Abstract
Some Escherichia coli serotypes cause diarrhea in infants and acute gastroenteritis. In this study, the incidence of Shiga toxin-producing (STEC) and enterohemorrhagic (EHEC) E. coli in 310 fresh raw beef samples and the presence of pathogenicity-associated virulence genes in the isolated strains were evaluated. The contamination rate reached 18.06% (STEC, 12.26%; EHEC, 5.81%). The highest rate of identified virulence genes was 8.38% for stx2 and 3.23% for stx2 and eae in STEC and EHEC, respectively. Vinegar N6 significantly lowered E. coli growth in beef samples, depending on its concentration (> 0.5%), treatment temperature (5 or 10 °C), and E. coli type (STEC, EHEC, or enteropathogenic), during 28 days of storage. However, no bactericidal effects were detected, unlike those observed for combined treatment with UV-C LED and vinegar N6. Treatment with vinegar N6 and UV-C LED together may significantly reduce E. coli growth in fresh beef, thereby improving food safety.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01260-x.
Keywords: Shiga toxin-producing E. coli, Enterohemorrhagic E. coli, Beef, Natural antimicrobial, Ultraviolet-C light-emitting diode
Introduction
Escherichia coli is an intestinal bacterium in humans and warm-blooded animals. Some specific serotypes, referred to as Shiga toxin-producing E. coli (STEC), cause diarrhea in infants and acute gastroenteritis (Ewing and Edwards, 1986). These pathogens characteristically produce Shiga toxin (stx), which contributes to hemorrhagic colitis and hemolytic uremic syndrome (HUS). A subset of STEC strains, enterohemorrhagic E. coli (EHEC), possesses the locus of enterocyte effacement, with genes, such as eae (intimin), which are important in the development of attaching and effacing lesions on intestinal epithelial cells (McDaniel et al., 1995). Moreover, STEC exhibits pathogenicity even in the absence of an adhesion factor (eae). In STEC O104, which is Shiga toxin 2-positive, the eae-negative strain causes food-borne outbreaks of diarrhea and HUS (Moore et al., 1995).
Cattle are the most important reservoirs of STEC and enteropathogenic E. coli (EPEC). These pathogens are transmitted to humans via the ingestion of contaminated meat. Screening of cattle for the presence of STEC led to the isolation of stx-negative EPEC strains (Salaheen et al., 2019). These E. coli strains may be transmitted to carcass surfaces during slaughter, particularly during skinning and evisceration operations, via the equipment or through the hands of individuals handling the carcasses (Dickson and Anderson, 1992). STEC has been isolated from beef samples consumed by infected patients (Riley et al., 1983). The prevalence of STEC in beef samples ranges from 22.6 to 34.0% (Kagambèga et al., 2012a; Lee et al., 2009).
The abovementioned pathogenic E. coli serotypes are among the most prevalent infectious pathogens, accounting for the largest burden of illness-related costs and considerable economic losses to food producers (Bhaskar, 2016). To address these problems, safe natural antibacterial substances are being explored for food preservation. Vinegar N6, which mainly consists of acetic acid and is label-friendly, is used in the food industry and exhibits antibacterial effects (Heir et al., 2019). For this reason, vinegar N6 was investigated as an antipathogenic E. coli agent for use in the processing and storage of fresh beef.
UV-C light-emitting diode (UV-C LED) treatment is a novel approach proposed for pathogen inactivation (Pinela and Ferreira, 2017). UV-C LEDs have emerged as viable replacements to traditional UV mercury lamps owing to several benefits, such as extended lifetime, eco-friendliness (no mercury use), range of available wavelengths, and no warm-up time (Song et al., 2018). Treatment with UV-C LED in combination with other agents is effective in reducing harmful bacteria in various foods.
This study was designed to (1) determine the presence of STEC in beef samples from Korea, (2) investigate the effect of vinegar N6 and UV-C LED treatment of fresh beef on the growth and survival of pathogenic E. coli (STEC, EHEC, and EPEC), and (3) analyze the morphological characteristics of STEC in response to vinegar N6 and UV-C LED treatment.
Materials and methods
Sample collection
Samples of fresh minced beef were collected from wholesale stores and typical markets in Seoul, Gyeonggi, Gangwon, Dangjin, Gwangju, Daegu, Gimhae, and Jeju—representative regions that were selected considering regional distribution of domestic livestock processing plants within Korea. The minced beef samples contained various parts of the trim remains after deboning and selection of lumps that were pulverized. All samples were collected at the beginning of each quarter (first Monday of January, April, July, and October) over 3 years and were continuously monitored to increase accuracy and minimize variations. A total of 310 samples—118 in 2019, 112 in 2020, and 80 in 2021—were collected, transferred to an ice box, and placed at 5 °C until analysis. The samples were tested immediately upon purchase, within the expiration date.
Isolation and identification of STEC strains
The microbiological approach described in the bacteriological analytical manual was used to identify STEC strains in beef samples according to the Food Code (Food and Food Additives Convention, 2021), with certain modifications. Aseptically cut meat samples (25 g) were added to sterile bags with 225 mL of mTSB broth (Oxoid, Basingstoke, UK) and homogenized for 2 min using a stomacher (Bag-Mixer 400; Interscience, Paris, France), followed by incubation for 24 h at 36 °C to get enriched bacterial cultures.
