and mortality. These data led to suspension of Centers for Medicare and Medicaid Services coverage for LVRS as a reimbursable treatment and the impetus to conduct the National Emphysema Treatment Trial (NETT).
The National Emphysema Treatment Trial
NETT was a prospective randomized controlled trial of optimal medical therapy versus optimal medical therapy plus bilateral LVRS. The two co-primary outcomes of NETT were survival and exercise capacity; the secondary outcomes included changes in FEV1, 6-min walk distance (6MWD), quality of life measured by St George Respiratory Questionnaire (SGRQ), and quality of well-being.8
The NETT screened 3,777 patients and enrolled 1,218 subjects for optimal medical therapy (n = 610) or optimal medical therapy plus bilateral LVRS (n = 608).9 Exercise performance at 6, 12, and 24 months improved > 10 W in 28, 22, and 15% of the subjects undergoing LVRS compared to 4, 5, and 3% who received optimal medical treatment alone, respectively. Subjects who received LVRS also had greater improvements in FEV1, 6MWD, dyspnea, and quality of life than those who received medical therapy alone. A subgroup of subjects with homogenous emphysema on chest computed tomography (CT) and FEV1 < 20% predicted or diffusing capacity of the lung for carbon monoxide (DLCO) < 20% predicted had excessive mortality and were identified during the conduct of NETT. Subjects meeting these features were excluded from further enrollment into NETT.10
Post hoc analyses identified 2 factors as key to selecting patients for LVRS with better outcomes: (1) the distribution of emphysema (upper-lobe predominant vs non–upper-lobe predominant) and (2) post-rehabilitation exercise performance (low exercise capacity: < 25 W females and < 40 W males vs high exercise: >25 W females and > 40 W males).9 Based on emphysema pattern on chest CT (upper lobe vs non–upper lobe) and exercise performance (high exercise vs low exercise), subjects were separated into 4 groups. The subjects with upper lobe–predominant emphysema and low exercise capacity had a significant improvement in mortality with LVRS compared to optimal medical therapy alone and were more likely to have a 10-W improvement in exercise capacity at 24 months and an 8-point improvement in SGRQ (Fig. 3). Subjects with upper lobe–predominant disease and high exercise had no improvement in mortality with LVRS but were more likely to have a 10-W improvement in exercise capacity and to have an 8-point improvement in SGRQ score. In subjects with non–upper lobe-predominant emphysema and low exercise capacity, LVRS did not improve survival or produce a 10-W improvement in exercise capacity, but subjects undergoing LVRS were more likely to have an 8-point improvement in SGRQ score. In subjects with non–upper lobe-predominant emphysema and high exercise, LVRS increased mortality compared to medical therapy and had no effect on achieving at least a 10-W improvement in exercise capacity or an 8-point improvement in SGRQ (Fig. 4).
Fig. 3.

Probability of death as function of the number of months after randomization (Kaplan-Meier estimates). High-risk subjects were those with FEV1 < 20% predicted and either homogeneous emphysema or diffusing capacity of the lung for carbon monoxide < 20% predicted. Low-baseline exercise capacity was defined as a maximal work load at or below the sex-specific 40th percentile (25 W for females and 40 W for males); high exercise capacity was defined as a work load greater than this threshold. From Reference 9, with permission.
Fig. 4.

Histograms of changes from baseline in exercise capacity (maximal work load) after 6-, 12-, and 24-months follow-up in subgroups of non–high-risk subjects. Baseline tests performed after pulmonary rehabilitation. Subjects too ill to complete the procedure or who declined were included in the missing category. The degree of bars shifted to the upper left indicates the degree of relative benefit of lung volume reduction surgery. High-risk subjects were defined as those with a FEV1 < 20% predicted and either homogeneous emphysema or diffusing capacity of the lung for carbon monoxide < 20% predicted value. A low-baseline exercise capacity was defined as a maximal work load at or below the sex-specific 40th percentile (25 W for females and 40 W for males); a high exercise capacity was defined as a work load above this threshold. From Reference 9, with permission.
In long-term follow-up of subjects enrolled in NETT with mean follow-up time of 4.3 y, long-term survival improved with LVRS.11 In those with upper lobe–predominant emphysema and baseline low exercise performance, improvements in exercise capacity were observed for up to 3 y with a significant improvement in SGRQ scores through 5 y with a marked decrease in mortality (Fig. 5). There was no survival advantage with LVRS in subjects with non–upper lobe-predominant emphysema and low exercise capacity although subjects in this subgroup were more likely to have an 8-point improvement in SGRQ at 2 y but had no improvement in exercise capacity compared to medical treatment alone (Fig. 6).
Fig. 5.
Kaplan-Meier estimates of the cumulative probability of death as a function of years after randomization to lung volume reduction surgery or medical treatment for A: all subjects and (B–D) non–high-risk and upper lobe–predominant subgroups of subjects. P values using the Fisher exact test for difference in the proportions of subjects who died during the 4.3 y (median) of follow-up. This was an intention-to-treat analysis. A: All subjects (n = 1,218); B: non–high-risk subjects (n = 1,078); C: upper lobe–predominant and low-baseline exercise capacity (n = 290); and D: upper-lobe predominant and high exercise capacity (n = 419). From Reference 11, with permission. LVRS = lung volume reduction surgery; RR = relative risk.
Fig. 6.

Improvement in exercise capacity (increase in maximum work of 10 W above the subject’s post-rehabilitation baseline) at 1, 2, and 3 y after randomization to lung volume reduction surgery or medical treatment for A: all subjects and (B–D) non–high-risk and upper lobe–predominant subgroups of subjects. Subjects who died or who did not complete the assessment were considered not improved. This was an intention-to-treat analysis. A: All subjects (n = 1,218); B: non–high-risk subjects (n = 1,078); C: upper-lobe predominant and low-baseline exercise capacity (n = 290); and D: upper-lobe predominant and high exercise capacity (n = 419). From Reference 11, with permission. LVRS = lung volume reduction surgery.
In subjects with non–upper lobe-low-predominant emphysema and high exercise, LVRS did not confer a survival advantage or improvement in exercise performance or improvement in quality of life as measured by SGRQ. The long-term follow-up of subjects in NETT reaffirmed that LVRS benefits subjects primarily with advanced upper lobe–predominant emphysema and demonstrated that this treatment provided durable significant improvements in clinical end points such as quality of life and lung function and reduction of dyspnea in those with baseline low exercise tolerance and upper lobe–predominant disease, a survival advantage.
The operative mortality in the 511 subjects who underwent LVRS in NETT was 5.5% at 90 d; the only predictor of operative mortality was the presence of non–upper lobe-predominant emphysema on chest CT12 (Table 1). During the 30-d postoperative, 58.7% of subjects had a complication following LVRS, with the most common complication being cardiac arrhythmia in 23.5% of subjects. Other important complications included re-intubation in 21.8%, pneumonia in 18.2%, ICU readmission in 11.7%, and tracheotomy in 8.2%. Since NETT, more recent studies have reported a lower FEV1 and body mass index (BMI) to increase LVRS operative mortality.13 The BMI, degree of air flow obstruction, level of dyspnea, and exercise capacity (BODE) has also been reported to be a predictor of survival following LVRS.14
Table 1.
National Emphysema Treatment Trial: Complications of Lung Volume Reduction Surgery
Ninety percent of subjects had an air leak in the first 30 d post LVRS in NETT.15 The median duration of air leak was 7 d, and 12% had an air leak after 30 d. Air leaks were common in whites, those with lower FEV1 or DLCO, presence of upper lobe–predominant emphysema, use of inhaled corticosteroids, and those with pleural adhesions. The presence of pleural adhesion was the greatest risk factor. Those with air leaks had a longer hospitalization and greater postoperative complications.
A post hoc analysis showed that subjects who had surgical resection of the most oligemic regions of the lung had the greatest improvements in lung function, exercise performance, SGRQ, and shortness of breath.16 In those with upper lobe–predominant emphysema with low exercise capacity post rehabilitation, resection of the most oligemic lung regions resulted in a significant improvement in survival. Successful outcomes with LVRS have been reported in select subjects with severely impaired DLCO when hyperinflation is severe but has approachable emphysematous targets for resection.17 Identification of target zones using 3D CT imaging can be used to select target zones for resection.18
Other post hoc analyses showed significant benefits of LVRS on reduction of exacerbations,19 increase in BMI,20 a reduction in rapid shallow breathing and an improvement in ventilator efficiency during exercise,21 and an improvement in O2/pulse during exercise, a surrogate marker of improvement in cardiac stroke volume.22 Others using cardiac magnetic resonance imaging have shown an improvement in cardiac index, left ventricular stroke volume index, and stroke work index following LVRS.23 Improvements in endothelial function measured by flow-mediated dilatation and a decrease in mean arterial pressure have also been shown following LVRS.24
In the time period following NETT, several experienced centers have reported substantial physiological and functional improvements with LVRS with acceptable morbidity and mortality.25,26 However, the numbers of patients receiving LVRS remain low.27 Several limiting factors have been identified why the numbers of patients undergoing LVRS remain low despite its reported benefits.27 These limitations include the perception of increased surgical complications, disruptions in the continuity of care, and the inability to obtain timely referrals.28,29 To achieve successful outcomes, a multidisciplinary team is key to select potential LVRS candidates and coordinate postoperative care.30 A prospective economic analysis in NETT reported that LVRS is costly relative to non-surgical health care programs.31
Nonsurgical Approaches to Lung Volume Reduction
Because of the effectiveness of LVRS to improve patient outcomes, multiple techniques that are less invasive to reduce the morbidity and mortality of surgical lung reduction have been proposed (Table 2). In broad terms, therapies that have been studied over the last 3 decades can be broken down into 3 major categories: (1) targeted lobar atelectasis of the most emphysematous disease lobe by the use of one-way endobronchial valves (EBVs) and pre-shaped nitinol lung coils, (2) remodeling of emphysematous tissue with the use of flowable adhesives and sclerosing agents or induction of airway inflammation and fibrosis with resultant atelectasis via thermal vapor ablation, and (3) placement of airway stents into emphysematous tissue to deflate gas trapped regions where medium and small lack sufficient lung elastic recoil to keep the airways open (Table 3).
