Abstract
Introduction
For preoperative analgesia during a variety of operations, the erector spinae plane block (ESPB) has grown in popularity. However, its effectiveness in lumbar surgery is still unknown. The purpose of this study was to investigate the potential benefits of ESPB in enhancing analgesic efficacy in elderly individuals following posterior lumbar spine surgery.
Methods
Patients aged 65 years or older who underwent elective posterior lumbar instrumented fusion (with or without decompression) at our institution between January 2019 and June 2022 were included. Demographic data, comorbidities, and results of preoperative screening were retrospectively collected. Propensity score matching (PSM) was performed in a ratio of 1:1 for control and ESPB groups. The primary outcome was opioid consumption at 24 h after surgery. Secondary outcomes was visual analog scale (VAS) pain scores at rest in the first 24 h. Additional secondary outcomes included number of patients requesting rescue analgesia, incidence of nausea and vomiting, time to the first request for analgesia via patient-controlled analgesia, and length of stay.
Results
A total of 382 patients were included, of whom 119 received ESPB. The mean age of the study patients was 70.6 years old, and 254 (66.5%) were male. After PSM, each group comprised 115 patients. Patients in the ESPB group showed a significantly lower opioid consumption at 24 h after surgery. Compared with the control group, VAS pain scores at rest in the first 24 h, number of patient-controlled intravenous analgesia (PCIA) pump compressions, ratio of patients requesting rescue analgesia, incidence of nausea and vomiting, and length of stay were significantly reduced in the ESPB group. There were no significant differences between the two groups regarding safety outcomes.
Conclusions
ESPB reduces short-term opioid consumption while providing safe and effective analgesia in elderly patients undergoing posterior lumbar surgery.
Keywords: Erector spinae plane block, Analgesic, Elderly patients, Posterior lumbar spine surgery, Propensity score matching
Key Summary Points
| Why carry out this study? |
| For preoperative analgesia during a variety of operations, the erector spinae plane block (ESPB) has grown in popularity. Its effectiveness in lumbar surgery is still unknown. The aim of this study is to assess the potential benefits of ESPB in enhancing analgesic efficacy in elderly individuals following posterior lumbar spine surgery. |
| What was learned from the study? |
| ESPB is a novel, effective, and promising technique in posterior lumbar spine surgery and should be used widely. |
Introduction
Degenerative lumbar spine illness is becoming an increasingly serious global public health issue as the world’s population ages [1, 2]. Treatment for lumbar pathologies is thought to cost close to $50 billion annually in the USA alone [3]. Lumbar degenerative disease is induced by the aging process and the gradual loss of lumbar spine function. Clinical symptoms are frequently exhibited as degenerative alterations of the articular cartilage and surrounding ligaments of the lumbar intervertebral disc and facet joints, lumbar and leg pain, and sciatica, which is characterized by a complex etiology, a protracted illness course, and a challenging therapy [4].
Currently, posterior lumbar canal decompression and bone graft fusion are the primary surgical treatments for lumbar degenerative disorders. Statistics indicate that the number of posterior lumbar decompressions has more doubled in the past 15 years [5]. As a result of the large wound surface, long incision, severe damage to the surgical area’s structure and surrounding tissues, and postoperative postural compression, patients frequently experience severe pain shortly after surgery, which has a negative effect not only on their postoperative satisfaction but also on their mental health. In addition, postoperative pain cannot be adequately managed, preventing it from cooperating with early functional activity of the waist and back muscles and lower limbs, hence increasing the risk of thrombosis and other significant complications and resulting in a poor prognosis [6–8]. Consequently, analgesia in posterior lumbar surgery has become a pressing issue for clinicians to address.
Although opioids are the most often used analgesics for postoperative analgesia, they are associated with adverse effects including dizziness, pruritus, nausea, vomiting, and respiratory depression [9]. With the invention and refinement of ultrasound-guided regional nerve block technology, peripheral somatic nerve block technology has increasingly become a vital component of perioperative multimode analgesia, which plays a significant role in a number of conventional surgical procedures [10, 11]. These methods have a high success rate, particularly when performed under ultrasound guidance, which increases vision and consequently minimizes the likelihood of problems [12]. Erector spinae plane block (ESPB), which was established in 2016, is a relatively new regional anesthesia treatment involving the injection of local anesthetic into the fascial plane between the transverse process of the spine and the erector spinae muscles [13]. ESPB has been demonstrated to provide analgesia effectively in orthopedic surgery [14], breast surgery [15], thoracic surgery [16], and abdominal surgery [17]. Prior research has paid less attention to the use of ESPB in posterior lumbar surgery for older individuals. To address these limitations, a well-designed randomized controlled trial is required, notwithstanding the difficulty of conducting such a study in older patients undergoing posterior lumbar spine surgery.
