Abstract
Artemisia iwayomogi (AI) is a perennial herb found in Korea. Its ground parts are dried and used in food and traditional medicine for treating hepatitis, inflammation, cholelithiasis, and jaundice. In this study, the anti-obesity effects of single compounds isolated from AI extracts on adipose tissue were investigated. Results demonstrated that caffeoylquinic acid analogs strongly inhibited adipocyte differentiation from 3T3-L1 preadipocytes and reduced neutral lipids in differentiated adipocytes. Accordingly, lipid accumulation in adipocytes decreased, and lipid droplets became granulated. Caffeoylquinic acid analogs suppressed the expression of adipocyte differentiation marker genes, namely, Cebpa, Lep, and Fabp4, but it induced the expression of Ucp1, Ppargc1a, and Fgf21, which are browning biomarkers. Therefore, caffeoylquinic acid analogs from AI inhibited preadipocyte differentiation and induced adipose tissue browning, suggesting that these compounds could be promising therapeutic agents for obesity.
Keywords: Artemisia iwayomogi, Caffeoylquinic acid, Adipose tissue, Preadipocyte, Adipogenesis
Introduction
Obesity characterized by an increased adipose tissue commonly occurs when energy uptake exceeds energy expenditure (Tseng et al., 2010). It is associated with various diseases, such as cardiovascular diseases, inflammation-related disorders, fatty liver diseases, osteoarthritis, hypertension, type 2 diabetes mellitus, and cancer (Bardou et al., 2013; Singla et al., 2010). However, the World Health Organization considered obesity as a preventable cause of death (WHO, 1997).
Caloric intake restriction and caloric consumption increase are considered the first treatments for obesity. Caloric intake restriction is a typical dietary restriction, and medications such as orlistat (Xenical®), phentermine (Lonamin®), lorcaserin (Belviq®), naltrexone plus bupropion (Contrave®), or liraglutide (Saxenda®) can be used. However, reducing caloric intake activates the reward system, causing difficulty in losing weight (Saper et al., 2002). Moreover, medication is limited because of adverse effects, such as tachycardia, insomnia, high blood pressure, tremor, headache, palpitation, and constipation (Devereux, 1998; Hanefeld and Sachse, 2002). Therefore, studies have been performed to find alternative ways for treating obesity.
Adipose tissue is classified into three types depending on its metabolic functions, origin, and distribution: white, brown, and beige (Chu and Gawronska-Kozak, 2017). White adipose tissue (WAT) stores surplus energy in a lipid form, whereas brown adipose tissue (BAT) generates heat by oxidizing stored lipids (Saely et al., 2012). Beige adipose tissue (BAT-like) is a subtype that refers to tissues with behavior similar to BAT in response to specific stimuli such as cold exposure or β3-adrenergic stimulation to WAT (Montanari et al., 2017). Adipocytes in BAT and BAT-like are packed with mitochondria that considerably express uncoupling protein 1 (UCP1), which can dissipate the proton gradient from the mitochondrial matrix to the mitochondrial intermembrane space; as a result, heat energy is generated (Kozak and Anunciado-Koza, 2008). Thus, these cells can burn fat.
Obesity can be treated by reducing the number of adipocytes or browning of WAT tissues (Kim and Plutzky, 2016; Spalding et al., 2008). Recent studies have discovered the mechanisms of inhibiting adipogenesis and WAT browning (Calderon-Dominguez et al., 2016; Rosen and MacDougald, 2006). Furthermore, adipogenesis can be inhibited by numerous compounds, such as echinomycin, Aster spathulifolius extract, and curcumin (Kim and Choung, 2016; Lone et al., 2016; Yamaguchi et al., 2017), while resveratrol, magnolol, capsaicin, and trans-anethole are proposed as browning-inducing agents (Kang et al., 2018; Parray et al., 2018; Wang et al., 2015; Yoneshiro et al., 2012).