Table 2.
Approaches to Bronchoscopic Lung Volume Reduction

Table 3.
Important Differences Between Pivotal Studies
One-Way Endobronchial Valves
Of all the bronchoscopic techniques proposed for lung volume reduction, one-way EBVs have been studied the longest and most extensively. They are the only device FDA (United States Food and Drug Administration) approved for lung reduction therapy in the United States for patients with emphysema and hyperinflation (Fig. 7). The EBVs are placed at the segmental, subsegmental, or lobar levels to block inspiration but permit expiration of air and secretions. The goal of this therapy is to achieve complete lobar occlusion and induce lobar atelectasis. There is a direct relationship between the degree of induced lobar atelectasis and improvement in lung function, exercise performance, and reduction of dyspnea.
Fig. 7.
Devices used in the prospective randomized controlled trial with endobronchial valves for treatment of advanced emphysema with severe hyperinflation.
There are 2 EBV products available in the United States that are FDA approved. They include the Spiration Valve System (Spiration, Olympus, Tokyo, Japan) that has an umbrella design with an occlusive cover stretched over a flexible metal frame (nitinol, composed of titanium and nickel) covered by a thin flexible film that allows expired air and secretions to escape around the outer edges of the device with the airway wall. The other device is the Zephyr EBV (Pulmonx, Redwood City, California), which is a cylindrical device with a one-way silicone duck-bill valve attached to a nickel-titanium (nitinol) self-expanding retainer covered with a silicone membrane that permits expired air and secretions to escape through the center of the valve.
The first prospective randomized controlled trial to study the effectiveness of one-way valve therapy for subjects with severe emphysema and hyperinflation was the EBV for Emphysema Palliation Trial (VENT).32 The primary end points of the study were percent change in FEV1 and 6MWD at 6 months. EBVs were placed targeting the lobe with the greatest percentage of emphysema and highest degree of heterogeneity (ie, difference between the emphysematous destruction in the target lobe, the ipsilateral non-targeted lobe).
The study reported that FEV1 increased by 4.3% in the EBV-treated group and decreased by 2.5% in the control group at 6 months. The mean increase in 6MWD was 5.8% or 19.1 m following EBV treatment compared to the controls. Furthermore, there were modest improvements in quality of life, reduction of dyspnea, and supplemental oxygen use that all favored the group that received the EBVs. Factors that were predictive of improvement were heterogeneity of emphysema between the lobes in the treated lung and the presence of complete fissures. The VENT study was also performed in 23 European centers where 111 subjects were randomized to EBV treatment and 62 to medical therapy. The European VENT study results were similar to the outcomes of the United States VENT study. Once again, the medium reduction in targeted lobe volume was greatest in those with complete fissures compared to those with incomplete fissures. These 2 studies indicate that when complete fissures are present and thus collateral ventilation is absent then EBV treatments are more capable of effectively reducing air trapping and thus improve lung function, reduce dyspnea, and improve exercise tolerance and quality of life.
Determination of intact fissures can be done by assessment of fissure integrity by chest CT examination or physiological measurement of flow across the fissure by use of a balloon-tipped catheter during bronchoscopy. The Chartis system (PulmonX, Redwood City, CA) measures collateral ventilation by inserting a balloon-tipped catheter outfitted with flow and pressor sensors into the targeted bronchus via the working channel of the bronchoscope. With inflation of the balloon-tipped catheter, flow and pressure are measured at its distal port, and cessation of flow over several minutes indicates a collateral negative status and identifies the patient as a viable candidate for EBV placement. Continuation of flow after several minutes of balloon occlusion indicates a collateral ventilation–positive status between the adjacent lobes and indicates a nonviable candidate for EBV treatment.
Following conclusions from the VENT trial that identified the importance of collateral ventilation–negative status or the presence of fissure integrity as a prerequisite for successful EBV placement, subsequent randomized controlled trials enrolled subjects only with collateral ventilation–negative status or intact fissures.
STELVIO was the first study to assess collateral ventilation status as a study inclusion criterion. In that study, subjects were randomized 2 to 1 to receive Zephyr EBV treatment or medical therapy. In subjects treated with the Zephyr EBV, 55% of subjects had a > 12% improvement in FEV1 compared to controls. The improvements in FEV1 were accompanied by significant improvements in 6MWD, quality of life measured by SGRQ, and a reduction in RV at 6 months following treatment compared to baseline.33
Similar results were reported in a larger European-based multi-center clinical trial that enrolled subjects exclusively with heterogeneous emphysema.34 At 6 months post Zephyr EBV treatment, EBV 56.3% versus standard of care 3.2% had a mean change in FEV1 at 6 months of 20.7 ± 9.6% and −8.6 ± 13.0%, respectively. A total of 89.8% of Zephyr EBV subjects had target lobe volume reduction > 350 mL, mean 1.09 ± 0.62 L. The authors reported that pneumothorax was the most common adverse event, occurring in 29% of EBV-treated subjects.
The Lung Function Improvement After Bronchoscopic Lung Volume Reduction with Pulmonx EBVs used in Treatment of Emphysema (LIBERATE) was the largest multi-centered controlled trial to follow subjects for at least one year post treatment with Zephyr EBVs.35 The study was also used as the premarket approval application to the FDA. LIBERATE recruited subjects with collateral ventilation–negative status and 15% emphysematous heterogeneity between lobes, with more than 50% destruction in the target lobe. Moreover, subjects had an FEV1 between 15–45%, total lung capacity (TLC) > 100% predicted, and RV > 175% of predicted with a 6MWD between 100–500 m following a supervised pulmonary rehabilitation program. The primary outcome was the percentage of subjects with an improvement in FEV1 > 15% at one year; secondary outcomes included improvements in post FEV1, quality of life measured by SGRQ, and 6MWD. One hundred and ninety subjects were enrolled into the trial; 128 received the Zephyr EBV, and 62 received usual medical care. Enrolled subjects had a mean FEV1 of 28% predicted and had an RV of 225% of predicted. At one-year post randomization, 47.7% of subjects that received the Zephyr EBV had improved FEV1 > 15% compared to 16.8% receiving usual medical care (Fig. 8). All secondary end points were significantly improved, favoring Zephyr EBV treatment compared to usual care. The between-group differences for FEV1 were 0.106 L, 39.3 m for 6MWD, and a reduction in SGRQ of −7.05 points. A significantly greater percentage of subjects achieved the minimal important clinical difference for improvement of quality of life in Zephyr valve–treated subjects compared to the usual care subjects measured by reduction in SGRQ (56% vs 30%), 6MWD improvement of 25 m (41% vs 19%), RV decline of 310 mL (61% vs 22%), and an improvement of 12% in FEV1 (56% vs 21%) (Fig. 9). These data demonstrated that subjects with predominant heterogenous emphysema with severe hyperinflation and negative collateral ventilation status had significant improvements in lung function, quality of life, and exercise performance that were durable for at least one year following total lobar occlusion with the Zephyr one-way EBV.
Fig. 8.

Primary and secondary outcomes of the LIBERATE randomized controlled trial with use of the Zephyr endobronchial valve to treat heterogenous emphysema. From Reference 35, with permission. 6MWD = 6 minute walk distance; SRGQ = St George’s Respiratory Questionnaire; EBV = endobronchial valve.
Fig. 9.
Responder rates for key outcomes in the LIBERATE study. From Reference 35, with permission. RV = residual volume; SGRQ = St George’s Respiratory Questionnaire; 6MWD = 6-min walk distance; mMRC = Modified Medical Research Council; BODE = body mass index, obstruction, dyspnea, and exacerbations; TLVR = targeted lung volume reduction.
The other EBV approved for treatment of patients with advanced emphysema and severe hyperinflation is the Spiration Valve System (SVS; Olympus, Tokyo, Japan). A multi-center study in China compared EBV placement with SVS to maximal medical therapy.36 The status of fissure integrity was assessed by CT analysis and considered to be intact if fissure integrity was > 90%. All subjects had > 15% heterogeneity scores between the target and ipsilateral non-targeted lobe, with the target lobe having at least 40% destruction by emphysema measured at –920 Hounsfield units. The primary end point for the study was change in FEV1 at 3 months; secondary end points included changes in quality of life measured by SGRQ, the percentage of subjects achieving targeted lung volume reduction (TLVR) of at least 350 mL measured by quantitative CT analysis and 6MWD, and changes in dyspnea scores. At 3 months, FEV1 improved by 0.104 L in the SVS EBV–treated group compared to 0.003 L in the control group. There was a significant improvement in quality of life measured by SGRQ 6 months after EBV treatment in the SVS EBV group compared to control, and TLVR of at least 350 mL was met in 52% of the SVS EBV group at 3 months and 66% of the SVS EBV group at 6 months.
