Abstract
COPD is a progressive inflammatory process affecting both the airways and alveolar structures of the lungs. Exacerbations of COPD are episodes of acute worsening of this inflammatory process, often triggered by infections. The most severe exacerbations are characterized by substantial air trapping and inspiratory muscle overload, which leads to hypercapnic respiratory failure. Pharmacologic therapies focus on intense bronchodilator administration (usually by aerosol), corticosteroids, and antibiotics. Respiratory life support technologies are often needed for severe exacerbations and range from carefully titrated supplemental O2 administration to positive-pressure ventilation (both invasive and noninvasive). Future life support strategies will likely involve extracorporeal life support technologies.
Keywords: COPD, COPD exacerbations, inspiratory muscle overload, air trapping (intrinsic PEEP), hypercapnic respiratory failure, positive-pressure ventilation (invasive and noninvasive)
Introduction
COPD is a chronic inflammatory disease of the airways (bronchitis), often with extension into alveolar spaces (emphysema).1,2 The development of COPD is usually a consequence of exposure to a variety of noxious stimuli (eg, tobacco smoke, indoor smoke) that prompt an inflammatory response involving macrophages, neutrophils, inflammatory mediators, and proteases in susceptible individuals.1,2 Clinical manifestations include dyspnea, cough, sputum production, and progressive functional decline punctuated by exacerbations described below.3
The underlying pathophysiology of COPD involves a progressive inflammatory narrowing of airways.2,4 This leads to increases in the inspiratory work of breathing and excessive inspiratory muscle loading.4,5 Narrow airways coupled with airway collapse and loss of elastic recoil from emphysema also result in an inability to fully exhale to functional residual capacity (dynamic hyperinflation or air trapping).2,5-7 This flattens the diaphragm, rendering it less able to generate inspiratory pressure. Muscle function can also be impaired by the systemic inflammatory effects of chronic diseases like COPD.8,9 Complicating all of this is emphysematous alveolar destruction1 and ventilation/perfusion (V̇/Q̇) mismatching resulting in impaired gas exchange and dead-space development.
COPD Exacerbations and the Development of Hypercapnic Respiratory Failure
Exacerbations, often triggered by infections,10,11 worsen all of the pathophysiologic features of COPD. First, increased muscle loading in combination with worsening muscle function and inefficient gas exchange can reach a tipping point resulting in muscle (pump) failure.12-18 Mathematically, this process can be represented by the pressure-time index (PTI):
Where PI = inspiratory pressure requirement, PImax = maximal inspiratory muscle pressure generation capability, and Ti/Ttot is the fraction of the ventilatory cycle time spent in inspiration.
In COPD exacerbations, all of these factors are impacted: PI increases because of narrow airways; PImax is diminished because of diaphragm impairment, and Ti/Ttot is increased because slower inspiratory flow may prolong the time spent in inspiration. Muscle (pump) failure occurs when the PTI exceeds 0.15.18 Muscle failure coupled with increased dead space results in reduced alveolar ventilation, PaCO2 elevation, and so-called hypercapnic respiratory failure (HCRF).19 Progressive HCRF may result in a respiratory arrest.
A second important physiologic effect of an exacerbation is that lung emptying is further impaired, leading to worsening air trapping.6,7,20 Mean and end-expiratory positive pressures (intrinsic PEEP) increase, which can compromise cardiac filling; impair inspiratory ventilator triggering of assisted breaths; produce more dead space; and, through further diaphragm flattening, create a worsening mechanical disadvantage for the inspiratory muscles.6,7,20-23
Finally, abnormal airway and vascular function lead to worsening V̇/Q̇ matching10,11,24 and impaired gas exchange. Overinflated regions can also compress more healthy regions of the lung, further worsening V̇/Q̇ matching.
Severity Definitions and Impact
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) consortium defines COPD exacerbations as mild, moderate, and severe.25 Mild exacerbations only require out-patient use of short-acting bronchodilators; moderate exacerbations require out-patient use of bronchodilators along with antibiotics and/or corticosteroids; severe exacerbations require emergency department care or hospitalization. Severe exacerbations are further classified by GOLD as “no-respiratory failure” if the PaCO2 is stable, no accessory muscle activity is present, and there is normal mentation; “non-life threatening COPD HCRF” is present if PaCO2 is elevated but < 60 mm Hg, pH > 7.25, some accessory muscle use is occurring but there is normal mentation; and “life threatening COPD HCRF” is present if PaCO2 is elevated and > 60 mm Hg, pH < 7.25, marked accessory muscle use is occurring, and abnormal mentation is present.
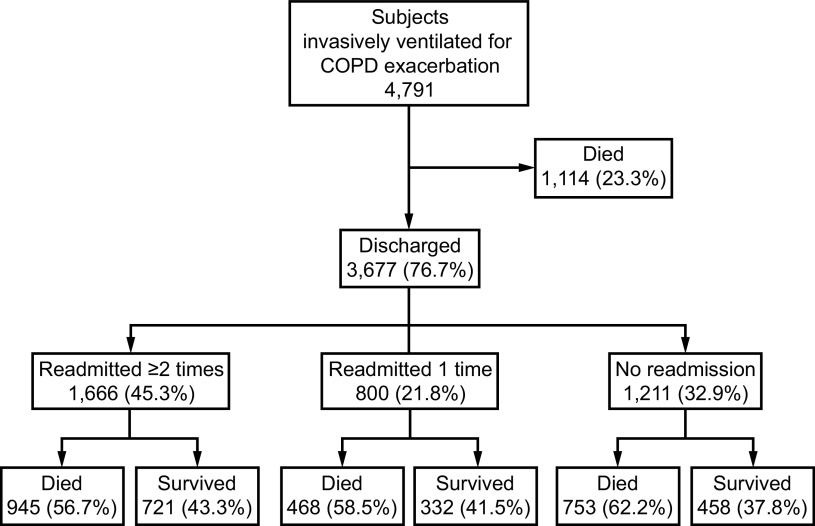
Exacerbations of COPD are common events resulting in over 1 million COPD-related hospitalizations in the United States in 2010.26 COPD HCRF is far less common but often requires respiratory life support technology and is associated with substantial short- and long-term mortality27 (Fig. 1).