Artemisia iwayomogi (AI) is a perennial herb found in Korea, and its ground parts are dried and used for various purposes. It has been used in food and traditional medicine for treating hepatitis, inflammation, cholelithiasis, and jaundice (Lee et al., 1998). Pharmacological studies have demonstrated that AI water extracts reduce allergic reactions and inflammatory cytokine production, while 95% ethanolic extracts activate fatty acid oxidation and reduce serum and liver lipids in rats by activating the AMP-activated protein kinase (AMPK)-mediated pathway (Cho et al., 2012; Choi et al., 2012). AMPK activation inhibits fatty acid synthesis and promotes fatty acid oxidation (Dobrzyn et al., 2004). Furthermore, AMPK activation induces adipose tissue browning and reduces fat mass (Yan et al., 2016). AI extract (AIE) may inhibit adipocyte differentiation (Choi et al., 2013). These findings suggest that AIE can be effective in regulating adipocytes. However, studies have yet to determine the ingredients from AI that will exert anti-adipogenic or browning effects on preadipocytes. Therefore, the present study was performed to demonstrate the effect of single compounds isolated from AIE on the differentiation and browning of 3T3-L1 adipocytes.
Materials and methods
Cell culture and differentiation
3T3-L1 preadipocytes (Korean Cell Line Bank, Seoul, Korea) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% bovine calf serum, 1% penicillin and streptomycin (Welgene, Gyeongsan, Gyeong-buk, Korea). The cells were cultured in a humidified 5% CO2 atmosphere at 37 °C. The differentiation of confluent cells was induced by culturing them in a differentiation medium consisting of 10 μg/mL insulin, 1 μM dexamethasone, 0.5 mM methylisobutylxanthine (Sigma-Aldrich, St. Louis, MO, USA), and 10% fetal bovine serum (FBS, Welgene) in DMEM. After 2 days, the differentiation medium was replaced with a maintenance medium consisting of 10 μg/mL insulin and 10% FBS in DMEM (Bae and Kim, 2020; Montanari et al., 2019).
Chemicals
A 95% ethyl alcohol extract of Artemisia iwayomogi was obtained from the Korea plant extract bank (Daejeon, Korea). Fourteen purified compounds from AIE were kindly provided by Dr. Wan Kyunn Whang (Chung-Ang University, Seoul, Korea), and used for cytotoxicity assay and oil red O (ORO) staining assay. 1,3-Di-O-caffeoylquinic acid (1,3-di-O-CQA), 3,4-di-O-caffeoylquinic acid (3,4-di-O-CQA), 3,5-di-O-caffeoylquinic acid (3,5-di-O-CQA), 3,5-di-O-caffeoylquinic acid methyl ester (3,5-di-O-CQA methyl ester), and 2,4-dihydroxy-6-methoxyacetophenone (2,4-dihydroxy-6-MAP) were purchased from Chemfaces (Wuhan, Hubei, China) for real-time quantitative PCR. Stock solutions were prepared by dissolving compounds in dimethyl sulfoxide (DMSO, Sigma-Aldrich).
Cytotoxicity assay
3T3-L1 preadipocytes were seeded in a 96-well plate at a density of 1 × 104 cell/100 μL per well, incubated for 72 h, and treated with each compound or AIE. After 3 days, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) was added to the culture at a concentration of 0.5 mg/mL and incubated in a CO2 incubator at 37 °C for 4 h. Then, 100 μL of 0.04 N HCl–isopropanol (Junsei, Tokyo, Japan) was added to dissolve the reduced purple formazan, and the degree of MTT reduced to formazan by viable cells was quantified by measuring the absorbance at 540 nm using an Emax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Cytotoxicity was represented as the percentage of cell viability compared with that of the control.
ORO staining
The control or chemical-treated cells were incubated for 7 days, washed with PBS, fixed with 10% formalin (Daejung, Siheung, Gyeonggi, Korea) at 25 °C for 30 min, and washed with deionized water (DW) twice. Then, 60% isopropanol was added to the cells and incubated for 5 min. A mixture of 0.6% ORO (Sigma-Aldrich) dye in isopropanol and water at a ratio of 6:4 was layered onto the cells for 20 min and washed with DW thrice. The images of the cells were captured under a microscope. For quantification, intracellular lipid droplets were quantified after ORO was extracted with 100% isopropanol, and absorbance at 492 nm was determined in doublet wells by using a microplate reader.
Real-time quantitative PCR
Total RNA was extracted using RNAiso Plus (Takara Bio Inc., Kusatsu, Shiga, Japan) in accordance with the manufacturer’s instructions. The mRNA levels were analyzed through quantitative real-time PCR (qRT-PCR) by using a TOPreal qPCR 2X PreMIX (Enzynomics, Daejeon, Korea). Gene expression was normalized to β-actin expression. qRT-PCR was performed using a CFX Connect™ real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The primer sequences are listed in Table 1.
Table 1.