The definitive study using SVS that served as the basis for FDA approval in the United States was the Improving Lung Function in Severe Heterogenous Emphysema With the Spiration Valve System (EMPROVE) study.37 One hundred seventy-two subjects with severe heterogenous emphysema (10% heterogeneity between the target and non-targeted lobe) were randomized 2:1 to SVS treatment (n = 113) versus usual medical care as the control therapy (n = 59). Mean FEV1 between-group difference between the SVS treatment and control groups at 6 and 12 months, respectively, was 0.101 L and 0.099 L (Fig. 10). At 6 months, the SVS-treated group had significant improvements in all secondary end points except 6MWD. Serious adverse events through 6 months were greater in the SVS group (31% vs 11.9%), primarily due to a 12.4% incidence of pneumothorax that required > 7 d of chest tube treatment or other surgical interventions to treat the persistent pneumothorax.
Fig. 10.
Primary (A) and secondary outcomes (B and C) of the EMPROVE randomized controlled trial with use of the SVS EBV to treat heterogenous emphysema. Data from References 11 and 37. SVS = Spiration Valve System; SGRQ = St George Respiratory Questionnaire; mMRC = Modified Medical Research Council.
The above 2 pivotal studies have some differences in terms of entry criteria, whether there was assessment of fissure integrity or collateral ventilation to predict treatment efficacy, the primary outcomes, and the duration of follow-up (Table 3). Despite these differences, both studies corroborate the importance of defining fissure integrity by chest CT or by assessment of collateral ventilation prior to placing EBVs. A recent meta-analysis has shown the importance of defining fissure integrity or collateral ventilation prior to the placement of EBVs on all outcomes in all the randomized trials completed to date.38
Patients with advanced homogeneous emphysema have few treatment options since LVRS is rarely performed in this subgroup and lung transplantation is less commonly available. The use of EBVs has been studied in homogeneous disease, although to a much lesser extent compared to patients with heterogeneous emphysema. One prospective randomized control trial utilized the Zephyr valve and enrolled subjects with severe homogeneous emphysema.39 Homogeneous emphysema was defined as heterogeneity score < 15% between the targeted and ipsilateral non-targeted lobe determined by quantitative chest CT analysis. To determine the target lobe for Zephyr EBV treatment, subjects had to have < 20% difference in perfusion between the right and left lungs as measured by perfusion scintigraphy. The primary end point for this study was change in FEV1 in 3 months, and secondary end points included 6MWD, quality of life measured by SGRQ, and change in dyspnea at 3 months compared to baseline values. The mean difference in FEV1 between treated and control groups at 3 months post randomization was 17% favoring treatment with the Zephyr EBV (Fig. 11). Similarly, improvements in quality of life, 6MWD, and reduction in RV were observed favoring the EBV-treated group. Follow-up of study subjects at one year showed durability of treatment effect favoring the EBV-treated group.40 These data overall suggest that in carefully selected patients with homogeneous emphysema, placement of EBVs may have benefit when the therapy is directed toward the least perfused lobe in patients with homogeneous distribution of emphysema suffering from significant hyperinflation.
Fig. 11.
Primary outcome of IMPACT with the use of the Zephyr EBV to treat subjects with homogenous emphysema and severe hyperinflation. Data from References 11 and 39. EBV = endobronchial valve; SoC = standard of care.
Complications Associated With Endobronchial Valve Placement
Although EBV placement has less morbidity and mortality than LVRS, significant complications are still associated with the intervention.33-37,39 The 2 most common complications include exacerbation of COPD and pneumothorax. Most studies that examined pneumothorax report rates of 25–34% in the periprocedural period (Fig. 12). This is believed due to treating patients with intact fissures or absence of collateral ventilation and performing complete lobar occlusion as the therapeutic goal. In this circumstance, complete or near-complete atelectasis of the targeted lobe can result in ipsilateral non-target lobe expansion at a rate and volume that exceed the plasticity of the non-treated lobe and result in a pneumothorax. Most, but not all, patients who develop a pneumothorax require chest tube placement; ex vacuo pneumothorax occurs in about 15% of the treated group and does not require chest tube placement. In most cases, patients who develop a pneumothorax have the same magnitude of benefits observed in patients treated with EBVs who do not develop a pneumothorax.35
Fig. 12.
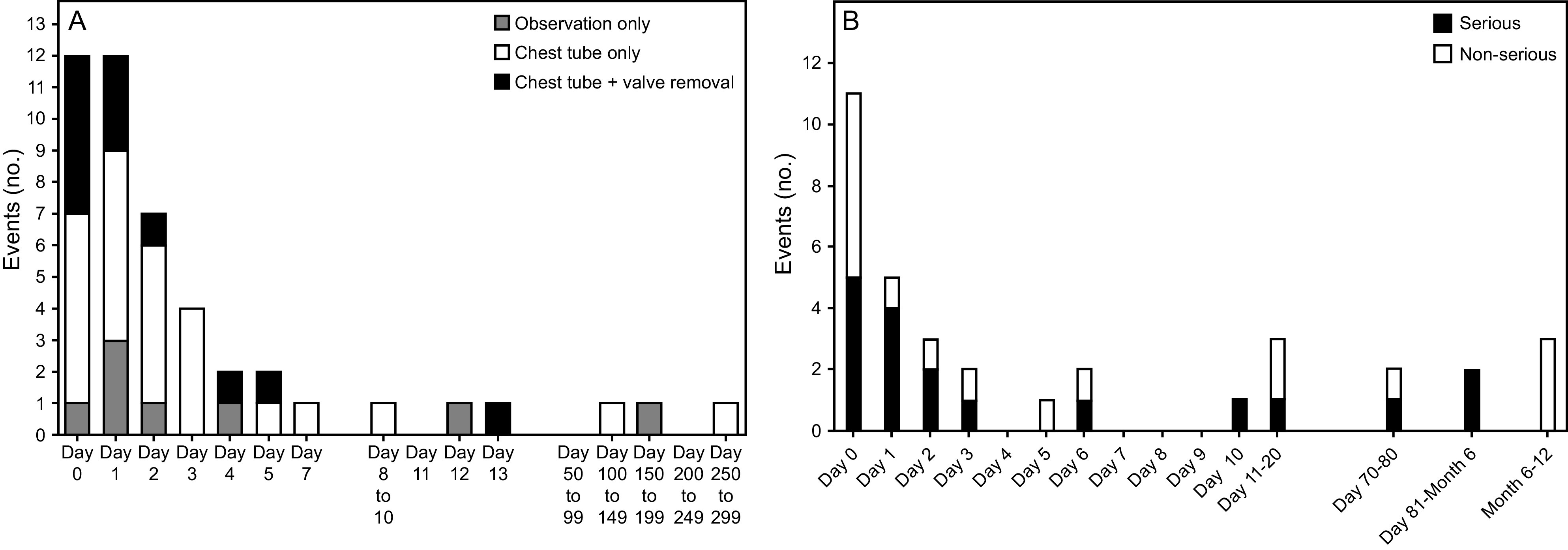
Pneumothorax rates and temporal relationship to EBV treatment in the LIBERATE and EMPROVE randomized controlled multi-center trials. From References 35 and 37, with permission.
The development of a pneumothorax usually occurs in the first 72 h post EBV implantation, necessitating hospitalization after the EBV procedure to monitor for development of pneumothorax and chest tube placement if required. Mortality does occur following EBV treatment; however, most of the mortality is associated with the development of a pneumothorax or its sequelae.35 Other complications reported include EBV expectoration or migration, pneumonia, respiratory insufficiency, and development of granulation tissue.41 Approximately 30–40% of patients may need a revision of the EBV procedure to maintain lobar atelectasis when followed over a period of 2–3 y post EBV implantation.42
Bronchoscopic Treatments for Patients With Non-Intact Fissures or Collateral Ventilation
EBVs are not possible in patients with non-intact fissures or who exhibit collateral ventilation between the target and non-targeted lobes. This is the case for approximately 70–80% of the patient population with severe emphysema. Various therapies have been looked at over the last several years to treat these patients who are not candidates for EBV placement due to the presence of collateral ventilation or non-intact fissures.
Self-Activating Coils for Lung Volume Reduction
Endobronchial coils are non-occlusive devices that are placed straight during bronchoscopy and then upon release assume a pre-shaped coiled configuration. The proposed mechanism of action is a combination of reduction in lung volume by the coils compressing emphysematous tissue and restoring lung elastic recoil. The lung coils function independently of collateral ventilation. Treatments to date usually involve placing 10 coils in each lung over 2 bronchoscopic procedures separated by at least one month.
The endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET) trial was the first prospective, randomized, multi-center controlled trial that compared lung coils to optimal medical treatment in43 subjects with either heterogeneous or homogeneous emphysema. The primary outcome was improvement in quality of life measured by SGRQ at 90 d; secondary end points included change in FEV1, TLC, RV, 6MWD, and reduction in dyspnea. In the coil-treated subject group, quality of life measured by SGRQ improved, and significant improvements were also seen in RV reduction and increases in FEV1 and 6MWD.
The Lung Volume Reduction Coil Treatment vs Usual Care in Patients with Severe Emphysema Study (REVOLENS) trial was a multi-center French prospective controlled trial that compared lung coils to maximal medical therapy in 100 subjects with severe bilateral emphysema with an FEV1 < 50% predicted and RV > 220% predicted. Compared to the control group, the patients who received lung coils had significant improvements in FEV1, RV, and SGRQ but not 6MWD at 12 months.