Fig. 1.
One-year outcomes in 4,791 subjects with COPD requiring invasive mechanical ventilation. Note an in-hospital mortality of 23% and an overall one-year mortality of 68%. From Reference 27, with permission.
Differential Diagnosis of Acute HCRF in a Patient With COPD
Making a diagnosis of COPD HCRF is usually straight forward—severe hypercapnia, dyspnea/cough/sputum, sometimes fever, and a toxic appearance in a patient with an underlying COPD diagnosis strongly suggests COPD HCRF. However, other causes of HCRF can also strike patients with COPD and include pneumothorax, pulmonary embolism, heart failure, and pleural disease.25 If any of these are possibilities, appropriate imaging, electrocardiography, and blood work may be indicated.
Pharmacologic Management of Acute COPD HCRF
Bronchodilators
Bronchodilators involve both short-acting β-agonists and short-acting muscarinic antagonists given in higher than usual doses in an attempt to balance maximal bronchodilator benefits versus cardiac effects (usually tachycardias) and tremors.25,28,29 The aerosol route is preferred over systemic administration to ensure optimal airway delivery and to reduce systemic effects. In general, a small-volume jet or vibrating mesh nebulizer is easier for severely dyspneic patients to use rather than pressurized metered dose inhalers. In the presence of an artificial airway, doubling or tripling of dosing is generally advised to overcome tube resistance.30 Intravenous (IV) dosing is also an option but usually not necessary. Although supporting evidence is sparse, GOLD recommends continuing any chronic long-acting bronchodilators (long-acting β-agonists or long-acting muscarinic antagonists) during an exacerbation.25
Corticosteroids
Corticosteroids have an extensive evidence base supporting important outcome benefits in COPD exacerbations.25,31-33 GOLD guidelines recommend initial doses of 40 mg prednisone,25 but optimal dosing regimens are controversial. In general, IV dosing is usually used in COPD HCRF although oral dosing is also effective. Inhaled corticosteroids have also been used effectively in COPD exacerbations but are generally reserved for patients capable of performing an acceptable inspiration and breath hold. Importantly, prolonged high dosing of corticosteroids is associated with increased pneumonia (and even sepsis) risk.33 GOLD guidelines emphasize that dosing strategies should thus always include a tapering regimen.
Antibiotics
Because COPD exacerbations are often initiated by a bacterial infection or complicated by a bacterial superinfection,25,34-37 antibiotics often are indicated, especially in the presence of sputum purulence. This is especially true in severe COPD HCRF, and, indeed, GOLD guidelines recommend antibiotics in all patients with COPD requiring mechanical ventilation.25 Various biomarkers such as procalcitonin to guide antibiotic use are controversial and are not used routinely.25 Importantly, pneumonia development can complicate severe exacerbations and should be managed with appropriate imaging studies and targeted antibiotic selection.
Respiratory Life Support for COPD HCRF
Supplemental Oxygen
HCRF in COPD is usually associated with some hypoxemia from 2 mechanisms discussed above: hypoventilation and V̇/Q̇ mismatch. Supplemental oxygen, generally with nasal cannulas, is often needed.25,38
When providing supplemental oxygen in HCRF, the SpO2 target needs to be carefully assessed. This is because in severe COPD with baseline resting hypercapnia excessive supplemental oxygen can actually worsen hypercapnia through a variety of mechanisms including alterations in V̇/Q̇ relationships (especially dead-space worsening) and the Haldane effect on hemoglobin-CO2 binding. Reduced alveolar ventilation may progress to respiratory arrest. Supplemental oxygen should thus be targeted to SpO2 values of 89–93% in most circumstances.38
In recent years, devices capable of delivering warmed and humidified gas through nasal cannulas at flows of up to 60 L/min have been developed.39-40 So-called heated, humidified high-flow nasal cannula (HFNC), these devices can deliver FIO2 ranging from 0.21–near 1.0. In addition to supplying oxygen, the continuous high flow can flush upper-airway dead space clear of exhaled CO2. This functionally reduces dead space by ≥ 100 mL and would, therefore, theoretically reduce ventilation needs and work of breathing. A modest CPAP effect may also be produced in the upper airway assisting breath initiation and flow delivery in the setting of auto-PEEP (see below).
Because of these effects, some have advocated a role for HFNC in the management of COPD HCRF in COPD.40 Indeed, small studies have demonstrated some benefit in those subjects not responding well to standard pharmacotherapy and simple supplemental O2. However, the role of HFNC as a possible precursor to or substitute for noninvasive ventilation (NIV) (see below) remains unclear.
Positive-Pressure Ventilation
In patients with COPD HCRF who are failing optimal medical therapy, respiratory life support with positive-pressure ventilation (PPV) becomes indicated. PPV can provide positive airway pressure during both inspiration and expiration and can be delivered through either a mask interface (NIV) or through an artificial airway (invasive PPV). Importantly, PPV is a supportive technology. It cannot cure the underlying disease process but rather can only buy time for definitive therapies to work while hopefully avoiding further lung injury.
Physiologic effects of PPV in COPD HCRF.
First, by providing positive pressure during inspiration, tidal volume (VT) can be provided that augment alveolar ventilation. This increases alveolar PO2 and clears alveolar PCO2 according to the following relationships:
(Equations 2, 3)
where PIO2 is inspired PO2, PAO2 is alveolar PO2, VA is alveolar ventilation, V̇O2 is total body oxygen consumption, PACO2 is alveolar PCO2, V̇CO2 is total body CO2 production, and k2 and k2 are constants.