Primer sequences used for qRT-PCR
| Primer | Direction | Sequence (5′–3′) |
|---|---|---|
| Actb | Forward | ACCCACACTGTGCCCATCTAC |
| Reverse | GCCATCTCCTGCTCGAAGTC | |
| Fabp4 | Forward | AACACCGAGATTTCCTT |
| Reverse | ACACATTCCACCACCAG | |
| Lep | Forward | GTGGTCGGAAGCCCTGAGATAG |
| Reverse | GGGCGATCACTCGATGGAA | |
| Tfam | Forward | ATGTGGAGCGTGCTAAAAGC |
| Reverse | GGATAGCTACCCATGCTGGAA | |
| Nrf1 | Forward | GCTAATGGCCTGGTCCAGAT |
| Reverse | CTGCGCTGTCCGATATCCTG | |
| Ppargc1a | Forward | ATGTGCAGCCAAGACTCTGTA |
| Reverse | CGCTACACCACTTCAATCCAC | |
| Fgf21 | Forward | CGTCTGCCTCAGAAGGACTC |
| Reverse | TCTACCATGCTCAGGGGGTC | |
| Ucp1 | Forward | CCT GCC TCT CTC GGA AAC AA |
| Reverse | GTA GCG GGG TTT GAT CCC AT | |
| Cepba | Forward | GTGTGCACGTCTATGCTAAACCA |
| Reverse | GCCGTTAGTGAAGAGTCTCAGTTTG | |
| Cidea | Forward | CGGGAATAGCCAGAGTCACC |
| Reverse | TGTGCATCGGATGTCGTAGG |
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM). Each experiment was performed at least thrice, and representative data were obtained. Statistical significance was assessed through one-way ANOVA followed by Tukey’s or Dunnett’s multiple comparison test by using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Data with p < 0.05 were considered significant.
Results and discussion
Cytotoxic effects of purified compounds derived from AIE
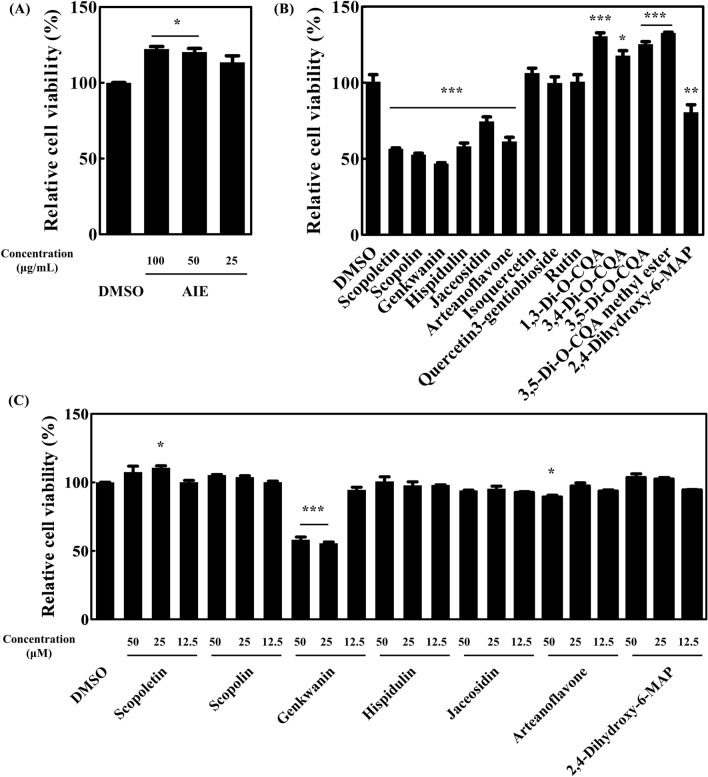
MTT assays were performed in 3T3-L1 cells to test the cytotoxic effects of AIE and 14 purified compounds isolated from AIE. AIE did not show any cytotoxicity at 100 μg/mL (Fig. 1(A)). In Fig. 1(B), scopoletin, scopolin, genkwanin, hispidulin, jaceosidin, arteanoflavone, and 2,4-dihydroxy-6-MAP exerted cytotoxic effects at 100 μM. Conversely, isoquercetin, quercetin 3-gentiobioside, rutin, 1,3-di-O-CQA, 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester had no cytotoxic effects. MTT assay was further conducted with cytotoxic compounds at lower concentration ranging from 12.5 to 50 μM (Fig. 1(C)). The result showed that genkwanin had cytotoxicity at 50 and 25 μM, and arteanoflavone at 50 μM. Scopoletin, scopolin, hispidulin, jaceosidin, and 2,4-dihydroxy-6-MAP showed no cytotoxic effects at 50 μM. Further experiments were proceeded with AIE and all 14 compounds at a concentration without cytotoxicity.