The Lung Volume Reduction Coil Treatment in Patients with Emphysema Study (RENEW) study was the largest multi-centered trial of lung coils compared to optimal medical therapy in 315 subjects with severe emphysema. Subjects had severe emphysema, either homogeneous or heterogeneous disease, with an FEV1 < 45% predicted and RV > 220% predicted.44 The lobe with the most emphysematous destruction was treated in heterogeneous emphysema, whereas upper lobes were targeted in those with homogeneous emphysema. The 6MWD improved 10.3 m in the coil group, whereas control group 6MWD declined 7.6 m. The between-group mean differences for coil-treated subjects compared to usual care demonstrated improvements in FEV1, SGRQ, and RV that were statistically significant, but group mean data were not clinically important (Fig. 13). Although data from the 3 randomized control trials of coil treatment demonstrate a modest improvement in 6MWD, FEV1, and improvement in quality of life measured by SGRQ, based on the results of this study the FDA did not approve the therapy for clinical use in the United States. However, it still is an available therapy in other countries.
Fig. 13.

Main outcomes of the RENEW lung coil study. From Reference 94, with permission. 6MWD = 6 minute walk distance, RV = residual volume, SGRQ = St George’s Respiratory Questionnaire.
Biologic Lung Reduction
These approaches use biodegradable adhesive or sclerosing agents designed to polymerize in the small airways and alveolar spaces that aim to reduce lung volume by scarring and/or remodeling the targeted emphysematous lung regions over a period of days to weeks. The most studied of these compounds is the AeriSeal (Aeris Therapeutics) emphysematous lung sealant, an agent that uses aminated polyvinyl alcohol and glutaraldehyde mixed with air to create the aerosol foam, which is delivered to targeted pulmonary segments and subsegments via bronchoscopy (Fig. 14). Procedure efficacy is not limited by the presence of collateral ventilation. Early pilot studies demonstrated physiologic and radiographic improvements, but the procedure was associated with a flu-like reaction 8–24 h after the procedure.45,46 The most common adverse events were shortness of breath, fever, and leukocytosis. Subjects also had elevated serum inflammatory markers such as C-reactive protein or erythrocyte sedimentation rate.47 In a pilot study of 20 subjects, 10 with homogeneous and 10 with upper lobe–predominant heterogeneous emphysema, treatment of 2 subsegments of each lung demonstrated improvements in FEV1 and reduction in air trapping.48
Fig. 14.

Example of a flowable adhesive (AeriSeal) used in the ASPIRE study for treatment of severe emphysema.
A large multi-center randomized control trial, the ASPIRE trial, was planned to determine the efficacy of AeriSeal treatment in severe upper lobe–predominant emphysema compared to optimal medical care.22 Financial problems led to premature closure of the study after 95 subjects were enrolled. However, 57 subjects (34 treated and 23 control) had efficacy data available at 3 months, and 34 subjects (21 treated and 13 control) had data at 6 months. At 3 months, the median improvement in FEV1 was 11.4% in the AeriSeal-treated group compared to a change of −2.1% in the control population. There were improvements in SGRQ in the AeriSeal-treated group compared to the control arm. However, 3 subjects in the AeriSeal group had 4 episodes of respiratory failure requiring invasive ventilation, and 2 deaths occurred in the AeriSeal-treated group compared to none in the control group. Forty-four percent of the AeriSeal-treated and 18% of the control subjects required hospitalization during the study. Most of the events were respiratory related.
Currently, the same AeriSeal compound is being studied to determine if it can be used as a treatment to close disruptions in the fissures of patients who are collateral-ventilation positive.43 Preliminary data report successful fissure closure in 80% of subjects who are collateral-ventilation positive following administration of the AeriSeal sealant, with an improvement in lung function and reduction in RV when AeriSeal fissure closure is followed by Zephyr valve placement.49 Prospective multi-center randomized phase 3 trials are being planned.
Placement of Airway Bypass Tracks
Airway bypass tracks aim to create artificial airway passages to deflate trapped air using paclitaxel drug-eluting stents to maintain patency of the bypass tracks. The Exhale Airway Stents for Emphysema (EASE) trial was a randomized sham-controlled study designed to investigate safety and efficacy of these endobronchial stents.50 EASE enrolled 315 subjects with severe homogeneous emphysema that were severely hyperinflated in a one-to-one fashion. The co-primary end points were an increase in FVC by 12% and a decrease in Modified Medical Research Council (mMRC) dyspnea scale of one point. No significant differences between the airway bypass stenting compared with sham procedure were found in any of the primary end points. There was no difference in lung volume or lung function at 6–12 months. Possible explanations included loss of stent function either from expectoration or occlusion by secretions or tissue debris.
Bronchoscopic Thermal Vapor Ablation
In bronchoscopic thermal vapor ablation (BTVA), heated water vapor is used to produce airway thermal injury that results in an inflammatory response followed by airway fibrosis and subsequent atelectasis that induce a reduction in air trapping. This technique is impervious to the presence of collateral ventilation or non-intact fissures. Other advantages are that no foreign body is left in the airway, and the reduction of lung volume is slow and happens over weeks to months so the development of pneumothorax is significantly less than seen with EBVs. A downside is that thermal injury can cause an inflammatory pneumonitis that can mimic the development of a bacterial pneumonia and sometimes can be hard to differentiate clinically.
In a pilot study of 44 subjects with upper lobe–predominant emphysema, BTVA treatment demonstrated a significant reduction in target lobe volume as well as significant improvements in FEV1, quality of life measured by SGRQ, and 6MWD.51 In a multi-center randomized control trial, in 70 subjects comparing BTVA to medical treatment (46 BTVA vs 24 control) with advanced upper lobe–predominant emphysema,52 the average between-groups difference in FEV1 at 6 months was 14.7%; the mean difference in SGRQ scores was –9.7 points. There was a significant improvement in FEV1 in the BTVA-treated group with an absolute increase of 130 mL. The incidence of COPD exacerbation requiring hospitalization was 24% in the BTVA-treated group compared to 4% in the control group, whereas pneumonia or pneumonitis occurred in 18% of the BTVA-treated group and 8% of the control group. A follow-up report in these patients to 12 months shows the durability of treatment effects.53 At this time, BTVA therapy has limited availability outside of the United States.
Airway-Predominant Treatments
Many patients with COPD suffer from abnormalities that predominantly afflict the airways such as chronic bronchitis, bronchiectasis, and frequent moderate and/or severe exacerbations that are not responsive to optimal medical treatment. These patients pose significant challenges, especially those with chronic bronchitis and refractory exacerbations. Chronic bronchitis is defined by cough with regular expectorated sputum over a defined period. Lung health depends on effective mucus clearance; in disease states, thick and viscoid mucus can lead to airway inflammation and infection. Patients with chronic bronchitis associated with COPD have a progressive decrease in lung function, impaired quality of life, reduced exercise tolerance, more frequent exacerbations, and increased mortality due to development of respiratory tract infections. Increased mucus production can occur throughout the tracheobronchial tree. Goblet cells have been reported to be found from the trachea down to the segmented airways. Mucus production in the large airways is more likely to be associated with cough and mucus production in contrast to mucus accumulation in the smaller conducting airways that is hallmarked by dyspnea but less cough and sputum production. Therefore, a high index of suspicion for mucus hypersecretion needs to be maintained in patients with COPD due to the diverse clinical problems that accompany its presence.
New interventions utilizing the bronchoscope have been proposed to reduce mucous hypersecretion by addressing airway goblet cell hyperplasia and the submucosal glands. Liquid nitrogen–metered cryospray, rheoplasty, and TLD are currently undergoing evaluation and are described below (Fig. 15).
Fig. 15.
Types of therapies under investigation for chronic bronchitis and exacerbation reduction.
Metered-Dose Nitrogen Cryospray
With this therapy, liquid nitrogen–metered cryospray is delivered to the central airways with the goal of ablating the epithelium to a depth of 0.1–0.5 mm. After this treatment, it is hoped that rapid generation of normal epithelium will occur without an influx of goblet cells or airway scarring and will serve as an effective treatment for treating chronic bronchitis.
With cryotherapy, the mechanism of action for tissue destruction is based on a development of ice crystals. Given that cells are approximately 70% or more water, exposure to extreme cold leads to ice crystal formation. The development of intracellular and extracellular ice formation leads to cell dehydration with cell shrinkage. Although instant cell death occurs, there is preservation of the extracellular matrix that enables healing with limited amounts of resultant scarring and fibrosis. It is hoped that rapid endobronchial tissue freezing using liquid nitrogen at −196°C with a predetermined meter cryospray will cryoablate abnormal surface epithelium with overgrown mucin-producing goblet cells and adjacent submucosa up to a depth of 0.5 mm. It is hoped that the result of this will facilitate normal bronchial epithelium regrowth with ciliated respiratory epithelial cells that will facilitate removal of mucins from the bronchial airways. In preclinical animal studies, radial cryotherapy ablated the airway surface but preserved the architecture of the airway smooth muscle and cartilaginous ring. This contrasted with hyperthermic treatment that caused denaturation and desiccation and tissue disruption of the submucosal area and cartilaginous ring.
In 16 subjects who underwent cyroablation and subsequent lobar and lung resection for lung cancer treatment, 2 h post liquid-nitrogen cryospray, an intact submucosal extracellular matrix with mild capillary congestion and minimal luminal-oriented acute inflammation and edema was pathologically observed.54 At 14 d post metered liquid-nitrogen cryospray, evidence of airway healing was present with pseudostratified respiratory epithelium and occasional goblet cells. Preservation of the submucosa and cartilage and absence of inflammation and fibrosis were also evident.