We use propensity score matching (PSM) approaches to control for sample selection bias and simulate the randomization procedure. In addition, it was found that PSM data were comparable to what a prospective randomized data set would have revealed [18]. The purpose of this study is to examine whether ESPB can enhance analgesic efficacy in elderly individuals following posterior lumbar spine surgery.
Methods
Study Design
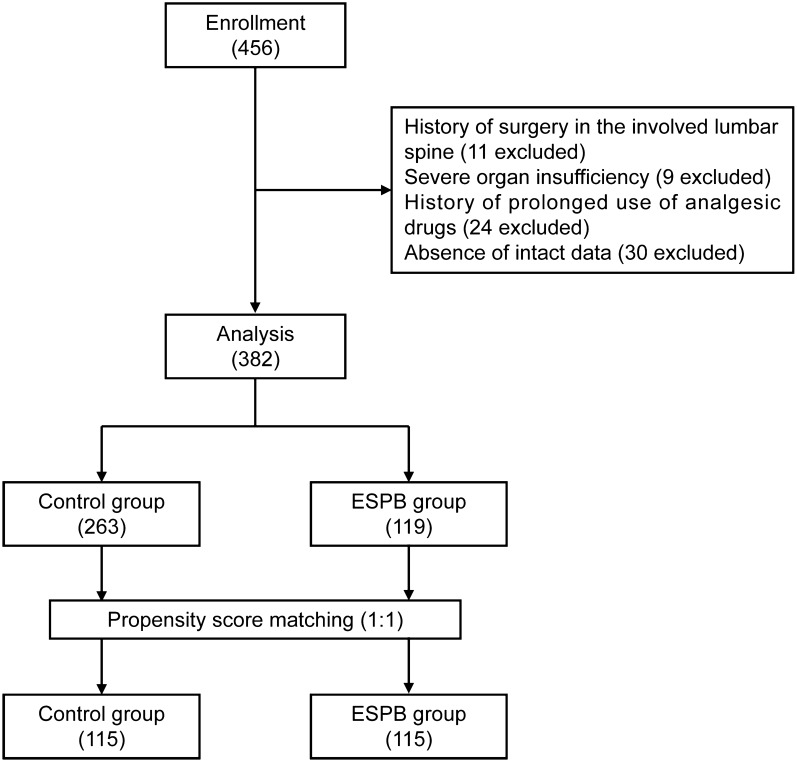
This study was conducted in compliance with the Helsinki declaration’s ethical principles and was approved by the institutional review board of Ganzhou People’s Hospital. As a result of the retrospective design, signed informed consents for participation were unavailable; therefore, the institutional review board at Ganzhou People’s Hospital waived the informed consent procedure for the current study. The electronic medical records of our hospital were reviewed to identify patients who met the following inclusion criteria: (1) aged 65 years or older, (2) posterior lumbar spine surgery under general anesthesia, (3) American Society of Anesthesiologists (ASA) physical status 1–3, (4) body mass index (BMI) less than 40 kg/m2. The exclusion criteria included (1) a previous history of surgery in the involved lumbar spine, (2) severe organ insufficiency, (3) a history of prolonged use of analgesic drugs, and (4) absence of complete data.
Data Collection
Data of selected patients were retrospectively retrieved from the medical database of our hospital. Demographic features included age, gender, BMI, marital status, smoking history, ASA physical status, and surgery time. Charlson comorbidity index (CCI) was calculated to obtain an overall assessment of preoperative comorbid condition. CCI included prior stroke, circulatory abnormalities (hypertension, coronary heart disease, prior myocardial infarction, and arrhythmia), type 2 diabetes, chronic obstructive pulmonary disease, dementia, pulmonary infection, Parkinson’s disease, digestive system disorders, chronic renal failure, rheumatologic disease, and osteoporosis [19]. Preoperative vital signs, as well as blood glucose (Glu), hemoglobin (HGB), serum albumin (ALB), platelet (PLT) count, white blood cell (WBC) count, international normalized ratio (INR), blood urea nitrogen (BUN), and left ventricular ejection fraction (LVEF), were recorded. An abnormal vital sign was defined according to the criteria of Zanker and Duque’s study [20].