Using real-time PCR, the enriched cultures were examined for the presence of virulence genes encoding Shiga toxins 1 and 2 (stx1 and stx2). stx1- and/or stx2-positive cultures were streaked on two plates of Sorbitol-MacConkey agar supplemented with 0.05 mg/L cefixime and 2.5 mg/L potassium tellurite (CT-SMAC; Oxoid) and BCIG agar (tryptone bile agar containing 5-bromo-4-chloro-3-indolyl-b-D-glucuronic acid; Oxoid), followed by incubation at 36 °C for 24 h. STEC colonies that appeared colorless or purple on CT-SMAC agar and green on BCIG agar were streaked on tryptic soy agar (TSA; Becton Dickinson, NJ, USA) and the plates were incubated at 36 °C for 24 h. At least five representative colonies were chosen, biochemically characterized as E. coli using the Vitek 2 system (BioMérieux, France), and verified using real-time PCR.
Real-time PCR assays for the detection of virulence genes
The STEC and EHEC isolates were subjected to multiplex real-time PCR for the detection of genes encoding virulence factors, namely stx1, stx2, and eae. Bacterial DNA was extracted from colonies cultured on TSA (Becton Dickinson) for 24 h at 36 °C. Colonies suspended in 1 mL of sterile distilled water were centrifuged for 1 min at 21,206×g (Gyrozen, Gyeonggi-do, Korea), and the supernatant was discarded. The pellet was resuspended in 200 μL of sterile distilled water, and the solution was boiled for 10 min on a hot plate at 100 °C (Daihan Scientific, Gangwon-do, Korea). Thereafter, the samples were centrifuged at 21,206×g for 5 min, and the supernatant was used as a DNA template. Real-time PCR assays were performed in a final reaction volume of 20 μL, containing 5 μL of template DNA with 0.2 mM of each primer (Table 1), 0.5 mM of each probe, and 10 μL of real-time PCR master mix (Kogenbiotech, Seoul, Korea). The real-time PCR assays were performed using CFX96 Real-Time PCR (Bio-Rad, CA, USA) on an ABI 7500 Real-Time PCR system (Applied Biosystems, CA, USA). After 2 min of pretreatment at 50 °C, the samples were subjected to 10 min of denaturation at 95 °C, followed by 35 cycles of denaturation and annealing at 95 °C for 15 s and 60 °C for 1 min, respectively. The amplified gene was displayed in an exponential log graph and assessed to be positive when the value of threshold cycle (Ct) was < 35.0, as suggested by the manufacturer. If a strain possessed stx1 and/or stx2 and was negative for eae, it was categorized as STEC; if it possessed stx1 and/or stx2 and was positive for eae, it was classified as EHEC.
Table 1.
Target genes and primer sequences used in real-time PCR assays for the identification of virulence genes of Shiga toxin-producing Escherichia coli (STEC)
| Target gene | Primer sequence | Amplicon size (bp) | References | |
|---|---|---|---|---|
| stx1 | Forward | TTT GTY ACT GTS ACA GCW GAA GCY TTA CG | 131 | Ministry of Food and Drug Safety (2021) |
| Reverse | CCC CAG TTC ARW GTR AGR TCM ACR TC | |||
| Probe | FAM-CTG GAT CTC AGT GGG CGT TCT TAT GTA A-TAMRA | |||
| stx2 | Forward | TTT GTY ACT GTS ACA GCW GAA GCY TTA CG | 128 | Ministry of Food and Drug Safety (2021) |
| Reverse | CCC CAG TTC ARW GTR AGR TCM ACR TC | |||
| Probe | VIC-TCG TCA GGC ACT GTC TGA AAC TGC TCC-TAMRA | |||
| eae | Forward | CCG ATT CCT CTG GTG ACGA | 105 | |
| Reverse | CCA CGG TTT ATC AAA CTG ATA ACG | This study | ||
| Probe | FAM-CGT CAT GGT ACG GGT AA-MGB | |||
Bacterial strains for inoculation
Strains of STEC (NCCP 13,720, stx1; NCCP 13,721, stx2) were obtained from the Korean National Culture Collection (NCCP). EHEC (NCTC 12,079, stx and eae), a representative E. coli O157:H7 strain, was purchased from National Collection of Type Cultures (NCTC, United Kingdom). Another strain, E. coli NCCP 13,715, was assigned to the EPEC pathotype, which is associated with human illness. The strains were maintained at − 80 °C in tryptic soy broth (TSB; Difco, Sparks, MD, USA) in beads. Each stock culture was thawed and inoculated in 10 mL of TSB; the culture was incubated at 36 °C for 24 h with shaking at 140 rpm in a shaking incubator (LSI-3016A; LabTech, Gyeonggi-do, Korea). After incubation, the viable cell count was approximately 9.0 log CFU/mL. One milliliter of the initial stationary growth phase culture was serially diluted in 9 mL of 0.85% NaCl. The two STEC strains (NCCP 13,720 and NCCP 13,721) were mixed and prepared as a cocktail; the final concentration of each strain was 3.0 log CFU/g in the beef samples.
Antibacterial effects of vinegar N6 against pathogenic E. coli in fresh beef samples
To simulate packaged beef products obtained after slaughter, cut chunks of meat that were subjected to deboning and selection were used. During transportation, the samples were placed in portable cooling containers at 4 °C, and the experiment was completed within 4 h of purchase. White distilled fermented vinegar N6 powder (Corbion, Amsterdam, Netherlands) was rehydrated in distilled water and used in a liquid form. This substance is applied to beef meat during storage before packing or sale.