Second, unloading inspiratory muscle and reducing patient work with PPV should facilitate muscle function recovery.41,42 According to Equation 1 above, PPV reduces the PTI loads by reducing the PI required by the patient.
Third, PPV can worsen air trapping and its effects.20,23 As noted above, air trapping is a common feature of COPD HCRF due to the long expiratory time constants of COPD lungs coupled with high minute ventilation requirements and significant dead space. Classically, the amount of intrinsic PEEP from air trapping can be quantified by an expiratory hold maneuver although the intrinsic PEEP may be underestimated in the setting of expiratory airway collapse. Because air trapping is often directly affected by minute ventilation in COPD HCRF, any attempts to increase ventilation and CO2 clearance with PPV places the patient with COPD HCRF at risk for worsening air trapping.
Fourth, interactive PPV can be difficult to synchronize with dyspnea-driven patient effort.43,44 This can be compounded by the effect of air trapping and intrinsic PEEP placing an inspiratory threshold load on inspiratory muscles, compromising the triggering process.21 All of these effects create the potential for patient discomfort and imposed muscle loading.
Finally, as in other forms of respiratory failure, PPV can produce maximal and tidal overstretch injury to alveolar structures of the lungs.45-47 Known as ventilator-induced lung injury (VILI), this phenomenon is most likely to occur in less injured regions of the lung subject to higher regional delivery of gas. In general, the development of VILI in COPD HCRF has similar determinants as other forms of acute respiratory failure: excessive maximal stretch (end-inspiratory transpulmonary pressures, often estimated by end-inspiratory airway plateau pressures, > 25–30 cm H2O) and excessive tidal stretch (VT > 8 mL/kg ideal body weight or drive pressures during inspiration exceeding 15 cm H2O).48
Considerations for setting PPV support (both invasive and noninvasive).
PPV settings involve several issues. First, the minute ventilation (frequency × VT; f × VT) must balance 2 competing goals: (1) the minute ventilation must be adequate for required gas exchange and (2) the applied pressures and volumes must minimize air trapping and avoid maximal and tidal overstretch risks of VILI. Achieving this balance may require permissive hypercapnia (eg, pH < 7.15) if cardiovascular function is acceptable.49 This may require neuromuscular blockade and controlled ventilation but is an appropriate trade-off for lung protection and minimization of air trapping.49
Second, whereas neuromuscular blockade and controlled ventilation may be necessary initially in intubated patients to provide safe and effective settings, an important subsequent goal of PPV is to support patients using interactive modes that allow patient activity.44 This will forestall muscle atrophy and minimize sedation. To this end, patient-triggered, pressure-targeted modes often synchronize better with patient efforts than fixed-flow modes.44 Assuring an appropriate expiratory time can be a particular problem with pressure support (PS) in COPD HCRF. Under these conditions, inappropriately low PS breath cycling criteria can excessively lengthen the inspiratory time and shorten the expiratory time, worsening air trapping.50-53 Newer approaches to optimize synchrony include proportional assist ventilation and neurally-adjusted ventilatory assist, but these have not been studied well in COPD HCRF.51,52
Third, the role of PEEP in managing COPD HCRF is complex. Unlike parenchymal lung injury, atelectasis and alveolar flooding are not cardinal features of COPD HCRF. Thus, the role of PEEP to maintain alveolar recruitment and reduce collapse-reopening VILI (atelectrauma) is less important. Instead, PEEP can be beneficial during COPD HCRF for 2 other reasons. As noted above, in the setting of substantial intrinsic PEEP, a significant triggering load is placed on the inspiratory muscles to initiate a breath. Modest amounts of applied PEEP (below the intrinsic PEEP level) can reduce the gradient between alveolar and circuit PEEP to facilitate triggering.21 Clinically, this can be appreciated by careful PEEP titration and observing improvement in the delay between effort initiation and ventilator response. In addition, in a minority of COPD HCRF patients with air trapping, modest levels of applied PEEP may be able to splint open collapsed airways and reduce air trapping. This can be appreciated by careful PEEP titration and noting reductions in airway pressures and end-expiratory lung volumes with the application of PEEP.54
NIV versus invasive PPV.
NIV has many of the same physiologic effects as invasive PPV through an artificial airway.55 However, there are important differences. First, despite sophisticated leak compensation features, NIV cannot always supply volumes and pressures comparable to invasive PPV. These leaks can also affect triggering sensitivity and patient-ventilator synchrony during flow delivery and breath cycling. Second, NIV is applied to the oronasal pharynx, which connects to the esophagus as well as the trachea. Despite the presence of gastro-esophageal sphincters, high positive esophageal pressures can lead to gastric distention. Third, the unprotected trachea, especially in the setting of a distended stomach, is exposed to a potential aspiration risk. Fourth, the absence of an artificial airway also limits the effectiveness of airway suctioning and pulmonary toilet. Fifth, with single-lumen circuits relying on controlled leaks for exhalation, the potential for CO2 rebreathing exists although this is less likely with newer devices. Finally, inspiratory pressure settings on many dedicated NIV systems are referenced to atmosphere, which can lead to confusion in clinicians more familiar with invasive PPV devices where inspiratory pressure is referenced to set expiratory pressure (PEEP).
There are important advantages to NIV versus invasive PPV.56-59 By using a noninvasive mask interface instead of an artificial translaryngeal airway, preservation of glottic function may, in fact, reduce aspiration risk from pharyngeal material. Intermittent breaks from NIV are possible, which allows talking/swallowing; and this, coupled with the absence of an irritating translaryngeal airway, may improve comfort and reduce sedation needs compared to invasive PPV. NIV can also be helpful in maintaining patency of upper-airway in patients with obstructive sleep apnea and reducing venous return and pulmonary edema in selected patients with congestive heart failure without the need for an artificial airway.