Outcomes
The primary outcome was opioid consumption at 24 h after surgery. Secondary outcomes was visual analog scale (VAS) pain scores at rest in the first 24 h, number of patient-controlled intravenous analgesia (PCIA) pump compressions, ratio of patients requesting rescue analgesia, incidence of nausea and vomiting, and length of stay. Additional secondary outcomes were adverse events, which were monitored from the beginning of anesthesia until 24 h after surgery.
Treatment Protocol in Perioperative Period
Visit the patient in the ward on the day before surgery, inquire and understand the general physical condition of the patient, and establish relevant preoperative preparation and improvement of relevant examinations. Communicate fully with patients and their families, and inform them about the anesthesia protocol and the use of PCIA pump.
After entering the operating room, all patients underwent tripartite verification, and peripheral venous access was routinely opened after verification. The drip rate of sodium lactate Ringer injection was set at 8 ml/kg/h. Vital signs were routinely monitored, and the depth of anesthesia was monitored with bispectral index (BIS) monitor (Covidien llc, USA). Before anesthesia induction, 0.5 mg atropine was injected intravenously to estimate the scope of surgical incision, and body surface markers were made in the median of the corresponding lumbar segment.
In the ESPB group, patients were placed in the prone position, and an ultrasonic high-frequency linear array probe (5–10 MHz) was installed with disposable sterile protective sleeve. The position of the spinous process of the third lumbar spine was determined by median sagittal long-axis scanning (Fig. 1). Then the probe was rotated to the horizontal position of the short axis, and the probe was moved laterally 2–3 cm to identify the pectoralis longissimus muscle and multifidus muscle successively. The puncture needle tip (22 G, 90 cm long) was positioned at the fascia space between the longissimus pectoralis and multifidus muscle. After blood was withdrawn, all patients received an injection of 0.33% ropivacaine 15 mL.
Fig. 1.
Ultrasound image of erector spinae plane block
Anesthesia induction was performed immediately after completion of nerve block. Anesthesia induction was performed in the two groups by intravenous injection of midazolam 0.05 mg/kg, sufentanil 0.5 μg/kg, etomidate 0.2 mg/kg, and cisatracurium sulfonate 0.15 mg/kg. After anesthesia induction, 4–6 L/min high-flow pure oxygen was given for 3 min under mask pressure. After the full effect of the induction drug, the patient’s body movement, pain and eyelash reflex disappeared, and the muscle relaxation was satisfactory, endotracheal intubation was performed under laryngeal exposure. After intubation, the anesthesia machine (Draeger Medical GmbH, Germany) was used for mechanical ventilation, and the tidal volume was set at 6–8 mL/kg to control respiratory parameters. The respiratory rate was 10–12 times/min, and the partial pressure of ETCO2 was maintained at 35–45 mmHg. Propofol 4–8 mg/kg/h, remifentanil 0.1–0.2 μg/kg/min, and cisatracurium 0.06–0.12 mg/kg/h were used to maintain BIS at 45–65. The infusion rate was adjusted according to BIS value and vital signs. The mean arterial pressure of the patients was maintained to be no less than 20% of that before surgery to ensure stable blood flow. Sufentanil was added periodically according to specific conditions and the infusion speed of anesthetic drugs was adjusted. All operations were performed by the same group of experienced surgeons who did not know the experimental grouping.
Statistical Analysis
Continuous data were evaluated for normality using the Shapiro–Wilk test and Q–Q plots. Variables with normal distribution were expressed as mean ± SD and compared with the independent t test; otherwise, they were expressed as median (interquartile range) and compared with Mann–Whitney U test. Categorical variables are presented as total numbers and percentages. Comparisons between groups were made using the χ2 test for categorical variables and the Mann–Whitney U test for continuous variables, as appropriate.
