Abstract
Ventilator-associated pneumonia (VAP) is the most common ICU acquired pneumonia among patients who are invasively intubated for mechanical ventilation. Patients with VAP suffer an increased mortality risk, financial burden, and length of stay in the hospital. The authors aimed to review the literature to describe the incidence, mortality, and microbiological evidence of VAP. We selected 13 peer-reviewed articles published from 1 January 2010 to 15 September 2022 from electronic databases for studies among adult or pediatric patients diagnosed with VAP expressed per thousand days admitted in the ICU. The VAP rates ranged from 7 to 43 per thousand days, varying among different countries of the world. A significant rate of mortality was observed in 13 studies ranging from 6.3 to 66.9%. Gram-negative organisms like Acinetobacter spp., Pseudomonas aeruginosa Gram-positive organisms like Staphylococcus aureus were frequently found. Our findings suggest an alarming situation of VAP among patients admitted to the intensive care units with increasing incidence and mortality. The review also found that VAP is more common in males and that there is a significant variation in the incidence and mortality rates of VAP among different countries. The findings of this review can inform the development of infection control and prevention strategies to reduce the burden of VAP. Thus, there is a crucial need for control and preventive measures like interventional studies and educational programs on staff training, hand-hygiene, and the appropriate use of ventilator bundle approach to curb this preventable threat that is increasing at an alarming rate.
Keywords: Adult, artificial, intensive care units, incidence pneumonia, ventilator-associated, respiration
Introduction
Highlights
Ventilator-associated pneumonia (VAP) is the most common ICU acquired pneumonia among patients who are invasively intubated for mechanical ventilation.
The VAP rates ranged from 7 to 43 per thousand days, varying among different countries of the world.
Our findings suggest an alarming situation of VAP among patients admitted to the ICUs with increasing incidence and mortality.
The review also found that VAP is more common in males and that there is a significant variation in the incidence and mortality rates of VAP among different countries.
Ventilator-associated pneumonia (VAP) or intubation-associated pneumonia is a pneumonia that arises more than 48–72 h after endotracheal intubation and is not incubating at the time of admission1. Infiltrates that are either new or progressive in nature, systemic infection (fever, altered white blood cell counts), changes in the characteristics of sputum, and the detection of a causative agent are seen in patients with VAP2. VAP is the most common ICU acquired pneumonia among patients who are invasively intubated for mechanical ventilation3. Patients having VAP have an attributable mortality of 13.5%. It leads to an increase in the length of stay at the hospital as well as an increase in financial burden.
While delayed antimicrobial medication delivery has been linked to higher mortality, early detection of VAP is essential. The hazards of overusing antibiotics, such as resistance to antibiotics and superinfections, should be weighed against the necessity of administering antibiotics quickly, especially in the ICU. VAP is tricky to diagnose, making finding the proper balance difficult. The perceived incidence and consequences of VAP vary greatly based on the definition used, and up to two-thirds of people diagnosed for VAP do not always genuinely have VAP because there is no realistic reference standard for the condition. There is an urgent need for improved approaches to diagnose VAP and guide the use of empirical antibiotics4.
For determining the presence of VAP and starting empiric antibiotics, clinicians often depend on diagnostic, radiological, and laboratory signs. Symptoms include fever, sputum production, hypoxemia, a chest radiography infiltrate that has developed or is new, a raised white blood cell count, and abnormal cultures from ETA or bronchoscopic sample procedures (bronchoalveolar lavage and protected specimen brush). Several of these have been incorporated into clinical models, with the clinical lung infection score (CPIS) being the most well-liked. Yet, despite the fact that these symptoms and tests are often used, nothing is known about how accurate they are at diagnosing VAP5.
The main objective of our review is to estimate the incidence, mortality, and etiological agents associated with VAP. This reliable and upgraded information would help in assessing the significance of the situation and providing evidence for patients, clinicians, and policy makers for planning infection control and other prevention strategies to control this preventable disease and lower the mortality associated with it for future.