Over the last 2 decades it has become apparent that mask NIV is a safe and effective technique that can buy time in early COPD HCRF for other therapies to work and thereby prevent the need for intubation and invasive PPV. Several meta-analyses have confirmed this role and have often shown that mortality can also be reduced as well.59 Our group has recently reported the results of a large observational study (N = 385) in emergency department NIV use in COPD HCRF and demonstrated an 86% success rate, with 73% of the failures (death or intubation) occurring in the first 24 h.60
Setting up NIV for COPD HCRF requires clinical skills to assure proper mask selection for comfort, appropriate inspiratory pressure settings for desired VT and synchrony, and often small amounts of PEEP for triggering ease. More recently NIV has also been applied immediately postextubation to prevent re-intubation. Several studies support this application, underscoring the role of NIV on both the front end and back end of COPD HCRF.61 As noted above, HFNC devices may also accomplish these goals in milder forms of COPD HCRF, but supporting data are limited.62
Extracorporeal Life Support
Extracorporeal life support (ECLS) technology involves removing blood from the venous system, pumping it through a gas exchange device that adds O2 and removes CO2, and then returns the blood to the patient. In medical ICUs caring for patients with COPD HCRF, the most common system accesses the great veins and returns blood to the right atrium, a venovenous (VV) system. Modern VV systems can pump up to 60–80% of the cardiac output through the device and can provide virtually all of the body’s CO2 removal needs while providing substantial (but not all) of the body’s oxygenation needs. As such, VV ECLS can be very effective at supporting patients with severe COPD HCRF. Studies evaluating outcomes in COPD HCRF with ECLS have not been done.
Advances in VV ECLS technology have resulted in much simpler systems using smaller catheters and much lower flows (eg, < 500 mL/min). Under these conditions, CO2 removal remains quite effective, but oxygenation capabilities are minimal. Labeled extracorporeal CO2 removal, these devices would seem ideally suited for HCRF from COPD or asthma where hypercapnia is the major issue and hypoxemia of less concern.63 Complications (especially clotting and bleeding) are fewer with extracorporeal CO2 removal than standard VV ECLS. Again, outcome studies are lacking.
A novel addition to extracorporeal CO2 removal technology is the use of a parallel circuit through the CO2 removal device that converts bicarbonate to CO2.64 This can as much as double the ultimate CO2 removal capabilities. Experience to date is limited to animals where it has proven safe and effective.
Summary
Exacerbations of COPD are common occurrences and are associated with significant morbidity and mortality. The most severe exacerbations are often associated with HCRF. Pharmacologic therapies focus on intense bronchodilator administration (usually by aerosol), corticosteroids, and antibiotics. Respiratory life support technologies are often needed for severe exacerbations and range from carefully titrated supplemental O2 administration to PPV (both invasive and noninvasive). Future management strategies will likely involve ECLS technologies.
Discussion
Carlin: Are there any studies out there looking at the dose, duration, and mode of administration of corticosteroid therapy that are useful for patients with hypercapnic respiratory failure requiring mechanical ventilation?
MacIntyre: About so much and about so long. Seriously, we really don’t know. I have no idea of what the right dose of steroids is. The commonly touted range, including what is listed in GOLD, is usually in the 1–2 mg/kg methylprednisolone equivalent. Until something comes along that says we should use either higher or lower I think that’s a reasonable place to go.
Carlin: That’s interesting. I think we can all relate to the use of corticosteroids with COVID respiratory failure. Studies have shown a dose of dexamethasone per day to be beneficial, yet I see some of my colleagues using 20–40 g per day. Were they wrong? It’s hard to tell.
MacIntyre: Yep. About so much and about so long. And you’d generally keep it going until you were convinced they would turn the corner and then you would come up with some kind of tapering schedule. Again, it doesn’t have a huge evidence base behind it other than to say you ought to taper it. Do you like the way I dodged that answer?
Carlin: It was a good dodge.
Haynes: I want to address the issue of asynchrony in patients on mechanical ventilation both who are on volume controlled ventilation and more pressure-targeted or partial ventilatory support. I always fancied myself as a very good clinician in terms of tailoring the ventilator settings to reduce asynchronies. And when we started using neurally-adjusted ventilatory assist (NAVA) I discovered right away that even though the ventilator graphics looked fine there was a lot of asynchrony present. The fact is that while ventilator graphics can show gross asynchrony, they’re not very sensitive to many forms of asynchrony.
MacIntyre: You are exactly right. It’s an inherent physical limitation. The sensor for our ventilators is up here at the proximal airway (nose), and the action is down here (diaphragm). As a consequence there are going to be inherent delays in sensing activity, and there will be inherent delays in delivering flow and pressure in synchrony with that patient’s effort. It is an inherent problem, and we’ve tried for years to come up with more sensitive triggers and flow controllers. This was the whole reason we went to pressure-targeted ventilation is that because it’s a variable flow it was supposed to be more synchronous with flow. It’s why proportional assist ventilation (PAV) came along as a way to try to match inspiratory flow efforts, timing. You mentioned NAVA. NAVA is very clever. It uses an electromyography sensor array that abuts the diaphragm in the esophagus. It’s designed to measure the electrical activity of the diaphragm (EAdi) and have the ventilator respond to that. You’re no longer measuring here and acting there; you’re going to put them together. And sure enough, NAVA works. It does what it’s supposed to do. It eliminates a lot of the delays and the out-of-phase flow reactions, and it puts a nice picture up on the screen for you. To date, however, I’m not aware of any outcome studies that say, OK, we made the patient more synchronous with this clever approach and have shortened their stay on the ventilator, have reduced ventilator pressures and risks for ventilator-induced lung injury, have increased their ventilator-free days, or have reduced mortality. These important clinical questions have to be answered. And the reason I think they have to be answered and answered satisfactorily is because (a) NAVA is very expensive. This device is only available on one machine; you have to buy the package to go with it, and then you have to buy the catheters; and (b) you need to have the skill to place the catheters and monitor them, troubleshoot, and make sure they stay in place. There are reasons why it hasn’t grabbed the world by storm. However, one good study that shows ventilator-free days are increased could change that. But right now I’m just aware of short-term clinical observation types of trials that show that it works. It does what it’s designed to do.