In a phase 2 prospective study examining the feasibility of metered cryospray for subjects with chronic bronchitis and COPD, significant improvements in quality of life as measured by SGRQ in the total score and subcomponents of subject symptoms, impacts, and activity were reported.55 These beneficial changes were more pronounced in subjects with a baseline SGRQ > 50 points. These data were reconfirmed in a recently reported study at 6 months post treatment in subjects with COPD with chronic bronchitis with a baseline SGRQ > 50 points on study entry. In patients treated with metered-dose nitrogen cryospray, a decrease in SGRQ of −5.7 points at 6 months was observed compared to the sham group that had an increase in SGRQ of +3.9 points. Subject symptoms, COPD Assessment Test (CAT) score, and cough questionnaire scores were all similarly improved following metered-nitrogen cryospray.
A phase 3 multi-center clinical trial is currently being conducted in 210 subjects with a 2:1 randomization to determine the efficacy of this therapy in alleviating patient symptoms as well as decreasing the presence of cough, sputum production, and rate of COPD exacerbations.56
Airway Rheoplasty for Chronic Bronchitis
Airway rheoplasty delivers short bursts of high-frequency electrical energy to the airway epithelium and submucosal tissues, thereby ablating mucous glands and goblet cells to repopulate the airway structures with more normal epithelium and submucosal tissues. Treatment is done under general anesthesia one lung at the time with progressive applications in 2 cm segments using an expandable basket catheter device working from more distal to proximal airways. Procedure time is about 20–30 min for one lung; treatments are separated by one-month intervals. Patients with pacemakers or irregular heart rhythms are not appropriate for this therapy since the application of the electrical energy is applied using a monopolar electrode with energy delivery gated to the cardiac cycle.
A pooled analysis of 2 separate studies of 30 subjects that had undergone bilateral bronchial rheoplasty reported adverse events, airway histology, and changes in CAT and SGRQ at 6 months (primary outcome) and 12 months.57 The mean goblet cell hyperplasia score was reduced as well as significant reductions in CAT (mean −7.9) and SGRQ (mean −14.6) scores at 6 months that remained reduced at 12 months. The most frequent nonserious, device- and/or procedure-related event through 6 months was mild hemoptysis.
A phase 3 prospective, randomized, parallel group, double-blind, sham-controlled, multi-center clinical trial is currently underway in 270 subjects, with the objective to assess the safety and effectiveness of bronchial rheoplasty for the treatment of the symptoms of chronic bronchitis in patients with COPD.58 The primary end point is change in CAT score; secondary end points include changes in distal airway volume measured by quantitative chest CT analysis, rates of moderate and severe exacerbations of COPD, changes in airway goblet cell number by histological changes, and changes in cough frequency measured by a cough meter.
Targeted Lung Denervation
TLD uses radio-frequency ablation to disrupt parasympathetic nerve transmission to and from the lungs. Patients with COPD have elevated basal sympathetic tone that increases airway contraction and simulated receptors that release larger amounts of acetylcholine that trigger greater mucus production. With TLD, radio-frequency energy is delivered to a sufficient depth from the inner surface of the right and left mainstem bronchi to ablate parasympathetic branches of the vagus nerve that are located on the outer surfaces of the main bronchi. The airway wall and adjacent structures are protected by a cooled water-filled balloon to avoid thermal-induced injury. The goal of TLD is to permanently disrupt airway cholinergic tone to produce airway bronchodilation of greater magnitude and durability than that provided by inhaled long-acting antimuscarinic bronchodilators. The procedure is conducted in one session under general anesthesia and takes about 40–60 min. A risk of gastrointestinal side effects was noted in the early phase clinical trials, and a procedural modification was initiated to measure the distance from the electrode to titrate the radio-frequency ablation energy applied. Distance from the outer margin of the esophageal wall to the TLD device radio-frequency ablation coil is measured using fluoroscopy via a contrast-filled esophageal balloon to titrate the energy applied to reduce the likelihood of esophageal injury. Subsequent treatment has shown this to be an effective method to attenuate development of gastrointestinal symptoms.
A first-in-human study in 22 subjects with COPD showed that TLD treatment was technically feasible when performed in a single lung using a 2-step sequential fashion and that subjects treated with a higher energy (22 W) had greater improvements in quality of life measured by SGRQ, FEV1, and 6MWD. A subsequent small study showed that the procedure could be safely done bilaterally in a single session. The AIRFLOW-2 study was a prospective randomized controlled multi-centered trial in 82 symptomatic subjects (MRC > 2 and CAT > 10) with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II–III COPD that compared TLD to standard care. At one year, subjects that received TLD had a significantly lower number of overall respiratory events (COPD exacerbations, cough, wheezing, chest infections, respiratory failure), and the risk of COPD exacerbations requiring hospitalization was significantly reduced compared to the control group. However, in contrast to the prior smaller study in 22 subjects, AIRFLOW-2 reported no significant effect on reduction of respiratory symptoms or physiological measures despite the reduction in moderate and severe exacerbations.
The results of AIRFLOW-2 were used to design a pivotal prospective randomized controlled multi-centered phase 3 trial of TLD with the primary aim to decrease the rate of moderate and severe exacerbations compared to usual care with sham bronchoscopy at one year.59 Enrollment criteria include subjects with GOLD stage II–III COPD with an exacerbation history of at least 2 moderate or one severe in the past year, a CAT score > 10, and on optimal medical therapy of dual or triple inhaled therapy for at least the past year. The study is planned to enroll the last subject in the second quarter of 2023.
Lung Transplantation for Patients With COPD
Annually, 1,000 patients with COPD will undergo lung transplantation, which is approximately 30.6% of all patients who undergo transplantation.60 Since the lung allocation severity scoring system was implemented, the number of patients undergoing lung transplantation for COPD are less than the number of patients receiving transplantation for interstitial lung diseases. Referral for consideration of lung transplantation in patients with COPD should be considered for those with progressive disease despite maximal medical treatment, those who are not candidates for lung reduction, have a BODE index 5–6, a PaCO2 > 50 mm Hg and/or PaCO2 < 60 mm Hg, and FEV1 < 25%61 (Table 4). Patients with COPD should be considered for listing for lung transplantation when FEV1 is < 15–20% predicted, BODE index > 7, > 3 severe exacerbations during the previous year, one severe exacerbation with hypercapnic respiratory failure, or have moderate to severe pulmonary hypertension.61 In the last several years, lung transplantation has been increasingly performed in patients who are older, have a higher BMI, prior chest surgery, reduced nutritional status, previous chronic infection, comorbid cardiovascular disease, or extrapulmonary comorbid conditions.62
Table 4.
Lung Transplantation for COPD
Lung transplantation in COPD predominately improves quality of life but not survival except for patients with COPD with severe alpha-1 antitryspin deficiency or those with high BODE scores63–70 (Table 5). The median survival post lung transplantation for COPD is 5.9 y.59 Greater than 70% of all lung transplants performed in patients with COPD are double lung transplantations; the remainder are single lung transplantations.71 Bilateral lung transplantation has previously been reported to increase survival in patients with COPD, especially those < 60 y of age.72,73
Table 5.
Outcomes of Lung Transplantation for COPD
Double lung transplantation in patients with COPD has been preferred over single lung transplantation because of concerns for native lung hyperinflation and lung cancer occurrence in the native lung.74,75 Lung cancer has an incidence of 5.2–6.1% in the native lung following single lung transplantation.74,76 Native lung hyperinflation occurs 15–30% of the time following single lung transplantation for COPD.77,78 By contrast, some studies report no impact of single lung transplantation on post-transplant morbidity and even improved survival with single lung transplantation in subjects with COPD.77,79,80
Lung transplantation is a limited option for many patients due to a shortage of donor organs. As a result, single versus double lung transplantation is a balance between individual patient needs versus the demands placed upon society to increase the eligible donor pool for potential recipients.81
Sequential Performance of LVRS or BLVR Prior to or Following Lung Transplantation
Because COPD is a progressive disease, LVRS or BLVR may be followed or preceded by lung transplantation. In patients with advanced emphysema and hyperinflation, LVRS or BLVR may be effective treatments to either delay the need for transplantation or serves to optimize the overall condition of patients who may eventually need lung transplantation.82-84 In some patients following single lung transplantation, subsequent performance of LVRS or BLVR may decrease native lung hyperinflation and improve lung function and performance status.85-90 In patients undergoing lung transplantation post LVRS, the incidence of renal dysfunction requiring dialysis, the use of extracorporeal membrane oxygenation, and postoperative bleeding requiring re-exploration may be higher.91,92 Based on some reports, prior BLVR has no impact on morbidity or survival post subsequent lung transplantation but may alter microbial colonization.92,93
Summary
Many advances have occurred over the past several decades in the development and study of surgical and bronchoscopic interventions to complement the current pharmacologic and non-pharmacologic medical treatments used to treat patients with COPD. None of these therapies replace medical treatments, but in additive fashion, they may bring relief to patients with COPD by reducing dyspnea, improving symptoms of cough and sputum production, and increasing functional performance. Although much has been accomplished, much more needs to be done to address the symptoms of dyspnea and sputum production and development of effective therapies to treat hyperinflation in patients with emphysema that are not candidates for LVRS or bronchoscopic lung reduction with EBVs.
