Abstract
Excess mortality is the difference between expected and observed mortality in a given period and has emerged as a leading measure of the COVID-19 pandemic’s mortality impact. Spatially and temporally granular estimates of excess mortality are needed to understand which areas have been most impacted by the pandemic, evaluate exacerbating factors, and inform response efforts. We estimated all-cause excess mortality for the United States from March 2020 through February 2022 by county and month using a Bayesian hierarchical model trained on data from 2015 to 2019. An estimated 1,179,024 excess deaths occurred during the first 2 years of the pandemic (first: 634,830; second: 544,194). Overall, excess mortality decreased in large metropolitan counties but increased in nonmetropolitan counties. Despite the initial concentration of mortality in large metropolitan Northeastern counties, nonmetropolitan Southern counties had the highest cumulative relative excess mortality by July 2021. These results highlight the need for investments in rural health as the pandemic’s rural impact grows.
Excess mortality estimates show increases in rural mortality during the second year of the COVID-19 pandemic in the United States.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has had a substantial impact on mortality in the United States (U.S.), leading to declines in life expectancy rarely observed since the end of World War II (1, 2). Estimates of excess mortality, which compare observed deaths to those expected in the absence of the pandemic, suggest that the true death toll of the pandemic is much larger than indicated by official COVID-19 deaths alone (3–7). Deaths attributable to the pandemic may have been assigned to causes other than COVID-19 for several reasons. Lack of access to testing in the community, combined with the inconsistent use of postmortem testing for suspected cases, likely resulted in a large share of undiagnosed COVID-19 infections and deaths, especially early in the pandemic (8–12). In addition, persons with comorbid conditions may have had their cause of death assigned to the comorbid condition rather than to COVID-19 (13). Excess deaths not assigned to COVID-19 may also reflect deaths indirectly related to the pandemic, including deaths associated with reductions in access to health care, hospital avoidance due to fear of COVID-19 infection, increases in drug overdoses, and economic hardship leading to housing and food insecurity (14–20). Last, excess mortality may also capture the offsetting effects of pandemic-related declines in mortality, such as reductions in influenza mortality associated with COVID-19 mitigation measures, declines in air pollution and related mortality, and fewer deaths occurring because people who might have died later in the pandemic had already died from COVID-19 (3, 12, 21–25).
For these reasons, it is beneficial to use excess mortality as a measure of the pandemic’s impact, particularly when examining geographic patterns in mortality. Estimates of excess mortality are more comparable spatially than COVID-19–assigned deaths alone, because states use different procedures to assign COVID-19 deaths and local death investigation systems may have different policies and resources that affect assignment of COVID-19 deaths (9, 26). Furthermore, because many COVID-19 deaths were not assigned to COVID-19 early in the pandemic, excess mortality is likely to provide a more accurate measure of the pandemic’s impact for purposes of resource allocation and evaluating health disparities (7, 27, 28). Thus, continued tracking of excess mortality across time and space helps to clarify the total impact of the pandemic, identify where its impacts have been greatest, and implement the most appropriate policy responses.
Prior studies of excess mortality in the U.S. have primarily focused on national- and state-level estimates (5, 6), but estimating the full impact of the COVID-19 pandemic at the county level is necessary to understand finer-grained geographic patterns of excess mortality. Although a prior study generated predictions of excess mortality for 1470 county sets for all months of 2020 combined (4), to the best of our knowledge, there are no estimates of excess mortality at the county-month level across the first 2 years of the pandemic. In addition, expanding these estimates to the second year of the pandemic is critical because the geographic impact of the pandemic has changed markedly since the first year because of changing national- and state-level policies, the availability of vaccines, and the emergence of additional variants.
In the present study, we use a Bayesian hierarchical model to estimate all-cause excess mortality by month for 3127 counties for the period from March 2020 to February 2022. In addition to generating county-month level estimates of excess mortality, we examine spatial patterning of these estimates across Census divisions and metropolitan (metro) and nonmetropolitan (nonmetro) areas between the first and second years of the pandemic.
RESULTS
Across 3127 counties in the U.S., 634,830 estimated excess deaths occurred during the first year of the pandemic (March 2020 to February 2021), and 544,194 estimated excess deaths occurred during the second year (March 2021 to February 2022). This equals a total of 1,179,024 excess deaths during the first 2 years of the pandemic.
Geographic patterns in relative excess mortality
Table 1 shows excess deaths and relative excess mortality across combinations of Census divisions and metro-nonmetro categories during each pandemic year. In this context, relative excess mortality refers to the ratio of the number of excess deaths that occurred during a period of the pandemic to the number of deaths that were expected on the basis of prepandemic trends. It indicates in percentage terms how much higher (or lower) mortality was during a period of the pandemic than would have been expected in the absence of the pandemic. In the entire U.S., relative excess mortality decreased in large metros from 24.0% of expected deaths in the first year to 17.1% in the second year. Meanwhile, relative excess mortality in nonmetro areas increased from 20.2% in the first year to 21.9% in the second year. The decrease in relative excess mortality in large metros between the first and second years was particularly notable in the Middle Atlantic (30.6 to 10.7%), New England (17.6 to 5.8%), and Pacific (26.4 to 16.3%) divisions. The increase in relative excess mortality in nonmetro areas was largest in the Pacific (6.6 to 19.1%), New England (6.0 to 12.7%), and Mountain (22.6 to 28.5%) divisions. The divisions that had the highest relative excess mortality in nonmetro areas during the second year were the Mountain (28.5%), South Atlantic (25.8%), East South Central (25.7%), and West South Central (25.0%) divisions.
Table 1. Excess mortality by census division and metro-nonmetro category, March 2020 to February 2022.
Relative excess mortality is the ratio of excess deaths to expected deaths, indicating the proportion increase in observed deaths compared to expected deaths during a period.