Haynes: I would say that perhaps the reason why people aren’t as excited about it is because they don’t appreciate the importance of asynchrony. As someone who’s fussed over asynchrony from early in my career, my personal experience with NAVA is that it’s amazing. Auto-PEEP doesn’t matter, bronchopleural fistula doesn’t matter, NAVA can eliminate both inspiratory and expiratory forms of asynchrony. I’ve asked patients, “do you feel better? nod your head yes or no” after changing the patient to NAVA, and they often nod affirmatively. You can also use NAVA in noninvasive ventilation (NIV) mode. We have extubated COPD patients while keeping the EAdi catheter in place, and then applied the NIV interface, and the patient breathes with NIV in the NAVA mode. To me this is a much better approach because the ventilator breathes with the patient instead of the patient trying to breathe with our ventilator settings. Even if you set the ventilator settings appropriately, when you walk away conditions might change; your static ventilator settings don’t adjust to the changing conditions, and in NAVA they do.
MacIntyre: I can’t argue with any of your points. You’re exactly right. I’ve had experience with NAVA, they’ve designed it well and it does what it’s supposed to do. It tracks effort more closely than any other technique we’ve ever had. But I’m going to reiterate what I said: it’s expensive, it requires some skill, and before it comes to prime time I would like to see it used in a patient population that’s having those kinds of issues and that using this approach truly does translate to a better outcome.
*Dean Hess: In ARDS we know that pendelluft occurs when the patient makes a strong inspiratory effort and, before the breath delivery from the ventilator, gas moves from the non-dependent to the dependent part of the lungs. It’s been suggested that this is a contributor to lung injury. As you were talking about the patient with COPD who has auto-PEEP and is making a strong inspiratory effort that does not trigger the ventilator, might there be pendelluft? And might that be harmful to the lungs?
MacIntyre: Thank you for bringing that up, Dean. In fact, that does happen. It hasn’t been well studied, but the phenomenon you’ve described and the reasons underlying why it happens I think are correct. It’s another argument to get patient effort more in sync with the ventilator, and not having the patient make these huge efforts. We didn’t get into the notion of self-inflicted lung injury (SILI) because of time, but these large efforts put patients at risk for this, and it is likely perpetuated by pendelluft in grossly heterogeneous lung injury. Addressing excessive effort is not simple and should focus on reducing inappropriate breathing stimuli (eg, acidosis, pain, anxiety, air trapping, asynchrony) and using sedation (even paralysis) as a last resort.
Carlin: If you have 100 patients with hypercapnic respiratory failure on the ventilator, in your experience how many do you think you would be able to manage by just adjusting the ventilator? No high doses of benzodiazepines or opioids and no paralytic agents. I don’t know how many times I have been managing a patient and overnight the patient is suddenly on significantly higher doses of these medications when I return to the hospital in the morning. What is your experience?
MacIntyre: I don’t have a short answer for you. It often depends on the time course of the disease. At the beginning of respiratory failure when patients are really agitated and dyspneic the role of a paralytic for a day or a little more actually makes a lot of sense to me. That doesn’t mean keep it going. As time passes, the focus changes to making the as patient as comfortable as possible with patient-triggered ventilation. You try and set the trigger sensitivity properly, you use PEEP like we just described, work on better flow synchrony, be careful with the cycling criteria. Then you want to make sure the patient has adequate pain control. I also think we need to recognize that the endotracheal tube (ETT) is not a very comfortable device. Indeed, when you’re only using 5 cm H2O of pressure support and the patient is pulling excessive VT, you have to wonder, do they really need the ETT? Maybe they’re giving just a little hint that it’s time to extubate. I’ll stick my neck out and say that I’m a proponent of earlier tracheostomy for just that reason. I think if we can get the ETT out of their throat that might reduce at least one factor that might stimulate excess drive
*Dean Hess: I’ll just add that narcotics are helpful not only for pain control, but for dyspnea as well.
MacIntyre: Thank you, Dean, I think that’s an important point. If you find yourself having to go to drugs to keep the patient calm I think opioids for pain relief are the first choice. Adding more propofol or precedex I think should be second choice.
Orr: I really enjoyed that. We’ve recently started using two tools in our ICU that I think are interesting in this patient population. One is P0.1, which is an old tool, and the other is electrical impedance tomography (EIT). And I’m just wondering whether you think those tools might be useful in this patient population. In ARDS it’s a different kettle of fish, but I see some parallels in that there are plusses and minuses to more PEEP, to low VT ventilation with permissive hypercapnia versus higher VT, heavier sedation, things like that. At times I feel like we’re flying fairly blind with pressure and flow. A good clinical exam hopefully helps, but do you think we need more information to adequately manage this patient population?