Methods
We organized this systematic review in accordance with the Cochrane Manual for Diagnostic Accuracy Level, PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)4 recommendations for Diagnostics Accuracy Level, and other reviews of Diagnostics Give Accurate information guidelines. Via PROSPERO, we validated the study procedures (CRD42019124907).
Search methods
Four authors independently conducted an extensive literature search of the four electronic databases, namely PubMed, Google Scholar, and PLOS ONE to identify all the peer-reviewed research articles published within the time frame of 1 January 2010 to 15 September 2022. The complete search strategy in detail for PubMed and PLOS ONE is given in the supporting file for the search strategy. All the databases were searched using relevant MeSH Terms in PubMed, as well as PLOS ONE and Google Scholar. The terms ‘Ventilator-associated Pneumonia’, ‘Healthcare-Associated Pneumonia’, were searched under MeSH terms. All the references to the studies qualified for the review were also thoroughly searched for additional relevant articles.
Selection criteria
Inclusion criteria
Randomized controlled trials and cohort studies that were published in English including information on at least the prevalence, incidence, or incidence rate of VAP among adults expressed as episodes per 1000 ventilator days were considered eligible for inclusion.
The search method has been briefly described in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)4 flowchart in Figure 1.
Figure 1.
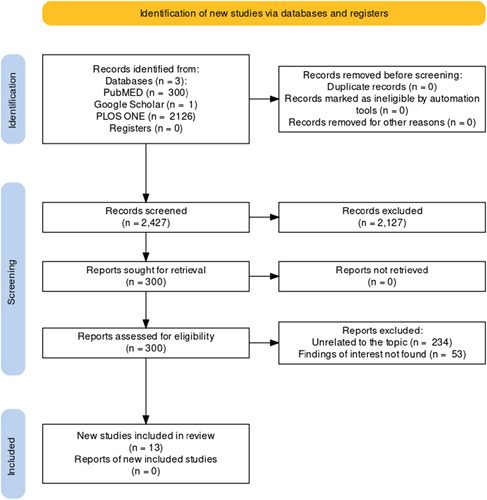
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart.
Exclusion criteria
Review articles, research protocols, case series/case reports, symposium/conference proceedings, commentaries/editorials/letters, views/opinions and articles that were not in English language as well as those whose full-text was not available were excluded from our review.
Data collection and analysis
According to prespecified inclusion and exclusion criteria, four independent authors screened the articles remaining after duplicates removal. Full-text articles were retrieved, and studies were shortlisted to be included in the review, which met the eligibility criteria. The disparities and confusions among the two authors were resolved by the consultation of a third author involved in the supervision of the study. The data was extracted using the data abstraction spreadsheet in Microsoft Excel version 2013(Microsoft Corp) under the following variables: Name of the author, country where the study was done, year of the publication, study design, sample size, inclusion criteria, exclusion criteria, primary outcome, secondary outcome, and duration of study. The work has been reported in line with AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines. Descriptive statistical analysis was done using IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0.: IBM Corp. Risk of bias analysis was done using Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020.
Risk of bias analysis
The Cochrane risk of bias tool for nonrandomized studies was used to assess the risk of bias for studies included in this review5. The analysis is graphically represented in Figure 2 and Figure 3.
Figure 2.

Risk of bias summary.
Figure 3.
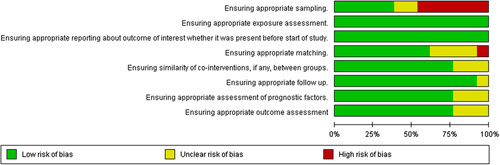
Risk of bias analysis.
Main results
The results of our systematic review are as follows:
Study selection
A total of 2427 articles were obtained after a thorough search through the databases. After the first scanning, a total of 300 articles remained for further processing. After the title and abstract screening, 234 of those papers were omitted because they did not follow the inclusion requirements. The full-text was obtained for the remaining 66 articles. Of the 66 full-text posts, 53 were rejected because the findings of interest were not found. Finally, 13 articles were included in the review.