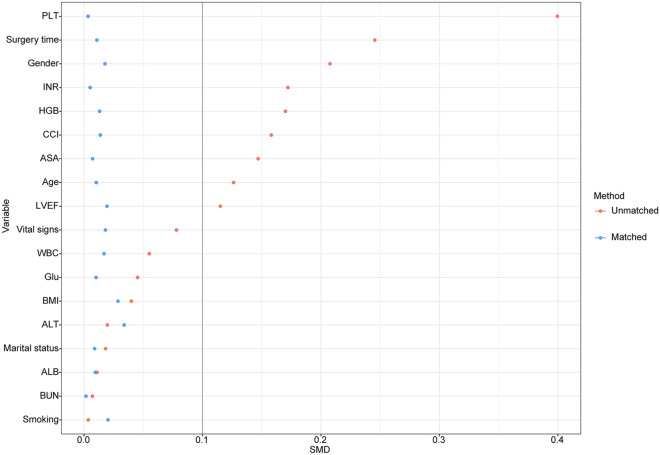
The total number of initial participants comprised the original cohort. PSM was used to create well-balanced groupings, notably the matched cohort, in addition to the original cohort. PSM-based propensity score adjustments were also applied to confirm the validity of our findings [21, 22]. The propensity score was calculated utilizing a non-parsimonious multivariable logistic regression model, with ESPB as the dependent variable and all baseline parameters as the independent factors. Patients in the ESPB group were matched with patients in the control group using the greedy nearest neighbor-matching method with a caliper width of 0.2. To assess the efficacy of the PSM, the standardized mean differences (SMD) were computed. SMD < 0.1 is considered a reasonable compromise between the groups [21].
All statistical analyses were performed using R software (version 4.1.1), and P < 0.05 was considered statistically significant.
Results
Demographic Data
We identified 456 elderly patients who underwent elective posterior lumbar instrumented fusion between January 2019 and June 2022. After screening, 382 patients were included in the final analysis; details are shown in the flowchart (Fig. 2). The mean age of the study patients was 70.6 years old, 254 (66.5%) were male, and 119 patients (31.2%) received ESPB. Before PSM, the majority of factors between the two groups were not balanced. Patients who underwent ESPB were more likely to be younger, male and have a lower ASA physical status, CCI score, and surgery time. Two hundred and thirty patients (115 per group) were selected after PSM. After PSM, the imbalanced covariates were balanced in the matched cohort (Table 1 and Fig. 3).
Fig. 2.
Screening of admissions for inclusion
Table 1.
Baseline characteristics of subjects in the original and matched cohorts
| Covariates | Original cohort (N = 382) | SMD | Matched cohort (N = 230) | SMD | ||
|---|---|---|---|---|---|---|
| Control group | ESPB group | Control group | ESPB group | |||
| N | 263 | 119 | 115 | 115 | ||
| Age (years) | 70.78 ± 4.82 | 70.20 ± 4.26 | 0.126 | 70.23 ± 4.49 | 70.19 ± 4.26 | 0.010 |
| Male (%) | 167 (63.5) | 87 (73.1) | 0.208 | 82 (71.3) | 83 (72.2) | 0.018 |
| BMI (kg/m2) | 22.84 ± 3.05 | 22.96 ± 3.06 | 0.040 | 23.05 ± 3.02 | 22.96 ± 3.07 | 0.029 |
| Marital status (%) | 0.018 | < 0.001 | ||||
| Married | 179 (68.1) | 82 (68.9) | 78 (67.8) | 78 (67.8) | ||
| Single | 84 (31.9) | 37 (31.1) | 37 (32.2) | 37 (32.2) | ||
| Smoking (%) | 80 (30.4) | 36 (30.3) | 0.004 | 34 (29.6) | 35 (30.4) | 0.020 |