MacIntyre: You brought up some measurements that are interesting. P0.1 is the pressure generated against a closed shutter after 100 ms of effort, and it is byproduct of most of our ventilators because patients can’t open the valve in 100 ms even if they wanted to. So, you can often get a P0.1 as a freebie if you have a ventilator that’s capable of reporting it and calculating it for you. I’ve never found it to be particularly helpful, however. One of the problems I have with it is there are two factors that impact P0.1: respiratory drive and inspiratory muscle strength, and it can be difficult separating which is the cause of a low value. EIT is a much more interesting subject to me. It is a poor man’s CT scan. It has an array of sensors that look like electrocardiogram sensors that are wrapped around the chest. Pulses of electricity are shot across the chest, and the impedance across the chest is measured by these different electrodes. From this a cross-sectional image of the chest can be obtained. Now, it’s only one slice through the chest, but you can get some nice pictures of distribution of both inspiration and the emptying patterns of the lung. You can also break it into regions. Most commonly this is done in quadrants: the 2 anterior and the 2 posterior quadrants. You can look for the effects of PEEP on distribution and the effects of VT on overdistention at the regional (at least quadrant) level, and you can look for Dean’s pendelluft.
*Dean Hess: That’s the way it was first shown.
MacIntyre: Yeah, it’s a very nice way to measure pendelluft. You can look at the potential for regional SILI as the lung expands as inflation proceeds and pendelluft develops. So, I think it has a tremendous future. When you go to a talk on EIT and they always have these really pretty pictures. However, I find when amateurs like me put the belt on to try to play with it I get funky pictures. I just get told that I need to learn how to use it, and I certainly believe that. To be fair, it’s expensive and hasn’t been shown to improve outcomes, the same criticisms I had of NAVA.
Haynes: I want to ask about ventilator-induced diaphragmatic dysfunction, either from keeping people on volume control too long or using too much pressure support?
MacIntyre: I’m not sure it’s from too much pressure support. I’ve been asking this question for a long time: how much effort is enough? I’ve asked people who I really respect like Martin Tobin, John Marini, and others, and the general gist I get from them is if the diaphragm is moving – it doesn’t have to have a huge load on it – if it’s moving it’s probably okay. You can have a fair amount of pressure control. Volume-assist control, what’s your backup rate and is the patient triggering? And is patient triggering enough to prevent atrophy and dysfunction? I don’t know. All the studies of ventilator-induced diaphragmatic dysfunction have been in subjects who were doing nothing. That doesn’t mean that the cure is to do everything, in fact, you may not have to. I’m going to hedge my bet here. I’m not sure what the right answer is, but I get the sense that if the diaphragm is just doing something you’re probably in better shape, and I’ll leave it at that.
*Dean Hess: I was just going to say that Jeremy has the tool to answer the question of auto-PEEP and difficulty triggering, because it was in fact EIT that was first used to illustrate pendelluft in an ARDS setting.
Orr: There’s only so much time in the day, and we have to ultimately decide where to focus our efforts. In ARDS you can see a little more of a straight line to the relevance of EIT, but in this patient population I don’t think there’s any available literature. It’s hard to know if it’s a good topic to look at or if we should focus on other areas such as optimizing use of tracheostomy and NIV.
MacIntyre: I love the idea of NAVA, and I love the idea of EIT, and I’m sorry my career is on the downside now because I think if I could stick around for another 20 years I would find that both of these technologies will be easier to use, cheaper to use, and be associated with better outcomes. A big breakthrough for NAVA will be surface electrodes. I know people have tried to develop these for years and there are issues with filtering the signal. But I can sense that it’s coming.
†Branson: I think ventilator-induced diaphragmatic dysfunction is the typical medical Goldilocks story; too little diaphragmatic work is bad and too much diaphragmatic work is bad. You didn’t touch on it, but there’s a lot of interest now in – I don’t want to call it diaphragmatic pacing because that’s not accurate – diaphragmatic stimulation either transabdominally or through an intravascular catheter. Do you have any thoughts there?
MacIntyre: I’ve heard about it and I can see where it might have some utility in someone who’s not spontaneously breathing on high rate volume assist control, so they’re not moving their diaphragm. And perhaps there it might make some sense. But if you have somebody who can trigger a ventilator on a regular basis, I’m having trouble seeing why a pacer would be of any utility. But again, you need to keep an open mind in this world, I’ve been proved wrong many times.
Orr: We are a site for the trial with the Lungpacer device and it was very interesting, although it is not totally clear why it might be better than a spontaneous breathing trial. The only way I can see that it would be better is if there is an important central fatigue component to diaphragm dysfunction. When you put someone on a spontaneous breathing trial with minimal pressure support, their respiratory drive should be pretty maximal. So why would stimulating a nerve do anything more than make them try to breathe on their own? Again, I don’t think we know how much of that is a factor in hypercapnic COPD.
MacIntyre: I don’t know either. And I’d love to see the study and know if it helps and then I’d like to rethink why it would help. Because as I said, unless it’s somebody who’s not breathing on their own I’m not sure I understand why it would be of benefit.
†Branson: We’re a site in Cincinnati, too, our pulmonary group. It is interesting to try and figure out what happens when – again, you’re not pacing the diaphragm you’re providing some tone. So they do it for 2 h a couple times a day and it doesn’t always trigger the ventilator. Neil, I had the same thought that you did, that this would be great if you paralyzed people, but then it’s not going to work. If you paralyze them it doesn’t matter how often you stimulate the diaphragm, it’s not going to result in a muscle contraction.
MacIntyre: Well, it all depends. Maybe electrical impulses across the diaphragm even if it doesn’t trigger the ventilator might have some utility, I don’t know.
†Branson: It is very interesting. To me it seems like in a patient population who’s at risk for prolonged mechanical ventilation will perhaps have the greatest utility, but we’ll find out in the next 5 years I hope.
MacIntyre: We spent the first half talking about excessive drive and SILI and the like, and now we’re turning around and saying, “Well maybe we ought to stimulate that nerve after all, I don’t know.”
Footnotes
Dean R Hess PhD RRT FAARC is Managing Editor of Respiratory Care.