| Excess deaths | Relative excess mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| March 2020 to February 2021 | March 2021 to February 2022 | March 2020 to February 2021 | March 2021 to February 2022 | |||||
| Posterior interval | Posterior interval | Posterior interval | Posterior interval | |||||
| Median | (90% Credible Interval) | Median | (90% Credible Interval) | Median | (90% Credible Interval) | Median | (90% Credible Interval) | |
| East North Central | ||||||||
| Large metro | 49,770 | (43,265–56,415) | 39,732 | (31,578–47,555) | 0.221 | (0.186–0.258) | 0.176 | (0.135–0.218) |
| Medium or small metro | 23,689 | (20,114–27,615) | 23,483 | (18,815–28,118) | 0.182 | (0.150–0.219) | 0.180 | (0.139–0.223) |
| Nonmetro | 17,492 | (14,615–20,401) | 19,374 | (15,813–22,991) | 0.175 | (0.142–0.210) | 0.193 | (0.152–0.237) |
| Total | 90,872 | (77,998–104,372) | 82,607 | (66,298–98,677) | 0.199 | (0.166–0.236) | 0.181 | (0.140–0.224) |
| East South Central | ||||||||
| Large metro | 12,278 | (10,658–13,959) | 12,730 | (10,638–14,873) | 0.221 | (0.186–0.259) | 0.227 | (0.183–0.276) |
| Medium or small metro | 16,134 | (13,878–18,414) | 17,555 | (14,766–20,389) | 0.211 | (0.176–0.248) | 0.227 | (0.184–0.273) |
| Nonmetro | 18,548 | (16,341–20,824) | 20,064 | (17,216–22,803) | 0.238 | (0.204–0.276) | 0.257 | (0.212–0.302) |
| Total | 46,980 | (41,033–53,111) | 50,293 | (42,774–57,883) | 0.224 | (0.190–0.260) | 0.237 | (0.195–0.283) |
| Middle Atlantic | ||||||||
| Large metro | 77,744 | (70,408–85,132) | 27,118 | (17,732–36,180) | 0.306 | (0.269–0.345) | 0.107 | (0.068–0.148) |
| Medium or small metro | 15,352 | (12,873–17,924) | 11,524 | (8,358–14,672) | 0.174 | (0.142–0.210) | 0.130 | (0.091–0.172) |
| Nonmetro | 5,392 | (4,435–6,417) | 6,478 | (5,249–7,672) | 0.166 | (0.132–0.204) | 0.199 | (0.155–0.244) |
| Total | 98,564 | (87,782–109,531) | 45,046 | (31,696–58,287) | 0.263 | (0.228–0.301) | 0.120 | (0.082–0.161) |
| Mountain | ||||||||
| Large metro | 22,423 | (19,991–24,945) | 21,145 | (17,929–24,329) | 0.265 | (0.230–0.304) | 0.246 | (0.201–0.294) |
| Medium or small metro | 15,634 | (13,461–17,789) | 17,610 | (14,826–20,368) | 0.211 | (0.176–0.247) | 0.233 | (0.189–0.280) |
| Nonmetro | 8,506 | (7,381–9,637) | 10,891 | (9,487–12,302) | 0.226 | (0.191–0.265) | 0.285 | (0.240–0.335) |
| Total | 46,586 | (40,955–52,228) | 49,673 | (42,356–56,997) | 0.237 | (0.203–0.274) | 0.249 | (0.205–0.296) |
| New England | ||||||||
| Large metro | 11,658 | (9,809–13,661) | 3,838 | (1,340–6,163) | 0.176 | (0.144–0.212) | 0.058 | (0.019–0.096) |
| Medium or small metro | 7,721 | (6,268–9,204) | 3,765 | (1,899–5,704) | 0.151 | (0.119–0.186) | 0.073 | (0.036–0.115) |
| Nonmetro | 1,176 | (555–1,817) | 2,513 | (1,790–3,237) | 0.060 | (0.028–0.096) | 0.127 | (0.087–0.170) |
| Total | 20,508 | (16,765–24,562) | 10,098 | (5,137–15,152) | 0.150 | (0.119–0.185) | 0.073 | (0.036–0.114) |
| Pacific | ||||||||
| Large metro | 64,554 | (57,648–71,672) | 39,880 | (31,028–48,514) | 0.264 | (0.229–0.302) | 0.163 | (0.123–0.206) |
| Medium or small metro | 17,460 | (14,370–20,882) | 21,126 | (16,927–25,212) | 0.156 | (0.125–0.193) | 0.188 | (0.145–0.232) |
| Nonmetro | 1,974 | (1,111–2,881) | 5,802 | (4,623–6,889) | 0.066 | (0.036–0.099) | 0.191 | (0.146–0.235) |
| Total | 84,016 | (72,995–95,495) | 66,750 | (52,732–80,806) | 0.217 | (0.184–0.255) | 0.172 | (0.131–0.217) |
| South Atlantic | ||||||||
| Large metro | 55,890 | (47,773–64,207) | 56,438 | (46,118–67,140) | 0.196 | (0.163–0.231) | 0.196 | (0.154–0.242) |
| Medium or small metro | 43,570 | (36,867–50,390) | 48,412 | (39,633–56,873) | 0.187 | (0.154–0.223) | 0.204 | (0.161–0.248) |
| Nonmetro | 21,878 | (19,501–24,581) | 23,231 | (19,999–26,420) | 0.245 | (0.213–0.284) | 0.258 | (0.215–0.305) |
| Total | 121,384 | (103,917–139,195) | 127,973 | (105,835–150,585) | 0.200 | (0.166–0.236) | 0.208 | (0.166–0.254) |
| West North Central | ||||||||
| Large metro | 11,832 | (10,029–13,708) | 10,508 | (8,171–12,870) | 0.185 | (0.153–0.221) | 0.164 | (0.123–0.208) |
| Medium or small metro | 11,183 | (9,405–13,024) | 9,351 | (6,988–11,648) | 0.181 | (0.148–0.217) | 0.150 | (0.108–0.194) |
| Nonmetro | 12,517 | (10,422–14,779) | 11,136 | (8,394–13,831) | 0.169 | (0.137–0.206) | 0.150 | (0.109–0.193) |
| Total | 35,518 | (29,891–41,461) | 31,074 | (23,777–38,178) | 0.178 | (0.146–0.214) | 0.155 | (0.114–0.197) |
| West South Central | ||||||||
| Large metro | 36,978 | (32,875–41,278) | 33,580 | (28,191–39,012) | 0.251 | (0.217–0.288) | 0.224 | (0.181–0.270) |
| Medium or small metro | 32,654 | (29,620–35,833) | 28,494 | (24,580–32,364) | 0.307 | (0.271–0.347) | 0.266 | (0.221–0.313) |
| Nonmetro | 20,110 | (18,007–22,284) | 18,608 | (15,893–21,309) | 0.273 | (0.238–0.312) | 0.250 | (0.206–0.297) |
| Total | 89,704 | (80,395–99,275) | 80,736 | (68,893–92,289) | 0.274 | (0.239–0.312) | 0.243 | (0.201–0.288) |
| United States | ||||||||
| Large metro | 343,160 | (302,459–384,568) | 244,887 | (193,122–296,515) | 0.240 | (0.206–0.277) | 0.171 | (0.130–0.215) |
| Medium or small metro | 183,352 | (156,925–210,142) | 181,466 | (147,296–214,867) | 0.196 | (0.163–0.232) | 0.192 | (0.151–0.236) |
| Nonmetro | 107,680 | (92,586–123,227) | 118,070 | (99,245–137,218) | 0.202 | (0.169–0.238) | 0.219 | (0.178–0.264) |
| Total | 634,830 | (552,081–718,370) | 544,194 | (439,021–648,996) | 0.219 | (0.185–0.256) | 0.187 | (0.145–0.231) |
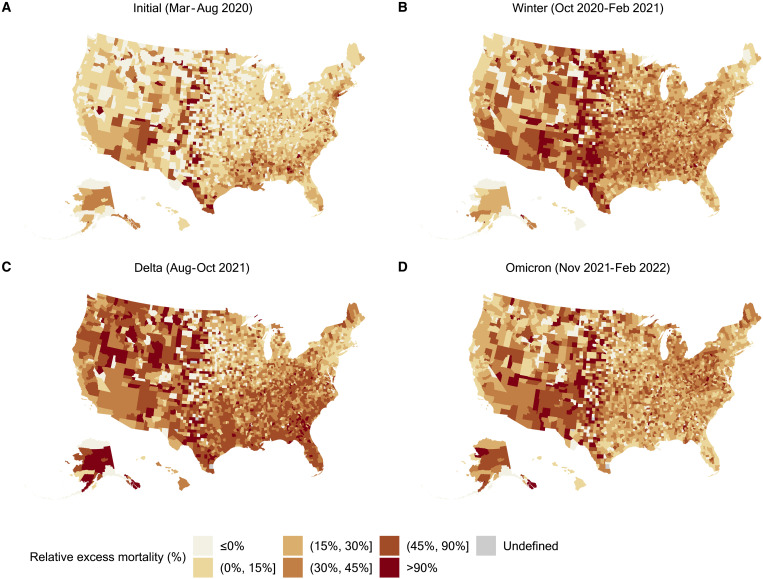
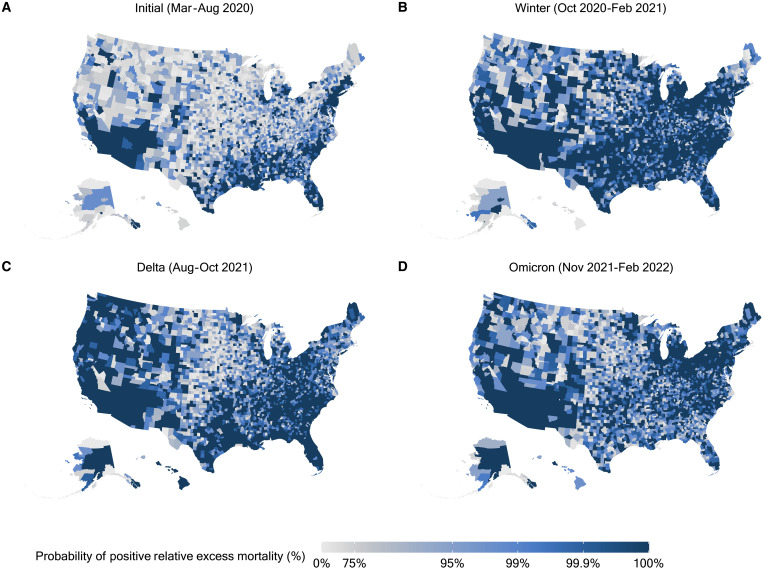
Figure 1 shows the evolution of relative excess mortality across four mortality peaks during the pandemic across U.S. counties. These maps show the extent of excess mortality and demonstrate how excess mortality shifted from coastal regions early in the pandemic into the rest of the country as the pandemic progressed. During the Delta peak, excess mortality became more concentrated throughout the South and West while the spatial distribution of excess mortality during the Omicron peak was less geographically consistent. Figure 2 shows the probability of counties having any positive relative excess mortality across four mortality peaks during the pandemic, demonstrating that during each wave of the pandemic, observed mortality fell above the range of values we would have expected in the absence of the COVID-19 pandemic.