| ASA (%) | 0.147 | < 0.001 | ||||
| I–II | 229 (87.1) | 109 (91.6) | 105 (91.3) | 105 (91.3) | ||
| > II | 34 (12.9) | 10 (8.4) | 10 (8.7) | 10 (8.7) | ||
| Surgery time (min) | 104.0 (92.5, 135.0) | 99.0 (89.0, 120.0) | 0.245 | 96.0 (86.0, 121.0) | 100.0 (89.0, 120.0) | 0.011 |
| CCI | 5.0 (3.0, 6.0) | 5.0 (2.0, 7.0) | 0.158 | 4.0 (3.0, 6.0) | 5.0 (2.0, 7.0) | 0.014 |
| Vital signs (%) | 0.078 | 0.018 | ||||
| Normal | 183 (69.6) | 87 (73.1) | 82 (71.3) | 83 (72.2) | ||
| Abnormal | 80 (30.4) | 32 (26.9) | 33 (28.7) | 32 (27.8) | ||
| Glu (mmol/L) | 5.90 (5.30, 6.50) | 5.80 (5.30, 6.70) | 0.045 | 5.80 (5.20, 6.40) | 5.80 (5.30, 6.70) | 0.010 |
| HGB (g/L) | 131.0 (119.0, 141.0) | 130.0 (99.0, 141.0) | 0.170 | 130.0 (100.0, 141.0) | 130.0 (100.0, 141.0) | 0.013 |
| ALB (g/L) | 42.09 ± 5.02 | 42.03 ± 5.03 | 0.011 | 42.05 ± 4.88 | 42.10 ± 5.02 | 0.009 |
| PLT (× 109/L) | 170.0 (127.0, 218.0) | 152.0 (109.5, 193.0) | 0.400 | 145.0 (107.0, 191.0) | 154.0 (112.0, 193.0) | 0.003 |
| WBC (× 109/L) | 5.50 (4.50, 6.90) | 5.50 (4.25, 6.75) | 0.055 | 5.30 (4.30, 6.10) | 5.50 (4.30, 6.40) | 0.017 |
| INR | 1.02 ± 0.10 | 1.04 ± 0.13 | 0.172 | 1.03 ± 0.11 | 1.03 ± 0.13 | 0.005 |
| ALT (U/L) | 21.0 (14.5, 29.0) | 22.0 (14.5, 28.0) | 0.020 | 22.0 (14.0, 28.0) | 20.0 (14.0, 28.0) | 0.034 |
| BUN (mmol/L) | 5.23 (4.25, 6.15) | 5.32 (4.40, 6.43) | 0.007 | 5.25 (4.29, 6.15) | 5.27 (4.39, 6.41) | 0.002 |
| LVEF (%) | 62.34 ± 8.39 | 61.29 ± 9.71 | 0.115 | 61.11 ± 9.08 | 61.29 ± 9.71 | 0.019 |
BMI body mass index, ASA American Society of Anesthesiologists, CCI Charlson comorbidity index, Glu blood glucose, HGB hemoglobin, ALB serum albumin, PLT platelet counts, WBC white blood cell count, INR international normalized ratio, BUN blood urea nitrogen, LVEF left ventricular ejection fraction
Fig. 3.
SMD between the Control and ESPB groups in each cohort
Primary Outcomes
In the matched cohort, as shown in Fig. 4, patients in the ESPB group showed a significantly lower opioid consumption (P < 0.001) at 24 h after surgery.
Fig. 4.
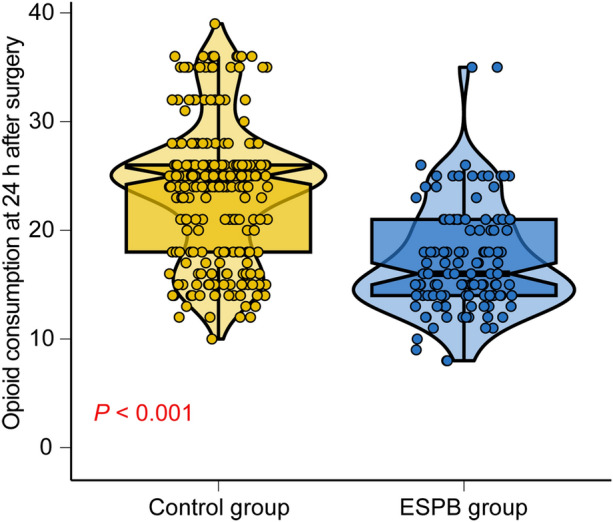
Comparison of opioid consumption at 24 h after surgery between different groups
Secondary Outcomes
Patients in the ESPB group showed similar independent length of hospital stay as compared with the control group (Table 2). Compared with the control group, VAS pain scores at rest in the first 24 h, number of PCIA pump compressions, ratio of patients requesting rescue analgesia, and incidence of nausea and vomiting (P < 0.05, Table 2).
Table 2.