Altogether, 13 studies conducted in various hospital settings among different adult patients and pediatric patients presented in the ICU were reviewed qualitatively. Studies included The US Centers for Disease Control and Prevention and CPIS as diagnostic criteria for VAP expressed per thousand ventilator days. Countries where studies were conducted includes the United States, Spain, Belgium, Taiwan, China, France, Lebanon, Portugal, Turkey, Bosnia and Herzegovina, and Nigeria. A description of the individual studies is provided in the Table 1 (available from the supplementary file Table 1). Detailed descriptive statistics are tabulated in Table 2.The statistical results showed the males ratio (71.60) is higher than female (45.29) and the highest incidence rate of ventilator-associated pneumonia (%), that is, 25.21% and the highest ICU mortality (%) rate, that is, 29.23%.
Table 1.
Table of studies included in this systematic review.
| Serial number | References | Title | Country | Sample size | Study design |
|---|---|---|---|---|---|
| 1 | Javier de Miguel-Díez 6 | Decreasing incidence and mortality among hospitalized patients suffering a ventilator-associated pneumonia - Analysis of the Spanish national hospital discharge database from 2010 to 2014. | Spain | 9336 | Retrospective cohort study |
| 2 | Lydie Decelle 7 | Ventilation-associated pneumonia after intubation in the prehospital or the emergency unit. | Belgium | 75 | Retrospective descriptive (case–control) study |
| 3 | Ting-Chang Hsieh 8 | Frequency of Ventilator-associated Pneumonia With 3-day Versus 7-day Ventilator Circuit Changes. | Taiwan | 397 | Retrospective cohort study |
| 4 | Xia Zhao 9 | Epidemiological and clinical characteristics of healthcare-associated infection in elderly patients in a large Chinese tertiary hospital: a 3-year surveillance study. | China | 134637 | Prospective cohort study |
| 5 | Charles-Hervé Vacheron 10 | Increased Incidence of Ventilator-Acquired Pneumonia in Coronavirus Disease 2019 Patients: A Multicentric Cohort Study. | France | 3758 | Prospective cohort study |
| 6 | Zeina A. Kanafani 11 | Ten-year surveillance study of ventilator-associated pneumonia at a tertiary care center in Lebanon. | Lebanon | 162 | Retrospective cohort study |
| 7 | Rui Dias Costa 12 | Hospital-Acquired Pneumonia in a Multipurpose Intensive Care Unit: One-Year Prospective Study. | Portugal | 60 | Prospective cohort study |
| 8 | Alicia J. Mangram 13 | Trauma-associated pneumonia: time to redefine ventilator-associated pneumonia in trauma patients. | United States | 1044 | Retrospective cohort study |
| 9 | I. Ucgun 14 | Effects of isolation rooms on the prevalence of hospital-acquired pneumonia in a respiratory ICU. | Turkey | 532 | Prospective cohort study |
| 10 | Alisa Krdzalic 15 | Influence of Remifentanil/Propofol Anesthesia on Ventilator-associated Pneumonia Occurence After Major Cardiac Surgery. | Bosnia and Herzegovina | 82 | Retrospective-prospective study |
| 11 | François Philippart 16 | Decreased Risk of Ventilator-Associated Pneumonia in Sepsis Due to Intra-Abdominal Infection. | France | 2623 | Retrospective cohort study |
| 12 | Anthony A. Iwuafor 17 | Incidence, Clinical Outcome and Risk Factors of Intensive Care Unit Infections in the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria. | Nigeria | 71 | Prospective cohort study |
| 13 | Qiao He 18 | The epidemiology and clinical outcomes of ventilator-associated events among 20 769 mechanically ventilated patients at intensive care units: an observational study. | China | 22343 | Analytical randomized study |
Table 2.