For applying on beef, vinegar N6 was prepared at different concentrations (0%, 0.5%, 1%, and 2% w/w) and applied according to the weight of each beef sample. The beef samples were inoculated with bacteria, mixed with vinegar N6, vacuum-packed, and stored as described below. Weighed (10 g) beef samples were aseptically placed on Petri dishes. The surfaces of the samples were evenly inoculated with 0.1 mL of diluted cultures of each pathogenic E. coli (STEC cocktail, E. coli O157:H7, and EPEC) at 3.0 log CFU/g. The volume of E. coli cell suspension required to achieve the target concentration was spotted on the surface of the sample using a pipette, and the suspension was evenly spread using a sterile spreader. The inoculated samples were fixed at 5 °C to stabilize bacteria on the sample surface for 30 min. Vinegar N6 (0–2%) was added to each sample. Equal volumes of vinegar of the same concentration were inoculated onto the samples using a pipette. To ensure that E. coli cells were evenly exposed to vinegar N6, the vinegar solution was evenly spread on the surface of the inoculated samples using a sterile spreader. The inoculated samples were aseptically vacuum-packed in Petri dishes using a vacuum packaging machine, ensuring the removal of oxygen to create anaerobic conditions. The samples were placed at 5 or 10 °C for 28 days.
At specific time intervals during storage, each sample (10 g) was homogenized in 90 mL of 0.85% NaCl for 2 min; 1 mL of the solution was diluted with 9 mL of 0.85% NaCl, and 0.1 mL aliquots of each sample were plated on eosin methylene blue agar (Difco) aerobically for 24 h at 36 °C. The colonies were counted using an automated colony counter (Scan 300, Interscience, Saint Nom, France). All experiments were performed in triplicate for each strain and at each temperature. Data were used to examine the influence of vinegar N6 on pathogen behavior (growth or survival) in fresh beef; the results are shown as log CFU/g.
Bactericidal treatment of meat with UV-C LED
To assess the combined effect of vinegar N6 and UV-C irradiation, meat samples were subjected to vinegar N6 treatment, followed by UV-C LED irradiation. As vinegar N6 exerted an antibacterial effect at 0.5% in the antibacterial test, this concentration was set as a control, and the samples were irradiated with UV-C LED to confirm whether the antibacterial ability against pathogens was increased. For control conditions, a beef sample contaminated with E. coli and treated with 0.5% vinegar N6 under the same conditions as in the antibacterial test was prepared and subjected to UV-C LED irradiation. An electronic printed circuit board (PCB) with 24 UV-C light-emitting diode modules (UV-C LED, 275 nm) manufactured by Seoulviosys Co., Ltd. (Gyeonggi-do, Korea) was used to connect the LED modules. The samples were treated in a stainless-steel chamber equipped with UV-C LED lights on a PCB attached to the ceiling. The intensity of a distance of 5 cm between the sample and UV-C LED, measured using a UV light instrument (UVC-254SD; Lutron Electronics Co., Inc., PA, USA), was 0.18 mW/cm2. The 3 min exposure resulted in a total fluence of 32.4 mJ/cm2. The UV dosage (mJ/cm2) was calculated for pulse irradiation using Eq. 1:
| 1 |
where I is UV irradiance (mW/cm2) and t is the irradiation duration.
Following UV-C LED treatment, the samples were vacuum-packed and stored at 5 or 10 °C for 28 days. The vacuum packaging conditions, storage conditions, sampling time, and method were the same as those for the antibacterial test of vinegar N6. All experiments were conducted in triplicate.
Specific growth rate of E. coli on fresh beef meat
DM Fit 1.0 curve-fitting software was used to successively fit the survival or growth of pathogenic E. coli as a function of time using the Baranyi equation (Institute of Food Research, Norwich, UK). The equations are as follows (Baranyi and Roberts, 1994):
| 2 |
| 3 |
where y is the logarithm of the cell number (log CFU/g), y0 is the initial cell number, ymax is the final cell number, A is the time variable, μmax is the specific growth rate or inactivation (log per day), q0 is the physiological state of the inoculum, and t is the sampling time. Each experiment was performed twice. The coefficient of determination (R2), obtained using the DM Fit software 3.5, was used to assess the goodness of fit. The specific growth rate (SGR) was calculated as reported by Baranyi and Roberts (1994).
Morphological analysis
Transmission electron microscopy (TEM) was performed to analyze membrane damage in STEC cells following treatments with vinegar N6 alone or in combination with UV-C LED. STEC suspended in TSB were subjected to one of the following three treatments: stationary-phase STEC cells (control) not subjected to any treatment, cells treated with 0.5% vinegar N6, and cells treated for 3 min with 0.5% vinegar N6 and UV-C LED. All bacterial samples were centrifuged for 5 min at 5000 rpm. The cell pellets, thus obtained, were fixed in a modified Karnovsky’s fixative and rinsed three times with 0.05 M sodium cacodylate buffer. After washing, the pellets were post-fixed using 2% osmium tetroxide and 0.1 M sodium cacodylate buffer. The fixed pellets were stained en bloc for 30 min at 4 °C with 0.5% uranyl acetate. The pellets were dehydrated using a graded ethanol series (30%, 50%, 70%, 80%, 90%, and 100%). The ethanol was replaced with propylene oxide. Spurr’s resin was used to embed the fixed cells, which were placed in an oven (Dosaka EM Co., Kyoto, Japan) at 70 °C for 24 h. Each specimen was cut into ultrathin slices using an ultramicrotome (MTX; RMC, AZ, USA) and double-stained for 7 min with 2% uranyl acetate, followed by 7 min incubation with Reynolds’ lead citrate (Reynolds, 1963). The sections were observed using TEM (LIBRA 120; Carl Zeiss, Oberkochen, Germany).