Richard D Branson MSc RRT FAARC is Editor-in-Chief of Respiratory Care.
Dr MacIntyre discloses relationships with Baxter and Inogen.
Dr MacIntyre presented a version of this paper at the 59th Respiratory Care Journal Conference, “COPD: Current Evidence and Implications for Practice,” held June 21–22, 2022, in St. Petersburg, Florida.
REFERENCES
- 1.Barnes PJ. Pathophysiology of COPD. In Crapo J, editor. Atlas of chronic obstructive pulmonary disease. Philadelphia: Springer Science; 2008:19-32. [Google Scholar]
- 2.Burrows B, Earle RH. Course and prognosis of chronic obstructive lung disease. N Engl J Med 1969;280(8):397-404. [DOI] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007;370(9589):786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278(25):1355-1360. [DOI] [PubMed] [Google Scholar]
- 5.Younes M. Load responses, dyspnea, and respiratory failure. Chest 1990;97(3 Suppl):59S-68S. [DOI] [PubMed] [Google Scholar]
- 6.Del Vecchio L, Polese G, Poggi R, Rossi A. “Intrinsic” positive end-expiratory pressure in stable patients with chronic obstructive pulmonary disease. Eur Respir J 1990;3(1):74-80. [PubMed] [Google Scholar]
- 7.Haluszka J, Chartrand DA, Grassino AE, Milic-Emili J. Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1990;141(5 pt. 1):1194-1197. [DOI] [PubMed] [Google Scholar]
- 8.Campellone JV. Respiratory muscle weakness in patients with critical illness neuromyopathies: a practical assessment. Crit Care Med 2007;35(9):2205-2206. [DOI] [PubMed] [Google Scholar]
- 9.Schols AM, Gosker HR. The pathophysiology of cachexia in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care 2009;3(4):282-287. [DOI] [PubMed] [Google Scholar]
- 10.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007;29(6):1224-1238. [DOI] [PubMed] [Google Scholar]
- 11.White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease. 6: The etiology exacerbations of chronic obstructive pulmonary disease. Thorax 2003;58(1):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldridge F, Davis JM. Effect of mechanical factors on respiratory work and ventilatory responses to CO2. J Appl Physiol 1959;14(5):721-726. [DOI] [PubMed] [Google Scholar]
- 13.Roussos C, Fixley M, Gross D, Macklem PT. Fatigue of the inspiratory muscles and their synergistic behavior. J Appl Physiol Respir Environ Exerc Physiol 1979;46(5):897-904. [DOI] [PubMed] [Google Scholar]
- 14.Bellemare F, Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol 1983;55(1 pt. 1):8-15. [DOI] [PubMed] [Google Scholar]
- 15.Cohen CA, Zagelbaum G, Gross D, Roussos C, Macklem PT. Clinical manifestations of inspiratory muscle fatigue. Am J Med 1982;73(3):308-316. [PubMed] [Google Scholar]
- 16.McKenzie DK, Allen GM, Butler JE, Gandevia SC. Task failure with lack of diaphragm fatigue during inspiratory resistive loading in human subjects. J Appl Physiol (1985) 1997;82(6):2011-2019. [DOI] [PubMed] [Google Scholar]
- 17.Zhu E, Petrof BJ, Gea J, Comtois N, Grassino AE. Diaphragm muscle fiber injury after inspiratory resistive breathing. Am J Respir Crit Care Med 1997;155(3):1110-1116. [DOI] [PubMed] [Google Scholar]
- 18.Bellemare F, Grassino A. Effect pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol Respir Environ Exerc Physiol 1982;53(5):1190-1195. [DOI] [PubMed] [Google Scholar]
- 19.Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J Suppl 2003;47:3s-14s. [DOI] [PubMed] [Google Scholar]
- 20.Milic-Emili J. Dynamic pulmonary hyperinflation and intrinsic PEEP: consequences and management in patients with chronic obstructive pulmonary disease. Recent Prog Med 1990;81(11):733-737. [PubMed] [Google Scholar]
- 21.MacIntyre NR, McConnell R, Cheng KC. Applied PEEP reduces the inspiratory load of intrinsic PEEP during pressure support. Chest 1997;111(1):188-193. [DOI] [PubMed] [Google Scholar]
- 22.Marini JJ, Culver BH, Butler J. Mechanical effect of lung inflation with positive pressure on cardiac function. Am Rev Respir Dis 1981;124(4):382-386. [DOI] [PubMed] [Google Scholar]
- 23.Pinsky MR, Guimond JG. The effects of positive end-expiratory pressure on heart-lung interactions. J Crit Care 1991;6(1):1-15. [Google Scholar]
- 24.West JB, Wagner PD. Pulmonary gas exchange. Am J Respir Crit Care Med 1998;157(4 pt. 2):S82-S87. [DOI] [PubMed] [Google Scholar]
- 25.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2022. https://goldcopd.org/2022-gold-reports
- 26.Jinjuvadia C, Jinjuvadia R, Mandapakala C, Durairajan N, Liangpunsakul S, Soubani AO. Trends in outcomes, financial burden, and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD) in the United States from 2002 to 2010. COPD 2017;14(1):72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajizadeh N, Goldfeld K, Crothers K. What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow-up study. Thorax 2015;70(3):294-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cell BR, MacNee W; ATS ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23(6):932-946. [DOI] [PubMed] [Google Scholar]
- 29.Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD; a systemic review and meta-analysis. Chest 2008;133(3):756-766. [DOI] [PubMed] [Google Scholar]
- 30.MacIntyre NR. Aerosol delivery through an artificial airway. Respir Care 2002;47(11):1279-1288. [PubMed] [Google Scholar]
- 31.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340(25):1941-1947. [DOI] [PubMed] [Google Scholar]
- 32.Alia I, de la Cal MA, Esteban A, Abella A, Ferrer R, Molina F, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilator support. Arch Intern Med 2011;171(21):1939-1946. [DOI] [PubMed] [Google Scholar]