Descriptive statistics of variables.
| Descriptive statistics of variables | |||||||
|---|---|---|---|---|---|---|---|
| Males (%) | Females (%) | Sample size of the study (n) | Overall age distribution in years (mean) | Length of stay in the intensive care unit in days (mean) | Incidence of ventilator-associated pneumonia (%) | Intensive care unit mortality (%) | |
| Mean | 64.88 | 35.12 | 13470.77 | 53.55 | 8.62 | 20.05 | 22.24 |
| Median | 66.35 | 33.65 | 532.00 | 57.50 | 8.50 | 17.29 | 20.30 |
| Percentiles | |||||||
| 25 | 54.71 | 28.40 | 78.50 | 50.70 | 8.65 | 16.22 | |
| 50 | 66.35 | 33.65 | 532.00 | 57.50 | 17.29 | 20.30 | |
| 75 | 71.60 | 45.29 | 6547.00 | 62.05 | 25.21 | 29.23 | |
Ventilator-associated pneumonia incidence rate
The VAP incidence rate ranged from 7 to 43 per thousand ventilator days (95% CI 20.05+13.189) as shown in Figure 4 showing a great difference between countries. The pooled mean of sex from our studies resulted with males consisting of 64.88% (95% CI 64.88+10.78) and females 35.12% (95% CI 35.12+10.78) as shown in the sex distribution graph in Figure 5. The highest VAP prevalence rate was reported from a database study of Spain. The lowest was from Portugal. The VAP rate reported from various studies are reported in the table of VAP prevalence rates.
Figure 4.
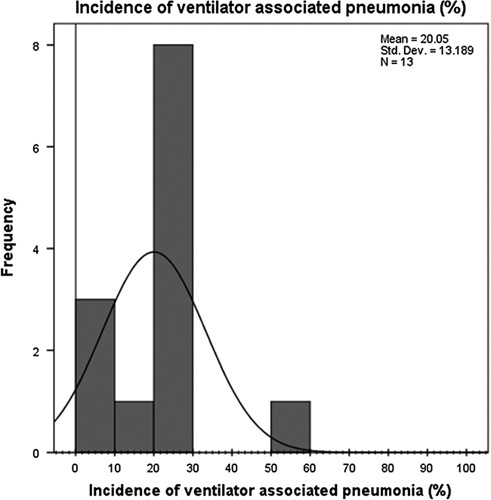
Incidence of ventilator-associated pneumonia graph. (Frequency is the frequency with which an incidence value [in percentage] was reported in our studies).
Figure 5.
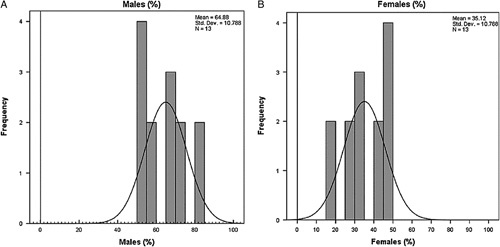
Sex distribution graph;(a) Males; Females (b); frequency is the frequency with which a particular gender [in percentage] was reported in our studies.
Mortality
Ten of the 13 articles included in the review reported the mortality rate in hospitalized patients. The mortality rate ranged from 6.3 to 66.9%. The highest mortality rate was reported from a study of Turkey19. No mortality rates were reported from studies of Lebanon, Bosnia, and Herzegovina, and one study of China6–8. The detailed description of the mortality rate reported from studies of different countries is graphically presented in Figure 6 and tabulated in Table 3.
Figure 6.
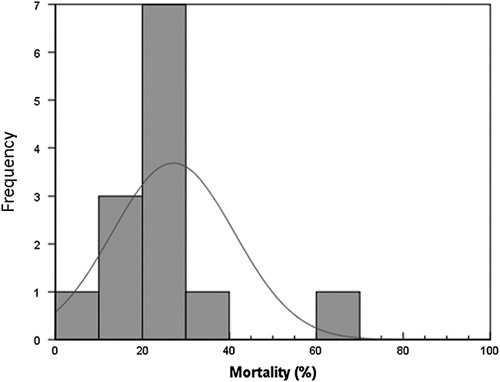
Percent mortality graph; only studies that reported mortality were included in this graph; frequency is the frequency with which a particular mortality value [in percentage] was reported in our studies.