Discussion
MacIntyre: Gerry, I’m afraid I don’t really understand why surgeons don’t want to do more bilateral transplants. The single lung just seems like a half-hearted approach.
Criner: Thanks for the question, Neil. We did a nested case control comparing bronchoscopic lung volume reduction (BLVR), which is uni-lobar treatment, and compared it to lung volume reduction surgery (LVRS), a bilateral sub-lobar treatment. The potency of the treatment effect ends up being the same with a nod toward more favorable outcome with BLVR compared to bilateral LVRS since physiological and functional outcomes were similar, but there was two thirds less morbidity and mortality. Surgeons do want to perform LVRS, but they don’t get the patients directed to them. As has been shown in Europe where BLVR has been approved for the last decade, when more BLVR cases are performed there are more patients referred for LVRS. In our institution before BLVR was approved, we were doing about 6 LVRS a year, and we have a large COPD population; we do about 150 lung transplants a year. But now that we are doing about 100–125 BLVRs annually, we’re now doing about 50–60 LVRS cases annually. Patients come seeking BLVR, but they end up not having intact fissures or are collateral-ventilation positive and then are referred for LVRS. I think you’ll see an uptick in LVRS cases as more patients are referred for BLVR.
MacIntyre: I was specifically thinking about transplants for COPD. The idea of putting a normal or even a stiff lung right next to a hyperinflated lung just doesn’t make a lot of physiologic sense to me.
Criner: Yes, the differences in compliance of a lung allograft compared to emphysematous native lung are remarkable, but it’s amazing how well people can do with a single lung transplant, especially the older patients. Our mean age for transplants at Temple is 68 y. It’s half the surgery, and it’s less ischemic injury, so there can be substantial benefits in outcomes versus risk in some older patients receiving single lung transplantation for advanced emphysema.
Mike Hess: With advances like this and with certain pharmaceutical companies leaving the COPD arena, is the future of COPD therapy more mechanical or pharmacologic?
Criner: I think it’s both. I think MeiLan mentioned there’s been some withdrawal or retreat from pharmacologic groups in terms of COPD investigation right now. But it’s similar to sports fans in Philadelphia; when you’re winning, the sports fans jump on the wagon; and when you’re losing, the fans drift away. I think there is some investigative exhaustion in the area of pharmacologic pathways in COPD from just looking at bronchodilators and inhaled corticosteroids; those agents are only going to get you so far. However, I think there is a substantial future in the pharmacologic investigation of other targets such as mucus hypersecretion. Mucus is an important contributor to disease. Besides mucus, other targets like oxidative injury to the airway and aberrant repair of the airway and lung parenchyma are other pharmacologic targets. I don’t think you have to look any further than lung cancer, which throughout my whole career was the disease where little could be done. But now I can say that there’s much more I can do for patients with lung cancer than I can do for some patients with COPD. I think this is the challenge for the medical community treating patients with COPD, to basically catch up to lung cancer and better characterize or phenotype our patient population for future targeted therapies.
MacIntyre: Gerry, while we’re on the topic of transplants, you didn’t mention anything about animal transplants. Where are we with that?
Criner: Xenografts?
MacIntyre: Any future there?
Criner: I think there is, but I think there’s a long way to go. Same thing with stem cell therapy; there’s probably a future, but I think the lungs will be more challenging than any other organ because it has that delicate balance of airways and vasculature and it’s a diseased cytoskeleton. The challenge will be how to make a successful engraftment onto a diseased cytoskeleton. It’s going to be difficult.
Haynes: With regards to bronchoscopic approaches, are there differences in outcomes at different centers? For example, Joel Cooper reintroduced LVRS with excellent results;1 and in response, multiple centers started to do it, and they subsequently realized that you may not get the same results if it’s not Dr Cooper performing the procedure. Does the same phenomenon apply to these bronchoscopic approaches?
Criner: Yes, it does. And for the most part, like most things, it’s patient selection. And if you’re a small center without comprehensive options for patients with advanced COPD and especially emphysema and you can’t offer other options to patients, you might do BLVR out of trying to do something to help a patient who otherwise might be better suited for LVRS or transplantation. I think patient selection is number one, and having a comprehensive program where you follow the patient and solve post-treatment complications are really the top 2 components of any successful program. In terms of technical ability, there are some differences, but it’s basically patient selection and patient follow-up that are the 2 most important things.
Orr: As far as lung volume measurements or DLCO, I think it’s not done by many. I do it routinely, but it’s not clearly there in the guidelines. What is it going to take to get it measured now that we know there’s treatment that can target that and you’re missing some of important phenotypic information if you’re not measuring it? What’s it going to take for us to put it in guidelines?
Criner: That’s a good argument. The variability of doing any kind of measurement of lung volume is more variable than doing standard spirometry. And industry has shied away from doing lung volumes routinely because of the argument that it’s harder to conduct that and set up a multi-center trial to be able to find a patient population and get reliable indices of treatment. But we did that in the National Emphysema Treatment Trial across 17 centers or more, and we’ve done that in multiple device trials worldwide and were able to standardize the lung volume and DLCO assessments.2 So, I think that the prior notion is wrong, and we don’t emphasize enough the measurement of lung volumes to look for key variables that we could use to select patients with COPD for the best treatment options. One of the problems we face is that most physicians coming out of pulmonary fellowships, especially interventional pulmonology graduates, aren’t extensively exposed to pulmonary function testing. I believe there is a deficit in our training of pulmonary physiology in our current pulmonologists.
Footnotes
Dr Criner discloses relationships with Pulmonx, PneumRX, Broncus, Aeris, and Olympus.
Dr Criner presented a version of this paper at the 59th Respiratory Care Journal Conference, COPD: Current Evidence and Implications for Practice, held June 21–22, 2022, in St Petersburg, Florida.
REFERENCES
- 1.Miravitlles M, Worth H, Soler Cataluña JJ, Price D, De Benedetto F, Roche N, et al. Observational study to characterize 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res 2014;15(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansfield C, Sutphin J, Shriner K, Criner GJ, Celli BR. Patient preferences for endobronchial valve treatment of severe emphysema. Chronic Obstr Pulm Dis 2018;6(1):51-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchetti N, Criner KT, Keresztury MF, Furukawa S, Criner GJ. The acute and chronic effects of bullectomy on cardiovascular function at rest and during exercise. J Thorac Cardiovasc Surg 2008;135(1):205-206, 206.e201. [DOI] [PubMed] [Google Scholar]
- 4.Travaline JM, Addonizio VP, Criner GJ. Effect of bullectomy on diaphragm strength. Am J Respir Crit Care Med 1995;152(5 Pt 1):1697-1701. [DOI] [PubMed] [Google Scholar]
- 5.Brantigan OC, Mueller E, Kress MB. A surgical approach to pulmonary emphysema. Am Rev Respir Dis 1959;80(1, Part 2):194-206. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109(1):106-116.discussion 116-109. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JD, Patterson GA, Sundaresan RS, Trulock EP, Yusen RD, Pohl MS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996;112(5):1319-1329.discussion 1329-1330. [DOI] [PubMed] [Google Scholar]
- 8.The National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116(6):1750-1761. [DOI] [PubMed] [Google Scholar]
- 9.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348(21):2059-2073. [DOI] [PubMed] [Google Scholar]
- 10.Fishman A, Fessler H, Martinez F, McKenna RJ, Jr, Naunheim K, Piantadosi S, et al. ; National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung volume reduction surgery. N Engl J Med 2001;345(15):1075-1083. [DOI] [PubMed] [Google Scholar]
- 11.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, et al. ; National Emphysema Treatment Trial Research Group. Long-term follow-up of patients receiving lung volume reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006;82(2):431-443. [DOI] [PubMed] [Google Scholar]
- 12.Naunheim KS, Wood DE, Krasna MJ, DeCamp MM, Ginsburg ME, McKenna RJ, et al. ; National Emphysema Treatment Trial Research Group. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg 2006;131(1):43-53. [DOI] [PubMed] [Google Scholar]
- 13.Greening NJ, Vaughan P, Oey I, Steiner MC, Morgan MD, Rathinam S, et al. Individualized risk in patients undergoing lung volume reduction surgery: the Glenfield BFG score. Eur Respir J 2017;49(6):1601766. [DOI] [PubMed] [Google Scholar]
- 14.Imfeld S, Bloch KE, Weder W, Russi EW. The BODE index after lung volume reduction surgery correlates with survival. Chest 2006;129(4):873-878. [DOI] [PubMed] [Google Scholar]
- 15.DeCamp MM, Blackstone EH, Naunheim KS, Krasna MJ, Wood DE, Meli YM, et al. ; NETT Research Group. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82(1):197-206.discussion 206-197. [DOI] [PubMed] [Google Scholar]