Fig. 1. Relative excess mortality across U.S. counties during four mortality peaks, March 2020 to February 2022.
(A to D) Each county in the map is colored according to its relative excess mortality (the ratio of excess deaths over expected deaths). Each of the four maps refers to one of the four peak periods of the pandemic, months of particularly high excess mortality. Relative excess mortality for counties with 0 expected deaths was classified as “Undefined.”
Fig. 2. Probability of positive excess mortality across U.S. counties during four mortality peaks, March 2020 to February 2022.
(A to D) Each county in the map is colored according to the posterior probability that the observed death count is higher than the expected one. We highlight counties where the probability of positive relative excess mortality is higher than 0.75. The four maps refer to the four peak periods of the pandemic, months of particularly high excess mortality.
Temporal trends in relative excess mortality
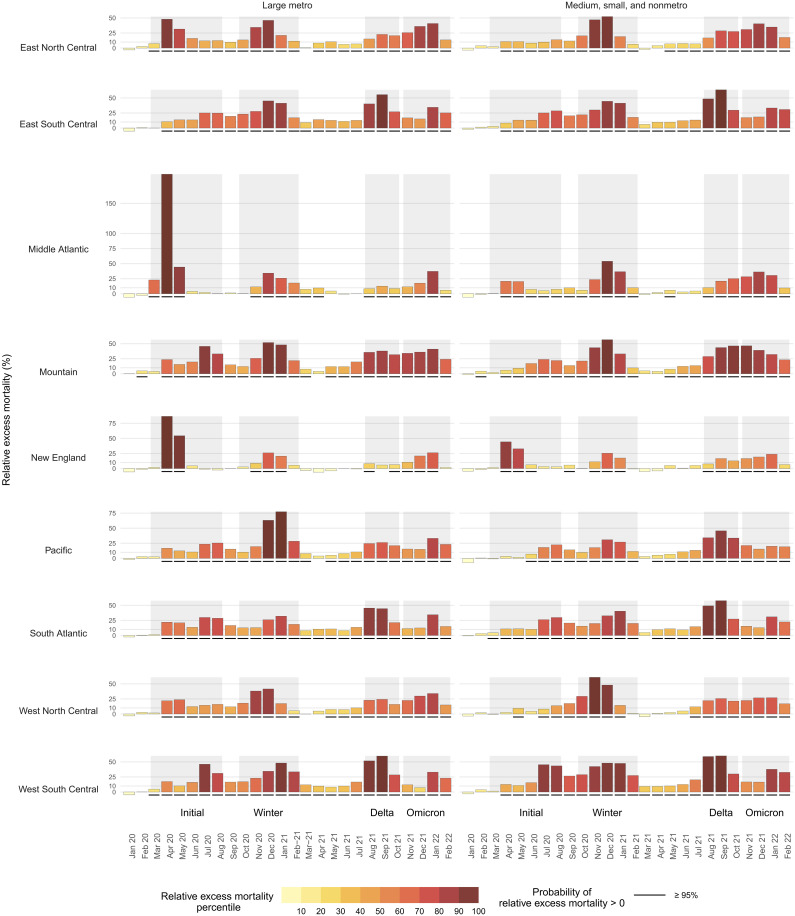
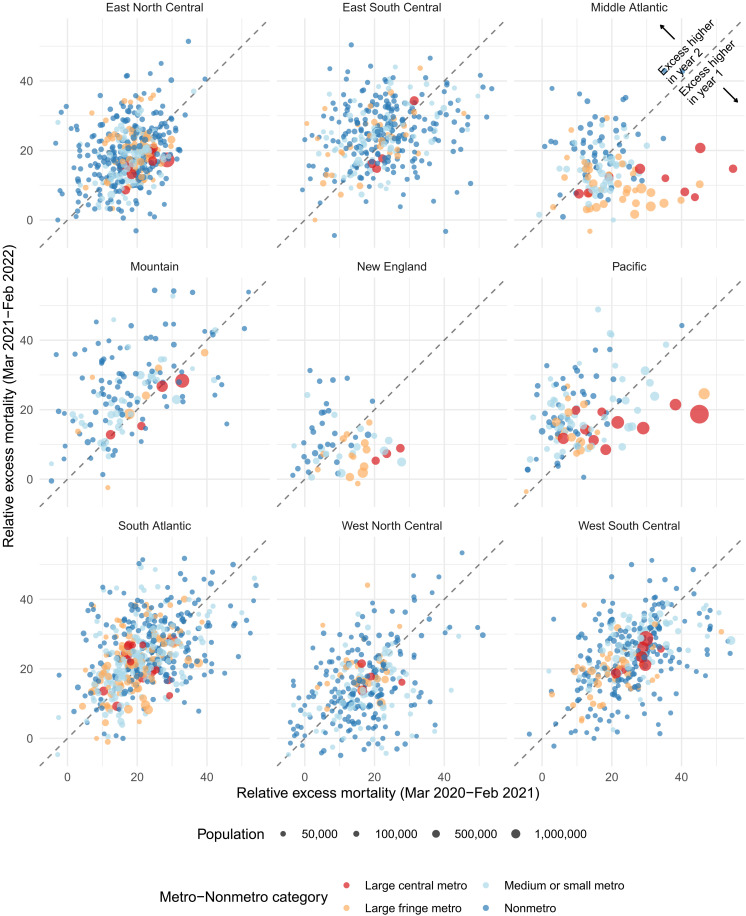
Throughout the pandemic, national trends in excess mortality reflect the aggregation of heterogeneous trends across disparate regions and metro and nonmetro areas. To explore subnational patterns, Fig. 3 shows temporal trends in relative excess mortality across combinations of Census divisions (New England, Middle Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, and Pacific) and metro-nonmetro categories (large metro versus medium metro, small metro, and nonmetro areas). The initial peak in excess mortality nationally was mostly driven by high excess mortality in large metros within the Middle Atlantic division. In contrast, the Winter peak spared this region and affected counties across the metro-nonmetro continuum in other divisions. As the pandemic progressed, there was a higher degree of concordance in temporal patterns across areas, which was especially evident during Delta and Omicron. Figure 4 further illustrates differences in the geography of the pandemic between the first and second years by directly comparing relative excess mortality in the 2 years across divisions and metro-nonmetro categories. Large metro counties mostly had greater relative excess mortality in the first year of the pandemic than they did in the second year. In contrast, nonmetro counties were more likely to have greater relative excess mortality in the second year compared with the first year. This pattern is indicative of the emergence of a rural mortality disadvantage in the second year of the pandemic.