Fig. 1.
Cytotoxic effects of AIE and purified compounds isolated from AIE. Cytotoxicity was represented as the percentage of cell viability compared with the DMSO control. (A) Cytotoxicity of AIE. Cytotoxicity of compounds isolated from AIE at 100 μM (B), 50, 25, and 12.5 μM (C). Data were expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the DMSO control
Effects of chemical compounds on 3T3-L1 preadipocyte differentiation
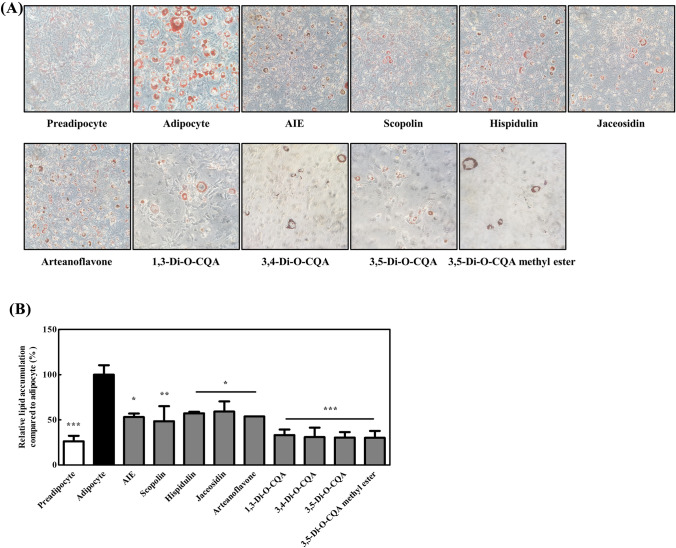
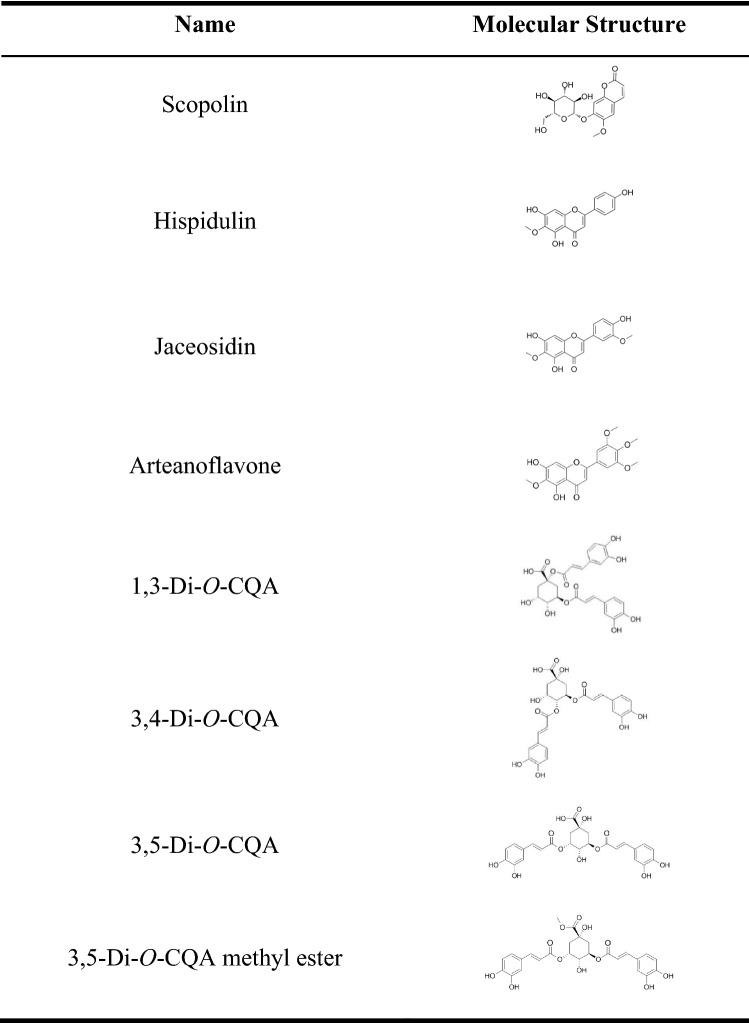
3T3-L1 cells were cultured in the differentiation media with each chemical compound for 2 days and transferred to maintenance media (Fig. 2) to investigate the effect of AIE and 14 chemical compounds on the differentiation of preadipocytes into white adipocytes. After 7 more days of exposure to AIE or each chemical compound, ORO staining was performed to visualize small lipid droplets and quantify triglyceride accumulation. The results showed that scopoletin, genkwanin, isoquercetin, quercetin-3-gentiobioside, rutin, and 2,4-dihydroxy-6-MAP did not reduce lipid accumulation, and no change was found in the microscopic image (data not shown). However, AIE, scopolin, hispidulin, jaceosidin, arteanoflavone, 1,3-di-O-CQA, 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester reduced lipid accumulation. The results confirm the previous studies showing anti-adipogenic effects of scopolin and hispidium (Lee et al., 2021; Park et al., 2020), and reveal that AIE and its isolated compounds including coumarin (scopolin), flavonoids (hispidulin, jaceosidin, and arteanoflavone), and CQAs inhibit preadipocyte differentiation. It is noteworthy that the four CQAs used in this experiment all have structural similarities and inhibitory effect on adipogenesis. The chemical structures of the eight compounds are presented in Table 2.