Table 3.
Mortality rates reported in each study.
| Serial number | References | Mortality rate (%) |
|---|---|---|
| 1 | Javier de Miguel-Díez 9 | 34.88 |
| 2 | Lydie Decelle 10 | 17.33 |
| 3 | Ting-Chang Hsieh 11 | 28.85 |
| 4 | Charles-Hervé Vacheron 12 | 28.50 |
| 5 | Rui Dias Costa 13 | 18.30 |
| 6 | Alicia J. Mangram 14 | 6.30 |
| 7 | I. Ucgun 19 | 66.30 |
| 8 | François Philippart 15 | 29.95 |
| 9 | Anthony A. Iwuafor 16 | 27.11 |
| 10 | Qiao He 17 | 15.10 |
Microbiology of VAP
Ten studies included data on microbiology, causing VAP, as shown in the table of microbiology of VAP. Acinetobacter baumannii caused the majority of VAP episodes followed by Pseudomonas aeruginosa.
Comparison among the microbiology of VAP of 13 a studies table of the microbiology of VAP showed Acinetobacter sp., followed by Pseudomonas aeruginosa as common gram-negative organisms causing VAP while Staphylococcus aureus as common gram-positive organisms. Studies also isolated resistant forms of gram-positive bacteria like MRSA (Methicillin resistant Staphylococcus Aureus). Candida sp. was the most common of the fungal isolates described in the studies by Iwuafor16, Diez9, and Zhao8 as shown in Figure 7.
Figure 7.
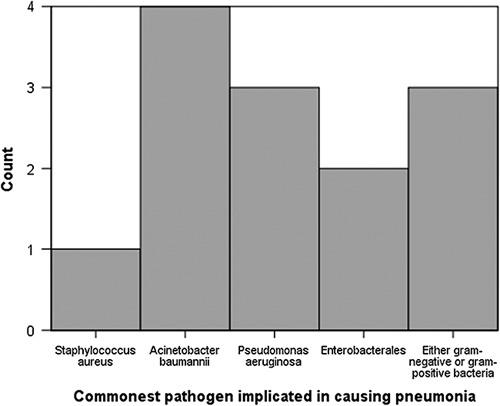
Commonest pathogen implicated in causing pneumonia. (Count represents the number of times an organism was reported as the most common in all studies)
Different countries across the globe whose studies have been included in this review are the United States, China, Taiwan, Nigeria, France, Portugal, Belgium, Portugal, Bosnia and Herzegovina, Spain, and Turkey.
Discussion
Our review highlights the situation of VAP among the populations of 11 different countries across the globe, from the United States in North America to Taiwan in Asia. We found a wide range of VAP burden with much variability in the VAP rate, ranging from 7 to 43 per thousand ventilator days among these countries, showing most studies with disturbing VAP situations. Mortality rates reported by the majority of the studies included in our review were caused by gram-negative organisms followed by gram-positive bacteria.
The economical differences, which can then lead to a lack of advancement and availability of health facilities in some regions can be a reason for the higher VAP rate in those countries. Differences in the study setting such as pediatric or adult ICU or surgical and medical ICU can also be another probable reason why there is a wide variation in the VAP rate. As there is no unanimous worldwide gold-standard definition for VAP, the criteria can also be a reason why sometimes VAP is over or under reported.
Patients diagnosed with many comorbidities especially neurologic, cardiorespiratory diseases as well as coronavirus disease 2019 are associated with a higher VAP rate8,10,12.
Finally, staff practices, be it the prehospital emergency team or the medical staff in the hospital and ICU premises, which do not follow infection prevention guidelines could cause a variability in the VAP rate with an increased rate in areas where guidelines are not properly followed by the staff14.