Fig. 3. Temporal trends in relative excess mortality by census division and metro-nonmetro category, March 2020 to February 2022.
The large metro category includes large central metros and large fringe metros. All nonlarge metro counties are classified as medium metros, small metros, and nonmetro areas. The shaded intervals behind the bars separate the different waves of the COVID-19 pandemic as follows: Initial (March 2020 to Aug 2020), Winter (October 2020 to February 2021), Delta (August 2021 to October 2021), and Omicron (November 2021 to February 2022). The height of each bar reflects relative excess mortality (excess deaths as a percentage of expected deaths). The color of the bars reflects each division-month position (percentile) in the overall distribution of relative excess mortality. Black, solid segments below the bars indicate units for which the posterior probability of positive excess mortality is above 95%.
Fig. 4. Comparison of relative excess mortality by pandemic year, March 2020 to February 2022.
Each point in the graph represents a county and reflects its relative excess mortality from March 2020 to February 2021 (horizontal axis) and its relative excess mortality from March 2021 to February 2022 (vertical axis). We excluded counties with less than 10,000 residents to make the relationship between the two variables clearer. The 45° line separates the plot into two parts. Points above the line saw higher excess mortality in the second year of the pandemic compared to the first. Points falling below the line instead saw lower excess mortality in the second year compared to the first.
Cumulative trends in relative excess mortality
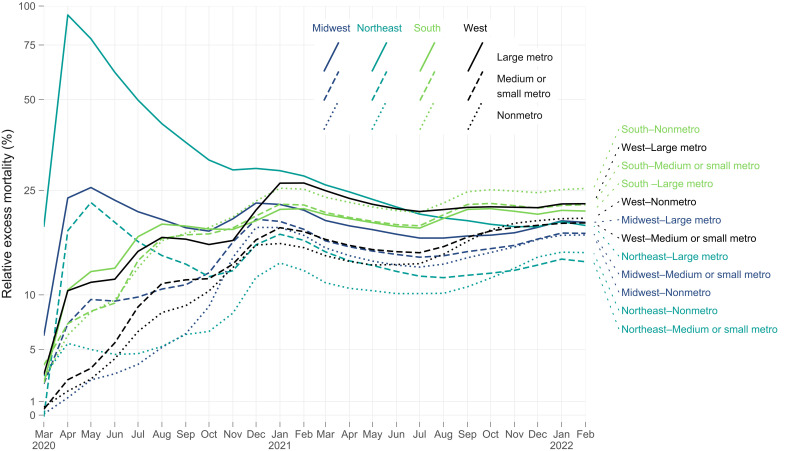
Figure 5 examines relative excess mortality in cumulative terms for combinations of Census regions (Northeast, Midwest, South, and West) and metro-nonmetro categories (large metro, medium and small metro, and nonmetro areas). In the initial months of the pandemic, large metros in the Northeast experienced exceptionally high relative excess mortality. However, the months that followed saw a prolonged period of intensification of relative excess mortality across the South and West. By July 2021, cumulative relative excess mortality in nonmetro counties in the South and large metro counties in the West had exceeded cumulative relative excess mortality in large metros in the Northeast. Cumulative relative excess mortality remained higher in the nonmetro South than in any other region through the end of February 2022. By the end of February 2022, cumulative relative excess mortality was lower in large metro, medium and small metro, and nonmetro areas in the Northeast than in large metro, medium and small metro, and nonmetro areas in the South and West. The regions with the highest cumulative relative excess mortality at the end of February 2022 were nonmetro areas in the South, large metros in the West, medium and small metros in the South, large metros in the South, and nonmetro areas in the West.
Fig. 5. Rolling cumulative relative excess mortality by census region and metro-nonmetro category, March 2020 to February 2022.
Each line represents the rolling cumulative relative excess mortality for a combination of metro-nonmetro category and Census region. Large metro includes large central metros and large fringe metros. Each Census region is represented by a different line color: dark blue for Midwest, teal for Northeast, yellow-green for South, and black for West. Each metro-nonmetro category is represented by a different line type: solid for large metro, dashed for medium or small metro, and dotted for nonmetro. The y axis greater than 1 has been log-transformed (base 10) to facilitate comparisons between categories at lower values of relative excess mortality. Rolling cumulative relative excess mortality is calculated as the sum of excess deaths divided by the sum of expected deaths for all months from March 2020 through a given month. Mean estimates for expected deaths were used for these calculations. For example, values for February 2022 reflect total excess deaths for 24 months of the pandemic, from March 2020 through February 2022. Decreasing cumulative relative excess mortality indicates months with relative excess mortality below average to date for a given combination of Census region and metro-nonmetro category. Increasing cumulative relative excess mortality indicates months with relative excess mortality above average to date for a given combination of Census region and metro-nonmetro category.
County-level trends in relative excess mortality
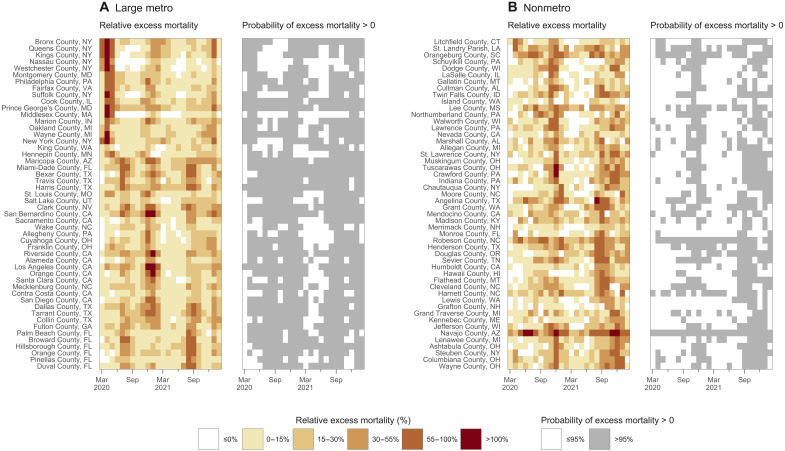
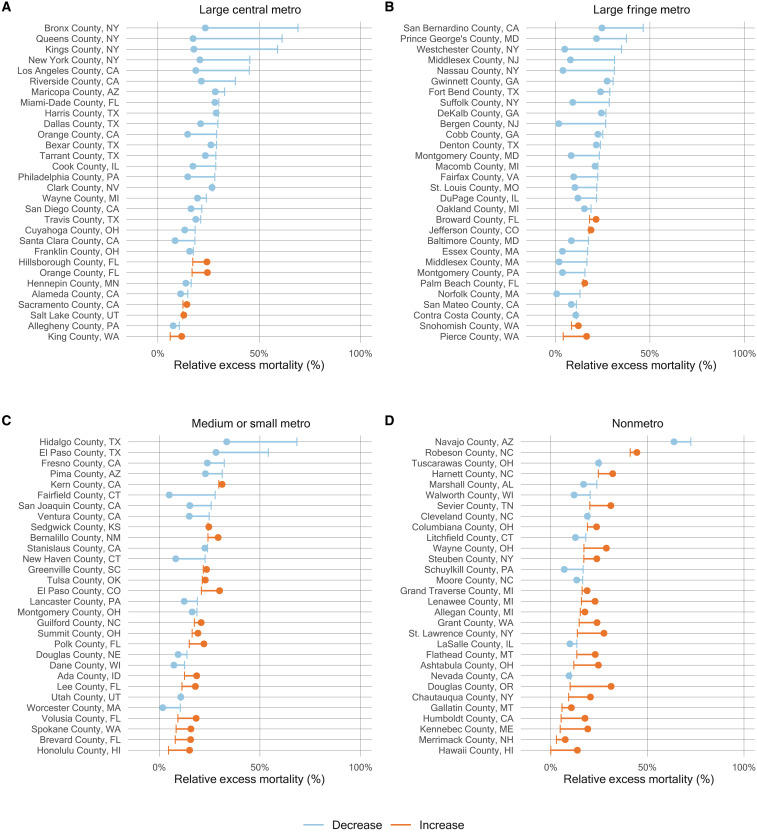
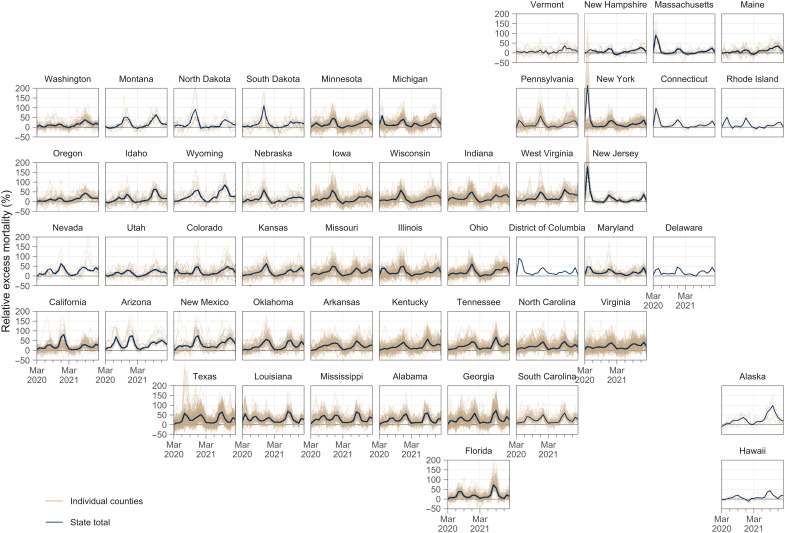
An emerging rural disadvantage is also visible when examining temporal trends for individual counties. Figure 6 shows temporal trends in relative excess mortality for the most populous counties among large metro and nonmetro counties. Among large metro counties, relative excess mortality was especially high in Northeastern counties in the early pandemic and in California counties during the Winter peak. In nonmetro counties, marked increases in mortality were observed during the second year of the pandemic, especially during the Delta peak. Figure 7 explores changes in excess mortality between the first and second years of the pandemic among the most populous counties in each metro-nonmetro category. In the most populous large metro counties, substantial declines in excess mortality were observed between the first and second years. For nonmetro counties, the opposite pattern was observed. These areas were generally spared in the first year, after which they experienced high excess mortality in the second year. Figure 8 displays temporal trends for each county alongside state trend lines. This figure reveals substantial variation in temporal trends in relative excess mortality across states along with substantial variation in relative excess mortality trends within states.
Fig. 6. Temporal trends in relative excess mortality among the most populous U.S. counties by metro-nonmetro category, March 2020 to February 2022.