- 16.Chandra D, Lipson DA, Hoffman EA, Hansen-Flaschen J, Sciurba FC, Decamp MM, et al. ; National Emphysema Treatment Trial Research Group. Perfusion scintigraphy and patient selection for lung volume reduction surgery. Am J Respir Crit Care Med 2010;182(7):937-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caviezel C, Schaffter N, Schneiter D, Franzen D, Inci I, Opitz I, et al. Outcome after lung volume reduction surgery in patients with severely impaired diffusion capacity. Ann Thorac Surg 2018;105(2):379-385. [DOI] [PubMed] [Google Scholar]
- 18.Caviezel C, Froehlich T, Schneiter D, Muehlematter U, Frauenfelder T, Guglielmetti LC, et al. Identification of target zones for lung volume reduction surgery using three-dimensional computed tomography rendering. ERJ Open Res 2020;6(3):00305-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washko GR, Fan VS, Ramsey SD, Mohsenifar Z, Martinez F, Make BJ, et al. ; National Emphysema Treatment Trial Research Group. The effect of lung volume reduction surgery on chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008;177(2):164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim V, Kretschman DM, Sternberg AL, DeCamp MM, Criner GJ; Group NETTR. Weight gain after lung reduction surgery is related to improved lung function and ventilatory efficiency. Am J Respir Crit Care Med 2012;186(11):1109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criner GJ, Belt P, Sternberg AL, Mosenifar Z, Make BJ, Utz JP, et al. ; National Emphysema Treatment Trial Research Group. Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest 2009;135(5):1268-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Come CE, Divo MJ, San José Estépar R, Sciurba FC, Criner GJ, Marchetti N, et al. ; NETT Research Group. Lung deflation and oxygen pulse in COPD: results from the NETT randomized trial. Respir Med 2012;106(1):109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jörgensen K, Houltz E, Westfelt U, Ricksten SE. Left ventricular performance and dimensions in patients with severe emphysema. Anesth Analg 2007;104(4):887-892. [DOI] [PubMed] [Google Scholar]
- 24.Clarenbach CF, Sievi NA, Brock M, Schneiter D, Weder W, Kohler M. Lung volume reduction surgery and improvement of endothelial function and blood pressure in patients with chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med 2015;192(3):307-314. [DOI] [PubMed] [Google Scholar]
- 25.Ginsburg ME, Thomashow BM, Bulman WA, Jellen PA, Whippo BA, Chiuzan C, et al. The safety, efficacy, and durability of lung volume reduction surgery: a 10-year experience. J Thorac Cardiovasc Surg 2016;151(3):717-724.e711. [DOI] [PubMed] [Google Scholar]
- 26.Stanifer BP, Ginsburg ME. Lung volume reduction surgery in the post-National Emphysema Treatment Trial era. J Thorac Dis 2018;10(Suppl 23):S2744-S2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelsattar ZM, Allen M, Blackmon S, Cassivi S, Mandrekar J, Nichols F, et al. Contemporary practice patterns of lung volume reduction surgery in the United States. Ann Thorac Surg 2021;112(3):952-960. [DOI] [PubMed] [Google Scholar]
- 28.Buttery S, Lewis A, Oey I, Hargrave J, Waller D, Steiner M, et al. Patient experience of lung volume reduction procedures for emphysema: a qualitative service improvement project. ERJ Open Res 2017;3(3):00031-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNulty W, Jordan S, Hopkinson NS. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Respir Res 2014;1(1):e000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathinam S, Oey I, Steiner M, Spyt T, Morgan MD, Waller DA. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery program. Eur J Cardiothorac Surg 2014;46(6):1021-1026. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey SD, Berry K, Etzioni R, Kaplan RM, Sullivan SD, Wood DE, et al. Cost effectiveness of lung volume reduction surgery for patients with severe emphysema. N Engl J Med 2003;348(21):2092-2102. [DOI] [PubMed] [Google Scholar]
- 32.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. ; VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363(13):1233-1244. [DOI] [PubMed] [Google Scholar]
- 33.Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373(24):2325-2335. [DOI] [PubMed] [Google Scholar]
- 34.Kemp SV, Slebos DJ, Kirk A, Kornaszewska M, Carron K, Ek L, et al. ; TRANSFORM Study Team. A multi-center randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196(12):1535-1543. [DOI] [PubMed] [Google Scholar]
- 35.Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et al. ; LIBERATE Study Group. A multi-center randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018;198(9):1151-1164. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Wang G, Wang C, Gao X, Jin F, Yang H, et al. The REACH trial: a randomized controlled trial assessing the safety and effectiveness of the spiration valve system in the treatment of severe emphysema. Respiration 2019;97(5):416-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Criner GJ, Delage A, Voelker K, Hogarth DK, Majid A, Zgoda M, et al. Improving lung function in severe heterogenous emphysema with the spiration valve system (EMPROVE): a multi-center, open-label, randomized controlled trial. Am J Respir Crit Care Med 2019;200(11):1354-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel M, Chowdhury J, Zhao H, Lu X, Roth S, Giovacchini CX, et al. Meta-analysis and systematic review of bronchoscopic lung volume reduction through endobronchial valves in severe emphysema. J Bronchology Interv Pulmonol 2022;29(3):224-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, et al. ; IMPACT Study Team. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT study. Am J Respir Crit Care Med 2016;194(9):1073-1082. [DOI] [PubMed] [Google Scholar]
- 40.Eberhardt R, Slebos DJ, Herth FJF, Darwiche K, Wagner M, Ficker JH, et al. ; IMPACT Study Team. Endobronchial valve (Zephyr) treatment in homogeneous emphysema: one-year results from the IMPACT randomized clinical trial. Respiration 2021;100(12):1174-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorelli A, D’Andrilli A, Bezzi M, Ibrahim M, Anile M, Diso D, et al. Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multi-center study. J Thorac Dis 2018;10(Suppl 27):S3315-S3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roodenburg SA, Klooster K, Hartman JE, Koster TD, van Dijk M, Slebos DJ. Revision bronchoscopy after endobronchial valve treatment for emphysema: indications, findings, and outcomes. Int J Chron Obstruct Pulmon Dis 2021;16:1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ing A, Sullivan C, Hersch N, Saghaie T, Williamson J. Reversal of collateral ventilation using endobronchial polymer sealant in a patient with emphysema undergoing endoscopic lung volume reduction (ELVR) with valves: a case report and proof of concept. J Bronchology Interv Pulmonol 2020;27(1):e14-e16. [DOI] [PubMed] [Google Scholar]
- 44.Sciurba FC, Criner GJ, Strange C, Shah PL, Michaud G, Connolly TA, et al. ; RENEW Study Research Group. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA 2016;315(20):2178-2189. [DOI] [PubMed] [Google Scholar]
- 45.Criner GJ, Pinto-Plata V, Strange C, Dransfield M, Gotfried M, Leeds W, et al. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. Am J Respir Crit Care Med 2009;179(9):791-798. [DOI] [PubMed] [Google Scholar]
- 46.Refaely Y, Dransfield M, Kramer MR, Gotfried M, Leeds W, McLennan G, et al. Biologic lung volume reduction therapy for advanced homogeneous emphysema. Eur Respir J 2010;36(1):20-27. [DOI] [PubMed] [Google Scholar]
- 47.Herth FJ, Gompelmann D, Stanzel F, Bonnet R, Behr J, Schmidt B, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal). Respiration 2011;82(1):36-45. [DOI] [PubMed] [Google Scholar]
- 48.Kramer MR, Refaely Y, Maimon N, Rosengarten D, Fruchter O. Bilateral endoscopic sealant lung volume reduction therapy for advanced emphysema. Chest 2012;142(5):1111-1117. [DOI] [PubMed] [Google Scholar]
- 49.Ing AJ, Jayapadman A, Kim WV, Ly C, Ho-Shon K, Lilburn P, et al. Reversal of collateral ventilation using endoscopic polymer foam in COPD patients undergoing endoscopic lung volume reduction with endobronchial valves: a controlled parallel group trial. Respirology 2022;27(12):1064-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah PL, Slebos DJ, Cardoso PF, Cetti E, Voelker K, Levine B, et al. ; EASE trial study group. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomized, sham-controlled, multi-center trial. Lancet 2011;378(9795):997-1005. [DOI] [PubMed] [Google Scholar]
- 51.Snell G, Herth FJ, Hopkins P, Baker KM, Witt C, Gotfried MH, et al. Bronchoscopic thermal vapor ablation therapy in the management of heterogeneous emphysema. Eur Respir J 2012;39(6):1326-1333. [DOI] [PubMed] [Google Scholar]
- 52.Herth FJ, Valipour A, Shah PL, Eberhardt R, Grah C, Egan J, et al. Segmental volume reduction using thermal vapor ablation in patients with severe emphysema: 6-month results of the multi-center, parallel-group, open-label, randomized controlled STEP-UP trial. Lancet Respir Med 2016;4(3):185-193. [DOI] [PubMed] [Google Scholar]
- 53.Shah PL, Gompelmann D, Valipour A, McNulty WH, Eberhardt R, Grah C, et al. Thermal vapor ablation to reduce segmental volume in patients with severe emphysema: STEP-UP 12 month results. Lancet Respir Med 2016;4(9):e44-e45. [DOI] [PubMed] [Google Scholar]
- 54.Slebos DJ, Breen D, Coad J, Klooster K, Hartman J, Browning R, et al. Safety and histological effect of liquid nitrogen–metered spray cryotherapy in the lung. Am J Respir Crit Care Med 2017;196(10):1351-1352. [DOI] [PubMed] [Google Scholar]
- 55.Garner JL, Shaipanich T, Hartman JE, Orton CM, Caneja C, Klooster K, et al. A prospective safety and feasibility study of metered cryospray for patients with chronic bronchitis in COPD. Eur Respir J 2020;56(6):2000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clinicaltrials.gov. RejuvenAir System Trial for COPD With Chronic Bronchitis (SPRAY-CB). Available at: https://clinicaltrials.gov/ct2/show/NCT03893370?term=spray+cb&cond=Chronic+Bronchitis&draw=2.