In a study of 22 Asian countries by Bonnel et al.18 , they concluded that VAP incidence was on the lower side in rich countries where the average income was higher compared to countries where people had a low-income (9 vs. 18.5 per 1000 ventilator days, respectively). Studies across the globe from the Middle East and Japan also varied between 8 and 12.6 per thousand ventilator days20.
The studies in our review show heterogenous results. Some studies resemble the rates reported as of the above studies, but other studies have a variedly higher incidence of VAP increasing the VAP burden. Some of the risk factors which were reported to increase the risk of VAP were old age, longer time on ventilation, infection with coronavirus disease 2019, and macroaspiration8,10,12. In those who had trauma, fractures of the ribs, lung contusions, and a failed attempt at intubation before reaching the hospital were associated with a greater risk of developing VAP14. Patients who had cardiac surgery with cardiopulmonary bypass and required a longer respiratory tie were also prone to a greater risk of developing VAP7.
Interestingly, in one of our studies, it was reported that the mortality associated with hospital-acquired pneumonia was higher if the initial cause of admission was HAP compared to patients who were admitted to the ICU for another reason and later developed HAP— 32 vs 8.6%, respectively13. This may be due to the fact that patients in the ICU are under higher vigilant supervision than any other ward in general, and any deterioration that may occur in these patients may be promptly diagnosed and treated by the ICU healthcare staff before the patients deteriorate. This may not be the case for patients who develop pneumonia elsewhere and seek medical attention once symptoms have deteriorated enough for them to be admitted to the ICU.
The highest incidence rate in our study among ICU admitted patients was reported from a database of the Spanish National Healthcare Discharge database, while the lowest was found in a Portuguese hospital11. Possible reasons for the high rate may be due to a greater number of males, a higher number of comorbidities, and a higher number of readmissions in the patients of the database; all factors associated with a higher risk of VAP. The study in a Portuguese hospital probably had a lower VAP rate because they had a smaller sample size and a shorter duration of study follow-up.
Our study showed the mortality rate ranged from 6.3 to 66.3%. The highest mortality rate was reported from among Turkish patients19. This rate is similar to a study among developing countries by Kharel S, et al.21 The mortality rate reported by the Infectious Diseases Society of America and the American Thoracic Society is 13%. In Europe, the 30-day mortality rate is reported to be 29.9%, which is generally lower than what the studies in our review reported22,23. This may be because our study has heterogenous population sample sizes with the highest number of patients from China. This may skew the direction from the values reported by American and European societies, which do not include Chinese and Turkish data into their results. The highest mortality rate reported among the Turkish patients19 was probably due to a lack of a specialized ICU unit and high antibiotic resistance in the cohort.
Data on microbiology shows that the most common organism responsible for the majority of the VAP cases in our studies was gram-negative Acinetobacter baumannii followed closely by Pseudomonas aeruginosa. This data closely resembles a recent analysis done by Kharel S, et al.21, which reported similar results on microbiology in VAP. An analysis done by Fathy, et al.24 in an Egyptian patients also showed common causative organisms, which included Pseudomonas aeruginosa and methicillin resistant Staph. aureus.
To curb and decrease the burden of VAP, heat humidifying systems can be employed25. Proper education of ICU and hospital staff as well as strict adherence to hand-hygiene protocols and proper handling of bronchial secretions in patients admitted to ICU can be used, which can be effective even in countries where the income is low. Ventilator bundle approaches such as elevation of the head of the bead to decrease aspiration of gastric secretions, prophylaxis against gastric ulcer disease during the ICU stay, prophylaxis against venous thromboembolism, and oral chlorhexidine contamination can be embraced, which can surely decrease the burden of VAP in patients in the ICU26.