Each cell in the heatmaps represents a county-month. (A) The 50 most populous large metro counties. (B) The 50 most populous nonmetro counties. Large metro includes large central metros and large fringe metros. In the shaded heatmaps colored from white to dark red, darker and redder colors indicate higher relative excess mortality. In the white-and-gray heatmaps, gray cells indicate county-months with a greater than 95% probability of positive excess mortality. Counties were sorted vertically on the basis of the month when the highest peak of excess mortality occurred. Counties at the top of the heatmaps thus had their highest relative excess mortality earlier in the pandemic. In contrast, those at the bottom had their highest relative excess mortality later in the pandemic.
Fig. 7. Change in relative excess mortality between the first and second pandemic years in the most populous U.S. counties by metro-nonmetro category, March 2020 to February 2022.
(A to D) Each line in the four panels represents a county. For each line, the end point that is a vertical line reflects relative excess mortality in the first year of the pandemic (March 2020 to February 2021), while the end point that is a dot indicates relative excess mortality in the second year of the pandemic (March 2021 to February 2022). The color of the line distinguishes between counties that saw a decline in relative excess mortality (blue) and those that saw an increase (orange). The 30 most populous counties were selected for each metro-nonmetro category.
Fig. 8. Temporal trends in relative excess mortality across U.S. counties in each state, March 2020 to February 2022.
Each plot presents data for a different state, and position in the figure reflects the approximate geographical position of each state within the U.S. State-level monthly time series of relative excess mortality are denoted by solid dark blue lines. Each light brown line represents the monthly time series for one county in that state. Counties with populations greater than 15,000 residents are depicted.
DISCUSSION
This study produced monthly estimates of excess mortality for 3127 counties in the U.S. from March 2020 through February 2022, identifying 1,179,024 excess deaths during the first 2 years of the pandemic. Between the first and second years of the pandemic, relative excess mortality decreased in large metros and increased in nonmetro areas. The increases in excess mortality in nonmetro areas occurred most markedly during the Delta wave of the pandemic. By the end of February 2022, nonmetro areas in the South had the highest cumulative relative excess mortality, surpassing large metros in the Northeast and other areas that were affected heavily in the early pandemic.
Prior studies of excess mortality have largely produced estimates for the year 2020 (3–6, 29), leaving patterns of excess mortality during 2021 and 2022 understudied. The Centers for Disease Control and Prevention (CDC) has reported an estimate of approximately 1.1 million excess deaths in the U.S. from March 2020 to February 2022, which is in line with our estimate (30).
Generating estimates of excess mortality at the county level has several potential benefits. First, because counties are the administrative unit for death investigation, excess mortality estimates have the potential to help identify counties where COVID-19 death rates differ from excess mortality rates and who might benefit from additional training and other resources around cause-of-death certification (31). These estimates may also be valuable for informing local public health workers, community organizations, and residents of the true impact of the pandemic, thus potentially increasing vaccination and uptake of other protective measures (32). These estimates may also be used to target federal and state emergency resources, such as funeral assistance support from the Federal Emergency Management Agency. Last, estimating excess mortality at the county level also enables analyses of social, structural, and policy factors affecting mortality associated with the pandemic, including across metro-nonmetro categories.
One major finding of this study is that the number of excess deaths in the second year of the pandemic was not substantially lower than the first year, which is noteworthy as vaccinations were available for much of 2021 and 2022. Despite the strong efficacy of vaccines, gaps in uptake likely contributed to high excess mortality in 2021 and 2022, which may persist into the future if these vaccination gaps are not closed. This finding may also reflect federal and state governments’ failure to invest in population-based strategies designed to protect the communities at greatest risk for COVID-19 death, such as financial support for family and medical leave especially for essential workers, improved ventilation of schools and workplaces, and vaccine and booster delivery programs organized in coordination with community partners (33).
A second major and related finding of this study is that excess mortality moved substantially from large metros in the first year of the pandemic to nonmetro areas during the second year. One factor that likely contributed to this change is vaccination. In urban areas, 75% of people aged 5 years and older were vaccinated as of January 2022 compared to only 59% of people aged 5 years and older in rural areas (34, 35). This urban-rural difference in vaccination rates more than doubled since April 2021, suggesting that differences in vaccination rates across metro-nonmetro categories may be playing an increasingly important role in the rural mortality disadvantage observed in the second year of the pandemic. Another factor that may be contributing to high rural excess mortality is insufficient rural health infrastructure related to funding gaps and workforce shortages (36). This may have affected access to COVID-19 vaccination and treatment, including oral antivirals and monoclonal antibody treatments (37, 38). Another consideration is the high prevalence of comorbidities among rural residents that likely increased risk for severe COVID-19 outcomes (39). Each of these factors may have contributed to the rural mortality disadvantage observed in this study. Additional research is needed to understand how the rural mortality disadvantage in excess mortality may differ by race and ethnicity and other demographic factors.