Comparison of the secondary outcomes of subjects in the matched cohorts
| Covariates | Control group | ESPB group | P |
|---|---|---|---|
| N | 115 | 115 | |
| VAS pain score | 3.0 (2.0, 4.0) | 2.0 (1.0, 2.0) | < 0.001 |
| Number of PCIA pump compressions | 8.0 (2.0, 14.0) | 2.0 (2.0, 5.0) | < 0.001 |
| Rescue analgesia (%) | 24 (20.9) | 6 (5.2) | < 0.001 |
| Nausea and vomiting (%) | 10 (8.7) | 2 (1.7) | 0.018 |
| Length of stay (days) | 7.0 (6.0, 8.0) | 7.0 (5.0, 7.0) | 0.097 |
VAS visual analog scale, PCIA patient-controlled intravenous analgesic
Safety Outcomes
There were no significant differences between the two groups regarding safety outcomes (Table 3). Hypotension comprised the largest proportion of postoperative complications in both groups. No adverse events related to either the ESPB or lack of ESPB were observed, including local anesthetic intoxication and hematoma.
Table 3.
Comparison of the safety outcomes of subjects in the matched cohorts
| Covariates | Control group | ESPB group | P |
|---|---|---|---|
| N | 115 | 115 | |
| Respiratory depression (%) | 4 (3.5) | 2 (1.7) | 0.683 |
| Hypotension (%) | 30 (26.1) | 20 (17.4) | 0.110 |
| Hypertension (%) | 5 (4.3) | 2 (1.7) | 0.446 |
| Bradycardia (%) | 22 (19.1) | 16 (13.9) | 0.287 |
| Tachycardia (%) | 3 (2.6) | 0 (0.0) | 0.247 |
| Desaturation (%) | 2 (1.7) | 0 (0.0) | 0.498 |
| Dizziness (%) | 6 (5.1) | 2 (1.6) | 0.280 |
Discussion
In the present study, we found that ESPB significantly decreased the 24-h opioid consumption following posterior lumbar spine surgery; it also decreased the VAS pain scores at rest in the first 24 h, number of PCIA pump compressions, ratio of patients requesting rescue analgesia, and incidence of nausea and vomiting. In addition, we observed that ESPB was less likely to cause postoperative adverse events and had a good safety profile.
PSM, which was utilized in the present research, is one of the primary statistical methods for decreasing selection bias. PSM offers a number of benefits over more conventional regression techniques for controlling confounding by indication in observational research [23]. Presumably, data following PSM more closely resemble what a prospective randomized data set would have revealed. In order to reduce potential confounding by unmeasured and unknown effects of changes in procedures of treatment and postoperative care over the course of the study period, we only enrolled qualifying cases from recent years. In this regard, our study is likely superior to other retrospective investigations in terms of evidence quality. Nonetheless, prospective randomized controlled trials are required to investigate this topic further.
Posterior lumbar surgery is traumatic, the surgical incision is long, the structures of the surgical area and related tissues are severely damaged, and it is easy for postoperative position compression to increase the level of pain-causing substances locally or in plasma, thereby continuously stimulating the central or peripheral receptors, causing peripheral or central nerve pain sensitization, lowering the pain threshold, exacerbating the degree of pain, and affecting the lumbosacral plexus [24]. A multimodal analgesic regimen is indicated for patients undergoing difficult spine surgery, and should include paracetamol and nonsteroidal anti-inflammatory medications or cyclooxygenase-2-specific inhibitors, with opioids utilized for rescue analgesia [25]. However, an opioid overdose can result in adverse consequences such as dizziness, pruritus, nausea, vomiting, and respiratory depression [9]. In close proximity to the anterior surface of the transverse processes, lumbar spinal nerve roots emerge from the intervertebral foramina and divide into ventral and dorsal rami. Local anesthetics are injected into the plane between the deep fascia of the erector spinae muscle and the vertebral transverse process during ESPB [26].
There have been some previous studies on the use of ESPB for postoperative analgesia. Adhikary et al. [14] included 79 patients with rib fractures due to trauma in a retrospective study, in which 77% of the patients with traumatic rib fractures underwent ESPB. The results showed that the patients’ vital capacity was significantly improved in the first 24 h of ESPB, and the analgesic time lasted for nearly 72 h. The need for opioids is reduced and hemodynamic stability is maintained. In a retrospective study [27], 23 out of 41 lumbar surgery patients only received general anesthesia, while the other 18 patients also received ESPB in addition to general anesthesia. At 24 h following surgery, the pain scores of the group receiving ESPB were consistently lower than those of the group receiving only general anesthetic. At 24 h after surgery, the dosage of fentanyl was similarly lower than in the general anesthetic group, indicating that ESPB can provide effective postoperative analgesia for 24 h in patients undergoing lumbar surgery. In a double-blind, prospective, randomized, controlled experiment, it was discovered that ESPB can also enhance the analgesic efficacy in patients having hip and proximal femur surgery, a finding that merits therapeutic advancement [28]. In accordance with the aforementioned findings, our experiment demonstrated that ESPB considerably reduced opioid intake, PCIA bolus demand, and the usage of rescue analgesics in patients who underwent spinal surgery.