Strengths and limitations of the study
The major strength of our study lies in its huge total sample size as well as its global nature, including countries from four different continents with data spanning more than a decade. This gives us a unique picture into an often ignored but highly prevalent issue in the ICU. The main limitation in our study was that we did not expound on detailed information about the different subgroups of VAP such as early-onset VAP vs. late-onset VAP and the subcategories of ventilator-associated events individually, such as infection-related ventilator-associated complications, possible ventilator-associated pneumonia, and ventilator-associated complications. The other limitation in our study was that we did not utilize literature in a language other than English. Lastly, we did not utilize articles that were not available as free-access articles, which may have had an effect on our results.
Conclusions
According to this comprehensive review, the traditional clinical signs for diagnosing VAP—fever, purulent discharges, leukocytosis, chest radiograph, cultures from three different sample procedures (protected specimen brush, ETA, and bronchoalveolar lavage), and CPIS—had low specificity. Relying only on the existence of any one of these signs might lead to incorrect diagnoses and probably inappropriate use of antibiotics. These findings underline the difficulty of identifying VAP and the demand for new tools to assist doctors in determining when to initiate and discontinue empiric antibiotics for potential VAP.
Ethical approval and Consent to participate
Not applicable.
Consent for publication
All authors have reviewed the final version of this paper and given full consent for submission and publication of this paper.
Sources of funding
This research received no funding from any source.
Author contributions
H.M. and M.S.: conceptualization; M.S., W.K.: methodology; H.M., M.S., and S.M.I.: formal analysis; M.S., W.K.: investigation; H.S., M.M.: data curation; M.S., H.M.: writing – original draft preparation; S.S.S. and M.S.: writing – review and editing; M.S. and H.M.: visualization; H.M.: supervision. All authors reviewed the final version and approved it for submission.
Conflicts of interest disclosure
All authors declare that they have no conflict of interest with regards to the content of this paper.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Hassan Mumtaz and Muhammad Saqib.
Availability of data and materials
Data used in this study is available upon reasonable request to the corresponding author.
Provenance and peer-review
Not commissioned, externally peer-reviewed.
Acknowledgments
None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 12 May 2023
Contributor Information
Hassan Mumtaz, Email: hassanmumtaz.dr@gmail.com.
Muhammad Saqib, Email: sizxudawar@gmail.com;muhammadsaqib.drkmc@gmail.com.
Wajiha Khan, Email: wajihamughal06@gmail.com.
Syed M. Ismail, Email: si8599389@gmail.com.
Hassan Sohail, Email: hassansohail768@gmail.com.
Muhammad Muneeb, Email: mohammadmuneeb2011@hotmail.com.
Shazia S. Sheikh, Email: shaziashazia565@gmail.com.
References
- 1. Froes F, Paiva JA, Amaro P, et al. Consensus document on nosocomial pneumonia. Rev Port Pneumol 2007;13:419–486. [PubMed] [Google Scholar]
- 2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 3. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Int Med 2013;173:2039–2046. [DOI] [PubMed] [Google Scholar]
- 4. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed) 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sterne JA, Hernán MA, McAleenan A, et al. Assessing risk of bias in a non-randomized study. Cochrane Handbook for Syst Rev Intervent 2019;4:621–41. [Google Scholar]
- 6. Kanafani ZA, El Zakhem A, Zahreddine N, et al. Ten-year surveillance study of ventilator-associated pneumonia at a tertiary care center in Lebanon. J Inf Public Health 2019;12:492–495. [DOI] [PubMed] [Google Scholar]