The study had several limitations. First, the study relied on publicly available data, which were subject to suppression of death counts fewer than 10 in a given county-month. We addressed this limitation by pooling information across different geographical levels through the use of hierarchical models and by taking advantage of the additional information provided by yearly death counts. However, our estimates remain uncertain in areas with small populations and few deaths. Second, our study examined all-cause mortality and did not explore differences in trends using cause-specific death rates. Assessing geographic and temporal differences in excess death rates by cause of death would allow for a deeper understanding of the mechanisms driving trends in excess mortality overall and is an important direction for future research. Similarly, our study assessed all-cause mortality in the overall population. Demographic stratification was outside of the scope of the current study, but we plan to examine county-level differences in excess mortality by gender, race and ethnicity, and education in future work. At this time, the estimates produced in this study could be linked to data on county-level demographic characteristics to examine differences in excess mortality associated with these factors. Third, we were not able to model age-specific or age-adjusted excess mortality at the county-month level because of suppression in public CDC data. However, the number of excess deaths across all ages combined is an important metric of the impact of the pandemic in a given area—even when its magnitude is partially explained by age distribution—because it captures the actual increases in mortality rather than the increases that would have occurred under a hypothetical standard age distribution (40). Furthermore, the effect of differing age structures across counties is at least partially mitigated in our study by (i) our statistical model of expected mortality, which captures differences in prepandemic mortality rates due to age and other time-invariant factors in a county-specific random intercept term and (ii) the use of a relative excess mortality metric, in which heightened mortality rates among older populations prepandemic and during the pandemic may offset one another when this ratio is calculated. Future research should investigate how age composition may contribute to county-level differences in excess mortality. Fourth, while we use the most up-to-date population estimates available from the U.S. Census Bureau, which take into consideration migration across counties, it is possible that migration during the COVID-19 pandemic altered counties’ sociodemographic makeup in mortality-relevant ways. The extent to which these changes would bias estimates of excess mortality depends on the differences between the mortality profile of in-migrating and out-migrating populations. Last, the primary objective of the present study was to generate descriptive estimates of excess mortality for each county over the course of the pandemic. Hence, we did not model the determinants of spatial-temporal variation in excess mortality. An important direction for future research will be to identify the key social, structural, and policy factors that contributed to differences in county excess mortality over time to gain insight into why some counties experienced more substantial mortality burdens during the pandemic than others.
In conclusion, this study provides the first county-level estimates of excess mortality by month in the U.S. during the first 2 years of the COVID-19 pandemic (March 2020 to February 2022). It reveals that the burden of excess mortality has moved substantially from large metros in the first year to nonmetro areas in the second year. Future research should use the estimates generated here to examine the factors associated with excess mortality throughout the pandemic, identify counties where COVID-19 death rates differ substantially from excess death rates, and study the mechanisms contributing to growing rural health disparities during the pandemic.
MATERIALS AND METHODS
Study design
We computed excess mortality during the COVID-19 pandemic in three steps:
1) We estimated expected mortality had the pandemic not occurred for each county-month.
2) Excess mortality was calculated as the difference between observed mortality and expected mortality.
3) To facilitate comparisons among counties and months, we converted excess mortality estimates to relative excess mortality, or the ratio of excess mortality to expected mortality. Relative excess mortality can be interpreted as the percentage of deaths over (or under) that which would have been expected had the pandemic not occurred.
We estimated all-cause excess mortality using a Bayesian hierarchical spatial model for 3127 counties for each month from March 2020 to February 2022. We trained the model on data from 2015 to 2019, evaluated the models’ out-of-sample predictive performance, and performed sensitivity analyses using alternative model specifications. To explore how excess mortality shifted across the U.S. during the pandemic’s first 2 years, we aggregated the county-month estimates in various ways temporally (pandemic waves and years) and geographically (Census divisions, Census regions, and metro-nonmetro categories).
Data
Monthly death counts at the county level were extracted from the CDC WONDER online tool. The tool includes information from death certificates filed in the 50 states and the District of Columbia and does not include residents of Puerto Rico, Guam, the Virgin Islands, and other U.S. territories. See Supplementary Text for further details about data extraction procedures. We extracted all-cause death counts from the Multiple Cause of Death database using the provisional counts for 2022 and the final counts for 2015–2021. To convert the number of deaths into rates, we used publicly available yearly county-level population estimates from the Census Bureau [2010–2020 (41) and 2021 (42)]. To (i) correct for the discrepancies between the 2015–2019 and the 2020–2021 population estimates arising from the use of two different decennial censuses as base and (ii) project the population forward to February 2022, we smoothed the population estimates from July 2015 to July 2021 with a linear spline in time with 4 degrees of freedom before interpolating and extrapolating monthly population estimates through February 2022. We checked that no major distortions in each county population time series were introduced and validated the degrees of freedom and the degree of the spline by comparing the predicted population for February 2022 with the official estimates.
We harmonized county Federal Information Processing Standards (FIPS) codes by reversing FIPS code changes implemented by the U.S. Census Bureau (code changes, merging of counties, or separation of counties) until we could ensure that FIPS code represented the same spatial units across all data sources (43). This harmonization procedure led to a total of 3127 units. For exploration of the results of our model, we grouped counties into four metro-nonmetro categories (large central metro, large fringe metro, medium or small metro, and nonmetro) based on the 2013 National Center for Health Statistics (NCHS) Rural-Urban Classification Scheme for Counties (44). For simplicity of comparison, in some analyses, we reduced the four metro-nonmetro categories into two or three groups. We also grouped counties into four Census regions (Northeast, Midwest, South, and West) and nine Census divisions (New England, Middle Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, and Pacific). Last, in some analyses, we stratified the Census regions and divisions by the metro-nonmetro categories, leading to more granular geographic units. See Supplementary Text for further details about the geographic classifications used in this study.
Within the 2-year period, we identified four temporal peaks representing periods where COVID-19 mortality in the U.S. was heightened. We identified peaks as periods where excess death rates rose steeply and then steeply declined. The following peaks were identified: Initial (March to August 2020), Winter (October 2020 to February 2021), Delta (August to October 2021), and Omicron (November 2021 to February 2022).
Statistical methods
To predict the monthly county-level number of deaths, we fit a Bayesian hierarchical model starting from the framework described in a prior paper (27) and adapting it to our specific application. Let yts be the number of deaths in spatial unit s at time t. Let Pts be the population of spatial unit s at time t. We assume a Poisson distribution for the number of monthly deaths yts and model the risk rts of dying using the following specification
where β0 is the global intercept. We include fixed effects for each month and Census division to capture seasonal effects. The linear predictor also includes both a linear effect (captured by βTime) and nonlinear effect f(·) of time (in months) since the start of the period (t = 1,2, … with time 1 corresponding to January 2015). For the nonlinear effect, we assume the following first-order autoregressive process (AR1) model
We model county-level intercepts using the modified Besag, York, and Mollie spatial model proposed by Riebler et al. (BYM2 model) (45). This model is the sum of a spatially unstructured random effect, and spatially structured effects us. bs is defined as
where and are standardized versions of us and vs to have variance equal to 1. The term 0 ≤ ϕ ≤ 1 is a mixing parameter which measures the proportion of the marginal variance explained by the spatially structured effect.