Statistical analysis
ANOVA was used to evaluate the effects of temperature (5 or 10 °C) and concentration (vinegar N6: 0.5%, 1%, or 2%). A randomized block design was employed for each ANOVA, with the other variable pairs set as a block. Tukey’s honestly significant difference (HSD) was used for pairwise comparisons to determine the significant effects of the combination treatment of vinegar N6 and UV-C LED. The level of significance was set at p < 0.05. Python (3.7.7) was used for all statistical analyses.
Results and discussion
Prevalence of STEC and EHEC in meat samples
Among the 310 beef samples, 38 samples (12.26%) were contaminated with STEC, which were positive for stx1 and/or stx2 and negative for eae—26 samples (8.39%) were positive only for stx2, 8 (2.59%) were positive for both stx1 and stx2, and 4 (1.29%) were positive only for stx1. For EHEC (harboring eae with stx1 and/or stx2) detected in beef, the detection rate was 5.81% (18 samples)—10 samples (3.23%) were positive only for stx2, 7 (2.26%) were positive for both stx1 and stx2, and 1 (0.32%) was positive only for stx1 (Table 2). The positivity rate for STEC did not have significant deviation over the three years, being 23 (21.2%), 18 (16.1%), and 13 (16.3%) in 2019, 2020, and 2021, respectively (data not shown), indicating the continuous presence of the risk.
Table 2.
Shiga toxin-producing Escherichia coli (STEC) and enterohemorrhagic Escherichia coli (EHEC) contamination (%) in fresh beef samples from Korea
| Pathotype | Virulence gene | Total (%) n = 310 | ||
|---|---|---|---|---|
| STEC | stx1 | stx2 | stx1: stx2 | 38 (12.26) |
| 4 (1.29) | 26 (8.39) | 8 (2.59) | ||
| EHEC | stx1: eae | stx2: eae | stx1: stx2: eae | 18 (5.81) |
| 1 (0.32) | 10 (3.23) | 7 (2.26) | ||
A detection rate of 22.6% was reported for STEC in fresh beef samples from South Korea (Lee et al., 2009). Variations in contamination levels may be due to national or regional characteristics of meat suppliers, processing conditions, and analytical parameters, such as volume, quantity, and duration (Kegode et al., 2008). In Switzerland, STEC was detected in up to 1.75% of minced beef samples (Fantelli and Stephan, 2001), whereas in France, Pradel et al. (2000) detected STEC in 4.0% of the tested beef samples.
In the present study, the detection rate of STEC strains without eae was 12.26%. This was significantly higher than the detection rate of 5.81% for EHEC harboring eae, indicating that STEC should be managed as a potential food poisoning bacterium. STEC strains negative for eae have been isolated from humans with HUS (Paton et al., 1999). As the most representative example, the absence of eae is a feature of O104 strains causing disease outbreaks in humans (O104:H4 German outbreak and O104:H21 Montana outbreak strains) (Feng et al., 2001; Scheutz et al., 2011).
The detection rate of EHEC varies according to region and time and shows sporadic data patterns. EHEC has been identified in beef at rates between 1.8 and 19.0% (Barlow et al., 2006; Willshaw et al., 1993). In Iran, the EHEC strains were identified in various host samples, including the feces and carcasses of various types of livestock, such as sheep, goats, camels, and birds, with the highest incidence reported in bovine samples (Jajarmi et al., 2017).
For both STEC and EHEC, the positive rate of stx2 was the highest, followed by that of both stx1 and stx2, and only stx1 (all EHEC strains included eae). These findings for samples from Korea corroborate with those of Lee et al. (2009), who reported that 9 of 14 EHEC isolates were positive for only stx2, 2 were positive for both stx1 and stx2 as well as stx2 and eaeA, and 1 was positive for both stx1 and eaeA. Moreover, previous research in another region revealed different ratios for the virulence genes. In Iran, among 149 cattle samples positive for STEC virulence genes based on PCR detection, stx1 alone was found in 46 (31%) samples, stx2 alone in 36 (24%) samples, whereas both the genes were detected in 67 (45%) samples (Kagambèga et al., 2012b). Differences in geographical locations, processing conditions, and analytical parameters contribute to the variability in the prevalence of virulence genes among STEC and EHEC isolates. stx2 is commonly associated with HUS, whereas stx1 is associated with diarrhea or asymptomatic infection (Burnens et al., 1992). Some strains harbor both stx1 and stx2, which may enhance the potency of Shiga toxins (Friedrich et al., 2002). Therefore, in a region with a high ratio of only stx2 and both stx1 and stx2, such as Korea, the risk of food poisoning caused by STEC and EHEC contamination is high, warranting the need for proper management.