- 33.Sivapalan P, Ingebrigtsen TS, Rasmussen DB, Sørensen R, Rasmussen CM, Jensen CB, et al. COPD exacerbations: the impact of long versus short courses or oral corticosteroids on mortality and pneumonia: nationwide data on 67,000 patients with COPD followed for 12 months. BMJ Open Respir Res 2019;6(1):e000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anthonisen NT, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106(2):196-204. [DOI] [PubMed] [Google Scholar]
- 35.Martinez FJ, Han MK, Flaherty K, Curtis J. Role of infection and antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Expert Rev anti Infect Ther 2006;4(1):101-124. [DOI] [PubMed] [Google Scholar]
- 36.Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puah MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;12:CD010257. [DOI] [PubMed] [Google Scholar]
- 37.Wedzicha J-C, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Resp J 2017;49(3):1600791. [DOI] [PubMed] [Google Scholar]
- 38.Piraino T, Madden M, Roberts KJ, Lamberti JE, Strickland SL. Management of adult patients with oxygen in the acute care setting. Resp Care 2022;67(1):115-128. [DOI] [PubMed] [Google Scholar]
- 39.Cortegiani A, Longhini F, Madotto F, Groff P, Scala R, Crimi C, et al. ; H. F.-AECOPD study investigators. High-flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multi-center non-inferiority randomized trial. Crit Care 2020;24(1):692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z, Zhu L, Zhan J, Liu L. The efficacy and safety of high-flow nasal cannula therapy in patients with COPD and type II respiratory failure: a meta-analysis and systematic review. Eur J Med Res 2021;26(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banner MJ, Kirby RR, MacIntyre NR. Patient and ventilatory work of breathing and ventilatory muscle loads at different levels of pressure support ventilation. Chest 1991;100(2):531-533. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein RS, De Rosie JA, Avendano MA, Dolmage TE. Influence of noninvasive positive-pressure ventilation on inspiratory muscles. Chest 1991;99(2):408-415. [DOI] [PubMed] [Google Scholar]
- 43.Kondili E, Prinianakis G, Georgopoulos D. Patient-ventilator interaction. Br J Anaesth 2003;91(1):106-119. [DOI] [PubMed] [Google Scholar]
- 44.Gilstrap D, MacIntyre N. Patient-ventilator interactions. Implications for clinical management. Am J Respir Crit Care Med 2013;188(9):1058-1068. [DOI] [PubMed] [Google Scholar]
- 45.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157(1):294-323. [DOI] [PubMed] [Google Scholar]
- 46.Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med 2016;37(4):633-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369(22):2126-2136. [DOI] [PubMed] [Google Scholar]
- 48.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372(8):747-755. [DOI] [PubMed] [Google Scholar]
- 49.Nassar B. Should we be permissive with hypercapnia? Ann Am Thorac Soc 2022;19(2):165-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jubran A, Van de Graaff WB, Tobin MJ. Variability of patient-ventilatory interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;152(1):129-136. [DOI] [PubMed] [Google Scholar]
- 51.MacIntyre NR. Design features of modern mechanical ventilators. Clin Chest Med 2016;37(4):607-613. [DOI] [PubMed] [Google Scholar]
- 52.Younes M. Proportional assist ventilation, a new approach to ventilatory support. Am Rev Respir Dis 1992;145(1):114-120. [DOI] [PubMed] [Google Scholar]
- 53.Sinderby C. Neurally adjusted ventilatory assist (NAVA). Minerva Anesthes 2002;68(5):378-380. [PubMed] [Google Scholar]
- 54.Caramez MP, Borges JB, Tucci MR, Okamoto VN, Carvalho CRR, Kacmarek RM, et al. Paradoxical responses to positive end-expiratory pressure in patients with airway obstruction during controlled ventilation. Crit Care Med 2005;33(7):1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hess DR. Noninvasive ventilation for acute respiratory failure. Respir Care 2013;58(6):950-972. [DOI] [PubMed] [Google Scholar]
- 56.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995;333(13):817-822. [DOI] [PubMed] [Google Scholar]
- 57.Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med 2014;174(12):1982-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stefan MS, Nathanson BH, Higgins TL, Steingrub JS, Lagu T, Rothberg MB, et al. Comparative effectiveness of noninvasive and invasive ventilation in critically ill patients with acute exacerbation of chronic obstructive pulmonary disease. Crit Care Med 2015;43(7):1386-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ram FS, Picot J, Lightowler J, Wedzicha JA. Noninvasive positive-pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004(1):Cd004104. [DOI] [PubMed] [Google Scholar]
- 60.Mosher CL, Weber JM, Bhargav S, Adagarla S, Neely ML, Palmer SM, MacIntyre NR. Timing of treatment outcomes and risk factors for failure of BPAP in patients hospitalized for COPD exacerbation. Resp Care 2022, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Resp J 2017;50(2):160426. [DOI] [PubMed] [Google Scholar]
- 62.Tan D, Walline JH, Ling B, Xu Y, Sun J, Wang B, et al. High-flow nasal cannula oxygen therapy versus noninvasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multi-center, randomized controlled trial. Crit Car 2020;24(1):489-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azzi M, Aboab J, Alviset S, Ushmorova D, Ferreira L, Ioos V, et al. Extracorporeal CO2 removal in acute exacerbation of COPD unresponsive to noninvasive ventilation. BMJ Open Respir Res 2021;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanella A, Castagna L, Salerno D, Scaravilli V, El Sayed Deab SAEA, Magni F, et al. Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med 2015;192(6):719-726. [DOI] [PubMed] [Google Scholar]