We specify minimally informative prior distributions for the fixed effects β0, the month-division–specific intercepts Monthm · Divisions m = 1,2, …,12, the linear time effect βTime, and the ρ parameter for the AR1 process. For the hyperparameters of the BYM2 model, ϕ and τb, we adopt priors that tend to regularize inference while not providing too strong information, the so-called penalized complexity (PC) priors introduced by Simpson et al. (46) In particular, for the SD , we select a prior so that Pr (σb > 1) = 0.01, implying that it is unlikely to have a spatial relative risk higher than exp(2) based solely on spatial or temporal variation. For ϕ, we set Pr (ϕ < 0.5) = 0.5 reflecting our lack of knowledge about which spatial component, the unstructured or structured, should dominate the spatial term bs. Last, we also adopt PC priors for the SD of the AR1 process such that Pr (σt > 1) = 0.01.
We fit the models using the Integrated Nested Laplace Approximation (INLA) method, through the R-INLA software package (47). We trained the model on the years 2015–2019. We experimented with a longer training window (2010–2019) but found no meaningful improvements in performance with respect to our final choice.
Suppressed observations
Death counts less than 10 were censored in the public data used for analysis. Between 2015 and 2019, 1312 distinct counties had at least 1 month of censored data, totaling 42,734 county-months. To address suppression of death counts below 10, we estimated censored death counts with a set of state-year–specific censored Poisson models using monthly dummies to capture seasonality and imputed the suppressed observations with the estimated counts. We exploited lower levels of censoring in year-level data to further adjust the total of imputed deaths by year and state to sum to the difference between the total of uncensored month-level deaths aggregated to the year level and the uncensored year total (obtained from a year-level data extract).
Model validation
We performed a cross-validation procedure to evaluate the out-of-sample validity of the predictions generated by our methods. We started by training the model on 2015–2016 data and predicting 2017–2019 death counts, we then trained the model on 2015–2017 data and predicted 2018–2019 death counts, and so on. We assessed the agreement between the predicted and observed deaths in the year(s) excluded from the training data and average over the cross-validation results using the following metrics: (i) the correlation between predicted and observed deaths and (ii) 90% coverage, defined as the probability that the observed deaths lie within the 90% credible interval estimated from the model. Results from this cross-validation procedure, stratified by metro-nonmetro category and Census region, are presented in table S1. All strata achieved correlation >0.96, and 90% coverage >0.86, with the vast majority >0.99 and >0.90, respectively. Sample output for the largest counties in each Census division and metro-nonmetro category are provided in fig. S1. The posterior distributions of the model’s parameters are presented in table S2 and figs. S2 and S3.
Concerns have been raised about the informativeness of the BYM model in some applications (48). To investigate whether this is a concern, we estimated a hierarchical model with no spatial component and computed the same cross-validation metrics as for the final model. The results are presented in table S3. We found no indication that the BYM model produced narrower posterior intervals and thus decided to retain this specification in the final model to facilitate comparisons with other recent work that used similar models (27).
This study used deidentified publicly available data and was exempted from review and informed consent by the Boston University Medical Center Institutional Review Board. Programming code was developed using R, version 4.1.0 (R Project for Statistical Computing) and Python, version 3.7.13 (Python Software Foundation).
Acknowledgments
We would like to thank R. Anderson and F. Ahmad (National Center for Health Statistics) for providing assistance with the provisional mortality files and M. Blangiardo (Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London) for input on the statistical modeling. We would also like to thank S. Myrick, M. Hansen, S. Lutze, and E. Coskun for technical and administrative support.
Funding: The content is solely the responsibility of the authors and does not necessarily represent the official views of the study sponsors. The authors gratefully acknowledge financial support from the following: Robert Wood Johnson Foundation (77521 to A.C.S.), the National Institute on Aging (R01-AG060115-04 to I.T.E. and R01-AG060115-04S1 to I.T.E. and A.C.S.), the W.K. Kellogg Foundation (P-6007864-2022 to A.C.S.), Boston University Center for Emerging Infectious Diseases Policy and Research (to A.C.S.), Agency for Healthcare Research and Quality (T32HS013853 to D.J.L.), and the National Institute of Child Health and Human Development (T32HD007242 to A.N.L.).
Author contributions: The authors made the following contributions to the study: Conceptualization: E.P. and A.C.S. Methodology: E.P., J.A.W., and A.C.S. Investigation: E.P., D.J.L., Z.Z., J.A.W., and R.R. Visualization: E.P., Z.Z., J.A.W., and A.C.S. Supervision: A.C.S. Writing—original draft: E.P., D.J.L., J.A.W., and A.C.S. Writing—review and editing: All authors.
Competing interests: A.C.S. reported receiving grants from Swiss Re and Johnson & Johnson outside the submitted work. The authors reported that they have no other conflicts of interests to disclose.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The underlying data used in the study are publicly available from the U.S. CDC and U.S. Census Bureau. The county-month estimates of excess mortality generated in this study and programming code for replicating the analyses can be downloaded from the following permanent repository: https://osf.io/9nrtx/ (DOI 10.17605/OSF.IO/9NRTX). The estimates are visualized in an RShiny App in data S1.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S3
Tables S1 to S3
Data S1
REFERENCES AND NOTES
- 1.Andrasfay T., Goldman N., Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations. Proc. Natl. Acad. Sci. U.S.A. 118, e2014746118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolf S. H., Masters R. K., Aron L. Y., Effect of the covid-19 pandemic in 2020 on life expectancy across populations in the USA and other high income countries: simulations of provisional mortality data. BMJ 373, n1343 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stokes A. C., Lundberg D. J., Elo I. T., Hempstead K., Bor J., Preston S. H., COVID-19 and excess mortality in the United States: A county-level analysis. PLOS Med. 18, e1003571 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackley C. A., Lundberg D. J., Ma L., Elo I. T., Preston S. H., Stokes A. C., County-level estimates of excess mortality associated with COVID-19 in the United States. SSM Popul. Health 17, 101021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossen L. M., Branum A. M., Ahmad F. B., Sutton P. D., Anderson R. N., Notes from the field: update on excess deaths associated with the COVID-19 pandemic - United States, January 26, 2020-February 27, 2021. MMWR Morb. Mortal. Wkly Rep. 70, 570–571 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolf S. H., Chapman D. A., Sabo R. T., Zimmerman E. B., Excess deaths from COVID-19 and other causes in the US, March 1, 2020, to January 2, 2021. JAMA 325, 1786–1789 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.C. J. Ruhm, Excess Deaths in the United States During the First Year of COVID-19 (National Bureau of Economic Research, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill J. R., DeJoseph M. E., The importance of proper death certification during the COVID-19 pandemic. JAMA 324, 27–28 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Iuliano A. D., Chang H. H., Patel N. N., Threlkel R., Kniss K., Reich J., Steele M., Hall A. J., Fry A. M., Reed C., Estimating under-recognized COVID-19 deaths, United States, March 2020-may 2021 using an excess mortality modelling approach. Lancet Reg. Health Am. 1, 100019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Chang H. H., Iuliano A. D., Reed C., Application of Bayesian spatial-temporal models for estimating unrecognized COVID-19 deaths in the United States. Spat. Stat. 50, 100584 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller J. T., McConnell K., Burow P. B., Pofahl K., Merdjanoff A. A., Farrell J., Impacts of the COVID-19 pandemic on rural America. Proc. Natl. Acad. Sci. U.S.A. 118, 2019378118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y.-H., Stokes A. C., Aschmann H. E., Chen R., DeVost S., Kiang M. V., Koliwad S., Riley A. R., Glymour M. M., Bibbins-Domingo K., Excess natural-cause deaths in California by cause and setting: March 2020 through February 2021. PNAS Nexus 1, pgac079 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elezkurtaj S., Greuel S., Ihlow J., Michaelis E. G., Bischoff P., Kunze C. A., Sinn B. V., Gerhold M., Hauptmann K., Ingold-Heppner B., Miller F., Herbst H., Corman V. M., Martin H., Radbruch H., Heppner F. L., Horst D., Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 11, 4263 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Hospital avoidance and unintended deaths during the COVID-19 pandemic. Am. J. Health Econ. 7, 405–426 (2021). [Google Scholar]
- 15.Anderson K. E., McGinty E. E., Presskreischer R., Barry C. L., Reports of forgone medical care among US adults during the initial phase of the COVID-19 pandemic. JAMA Netw. Open 4, e2034882 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokes A. C., Lundberg D. J., Bor J., Elo I. T., Hempstead K., Preston S. H., Association of health care factors with excess deaths not assigned to COVID-19 in the US. JAMA Netw. Open 4, e2125287 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkow N. D., Collision of the COVID-19 and addiction epidemics. Ann. Intern. Med. 173, 61–62 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubey M. J., Ghosh R., Chatterjee S., Biswas P., Chatterjee S., Dubey S., COVID-19 and addiction. Diabetes Metab. Syndr. 14, 817–823 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raifman J., Bor J., Venkataramani A., Association between receipt of unemployment insurance and food insecurity among people who lost employment during the COVID-19 pandemic in the United States. JAMA Netw. Open 4, e2035884 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandoval-Olascoaga S., Venkataramani A. S., Arcaya M. C., Eviction moratoria expiration and COVID-19 infection risk across strata of health and socioeconomic status in the United States. JAMA Netw. Open 4, e2129041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention, 2020-2021 Flu Season Summary (Centers for Disease Control and Prevention, 2023); www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm.