Antimicrobial activity of vinegar N6 against pathogenic E. coli in raw beef meat
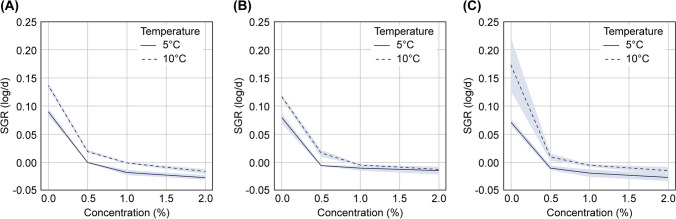
As a control strategy for these pathogens, in this study, the antibacterial efficacy of vinegar N6, a “clean label” natural fermented vinegar used as a preservative in the processing or storage of raw beef products, was investigated. A significant correlation was noted between the concentration of vinegar N6 and temperature for STEC (p = 2.12e − 06, Fig. 1A and Supplementary Table S1). At both temperatures, the SGR decreased significantly upon vinegar N6 treatment at concentrations over 0.5% (both p = 0.001, Fig. 1 and Supplementary Table S2). At 5 °C, the SGR of STEC decreased from 0.089 log/d (without vinegar N6) to − 0.001 log/d (with 0.5% vinegar N6), and the pathogen population was maintained at initial levels throughout the storage period. At 10 °C, the SGR of STEC decreased significantly from 0.137 log/d (without vinegar N6) to 0.019 log/d (with 0.5% vinegar N6) (p = 0.001), although this was not indicative of the STEC-killing efficacy. Taken together, these results indicate that vinegar N6 was effective at concentrations of at least 0.5%. At 5 °C, the absolute SGR was lower upon treatment with 1% and 2% vinegar N6 than it was upon treatment with 0.5% vinegar N6. However, a significant difference was observed only upon 2% vinegar N6 treatment (p = 0.001). Similarly, at 10 °C, there was a significant difference between only 2% vinegar N6 and 0.5% vinegar N6 treatments (p = 0.001). The results revealed a complete inhibitory effect at 2% vinegar N6 at both temperatures; however, the antibacterial effect was similar to that of 0.5% and 1% vinegar N6, which indicated that 0.5% is a reasonable concentration, considering the efficient antibacterial effect.
Fig. 1.
Variations in the growth rate of pathogenic Escherichia coli with temperature and vinegar N6 concentration (0–2%). (A) STEC; (B) E. coli O157:H7; (C) EPEC. STEC, Shiga toxin-producing E. coli; EPEC, enteropathogenic E. coli; SGR, specific growth rate
Similarly, for E. coli O157:H7, a significant reduction in SGR upon treatment with > 0.5% vinegar N6 concentration was observed at both temperatures (p < 0.05, Fig. 1B and Supplementary Table S3). When 0.5% vinegar N6 was applied, the SGR of E. coli O157:H7 decreased significantly from 0.078 and 0.103 (control, log/d) to − 0.007 and 0.015 (0.5% vinegar N6, log/d) at 5 and 10 °C, respectively. Unlike STEC, the SGR of E. coli O157:H7 was lower at both 1% and 2% vinegar N6 than at 0.5% under each temperature treatment; however, these changes were not significant. Furthermore, no significant difference in SGR reduction was observed between 1 and 2% vinegar N6 treatments for E. coli O157:H7 at both temperatures (p > 0.05). Therefore, the antimicrobial efficacy of vinegar N6 was similar at concentrations of 0.5% and above 1%.
For EPEC, vinegar N6 concentration and storage temperature had a significant effect, but the degree of significance was the lowest among the three pathogens (p = 0.000004, Fig. 1C and Supplementary Table S4). At 5 °C, the SGR of EPEC treated with 0.5% vinegar N6 (SGR = − 0.009 log/d) was lower than that of EPEC not treated with vinegar N6 (SGR = 0.073 log/d). Similar to the findings for STEC and E. coli O157:H7, the SGR of EPEC differed significantly between the control and 0.5% vinegar N6 groups (p = 0.001) at 5 °C. When 0.5% vinegar N6 was added to beef at 10 °C, the SGR decreased significantly from 0.174 to 0.010 (log/d), maintaining initial levels without EPEC growth. Therefore, although all three types of pathogenic E. coli responded significantly to temperature and antibacterial vinegar N6 concentration, STEC responded to the greatest extent, followed by E. coli O157:H7 and EPEC.
Altogether, the findings in this study suggest that vinegar N6 is a label-friendly chemical that significantly suppresses the growth of pathogenic E. coli, enabling primary prevention when raw beef is maintained at cold temperatures. Being acetate-rich, vinegar N6 dose-dependently inhibited the growth of pathogenic E. coli in beef samples. Acetate exerts antibacterial effects against E. coli, which is mediated via the entry of undissociated acetic acid through the bacterial membrane, leading to intracellular acidification (Adams and Hall, 1988). A decrease in cytosolic pH is an early event that potently triggers apoptosis in thymineless death (Ketcham et al., 2022) Although direct comparisons among studies are challenging owing to differences in experimental parameters, the ability of acetate to prolong lag time and decrease the growth rate of pathogenic E. coli has been validated. Thus, vinegar N6 is a promising agent for protection against bacterial contamination. Nevertheless, further research is required to determine whether the antimicrobial activities of vinegar N6 improve the overall quality and shelf-life of treated meats.
Combination of vinegar N6 and UV-C LED irradiation control pathogenic E. coli in raw beef samples
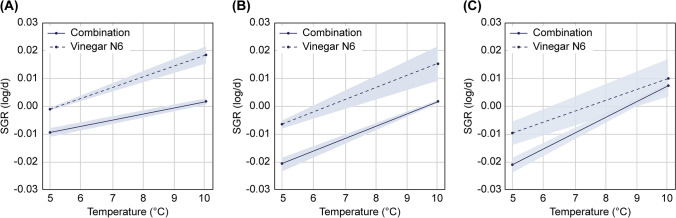
Although 0.5% vinegar N6 suppressed the growth of pathogenic E. coli, all three strains survived for up to 28 days, suggesting that additional treatment is required to limit pathogen survival during storage. Thus, the additive effect of bactericidal UV-C LED irradiation with vinegar N6 was investigated. The combination of UV-C LED (275 nm)-induced killing and vinegar N6-induced growth inhibition represents a viable strategy for control.