- 7. Krdzalic A, Kosjerina A, Jahic E, et al. Influence of Remifentanil/Propofol anesthesia on ventilator-associated pneumonia occurence after major cardiac surgery. Med Arch 2013;67:407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao X, Wang L, Wei N, et al. Epidemiological and clinical characteristics of healthcare-associated infection in elderly patients in a large Chinese tertiary hospital: a 3-year surveillance study. BMC Infect Dis 2020;20:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Miguel-Diez J, Lopez-de-Andres A, Hernandez-Barrera V, et al. Decreasing incidence and mortality among hospitalized patients suffering a ventilator-associated pneumonia: analysis of the Spanish national hospital discharge database from 2010 to 2014. Medicine 2017;96:e7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Decelle L, Thys F, Zech F, et al. Ventilation-associated pneumonia after intubation in the prehospital or the emergency unit. Eur J Emerg Med 2013;20:61–63. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh TC, Hsia SH, Wu CT, et al. Frequency of ventilator-associated pneumonia with 3-day versus 7-day ventilator circuit changes. Pediat Neonatol 2010;51:37–43. [DOI] [PubMed] [Google Scholar]
- 12. Vacheron CH, Lepape A, Savey A, et al. Increased incidence of ventilator-acquired pneumonia in coronavirus disease 2019 patients: a multicentric cohort study. Crit Care Med 2022;50:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa RD, Baptista JP, Freitas R, et al. Hospital-acquired pneumonia in a multipurpose intensive care unit: one-year prospective study. Acta Med Port 2019;32:746–53. [DOI] [PubMed] [Google Scholar]
- 14. Mangram AJ, Sohn J, Zhou N, et al. Trauma-associated pneumonia: time to redefine ventilator-associated pneumonia in trauma patients. Am J Surg 2015;210:1056–1061; discussion 61-2. [DOI] [PubMed] [Google Scholar]
- 15. Philippart F, Bouroche G, Timsit JF, et al. Decreased risk of ventilator-associated pneumonia in sepsis due to intra-abdominal infection. PLoS ONE 2015;10:e0137262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iwuafor AA, Ogunsola FT, Oladele RO, et al. Incidence, clinical outcome and risk factors of intensive care unit infections in the lagos university teaching hospital (LUTH), Lagos, Nigeria. PLoS ONE 2016;11:e0165242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He Q, Wang W, Zhu S, et al. The epidemiology and clinical outcomes of ventilator-associated events among 20,769 mechanically ventilated patients at intensive care units: an observational study. Crit Care 2021;25:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonell A, Azarrafiy R, Huong VTL, et al. A systematic review and meta-analysis of ventilator-associated pneumonia in adults in Asia: an analysis of national income level on incidence and etiology. Clin Infect Dis 2019;68:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ucgun I, Dagli CE, Kiremitci A, et al. Effects of isolation rooms on the prevalence of hospital acquired pneumonia in a respiratory ICU. Eur Rev Med Pharmacol Sci 2013;17(Suppl 1):2–8. [PubMed] [Google Scholar]
- 20. Ali HS, Khan FY, George S, et al. Epidemiology and outcome of ventilator-associated pneumonia in a heterogeneous ICU population in Qatar. Biomed Res Int 2016;2016:8231787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kharel S, Bist A, Mishra SK. Ventilator-associated pneumonia among ICU patients in WHO Southeast Asian region: a systematic review. PLoS ONE 2021;16:e0247832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin-Loeches I, Rodriguez AH, Torres A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr Opin Crit Care 2018;24:347–352. [DOI] [PubMed] [Google Scholar]
- 23. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of america and the american thoracic society. Clin. Infect. Dis. 2016;63:e61–ee111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fathy A, Abdelhafeez R, Abdel-Hady E-G. Abd Elhafez SAJEJoCD, Tuberculosis. Analysis of Ventilator associated Pneumonia (VAP) studies in Egyptian University Hospitals 2013;62:17–25. [Google Scholar]
- 25. Memish ZA, Oni GA, Djazmati W, et al. A randomized clinical trial to compare the effects of a heat and moisture exchanger with a heated humidifying system on the occurrence rate of ventilator-associated pneumonia. Am J Infect Control 2001;29:301–305. [DOI] [PubMed] [Google Scholar]
- 26. Rosenthal VD, Guzman S, Crnich C. Impact of an infection control program on rates of ventilator-associated pneumonia in intensive care units in 2 Argentinean hospitals. Am J Infect Control 2006;34:58–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study is available upon reasonable request to the corresponding author.