Fig. 2.
Effects of chemical compound on preadipocyte differentiation. 3T3-L1 cells were cultured under differentiation for 2 days, transferred to maintenance media, and cultured for > 7 days before ORO staining. The cells were treated with each chemical compound after they were cultured with differentiation media on day 0. (A) Representative light microscopy images of 3T3-L1 cells with ORO staining (×400). (B) Quantification of intracellular lipid droplets in the cells treated with AIE, scopolin, hispidulin, jaceosidin, arteanoflavone, 1,3-di-O-CQA, 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester. Data were expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the adipocyte control
Table 2.
Chemical structures of the compounds with anti-adipogenesis activity
Effects of chemical compounds on adipocyte browning
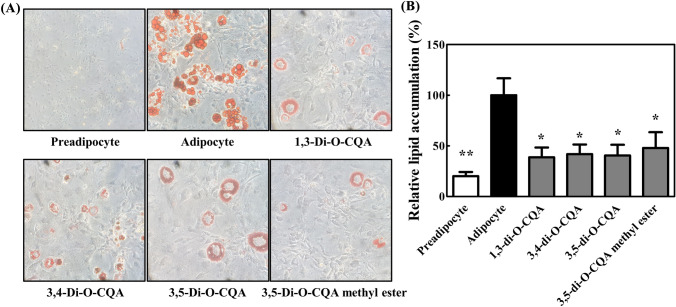
3T3-L1 cells were cultured under differentiation media for 2 days and transferred to the maintenance media containing AIE or chemical compounds (Fig. 3) to test the effect of AIE and fourteen chemical compounds on adipocyte browning. After 7 days of exposure to each chemical compound, ORO staining was performed to visualize small lipid droplets and quantify triglyceride accumulation. The results showed that lipid accumulation did not change with AIE, scopoletin, scopolin, genkwanin, hispidulin, jaceosidin, arteanoflavone, isoquercetin, quercetin-3-gentiobioside, rutin, and 2,4-dihydroxy-6-MAP (data not shown); conversely, 1,3-di-O-CQA, 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester successfully inhibited the formation of lipid accumulation, as measured by ORO staining (Fig. 3). CQAs did not only show anti-adipogenesis effect on preadipocytes but also inhibited lipid accumulation in differentiated adipocytes, while AIE did not show inhibitory effect on lipid droplet accumulation in differentiated adipocytes. The data suggest that CQA concentration present in AIE may not be sufficient to inhibit lipid accumulation.
Fig. 3.
Effects of chemical compounds on lipid accumulation. 3T3-L1 cells were cultured under differentiation for 2 days, transferred to maintenance media, and cultured for > 7 days before ORO staining. The cells were treated with each chemical compound after they were cultured in the maintenance media on day 2. (A) Representative light microscopy images of 3T3-L1 cells with ORO staining (×400). (B) Quantification of intracellular lipid droplet. Data were expressed as mean ± SEM. *p < 0.05; **p < 0.01 compared with the adipocyte control
The four effective compounds have similar structures to CQA. CQA is a natural phenolic compound composed of quinic acid core and acylation with caffeoyl acid. CQAs have antioxidant (Rebollo-Hernanz et al., 2019), hepatoprotective (Choi et al., 2005), and antiobesity (Cho et al., 2010), and antidiabetic (Johnston et al., 2005) effects. Miyamae et al. showed that the number of caffeoyl groups, double bonds in caffeoyl acid, and carboxyl group in quinic acid are important for activity in ATP production (Miyamae et al., 2011). However, structure–activity relationship had no significant differences in the regulation of lipid accumulation by CQA analogs tested in this study. Rather, the four CQAs commonly inhibited all phases of adipocyte differentiation and lipid accumulation strongly.