Despite the importance of our findings, there are significant limits to acknowledge. As a result of the retrospective study design, selection bias could not be eliminated. We applied PSM methodologies to confirm the validity of our findings. Second, the dermatomal extent of the sensory block was not determined. In addition, intraoperative opioids may impede the outcome evaluation. Considering the pharmacokinetic characteristics of sufentanil, this effect does not appear to last more than 4 h. We did not conduct a long-term follow-up since the effect of nerve blocks on long-term pain outcomes is expected to be limited.
Conclusion
ESPB reduces short-term opioid consumption while providing safe and effective analgesia in elderly patients undergoing posterior lumbar surgery. ESPB may be recommended for these patients given their opioid retention effects.
Acknowledgements
The authors thank the participants of the study.
Declarations
Funding
This work was supported by the Science and Technology Plan of Jiangxi Provincial Administration of Traditional Chinese Medicine (SZYYB20224730) and the Science and Technology Plan of Jiangxi Provincial Health Commission (SKJP220202171). The Rapid Service Fee was funded by the corresponding authors.
Author Contributions
Jianqin Zhu designed the research, analyzed the data, and wrote the manuscript; Guiming Huang and Yuting Zhu analyzed and interpreted the data; Cheng Peng designed the research, analyzed the data, and corrected the manuscript.
Disclosures
The authors have no conflict of interest to declare.
Ethics and Compliance Guidelines
This study was approved by the ethics committee of Ganzhou People’s Hospital (approval number TYZKY202201801). This study was conducted in accordance with the principles of the Declaration of Helsinki of 1964 and its amendments. All participants were informed of the experimental protocol and signed the informed consent in the study.
Data Availability
All data are available and the correspondent can be contacted if requested.
References
- 1.Strömqvist F, Strömqvist B, Jönsson B, Karlsson MK. Surgical treatment of lumbar disc herniation in different ages-evaluation of 11,237 patients. Spine J. 2017;17(11):1577–1585. doi: 10.1016/j.spinee.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Ravindra VM, Senglaub SS, Rattani A, et al. Degenerative lumbar spine disease: estimating global incidence and worldwide volume. Glob Spine J. 2018;8(8):784–794. doi: 10.1177/2192568218770769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogt MT, Kwoh CK, Cope DK, Osial TA, Culyba M, Starz TW. Analgesic usage for low back pain: impact on health care costs and service use. Spine (Phila Pa 1976) 2005;30(9):1075–1081. doi: 10.1097/01.brs.0000160843.77091.07. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser MG, Eck JC, Groff MW, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology. J Neurosurg Spine. 2014;21(1):2–6. doi: 10.3171/2014.4.SPINE14257. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhakar NK, Chadwick AL, Nwaneshiudu C, et al. Management of postoperative pain in patients following spine surgery: a narrative review. Int J Gen Med. 2022;2(15):4535–4549. doi: 10.2147/IJGM.S292698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenders N, Rushton A, Verra ML, Willems PC, Hoogeboom TJ, Staal JB. Pain and disability after first-time spinal fusion for lumbar degenerative disorders: a systematic review and meta-analysis. Eur Spine J. 2019;28(4):696–709. doi: 10.1007/s00586-018-5680-3. [DOI] [PubMed] [Google Scholar]
- 8.Kalogera E, Dowdy SC. Enhanced recovery after surgery and acute postoperative pain management. Clin Obstet Gynecol. 2019;62(4):656–665. doi: 10.1097/GRF.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 9.Dinges HC, Otto S, Stay DK, et al. Side effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: a systematic review and network meta-analysis. Anesth Analg. 2019;129(4):1153–1162. doi: 10.1213/ANE.0000000000003887. [DOI] [PubMed] [Google Scholar]