- 57.Valipour A, Fernandez-Bussy S, Ing AJ, Steinfort DP, Snell GI, Williamson JP, et al. Bronchial rheoplasty for treatment of chronic bronchitis. Twelve-month results from a multi-center clinical trial. Am J Respir Crit Care Med 2020;202(5):681-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clinicaltrials.gov. Clinical study of the RheOx bronchial rheoplasty system in treating the symptoms of chronic bronchitis. Available at: https://clinicaltrials.gov/ct2/show/NCT04677465?term=chronic+bronchitis&cond=rheoplasty&draw=2&rank=1.
- 59.Clinicaltrials.gov. Evaluation of the Safety and Efficacy of TLD in Patients With COPD (AIRFLOW-3). Available at: https://clinicaltrials.gov/ct2/show/NCT03639051?term=AIRFLOW&cond=COPD&draw=2&rank=9.
- 60.Chambers DC, Cherikh WS, Goldfarb SB, Hayes D, Kucheryavaya AY, Toll AE, et al. ; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018;37(10):1169-1183. [DOI] [PubMed] [Google Scholar]
- 61.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34(1):1-15. [DOI] [PubMed] [Google Scholar]
- 62.Arjuna A, Olson MT, Walia R. Current trends in candidate selection, contraindications, and indications for lung transplantation. J Thorac Dis 2021;13(11):6514-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. ; International Society of Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31(10):1073-1086. [DOI] [PubMed] [Google Scholar]
- 64.Marchetti N, Criner GJ. Surgical approaches to treating emphysema: lung volume reduction surgery, bullectomy, and lung transplantation. Semin Respir Crit Care Med 2015;36(4):592-608. [DOI] [PubMed] [Google Scholar]
- 65.Stavem K, Bjørtuft Ø, Borgan Ø, Geiran O, Boe J. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant 2006;25(1):75-84. [DOI] [PubMed] [Google Scholar]
- 66.Tanash HA, Riise GC, Hansson L, Nilsson PM, Piitulainen E. Survival benefit of lung transplantation in individuals with severe α1-anti-trypsin deficiency (PiZZ) and emphysema. J Heart Lung Transplant 2011;30(12):1342-1347. [DOI] [PubMed] [Google Scholar]
- 67.Tanash HA, Riise GC, Ekström MP, Hansson L, Piitulainen E. Survival benefit of lung transplantation for chronic obstructive pulmonary disease in Sweden. Ann Thorac Surg 2014;98(6):1930-1935. [DOI] [PubMed] [Google Scholar]
- 68.Eskander A, Waddell TK, Faughnan ME, Chowdhury N, Singer LG. BODE index and quality of life in advanced chronic obstructive pulmonary disease before and after lung transplantation. J Heart Lung Transplant 2011;30(12):1334-1341. [DOI] [PubMed] [Google Scholar]
- 69.Lahzami S, Bridevaux PO, Soccal PM, Wellinger J, Robert JH, Ris HB, et al. Survival impact of lung transplantation for COPD. Eur Respir J 2010;36(1):74-80. [DOI] [PubMed] [Google Scholar]
- 70.Thabut G, Ravaud P, Christie JD, Castier Y, Fournier M, Mal H, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177(10):1156-1163. [DOI] [PubMed] [Google Scholar]
- 71.ISHLT: The International Society for Heart and Lung Transplantation. Slide Sets - Overall Lung Transplantation Statistics. Available at: https://ishltregistries.org/registries/slides.asp. Accessed May 25, 2023.
- 72.Thabut G, Christie JD, Ravaud P, Castier Y, Brugière O, Fournier M, et al. Survival after bilateral versus single lung transplantation for patients with chronic obstructive pulmonary disease: a retrospective analysis of registry data. Lancet 2008;371(9614):744-751. [DOI] [PubMed] [Google Scholar]
- 73.Pochettino A, Kotloff RM, Rosengard BR, Arcasoy SM, Blumenthal NP, Kaiser LR, et al. Bilateral versus single lung transplantation for chronic obstructive pulmonary disease: intermediate-term results. Ann Thorac Surg 2000;70(6):1813-1818.discussion 1818-1819. [DOI] [PubMed] [Google Scholar]
- 74.Dickson RP, Davis RD, Rea JB, Palmer SM. High frequency of bronchogenic carcinoma after single-lung transplantation. J Heart Lung Transplant 2006;25(11):1297-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez FJ, Alvarez E, Moreno P, Poveda D, Ruiz E, Fernandez AM, et al. The influence of the native lung on early outcomes and survival after single lung transplantation. PLoS One 2021;16(4):e0249758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minai OA, Shah S, Mazzone P, Budev MM, Sahoo D, Murthy S, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol 2008;3(12):1404-1409. [DOI] [PubMed] [Google Scholar]
- 77.Weill D, Torres F, Hodges TN, Olmos JJ, Zamora MR. Acute native lung hyperinflation is not associated with poor outcomes after single lung transplant for emphysema. J Heart Lung Transplant 1999;18(11):1080-1087. [DOI] [PubMed] [Google Scholar]
- 78.Yonan NA, el-Gamel A, Egan J, Kakadellis J, Rahman A, Deiraniya AK. Single lung transplantation for emphysema: predictors for native lung hyperinflation. J Heart Lung Transplant 1998;17(2):192-201. [PubMed] [Google Scholar]
- 79.Benvenuto LJ, Costa J, Piloni D, Aversa M, Anderson MR, Shah L, et al. Right single lung transplantation or double lung transplantation compared with left single lung transplantation in chronic obstructive pulmonary disease. J Heart Lung Transplant 2020;39(9):870-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mal H, Brugière O, Sleiman C, Rullon I, Jebrak G, Groussard O, et al. Morbidity and mortality related to the native lung in single lung transplantation for emphysema. J Heart Lung Transplant 2000;19(2):220-223. [DOI] [PubMed] [Google Scholar]
- 81.Ramos KJ, Harhay MO, Mulligan MS. Which shall I choose? Lung transplantation listing preference for individuals with interstitial lung disease and chronic obstructive pulmonary disease. Ann Am Thorac Soc 2019;16(2):193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bavaria JE, Pochettino A, Kotloff RM, Rosengard BR, Wahl PM, Roberts JR, et al. Effect of volume reduction on lung transplant timing and selection for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1998;115(1):9-17.discussion 17-18. [DOI] [PubMed] [Google Scholar]
- 83.Senbaklavaci O, Wisser W, Ozpeker C, Marta G, Jaksch P, Wolner E, et al. Successful lung volume reduction surgery brings patients into better condition for later lung transplantation. Eur J Cardiothorac Surg 2002;22(3):363-367. [DOI] [PubMed] [Google Scholar]
- 84.Slama A, Taube C, Kamler M, Aigner C. Lung volume reduction followed by lung transplantation-considerations on selection criteria and outcome. J Thorac Dis 2018;10(Suppl 27):S3366-S3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reece TB, Mitchell JD, Zamora MR, Fullerton DA, Cleveland JC, Pomerantz M, et al. Native lung volume reduction surgery relieves functional graft compression after single lung transplantation for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 2008;135(4):931-937. [DOI] [PubMed] [Google Scholar]
- 86.Anderson MB, Kriett JM, Kapelanski DP, Perricone A, Smith CM, Jamieson SW. Volume reduction surgery in the native lung after single lung transplantation for emphysema. J Heart Lung Transplant 1997;16(7):752-757. [PubMed] [Google Scholar]
- 87.Crespo MM, Johnson BA, McCurry KR, Landreneau RJ, Sciurba FC. Use of endobronchial valves for native lung hyperinflation associated with respiratory failure in a single lung transplant recipient for emphysema. Chest 2007;131(1):214-216. [DOI] [PubMed] [Google Scholar]
- 88.Venuta F, De Giacomo T, Rendina EA, Della Rocca G, Flaishman I, Guarino E, et al. Thoracoscopic volume reduction of the native lung after single lung transplantation for emphysema. Am J Respir Crit Care Med 1998;157(1):292-293. [DOI] [PubMed] [Google Scholar]
- 89.Kemp SV, Carby M, Cetti EJ, Herth FJ, Shah PL. A potential role for endobronchial valves in patients with lung transplant. J Heart Lung Transplant 2010;29(11):1310-1312. [DOI] [PubMed] [Google Scholar]
- 90.Perch M, Riise GC, Hogarth K, Musani AI, Springmeyer SC, Gonzalez X, et al. Endoscopic treatment of native lung hyperinflation using endobronchial valves in single lung transplant patients: a multinational experience. Clin Respir J 2015;9(1):104-110. [DOI] [PubMed] [Google Scholar]
- 91.Shigemura N, Gilbert S, Bhama JK, Crespo MM, Zaldonis D, Pilewski JM, et al. Lung transplantation after lung volume reduction surgery. Transplantation 2013;96(4):421-425. [DOI] [PubMed] [Google Scholar]
- 92.Slama A, Ceulemans L, Hedderich C, Boehm P, Van Slambrouck J, Schwarz S, et al. Lung volume reduction followed by lung transplantation in emphysema-a multi-center matched analysis. Transpl Int 2022;35:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuehner T, Clajus C, Fuge J, Jonigk D, Welte T, Haverich A, et al. Lung transplantation after endoscopic lung volume reduction. Respiration 2015;90(3):243-250. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Cooper JD, Patterson GA, Sundaresan RS, Trulock EP, Yusen RD, Pohl MS, Lefrak SS. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996;112(5):1319-1329. [DOI] [PubMed] [Google Scholar]
- 2.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348(5):2059-2073. [DOI] [PubMed] [Google Scholar]