The results for the three pathogenic E. coli strains are shown in Fig. 2. The UV-C LED treatment and temperature affected the fluctuations in the SGR of STEC, E. coli O157:H7, and EPEC (p = 5.193e − 7, 0.000017, and 0.000055, respectively). The most significant difference was observed for STEC, followed by that for E. coli O157:H7 and EPEC, similar to the findings in the vinegar N6 experiments. When UV-C LED irradiation was combined with vinegar N6 treatment, the SGR of STEC significantly decreased from − 0.001 and 0.019 (log/d) to − 0.010 and 0.002 (log/d) at 5 and 10 °C, respectively (Table 3). Similar to the findings for STEC, there was a significant difference between the effects of vinegar N6 alone and the vinegar N6 and UV-C LED combination (p < 0.05) on E. coli O157:H7 at both temperatures. No significant difference was observed in the SGR of EPEC between vinegar N6 alone and vinegar N6 combined with UV-C treatments at both temperatures. Nevertheless, the absolute SGR of EPEC decreased under LED treatment, indicative of an additive antibacterial effect of UV-C. Our findings revealed the beneficial effect of UV-C LED and vinegar N6 on E. coli inactivation, with the maximum effect of this combination observed for STEC.
Fig. 2.
Efficacy of treatment of raw beef samples with UV-C light-emitting diode and 0.5% vinegar N6 combination in reducing the specific growth rate of pathogenic Escherichia coli. (A) STEC; (B) E. coli O157:H7; (C) EPEC. STEC, Shiga toxin-producing E. coli; EPEC, enteropathogenic E. coli; SGR, specific growth rate
Table 3.
Specific growth rate of pathogenic Escherichia coli in raw beef treated with vinegar N6 alone or with UV-C LED and vinegar N6
| Temp (°C) | Treatment | STECa | p | E. coli O157:H7 | p | EPECb | p |
|---|---|---|---|---|---|---|---|
| 5 | Vinegar N6 | − 0.001 ± 0.000 | 0.003 | − 0.007 ± 0.001 | 0.009 | − 0.009 ± 0.004 | 0.062 |
| Combination | − 0.010 ± 0.001c | − 0.020 ± 0.002c | − 0.020 ± 0.003 | ||||
| 10 | Vinegar N6 | 0.019 ± 0.003 | 0.001 | 0.015 ± 0.006 | 0.009 | 0.010 ± 0.007 | 0.900 |
| Combination | 0.002 ± 0.001c | 0.002 ± 0.001c | 0.009 ± 0.002 |
Values (n = 3) are presented as mean ± standard deviation
aShiga toxin-producing Escherichia coli
bEnterohemorrhagic Escherichia coli
cMeans within columns under identical conditions with different superscripts are significantly different (p < 0.05)
The use of UV-C LED irradiation has several benefits over chemical and physical treatments, which are frequently employed, including shorter contact periods, no by-product formation, and no toxicity. The performance of UV-C LEDs will continue to improve with advances in semiconductor and reactor designs, making the introduction of the technology in the food industry realistic. LEDs have several advantages over UV lamps; they emit light of various wavelengths, are mercury-free, have a small and stable structure, have a quicker start-up time, and operate at lower voltages (Würtele et al., 2011). Pathogen inactivation may be improved owing to varied wavelength outputs and compact reactor structure. Furthermore, the increased efficiency may contribute to power savings.
The disinfection efficacy of UV LEDs at different wavelengths has been investigated in several studies. UV inactivation of bacteria is dose-dependent (Song et al., 2016); E. coli inactivation is affected by the incident irradiance and exposure period at longer wavelengths (UV-LED 275, UV-LED 285, and UV-LED 295). Increased irradiance and short duration of exposure result in higher inactivation efficacy against certain microbes, such as E. coli, suggesting that UV-induced E. coli inhibition is influenced by biological mechanisms besides photochemical reactions (Harm, 1980; Song et al., 2016). Therefore, as a new technology, UV-LED induces a higher rate of log inactivation. For T7 bacteriophage and E. coli inhibition, UV LED emitting 275 nm was more efficient than that that emitting 255 nm. Oguma et al. (2002) reported that MP UV emissions at wavelengths ranging from 220 to 300 nm inhibit photorepair in E. coli, presumably by disturbing the endogenous photolyase, a DNA repair enzyme.