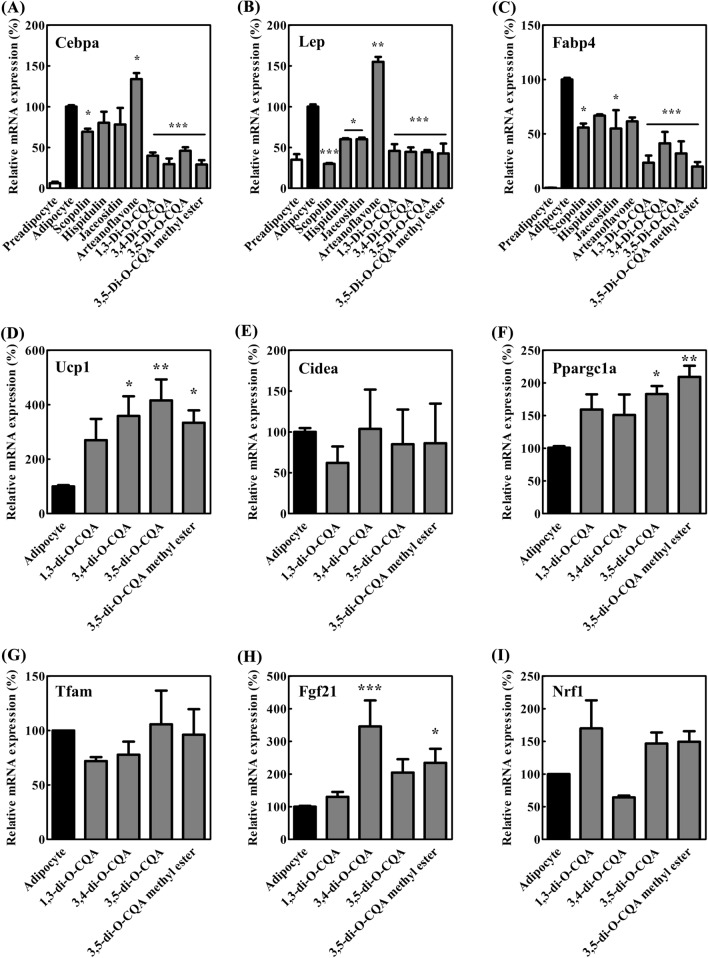
Changes in the expression of adipogenesis-related genes and brown adipose tissue marker genes
The mRNA expression levels of Cebpa, Lep, and Fabp4, which are biomarkers of differentiation, were analyzed to confirm the inhibitory effect of each chemical on adipocyte differentiation. In Fig. 4, the expression levels of Cebpa, Lep, and Fabp4 were increased upon adipocyte differentiation compared with those upon preadipocyte differentiation. However, the expression of adipogenesis-related genes was all decreased when the cells were treated with scopolin, 1,3-di-O-CQA, 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester. C/ebp-α is a CCAAT/enhancer binding protein α that performs a crucial role in the control of differentiation of preadipocytes into adipocytes by regulating the expression of characteristic adipocyte genes, such as leptin, adipocyte fatty acid binding protein 2 (Fabp4, aP2), and lipoprotein (Rosen and MacDougald, 2006). In the present study, the mRNA expression of C/ebp-α was decreased; mRNA downstream genes, namely, leptin and fatty acid binding protein 2, were also decreased because of the treatment with scopolin and CQA analogs. These results confirmed that four CQA analogs and scopolin repressed adipogenesis.
Fig. 4.