- 10.Oh SK, Lim BG, Won YJ, Lee DK, Kim SS. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: a systematic review and meta-analysis. J Clin Anesth. 2022;78:110647. doi: 10.1016/j.jclinane.2022.110647. [DOI] [PubMed] [Google Scholar]
- 11.Bonvicini D, Tagliapietra L, Giacomazzi A, Pizzirani E. Bilateral ultrasound-guided erector spinae plane blocks in breast cancer and reconstruction surgery. J Clin Anesth. 2018;44:3–4. doi: 10.1016/j.jclinane.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Zhu RL, Yue L, et al. Bilateral ultrasound-guided erector spinae plane block versus wound infiltration for postoperative analgesia in lumbar spinal fusion surgery: a randomized controlled trial. Eur Spine J. 2023;32(1):301–312. doi: 10.1007/s00586-022-07453-y. [DOI] [PubMed] [Google Scholar]
- 13.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 14.Adhikary SD, Liu WM, Fuller E, Cruz-Eng H, Chin KJ. The effect of erector spinae plane block on respiratory and analgesic outcomes in multiple rib fractures: a retrospective cohort study. Anaesthesia. 2019;74(5):585–593. doi: 10.1111/anae.14579. [DOI] [PubMed] [Google Scholar]
- 15.Leong RW, Tan ESJ, Wong SN, Tan KH, Liu CW. Efficacy of erector spinae plane block for analgesia in breast surgery: a systematic review and meta-analysis. Anaesthesia. 2021;76(3):404–413. doi: 10.1111/anae.15164. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Wang W, Xie W, Chen Z, Liu Y. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: a systematic review and meta-analysis. J Clin Anesth. 2020;66:109900. doi: 10.1016/j.jclinane.2020.109900. [DOI] [PubMed] [Google Scholar]
- 17.Bhushan S, Huang X, Su X, Luo L, Xiao Z. Ultrasound-guided erector spinae plane block for postoperative analgesia in patients after liver surgery: a systematic review and meta-analysis on randomized comparative studies. Int J Surg. 2022;103:106689. doi: 10.1016/j.ijsu.2022.106689. [DOI] [PubMed] [Google Scholar]
- 18.Reiffel JA. Propensity score matching: the ‘devil is in the details’ where more may be hidden than you know. Am J Med. 2020;133(2):178–181. doi: 10.1016/j.amjmed.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: a critical review of clinimetric properties. Psychother Psychosom. 2022;91(1):8–35. doi: 10.1159/000521288. [DOI] [PubMed] [Google Scholar]
- 20.Zanker J, Duque G. Rapid geriatric assessment of hip fracture. Clin Geriatr Med. 2017;33(3):369–382. doi: 10.1016/j.cger.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushimoto S, Gando S, Saitoh D, et al. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care. 2013;17(6):R271. doi: 10.1186/cc13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112–1117. doi: 10.1093/ejcts/ezy167. [DOI] [PubMed] [Google Scholar]
- 24.Chin KJ, Adhikary S, Forero M. Is the erector spinae plane (ESP) block a sheath block? A reply. Anaesthesia. 2017;72(7):916–917. doi: 10.1111/anae.13926. [DOI] [PubMed] [Google Scholar]
- 25.Peene L, Le Cacheux P, Sauter AR, et al. Pain management after laminectomy: a systematic review and procedure-specific post-operative pain management (PROSPECT) recommendations. Eur Spine J. 2021;30(10):2925–2935. doi: 10.1007/s00586-020-06661-8. [DOI] [PubMed] [Google Scholar]
- 26.Restrepo-Garces CE, Chin KJ, Suarez P, Diaz A. Bilateral continuous erector spinae plane block contributes to effective postoperative analgesia after major open abdominal surgery: a case report. Case Rep. 2017;9(11):319–321. doi: 10.1213/XAA.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 27.Ueshima H, Inagaki M, Toyone T, Otake H. Efficacy of the erector spinae plane block for lumbar spinal surgery: a retrospective study. Asian Spine J. 2019;13(2):254–257. doi: 10.31616/asj.2018.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tulgar S, Kose HC, Selvi O, et al. Comparison of ultrasound-guided lumbar erector spinae plane block and transmuscular quadratus lumborum block for postoperative analgesia in hip and proximal femur surgery: a prospective randomized feasibility study. Anesth Essays Res. 2018;12(4):825–831. doi: 10.4103/aer.AER_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available and the correspondent can be contacted if requested.