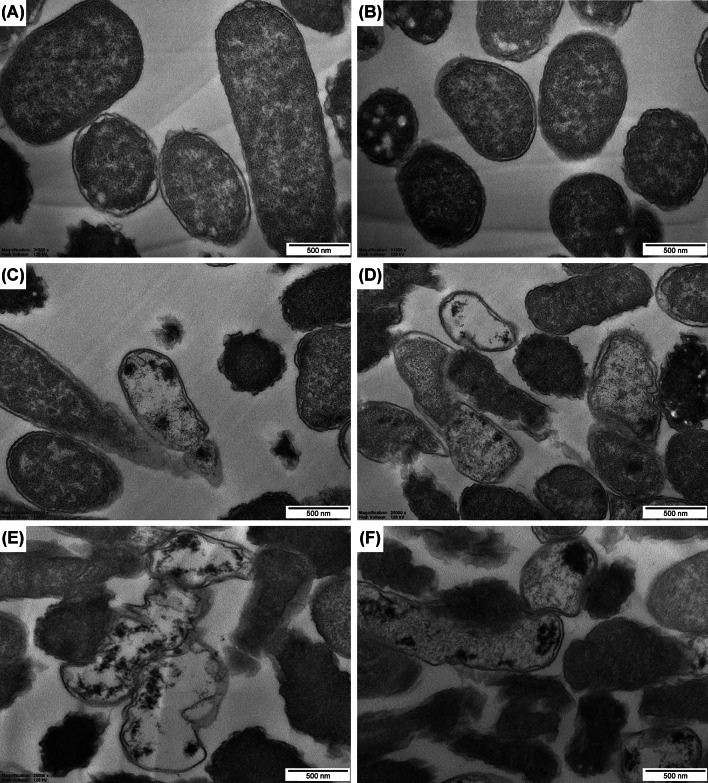
TEM micrographs of STEC treated with vinegar N6 and UV-C LED
TEM was utilized to examine the morphological changes in STEC cells following exposure to vinegar N6 and UV-C LED treatments. The growth-ceased cells (control) exhibited normal morphology with a typical surface and cytoplasm (Fig. 3A, B). In contrast, destruction of the membrane and cell death were observed following vinegar N6 treatment because the cells could not retain their structures, such as the wall, cortex, and coat (Fig. 3C, D). These effects could be attributable to acetate, which is a key component of vinegar N6. The ratio of normal cells was slightly higher than that of cells treated with vinegar N6 in combination with UV-C LED. The morphological damage caused by the combined treatment was greater than that caused by vinegar N6 treatment alone (Fig. 3E, F). UV-C LED treatment caused STEC cells to lose their structure and wall. The altered cell morphology included partial breakdown of membranes and outflow of cytoplasmic contents. These findings suggest that UV-C LED irradiation suppressed the survival of STEC cells.
Fig. 3.
Transmission electron microscopy of STEC cells. (A, B) Stationary cells (control); (C, D) Cells treated with 1% vinegar N6; (E, F) Cells subjected to combined treatment with vinegar N6 and UV-C LED for 3 min. STEC, Shiga toxin-producing E. coli
The combined treatment with 0.5% vinegar N6 and UV-C LED under the tested conditions provides an effective strategy for suppressing the growth of pathogenic E. coli in beef meat. Morphological analysis of STEC treated with vinegar N6 alone or in combination with UV-C LED revealed cell damage, with the degree of damage being greater under combined treatment. The current findings indicate that UV-C LEDs can inactivate microorganisms that contaminate beef products. Mechanistically, the combined treatment with vinegar N6 and UV-C LED enhanced growth inhibition and killing of pathogenic E. coli in beef samples, which should ensure safety during the production and supply of meat. This strategy has potential in the meat production market, and should promote the provision of safe food to consumers. However, as the antibacterial effect is influenced by temperature and other factors, additional research is warranted. Moreover, whether the sterilizing effect of UV-C LED alone is sufficient remains to be investigated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This study was funded by the Pulmuone Institute of Technology as a part of their annual research budget; hence, no grant number is available.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eun Young Ro, Email: eyro@pulmuone.com.
Mi Ri Choi, Email: miri.choi@pulmuone.com.
Young Min Park, Email: ymparka@pulmuone.com.
Sang Gu Kim, Email: sgkimn@pulmuone.com.
Sang Yun Lee, Email: sylee@pulmuone.com.
References
- Adams M, Hall CJ. Growth inhibition of food-borne pathogens by lactic and acetic acids and their mixtures. International Journal of Food Science & Technology. 1988;23:287–292. doi: 10.1111/j.1365-2621.1988.tb00581.x. [DOI] [Google Scholar]
- Baranyi J, Roberts TA. A dynamic approach to predicting bacterial growth in food. International Journal of Food Microbiology. 1994;23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Barlow RS, Gobius KS, Desmarchelier PM. Shiga toxin-producing Escherichia coli in ground beef and lamb cuts: Results of a one-year study. International Journal of Food Microbiology. 2006;111:1–5. doi: 10.1016/j.ijfoodmicro.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Bhaskar S. Foodborne diseases—disease burden. In: Dudeja P, Gupta RK, Minhas AS, editors. Food safety in the 21st century. Cambridge: Academic Press; 2016. pp. 1–10. [Google Scholar]
- Burnens AP, Boss P, Ørskov F, et al. Occurrence and phenotypic properties of verotoxin producing Escherichia coli in sporadic cases of gastroenteritis. European Journal of Clinical Microbiology and Infectious Diseases. 1992;11:631–634. doi: 10.1007/BF01961673. [DOI] [PubMed] [Google Scholar]
- Dickson JS, Anderson ME. Microbiological decontamination of food animal carcasses by washing and sanitizing systems: A review. Journal of Food Protection. 1992;55:133–140. doi: 10.4315/0362-028X-55.2.133. [DOI] [PubMed] [Google Scholar]
- Ewing WH, Edwards PR. Edwards and Ewing’s identification of enterobacteriaceae. 4. New York: Elsevier; 1986. [Google Scholar]
- Fantelli K, Stephan R. Prevalence and characteristics of shigatoxin-producing Escherichia coli and Listeria monocytogenes strains isolated from minced meat in Switzerland. International Journal of Food Microbiology. 2001;70:63–69. doi: 10.1016/S0168-1605(01)00515-3. [DOI] [PubMed] [Google Scholar]
- Feng P, Weagant SD, Monday SR. Genetic analysis for virulence factors in Escherichia coli O104: H21 that was implicated in an outbreak of hemorrhagic colitis. Journal of Clinical Microbiology. 2001;39:24–28. doi: 10.1128/JCM.39.1.24-28.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Food Additives Convention. General test methods, microbial test methods, enterohaemorrhagic E. coli. Available from: https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=387. Accessed Dec. 20, 2021