- 22.Shiels M. S., Haque A. T., Haozous E. A., Albert P. S., Almeida J. S., García-Closas M., Nápoles A. M., Pérez-Stable E. J., Freedman N. D., Berrington de González A., Racial and ethnic disparities in excess deaths during the COVID-19 pandemic, March to December 2020. Ann. Intern. Med. 174, 1693–1699 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venter Z. S., Aunan K., Chowdhury S., Lelieveld J., COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. U.S.A. 117, 18984–18990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faust J. S., Du C., Mayes K. D., Li S.-X., Lin Z., Barnett M. L., Krumholz H. M., Mortality from drug overdoses, homicides, unintentional injuries, motor vehicle crashes, and suicides during the pandemic, March-August 2020. JAMA 326, 84–86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruhm C. J., Pandemic and recession effects on mortality in The US during the first year of COVID-19. Health Aff. (Millwood) 41, 1550–1558 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Council of State and Territorial Epidemiologists, “Interim Guidance for Public Health Surveillance Programs for Classification of Covid-19-associated Deaths among Covid-19 Cases” (2021); https://cdn.ymaws.com/www.cste.org/resource/resmgr/pdfs/pdfs2/20211222_interim-guidance.pdf.
- 27.Konstantinoudis G., Cameletti M., Gómez-Rubio V., Gómez I. L., Pirani M., Baio G., Larrauri A., Riou J., Egger M., Vineis P., Blangiardo M., Regional excess mortality during the 2020 COVID-19 pandemic in five European countries. Nat. Commun. 13, 482 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-19 Excess Mortality Collaborators , Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet 399, 1513–1536 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberger D. M., Chen J., Cohen T., Crawford F. W., Mostashari F., Olson D., Pitzer V. E., Reich N. G., Russi M., Simonsen L., Watkins A., Viboud C., Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern. Med. 180, 1336–1344 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention, Excess Deaths Associated with COVID-19: Provisional Death Counts for Coronavirus Disease (COVID-19) (Centers for Disease Control and Prevention, 2022); www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm.
- 31.Stokes A. C., Lundberg D. J., Bor J., Bibbins-Domingo K., Excess deaths during the COVID-19 pandemic: Implications for US death investigation systems. Am. J. Public Health 111, S53–S54 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilinski A., Emanuel E., Salomon J. A., Venkataramani A., Better late than never: Trends in COVID-19 infection rates, risk perceptions, and behavioral responses in the USA. J. Gen. Intern. Med. 36, 1825–1828 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.E. Wrigley-Field, K. M. Berry, A. C. Stokes, J. P. Leider, "Pandemic of the unvaccinated"? At midlife, white people are less vaccinated but still at less risk of Covid-19 mortality in Minnesota. medRxiv 2022.03.02.22271808 (2022). 10.1101/2022.03.02.22271808.
- 34.Saelee R., Zell E., Murthy B. P., Castro-Roman P., Fast H., Meng L., Shaw L., Gibbs-Scharf L., Chorba T., Harris L. Q., Murthy N., Disparities in COVID-19 vaccination coverage between urban and rural counties - United States, December 14, 2020-January 31, 2022. MMWR Morb. Mortal. Wkly Rep. 71, 335–340 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murthy B. P., Sterrett N., Weller D., Zell E., Reynolds L., Toblin R. L., Murthy N., Kriss J., Rose C., Cadwell B., Wang A., Ritchey M. D., Gibbs-Scharf L., Qualters J. R., Shaw L., Brookmeyer K. A., Clayton H., Eke P., Adams L., Zajac J., Patel A., Fox K., Williams C., Stokley S., Flores S., Barbour K. E., Harris L. Q., Disparities in COVID-19 vaccination coverage between urban and rural counties - United States, December 14, 2020-April 10, 2021. MMWR Morb. Mortal. Wkly Rep. 70, 759–764 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dearinger A. T., COVID-19 reveals emerging opportunities for rural public health. Am. J. Public Health 110, 1277–1278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D. A. Wood, A. Aleem, D. Davis, Providing Access To Monoclonal Antibody Treatment Of Coronavirus (COVID-19) Patients In Rural And Underserved Areas (StatPearls, StatPearls Publishing, 2022). [PubMed] [Google Scholar]
- 38.Hernandez I., Dickson S., Tang S., Gabriel N., Berenbrok L. A., Guo J., Disparities in distribution of COVID-19 vaccines across US counties: A geographic information system-based cross-sectional study. PLOS Med. 19, e1004069 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denslow S., Wingert J. R., Hanchate A. D., Rote A., Westreich D., Sexton L., Cheng K., Curtis J., Jones W. S., Lanou A. J., Halladay J. R., Rural-urban outcome differences associated with COVID-19 hospitalizations in North Carolina. PLOS ONE 17, e0271755 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilinski A., Thompson K., Emanuel E., COVID-19 and excess all-cause mortality in the US and 20 comparison countries, June 2021-March 2022. JAMA 329, 92–94 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Census Bureau, Datasets: 2010-2020: Counties: Totals (U.S. Census Bureau); www2.census.gov/programs-surveys/popest/datasets/2010-2020/counties/totals/.
- 42.U.S. Census Bureau, Datasets: 2020-2021: Counties: Totals (U.S. Census Bureau); www2.census.gov/programs-surveys/popest/datasets/2020-2021/counties/totals/.
- 43.U.S. Census Bureau, Substantial Changes to Counties and County Equivalent Entities: 1970-Present (U.S. Census Bureau); www.census.gov/programs-surveys/geography/technical-documentation/county-changes.2020.html.
- 44.U.S. Department of Agriculture, Economic Research Service, 2013 Rural-Urban Continuum Codes (U.S. Department of Agriculture); www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx.
- 45.Riebler A., Sørbye S. H., Simpson D., Rue H., An intuitive Bayesian spatial model for disease mapping that accounts for scaling. Stat. Methods Med. Res. 25, 1145–1165 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Simpson D., Rue H., Riebler A., Martins T. G., Sørbye S. H., Penalising model component complexity: A principled, practical approach to constructing priors. Statist. Sci. 32, 1–28 (2017). [Google Scholar]
- 47.Rue H., Martino S., Chopin N., Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Series B Stat. Methodol. 71, 319–392 (2009). [Google Scholar]
- 48.Quick H., Song G., Tabb L. P., Evaluating the informativeness of the Besag-York-Mollié CAR model. Spat. Spatiotemporal Epidemiol. 37, 100420 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S3
Tables S1 to S3
Data S1