Changes in the expression of adipogenesis-related genes and brown adipose tissue marker genes. (A) Cebpa, (B) Lep, (C) Fabp4, (D) Ucp1, (E) Cidea, (F) Ppargc1a, (G) Tfam, (H) Fgf21, and (I) Nrf1. mRNA expression was measured through quantitative real-time PCR. The signals were normalized to β-actin internal control. Data were expressed as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control
To investigate the inducing effect of CQA analogs on adipocyte browning, mRNA expression of brown adipose tissue markers was tested. The results showed that 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester increased the mRNA expression of brown adipose tissue markers, namely, Ucp1, Ppargc1a, and Fgf21 (Fig. 4). However, other browning markers, such as Tfam, Cidea, and Nrf1, had no significant changes upon treatment with CQA analogs (Fig. 4). UCP1 is an uncoupling protein 1 that creates a hole through which protons become dispersed into the mitochondrial matrix, thereby generating heat by nonshivering thermogenesis. Increasing thermogenesis leads to an increased energy consumption, which likely yields significant weight loss (Harper et al., 2008). UCP1 expression is induced by several factors, including peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) and Fibroblast growth factor 21 (FGF21) (Song et al., 2017). FGF21 enhances PGC-1α in adipose tissue and in turn, PGC-1α induces UCP1 expression (Song et al., 2017). In this study, the gene expression levels of Fgf21, Ppargc1a, and Ucp1 were significantly increased by 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester treatment. Thus, CQA analogs isolated from the AIE possibly induce adipocyte browning through the PGC-1α-stimulating pathway. In our experiment, some browning markers Ucp1, Ppargc1a, and Fgf21 were reduced by CQA analogs while other browning markers Tfam, Cidea, and Nrf1 were not regulated by CQA analogs. The selective regulation of gene expression would help elucidate the mechanism of action of CQA analogs in adipocyte modulation.
In conclusion, 1,3-di-O-CQA, 3,4-di-O-CQA, 3,5-di-O-CQA, and 3,5-di-O-CQA methyl ester of AIE regulate adipogenesis. Future studies should be performed to test their lipid-regulating effects on animal models.
Acknowledgements
This research is supported by National Research Foundation of Korea (NRF) Grant funded by Ministry of Science and ICT (2021R1F1A1061287).
Declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Su-Young Han and Jisu Kim have contributed equally to this work.
References
- Bae IS, Kim SH. Sinapic acid promotes browning of 3T3-L1 adipocytes via p38 MAPK/CREB pathway. BioMed Research International. 2020;2020:5753623. doi: 10.1155/2020/5753623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933–947. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- Calderon-Dominguez M, Mir JF, Fucho R, Weber M, Serra D, Herrero L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte. 2016;5:98–118. doi: 10.1080/21623945.2015.1122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A-S, Jeon S-M, Kim M-J, Yeo J, Seo K-I, Choi M-S, Lee M-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food and Chemical Toxicology. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Cho SY, Jeong HW, Sohn JH, Seo D-B, Kim WG, Lee S-J. An ethanol extract of Artemisia iwayomogi activates PPARδ leading to activation of fatty acid oxidation in skeletal muscle. PLoS ONE. 2012;7:e33815. doi: 10.1371/journal.pone.0033815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park J-K, Lee K-T, Park K-K, Kim W-B, Lee J-H, Jung H-J, Park H-J. In vivo antihepatotoxic effects of Ligularia fischeri var. spiciformis and the identification of the active component, 3,4-dicaffeoylquinic acid. Journal of Medicinal Food. 2005;8:348–352. doi: 10.1089/jmf.2005.8.348. [DOI] [PubMed] [Google Scholar]
- Choi Y-G, Yeo S, Kim S-H, Lim S. Anti-inflammatory changes of gene expression by Artemisia iwayomogi in the LPS-stimulated human gingival fibroblast: microarray analysis. Archives of Pharmacal Research. 2012;35:549–563. doi: 10.1007/s12272-012-0319-0. [DOI] [PubMed] [Google Scholar]
- Choi Y, Yanagawa Y, Kim S, Whang WK, Park T. Artemisia iwayomogi extract attenuates high-fat diet-induced obesity by decreasing the expression of genes associated with adipogenesis in mice. Evidence-Based Complementary and Alternative Medicine. 2013: 11 (2013) [DOI] [PMC free article] [PubMed]
- Chu DT, Gawronska-Kozak B. Brown and brite adipocytes: same function, but different origin and response. Biochimie. 2017;138:102–105. doi: 10.1016/j.biochi.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Devereux RB. Appetite suppressants and valvular heart disease. The New England Journal of Medicine. 339: 765–767 (1998) [DOI] [PubMed]
- Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2002;4:415–423. doi: 10.1046/j.1463-1326.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- Harper M-E, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annual Review of Nutrition. 2008;28:13–33. doi: 10.1146/annurev.nutr.28.061807.155357. [DOI] [PubMed] [Google Scholar]
- Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Letters. 2005;579:1653–1657. doi: 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- Kang NH, Mukherjee S, Min T, Kang SC, Yun JW. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie. 2018;151:1–13. doi: 10.1016/j.biochi.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Choung SY. Inhibitory effects of Aster spathulifolius extract on adipogenesis and lipid accumulation in 3T3-L1 preadipocytes. Journal of Pharmacy and Pharmacology. 2016;68:107–118. doi: 10.1111/jphp.12485. [DOI] [PubMed] [Google Scholar]
- Kim SH, Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes & Metabolism Journal. 2016;40:12–21. doi: 10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak L, Anunciado-Koza R. UCP1: its involvement and utility in obesity. International Journal of Obesity. 2008;32:S32–S38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Lee SM, Cho TS, Shim SB, Yoon KW, Lee JC. A study on the hepatoprotective effect of PS-1 from Artemisia iwayomogi. Biomolecules & Therapeutics. 1998;6:119–129. [Google Scholar]
- Lee D, Kwak HJ, Kim BH, Kim SH, Kim D-W, Kang KS. Combined anti-adipogenic effects of hispidulin and p-synephrine on 3T3-L1 adipocytes. Biomolecules. 2021;11:1764. doi: 10.3390/biom11121764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. Journal of Nutritional Biochemistry. 2016;27:193–202. doi: 10.1016/j.jnutbio.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Miyamae Y, Kurisu M, Han J, Isoda H, Shigemori H. Structure–activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chemical and Pharmaceutical Bulletin. 2011;59:502–507. doi: 10.1248/cpb.59.502. [DOI] [PubMed] [Google Scholar]
- Montanari T, Pošćić N, Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Obesity Reviews. 2017;18:495–513. doi: 10.1111/obr.12520. [DOI] [PubMed] [Google Scholar]
- Montanari T, Boschi F, Colitti M. Comparison of the effects of browning-inducing capsaicin on two murine adipocyte models. Frontiers in Physiology. 2019;10:1380. doi: 10.3389/fphys.2019.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Lee CG, Kim J, Lim E, Yeo S, Jeong S-Y. Scopolin prevents adipocyte dierentiation in 3T3-L1 preadipocytes and weight gain in an ovariectomy-induced obese mouse model. International Journal of Molecular Sciences. 2020;21:8699. doi: 10.3390/ijms21228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parray HA, Lone J, Park JP, Choi JW, Yun JW. Magnolol promotes thermogenesis and attenuates oxidative stress in 3T3-L1 adipocytes. Nutrition. 2018;50:82–90. doi: 10.1016/j.nut.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Rebollo-Hernanz M, Zhang Q, Aguilera Y, Martín-Cabrejas MA, Gonzalez de Mejia E. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants. 2019;8:279. doi: 10.3390/antiox8080279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nature Reviews Molecular Cell Biology. 2006;7:885. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58:15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/S0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: a review. World Journal of Diabetes. 2010;1:76. doi: 10.4239/wjd.v1.i3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N-J, Chang S-H, Li DY, Villanueva CJ, Park KW. Induction of thermogenic adipocytes: molecular targets and thermogenic small molecules. Experimental & Molecular Medicine. 2017;49:e353. doi: 10.1038/emm.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T. Dynamics of fat cell turnover in humans. Nature. 2008;453:783. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Tseng Y-H, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nature Reviews Drug Discovery. 2010;9:465. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, Rodgers B, Jiang Q, Dodson MV, Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. International Journal of Obesity. 2015;39:967. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity. Geneva: WHO; 1997. [PubMed] [Google Scholar]
- Yamaguchi J, Tanaka T, Saito H, Nomura S, Aburatani H, Waki H, Kadowaki T, Nangaku M. Echinomycin inhibits adipogenesis in 3T3-L1 cells in a HIF-independent manner. Scientific Reports. 2017;7:6516. doi: 10.1038/s41598-017-06761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Audet-Walsh É, Manteghi S, Dufour CR, Walker B, Baba M, St-Pierre J, Giguère V, Pause A. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes & Development. 2016;30:1034–1046. doi: 10.1101/gad.281410.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. The American Journal of Clinical Nutrition. 2012;95:845–850. doi: 10.3945/ajcn.111.018606. [DOI] [PubMed] [Google Scholar]