- Friedrich AW, Bielaszewska M, Zhang WL, et al. Escherichia coli harboring Shiga toxin 2 gene variants: Frequency and association with clinical symptoms. Journal of Infectious Diseases. 2002;185:74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- Harm W. Biological effects of ultraviolet radiation. 1. Cambridge: University Press; 1980. [Google Scholar]
- Heir E, Liland KH, Carlehög M, et al. Reduction and inhibition of Listeria monocytogenes in cold-smoked salmon by verdad N6, a buffered vinegar fermentate, and UV-C treatments. International Journal of Food Microbiology. 2019;291:48–58. doi: 10.1016/j.ijfoodmicro.2018.10.026. [DOI] [PubMed] [Google Scholar]
- Jajarmi M, Imani Fooladi AAI, Badouei MA, et al. Virulence genes, Shiga toxin subtypes, major O-serogroups, and phylogenetic background of Shiga toxin-producing Escherichia coli strains isolated from cattle in Iran. Microbial Pathogenesis. 2017;109:274–279. doi: 10.1016/j.micpath.2017.05.041. [DOI] [PubMed] [Google Scholar]
- Kagambèga A, Martikainen O, Lienemann T, et al. Diarrheagenic Escherichia coli detected by 16-plex PCR in raw meat and beef intestines sold at local markets in Ouagadougou, Burkina Faso. International Journal of Food Microbiology. 2012;153:154–158. doi: 10.1016/j.ijfoodmicro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Kagambèga A, Martikainen O, Siitonen A, et al. Prevalence of diarrheagenic Escherichia coli virulence genes in the feces of slaughtered cattle, chickens, and pigs in Burkina Faso. Microbiologyopen. 2012;1:276–284. doi: 10.1002/mbo3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegode RB, Doetkott DK, Khaitsa ML, et al. Occurrence of Campylobacter species, Salmonella species and generic Escherichia coli in meat products from retail outlets in the Fargo metropolitan area. Journal of Food Safety. 2008;28:111–125. doi: 10.1111/j.1745-4565.2007.00099.x. [DOI] [Google Scholar]
- Ketcham A, Freddolino PL, Tavazoie S. Intracellular acidification is a hallmark of thymineless death in E. coli. PLoS Genetics. 2022;18:e1010456. doi: 10.1371/journal.pgen.1010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GY, Jang HI, Hwang IG, et al. Prevalence and classification of pathogenic Escherichia coli isolated from fresh beef, poultry, and pork in Korea. International Journal of Food Microbiology. 2009;134:196–200. doi: 10.1016/j.ijfoodmicro.2009.06.013. [DOI] [PubMed] [Google Scholar]
- McDaniel TK, Jarvis KG, Donnenberg MS, et al. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Food and Drug Safety. Korean food code. MFDS Notification; No. 4.16; MFDS: Cheongju, Korea (2021)
- Moore K, Damrow T. Outbreak of acute gastroenteritis attributable to Escherichia coli serotype O104: H21—Helena. Morbidity and Mortality Weekly Report. 1995;44:501–503. [PubMed] [Google Scholar]
- Oguma K, Katayama H, Ohgaki S. Photoreactivation of Escherichia coli after low- or medium-pressure UV disinfection determined by an endonuclease sensitive site assay. Applied and Environmental Microbiology. 2002;68:6029–6035. doi: 10.1128/AEM.68.12.6029-6035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton AW, Woodrow MC, Doyle RM, et al. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. Journal of Clinical Microbiology. 1999;37:3357–3361. doi: 10.1128/JCM.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinela J, Ferreira ICFR. Nonthermal physical technologies to decontaminate and extend the shelf-life of fruits and vegetables: Trends aiming at quality and safety. Critical Reviews in Food Science and Nutrition. 2017;57:2095–2111. doi: 10.1080/10408398.2015.1046547. [DOI] [PubMed] [Google Scholar]
- Pradel N, Livrelli V, De Champs C, et al. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. Journal of Clinical Microbiology. 2000;38:1023–1031. doi: 10.1128/JCM.38.3.1023-1031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley LW, Remis RS, Helgerson SD, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. New England Journal of Medicine. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Salaheen S, Kim SW, Karns JS, et al. Metagenomic analysis of the fecal microbiomes from Escherichia coli O157: H7-shedding and non-shedding cows on a single dairy farm. Food Control. 2019;102:76–80. doi: 10.1016/j.foodcont.2019.03.022. [DOI] [Google Scholar]
- Scheutz F, Nielsen EM, Frimodt-Møller J, et al. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104: H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Eurosurveillance. 2011;16:19889. doi: 10.2807/ese.16.24.19889-en. [DOI] [PubMed] [Google Scholar]
- Song K, Mohseni M, Taghipour F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Research. 2016;94:341–349. doi: 10.1016/j.watres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Song K, Taghipour F, Mohseni M. Microorganisms inactivation by continuous and pulsed irradiation of ultraviolet lightemitting diodes (UV-LEDs) Chemical Engineering Journal. 2018;343:362–370. doi: 10.1016/j.cej.2018.03.020. [DOI] [Google Scholar]
- Willshaw GA, Smith HR, Roberts D, et al. Examination of raw beef products for the presence of Vero cytotoxin producing Escherichia coli, particularly those of serogroup O157. Journal of Applied Bacteriology. 1993;75:420–426. doi: 10.1111/j.1365-2672.1993.tb02797.x. [DOI] [PubMed] [Google Scholar]
- Würtele MA, Kolbe T, Lipsz M, et al. Application of GaN-based ultraviolet-C light emitting diodes–UV LEDs–for water disinfection. Water Research. 2011;45:1481–1489. doi: 10.1016/j.watres.2010.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.